Abstract
Calcium-dependent protein kinase (CDPKs) is one of the calcium-sensing proteins in plants. They are likely to play important roles in growth and development and abiotic stress responses. However, these functions have not been explored in sweet potato. In this study, we identified 39 CDPKs in cultivated hexaploid sweet potato (Ipomoea batatas, 2n = 6x = 90), 35 CDPKs in diploid relative Ipomoea trifida (2n = 2x = 30), and 35 CDPKs in Ipomoea triloba (2n = 2x = 30) via genome structure analysis and phylogenetic characterization, respectively. The protein physiological property, chromosome localization, phylogenetic relationship, gene structure, promoter cis-acting regulatory elements, and protein interaction network were systematically investigated to explore the possible roles of homologous CDPKs in the growth and development and abiotic stress responses of sweet potato. The expression profiles of the identified CDPKs in different tissues and treatments revealed tissue specificity and various expression patterns in sweet potato and its two diploid relatives, supporting the difference in the evolutionary trajectories of hexaploid sweet potato. These results are a critical first step in understanding the functions of sweet potato CDPK genes and provide more candidate genes for improving yield and abiotic stress tolerance in cultivated sweet potato.
1. Introduction
Ca2+ is an important second messenger in plants, and signaling pathways mediated by Ca2+ have been shown to play an important role in plant growth and development in response to abiotic and biotic stresses [1,2]. When cells sense external changes, the Ca2+ concentration in the cytoplasm changes, resulting in a series of physiological and biochemical reactions to plant tolerance improvement. There are three families of calcium-sensing proteins in plants, including calmodulin (CaM) and calmodulin-like protein (CaML), calcium-dependent protein kinase (CDPK), and calcineurin B-like proteins (CBLs)/CBL-interacting proteins (CIPK) [3,4,5,6,7]. Among these sensors, CDPKs are Ser/Thr protein kinases, serving as special sensors as they can directly convert upstream Ca2+ signals into downstream protein phosphorylation events [8]. Genome-wide analysis led to the identification of CDPK genes in various plant species. There are 34 genes in Arabidopsis thaliana [3], 31 in rice (Oryza sativa) [9], 20 in wheat (Triticum aestivum) [10], 35 in maize (Zea mays) [11], 29 in poplar (Populus trichocarpa) [12], and 19 in grape (Vitis spp.) [13].
CDPKs (CPKs) are single peptide chains with four typical domains: variable region, catalytic region (kinase region), linker region (autoinhibitory region), and regulatory region (calmodulin-like region/CaM-LD) [3,14]. The variable region is poorly conserved and generally contains 20 to 200 amino acid residues that may be involved in substrate recognition. Most CDPKs contain a palmitoylation site (cysteine residue at position 4 or 5) and a myristoylation site (glycine residue at position 2) related to membrane localization at the N-terminus [15]. The catalytic region includes all 11 highly conserved subdomains of eukaryotic Ser/Thr protein kinases, including the conserved Lys residue located in the second subdomain, which may be the binding site for ATP [16,17]. The linker region is composed of the most conserved 31 amino acids rich in basic amino acids, which can bind to the catalytic region to inhibit kinase activity, and also has a binding site to the regulatory region [17,18] which generally contains 1 to 4 (mostly 4) EF chiral structures that bind to Ca2+ [19].
Recent studies have shown that CDPK genes are involved in plant growth and development along with abiotic and biotic stress through hormone signaling [20,21,22,23,24,25,26]. In Medicago truncatula, silencing CDPK1 expression resulted in significantly reduced root hair and root cell lengths [27]. Overexpression of BnaCPK2 induced ROS accumulation and cell death [28]. AtCPK17 and AtCPK34 transduced Ca2+ signals to increase the rate of pollen tube tip growth [29]. PnCDPK1 was involved in Pharbitis nil flowering [30]. As a regulatory component, CPK28 from Arabidopsis thaliana regulated stem elongation and vascular development [31]. OsCPK7 was induced by cold and salt stress, and its overexpression increased cold, salt, and drought tolerance [32]. AtCPK32 interacted with ABF4 and its overexpression affected ABA sensitivity [33]. The expression of OsCDPK13 was increased in leaf sheath segments under gibberellin treatment or cold stress, suggesting that OsCDPK13 might be an important signaling component of rice seedlings in response to gibberellins under cold stress conditions [34]. However, little is known about CDPKs in sweet potato.
Sweet potato (Ipomoea batatas (L.) Lam.), as an important food and feed crop, as well as an industrial and energy raw material [35], ranks 8th in terms of world food production [36]. Sweet potato is hexaploid (2n = 6x = 90) [37], with complex genomes, hybrid incompatibility, lack of germplasm resources, susceptibility to diseases and insect pests, etc. It is of great significance to enhance the adaptability of sweet potato to saline-alkali land and improve the yield of sweet potato through molecular mechanisms. Since 2017, the genome of sweet potato has been sequenced, assembled, and released, including a hexaploid sweet potato Taizhong 6 [37] and two diploid species Ipomoea trifida NCNSP0306 (2n = 2x = 30) and Ipomoea triloba NCNSP0323 (2n = 2x = 30) (Figure S1) [38]. Using whole-genome sequence data in sweet potato to identify and analyze important gene families is feasible.
In this research, a total of 109 CDPKs (i.e., 39 in I. batatas, 35 in I. trifida, and 35 in I. triloba) were identified from the cultivated hexaploid sweet potato and its two diploid relatives. They were classified into five subgroups. We systematically investigated the protein physiological property, chromosome localization, phylogenetic relationship, conserved motifs, cis-elements of the promoter, and protein interaction network of CDPKs in sweet potato. Moreover, the tissue specificity and expression pattern analysis for hormone response and abiotic stress of CDPKs were analyzed by qRT-PCR and RNA-seq. The evolution, different functions on development, hormone crosstalk, and abiotic stress response were also discovered between sweet potato and its two diploid relatives.
2. Results
2.1. Genome-Wide Identification and Characteristic of CDPKs Family in Sweet Potato and Its Two Diploid Relatives
In order to identify all CDPKs in sweet potato and its two diploid relatives, two typical strategies (i.e., hmmersearch, SMART, and CD-search databases) were employed. A total of 39, 35, and 35 CDPKs were identified in I. batatas, I. trifida, and I. triloba, respectively (named after “Ib”, “Itf”, “Itb”). The physicochemical properties of CDPKs were analyzed using the sequences from I. batatas (Table 1). The CDS length of IbCDPKs varied from 1056 bp (IbCDPK25.4) to 3513 bp (IbCDPK16). The amino acid lengths of IbCDPKs were distributed from 351 aa (IbCDPK25.4) to 1170 aa (IbCDPK16), the molecular weight (MW) ranged from 38.732 kDa to 130.824 kDa, and the isoelectric point (pI) varied from 4.75 (IbCDPK25.4) to 9.28 (IbCDPK28). Most IbCDPKs contained 4 EF-hands, except IbCDPK12.3, IbCDPK18, IbCDPK24, IbCDPK25.4, IbCDPK29.3, IbCDPK32, IbCDPK35. Ten IbCDPKs have no myristoylation sites in N-terminus (i.e., IbCDPK 1, IbCDPK11.2, IbCDPK11.3, IbCDPK12.1, IbCDPK12.2, IbCDPK12.3, IbCDPK24, IbCDPK25.1, IbCDPK29.1, IbCDPK35) and eight IbCDPKs have no palmitoylation sites (i.e., IbCDPK1, IbCDPK11.1, IbCDPK12.1, IbCDPK12.3, IbCDPK24, IbCDPK25.1, IbCDPK29.1, IbCDPK33.1).

Table 1.
Characterization of IbCDPKs in sweet potato.
All the CDPKs were separately mapped on 15 chromosomes of I. batatas, I. trifida, and I. triloba (Figure 1). In the I. batatas genome, 39 IbCDPKs genes were distributed across every chromosome except LG4. In the I. trifida and I. triloba genome, 35 IbCDPKs genes were distributed across every chromosome except Chr13. In I. batatas, four IbCDPKs were detected on LG1, three on LG2, three on LG3, three on LG5, four on LG6, three on LG7, one on LG8, three on LG9, two on LG10, one on LG11, two on LG12, one on LG13, six on LG14, and three on LG15 (Figure 1A). The numbers of Itf/ItbCDPKs located on the chromosomes were the same in two diploid relatives. One CDPK was detected on Chr01, Chr02, and Chr11; two on Chr04, Chr06, Chr07, Chr08, and Chr14; three on Chr03, Chr10, and Chr12; four on Chr05 and Chr15; and five on Chr09 (Figure 1B,C). The results indicated that the distribution of CDPKs was different on chromosomes in sweet potato and its two diploid relatives, whereas it was similar in two diploid relatives.

Figure 1.
Chromosomal localization and distribution of CDPK genes in I. batatas (A), I. trifida (B), and I. triloba (C). The bars on the left margin represent chromosomes. The chromosome numbers are displayed on the left side of the chromosomes, and the gene names are displayed on the right side. Detail chromosomal location information is listed in Table S1.
