Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by (mal)adaptive remodeling of the pulmonary vasculature, which is associated with inflammation, fibrosis, thrombosis, and neovascularization. Vascular remodeling in PAH is associated with cellular metabolic and inflammatory reprogramming that induce profound endothelial and smooth muscle cell phenotypic changes. Multiple signaling pathways and regulatory loops act on metabolic and inflammatory mediators which influence cellular behavior and trigger pulmonary vascular remodeling in vivo. This review discusses the role of bioenergetic and inflammatory impairments in PAH development.
1. Introduction
Pulmonary hypertension (PH) is a progressive disease characterized by increased pulmonary vascular resistance (PVR) leading to right heart hypertrophy and ultimately death due to right heart failure [1]. Hemodynamically, PH is defined as a mean pulmonary artery pressure (mPAP) of >20 mmHg, pulmonary artery wedge pressure ≤ 15 mmHg and PVR ≥ 3.0 Wood units [2]. Recently, clinical aspects associated with pathophysiology, etiologies, clinical presentation, hemodynamic characteristics, and therapeutic management conditions were re-evaluated and revised during the 6th World Symposium on Pulmonary Hypertension (WSPH) [2]. PH is classified in 5 groups: (1) pulmonary arterial hypertension (PAH), which comprises diverse diseases that cause similar pathological changes within the pulmonary vasculature, that will be described below; (2) left heart diseases like systolic or diastolic heart failure and left sided valvular diseases; (3) lung parenchyma or hypoxia related diseases; (4) chronic thromboembolic pulmonary hypertension (CTEPH) and other pulmonary obstructive processes; (5) diseases with multifactorial mechanisms or unclear mechanisms, including hematological disorders and, systemic and metabolic disorders [2,3].
PAH is characterized by increases in PVR that are mostly due to (mal)adaptive remodeling of the pulmonary vasculature, which is associated with inflammation, fibrosis, thrombotic lesions, medial hypertrophy, and intimal proliferation. Plexiform lesions and remodeling of the small pre-capillary pulmonary arterioles constitute the hallmarks of the disease [4].
Due to its complexity and being constituted by several associated clinical disorders, PAH is subdivided in: idiopathic PAH (group 1.1); heritable PAH (group 1.2); drug- and toxin-induced PAH (group 1.3); PAH associated with various conditions including connective tissue diseases, such as HIV infection, portal hypertension, and congenital heart disease (group 1.4); PAH in long-term responders to calcium channel blockers (group 1.5); PAH with venous/capillary involvement (group 1.6); and persistent PH of the newborn (group 1.7) [5]. Genetic mutations in Bone Morphogenetic Protein Receptor Type 2 (BMPR2) remain the most common cause of PAH, account for ~80% of hereditary (HPAH) and ~20% idiopathic PAH (IPAH) [6]. Besides that, other hereditary causes of PAH involve TGF-β superfamily genes including ALK1/ACVRL1 (a heterodimeric partner of BMPR2), BMP9 (a BMPR2 family member), ENG (a coreceptor for BMPR2 signaling), and SMAD1, 4, and 9 (downstream BMP signaling molecules) have been linked to both HPAH and IPAH [7]. Recently, two other genes have been associated: CAV1 (involved in BMPR2 membrane localization and signaling) [8] and KCNK3 (a potassium channel that regulates resting membrane potential) [2,9].
Although in the last few decades there has been progress in understanding of PAH, cure of the disease remains an unmet challenge, as this group is the most aggressive form of PH, with poor survival and limited treatment options. According to Registry to Evaluate Early and Long-term PAH disease management study (REVEAL) there is an estimated five-year survival of 57% from the time of diagnostic right heart catheterization [2]. Thus, it is extremely necessary to understand the cellular and molecular mechanisms underlying this disease, in order to identify new intervention mechanisms.
In this review, we will summarize current data on the mechanisms that control metabolic and inflammatory events in endothelial cells (ECs) and smooth muscle cells (SMCs) as essential pathways leading to pulmonary vascular remodeling in PAH.
2. Metabolic Changes Related to Vascular Remodeling
Although the mechanisms involved with dysfunction in energy metabolism are not fully understood in PAH, it is known that metabolic shifts mediating abnormalities in PAH PAECs and PASMCs are directly linked to an imbalance between glycolysis, glucose oxidation, and fatty acid oxidation [10,11,12,13,14]. In cells, during energy substrate metabolism, glucose is metabolized to pyruvate in the cytosol and can follow different paths: (1) in mitochondria, pyruvate undergoes oxidative phosphorylation (OXPHOS) entering the tricarboxylic acid cycle (TCA), where it will generate 32–36 ATPs per glucose molecule, or (2) it can be converted into lactate (anaerobic glycolysis), which will generate only 2 ATPs per glucose molecule.
2.1. Glycolysis and the Warburg Effect
Despite the abundance of oxygen in the blood and the direct and unlimited access of blood vessel-lining ECs to oxygen, ECs prefer to use aerobic glycolysis as the major metabolic pathway to generate energy, which might be beneficial when ECs sprout into avascular tissue. This effect is known as the Warburg phenomenon, where cells exhibit a metabolic change in which mitochondrial glucose oxidation is suppressed [15]. Uebelhoer et al. [15] discussed in their paper some advantages for ECs to preferentially use glycolysis over OXPHOS, as a low-oxidative metabolism limits the formation of reactive oxygen species (ROS) and potential ROS-mediated damage. In addition, glycolysis can generate ATP much more rapidly, facilitating adaptation to quickly changing energy-demands of proliferating/migrating cells and enabling more oxygen availability for perivascular cells.
In similar fashion to cancer, proliferating cells in PAH switch their metabolism to glycolysis and that has been associated with vascular expansion. For example, the endothelium doubles glycolytic rates when switching from quiescence to an angiogenic phenotype [16]. Supporting this notion, PDG-PET, which which uses 18F-fluorodeoxyglucose (18FDG), a glucose analogue, as a radiotracer for positron emission tomography (PET) has been applied to monitor the pulmonary vasculature during PAH progression. Studies observed that 18FDG uptake is increased in the lungs of patients with idiopathic PAH (PAH) [12,17] reflecting a shift towards glycolytic metabolism. Some analyses in IPAH lungs, however, concluded that 18FDG uptake is not homogeneous. In some patients with IPAH 18FDG uptake was 3-fold that of control group, whereas others were in the range of controls, suggesting that this variability may be due to extensive morphological heterogeneity within individual lungs and between lungs of patients with IPAH [18]. In animal models, this technique detected an increase of glucose transporter 1 (GLUT1) mRNA, induced by HIF 1α in both endothelium and PASMCs, but not in airway cells or macrophages in rates with MCT induced PH [12]. Based on this information, it is suggested that 18FDG PET imaging may be a useful clinical tool to assess the subpopulation of IPAH patients with increased pulmonary glucose metabolism, making it possible to explore the efficacy and dose-response relationships of new treatments targeting PAH [18].
Undoubtedly, alterations in the function of different metabolic enzymes in PAH can be considered potential biomarkers for the diagnosis and prognosis of the disease. For instance, hexokinase-1 has also been identified to be up-regulated in early passage PASMCs derived from MCT animals, indicating metabolic shift in these cells [12]. Hexokinase binds to the voltage-dependent anion channel (VDAC) in the mitochondrial membrane, preventing the release of cytochrome C, thus contributing to the inhibition of apoptosis and promoting cell proliferation [19]. The enzyme PFKBF3, which catalyzes the conversion of fructose 1,6-bisphosphate (F1,6P2) to fructose 2,6-bisphosphate (F2,6P2) in vascular cells, also participates in glycolysis-associated angiogenesis [16,20] and upregulation of PFKFB3 mediates cell proliferation and collagen synthesis in PASMCs [21]. Besides that, the increase in PFKFB3 promotes an immunological response resulting in inflammasome activation [22]. The accumulated pyruvate, generated by PFKFB3 enhances the ability to inhibit prolyl hydroxylase domain proteins, reduces the degradation of hypoxia-inducible factors 1α (HIF-1α) in cells, promotes the expression of IL-1β, CXCL12 and platelet-derived growth factor B (PDGFB), and ultimately leads to PASMC abnormal proliferation and migration [22].
Enolase (ENO), the ninth enzyme of glycolysis, which catalyzes the dehydration of 2-phospho-D-glycerate (2-PG) to phosphoenolpyruvate (PEP), is elevated in PAH PASMCs but not in PAECs and fibroblasts or in IPAH suggesting potentially different pathogenetic mechanisms between IPAH and HPAH [23]. Interestingly, ENO antibodies were found in the serum of systemic sclerosis patients with PAH. These antibodies induce VSMCs contraction indicating that ENO1 is as possible regulator metabolic reprogramming in PAH associated with scleroderma [24,25]. Another abnormality occurs in the last step of glycolysis, the conversion of phosphoenolpyruvate to pyruvate and ATP by pyruvate kinase (PK). Some reports observed an increase of pyruvate kinase muscle 2 (PKM2) in PAECs and blood outgrowth ECs (BOECs) of PAH patients and in the Sugen/ hypoxia model, while the PKM1 did not have its activity altered, inferring that polypyrimidine tract binding protein 1 (PTBP1) could be responsible for the selective splicing of PKM2/PKM1 isoform [26,27]. It is important to mention that PKM2 transcription is regulated by HIF1α through its binding to HIF response elements promoter [28]. When the dimeric form of PKM2 is translocated into the nucleus, it phosphorylates nuclear proteins such as STAT3 and β-catenin [29,30,31]. It is known that STAT3 is the main member of the STAT family associated with cardiovascular diseases [32,33,34], and STAT3 activation in PAECs changes proliferative and survival phenotypes [35,36,37].
Increases in PTBP1 expression have been described in cancer cells and are correlated with decreases in the production of the small non-coding RNA miRNA-124, suggesting that in PAH, miR-124/PTBP1/PKM2 promotes glycolysis, increases cell proliferation, and causes apoptosis resistance [38]. In this respect, genetic manipulation by miR124 mimic or PTBP1-silencing regulated the splicing ratios in the pulmonary artery (PA) adventitia which harbors activated fibroblasts (PH-Fibs), preventing cell proliferation, and controlling metabolic derangements [39]. Noteworthy, miRNAs have been associated with the post-transcriptional regulation of genes that lead to proliferation, migration, and pro-inflammatory cytokines, such as IL-6, CCL2/monocyte chemoattractant protein 1 (MCP-1), CCL12/stromal cell derived factor 1, and IL-1β in SMCs, PAECs and fibroblasts in PAH [40,41,42]. For instance, PTBP1 acts in SMC and EC communication, rescues the activation of chromatin remodeling genes by BMPR2, such as Notch1, FOXO3, p21 and p27, which contributes to endothelial regeneration and homeostasis in cells exposed to environmental damage [43].
Although increases in glycolysis and lactate levels are well established in PAH [10,11,44,45] and that some studies demonstrated that lactate is a potent activator of proinflammatory genes in macrophages, which stimulates PASMC, PAEC and fibroblast growth [46,47]. Zhao and cols [11] using metabolomics and microarray data, did not detect glycolytic shift or significant changes in lactate levels (characteristic of the Warburg effect) in the lungs of patients with advanced PAH. Furthermore, high levels of glucose, sorbitol and fructose are observed in the lungs of patients with PAH. Possibly, in late-stage PAH, the sorbitol pathway uses excess glucose and, consequently, the potential formation of glycation products can generate free radicals which trigger tissue damage and its association with the PH phenotype. Another possible destination for the glucose consumption suggested by these authors is the pentose phosphate pathway (PPP) [11].
2.2. Pentose Phosphate Pathway
Although the PPP interfaces with multiple metabolic pathways, little is known about its role in PAH. The PPP is critical because it not only generates pentose phosphates to supply nucleic acid synthesis, but also provides NADPH, which is required for both the synthesis of fatty acids and cell survival under stress conditions. In this context, generation of isocitrate dehydrogenase 1 and 2 (IDH) through increased NADPH activity in PAs and lungs of PAH patients (idiopathic and heritable) has been observed [48,49]. Importantly, NADPH provides a substrate for NADPH oxidase (NOX) to generate ROS. Usually, free NADPH is reduced by glutathione (GSH) or thioredoxin (TRX) redutases–GSH/TRKs- as an antioxidant resource. Under pathological conditions, when metabolic pathways are altered (glycolysis, PPP, and suppression of mitochondrial respiration), vascular NOXs (especially NOX4 and NOX2) have an impact on cell proliferation, apoptosis resistance, as well as activation of cytokines and chemokines that favor the recruitment of immune cells causing vascular injury [50,51].
