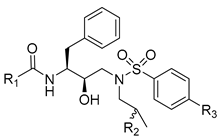
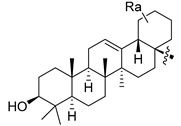
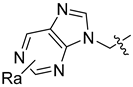
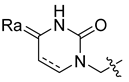
Abstract
The effective antiviral agents that treat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are urgently needed around the world. The 3C-like protease (3CLpro) of SARS-CoV-2 plays a pivotal role in virus replication; it also has become an important therapeutic target for the infection of SARS-CoV-2. In this work, we have identified Darunavir derivatives that inhibit the 3CLpro through a high-throughput screening method based on a fluorescence resonance energy transfer (FRET) assay in vitro. We found that the compounds 29# and 50# containing polyphenol and caffeine derivatives as the P2 ligand, respectively, exhibited favorable anti-3CLpro potency with EC50 values of 6.3 μM and 3.5 μM and were shown to bind to SARS-CoV-2 3CLpro in vitro. Moreover, we analyzed the binding mode of the DRV in the 3CLpro through molecular docking. Importantly, 29# and 50# exhibited a similar activity against the protease in Omicron variants. The inhibitory effect of compounds 29# and 50# on the SARS-CoV-2 3CLpro warrants that they are worth being the template to design functionally improved inhibitors for the treatment of COVID-19.
1. Introduction
The pandemic of COVID-19 caused by infection with SARS-CoV-2 has had an unprecedented impact on the health and economic development of the world, which has led to over 611 million cumulative cases as of 23 September 2022 (Coronavirus disease (COVID-19) (who.int)). SARS-CoV-2 is a positive-sense enveloped RNA virus with a single-strand, which shares about 80% of its RNA genome identity with SARS-CoV of the genus Betacoronaviru [1,2]. According to the World Health Organization (WHO), there are no clinically effective vaccines and targeted therapeutics for SARS-CoV-2 infections. Up to now, although several medications are currently being evaluated in clinical trials, and some kinds of vaccines have been used to prevent the occurrence of SARS-CoV-2, it will take a long time to effectively cure patients or vaccinate the world population faced with viral mutants. Therefore, effective and curative treatment measures are still urgently needed for COVID-19.
SARS-CoV-2 is an RNA virus that is ~30,000 nt in length. Its two overlapping open reading frames (ORF)1a and ORF1b, located at two-thirds of the viral genome from the 5′ terminus, encode two polyproteins, pp1a and pp1ab [1,3]. These replicase polyproteins will be cleaved by two different proteins, the papain-like cysteine protease (PLpro) and 3CLpro, encoded in the ORF1a to generate 16 nonstructural proteins (NSPs) for viral genome transcription and replication [4]. The 3CLpro, also referred to as the main protease (Mpro), plays an important role in the maturation of SARS-CoV-2, and the inhibition of the activity of the 3CLpro is expected to block the viral replication. Meanwhile, the sequence alignment shows that SARS-CoV-2 3CLpro is about 96% and 50% identical to the Mpro of SARS-CoV and MERS-CoV, respectively, which also reveals a structural similarity of the 3CLpro among the three CoVs [1]. The 3CLpro cleaves 11 out of 14 of the conserved sites, and it prefers to recognize the substrates involving the Leu-Gln ≤ ↓ (Ser, Ala, Gly) sequence; additionally, there are no known human proteases with a similar cleavage specificity [5,6]. Overall, the conservation features of the 3CLpro and its functional importance in the viral life cycle have turned it into an attractive antiviral target for the development of broad-spectrum, less toxic, and effective anti-CoVs drugs.