2.2. Phylogenetic Relationship of CDPKs in Sweet Potato and Its Two Diploid Relatives
To study the evolutionary relationship of CDPKs in I. batatas, I. trifada, I. triloba, and Arabidopsis, we constructed a phylogenetic tree for 143 CDPKs (i.e., 39 in I. batatas, 35 in I. trifida, 35 in I. triloba, and 34 in Arabidopsis) (Figure 2). All CDPKs were unevenly distributed and divided into five subgroups (group I to V) according to the evolutionary distance. The specific distribution of CDPKs was as follows (total: I. batatas, I. trifida, I. triloba, Arabidopsis): group I (54:16, 14, 14, 10); group II (42:11, 9, 9, 13); group III (32:8, 8, 8, 8); group IV (12:3, 3, 3, 3), and group V (3:1, 1, 1, 0) (Figure 2; Table S1). We named IbCDPKs, ItfCDPKs, and ItbCDPKs based on their homology with homologs in Arabidopsis, and AtCDPK15/19/21/22/23/27/31 from Arabidopsis have no homologous protein in I. batatas, I. trifada, I. triloba, and only Ib/Itf/ItbCDPK35 have no homologous proteins in Arabidopsis. One additional IbCDPK sequence (IbCDPK25.4) was identified in I. batatas that had no homology protein in I. trifida and I. triloba. Two sequences (IbCDPK34.1 and IbCDPK34.2) in I. batatas were homologous with ItfCDPK34 and ItbCDPK34. We speculated that large differences in number and type of CDPKs divided in five subgroups between Arabidopsis and sweet potato and its two diploid relatives were due to species specificity. Moreover, the discrepancy showed in sweet potato and its two diploid relatives might be due to chromosomal hybridization during evolution.
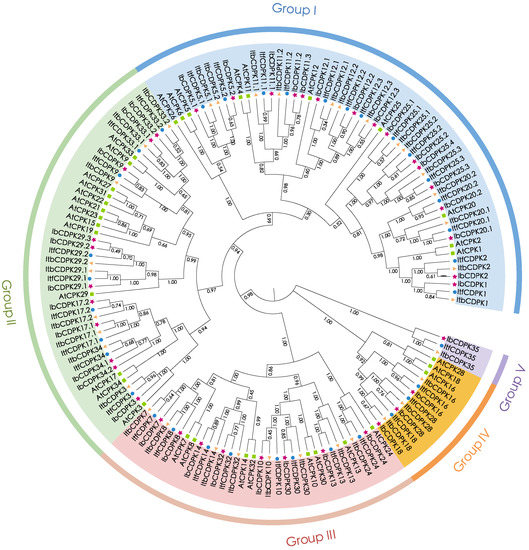
Figure 2.
Phylogenetic analysis of the CDPK family in I. batatas, I. trifida, I. triloba, and Arabidopsis. A total of 143 CDPKs were divided into five subgroups (group I to V) according to the evolutionary distance. The pink pentagrams, blue cycles, yellow triangles, and green squares represent IbCDPKs in I. batatas, ItfCDPKs in I. trifida, ItbCDPKs in I. triloba, and AtCPKs in Arabidopsis, respectively.
2.3. Conserved Motif and Exon-Intron Structure Analysis of CDPKs in Sweet Potato and Its Two Diploid Relatives
To illustrate the structural characteristics of the 109 CDPKs from I. batatas, I. trifida, and I. triloba, we performed motif and domain analysis using the MEME website (Figure 3). A total of 10 motifs were identified (Figure 3A and Figure S2). Overall, the protein structure of this family was relatively conserved, and most of the CDPKs contained six protein kinase domains and four EF-hands (three EF-hand_1 and one EF-hand_2) except ItbCDPK13. Most CDPKs contained even numbers of EF-hand except IbCDPK11.2, -12.3, -24, -25.3, -29.3, ItfCDPK5.1, and ItbCDPK20.1, -20.2, -25.1. The composition of the EF-hands of Ib/Itf/ItbCDPKs in group V was different, with two EF-hand_1 and no EF-hand_2. The number of protein kinase domains and EF-hands contained in individual protein varied in sweet potato and its two diploid relatives. IbCDPK11.3, -12.1, -20.2, -25.4 in group I, -9, ItbCDPK29.1, IbCDPK29.2, -29.3, -33.1 in group II, IbCDPK13 in group III, and IbCDPK28 in group IV lacked at least one protein kinase domain. In group I, ItfCDPK5.1, ItbCDPK20.1, and ItbCDPK25.1 contained two EF-hand_1 and one EF-hand_2. The C-terminus of IbCDPK12.3 and the N-terminus of ItbCDPK20.2 contained one more EF-hand. Ib/ItbCDPK11.1 lacked one and two protein kinase domains, respectively. Ib/Itf/ItbCDPK25.2 lacked one, two, and two protein kinase domains, respectively. In group II, the composition of the EF-hand of ItbCDPK3 was distinct, with two EF-hand_1 and two EF-hand_2. IbCDPK24 in group III lacked two protein kinase domains and one EF-hand_1. IbCDPK32 lacked one protein kinase domain and two EF-hands. Itf/ItbCDPK34 lacked two and three protein kinase domains, respectively. In group V, Ib/ItfCDPK35 lacked two protein kinase domains and two EF-hands, and ItbCDPK35 lacked three protein kinase domains and two EF-hands.
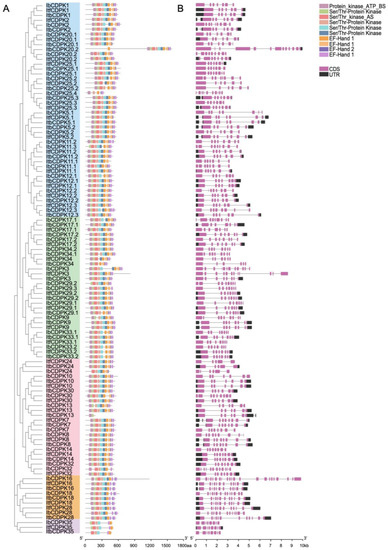
Figure 3.
Conserved motifs and exon–intro structure analysis of IbCDPKs, ItfCDPKs, and ItbCDPKs family. (A) Ten conserved motifs of CDPKs are shown in different colors. (B) Exon–intron structures of IbCDPKs, ItfCDPKs, and ItbCDPKs. The purple boxes, black boxes, and black lines represent the CDS, UTRs, and introns, respectively.
The exon–intron structures of IbCDPKs varied from those of Itf/ItbCDPKs, with the coding DNA sequence (CDS) composition ranging from five to twenty-three exons (Figure 3B). Ib/Itf/ItbCDPKs contained seven to ten, six to eight, and six to thirteen exons in group I; eight to twelve, seven to nine, and six to eight exons in group II; six to twelve, five to eight, and five to eight exons in group III; thirteen to twenty-three, twelve, and twelve exons in group IV; eight, eight, and eight exons in group V, respectively (Figure 3B).
The number of exon–intron structures was different in both sweet potato and its two diploid relatives and varied from I. trifida and I. triloba also. Some CDPKs contained same exon-intron in I. trifida and I. triloba but less than those in I. batata (i.e., Ib/Itf/ItbCDPK2, -CDPK5.1, -CDPK11.1, -CDPK11.2, -CDPK12.2, -CDPK12.3, -CDPK25.1, -CDPK25.2 in group I, -CDPK9, -CDPK29.2 in group II; -CDPK8, -CDPK24, -CDPK30, -CDPK32 in group III; all CDPKs in group IV) (Figure 3B). In group I, ItfCDPK20.2 contained eight exons and its homologous gene (ItbCDPK20.2) in I. triloba contained thirteen exons, whereas IbCDPK20.2 contained nine exons. In group II, IbCDPK3 contained twelve exons, ItbCDPK3 contained nine exons, whereas IbCDPK3 contained eight exons. IbCDPK17.1 contained nine exons, ItbCDPK17.1 contained seven exons, whereas ItfCDPK17.1 contained eight exons (Figure 3B).
2.4. Cis-Element Analysis in the Promoter of IbCDPKs in Sweet Potato
Cis-acting elements are nucleotide sequences that are found upstream or downstream of genes and can regulate their transcription levels. They work through combining with some specific transcription factors when plants respond to various development processes and stresses. To reveal how CDPKs function in growth and development and abiotic stress adaption in sweet potato, 2000 bp upstream sequences of 39 IbCDPKs in I. batatas were extracted and the cis-element analysis was performed using PlantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 30 January 2022). According to the prediction function, all cis-elements were divided into core elements and binding sites, development, light-responsive, hormonal-responsive, and abiotic/biotic stress-responsive elements (Figure 4). The degree of red colors represented the number of cis-elements upstream of the IbCDPKs.
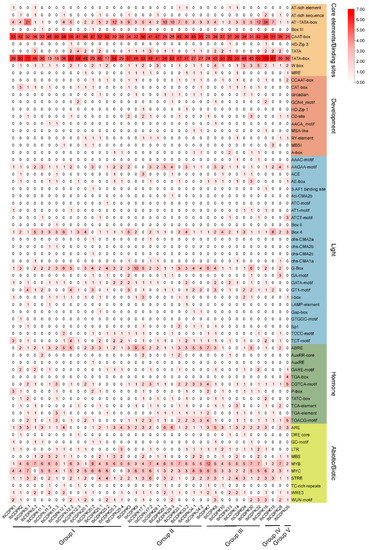
Figure 4.
Cis-elements analysis of IbCDPKs. The cis-elements were divided into five groups. The degree of red colors represents the number of cis-elements upstream of the IbCDPKs.