Studies have proposed that BMPR2 mutation stimulates PPP upregulation, which is driven by downstream ribose-5-phosphate isomerase upregulation that overcomes decreases in glucose-6-phosphate dehydrogenase (G6PD) expression and directly enhances nucleotide synthesis and salvage [48]. Alternatively, molecular mechanisms that stimulate G6PD expression and activity in hypoxic and/or endothelin-1 suppress contractile protein expression in cells and promote PASMC, EC and fibroblast proliferation [50,52] which contributes to pulmonary vascular remodeling. G6PD inhibition stimulated by protein kinase G1 (PKG1α) signaling triggered several pathways, such as (1) increases in contractile protein expression [53], (2) reduction in TNFα activation [54], contributing to reduction of pro-inflammatory signals in PA in hypoxia, (3) stimulation of apoptosis in PASMC and other cells through signaling that induces calcium release mediated by IP3 [50].
2.3. Krebs Cycle
Another disruption associated with PAH is the conversion of pyruvate into acetyl coenzyme A, which inhibits the Krebs cycle. The Krebs cycle is a sequence of reactions that occur within the mitochondria of eukaryotic cells through the release of stored energy by oxidation of acetyl-CoA derived from carbohydrates, lipids, and proteins, which is used as a synthesis of fatty acids, steroids, cholesterol, and amino acids for protein construction and the purines and pyrimidines used in DNA synthesis.
In PAH, mitochondria are the focus of several metabolic changes that result in the dysregulation of several enzymes involved in fatty acid metabolism. For example, SLC25A1, a mitochondrial citrate carrier, which exports citrate from mitochondria to the cytoplasm, has an altered export pathway in PAH. Lower levels of SLC25A1 were shown in PAH PAECs, suggesting that the concentration of mitochondrial citrate may be higher than cytosolic citrate in these cells [14]. Furthermore, citrate increases were shown in PAH lungs [11], and isocitrate and cis-aconitate are also increased in PAH plasma [14]. Notably, these metabolic changes lead to mitochondrial dysfunction that favors cells proliferation and contributes to vascular remodeling in PH.
Pathological activation of PDKs leads to phosphorylation and inhibition of pyruvate dehydrogenase (PDH) resulting in inhibition of acetyl CoA production, reduced electron flux and ROS generation in the mitochondria that is associated with vasoconstriction [12,44,45]. It is suggested that in PAH, the “pseudohypoxic” cellular environment created activates HIF, which mediates several metabolic adaptations. Once HIF is activated, it transactivates two inhibitory PDK isoforms, PDK1 and PDK3 [12,30,55]. Hence, potential mechanisms of PDH inhibition have been extensively studied in PAH. For example, the lungs of patients with PAH have reduced expression of mitochondrial deacetylase SIRT3 and SIRT3 deficiency in mice promotes pulmonary hypertension [56] in a mechanism that involves inhibition of PDH function [45].
Besides that, mitochondria from pulmonary arteries and plexiform lesions in patients with PAH are deficient in complex I and superoxide dismutase 2 (SOD2) expression [44]. Studies using genomic bisulfite sequencing demonstrated selective hypermethylation of a CpG island in an enhancer region of intron 2 and another in the promoter, suggesting epigenetic dysregulation of SOD2 possibly associated with upregulation of DNA methyltransferases 1 and 3B [57]. Notably, the restoration of both SOD2 expression and the proliferation-to-apoptosis ratio were reversed by the DNA methyltransferase inhibitor 5-aza-2’-deoxycytidine, suggesting that differential methylation occurs selectively in pulmonary arteries versus aortic SMCs [57].
2.4. Randle Cycle
The Randle cycle is characterized by competition between glucose and fatty acids for oxidation. In some organs, like the heart, fatty acid oxidation is the major source of ATP production, while glucose metabolism is the secondary source. During the Randle cycle, the production of citrate occurs through fatty acid oxidation, where citrate inhibits phosphofructokinase, causing accumulation of glucose-6-phosphate as a result of hexokinase inhibition which ultimately decreases the production of pyruvate. Randle cycle also involves inhibition of pyruvate dehydrogenase generated during fatty acid oxidation. This metabolic shift occurs in PAH and likely contributes to pathogenesis both in the heart and in the pulmonary vasculature [58,59]. Likewise, in an animal model of PH, inhibition of fatty acid oxidation by blocking the enzyme malonylcoenzyme A decarboxylase (MDC) resulted in attenuation of the disease [60].
2.5. Glutamine Metabolism
During the production of energy, glutamine is one of the metabolite fuels that can be hydrolyzed to glutamate and subsequently converted to α-ketoglutarate (α-KG) in mitochondria. α-KG can enter the Krebs’ cycle and replenish metabolic intermediates thereby supporting the biosynthetic demands for NADPH and fatty acids. This replenishment of the metabolic intermediates is called anapleurosis, which favors cell growth through the production of ATP. Regarding the metabolic flux, aerobic glycolysis and glutaminolysis are similar pathways. For example, glutamine transporters (SLC1A5 and SLC7A5) [61] are upregulated during glutaminolysis in a similar fashion to GLUT1 transporter in aerobic glycolysis [59,62]. In glutaminolysis, lactate is generated by malic enzyme (Me) [63] and Me2 exerts an important role in glutamate metabolism [64] associated to PAH [48].
Alterations in glutamine metabolism have been found in the pulmonary vasculature of patients with PAH [58]. Increases in glutamine metabolism was demonstrated in pulmonary microvascular ECs from BMPR2-mutant mice [65]. Furthermore, upregulation of the glutamine transporter, SLC1A5, was observed in the hypertrophic right ventricle of PAH patients and in the right ventricle of the MCT rat model of PH [66,67]. It is suggested that like in cancer cells, pulmonary vascular cells in PAH use glutamate, through glutamine hydrolysis in an anapleurotic reaction to generate α-ketoglutarate for the tricarboxylic acid cycle [58,68] for the biosynthetic demands for nicotinamide adenine dinucleotide phosphate (NADPH) and fatty acids during rapid cell growth. In concordance, a wide range of glutamate receptors (NMDARs but also AMPA receptors and metabotropic glutamate receptors) expressed in vascular cells could mediate crucial roles in the function and development of the vascular system, such as regulating endothelial barrier permeability and angiogenesis [69].
Data suggest that the increase in glutaminolysis seen in PAH is a result of the vascular extracellular matrix (ECM) stiffness and the activation of transcriptional coactivators YAP/TAZ in response to a metabolic adaption needed to maintain the proliferative state of lung cells [68]. Furthermore, repurposing of the YAP inhibitor verteporfin and CB-839 (GLS-1 inhibitor) for treatment of PH, either separately or together with possible optimization for tissue-specific delivery, is a potential new therapeutic opportunity for this disease [68].
3. Mediators That Alter Metabolic Function in PAH
3.1. Hypoxia-Inducible Factor
The most important transcription factors related to hypoxic responses are the hypoxia-inducible factors 1 and 2 (HIF1 and HIF2) [70]. Under normoxia, HIF activity is regulated through the oxygen sensor prolyl-4- hydroxylase domain-containing enzymes (PHDs) that use oxygen to hydroxylate specific proline residues of HIF-α. In this situation, HIF-α bind to von Hippel-Lindau (VHL) ubiquitin E3 ligase, leading to ubiquitination and its subsequent degradation by the proteasome [71,72,73]. Therefore, under low concentration of cellular oxygen, HIF-α subunit is activated and translocated to the nucleus where upon dimerization with the HIF-β subunit forms the heterodimeric complex, which binds to the specific DNA binding regions (hypoxia-responsive elements, or HREs) resulting in transcription of its target genes [74].
Several processes that trigger vasoconstriction and vascular remodeling are associated with hypoxia, which makes HIF an essential element in these responses. In cancer cells, HIF promotes numerous roles related to cell proliferation and metabolism. Likewise, the involvement of HIF in PAH pathogenesis is also extensively studied [71,75,76,77].
In PAH, HIF acts on different signaling pathways, where it is suggested that HIF-1α is mostly expressed in PASMCs, while HIF-2α and HIF-3α are expressed in PAECs, and pulmonary fibroblasts, respectively [78,79]. The contribution of HIF in the genesis of PAH and vascular remodeling, was elegantly demonstrated by Dai and cols (2016) [71] through disruption of PHD2 activity in ECs and hematopoietic cells. These authors observed that ablation of Egln1 (encoding PHD2) in ECs and hematopoietic cells (HCs; Egln1Tie2) in mice was sufficient to establish a phenotype strikingly different from the traditional models of chronic hypoxia (hypoxia- induced PH alone or hypoxia/Sugen 5416- induced PH) described by many authors [80,81,82,83]. These Egln1 tie2 Cre mice developed an irreversible obliterative vascular remodeling and pathophysiology similar to described in patients with severe PAH (idiopathic PAH), an effect that could be reverted using a HIF-2α translation inhibitor C76 (compound 76). Furthermore compound 76 also reversed PH induced with Sugen 5416/hypoxia and monocrotaline-induced in rat models PH [84].
A similar approach developed by Kapitsinou and cols (2016) [75] demonstrated that the PH response due to loss of endothelial PHD2 through genetic ablation of Phd2 individually or in conjunction with either Hif1a or Hif2a, is dependent on HIF-2 but not HIF-1; these data also suggested that HIF-2 can regulate BMPR-II levels [75]. In concordance, HIF2α increases in lung vascular endothelial cells (LVECs) isolated from IPAH patients has been implicated in endothelial-to-mesenchymal transition associated with vascular lesions and remodeling [85].
It is suggested that one of the factors that may contribute to hypoxic pulmonary vasoconstriction that occur in PAH is the increase of arginase expression in endothelial cells stimulated by HIF 2α, triggering alterations in normal nitric oxide homeostasis [86]. As arginase is an indirect collagen synthesis precursor, possible decreases of NO bioavailability causes dysregulation of vascular tone through upregulation of adhesion molecules in the endothelium, which induces immune cell recruitment to the vascular wall, and stimulates SMC proliferation and migration [87], potentially due to increases arginine metabolite availability, such as polyamines [50]. HIF2α activation in ECs also up-regulates endothelin-1 (EDN1), a vasoconstrictor molecule, through signaling mechanisms that involve inhibition of the binding of vasodilator apelin (APLN) to its receptor (APLNR) [75].
The role of HIF-1α expression and activity during vascular remodeling in PAH has also been extensively studied. HIF 1α is increased in EC plexiform lesions in the lungs of IPAH patients [88,89]. In the metabolic context, both HIF-1 and HIF-2 contribute to altered metabolic phenotypes in PAECs by modulating the expression of distinct mitochondrial enzymes such as pyruvate dehydrogenase kinase 1 (PDK1), hexokinase 1,2 (HK1,2), lactate dehydrogenase A (LDHA), and glucose transporter 1,3 (GLUT1,3). All these enzymes regulate anaerobic glycolysis and the Warburg effect (aerobic glycolysis) during PH pathogenesis [90]. In SMCs, HIF-1α is decreased in PAH patients and myosin light chain phosphorylation (pMLC), a central determinant of vascular tone, is increased in patients with PAH, suggesting that in these cells HIF-1α works inversely to promote pulmonary vascular contractility [78]. Barnes and cols [78] discuss that the increases in HIF-1α found by other reports do not correspond to isolated studies of SMC, but in those associated with total lung tissue or ECs. In animal models, systemic loss of a single HIF-1α allele (Hif1a+/−) [91] or deficiency of HIF-1α in SMCs (HIF-1α -SMM-Cre) in mice exposed to hypoxia can cause lower right ventricular systolic pressure (RVSP) and less pulmonary vascular remodeling when compared to wild type hypoxic controls, indicating that HIF-1α inactivation in SMCs attenuates hypoxia-induced PH [77]. Among the various signaling stimuli promoted by HIF, one of the factors associated with pulmonary vasoconstriction is an increase in intracellular calcium concentration mediated by the transient receptor potential channel (TRPC). This assertion was based on the fact that PASMCs from hypoxia-exposed rats show an increased expression of TRPC1 and TRPC6 mRNA and proteins, and these channels were also increased in PASMCs from normoxic animals cultured under hypoxic conditions (4% O2; 60 h) [92], validating that the presence of HIF-1α in PASMCs is necessary for the development of PH. Interestingly, increases in endothelial calcium flux are linked to EC-SMC crosstalk, where inositol 3-phosphate (IP3) released by the SMC activates the IP3 receptor in EC in internal elastic lamina microdomains [93,94,95], called the myoendothelial junction (MEJ), triggering the stimulation of vasodilator pathways, such as the NO and endothelial-derived hyperpolarizing factor (EDHF) pathway [96,97,98,99,100]. Increases in calcium, mediated by IP3, will elicit endothelial vasoconstriction [101], which will contribute to vascular remodeling.