Several crystal structures of the unliganded SARS-CoV-2 3CLpro and its complex with inhibitors have been resolved [7,8,9]. The studies have suggested that these compounds, N3 and ebselen [8], 11a and 11b [9], and improved α-ketoamide inhibitors [7], show the potential to develop as promising drug candidates against COVID-19. The availability of the crystal structure of SARS-CoV-2 3CLpro also promotes many initial computational studies to screen for compounds or existing drugs against COVID-19 [10,11,12,13,14,15]. At present, several studies have been reported to identify the potential inhibitors targeting the 3CLpro of SARS-CoV-2. The oral SARS-CoV-2 3CLpro inhibitor nirmatrelvir has been approved by the FDA in combined use with ritonavir (Paxlovid) for Covid 19 treatment [16,17,18]. The study of the molecular complexation between each inhibitor and SARS-CoV-2 3CLpro indicated that both drugs interact well with the residues at the active site of SARS-CoV-2 3CLpro [19]. It is reported that several HIV-1 protease inhibitors (HIV-1 PIs) approved by the FDA are active against SARS-CoV-2 and may have demonstrated antiviral activity towards the 3-chymotrypsin-like protease (3CLpro) in the emergency phase of the COVID-19 pandemic. Darunavir, a protease inhibitor used in HIV therapy [20,21], with cobicistat as a pharmaco-enhancer, has also been proposed as a COVID-19 treatment [22]. However, evidence for the efficacy of DRV/c in COVID-19 patients is scarce. Inspired by the above, we test several kinds of HIV-1 protease inhibitors, which were designed and synthesized in our laboratory in this study, and found that Darunavir derivatives 29# and 50# containing polyphenol and caffeine derivative as the P2 ligand, respectively, exhibited favorable anti-SARS-CoV-2 potency with IC50 values of 6.3 and 3.5 μM in vitro, as well as EC50 values of 8.9 and 13.5 μM at the cellular level, respectively. Meanwhile, 29# and 50# have been identified and showed high-binding affinities to SARS-CoV-2 3CLpro, which further suggested their strong potential to develop as COVID-19 drugs. Our results suggest that compounds 29# and 50# are worthy to act as hit compounds that will further improve functionally for the treatment of the SARS-CoV-2 infection.
2. Results
2.1. Establishing a High-Throughput Screening System with FRET Assay Targeting SARS-CoV-2 3CLpro
To identify candidate inhibitors accurately and quickly, we first established a high-throughput screening system with a FRET assay targeting SARS-CoV-2 3CLpro in vitro [9,23]. The full-length gene encoding SARS-CoV-2 3CLpro was codon-optimized and inserted into the pET-28a(+) vector, whereafter it was transformed into the BL21(DE3) Escherichia Coli strain and high-yieldingly expressed with a His-tag in its C-terminus. The recombinant 3CLpro was purified with a Ni-NTA column to high purity (Figure 1). The enzymatic activity of the 3CLpro was then evaluated by a fluorescently labeled peptide substrate, MCA-AVLQ↓SGFR-Lys(Dnp)-Lys-NH2, based on the auto-cleavage sequence of the 3CLpro N-terminal. Additionally, the FRET assay had an obvious difference between the negative group (substrate + inactivating 3CLpro) and workgroup (substrate + activating 3CLpro). The assay was further optimized for high-throughput screening by the assessment of its statistical parameters, as previously described [24], to determine the performance and robustness of this assay. The results showed that all the Z factors (0.84), %CV (13%), and S/N(22.3), met the criteria required for the high-throughput screening (HTS) assay (Figure 1). Together, we successfully established a high-sensitivity and high-throughput screening system with the FRET assay to find the inhibitors targeting the recombinant 3CLpro.
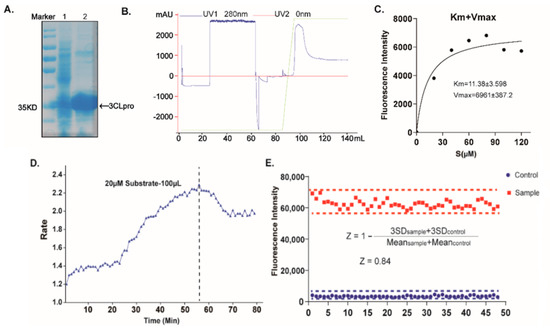
Figure 1.
Establishing a high-throughput screening system with FRET assay. (A) SDS–PAGE analysis of the 3CLpro. Lane 1 represents the molecular mass markers, 35 kDa. Lane 2 shows the cell lysate, and lane 3 shows the 3CLpro with the tag, respectively. (B) Purification flow-through curve. (C) Measurements of kinetic parameters of the 3CLpro. (D) Dependence of the 3CLpro reaction rate on the reaction time. (E) Summary of statistical parameters to assess the robustness of the HTS assay.
2.2. Screening of DRV Derivatives against SARS-CoV-2 3CLpro
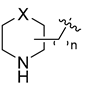
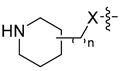
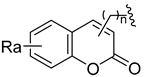
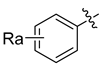
Using the high-throughput FRET assay, we screened a collection of DRV derivatives to identify potential 3CLpro inhibitors (Table 1). These DRV derivatives are grouped based on their structure, including pentacyclic triterpenoids [25], purine or pyrimidine bases [26,27], morpholines [28], piperidines, coumarin derivatives [29], phenols or polyphenols [30,31], caffeine derivatives, and so on.