The majority of 39 CDPKs possessed a large number of core promoter elements and binding sites, such as AT-TATA-box, TATA-box, and CAAT-box (Figure 5). TATA-box and CAAT-box are the binding sites of RNA polymerase and are involved in the transcription initiation and frequency of genes [39]. Some development elements (i.e., CCAAT-box, CAT-box, circadian, O2-site, RY-element, and A-box) were found in IbCDPKs (Figure 4). Light-responsive elements (i.e., AAGAA-motif, Box 4, G-box, CATA-motif, GT1-motif, Sp1, TCCC-motif, and TCT-motif) were found in most of IbCDPKs (Figure 4). In addition, hormonal-responsive elements (i.e., ABA-responsive element ABRE, GARE-motif, MeJA-responsive elements CGTCA-motif and TGACG-motif, GA-responsive elements P-box and TATC-box, SA-responsive element TCA, auxin-responsive element TGA-element) were found. The majority of IbCDPKs except IbCDPK1, IbCDPK12.3, and IbCDPK13, processed at least two hormone-responsive elements (Figure 4). These results indicated that IbCDPKs might be involved in the crosstalk between different hormone signaling pathways. Furthermore, anaerobic induction-responsive element ARE, low temperature-responsive element LTR, drought-responsive elements MBS, MYB and MYC, stress-responsive element STRE, injury and defensive-responsive elements WRE3, and WUN-motif were found in most IbCDPKs (Figure 4). All IbCDPKs processed at least three drought-responsive elements. These results suggested that IbCDPKs might be involved in the crosstalk between hormone signaling pathways to regulate the growth and development and stress adaption in sweet potato, particularly in drought stress.
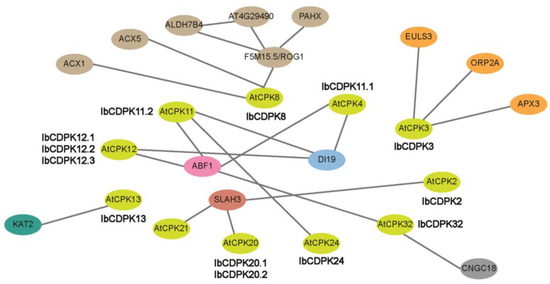
Figure 5.
Protein interaction network of IbCDPKs in I. batatas according to orthologues in Arabidopsis. Network nodes represent proteins, green nodes represent AtCPKs and other colored nodes represent interacting proteins. Lines represent protein–protein interaction which was experimentally determined.
2.5. Protein Interaction Network of IbCDPKs
To explore the potential regulatory network of IbCDPKs, we constructed an IbCDPKs interaction network based on Arabidopsis orthologous proteins (Figure 5). We speculated IbCDPK11 might interact with IbCDPK24. They also interacted with JA biosynthesis-related protein (i.e., ACX1 and ACX5), ABA-responsive element-binding factor 1 (ABF1), potassium channel protein (KAT2), and stomatal movement protein (ELUS3). They could interact with L-ascorbate peroxidase 3 (APX3) and catalase (F5M15.5/ROG1) to scavenge hydrogen peroxide in plants. IbCDPK11.1, IbCDPK11.2, IbCDPK12.1, IbCDPK12.2, and IbCDPK12.3 might interact with DI19 (DEHYDRATION-INDUCED 19) in response to drought stress. IbCDPK2 might generate a complex with IbCDPK20.1/20.2 through SALH3. SALH3, encoding S-type anion channel protein, was an essential negative regulator of inward potassium channels in guard cells. IbCDPK2, IbCDPK3, IbCDPK13, and IbCDPK20.1/20.2 may be essential for efficient stomatal movement in guard cells. IbCDPK3 might also interact with ORP2A to be involved in the transport of sterols. IbCDPK32 could interact with CNGC18 to regulate pollen growth in sweet potato. IbCDPK3 and IbCDPK8 might play important roles in disease resistance through their involvement in stomatal movement and JA biosynthesis. These results indicated that IbCDPKs might play an important role in plant growth and development, such as stomatal movement, icon transport, and participate in hormone signaling pathways (i.e., JA and ABA) in response to abiotic and biotic stresses. Therefore, interacting proteins of IbCDPKs are still worth exploring.
2.6. Expression Analysis of CDPKs in Sweet Potato and Its Two Diploid Relatives
2.6.1. Expression Analysis in Various Tissues
To investigate the potential biological functions of IbCDPKs in growth and development, the expression level in five representative tissues (i.e., leaf, petiole, stem, pigmented root, and tuberous root) of I. batatas was analyzed using real-time quantitative PCR (qRT-PCR) (Figure 6). In general, different subgroups did not exhibit regular expression patterns in five tissues. Some IbCDPKs showed tissue-specific expression. Almost half of the IbCDPKs (i.e., IbCDPK1, -12.3, -20.2, -25.1, -25.2, -25.3, -25.4, -3, -17.2, -29.2, -33.2, -34.2, -7, -13, -14, -24, and -18) were preferably expressed in the leaf. Five IbCDPKs (i.e., IbCDPK2, 11.2, -9, -29.3, and -30) were highly expressed in the petiole. IbCDPK5.2 was highly expressed in the stem. Six IbCDPKs (i.e., IbCDPK11.3, -12.1, -34.1, -10, -32, and -35) showed a higher gene expression in pigmented root as compared with other tissues. Two IbCDPKs (i.e., IbCDPK12.3, IbCDPK18) showed low expression in all tissues. The majority of IbCDPKs showed a relatively higher gene expression in at least two tissues. IbCDPK5.1 showed a higher expression level in the petiole and stem. IbCDPK12.2, -20.1, -29.1, -8, and -16 showed a higher expression level in the leaf and pigmented root. IbCDPK5.1 showed a higher expression level in the petiole and stem. IbCDPK11.1 showed a higher expression level in the leaf, petiole, and pigmented root. IbCDPK12.2, -20.1, -29.1, -8, and -16 showed a higher expression level in the leaf and pigmented root. IbCDPK33.1 showed a higher expression level in the leaf and petiole. IbCDPK28 showed a higher expression level in the tuberous root. These results indicated that IbCDPKs might function differently in different tissues of sweet potato.
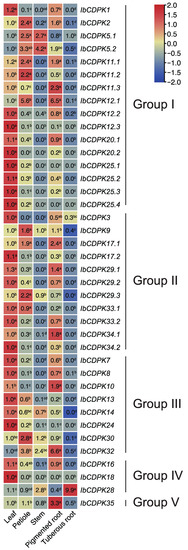
Figure 6.
Gene expression patterns of CDPKs in leaf, petiole, stem, pigmented root, and tuberous root of I. batatas. The values were determined by RT-qPCR from three biological replicates consisting of pools of three plants, and the results were analyzed using the comparative CT method. The fold change is shown in the boxes. Different lowercase letters indicate significant differences (p < 0.05; Student’s t-test).
Variation in gene expression between homolog CDPKs was also observed. RNA-seq data of six tissues (i.e., flower, flower bud, leaf, root 1, root 2, and stem) were used to analyze the expression patterns of ItfCDPKs and ItbCDPKs in I. trifida and I. triloba, respectively [38] (Figure 7). In I. trifida, eight ItfCDPKs (i.e., ItfCDPK5.2, -12.1, -9, -10, -14, -18, -28, and -35) were highly expressed in the root (root1 and root2). Five ItfCDPKs (i.e., ItfCDPK12.2, -29.1, -29.2, -33.1, and -32) were highly expressed in the stem. No ItfCDPKs were highly expressed in the leaf. Only two ItfCDPKs (i.e., ItfCDPK1 and ItfCDPK2) were highly expressed in the flower. Fourteen of thirty-five ItfCDPKs (i.e., ItfCDPK11.1, -11.2, -20.1, -20.2, -25.1, -25.2, -25.3, -17.1, -17.2, -33.2, -34, -7, -24, and -16) were highly expressed in flowerbud. Furthermore, ItfCDPK12.3, -8, and -30 showed low expression in all tissues, and ItfCDPK12.3 showed the lowest expression in the flower, while ItfCDPK30 had the lowest expression in the flower bud. ItfCDPK5.1 and ItfCDPK3 were highly expressed in the stem and flower, while ItfCDPK13 was highly expressed in the stem and flowerbud (Figure 7A).
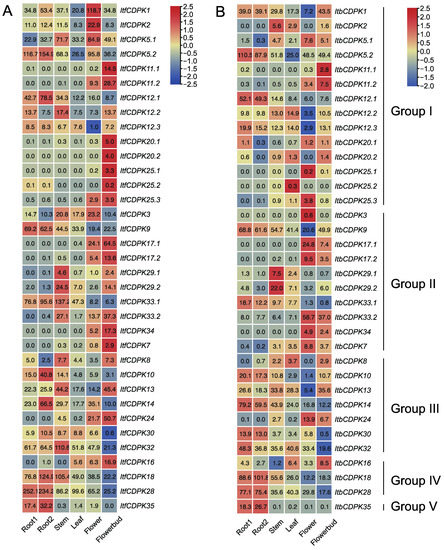
Figure 7.
Gene expression patterns of ItfCDPKs (A) and ItbCDPKs (B) in root 1, root 2, stem, leaf, flower and flower bud of I. trifida as determined by RNA-seq. Log2 (FPKM + 1) is shown in the boxes.
In I. triloba, the expression pattern of ItbCDPKs was similar to that of ItfCDPKs in I. trifida except for individual genes. ItbCDPK1 showed low expression in the flower but not the leaf. ItbCDPK2 was highly expressed in the stem but not flower. ItbCDPK12.2 and ItbCDPK25.2 were highly expressed in the leaf (Figure 7B). These results suggested that CDPKs had similar expression patterns and they might play the same role in the growth and development of I. trifida and I. triloba.