3.2. Cell Growth Factors
HIF-dependent genes regulate growth factors released by endothelium such as vascular endothelial growth factor (VEGF) (see [88]), epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2) and platelet-derived growth factor (PDGF), which have a direct proliferative effect on neighboring PASMCs [102,103,104]. Similarly, the absence of HIF-1α in embryonic stem cells impairs cellular proliferation and VEGF expression during hypoxia [105]. In the hypoxia-induced PH model, PDGF activates the PI3Ks/AKT pathway resulting in cyclic adenosine monophosphate (cAMP) response element-binding protein depletion in PASMCs, which in turn induces switching of PASMCs from a contractile to a synthetic phenotype. This switch promotes cell proliferation, migration, and dedifferentiation, leading to pulmonary arterial remodeling [106]. PDGF-treated PASMCs exhibit significant increases in calpain activity, cell proliferation, and increased collagen-I protein level and this effect is abrogated in PASMCs lacking calpain-2 [107]. Postnatal deletion of PDGFR-β+/SMC marker+ progenitors completely prevent PH and right ventricle hypertrophy (RVH), attenuating pulmonary vascular remodeling in animal models of hypoxia induced PH [90,108]. Moreover, HIF-1α, HIF-2α or PDGF factor B (PDGF-B) knockout mice exposed to hypoxia have decreased PDGF-B mRNA accumulation in macrophages, and are protected against PH, RVH and pulmonary vascular muscularization [109].
Not only PDGF, but also Interleukin-6 (IL-6), endothelin-1 (ET1) and angiotensin II (Ang II) activate the signal transducer and activator of transcription-3 (STAT3), all of which are dysregulated in PAH patients. In a monocrotaline (MCT)-induced PH rat model a reciprocal relationship between loss of caveolin-1 (Cav-1) in PAECs and hyperactivation of phosphorylated STAT3 (pSTAT3) was found, and that was associated with an increase of proliferating cell nuclear antigen (PCNA) mRNA, indicating cell proliferation [35]. The inhibition of Janus kinase II (JAK2) impairs STAT3 activation in PAECs from PAH patients, reducing growth factors, decreasing proliferation and migration rates and promoting cell survival [36]. Furthermore, in PAECs of an ovine model of persistent PH of the newborn (PPHN), pSTAT3 binds to the eNOS promoter, decreasing its activity, thereby decreasing eNOS protein levels and NO production [37]. Together, these events contribute to migration, proliferation, and resistance to apoptosis in ECs and SMCs, which are hallmarks of vascular remodeling (Figure 1).
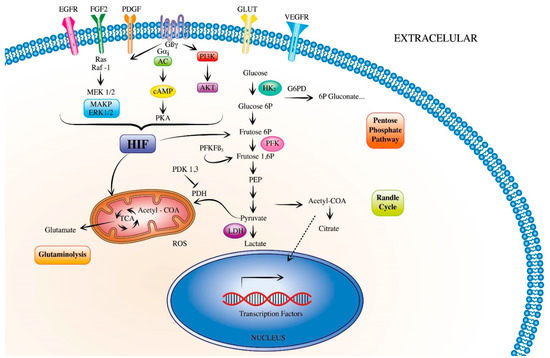
Figure 1.
Metabolic signaling pathways promoting pulmonary hypertension. During the development of pulmonary hypertension, vascular cells release growth factors, which activate the hypoxia-inducible factors (HIF). HIF triggers signaling pathways that have a direct proliferative effect in both endothelial and smooth muscle cells. HIF can also modulate metabolic pathways such as glycolysis, pentose phosphate pathway, randle cycle, glutaminolysis and tricarboxylic acid cycle, which activate several downstream pathways. (EGFR, epidermal growth factor receptor; FGF2, fibroblast growth factor 2; PDGF, platelet-derived growth factor; GLUT, glucose transporter; HIF, hypoxia-inducible factors; ROS, reactive oxygen species).
4. Role of Mitochondria in the Metabolic Reprogramming of PAH
Mitochondria are powerhouses that generate the majority of cellular energy but have other essential metabolic functions such as amino acid, lipid, and nucleotide synthesis, iron-sulfur cluster biogenesis, intermediate metabolite biogenesis and calcium homeostasis [110,111,112,113,114]. Importantly, mitochondrial signaling pathways can regulate innate immunity, programmed cell death as well as maintain cell survival and proliferation. These pleotropic functions make them essential to maintaining health of the organism. Thus, mitochondrial dysfunction is the primary cause of a number of metabolic diseases that are classified according to 3 types of genetic abnormalities, mitochondria DNA defects, nuclear DNA defects, or communication between mitochondria and nuclear DNA defects [115]. Multiple types of mitochondrial dysfunction have been identified in PAH and have contributed to the metabolic theory of PAH pathogenesis. However, as discussed below, activation or perturbation of any particular mitochondrial process, i.e., mitochondrial dynamics or bioenergetics, does not occur in isolation as modulation of these pathways may occur simultaneously or sequentially.
4.1. Mitochondria Dynamics
Mitochondria are arranged in dynamic networks that can rapidly undergo structural changes in response to changes in the cellular environment. These structural changes include mitochondrial division (fission), fusion and trafficking, a process termed mitochondrial dynamics [116]. Mitochondrial fission results in separation into small disconnected fragments while fusion produces larger interconnected networks that are connected to the endoplasmic reticulum. The balance between fission and fusion is a tightly controlled process and alteration of this balance affects mitochondrial quality control as well as cell apoptosis and proliferation. Mitochondrial dynamics are altered in PH since PASMCs isolated from PH patients have increased activation of dynamin related protein-1 (Drp1) and decreased expression of mitofusin-2 (Mfn2), hallmarks of mitochondrial fragmentation. Altered regulation of these proteins is associated with both increased mitochondria fragmentation and cell proliferation [117,118]. Hypoxic exposure of human PASMCs also increases Drp1 activation and mitochondrial fragmentation. Decreased membrane potential and respiration further indicated mitochondria dysfunction under these conditions. Pharmacological inhibition of Drp1 reversed the effects of hypoxia on mitochondrial structure, function and HPASMC proliferation [119].
ECs isolated from the Sugen/Hypoxia rat model also have increased mitochondrial fragmentation, proliferation, ROS, and intracellular calcium ([Ca2+]i) release via the transient receptor potential vanilloid-4 (TRPV4) calcium channel. Quenching ROS or inhibition of TRPV4 prevented mitochondrial fragmentation. Furthermore, quenching ROS prevented the [Ca2+]i increase indicating a mechanistic link between ROS generation, calcium signaling and mitochondrial dynamics [120,121]. Although activation of fission and ROS generation often occur in conjunction, the mechanism by which ROS alters mitochondrial structure in pulmonary vascular cells is not completely elucidated [121,122,123]. In addition to coordinated signaling between ROS and mitochondrial dynamics, other mitochondrial processes, i.e., biogenesis and mitophagy, are interrelated with changes in mitochondrial dynamics.
4.2. Mitochondrial Biogenesis
Mitochondrial biogenesis is the process by which mitochondrial grow and divide to increase their mass, a process that requires coordination between fission and fusion. Mitochondria can autoreplicate their genome, which encodes the 13 subunits of the electron transport chain and the 22 tRNAs and 2rRNAs necessary for their translation. Over 1000 mitochondrial proteins are transcribed by nuclear DNA and imported into mitochondria to perform many mitochondrial functions. This process requires communication between the mitochondria and nucleus. Mitochondrial biogenesis is regulated by environmental stresses such as oxidative stress and cell division and proliferation as occurs in PH [124]. Dysregulated mitochondrial biogenesis is an early indicator of endothelial dysfunction, which is characterized by modified cell metabolism, reduced nitric oxide (NO) availability, and use [125,126,127,128] and increased oxidative stress. Generation of excess mitochondrial ROS disrupts endothelial nitric oxide synthase (eNOS) signaling, which further exacerbates ROS production and leads to dysfunctional mitochondria [129,130,131,132,133].
Both mitochondrial biogenesis and oxidative metabolism are regulated by peroxisome proliferator activated receptor γ coactivator-1 alpha (PGC-1α). Upregulation of PGC-1α attenuates oxidative damage [134] and promotes mitochondrial biogenesis, while downregulation induces mitochondrial dysfunction [135]. Hypoxia leads to perturbed endothelial cell function by decreasing PGC-1α expression causing mitochondria dysfunction manifested by decreased membrane potential and ATP production. Conversely, increasing PGC-1α expression mitigates the effects of hypoxia on the endothelium by decreasing ROS generation and inflammation, and by increasing eNOS activation and ATP production [136]. Furthermore, restoration of eNOS signaling using NO derivatives recovers PGC-1α expression and promotes mitochondria biogenesis [137,138]. Hence, defective mitochondria biogenesis disrupts multiple mitochondrial pathways in PAECs. However, defective mitochondria biogenesis also affects PASMCs function in PH. Decreased expression of PGC-1α leads to increased PASMC proliferation, fission and ROS generation, and decreased mitochondrial mass and impaired bioenergetics [139].
4.3. Mitophagy
Mitophagy is the process by which damaged mitochondria are degraded and removed via the autophagy pathway in order to maintain cell survival. Increased cellular levels of LC3B-I and LC3B-II are markers of autophagy activation [140]. Coordinated signaling between mitophagy and mitochondrial dynamics maintain mitochondria health and homeostasis. Mitochondria fragmentation, mediated by Drp1, is involved in quality control by promoting removal of damaged mitochondria. Conversely, MFN1 and MFN2 mediated fusion mitigates mitochondria stress by allowing healthy mitochondria to complement damaged mitochondria [141]. Mitophagy is involved in the pathogenesis of PH as fission is elevated in PASMCs isolated from PH patients, which suggests an impairment in the mitophagic clearance of dysfunctional mitochondria [142,143]. Furthermore, lungs from both IPAH and non-IPAH pulmonary hypertension patients have increased LC3B-I/II and autophagosomes [144]. However, the role of mitophagy in PH is not completely understood as mitophagy is detrimental or protective depending on the PH model, chronic hypoxia, Sugen/Hypoxia or MCT.
Mice exposed to chronic hypoxia have increased LC3B expression in both PAECs and PASMCs. Hypoxia also increases LC3B-I/II expression and proliferation in human PAECs and PASMCs. LC3B elevation under these conditions is part of a cellular attempt to mitigate PH as deletion of LC3B, in knockout mice or using siRNA in cells, leads to both decreased vascular resistance and vascular cell proliferation [145]. In rats exposed to chronic hypoxia, LCB3 levels are not elevated. However, when these hypoxic rats are treated with 17b-estradiol, LC3B-II levels increase and are associated with protection against vascular remodeling [146]. LC3B also mitigates vascular remodeling in rats exposed to Sugen/Hypoxia. While LC3B expression is increased in the pulmonary endothelium of these animals, treatment with rapamycin further enhances the expression but decreases PAECs proliferation [147]. In contrast to the chronic hypoxia models, MCT-induced PH in rats leads to increased LC3B expression that is not associated with protection against vascular remodeling. In this model, autophagy is inhibited by choloroquine treatment but rat PASMCs have higher apoptosis and decreased proliferation [148].
4.4. Calcium Signaling and Bioenergetics
Mitochondria have an important role as cellular oxygen sensors and changes in oxygen levels alter mitochondrial function and promote vasoconstriction of the pulmonary vasculature. Hypoxia alters mitochondrial ROS (mROS) generation, which leads to inhibition of voltage-gated potassium channels causing the cell membrane to depolarize which allows an influx of Ca2+ into the cytoplasm [118]. This increase in Ca2+ causes PASMCs to contract [147]. Chronic hypoxia also activates ER stress, which decreases the influx of Ca2+ from the ER to the mitochondria. This decrease in Ca2+ suppresses mitochondria function by inhibiting Ca2+ dependent enzymes such as PDH and α-ketogutarate (α-KG) but also decreases mROS, increases mitochondria membrane potential and suppresses apoptosis [148,149,150]. The effects of chronic hypoxia on mitochondrial enzymes leads to inhibition of glucose oxidation and efficient ATP production and promotes uncoupled glycolysis in the cytosol [17,117,151].