Table 1.
HIV-1 protease inhibitors with different types in the P2 ligand.
The primary screening of these derivatives was conducted at a single concentration of 20 μM. We used nirmatrelvir, ebselen, and darunavir as comparisons, respectively, and DMSO-treated wells served as an intraplate negative control for the data normalization [8]. At least triplicate repeats were performed for each tested compound and the control. The results show that nirmatrelvir and ebselen can strongly inhibit 3CLpro activity, as reported(Figure 2). The DRV exhibited no inhibitory activity using this assay, which is consistent with the efficacy of the DRV/c in clinics [22]. The initial hits were defined as those active compounds that displayed cleaving efficiency with a significant difference compared with that of the control group, which identified two compounds (29# and 50#) with significant inhibition against SARS-CoV-2 3CLpro. Among the hits, 29# contains a polyphenol P2 ligand, and 50# contains a caffeine P2 ligand.
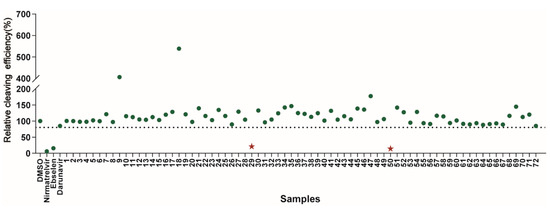
Figure 2.
Results of the high-throughput screening. Twenty µM of the compounds was pre-incubated with 100 nM of SARS-CoV-2 3CLpro for 30 min at 30 °C, and then a 10 µM FRET substrate was added to the reaction mixture to initiate the reaction. The reaction was monitored for 2 h. The initial velocity was calculated by linear regression using the data points from the first 15 min of the reaction. The calculated initial velocity with each compound was normalized to DMSO control. The results are the average of three repeats. The asterisk indicates 29# contains a polyphenol P2 ligand, and 50# contains a caffeine P2 ligand.
2.3. The Inhibitory Activity of Hits against SARS-CoV-2 3CLpro In Vitro
To verify the screening results, we further characterized the inhibitory activities of these hits against SARS-CoV-2 3CLpro in a dose-response experiment to determine their IC50 values. The FRET values were measured, and the inhibitory rates were calculated for the inhibitor treatments compared to the DMSO control, which was then plotted as dose-response curves for determining the inhibitor efficacies of the IC50 values. The results showed that nirmatrelvir and ebselen could dose-dependently inhibit the enzymatic activity of the 3CLpro with an IC50 value of 3.24 μM and 3.60 μM (Figure 3), which are comparable to the reported IC50 value. We also observed a gradual decrease in the cleavage efficacy of the 3CLpro under the treatment of our hits (29# and 50#) with an IC50 value of 6.30 μM and 3.50 μM, respectively. As a counter screen, we measured the activity of 29# and 50# against Cathepsin L, a member of the lysosomal cysteine protease [32]. The result showed that both the compounds exhibited a very limited inhibitory effect (Figure S1), providing evidence to support the specific activity of 29# and 50# against the 3CLpro.
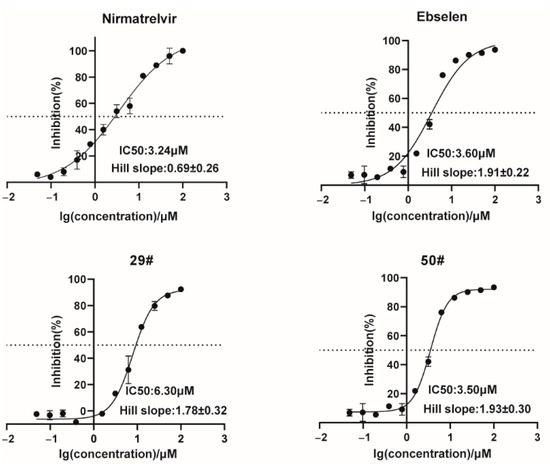
Figure 3.
Dose-response inhibition curves of selected hits against the 3CLpro. The hits with different concentrations were pre-incubated with 100 nM of SARS-CoV-2 3CLpro for 30 min at 30 °C, and then a 10 µM FRET substrate was added to the reaction mixture to initiate the reaction. The reaction was monitored for 2 h. The initial velocity was calculated by linear regression using the data points from the first 15 min of the reaction. The calculated initial velocity with each compound was normalized to the DMSO control. The results are an average ±standard deviation of three repeats.