2.6.2. Expression Analysis of Hormone Response
Plant hormones regulate various processes of plant growth, development, and environmental adaptation. They both independently and cooperatively regulate plant seed germination [40,41,42], vegetative growth [43,44,45], reproductive growth [46], embryonic development [47], seed maturation [48,49], as well as the tolerance to biotic [50,51,52,53,54,55,56] and abiotic stresses [57,58]. Thus, we performed qRT-PCR to evaluate the expression level of IbCDPKs in response to hormones, including ABA, GA, IAA, and MeJA. Under ABA treatment, most IbCDPKs were induced, specifically IbCDPK5.1, -29.3, and -33.2 which were up-regulated by 136.8-fold, 162.3-fold, and 222.3-fold, respectively. Two IbCDPKs (i.e., IbCDPK1 and IbCDPK9) were repressed. Thirty IbCDPKs peaked within 12 h, while only seven IbCDPKs (i.e., IbCDPK12.3, -25.1, -25.4, -33.1, -34.2, -16, -35) peaked at 48 h (Figure 8A). Under GA treatment, almost all IbCDPKs were significantly induced at 1 h, with IbCDPK17.2 and IbCDPK33.1 showing the highest folds, by 400.2-fold and 349.5-fold, respectively, while IbCDPK12.3 was repressed (Figure 8B). Under IAA treatment, twelve IbCDPKs (i.e., IbCDPK5.2, -25.1, -25.2, -25.3, -25.4, -17.2, -29.1, -29.2, -29.3, -13, -24, -18) were down-regulated. The expression levels of the most induced IbCDPKs (i.e., IbCDPK2, -11.1, -11.2, -11.3, -12.1, -12.2, -12.3, -20.1, -20.2, -3, -17.1, -33.1, -34.1, -34.2, -7) peaked at 6 h or 12 h. Only IbCDPK28 peaked at 1 h (Figure 8C). Under MeJA treatment, twenty-five IbCDPKs were significantly up-regulated (two peaked at 1 h, twenty peaked at 3 h, and three peaked at 48 h), but twelve IbCDPKs (i.e., IbCDPK1, -5.1, -5.2, -25.4, -3, -9, -17.1, -34.1, -30, -32, -18, -28) were repressed (Figure 8D). All IbCDPKs were induced by at least two hormones. These results suggested that IbCDPKs showed different expression patterns in response to various hormone treatments and might participate in the crosstalk between multiple hormones.
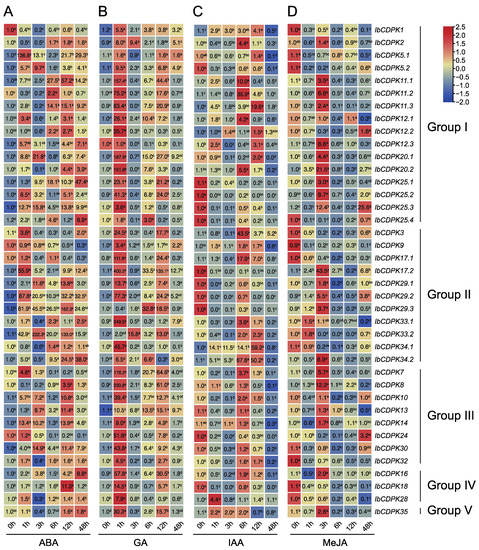
Figure 8.
Gene expression patterns of IbCDPKs in response to different phytohormones, i.e., (A) ABA, (B) GA, (C) IAA, and (D) MeJA of I. batatas. The values were determined by RT-qPCR from three biological replicates consisting of pools of three plants, and the results were analyzed using the comparative CT method. The expression of 0 h in each treatment was considered “1”. The fold change is shown in the boxes. Different lowercase letters indicate significant differences (p < 0.05; Student’s t-test).
We also analyzed the expression patterns of ItfCDPKs and ItbCDPKs using the RNA-seq data of I. trifida and I. triloba under ABA, IAA, GA, and BAP treatments [38]. In I. trifida, compared with the control (Itf_Control), CDPKs were induced by at least one hormone except for ItfCDPK25.1. Under ABA treatment, ItfCDPK11.1, -17.1, -29.2, -33.2, and -34 were significantly induced while ItfCDPK1, -3, -16, and -28 were repressed. Under IAA treatment, ItfCDPK20.2 was up-regulated. ItfCDPK5.2, -12.1, -12.3, -25.2, -9, -33.1, -13, and -24 were down-regulated. ItfCDPK20.1, ItfCDPK25.3, and ItfCDPK7 were induced significantly, whereas ItfCDPK14 and ItfCDPK18 were repressed by GA treatment. Under BAP treatment, ItfCDPK11.2, -12.1, -12.2, -12.3, -9, -17.1, -33.1, -10, -30, and -18 were up-regulated (Figure 9A). In I. triloba, compared with Itb_Control, ItbCDPK1, -11.2, -12.1, -12.3, -20.1, -9, -33.1, -33.2, -34, -10, -30, and -32 showed different expression patterns under hormone treatment compared with those in I. trifida. ItbCDPK1 was up-regulated by ABA while ItbCDPK11.2 was repressed by BAP. ItbCDPK12.1 was induced by ABA and repressed by BAP. ItbCDPK12.3 was up-regulated by GA. ItbCDPK20.1 was induced by ABA. ItbCDPK9 was induced by ABA but repressed by BAP. ItbCDPK33.1 was induced by IAA but repressed by BAP. ItbCDPK33.2 was induced by GA but not ABA. ItbCDPK34 was not responsive to any hormone. ItbCDPK10 was up-regulated by ABA but not BAP and down-regulated by IAA. ItbCDPK30 was induced by ABA but not BAP. ItbCDPK32 was induced but ItfCDPK32 was repressed by ABA (Figure 9B). Furthermore, IbCDPKs and their homologous CDPKs in I. trifida and I. triloba showed different expression patterns in response to ABA, GA, IAA, and MeJA. These results indicated that CDPKs might function in developmental processes through various hormone signaling pathways between sweet potato and its two diploid relatives.
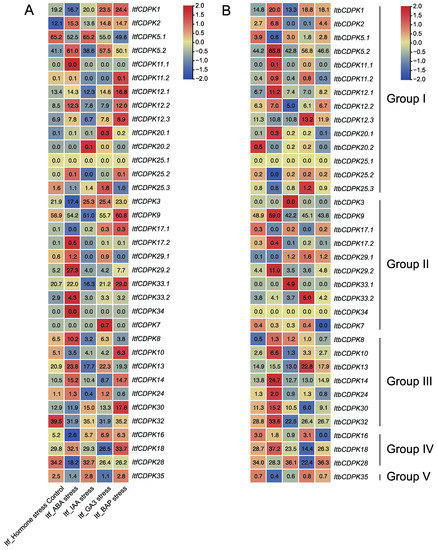
Figure 9.
Gene expression patterns of ItfCDPKs (A) and ItbCDPKs (B) in response to different phytohormone (ABA, IAA, GA3, and BAP) in I. trifida and I. triloba as determined by RNA-seq. Log2 (FPKM + 1) is shown in the boxes.
2.6.3. Expression Analysis under Abiotic Stresses
To evaluate the possible function of IbCDPKs, the level of transcript accumulation was determined using quantitative real-time PCR in leaf tissues at 0, 1, 3, 6, 12, and 48 h under NaCl, PEG, H2O2, and cold treatments (Figure 10). The result illustrated a variable transcript accumulation at different time points post stresses. Under salt stress, the majority of IbCDPKs were up-regulated with four genes, IbCDPK11.1, IbCDPK17.2, IbCDPK33.2, and IbCDPK34.2, induced by more than 100-fold. They peaked at 1 h except IbCDPK12.2 (48 h), IbCDPK33.2 (48 h), and IbCDPK34.2 (6 h). Only three IbCDPKs (i.e., IbCDPK25.4, IbCDPK34.1, and IbCDPK16) were down-regulated (Figure 10A). Under drought stress, more than half of the IbCDPKs were up-regulated, but thirteen IbCDPKs (i.e., IbCDPK12.2, -20.1 -20.2, -25.3, -25.4, -3, -17.1, -29.1, -29.3, -34.1, -34.2, -24, and -30) were repressed (Figure 10B). Under oxidation stress, only five IbCDPKs (i.e., IbCDPK1, -5.1, -17.1, -29.2, and -14) were repressed. IbCDPK17.2 and IbCDPK7 were up-regulated by more than 400-fold. The expression levels of induced IbCDPKs peaked at 12 h or 48 h (Figure 10C). Under cold stress, thirty-three IbCDPKs were induced with IbCDPK20.2 up-regulated by 102.4-fold, whereas six IbCDPKs (i.e., IbCDPK11.1, -3, -29.1, -29.3, -34.1, and -24) were significantly repressed (Figure 10D). In general, twenty IbCDPKs were induced by all four abiotic stress treatments in sweet potato, while only IbCDPK34.1 was down-regulated in three abiotic stress treatments (NaCl, PEG, and cold). These results indicated IbCDPKs might be key players in abiotic stress resistance.
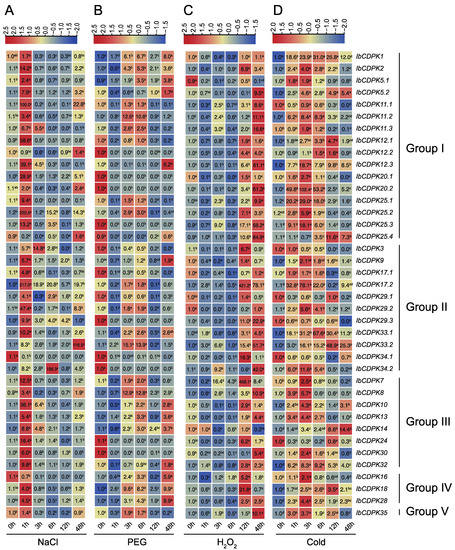
Figure 10.