The UCP2 protein is a member of a family of 5 uncoupling proteins which are anion transporters located on the inner mitochondria membrane and functions to dissipate protons generated from the electron transport chain. However, UCP2 does not exhibit uncoupling activity in vascular mitochondria but functions as a Ca2+ channel and regulates Ca2+ influx from the endoplasmic reticulum to mitochondria [152,153,154]. PASMCs isolated from UCP2 deficient mice have hyperpolarized mitochondria, reduced activity of Ca2+ dependent mitochondrial enzymes and resistance to apoptosis [149].
PAECs from PH patients have decreased expression of UPC2 and increased markers of mitophagy [149]. Targeted knockout of UCP2 in ECs using cre-inducible floxed mice spontaneously induces PH that is worsened by intermittent hypoxia. Mitophagy also increased as determined by increased LC3B-II lipidation. siRNA deletion of UCP2 in mouse lung ECs exposed to chemical hypoxia also resulted in increased mitophagy and decreased mitochondrial biogenesis. Notably, mitochondria membrane potential was decreased, and apoptosis was increased [155]. Interestingly, PAEC apoptosis is proposed as an early event in PH development that potentially leads to emergence of apoptosis resistant ECs that are involved in vascular remodeling [156].
5. The Effects of Inflammatory Mediators on Vascular Remodeling
Although the current understanding of the impact of the immune response in PAH is still evolving, pathological studies have shown that a large number of inflammatory cells such as B lymphocytes, T lymphocytes, mast cells, and macrophages infiltrate the vessels of PAH patients [157]. However, how the vessel layers (intima, media and adventitia) act in conjunction to promote vascular changes in response to a variety of stimuli is not yet well defined. Adventitial cells can detect and direct responses to a wide variety of stimuli through paracrine communication with other adventitial cells or with cells from neighboring tissues. The production/expression of various cytokines/chemokines, their receptors and adhesion molecules by adventitial fibroblasts and recruited monocytes, has been documented as having a central role in PH vascular remodeling [43,157,158,159,160].
Some authors consider that IPAH is, in part, an autoimmune inflammatory disease, in which activated B cells in PAH can produce a variety of autoantibodies such as anti-endothelial cell antibodies. These antibodies accumulate in the intima of the pulmonary artery, which can induce endothelial cell apoptosis and adhesion molecule expression, leading to excessive proliferation of ECs and pulmonary vascular remodeling [161]. Recent studies have pointed out that cytotoxic T lymphocytes and helper T lymphocytes are found in PAH pulmonary artery plexiform lesions, and both play a role in promoting the process of vascular remodeling [162]. In contrast, regulatory T lymphocytes have immunosuppressive effects, which can regulate the inflammatory response within the pulmonary artery wall and inhibit vascular remodeling. In addition, mast cells participate in the pulmonary vascular remodeling of PAH by secreting chymotrypsin and tryptase. Tryptase can act on its target receptor and induce PAEC proliferation by activating the extracellular regulatory protein kinase signaling pathway, leading to changes in the structure and elasticity of the pulmonary artery [163].
Activated macrophages can also present antigens to T lymphocytes, leading to the activation of T lymphocytes and the production of T lymphocyte-mediated factors, thereby further promoting the occurrence and development of PAH-related inflammatory infiltration. In PAH patients there is an increased number of activated macrophages around the pulmonary blood vessels that could induce the release of IL-1, IL-6, IL-10, TNF-α, CCL2 (MCP-1) CXCL1 (fractalkine) and CCL5 (RANTES) [157,164,165]. A schematic mechanism of vascular remodeling led by IL-6 overexpression was proposed by Furaya and cols (2010) [166] and Steiner and cols (2009) [167] using animal models. These authors hypothesized that proliferation and resistance to apoptosis in SMC is a consequence of IL-6-stimulated VEGF upregulation, which also induces BMPR2 and TGFβR downregulation. In ECs, exposure to IL-6 triggers apoptosis through the inhibition of Tie2 to a mechanism that disrupts Ang-1 expression in SMC. This stimulus induces the recruitment of inflammatory cells, such as lymphocytes and monocytes, further increasing the production of cytokines and IL-6 by ECs and SMCs. Furthermore, in patients and PAH experimental models, epigenetic changes stimulate and induce the proliferation of vascular fibroblasts, and this change is related to the activation of macrophages and the activation of a large number of pro-inflammatory cytokines mediated by HDAC1 [168].
Pro-inflammatory macrophages stimulate hexokinase 1 expression by upregulating NLRP3 inflammasome activation [169]. Inflammasome activation consists of a series of chain reactions starting with caspase-1 activation and promoting pro-IL-18 and pro-IL-1 maturation. Although it is still controversial whether glycolysis favors positively or negatively the activity of NLRP3 inflammasome. Not only hexokinase, but also PKM2 and other glycolytic regulators have been associated with NLRP3 inflammasome activity [169,170,171,172]. It has been seen that during vascular endothelial dysfunction, ECs promotes activation of programmed cell death through upregulation of caspase-1 [173]. Damage signal molecules released by PAEC can stimulate the production of IL-1β, and the combination of IL-1β and IL-1R1 can lead to the activation of NF-κB and the synthesis of IL-6 and TNF-α, which stimulate PASMC proliferation [174]. Studies have shown that after IL-1R1 knockout, IL-1β-mediated PASMC proliferation is inhibited [175] and that IL-1 antagonists can effectively reduce vascular inflammation while significantly slowing pulmonary vascular remodeling [176].
It is increasingly clear that in the vascular microenvironment of PAH, glycolysis, fatty acid oxidation and the production of reactive oxygen species involved in metabolic changes are affected by a variety of complex interactions, not only between ECs and SMCs, but also fibroblasts and macrophages. These studies show that bridging the research of metabolism and inflammation will help us to understand the pathogenesis of PAH more deeply and comprehensively, and the relationship between dysregulated immune response and metabolic changes in PAH will be an important goal in future research (see Figure 2).
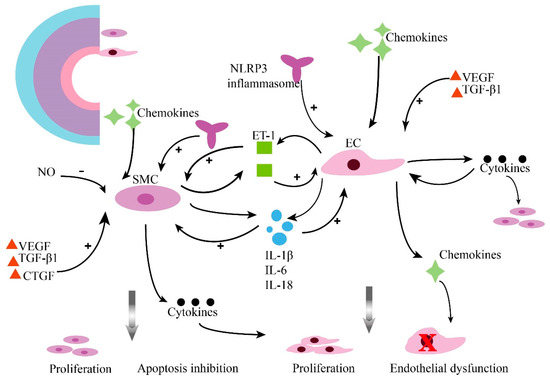
Figure 2.
Inflammation contributes to pulmonary vascular remodeling in PAH. Pulmonary vascular lesions subsist in a tremendously inflammatory microenvironment. Pulmonary arterial smooth muscle cells (PASMCs) and pulmonary arterial endothelial cells (PAECs) crosstalk, resulting in changed sensitivity to inflammatory factors and their promoted potential to promote the inflammatory response. (ET-1, endothelin 1; SMC, smooth muscle cells; EC, endothelial cells; NO, nitric oxide; VEGF, vascular endothelial growth factor; TGF-β1, transforming growth factor-β1; IL-1β, interleukin 1-beta; IL-6, interleukin 6; IL-18, interleukin-18; CTGF, connective tissue growth factor; NLRP3, Nod-like receptor protein 3).
6. Clinical Trials Targeting Metabolic and Inflammatory Signaling in PAH
6.1. Animal Models of PH
Preclinical rodent models of PH have been an essential tool used to identify pathways and molecules to target for PAH therapeutic intervention. The three major models used to induce PH in rodents are hypoxia induced PH (HPH), Sugen-hypoxia (SuHyp) and monocrotaline (MCT). These models result in mild (i.e., HPH) to severe (i.e., MCT or SuHyp) PH and display different disease characteristics based on the type of rodent, which has been extensively described by other groups [177,178]. Importantly, because of the heterogeneity of the human disease, no one model completely recapitulates the disease characteristics observed in patients. Nevertheless, these preclinical models are a critical means to discovering novel mechanisms to treat PAH and have led to clinical trials involving drugs that target multiple signaling mediators and cellular processes.
6.2. Standard PAH Interventions
Currently, therapies for PAH are categorized into four classes of drugs that were originally developed as vasodilators: (1) prostacyclin synthetics and analogs, (2) endothelin receptor antagonists (ERAs), (3) phosphodiesterase type 5 inhibitors (PDE5i) and (4) soluble guanylate cyclase (sGC) stimulators [179]. Unfortunately, none of these drugs can effectively reverse the disease, and in many cases, lung or lung and heart transplantation may be the only life-saving option for the many patients with poor prognosis. In addition, these therapies are not selective for the pulmonary circulation, and, whereas some were subsequently shown to limit the proliferative potential of vascular cells, they do not act directly on mechanisms of hyperproliferative and apoptosis-resistant remodeling of the pulmonary arteries [179].
6.3. Current Clinical Trials
Because PAH is a complex disease that involves signaling of many pathways and signaling mediators, interventions have targeted multiple pathways, which have been extensively reviewed by Sommer and cols. [3]. Studies involving compounds such as dichloroacetate (DCA), which normalizes glucose oxidation, inhibiting pyruvate dehydrogenase kinase, and trimetazidine and ranolazine, which act by modulating the balance between fatty acid and glucose oxidation, have shown promise in the treatment of PAH [45,180]. For instance, in a small trial (ClinicalTrials.gov identifier NCT01083524) dichloroacetate treatment improved hemodynamics and functional capacity in genetically susceptible patients with PAH, without clinically significant change in QT intervals, cardiac rhythm, and liver, bone marrow, and renal functions [45]. However, all patients did not respond to DCA, likely due to it is rapid clearance and small therapeutic window. Still, these studies were an important step in suggesting metabolic reprogramming as a fundamental feature of the molecular pathogenesis of PAH and reinforces the importance of understanding metabolic disturbances in disease initiation, maintenance, and mitigation via the use of therapeutic interventions. While there are multiple clinical trials underway to target various cellular processes, herein we have summarized the trials that specifically involve drugs that target metabolic and inflammatory pathways (Table 1).

Table 1.
Current PAH Clinical Trials Targeting Metabolic and Inflammation Pathways.
7. Conclusions
Several events contribute to the pathogenesis of PAH and pulmonary vascular remodeling. It is clear, however, that metabolic and inflammatory dysregulation are central to the pathogenesis of PAH. Questions remain whether these disturbances play a causal or a compensatory (mal)adaptive role in the disease. There are still multiple opportunities to better understand the role of pulmonary vascular EC-SMC behavior in the maintenance of vascular homeostasis in the response to injury and in the development of pulmonary vascular remodeling. Finally, the therapeutic role of strategies counteracting metabolic and inflammatory dysregulation in PAH needs to be established.
Author Contributions
M.T.G. and R.F.M. conceived the original scope of this manuscript. M.T.G., Y.B., S.R.P., L.Z. and A.D.L. wrote specific sections. M.T.G., A.D.L., Y.B., S.R.P. and R.F.M. critically reviewed the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01 HL127342, R01HL111656–RFM), National Nature Science Foundation of China (No. 81803530–YB), and Indiana Clinical and Translational Sciences Institute (No. UL1TR002529- from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award–ADL).