2.4. Binding of Hits to SARS-CoV-2 3CLpro
We next performed a bio-layer interferometry (BLI) kinetic binding assay to assess the binding capacity of these hits to SARS-CoV-2 3CLpro in vitro in expectation of exploring the possible mechanisms of these hits against SARS-CoV-2 3CLpro. The purified SARS-CoV-2 3CLpro was immobilized on the biosensor surface and then incubated with different concentrations of the hits. Meanwhile, a binding assay tested the fitting association and dissociation response curves of the inhibitors after different concentration hits and made a blank control containing only the sample buffer for reference.
The BLI results revealed that 29# and 50# bind to SARS-CoV-2 3CLpro in a dose-dependent manner (Figure 4 and Table 2). The kinetic affinity constant values (KD) of the hits were determined. 29# and 50# exhibited high affinity with the KD values of 52 μM and 62 μM, respectively. These results further support and explain the indirect function of the nominated hits that were provided by the enzymatic inhibition assay in vitro on the anti-SARS-CoV-2 3CLpro activity. To rule out the possibility of non-specific binding, we measured the binding affinity of 29# and 50# with the Norovirus RNA-dependent RNA polymerase (RdRp) using the BLI assay (Figure S2). The results showed either a large KD value or a not-detected KD value, suggesting the specific binding of 29# and 50# to SARS-CoV-2 3CLpro.
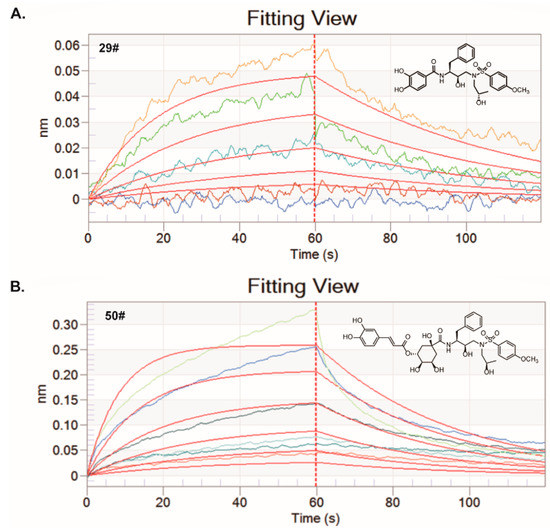
Figure 4.
BLI assays for the binding of the hits to the 3CLpro protein in vitro. Both of the hits at different serially diluted concentrations were titrated into a fixed concentration of the labeled 3CLpro protein (100 nM). (A) The kinetic fitting curves for the interaction between 29# and 3CLpro protein was analyzed by BLI assay. Five different concentrations including 6.25, 12.5, 25, 50, and 100 µM were set. (B) The kinetic fitting curves for the interaction between 50# and 3CLpro protein was analyzed by BLI assay. Six different concentrations including 6.25, 12.5, 25, 50, 100 and 200 µM were set.

Table 2.
Kinetics of 29# and 50# binding to the 3CLpro in the ForteBio’s Octet System.
2.5. Modes of Interaction for SARS-CoV-2 3CLpro with Hits
To explore the binding mode of the identified inhibitors, molecular docking studies of the DRV and its two derivatives (29# and 50#) against SARS-CoV-2 3CLpro were performed by AutoDock Vina. The calculated binding energies (DGADV) of the DRV, 29#, and 50# were −7.1 kcal/mol, −7.8, and −7.6 kcal/mol, respectively. Due to their common structural skeleton, all the compounds presented a similar arrangement within the binding cavity of SARS-CoV-2 3CLpro (Figure 5). As shown in Figure 5A, the binding pocket of the DRV is located in the catalytic cavity of SARS-CoV-2 3CLpro. The benzene ring of the DRV formed a π–π interaction with H41, one of the catalytic dyad residues that are important for the proteolytic activity of SARS-CoV-2 3CLpro. Compared with the DRV, 29# formed two additional H-bonds with the 3CLpro active site residues, T25 and N142 (Figure 5B), respectively. As for inhibitor 50#, after the introduction of the o-dihydroxybenzene group (Figure 5C), 50# formed a H-bond with E166, a key residue for the dimerization of the 3CLpro [8]. Additionally, another H-bond interaction was observed between 50# and the active site residue, Q192. This may account for the stronger inhibitory activity of DRV derivatives 29# and 50#. Taken together, the data suggest a direct interaction between the hits and SARS-CoV-2 3CLpro.