Gene expression patterns of IbCDPKs in response to abiotic stresses, i.e., (A) NaCl, (B) PEG, (C) H2O2, and (D) cold, of I. batatas. The values were determined by RT-qPCR from three biological replicates consisting of pools of three plants, and the results were analyzed using the comparative CT method. The expression of 0 h in each treatment was considered “1”. The fold change is shown in the boxes. Different lowercase letters indicate significant differences (p < 0.05; Student’s t-test).
In addition, we also analyzed the expression patterns of CDPKs using the RNA-seq data of I. trifida and I. triloba under cold, heat, drought, and salt treatments [38]. In I. trifida, under cold treatment, compared with the control, in group I, ItfCDPK1, -2, -5.2, -11.1, -12.1, -20.1, -20.2, -25.1, -25.2 were induced and ItfCDPK5.1, -12.2, -12.3, -25.3 were repressed. In group II, ItfCDPK17.1 and ItfCDPK33.1 were induced and ItfCDPK3, -9, and -17.2 were repressed. ItfCDPK10, ItfCDPK14, and ItfCDPK32 were induced while ItfCDPK8 and ItfCDPK13 were repressed. ItfCDPK28 was induced. The results suggested these ItfCDPKs might be involved in the response to cold stress. ItfCDPK2, -12.1, -12.3, -25.2, -25.3 in group I, -16 and -28 in group IV were induced by heat treatment. ItfCDPK20.2, -9, -17.2, -29.2, -33.2, -7, -24 and -35 were induced by salt stress, significantly. Under drought stress, ItfCDPK11.2, -12.2, -29.2, and -30 showed higher expression levels, while ItfCDPK12.1, -12.3, -20.1, -20.2, -29.1, -33.1, -24, -18, and -28 showed lower expression levels. ItfCDPK2 was induced by cold, heat, salt stresses. ItfCDPK5.2 was induced by cold, salt, and drought stresses. ItfCDPK12.1 and ItfCDPK28 were induced by cold and heat stresses. ItfCDPK29.2 was induced by salt and drought stresses. ItfCDPK30 was induced by cold, heat, salt, and drought (Figure 11A). In I. triloba, ItbCDPK2 showed an opposite expression pattern in cold, heat, salt, and drought stresses compared with ItfCDPK2. ItbCDPK12.2 was induced by cold stress. ItbCDPK20.1 was repressed by cold stress. ItbCDPK20.2 was repressed in salt stress. ItbCDPK3 and ItbCDPK9 were induced by cold stress. ItbCDPK17.1 and ItbCDPK17.2 were repressed by cold stress. ItbCDPK7 was repressed by salt stress. ItbCDPK16 was induced by cold stress and repressed by heat stress. ItbCDPK18 was induced by drought stress (Figure 11B). These results indicated that CDPKs showed commonalities and differences in response to abiotic stresses in I. trifida and I. triloba.
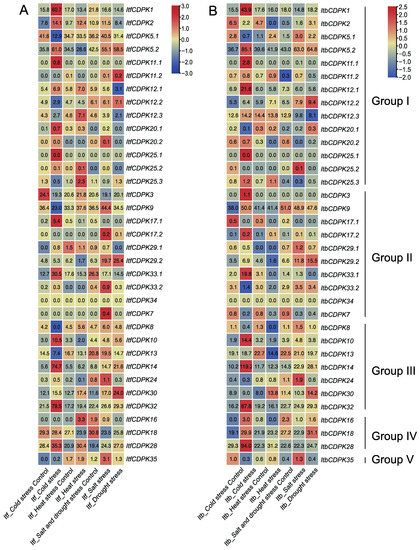
Figure 11.
Gene expression patterns of ItfCDPKs (A) and ItbCDPKs (B) under abiotic stress (cold, heat, salt, and drought) in I. trifida and I. triloba as determined by RNA-seq. Log2 (FPKM+1) is shown in the boxes.
3. Discussion
CDPKs are essential in the regulation of plant growth and development, as well as in response to biotic and abiotic stresses. In the previous study, CDPKs were identified in Arabidopsis, rice, wheat, maize, polar, pear, and grape. However, the functional roles of the CDPKs family are still poorly understood in sweet potato. The modern cultivated sweet potato (I. batatas) is an autohexaploid (2n = 6x = 90) varying from I. trifida NCNSP0306 (2n = 2x = 30) and I. triloba NCNSP0323 (2n = 2x = 30) (Figure S1) and is an important crop because of its tuberous roots [38]. In this work, we characterized the CDPKs family in sweet potato and its two diploid relatives using whole-genome sequence data. To investigate physicochemical properties of CDPKs, the protein physiological properties, chromosome localization, phylogenetic relationships, conserved motifs, and protein interaction networks were predicted. We also analyzed the expression patterns of CDPKs in different tissues and relatives cross-talking of multiple stress signaling pathways. Genome-wide identification of CDPKs in sweet potato and its two diploid relatives will facilitate further genetic studies of growth, development, and stress resistance.
3.1. Evolution of the CDPK Gene Family in Sweet Potato and Its Two Diploid Relatives
A total of 109 CDPKs (i.e., 39 in I. batatas, 35 in I. trifida, and 35 in I. triloba) were identified from the cultivated hexaploid sweet potato and its two diploid relatives. According to the evolutionary distance to AtCPKs, these CDPKs were classified into five subgroups (group I to V), with one more group than CDPKs in other species (group I to IV) [3,9,10,11,12]. Ib/Itf/ItbCDPK35 in group V have no homologous protein in Arabidopsis, meaning group V in sweet potato is unique. We also observed that the number of CDPKs varied between sweet potato and its two diploid relatives. Thirty-nine IbCDPKs were distributed across the genome in I. batatas (Figure 1A). While the number of CDPKs identified in I. trifida was the same as that in I. triloba but was less than that in I. batatas., supporting the distinct origin during the evolution of hexaploid sweet potato.
In this study, a total of 10 motifs were identified in the 109 CDPKs from I. batatas, I. trifida, and I. triloba (Figure 3A and Figure S2), including six protein kinase domains and four EF-hands. Moreover, these motifs were highly conserved in I. batatas, I. trifida, and I. triloba. The majority of CDPKs contained a protein kinase domain and EF-hand except for ItbCDPK13, which contained a protein kinase domain only. The EF-hand in the C-terminus is a Ca2+ binding site [59], which means IbCDPK13 might be Ca2+ insensitive. There was no significant difference in each group except for Ib/Itf/ItbCDPK35 in group V. The number of protein kinase domains and EF-hands varied in sweet potato and its two diploid relatives, especially in group I (i.e., ItfCDPK5.1, Ib/ItbCDPK11.1, IbCDPK11.3, IbCDPK12.1, IbCDPK12.3, ItbCDPK20.1, Ib/ItbCDPK20.2, ItbCDPK25.1, Ib/Itf/ItbCDPK25.2, and IbCDPK25.4) (Figure 3A).
Besides, the exon-intron structures of some homologous CDPKs were different between sweet potato and its two diploid relatives and varied from I. trifida and I. triloba also. Some CDPKs contained the same number of exons and introns in I. trifida and I. triloba but less than that in I. batata, while some CDPKs contained different exons-introns in I. batatas, I. trifida, and I. triloba. These results suggested that CDPKs produced more changes during the evolution from diploid to hexaploid, while the structures of other groups were relatively conserved. Moreover, CDPKs in group I might play more critical roles in plant growth and development and response to environmental stress.
3.2. Different Functions of CDPKs on Growth and Development between Sweet Potato and Its Two Diploid Relatives
CDPKs could be detected on roots, stems, leaves, fruits, and seeds of plants. The expression levels of OsCDPK2 increased with the seed development period. The expression patterns of OsCDPK2 were opposite in green leaves exposed to light and darkness. OsCDPK2 might function in seed development and in response to light in leaves [60]. PiCDPK1 and PiCDPK2 were specifically expressed at the pollen stage in Petunia hybrida. Overexpression of PiCDPK1 disturbed the growth polarity of pollen tubes, while overexpression of PiCDPK2 inhibits the elongation ability of pollen tubes but had no effect on the growth polarity of pollen tubes [52]. PnCDPK1 was accumulated mainly in petals and sepals, which means that PnCDPK1 may be an important component in the signal transduction pathways for flower morphogenesis [30]. Here, the expression levels of CDPKs in different tissues of I. batatas, I. trifida, and I. triloba were shown (Figure 6 and Figure 7). In I.batatas, no similar expression trends were observed between subgroups in five tissues. The majority of IbCDPKs were highly expressed in leaf, petiole, and pigmented root. Many IbCDPKs showed tissue-specific expression, and some IbCDPKs showed higher expression levels in the same tissue at the same time except for in tuberous root. Interestingly, only IbCDPK28 showed the highest expression in tuberous root (Figure 6). IbCDPK28 might be a key regulator in the development of tuberous root. In I. trifida, most ItfCDPKs in group I and group II might play key roles in flower and flowerbud growth and development. ItfCDPKs in group III might play roles in stem and flower and ItfCDPKs in group IV and V might be involved in root growth and development (Figure 7A). In I. triloba, the expression pattern of ItbCDPKs was similar to that of ItfCDPKs in I. trifida except for ItbCDPK1, -2, -12.2, and -25.2 (Figure 7B). Some homologous CDPKs showed similar or opposite tissue-specific expression (Figure 6 and Figure 7). Ib/Itf/ItbCDPK5.1 were highly expressed in stem and Ib/Itf/ItbCDPK18 were highly expressed in leaf. IbCDPK3 was lowly expressed, whereas ItfCDPK3 was highly expressed in leaf. Diverse gene expression patterns among homologous CDPKs across different tissues suggest that CDPK proteins might be specialized for different biological responses.