Acknowledgments
We are grateful to Alexandre Magalhães for designing the figures.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| [Ca2+]i | intracellular calcium concentration |
| Ang II | angiotensin II |
| BMPR2 | Bone Morphogenetic Protein Receptor Type 2 |
| BOECs | blood outgrowth endothelial cells |
| cAMP | cyclic adenosine monophosphate |
| Cav-1 | caveolin-1 |
| Drp1 | dynamin related protein-1 |
| ECs | endothelial cells |
| EGF | epidermal growth factor |
| ENO | enolase |
| eNOS | endothelial nitric oxide synthase (eNOS) |
| ERAs | endothelin receptor antagonists |
| ERK | extracellular signal-regulated kinase |
| ET1 | endothelin-1 |
| FGF2 | fibroblast growth factor 2 |
| G6PD | glucose-6-phosphate dehydrogenase |
| GLUT | glucose transporter |
| HIF1 | hypoxia-inducible factors 1 |
| HIF2 | hypoxia-inducible factors 2 |
| HK1,2 | hexokinase 1,2 |
| HLMVECs | human lung microvascular endothelial cells |
| HPAH | hereditary pulmonary arterial hypertension |
| HREs | hypoxia-responsive elements |
| IL18 | interleukin 18 |
| IL-6 | interleukin-6 |
| IPAH | idiopathic pulmonary arterial hypertension |
| JAK2 | Janus kinase II |
| LDHA | lactate dehydrogenase A |
| MCT | monocrotaline |
| Mfn2 | mitofusin-2 |
| MIF | migration inhibitory factor |
| miRNAs | microRNA |
| MMP | metalloproteinases |
| mPAP | mean pulmonary artery pressure |
| mROS | mitochondrial ROS |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-κB | nuclear factor-kappa B |
| NO | nitric oxide |
| OXPHOS | oxidative phosphorylation |
| PAECs | pulmonary artery endothelial cell |
| PAH | pulmonary arterial hypertension |
| PASMCs | pulmonary artery smooth muscle cells |
| PCNA | proliferating cell nuclear antigen |
| PDE5i | phosphodiesterase type 5 inhibitors |
| PDGF | platelet-derived growth factor |
| PDH | pyruvate dehydrogenase |
| PDK | pyruvate dehydrogenase kinase |
| PFKFB3 | 6-phosphofructo-2-kinase/fructose-2,6- bisphosphatase 3 |
| PGC-1α | peroxisome proliferator activated receptor gamma coactivator 1-alpha |
| PGI2 | prostaglandin 2 |
| PH | pulmonary hypertension |
| PKM | pyruvate kinase muscle |
| PPHN | persistent pulmonary hypertension of the newborn |
| PPP | pentose phosphate pathway |
| PTBP1 | polypyrimidine tract binding protein 1 |
| PVR | pulmonary vascular resistance |
| REVEAL | registry to evaluate early and long-term disease management in PAH |
| ROS | reactive oxygen species |
| RVH | right ventricle hypertrophy |
| RVSP | right ventricular systolic pressure |
| sGC | soluble guanylate cyclase |
| SIRT3 | sirtuin 3 |
| SMCs | smooth muscle cells |
| SOD | superoxide dismutase |
| STAT3 | signal transducers and activators of transcription-3 |
| TCA | tricarboxylic acid cycle |
| TGF-β1 | transforming growth factor-β1 |
| TIMP-1 | tissue inhibitors of metalloproteinases-1 |
| TNF-α | tumor necrosis factor alpha |
| TRPC | transient receptor potential |
| TRPV4 | transient receptor potential vanilloid-4 |
| UCP2 | uncoupling protein 2 |
| VEGF | vascular endothelial growth factor |
| VSMCs | vascular smooth muscle cells |
| α-KG | α-ketoglutarate |
References
- Dabral, S.; Tian, X.; Kojonazarov, B.; Savai, R.; Ghofrani, H.A.; Weissmann, N.; Florio, M.; Sun, J.; Jonigk, D.; Maegel, L.; et al. Notch1 signalling regulates endothelial proliferation and apoptosis in pulmonary arterial hypertension. Eur. Respir. J. 2016, 48, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S. Evaluation and classification of pulmonary arterial hypertension. J. Thorac. Dis. 2019, 11 (Suppl. S14), S1789–S1799. [Google Scholar] [CrossRef] [PubMed]
- Sommer, N.; Ghofrani, H.A.; Pak, O.; Bonnet, S.; Provencher, S.; Sitbon, O.; Rosenkranz, S.; Hoeper, M.M.; Kiely, D.G. Current and future treatments of pulmonary arterial hypertension. Br. J. Pharmacol. 2021, 178, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Maron, B.A. Molecular Mechanisms of Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2016, 17, 761. [Google Scholar] [CrossRef] [PubMed]
- Mandras, S.A.; Mehta, H.S.; Vaidya, A. Pulmonary Hypertension: A Brief Guide for Clinicians. Mayo Clin. Proc. 2020, 95, 1978–1988. [Google Scholar] [CrossRef]
- Tielemans, B.; Delcroix, M.; Belge, C.; Quarck, R. TGFβ and BMPRII signalling pathways in the pathogenesis of pulmonary arterial hypertension. Drug Discov. Today 2019, 24, 703–716. [Google Scholar] [CrossRef]
- Austin, E.D.; Loyd, J.E. The genetics of pulmonary arterial hypertension. Circ. Res. 2014, 115, 189–202. [Google Scholar] [CrossRef]
- Austin, E.D.; Ma, L.; LeDuc, C.; Berman Rosenzweig, E.; Borczuk, A.; Phillips, J.A., III; Palomero, T.; Sumazin, P.; Kim, H.R.; Talati, M.H.; et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ. Cardiovasc. Genet. 2012, 5, 336–343. [Google Scholar] [CrossRef]
- Ma, L.; Roman-Campos, D.; Austin, E.D.; Eyries, M.; Sampson, K.S.; Soubrier, F.; Germain, M.; Trégouët, D.-A.; Borczuk, A.; Rosenzweig, E.B.; et al. A novel channelopathy in pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 351–361. [Google Scholar] [CrossRef]
- Archer, S.L. Pyruvate Kinase and Warburg Metabolism in Pulmonary Arterial Hypertension: Uncoupled Glycolysis and the Cancer-Like Phenotype of Pulmonary Arterial Hypertension. Circulation 2017, 136, 2486–2490. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, J.; Lu, C.; Hsin, M.; Mura, M.; Wu, L.; Chu, L.; Zamel, R.; Machuca, T.; Waddell, T.; et al. Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS ONE 2014, 9, e88727. [Google Scholar] [CrossRef] [PubMed]
- Marsboom, G.; Wietholt, C.; Haney, C.R.; Toth, P.T.; Ryan, J.J.; Morrow, E.; Thenappan, T.; Bache-Wiig, P.; Piao, L.; Paul, J.D.; et al. Lung ¹⁸F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 185, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L. Mitochondrial dynamics—Mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Comhair, S.A.A.; Chen, R.; Hu, B.; Hou, Y.; Zhou, Y.; Mavrakis, L.A.; Janocha, A.J.; Li, L.; Zhang, D.; et al. Integrative proteomics and phosphoproteomics in pulmonary arterial hypertension. Sci. Rep. 2019, 9, 18623. [Google Scholar] [CrossRef]
- Uebelhoer, M.; Iruela-Arispe, M.L. Cross-talk between signaling and metabolism in the vasculature. Vasc. Pharmacol. 2016, 83, 4–9. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Carmeliet, P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013, 18, 634–647. [Google Scholar] [CrossRef]
- Oikawa, M.; Kagaya, Y.; Otani, H.; Sakuma, M.; Demachi, J.; Suzuki, J.; Takahashi, T.; Nawata, J.; Ido, T.; Watanabe, J.; et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J. Am. Coll. Cardiol. 2005, 45, 1849–1855. [Google Scholar] [CrossRef]
- Zhao, L.; Ashek, A.; Wang, L.; Fang, W.; Dabral, S.; Dubois, O.; Cupitt, J.; Pullamsetti, S.S.; Cotroneo, E.; Jones, H.; et al. Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: Potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation 2013, 128, 1214–1224. [Google Scholar] [CrossRef]
- Pedersen, P.L. Voltage dependent anion channels (VDACs): A brief introduction with a focus on the outer mitochondrial compartment′s roles together with hexokinase-2 in the “Warburg effect” in cancer. J. Bioenerg. Biomembr. 2008, 40, 123–126. [Google Scholar] [CrossRef]
- Xu, Y.; An, X.; Guo, X.; Habtetsion, T.G.; Wang, Y.; Xu, X.; Kandala, S.; Li, Q.; Li, H.; Zhang, C.; et al. Endothelial PFKFB3 plays a critical role in angiogenesis. Arter. Thromb. Vasc. Biol. 2014, 34, 1231–1239. [Google Scholar] [CrossRef]
- Kovacs, L.; Cao, Y.; Han, W.; Meadows, L.; Kovacs-Kasa, A.; Kondrikov, D.; Verin, A.D.; Barman, S.A.; Dong, Z.; Huo, Y.; et al. PFKFB3 in Smooth Muscle Promotes Vascular Remodeling in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2019, 200, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Finucane, O.M.; Sugrue, J.; Rubio-Araiz, A.; Guillot-Sestier, M.V.; Lynch, M.A. The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1β-dependent manner in macrophages. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Dai, J.; Zhou, Q.; Chen, J.; Rexius-Hall, M.L.; Rehman, J.; Zhou, G. Alpha-enolase regulates the malignant phenotype of pulmonary artery smooth muscle cells via the AMPK-Akt pathway. Nat. Commun. 2018, 9, 3850. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Tamby, M.C.; Camoin, L.; Guilpain, P.; Bérezné, A.; Tamas, N.; Broussard, C.; Hotellier, F.; Humbert, M.; Simonneau, G.; et al. Antifibroblast antibodies from systemic sclerosis patients bind to {alpha}-enolase and are associated with interstitial lung disease. Ann. Rheum. Dis. 2010, 69, 428–433. [Google Scholar] [CrossRef]
- Bussone, G.; Tamby, M.C.; Calzas, C.; Kherbeck, N.; Sahbatou, Y.; Sanson, C.; Ghazal, K.; Dib, H.; Weksler, B.B.; Broussard, C.; et al. IgG from patients with pulmonary arterial hypertension and/or systemic sclerosis binds to vascular smooth muscle cells and induces cell contraction. Ann. Rheum. Dis. 2012, 71, 596–605. [Google Scholar] [CrossRef]
- Caruso, P.; Dunmore, B.J.; Schlosser, K.; Schoors, S.; Dos Santos, C.; Perez-Iratxeta, C.; Lavoie, J.R.; Zhang, H.; Long, L.; Flockton, A.R.; et al. Identification of MicroRNA-124 as a Major Regulator of Enhanced Endothelial Cell Glycolysis in Pulmonary Arterial Hypertension via PTBP1 (Polypyrimidine Tract Binding Protein) and Pyruvate Kinase M2. Circulation 2018, 136, 2451–2467. [Google Scholar] [CrossRef]
- Desai, S.; Ding, M.; Wang, B.; Lu, Z.; Zhao, Q.; Shaw, K.; Yung, W.A.; Weinstein, J.N.; Tan, M.; Yao, J. Tissue-specific isoform switch and DNA hypomethylation of the pyruvate kinase PKM gene in human cancers. Oncotarget 2013, 5, 8202–8210. [Google Scholar] [CrossRef]
- Luo, W.; Hu, H.; Chang, R.; Zhong, J.; Knabel, M.; O’Meally, R.; Cole, R.N.; Pandey, A.; Semenza, G.L. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 2011, 145, 732–744. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.; Yang, J.J.; Liu, X.; Liu, Z.R. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol. Cell 2012, 45, 598–609. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Tamada, M.; Suematsu, M.; Saya, H. Pyruvate kinase M2: Multiple faces for conferring benefits on cancer cells. Clin. Cancer Res. 2012, 18, 5554–5561. [Google Scholar] [CrossRef] [PubMed]
- Paulin, R.; Courboulin, A.; Meloche, J.; Mainguy, V.; Dumas de la Roque, E.; Saksouk, N.; Côté, J.; Provencher, S.; Sussman, M.A.; Bonnet, S. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation 2011, 123, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Paulin, R.; Meloche, J.; Bonnet, S. STAT3 signaling in pulmonary arterial hypertension. Jakstat 2012, 1, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Erzurum, S.C. Endothelial cell energy metabolism, proliferation, and apoptosis in pulmonary hypertension. Compr Physiol. 2010, 1, 357–372. [Google Scholar]
- Jasmin, J.F.; Mercier, I.; Dupuis, J.; Tanowitz, H.B.; Lisanti, M.P. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation 2006, 114, 912–920. [Google Scholar] [CrossRef]
- Masri, F.A.; Xu, W.; Comhair, S.A.; Asosingh, K.; Koo, M.; Vasanji, A.; Drazba, J.; Anand-Apte, B.; Erzurum, S.C. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L548–L554. [Google Scholar] [CrossRef]