3.3. Different Functions of CDPKs on Hormone Crosstalk between Sweet Potato and Its Two Diploid Relatives
Protein interaction prediction was performed to further reveal the potential function of IbCDPKs (Figure 5). IbCDPKs might interact with each other, such as IbCDPK11 and IbCDPK24, although it has not been reported that CDPKs could form homodimers. They might interact with JA biosynthesis-related proteins (i.e., ACX1 and ACX5) or ABA-responsive element-binding factor 1 (ABF1). IbCDPK11.1, -11.2, -12.1, -12.2, and -12.3 might interact with DI19 in response to drought stress. In rice, OsDi19-4 regulated the expression of OsASPG1 and OsNAC18, two ABA-responsive genes, by directly binding to their promoters. The regulation was further enhanced by the increased phosphorylation of OsDi19-4 after the treatment of ABA [53]. Furthermore, All IbCDPKs were induced by at least two hormones (Figure 8). The majority of IbCDPKs possessed at least two hormone-responsive elements, such as ABA-responsive elements (ABRE, GARE-motif), MeJA-responsive elements (CGTCA-motif and TGACG-motif), GA-responsive elements (P-box and TATC-box), SA-responsive element (TCA), auxin-responsive elements (TGA-element) (Figure 4). Moreover, most of them peaked within 12 h (Figure 8), indicating that IbCDPKs could sense fluctuations quickly in hormone levels. SLAC1 was regulated by two AtCDPK protein kinases (CPK21 and CPK23), with distinct Ca2+ affinities in response to drought stress through ABA signaling pathways [54]. In Arabidopsis, disruption of the CPK6 gene impaired MeJA-induced stomatal closure. MeJA-induced transient cytosolic free calcium concentration increments were reduced in the cpk6-1 mutant. MeJA failed to activate slow-type anion channels in the cpk6-1 guard cells. AtCPK6 functions as a positive regulator of MeJA signaling in Arabidopsis guard cells [26]. Overexpression of ZmCPK4 in the transgenic Arabidopsis enhanced ABA sensitivity in seed germination, seedling growth, and stomatal movement. The transgenic plants also enhanced drought stress tolerance [55]. Thus, IbCDPKs might also participate in hormone signaling pathways in response to environmental stress.
However, some of their homologous CDPKs in I. trifida and I. triloba showed different expression patterns in response to ABA, GA, IAA, and MeJA. Under IAA treatment, ItfCDPKs and ItbCDPKs were insensitive. For example, Ib/Itf/ItbCDPK1, -5.1, -25.3, -16, and -35 under ABA treatment, -12.3, -9, and -10 under IAA treatment, and -18 under GA treatment showed opposite expression trends (Figure 8; Figure 11). In addition, expression patterns of ItfCDPKs were not exactly the same as those of ItbCDPKs (i.e., Itf/ItbCDPK30, -32 under ABA treatment, -33.1 under IAA treatment, -2 under GA treatment, and -30 under BAP treatment) (Figure 11). These results indicated that CDPKs participated in multiple hormones crosstalk, and homologous CDPK genes were involved in different hormonal pathways in sweet potato and its two diploid relatives.
3.4. Different Functions of CDPKs on Multiple Abiotic Stress Response between Sweet Potato and Its Two Diploid Relatives
There have been many reports that CDPKs were related to abiotic stress resistance [32,33,34]. In Chenopodium glaucum, CgCDPK interacted with CgbHLH001 in the signal transduction pathway in response to salt and drought stress [56]. Overexpression of SiCDPK24 in Arabidopsis enhanced drought resistance and improved the survival rate under drought stress [61]. In this study, we analyzed the level of transcript accumulation using qRT-PCR at different time points post treatments (Figure 10). Under NaCl, H2O2, and cold stresses, the expression of IbCDPKs peaked at 1 h (Figure 10A), 12/48 h (Figure 10C), and 3 h (Figure 10D), meaning that the regulation of IbCDPKs was mainly activated on the prophase of NaCl and cold treatments, and the anaphase of H2O2 treatment, respectively. In general, thirty-eight IbCDPKs were up-regulated by at least two abiotic stresses, consistent with CDPK genes in other species. The majority of IbCDPKs were induced by NaCl, H2O2, and cold stresses. IbCDPK25.3 was up-regulated 12.2-fold at 1 h under NaCl stress, 68.2-fold at 48 h under H2O2, and 18.1-fold at 3 h under cold stress, respectively. IbCDPK33.2 was induced under four stresses, with 116.5-fold at 48 h under NaCl stress, 15.1-fold at 3 h under PEG stress, 51.7-fold at 48 h under H2O2 stress, and 48.9-fold at 12 h under cold stress, respectively (Figure 9). In protein interaction prediction, IbCDPKs might interact with a potassium channel protein (KAT2), stomatal movement protein (ELUS3) (Figure 5). These results indicated that IbCDPKs might be key players in response to abiotic stresses by regulating stomatal movement and icon transport.
In addition, the expression patterns of homologous CDPKs in diploid I. trifida and I. triloba using RNA-seq were distinct (Figure 11). The numbers of Itf/ItbCDPKs induced by salt and cold stresses were less than those of IbCDPKs, which may be due to the fact that only one time point was detected.
Differences in expression patterns of CDPKs in sweet potato and its two diploid relatives might provide potential candidate genes for further functional characterization and for improving abiotic stress tolerance of sweet potato and other species.
4. Materials and Methods
4.1. Identification of CDPKs
The whole-genome sequences of I. batatas, I. trifida, and I. triloba were downloaded from Ipomoea Genome Hub (https://ipomoea-genome.org/, accessed on 15 January 2022) and Sweetpotato Genomics Resource (http://sweetpotato.plantbiology.msu.edu/, accessed on 15 January 2022). To accurately identify all CDPKs family members, two different screening methods were combined. Firstly, the HMMER 3.0 software was used to identify potential CDPKs through the Hidden Markov Model profiles (hmmsearch, E value ≤ 1 × 10−5) of Pkinase domain (PF00069) and EF-hand 7 (PF13499), which were extracted from the Pfam databases (http://pfam.xfam.org/ accessed on 30 November 2021). Next, all putative CDPKs were retested using SMART (http://smart.embl-heidelberg.de/ accessed on 1 December 2021) and CD-search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi accessed on 1 December 2021).
4.2. Chromosomal Distribution of CDPKs
The IbCDPKs, ItfCDPKs, and ItbCDPKs were separately mapped to the I. batatas, I. trifida, and I. triloba chromosome based on the chromosomal location provided in the Ipomoea Genome Hub (https://ipomoea-genome.org/ accessed on 15 January 2022) and Sweetpotato Genomics Resource (http://sweetpotato.plantbiology.msu.edu/ accessed on 15 January 2022). The visualization was generated by the TBtools software (v.1.098696) (South China Agricultural University, Guangzhou, China) [62].
4.3. Protein Properties Prediction of CDPKs
The MW, pI, and the number of EF-hands of CDPKs were calculated by ExPASy (https://www.expasy.org/ accessed on 23 December 2021). The N-myristoylation and Palmitoylation sites of CDPKs were predicted by GPS-Lipid 1.0 with a high threshold (http://lipid.biocuckoo.org/ accessed on 24 December 2021) [63].
4.4. Phylogenetic Analysis of CDPKs
The phylogenetic analysis of CDPKs from I. batatas, I. trifida, I. triloba, and Arabidopsis was performed using ClustalW in MEGA X [64] with default parameters, the maximum likelihood method, and the Poisson correction model. Bootstrapping was performed with 1000 replicates. Then, the phylogenetic tree was constructed by iTOL (http://itol.embl.de/ accessed on 13 January 2022).
4.5. Domain Identification and Conserved Motifs Analysis of CDPKs
The conserved motifs of CDPKs were analyzed by MEME (https://meme-suite.org/meme/ accessed on 29 January 2022), the maximum number of motifs parameter was set to 10.
4.6. Exon–Intron Structures and Promoter Analysis of CDPKs
The exon-intron structures of CDPKs were obtained by GSDS 2.0 (http://gsds.gao-lab.org/ accessed on 29 January 2022) and were visualized by the TBtools software. The cis-elements in the approximately 2000 bp promoter region of CDPKs were predicted by PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ accessed on 30 January 2022) [65].
4.7. Protein Interaction Network of CDPKs
The protein interaction network of CDPKs was predicted by STRING (https://cn.string-db.org/, accessed on 25 January 2022) based on Arabidopsis homologous proteins. The network map was built by Adobe Illustrator CC2019 software (Adobe Systems Incorporated, San Jose, CA, USA).
4.8. qRT-PCR Analysis of CDPKs
The salt-tolerant sweet potato (I. batatas) line ‘ND98’ was used for qRT-PCR analysis in this study [45]. In vitro-grown ND98 plants were cultured on Murashige and Skoog (MS) medium at 27 ± 1 °C under a photoperiod consisting of 13 h of cool-white fluorescent light at 54 μmol m–2 s–1 and 11 h of darkness. Sweet potato plants were cultivated in a field at the campus of China Agricultural University, Beijing, China.