- Sud, N.; Black, S.M. Endothelin-1 impairs nitric oxide signaling in endothelial cells through a protein kinase Cdelta-dependent activation of STAT3 and decreased endothelial nitric oxide synthase expression. DNA Cell Biol. 2009, 28, 543–553. [Google Scholar] [CrossRef]
- Yang, W.; Lu, Z. Pyruvate kinase M2 at a glance. J. Cell Sci. 2015, 128, 1655–1660. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Li, M.; Plecitá-Hlavatá, L.; D’Alessandro, A.; Tauber, J.; Riddle, S.; Kumar, S.; Flockton, A.; McKeon, B.A.; et al. Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension Is Regulated Through a MicroRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein 1)/Pyruvate Kinase Muscle Axis. Circulation 2017, 136, 2468–2485. [Google Scholar] [CrossRef]
- Bienertova-Vasku, J.; Novak, J.; Vasku, A. MicroRNAs in pulmonary arterial hypertension: Pathogenesis, diagnosis and treatment. J. Am. Soc. Hypertens. 2015, 9, 221–234. [Google Scholar] [CrossRef]
- Caruso, P.; Dempsie, Y.; Stevens, H.C.; McDonald, R.A.; Long, L.; Lu, R.; White, K.; Mair, K.M.; McClure, J.D.; Southwood, M.; et al. A role for miR-145 in pulmonary arterial hypertension: Evidence from mouse models and patient samples. Circ. Res. 2012, 111, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Blanco, F.J.; Stevens, H.; Lu, R.; Caudrillier, A.; McBride, M.; McClure, J.D.; Grant, J.S.; Thomas, M.; Frid, M.G.; et al. MicroRNA-143 Activation Regulates Smooth Muscle and Endothelial Cell Crosstalk in Pulmonary Arterial Hypertension. Circ. Res. 2015, 117, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Tuder, R.M.; El Kasmi, K.C. Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J. Appl. Physiol. 2015, 119, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Fang, Y.H.; Ryan, J.J.; Piao, L. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm. Circ. 2013, 3, 144–152. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Gurtu, V.; Webster, L.; Barnes, G.; Watson, G.; Howard, L.; Cupitt, J.; Paterson, I.; Thompson, R.B.; Chow, K.; et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci. Transl. Med. 2017, 9, 413. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Plecitá-Hlavatá, L.; Ježek, J.; Ježek, P. Aglycemia keeps mitochondrial oxidative phosphorylation under hypoxic conditions in HepG2 cells. J. Bioenerg. Biomembr. 2015, 47, 467–476. [Google Scholar] [CrossRef]
- Fessel, J.P.; Hamid, R.; Wittmann, B.M.; Robinson, L.J.; Blackwell, T.; Tada, Y.; Tanabe, N.; Tatsumi, K.; Hemnes, A.R.; West, J.D.; et al. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm. Circ. 2012, 2, 201–213. [Google Scholar] [CrossRef]
- Yao, C.; Yu, J.; Taylor, L.; Polgar, P.; McComb, M.E.; Costello, C.E. Protein Expression by Human Pulmonary Artery Smooth Muscle Cells Containing a BMPR2 Mutation and the Action of ET-1 as Determined by Proteomic Mass Spectrometry. Int. J. Mass Spectrom. 2015, 378, 347–359. [Google Scholar] [CrossRef]
- D’Alessandro, A.; El Kasmi, K.C.; Plecitá-Hlavatá, L.; Ježek, P.; Li, M.; Zhang, H.; Gupte, S.A.; Stenmark, K.R. Hallmarks of Pulmonary Hypertension: Mesenchymal and Inflammatory Cell Metabolic Reprogramming. Antioxid. Redox. Signal 2018, 28, 230–250. [Google Scholar] [CrossRef]
- Dorfmüller, P.; Chaumais, M.C.; Giannakouli, M.; Durand-Gasselin, I.; Raymond, N.; Fadel, E.; Mercier, O.; Charlotte, F.; Montani, D.; Simonneau, G.; et al. Increased oxidative stress and severe arterial remodeling induced by permanent high-flow challenge in experimental pulmonary hypertension. Respir. Res. 2011, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Chettimada, S.; Gupte, R.; Rawat, D.; Gebb, S.A.; McMurtry, I.F.; Gupte, S.A. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: Implication in pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L287–L300. [Google Scholar] [CrossRef] [PubMed]
- Chettimada, S.; Rawat, D.K.; Dey, N.; Kobelja, R.; Simms, Z.; Wolin, M.S.; Lincoln, T.M.; Gupte, S.A. Glc-6-PD and PKG contribute to hypoxia-induced decrease in smooth muscle cell contractile phenotype proteins in pulmonary artery. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L64–L74. [Google Scholar] [CrossRef] [PubMed]
- Lakhkar, A.; Dhagia, V.; Joshi, S.R.; Gotlinger, K.; Patel, D.; Sun, D.; Wolin, M.S.; Schwartzman, M.L.; Gupte, S.A. 20-HETE-induced mitochondrial superoxide production and inflammatory phenotype in vascular smooth muscle is prevented by glucose-6-phosphate dehydrogenase inhibition. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1107–H1117. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, S.C.; Chen, K.F.; Lai, Y.Y.; Tsai, S.J. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J. Biol. Chem. 2008, 283, 28106–28114. [Google Scholar] [CrossRef]
- Paulin, R.; Dromparis, P.; Sutendra, G.; Gurtu, V.; Zervopoulos, S.; Bowers, L.; Haromy, A.; Websterm, L.; Provencher, S.; Bonnet, S.; et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab. 2014, 20, 827–839. [Google Scholar] [CrossRef]
- Archer, S.L.; Marsboom, G.; Kim, G.H.; Zhang, H.J.; Toth, P.T.; Svensson, E.C.; Dyck, J.R.; Gomberg-Maitland, M.; Thébaud, B.; Husain, A.N.; et al. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: A basis for excessive cell proliferation and a new therapeutic target. Circulation 2010, 121, 2661–2671. [Google Scholar] [CrossRef]
- Egnatchik, R.A.; Brittain, E.L.; Shah, A.T.; Fares, W.H.; Ford, H.J.; Monahan, K.; Kang, C.J.; Kocurek, E.G.; Zhu, S.; Luong, T.; et al. Dysfunctional BMPR2 signaling drives an abnormal endothelial requirement for glutamine in pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 186–199. [Google Scholar] [CrossRef]
- Fang, Y.H.; Piao, L.; Hong, Z.; Toth, P.T.; Marsboom, G.; Bache-Wiig, P.; Rehman, J.; Archer, S.L. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: Exploiting Randle’s cycle. J. Mol. Med. 2012, 90, 31–43. [Google Scholar] [CrossRef]
- Sutendra, G.; Bonnet, S.; Rochefort, G.; Haromy, A.; Folmes, K.D.; Lopaschuk, G.D.; Dyck, J.R.B.; Michelakis, E.D. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci. Transl. Med. 2010, 2, 44ra58. [Google Scholar] [CrossRef]
- Dang, C.V. Glutaminolysis: Supplying carbon or nitrogen or both for cancer cells? Cell Cycle 2010, 9, 3884–3886. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Fang, Y.H.; Cadete, V.J.; Wietholt, C.; Urboniene, D.; Toth, P.T.; Marsboom, G.; Zhang, H.J.; Haber, I.; Rehman, J.; et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: Resuscitating the hibernating right ventricle. J. Mol. Med. 2010, 88, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Fang, Y.H.; Parikh, K.; Ryan, J.J.; Toth, P.T.; Archer, S.L. Cardiac glutaminolysis: A maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J. Mol. Med. 2013, 91, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Pongratz, R.L.; Kibbey, R.G.; Shulman, G.I.; Cline, G.W. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J. Biol. Chem. 2007, 282, 200–207. [Google Scholar] [CrossRef]
- Xu, W.; Janocha, A.J.; Erzurum, S.C. Metabolism in Pulmonary Hypertension. Annu. Rev. Physiol. 2021, 83, 551–576. [Google Scholar] [CrossRef]
- McCurdy, C.E.; Schenk, S.; Hetrick, B.; Houck, J.; Drew, B.G.; Kaye, S.; Lashbrook, M.; Bergman, B.C.; Takahashi, D.L.; Dean, T.A.; et al. Maternal obesity reduces oxidative capacity in fetal skeletal muscle of Japanese macaques. JCI Insight 2016, 1, e86612. [Google Scholar] [CrossRef]
- Hernandez-Saavedra, D.; Sanders, L.; Freeman, S.; Reisz, J.A.; Lee, M.H.; Mickael, C.; Kumar, R.; Kassa, B.; Gu, S.; D’Alessandro, A.; et al. Stable isotope metabolomics of pulmonary artery smooth muscle and endothelial cells in pulmonary hypertension and with TGF-beta treatment. Sci. Rep. 2020, 10, 413. [Google Scholar] [CrossRef]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Hao, J.; Tai, Y.; Tang, Y.; Zhang, Y.-Y.; et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef]
- Dumas, S.J.; Bru-Mercier, G.; Courboulin, A.; Quatredeniers, M.; Rücker-Martin, C.; Antigny, F.; Nakhleh, M.K.; Ranchoux, B.; Gouadon, E.; Vinhas, M.-C.; et al. NMDA-Type Glutamate Receptor Activation Promotes Vascular Remodeling and Pulmonary Arterial Hypertension. Circulation 2018, 137, 2371–2389. [Google Scholar] [CrossRef]
- Waypa, G.B.; Schumacker, P.T. Roles of HIF1 and HIF2 in pulmonary hypertension: It all depends on the context. Eur. Respir. J. 2019, 54, 1901929. [Google Scholar] [CrossRef]
- Dai, Z.; Li, M.; Wharton, J.; Zhu, M.M.; Zhao, Y.Y. Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2α. Circulation 2016, 133, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Slemc, L.; Kunej, T. Transcription factor HIF1A: Downstream targets, associated pathways, polymorphic hypoxia response element (HRE) sites, and initiative for standardization of reporting in scientific literature. Tumour Biol. 2016, 37, 14851–14861. [Google Scholar] [CrossRef]
- Kapitsinou, P.P.; Rajendran, G.; Astleford, L.; Michael, M.; Schonfeld, M.P.; Fields, T.; Shay, S.; French, J.L.; West, J.; Haase, V.J. The Endothelial Prolyl-4-Hydroxylase Domain 2/Hypoxia-Inducible Factor 2 Axis Regulates Pulmonary Artery Pressure in Mice. Mol. Cell Biol. 2016, 36, 1584–1594. [Google Scholar] [CrossRef]
- Dai, J.; Zhou, Q.; Tang, H.; Chen, T.; Li, J.; Raychaudhuri, P.; Yuan, J.X.-J.; Zhou, G. Smooth muscle cell-specific FoxM1 controls hypoxia-induced pulmonary hypertension. Cell Signal 2018, 51, 119–129. [Google Scholar] [CrossRef]
- Ball, M.K.; Waypa, G.B.; Mungai, P.T.; Nielsen, J.M.; Czech, L.; Dudley, V.J.; Beussink-Nelson, M.L.; Dettman, R.; Berkelhamer, S.K.; Steinhorn, R.H.; et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am. J. Respir. Crit. Care Med. 2014, 189, 314–324. [Google Scholar] [CrossRef]
- Barnes, E.A.; Chen, C.H.; Sedan, O.; Cornfield, D.N. Loss of smooth muscle cell hypoxia inducible factor-1α underlies increased vascular contractility in pulmonary hypertension. FASEB J. 2016, 31, 650–662. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, S.; Malcolm, K.C.; Miller, S.M.; Hendry-Hofer, T.; Schaack, J.B.; White, C.W. Differential regulation of pulmonary vascular cell growth by hypoxia-inducible transcription factor-1α and hypoxia-inducible transcription factor-2α. Am. J. Respir. Cell Mol. Biol. 2013, 49, 78–85. [Google Scholar] [CrossRef]
- Shimoda, L.A.; Semenza, G.L. HIF and the lung: Role of hypoxia-inducible factors in pulmonary development and disease. Am. J. Respir. Crit. Care Med. 2011, 183, 152–156. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Fagan, K.A.; Frid, M.G. Hypoxia-induced pulmonary vascular remodeling: Cellular and molecular mechanisms. Circ. Res. 2006, 99, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Ciuclan, L.; Bonneau, O.; Hussey, M.; Duggan, N.; Holmes, A.M.; Good, R.; Stringer, R.; Jones, P.; Morrell, N.W.; Jarai, G.; et al. A novel murine model of severe pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2011, 184, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Vitali, S.H.; Hansmann, G.; Rose, C.; Fernandez-Gonzalez, A.; Scheid, A.; Mitsialis, S.A.; Kourembanas, S. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: Long-term follow-up. Pulm. Circ. 2014, 4, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhu, M.M.; Peng, Y.; Machireddy, N.; Evans, C.E.; Machado, R.; Zhang, X.; Zhao, Y.-Y. Therapeutic Targeting of Vascular Remodeling and Right Heart Failure in Pulmonary Arterial Hypertension with a HIF-2alpha Inhibitor. Am. J. Respir. Crit. Care Med. 2018, 198, 1423–1434. [Google Scholar] [CrossRef]