For expression analysis in various tissues, total RNA was extracted from the pigmented root, tuberous root, stems, leaves, and petioles tissues of 3-month-old field-grown ND98 plants using the TRIzol method (Invitrogen). For expression analysis of hormone and abiotic treatment, the leaves were sampled at 0, 1, 3, 6, 12, and 48 h after being treated with 200 mM NaCl, 20% polyethylene glycol (PEG) 6000, 10 mM H2O2, 4 °C, 100 μM ABA, 100 μM GA, 100 μM MeJA, and 100 μM IAA, respectively. Three independent biological replicates were taken, each with three plants. qRT-PCR was conducted using the SYBR detection protocol (TaKaRa, Kyoto, Japan) on a 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The reaction mixture was composed of first-strand cDNA, primer mix, and SYBR Green M Mix (TaKaRa; code RR420A) to a final volume of 20 μL. A sweet potato actin gene (GenBank AY905538) was used as an internal control. The relative gene expression levels were quantified with the comparative CT method [66]. The specific primers of qRT-PCR analysis are listed in Table S2. The heat maps of gene expression profiles were constructed using TBtools software (v.1.098696) [62].
4.9. Transcriptome Analysis
The RNA-seq data of ItfCDPKs and ItbCDPKs in I. trifida and I. triloba were downloaded from the Sweetpotato Genomics Resource (http://sweetpotato.plantbiology.msu.edu/ accessed on 20 January 2022). The expression levels of CDPKs were calculated as fragments per kilobase of exon per million fragments mapped (FPKM). The heat maps were constructed by TBtools software (v.1.098696) [62].
5. Conclusions
Here, we identified 39, 35, and 35 CDPKs in cultivated hexaploid sweet potato and its two diploid relatives, I. trifida and I. triloba, respectively. There were differences in chromosome localization, phylogenetic relationship, and gene structure of these 109 CDPKs. The expression profiles of the identified CDPKs indicated that CDPKs showed tissue specificity and various expression patterns in sweet potato and its two diploid relatives. These results indicated that homologous CDPKs might be involved in distinct hormone crosstalk and abiotic stress responses to regulate plant growth and development. Moreover, the identification of interacting proteins of each CDPK might be their phosphorylation targets to help identify the mechanism. This work provided valuable insights into the structure and function of CDPK genes and provided more potential candidate genes for improving field and abiotic stress tolerance in sweet potato and its two diploid relatives.
Supplementary Materials
The following are available online at: https://www.mdpi.com/article/10.3390/ijms23063088/s1.
Author Contributions
H.Z. (Huan Zhang) and S.H. conceived and designed the research; X.L., L.Z., S.G. and N.Z. performed the experiments; H.Z. (Huan Zhang), X.L. and L.Z. analyzed the data; X.L. and L.Z. wrote the paper; S.H., Q.L. and H.Z. (Hong Zhai) revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the National Natural Science Foundation of China (grant no. 31901584), the National Key R&D Program of China (2019YFD1001301/2019YFD1001300), the Beijing Natural Science Foundation (grant no. 6212017), China Agriculture Research System of MOF and MARA (CARS-10, Sweetpotato), the Beijing Food Crops Innovation Consortium Program (BAIC09-2022), and Sanya Yazhou Bay Science and Technology City (SYND-2022-09).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sanders, D.; Pelloux, J.; Brownlee, C.; Harper, J.F. Calcium at the crossroads of signaling. Plant Cell 2002, 14, S401–S417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudsocq, M.; Willmann, M.R.; McCormack, M.; Lee, H.; Shan, L.B.; He, P.; Bush, J.; Cheng, S.H.; Sheen, J. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature 2010, 464, 418–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.H.; Willmann, M.R.; Chen, H.C.; Sheen, J. Calcium signaling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002, 129, 469–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.B.; Poovaiah, B.W. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003, 8, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Luan, S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009, 14, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.W.; Snedden, W.A. Calmodulin-Related Proteins Step Out from the Shadow of Their Namesake. Plant Physiol. 2013, 163, 486–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.P.; Munyampundu, J.P.; Xu, Y.P.; Cai, X.Z. Phylogeny of Plant Calcium and Calmodulin-Dependent Protein Kinases (CCaMKs) and Functional Analyses of Tomato CCaMK in Disease Resistance. Front. Plant Sci. 2015, 6, 1075. [Google Scholar] [CrossRef] [Green Version]
- Poovaiah, B.W.; Du, L.; Wang, H.; Yang, T. Recent advances in calcium/calmodulin-mediated signaling with an emphasis on plant-microbe interactions. Plant Physiol. 2013, 163, 531–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, S.; Agarwal, P.; Arora, R.; Kapoor, S.; Tyagi, A.K. Expression analysis of calcium-dependent protein kinase gene family during reproductive development and abiotic stress conditions in rice (Oryza sativa L. ssp. indica). Mol. Genet. Genom. 2007, 278, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Zhu, Y.F.; Tan, X.M.; Wang, X.; Wei, B.; Guo, H.Z.; Zhang, Z.L.; Chen, X.B.; Zhao, G.Y.; Kong, X.Y.; et al. Evolutionary and functional study of the CDPK gene family in wheat (Triticum aestivum L.). Plant Mol. Biol. 2008, 66, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Liu, J.; Yang, X.; Ma, R. Genome-wide identification of the maize calcium-dependent protein kinase gene family. Appl. Biochem. Biotechnol. 2013, 169, 2111–2125. [Google Scholar] [CrossRef] [PubMed]
- Valmonte, G.R.; Arthur, K.; Higgins, C.M.; MacDiarmid, R.M. Calcium-dependent protein kinases in plants: Evolution, expression and function. Plant Cell Physiol. 2014, 55, 551–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Han, Y.T.; Zhao, F.L.; Hu, Y.; Gao, Y.R.; Ma, Y.F.; Zheng, Y.; Wang, Y.J.; Wen, Y.Q. Genome-wide Identification and Expression Analysis of the CDPK Gene Family in Grape, Vitis spp. BMC Plant Biol. 2015, 15, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, J.E.; Breton, G.; Harmon, A. Decoding Ca2+ signals through plant protein kinases. Annu. Rev. Plant Biol. 2004, 55, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.L.; Busconi, L. Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J. 2000, 24, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Hrabak, E.M.; Chan, C.W.M.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.; Luan, S.; Nimmo, H.G.; Sussman, M.R.; et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef] [Green Version]
- Sheen, J. Ca2+-Dependent Protein Kinases and Stress Signal Transduction in Plants. Science 1997, 274, 1900–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, J.F.; Huang, J.F.; Lloyd, S.J. Genetic Identification of an Autoinhibitor in CDPK, a Protein-Kinase with a Calmodulin-Like Domain. Biochemistry 1994, 33, 7267–7277. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wang, L.; Xie, W.; Wan, B.; Li, X.; Lin, Y. Expression profile of calcium-dependent protein kinase (CDPKs) genes during the whole lifespan and under phytohormone treatment conditions in rice (Oryza sativa L. ssp. indica). Plant Mol. Biol. 2009, 70, 311–325. [Google Scholar] [CrossRef]
- Mori, I.C.; Murata, Y.; Yang, Y.Z.; Munemasa, S.; Wang, Y.F.; Andreoli, S.; Tiriac, H.; Alonso, J.M.; Harper, J.F.; Ecker, J.R.; et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 2006, 4, 1749–1762. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.Y.; Yu, X.C.; Wang, X.J.; Zhao, R.; Li, Y.; Fan, R.C.; Shang, Y.; Du, S.Y.; Wang, X.F.; Wu, F.Q.; et al. Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 2007, 19, 3019–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.Y.; Wu, W.H. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol. Biol. 2007, 65, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Weckwerth, P.; Ehlert, B.; Romeis, T. ZmCPK1, a calcium-independent kinase member of the Zea mays CDPK gene family, functions as a negative regulator in cold stress signalling. Plant Cell Environ. 2015, 38, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, Y.; Yu, X.; Liu, J.; Yang, L.; Gao, Y.; Ke, G.; Zhou, M.; Mu, B.; Xiao, S.; et al. Overexpression of two CDPKs from wild Chinese grapevine enhances powdery mildew resistance in Vitis vinifera and Arabidopsis. New Phytol. 2021, 230, 2029–2046. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhu, L.; Zhang, X.; Guan, Q.; Xiao, S.; Min, L.; Zhang, X. GhCPK33 Negatively Regulates Defense against Verticillium dahliae by Phosphorylating GhOPR3. Plant Physiol. 2018, 178, 876–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munemasa, S.; Hossain, M.A.; Nakamura, Y.; Mori, I.C.; Murata, Y. The Arabidopsis Calcium-Dependent Protein Kinase, CPK6, Functions as a Positive Regulator of Methyl Jasmonate Signaling in Guard Cells. Plant Physiol. 2011, 155, 553–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivashuta, S.; Liu, J.Y.; Liu, J.Q.; Lohar, D.P.; Haridas, S.; Bucciarelli, B.; VandenBosch, K.A.; Vance, C.P.; Harrison, M.J.; Gantt, J.S. RNA interference identifies a calcium-dependent protein kinase involved in Medicago truncatula root development. Plant Cell 2005, 17, 2911–2921. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.H.; Zhang, H.F.; Wei, X.Y.; Yang, L.; Yang, B.; Zhang, L.; Li, J.; Jiang, Y.Q. Functional characterization of calcium-dependent protein kinase (CPK) 2 gene from oilseed rape (Brassica napus L.) in regulating reactive oxygen species signaling and cell death control. Gene 2018, 651, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.; Romanowsky, S.M.; Barron, Y.D.; Garg, S.; Azuse, C.L.; Curran, A.; Davis, R.M.; Hatton, J.; Harmon, A.C.; Harper, J.F. Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 2009, 59, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, K.; Pawelek, A.; Kopcewicz, J.; Szmidt-Jaworska, A. The calcium-dependent protein kinase (PnCDPK1) is involved in Pharbitis nil flowering. J. Plant Physiol. 2012, 169, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Matschi, S.; Werner, S.; Schulze, W.X.; Legen, J.; Hilger, H.H.; Romeis, T. Function of calcium-dependent protein kinase CPK28 of Arabidopsis thaliana in plant stem elongation and vascular development. Plant J. 2013, 73, 883–896. [Google Scholar] [CrossRef]