- Tang, H.; Babicheva, A.; McDermott, K.M.; Gu, Y.; Ayon, R.J.; Song, S.; Wang, Z.; Gupta, A.; Zhou, T.; Sun, X.; et al. Endothelial HIF-2α contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L256–L275. [Google Scholar]
- Cowburn, A.S.; Crosby, A.; Macias, D.; Branco, C.; Colaço, R.D.; Southwood, M.; Toshner, M.; Alexander, L.E.C.; Morrell, N.; Chilvers, E.; et al. HIF2α-arginase axis is essential for the development of pulmonary hypertension. Proc. Natl. Acad. Sci. USA 2018, 113, 8801–8806. [Google Scholar] [CrossRef]
- Lerman, A.; Burnett, J.C., Jr. Intact and altered endothelium in regulation of vasomotion. Circulation 1992, 86 (Suppl. S6), III12–III19. [Google Scholar]
- Tuder, R.M.; Chacon, M.; Alger, L.; Wang, J.; Taraseviciene-Stewart, L.; Kasahara, Y.; Cool, C.D.; Bishop, E.; Geraci, M.; Semenza, G.L.; et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: Evidence for a process of disordered angiogenesis. J. Pathol. 2001, 195, 367–374. [Google Scholar] [CrossRef]
- Fijalkowska, I.; Xu, W.; Comhair, S.A.; Janocha, A.J.; Mavrakis, L.A.; Krishnamachary, B.; Zhen, L.; Mao, T.; Richter, A.; Erzurum, S.C.; et al. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am. J. Pathol. 2010, 176, 1130–1138. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Mamazhakypov, A.; Weissmann, N.; Seeger, W.; Savai, R. Hypoxia-inducible factor signaling in pulmonary hypertension. J. Clin. Investig. 2020, 130, 5638–5651. [Google Scholar] [CrossRef]
- Yu, A.Y.; Shimoda, L.A.; Iyer, N.V.; Huso, D.L.; Sun, X.; McWilliams, R.; Beaty, T.; Sham, J.; Wiener, C.M.; Sylvester, J.T.; et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J. Clin. Investig. 1999, 103, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Lyle, M.A.; Davis, J.P.; Brozovich, F.V. Regulation of Pulmonary Vascular Smooth Muscle Contractility in Pulmonary Arterial Hypertension: Implications for Therapy. Front Physiol. 2017, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Isakson, B.E.; Ramos, S.I.; Duling, B.R. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ. Res. 2007, 100, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, J.; Taylor, M.S.; Bonev, A.D.; Hannah, R.M.; Solodushko, V.; Shui, B.; Tallini, Y.; Kotlikoff, M.I.; Nelson, M.T. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc. Natl. Acad. Sci. USA 2008, 105, 9627–9632. [Google Scholar] [CrossRef]
- Nausch, L.W.; Bonev, A.D.; Heppner, T.J.; Tallini, Y.; Kotlikoff, M.I.; Nelson, M.T. Sympathetic nerve stimulation induces local endothelial Ca2+ signals to oppose vasoconstriction of mouse mesenteric arteries. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H594–H602. [Google Scholar] [CrossRef]
- Dora, K.A.; Doyle, M.P.; Duling, B.R. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc. Natl. Acad. Sci. USA 1997, 94, 6529–6534. [Google Scholar] [CrossRef]
- Saliez, J.; Bouzin, C.; Rath, G.; Ghisdal, P.; Desjardins, F.; Rezzani, R.; Rodella, L.F.; Vriens, J.; Nilius, B.; Feron, O.; et al. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 2008, 117, 1065–1074. [Google Scholar] [CrossRef]
- Straub, A.C.; Billaud, M.; Johnstone, S.R.; Best, A.K.; Yemen, S.; Dwyer, S.T.; Looft-Wilson, R.; Lysiak, J.J.; Gaston, B.; Palmer, L.; et al. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 399–407. [Google Scholar] [CrossRef]
- Straub, A.C.; Lohman, A.W.; Billaud, M.; Johnstone, S.R.; Dwyer, S.T.; Lee, M.Y.; Bortz, P.S.; Best, A.K.; Columbus, L.; Gaston, B.; et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 2012, 491, 473–477. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Fisslthaler, B.; Suzuki, M.; Janssens, A.; Voets, T.; Morisseau, C.; Hammock, B.D.; Flemming, I.; Busse, R.; et al. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ. Res. 2005, 97, 908–915. [Google Scholar] [CrossRef]
- Yashiro, Y.; Duling, B.R. Integrated Ca(2+) signaling between smooth muscle and endothelium of resistance vessels. Circ. Res. 2000, 87, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.; Fanburg, B.L.; Beasley, D. Hypoxia and hypoxia-inducible factor-1alpha promote growth factor-induced proliferation of human vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2528–H2534. [Google Scholar] [CrossRef] [PubMed]
- Budhiraja, R.; Tuder, R.M.; Hassoun, P.M. Endothelial dysfunction in pulmonary hypertension. Circulation 2004, 109, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Morrell, N. Pathways in pulmonary arterial hypertension: The future is here. Eur. Respir. Rev. 2012, 21, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef]
- Li, M.X.; Jiang, D.Q.; Wang, Y.; Chen, Q.Z.; Ma, Y.J.; Yu, S.S.; Wang, Y. Signal Mechanisms of Vascular Remodeling in the Development of Pulmonary Arterial Hypertension. J. Cardiovasc. Pharmacol. 2016, 67, 182–190. [Google Scholar] [CrossRef]
- Kovacs, L.; Han, W.; Rafikov, R.; Bagi, Z.; Offermanns, S.; Saido, T.C.; Black, S.M.; Su, Y. Activation of Calpain-2 by Mediators in Pulmonary Vascular Remodeling of Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2016, 54, 384–393. [Google Scholar] [CrossRef]
- Sheikh, A.Q.; Saddouk, F.Z.; Ntokou, A.; Mazurek, R.; Greif, D.M. Cell Autonomous and Non-cell Autonomous Regulation of SMC Progenitors in Pulmonary Hypertension. Cell Rep. 2018, 23, 1152–1165. [Google Scholar] [CrossRef]
- Ntokou, A.; Dave, J.M.; Kauffman, A.C.; Sauler, M.; Ryu, C.; Hwa, J.; Herzog, E.L.; Singh, I.; Saltzman, W.M.; Greif, D.M. Macrophage-derived PDGF-B induces muscularization in murine and human pulmonary hypertension. JCI Insight 2021, 6, e139067. [Google Scholar] [CrossRef]
- Ito, K.; Suda, T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat. Rev. Mol. Cell Biol. 2014, 15, 243–256. [Google Scholar] [CrossRef]
- Kasahara, A.; Scorrano, L. Mitochondria: From cell death executioners to regulators of cell differentiation. Trends Cell Biol. 2014, 24, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Sciacovelli, M.; Guzzo, G.; Morello, V.; Frezza, C.; Zheng, L.; Nannini, N.; Calabrese, F.; Laudiero, G.; Esposito, F.; Landriscina, M.; et al. The mitochondrial chaperone TRAP1 promotes neoplastic growth by inhibiting succinate dehydrogenase. Cell Metab. 2013, 17, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417. [Google Scholar] [PubMed]
- di Mauro, S. DVD. Diseases of Mitochondrial Metabolism. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects; Lippincott-Raven: Philadelphia, PA, USA, 1999. [Google Scholar]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Dasgupta, A.; Huston, J.; Chen, K.H.; Archer, S.L. Mitochondrial dynamics in pulmonary arterial hypertension. J. Mol. Med. 2015, 93, 229–242. [Google Scholar] [CrossRef]
- Ryan, J.J.; Archer, S.L. Emerging concepts in the molecular basis of pulmonary arterial hypertension: Part I: Metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation 2015, 131, 1691–1702. [Google Scholar]
- Parra, V.; Bravo-Sagua, R.; Norambuena-Soto, I.; Hernández-Fuentes, C.P.; Gómez-Contreras, A.G.; Verdejo, H.E.; Mellado, R.; Chiong, M.; Lavandero, S. Inhibition of mitochondrial fission prevents hypoxia-induced metabolic shift and cellular proliferation of pulmonary arterial smooth muscle cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2891–2903. [Google Scholar] [CrossRef]
- Suresh, K.; Servinsky, L.; Jiang, H.; Bigham, Z.; Yun, X.; Kliment, C.; Huetsch, J.; Damarla, M.; Shimoda, L.A. Reactive oxygen species induced Ca(2+) influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L893–L907. [Google Scholar]
- Suresh, K.; Servinsky, L.; Jiang, H.; Bigham, Z.; Zaldumbide, J.; Huetsch, J.C.; Kliment, C.; Acoba, M.G.; Kirsch, B.J.; Claypool, S.M.; et al. Regulation of mitochondrial fragmentation in microvascular endothelial cells isolated from the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L639–L652. [Google Scholar] [CrossRef]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.H.; Rossignol, R.; Dieteren, C.E.; Murphy, M.P.; Koopman, W.J. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar]
- Elinoff, J.M.; Rame, J.E.; Forfia, P.R.; Hall, M.K.; Sun, J.; Gharib, A.M.; Abd-Elmoniem, K.; Graninger, G.; Harper, B.; Danner, R.L.; et al. A pilot study of the effect of spironolactone therapy on exercise capacity and endothelial dysfunction in pulmonary arterial hypertension: Study protocol for a randomized controlled trial. Trials 2013, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Farrero, M.; Blanco, I.; Batlle, M.; Santiago, E.; Cardona, M.; Vidal, B.; Castel, M.A.; Sitges, M.; Barberà, J.A.; Perez-Villa, F. Pulmonary hypertension is related to peripheral endothelial dysfunction in heart failure with preserved ejection fraction. Circ. Heart Fail. 2014, 7, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Good, R.B.; Gilbane, A.J.; Trinder, S.L.; Denton, C.P.; Coghlan, G.; Abraham, D.J.; Holmes, A.M. Endothelial to Mesenchymal Transition Contributes to Endothelial Dysfunction in Pulmonary Arterial Hypertension. Am. J. Pathol. 2015, 185, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Bhunia, A.; Oh, Y.J.; Chang, F.; Bergman, Y.; Kim, J.H.; Serbo, J.; Boronina, T.N.; Cole, R.N.; Van Eyk, J.; et al. OxLDL triggers retrograde translocation of arginase2 in aortic endothelial cells via ROCK and mitochondrial processing peptidase. Circ. Res. 2014, 115, 450–459. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Blake, R.; Trounce, I.A. Mitochondrial dysfunction and complications associated with diabetes. Biochim. Biophys. Acta 2014, 1840, 1404–1412. [Google Scholar] [CrossRef]
- Makarenko, V.V.; Usatyuk, P.V.; Yuan, G.; Lee, M.M.; Nanduri, J.; Natarajan, V.; Kumar, G.K.; Prabhakar, N.R. Intermittent hypoxia-induced endothelial barrier dysfunction requires ROS-dependent MAP kinase activation. Am. J. Physiol. Cell Physiol. 2014, 306, C745–C752. [Google Scholar] [CrossRef]
- Marzetti, E.; Csiszar, A.; Dutta, D.; Balagopal, G.; Calvani, R.; Leeuwenburgh, C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: From mechanisms to therapeutics. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H459–H476. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.R. Mitochondrial bioenergetics and therapeutic intervention in cardiovascular disease. Pharmacol. Ther. 2014, 141, 13–20. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Song, I.S.; Lee, S.Y.; Jeong, S.H.; Lee, S.R.; Heo, H.J.; Thu, V.T.; Kim, N.; Ko, K.S.; Rhee, B.D.; et al. B7-H4 downregulation induces mitochondrial dysfunction and enhances doxorubicin sensitivity via the cAMP/CREB/PGC1-α signaling pathway in HeLa cells. Pflugers Arch. 2014, 466, 2323–2338. [Google Scholar] [CrossRef]
- Ye, J.X.; Wang, S.S.; Ge, M.; Wang, D.J. Suppression of endothelial PGC-1α is associated with hypoxia-induced endothelial dysfunction and provides a new therapeutic target in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L1233–L1242. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Eis, A.; Alexander, M.; Michalkiewicz, T.; Teng, R.J.; Lakshminrusimha, S.; Konduri, G.G. Decreased endothelial nitric oxide synthase expression and function contribute to impaired mitochondrial biogenesis and oxidative stress in fetal lambs with persistent pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L40–L49. [Google Scholar] [CrossRef]