- Saijo, Y.; Kinoshita, N.; Ishiyama, K.; Hata, S.; Kyozuka, J.; Hayakawa, T.; Nakamura, T.; Shimamoto, K.; Yamaya, T.; Izui, K. A Ca2+-dependent protein kinase that endows rice plants with cold- and salt-stress tolerance functions in vascular bundles. Plant Cell Physiol. 2001, 42, 1228–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.I.; Park, H.J.; Park, J.H.; Kim, S.; Im, M.Y.; Seo, H.H.; Kim, Y.W.; Hwang, I.; Kim, S.Y. Arabidopsis calcium-dependent protein kinase AtCPK32 interacts with ABF4, a transcriptional regulator of abscisic acid-responsive gene expression, and modulates its activity. Plant Physiol. 2005, 139, 1750–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.X.; Shen, S.H.; Yang, S.H.; Komatsu, S. OsCDPK13, a calcium-dependent protein kinase gene from rice, is induced in response to cold and gibberellin. Plant Physiol. Bioch. 2003, 41, 369–374. [Google Scholar] [CrossRef]
- Liu, Q.C. Sweet potato Omics and Biotechnology in China. Plant Omics 2011, 4, 295–301. [Google Scholar] [CrossRef]
- Bach, T.J. Some New Aspects of Isoprenoid Biosynthesis in Plants-a Review. Lipids 1995, 30, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Moeinzadeh, M.H.; Kuhl, H.; Helmuth, J.; Xiao, P.; Haas, S.; Liu, G.L.; Zheng, J.L.; Sun, Z.; Fan, W.J.; et al. Haplotype-resolved sweet potato genome traces back its hexaploidization history. Nat. Plants 2017, 3, 696–703. [Google Scholar] [CrossRef]
- Wu, S.; Lau, K.H.; Cao, Q.H.; Hamilton, J.P.; Sun, H.H.; Zhou, C.X.; Eserman, L.; Gemenet, D.C.; Olukolu, B.A.; Wang, H.Y.; et al. Genome sequences of two diploid wild relatives of cultivated sweetpotato reveal targets for genetic improvement. Nat. Commun. 2018, 9, 2580. [Google Scholar] [CrossRef] [Green Version]
- Chalker, D.L.; Sandmeyer, S.B. Sites of Rna Polymerase-Iii Transcription Initiation and Ty3 Integration at the U6 Gene Are Positioned by the Tata Box. Proc. Natl. Acad. Sci. USA 1993, 90, 4927–4931. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.H.; Zhao, X.Y.; Liu, Y.B.; Zhang, C.L.; O’Neill, S.D.; Zhang, X.S. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009, 59, 448–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Schumaker, K.S.; Guo, Y. Sumoylation of transcription factor MYB30 by the small ubiquitin-like modifier E3 ligase SIZ1 mediates abscisic acid response in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2012, 109, 12822–12827. [Google Scholar] [CrossRef] [Green Version]
- Ju, L.; Jing, Y.X.; Shi, P.T.; Liu, J.; Chen, J.S.; Yan, J.J.; Chu, J.F.; Chen, K.M.; Sun, J.Q. JAZ proteins modulate seed germination through interaction with ABI5 in bread wheat and Arabidopsis. New Phytol. 2019, 223, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.A.; Wang, L.J.; Xu, Z.H.; Xia, Z.A. Foliar modifications induced by inhibition of polar transport of auxin. Cell Res. 1999, 9, 27–35. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, J.Q.; Zhai, Q.Z.; Zhou, W.K.; Qi, L.L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.L.; Qi, J.; et al. The Basic Helix-Loop-Helix Transcription Factor MYC2 Directly Represses PLETHORA Expression during Jasmonate-Mediated Modulation of the Root Stem Cell Niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.Y.; Wabnik, K.; Niu, T.T.; Li, H.B.; Yu, Q.Q.; Pollmann, S.; Vanneste, S.; Govaerts, W.; Rolcik, J.; Geisler, M.; et al. WOX5-IAA17 Feedback Circuit-Mediated Cellular Auxin Response Is Crucial for the Patterning of Root Stem Cell Niches in Arabidopsis. Mol. Plant 2014, 7, 277–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, C.B.; Xiao, L.H.; Huaa, K.; Zou, C.; Zhao, Y.; Bressan, R.A.; Zhu, J.K. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Natl. Acad. Sci. USA 2018, 115, 6058–6063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.L.; Zhou, L.M.; Huang, M.K.; He, X.M.; Yang, Y.H.; Liu, X.; Li, Y.G.; Hou, X.L. Gibberellins play an essential role in late embryogenesis of Arabidopsis. Nat. Plants 2018, 4, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Y.; Chen, M.X.; Chen, Q.F.; Xiao, S.; Chye, M.L. Arabidopsis acyl-CoA-binding protein ACBP1 participates in the regulation of seed germination and seedling development. Plant J. 2013, 74, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, C.Z.; Ye, Q.; Wu, W.H.; Chen, Y.F. Arabidopsis WRKY6 Transcription Factor Acts as a Positive Regulator of Abscisic Acid Signaling during Seed Germination and Early Seedling Development. PLoS Genet. 2016, 12, e1005833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.P.; Kuo, C.H.; Lu, H.H.; Lo, H.S.; Yeh, K.W. The Sweet Potato NAC-Domain Transcription Factor IbNAC1 Is Dynamically Coordinated by the Activator IbbHLH3 and the Repressor IbbHLH4 to Reprogram the Defense Mechanism against Wounding. PLoS Genet. 2016, 12, e1006397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Q.; Zhai, H.; Gao, S.P.; Yang, L.; Wang, Z.; Xu, Y.T.; Huo, J.X.; Ren, Z.T.; Zhao, N.; et al. IbBBX24 Promotes the Jasmonic Acid Pathway and Enhances Fusarium Wilt Resistance in Sweet Potato. Plant Cell 2020, 32, 1102–1123. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.M.; Dowd, P.E.; Gilroy, S.; McCubbin, A.G. Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell 2006, 18, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yu, C.; Xu, S.; Zhu, Y.; Huang, W. OsDi19-4 acts downstream of OsCDPK14 to positively regulate ABA response in rice. Plant Cell Environ. 2016, 39, 2740–2753. [Google Scholar] [CrossRef] [PubMed]
- Geiger, D.; Scherzer, S.; Mumm, P.; Marten, I.; Ache, P.; Matschi, S.; Liese, A.; Wellmann, C.; Al-Rasheid, K.A.S.; Grill, E.; et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA 2010, 107, 8023–8028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.S.; Zhang, D.; Wang, L.; Pan, J.W.; Liu, Y.; Kong, X.P.; Zhou, Y.; Li, D.Q. A maize calcium-dependent protein kinase gene, ZmCPK4, positively regulated abscisic acid signaling and enhanced drought stress tolerance in transgenic Arabidopsis. Plant Physiol. Bioch. 2013, 71, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, G.; Wang, C.; He, Z.Z.; Lan, X.X.; Zhang, S.Y.; Lan, H.Y. The bHLH transcription factor CgbHLH001 is a potential interaction partner of CDPK in halophyte Chenopodium glaucum. Sci. Rep. 2017, 7, 8441. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Jia, W.S.; Zhang, J.H. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 2008, 54, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, W.Y.; Li, Z.H.; Deng, X.W.; Wu, W.H.; Xue, Y.B. F-Box Protein DOR Functions As a Novel Inhibitory Factor for Abscisic Acid-Induced Stomatal Closure under Drought Stress in Arabidopsis. Plant Physiol. 2008, 148, 2121–2133. [Google Scholar] [CrossRef] [Green Version]
- Schafer, B.W.; Heizmann, C.W. The S100 family of EF-hand calcium-binding proteins: Functions and pathology. Trends Biochem. Sci. 1996, 21, 134–140. [Google Scholar] [CrossRef]
- Frattini, M.; Morello, L.; Breviario, D. Rice calcium-dependent protein kinase isoforms OsCDPK2 and OsCDPK11 show different responses to light and different expression patterns during seed development. Plant Mol. Biol. 1999, 41, 753–764. [Google Scholar] [CrossRef]
- Yu, T.F.; Zhao, W.Y.; Fu, J.D.; Liu, Y.W.; Chen, M.; Zhou, Y.B.; Ma, Y.Z.; Xu, Z.S.; Xi, Y.J. Genome-Wide Analysis of CDPK Family in Foxtail Millet and Determination of SiCDPK24 Functions in Drought Stress. Front. Plant Sci. 2018, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Xie, Y.B.; Zheng, Y.Y.; Li, H.Y.; Luo, X.T.; He, Z.H.; Cao, S.; Shi, Y.; Zhao, Q.; Xue, Y.; Zuo, Z.X.; et al. GPS-Lipid: A robust tool for the prediction of multiple lipid modification sites. Sci. Rep. 2016, 6, 28249. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C-T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).