- Simon, M.A.; Vanderpool, R.R.; Nouraie, M.; Bachman, T.N.; White, P.M.; Sugahara, M.; Gorcsan, J.; Parsley, E.L.; Gladwin, M.T. Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction. JCI Insight 2016, 1, e89620. [Google Scholar] [CrossRef]
- Yeligar, S.M.; Kang, B.Y.; Bijli, K.M.; Kleinhenz, J.M.; Murphy, T.C.; Torres, G.; Martin, A.S.; Sutliff, R.L.; Hart, C.M. PPARγ Regulates Mitochondrial Structure and Function and Human Pulmonary Artery Smooth Muscle Cell Proliferation. Am. J. Respir. Cell Mol. Biol. 2018, 58, 648–657. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Aggarwal, S.; Mannam, P.; Zhang, J. Differential regulation of autophagy and mitophagy in pulmonary diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L433–L452. [Google Scholar] [CrossRef]
- Marsboom, G.; Toth, P.T.; Ryan, J.J.; Hong, Z.; Wu, X.; Fang, Y.H.; Thenappan, T.; Piao, L.; Zhang, H.J.; Pogoriler, J.; et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012, 110, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Smith, A.; Guo, L.; Alastalo, T.P.; Li, M.; Sawada, H.; Liu, X.; Chen, Z.-H.; Ifedigbo, E.; Jin, Y.; et al. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2011, 183, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Lahm, T.; Albrecht, M.; Fisher, A.J.; Selej, M.; Patel, N.G.; Brown, J.A.; Justice, M.J.; Brown, M.B.; Van Demark, M.; Trulock, K.M.; et al. 17β-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am. J. Respir. Crit. Care Med. 2012, 185, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Kato, F.; Sakao, S.; Takeuchi, T.; Suzuki, T.; Nishimura, R.; Yasuda, T.; Tanabe, N.; Tatsumi, K. Endothelial cell-related autophagic pathways in Sugen/hypoxia-exposed pulmonary arterial hypertensive rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L899–L915. [Google Scholar] [CrossRef]
- Long, L.; Yang, X.; Southwood, M.; Lu, J.; Marciniak, S.J.; Dunmore, B.J.; Morrell, N.W. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ. Res. 2013, 112, 1159–1170. [Google Scholar] [CrossRef]
- Sutendra, G.; Dromparis, P.; Kinnaird, A.; Stenson, T.H.; Haromy, A.; Parker, J.M.R.; McMurtry, M.S.; Michelakis, E.D. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene 2012, 32, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Denton, R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 2009, 1787, 1309–1316. [Google Scholar] [CrossRef]
- Dromparis, P.; Paulin, R.; Sutendra, G.; Qi, A.C.; Bonnet, S.; Michelakis, E.D. Uncoupling protein 2 deficiency mimics the effects of hypoxia and endoplasmic reticulum stress on mitochondria and triggers pseudohypoxic pulmonary vascular remodeling and pulmonary hypertension. Circ. Res. 2013, 113, 126–136. [Google Scholar] [CrossRef]
- Sutendra, G.; Dromparis, P.; Wright, P.; Bonnet, S.; Haromy, A.; Hao, Z.; McMurtry, M.S.; Michalak, M.; Vance, J.E.; Sessa, W.C.; et al. The role of Nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension. Sci. Transl. Med. 2011, 3, 88ra55. [Google Scholar] [CrossRef]
- Hagan, G.; Southwood, M.; Treacy, C.; Ross, R.M.; Soon, E.; Coulson, J.; Sheares, K.; Screaton, N.; Pepke-Zaba, J.; Morrell, N.W.; et al. (18)FDG PET imaging can quantify increased cellular metabolism in pulmonary arterial hypertension: A proof-of-principle study. Pulm. Circ. 2011, 1, 448–455. [Google Scholar] [CrossRef]
- Graier, W.F.; Trenker, M.; Malli, R. Mitochondrial Ca2+, the secret behind the function of uncoupling proteins 2 and 3? Cell Calcium 2008, 44, 36–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trenker, M.; Malli, R.; Fertschai, I.; Levak-Frank, S.; Graier, W.F. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 2007, 9, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Waldeck-Weiermair, M.; Malli, R.; Naghdi, S.; Trenker, M.; Kahn, M.J.; Graier, W.F. The contribution of UCP2 and UCP3 to mitochondrial Ca(2+) uptake is differentially determined by the source of supplied Ca(2+). Cell Calcium 2010, 47, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Haslip, M.; Dostanic, I.; Huang, Y.; Zhang, Y.; Russell, K.S.; Jurczak, M.J.; Mannam, P.; Giordano, F.; Erzurum, S.C.; Lee, P.J. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1166–1178. [Google Scholar] [CrossRef]
- Jin, Y.; Choi, A.M. Cross talk between autophagy and apoptosis in pulmonary hypertension. Pulm. Circ. 2012, 2, 407–414. [Google Scholar] [CrossRef]
- Savai, R.; Pullamsetti, S.S.; Kolbe, J.; Bieniek, E.; Voswinckel, R.; Fink, L.; Scheed, A.; Ritter, C.; Dahal, B.K.; Vater, A.; et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 897–908. [Google Scholar] [CrossRef]
- El Kasmi, K.C.; Pugliese, S.C.; Riddle, S.R.; Poth, J.M.; Anderson, A.L.; Frid, M.G.; Li, M.; Pullamsetti, S.S.; Savai, R.; Nagel, M.A.; et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J. Immunol. 2014, 193, 597–609. [Google Scholar] [CrossRef]
- Frid, M.G.; Brunetti, J.A.; Burke, D.L.; Carpenter, T.C.; Davie, N.J.; Reeves, J.T.; Roedersheimer, M.T.; van Rooijen, N.; Stenmark, K.R. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am. J. Pathol. 2006, 168, 659–669. [Google Scholar] [CrossRef]
- Pugliese, S.C.; Poth, J.M.; Fini, M.A.; Olschewski, A.; El Kasmi, K.C.; Stenmark, K.R. The role of inflammation in hypoxic pulmonary hypertension: From cellular mechanisms to clinical phenotypes. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L229–L252. [Google Scholar] [CrossRef]
- Kuebler, W.M.; Bonnet, S.; Tabuchi, A. Inflammation and autoimmunity in pulmonary hypertension: Is there a role for endothelial adhesion molecules? (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 2045893218757596. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Tamosiuniene, R.; Nicolls, M.R. Challenges and opportunities in treating inflammation associated with pulmonary hypertension. Expert Rev. Cardiovasc. Ther. 2016, 14, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Akers, I.A.; Parsons, M.; Hill, M.R.; Hollenberg, M.D.; Sanjar, S.; Laurent, G.J.; McAnulty, R. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L193–L201. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Chang, M.S.; Lee, C.; Liang, O.D.; Liu, X.; Fernandez-Gonzalez, A.; Mitsialis, S.A.; Kourembanas, S. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 2011, 123, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Dorfmüller, P.; Zarka, V.; Durand-Gasselin, I.; Monti, G.; Balabanian, K.; Garcia, G.; Capron, F.; Coulomb-Lherminé, A.; Marfaing-Koka, A.; Simonneau, G.; et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2002, 165, 534–539. [Google Scholar] [CrossRef]
- Furuya, Y.; Satoh, T.; Kuwana, M. Interleukin-6 as a potential therapeutic target for pulmonary arterial hypertension. Int. J. Rheumatol. 2010, 2010, 720305. [Google Scholar] [CrossRef]
- Steiner, M.K.; Syrkina, O.L.; Kolliputi, N.; Mark, E.J.; Hales, C.A.; Waxman, A.B. Interleukin-6 overexpression induces pulmonary hypertension. Circ. Res. 2009, 104, 236–244. [Google Scholar] [CrossRef]
- Li, M.; Riddle, S.R.; Frid, M.G.; El Kasmi, K.C.; McKinsey, T.A.; Sokol, R.J.; Strassheim, D.; Meyrick, B.; Yeager, M.E.; Flockton, A.R.; et al. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J. Immunol. 2011, 187, 2711–2722. [Google Scholar] [CrossRef]
- Moon, J.S.; Hisata, S.; Park, M.A.; DeNicola, G.M.; Ryter, S.W.; Nakahira, K.; Choi, A.M. mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. Cell Rep. 2015, 12, 102–115. [Google Scholar] [CrossRef]
- Xie, M.; Yu, Y.; Kang, R.; Zhu, S.; Yang, L.; Zeng, L.; Sun, X.; Yang, M.; Billiar, T.R.; Wang, H.; et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 2016, 7, 13280. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef]
- Meyers, A.K.; Zhu, X. The NLRP3 Inflammasome: Metabolic Regulation and Contribution to Inflammaging. Cells 2020, 9, 1808. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Zhang, Y.; Xu, Y.; Yang, W.Y.; Jiang, X.; Sha, X.; Cheng, X.; Wang, J.; Qin, X.; Yu, J.; et al. Caspase-1 Inflammasome Activation Mediates Homocysteine-Induced Pyrop-Apoptosis in Endothelial Cells. Circ. Res. 2016, 118, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Abu-Remaileh, M.; Akkawi, R.; Knani, I.; Udi, S.; Pacold, M.E.; Tam, J.; Aqeilan, R.I. WWOX somatic ablation in skeletal muscles alters glucose metabolism. Mol. Metab. 2019, 22, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Parpaleix, A.; Amsellem, V.; Houssaini, A.; Abid, S.; Breau, M.; Marcos, E.; Sawaki, D.; Delcroix, M.; Quarck, R.; Maillard, A.; et al. Role of interleukin-1 receptor 1/MyD88 signalling in the development and progression of pulmonary hypertension. Eur. Respir. J. 2016, 48, 470–483. [Google Scholar] [CrossRef]
- Christersdottir, T.; Pirault, J.; Gisterå, A.; Bergman, O.; Gallina, A.L.; Baumgartner, R.; Lundberg, A.M.; Eriksson, P.; Yan, Z.-Q.; Paulsson-Berne, G.; et al. Prevention of radiotherapy-induced arterial inflammation by interleukin-1 blockade. Eur. Heart J. 2019, 40, 2495–2503. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.Y.; Potus, F.; Chan, W.; Archer, S.L. Models and Molecular Mechanisms of World Health Organization Group 2 to 4 Pulmonary Hypertension. Hypertension 2018, 71, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.L.; Yeager, M.E. Animal Models of Pulmonary Hypertension: Matching Disease Mechanisms to Etiology of the Human Disease. J. Pulm. Respir. Med. 2014, 4, 198. [Google Scholar]
- Sutendra, G.; Michelakis, E.D. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014, 19, 558–573. [Google Scholar] [CrossRef]
- Qi, L.; Lv, T.; Cheng, Y.; Yu, M.; Han, H.; Kong, H.; Xie, W.; Wang, H.; Zhang, Y.; Huang, Z. Fasudil dichloroacetate (FDCA), an orally available agent with potent therapeutic efficiency on monocrotaline-induced pulmonary arterial hypertension rats. Bioorg. Med. Chem. Lett. 2019, 29, 1812–1818. [Google Scholar] [CrossRef]
- Howard, L.S.G.E.; He, J.; Watson, G.M.J.; Huang, L.; Wharton, J.; Luo, Q.; Kiely, D.G.; Condliffe, R.; Pepke-Zaba, J.; Morrell, N.W.; et al. Supplementation with Iron in Pulmonary Arterial Hypertension. Two Randomized Crossover Trials. Ann. Am. Thorac. Soc. 2021, 18, 981–988. [Google Scholar] [CrossRef]
- Han, Y.; Forfia, P.; Vaidya, A.; Mazurek, J.A.; Park, M.H.; Ramani, G.; Chan, S.Y.; Waxman, A.B. Ranolazine Improves Right Ventricular Function in Patients With Precapillary Pulmonary Hypertension: Results From a Double-Blind, Randomized, Placebo-Controlled Trial. J. Card. Fail. 2021, 27, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, R.T.; Badesch, D.; Chung, L.; Domsic, R.T.; Medsger, T.; Pinckney, A.; Keyes-Elstein, L.; D’Aveta, C.; Spychala, M.; White, R.J.; et al. Safety and Efficacy of B-Cell Depletion with Rituximab for the Treatment of Systemic Sclerosis Associated Pulmonary Arterial Hypertension: A Multi-center, Double-blind, Randomized, Placebo-controlled Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 209–221. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).