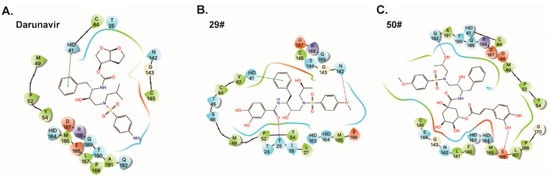
Figure 5.
Predicted binding poses for darunavir (A) and its two derivatives (B,C). Residues within 5 Å of the compounds were shown on the 2D ligand interaction diagram. For clarity, only polar hydrogen atoms were shown. Here, the color code is that dark blue is positively charged, red is negatively charged, cyan is polar, and green is hydrophobic. Hydrogen bonding is depicted as the magenta arrows, π-cation interactions are depicted as the red line, and π–π interactions are depicted as the green line.
In addition, in the predicted binding poses of compounds 50# and 29#, the phenolic hydroxyl groups at the P2 position formed H-bonds with the 3CLpro active site residues, such as N142, T25, and E66. This phenolic hydroxyl group may be largely absent in those series of compounds that show no activity against SARS-CoV-2 3CLpro. Thus, we speculated that the introduction of the phenolic hydroxyl group at the P2 position may improve the inhibitory effect of the compounds.
2.6. The Inhibitory Activity of Hits against SARS-CoV-2 3CLpro at Cellular Level
To further confirm and characterize the inhibitory activities of these hits against SARS-CoV-2 3CLpro at the cellular level, we performed a cell-based bioluminescence resonance energy transfer (BRET) assay, as previously described [33]. HEK293T cells were transfected with plasmids expressing pEYFP-linker-Rluc and SARS-CoV-2 3CLpro before being treated with increasing doses of the inhibitors. The BRET values were then measured, and the inhibitory rates were calculated for the inhibitor treatments compared to the DMSO control, which was then plotted as dose-response curves for determining the inhibitor efficacies of the EC50 values (Figure 6A). From the results, the EC50 values of the two hits ranged from 8.9 to 13.5 μM, with the positive control, ebselen, at 9.2 μM. Meanwhile, we also evaluated the cytotoxicity of the inhibitor hits using a Cell Counting Kit-8 (CCK-8) assay. The results show that the CC50 values of the two hits are higher than 24 μM (Figure 6C), which generally rules out the false positives due to significant cell toxicity. Ebselen has extremely low cytotoxicity (the median lethal dose in rats is >4600 mg kg−1 when taken orally), and darunavir is the latest protease inhibitor approved by the FDA; both are safe in humans and have been evaluated in a number of clinical trials [7,34]. It should be noted that the relatively low selectivity indexes of 29# and 50# suggest that their activity might be in part due to cytotoxicity; therefore, further optimization is clearly needed for future development.
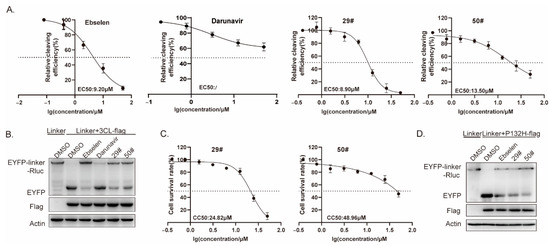
Figure 6.
Effect of hits on 3CL Omicron variants. (A) HEK293T cells were co-transfected with the 3CL-flag and EYFP-linker-Rluc plasmid DNA. At 12 h post-transfection, the cells were re-seeded in 96-well plates (104/well) and treated with the serially diluted hits. After 36 h, the BRET ratio was measured. (B,D) HEK293T cells (4 × 105) were co-transfected with the 3CL-flag(or P132H-flag) and EYFP-linker-Rluc plasmid DNA. At 12 h post-transfection, the cells were treated with the hits (20 μM). After 48 h post-transfection, cells were collected for a Western blot. (C) CC50 values in the cells were measured using CCK-8 kits. All data are expressed as the mean ± SD of the triplicate assays.
Next, we explored a mutation, P132H, in the 3CLpro, which was included in the SARS-CoV-2 Omicron variant. Based on the predicted binding model, this mutation most unlikely affected the binding of the two hits to the 3CLpro; therefore, no difference in the susceptibility was expected. Whereafter, we performed a Western blot assay to validate the inhibition of the two hits on the SARS-CoV-2 3CLpro enzymatic activity. In line with it, we found a similar effect of the hits on the cleavage efficiency of the 3CLpro containing the mutation P132H (Figure 6D).
3. Discussion
The Coronaviruses such as SARS, MERS, and SARS-CoV-2 that broke out in 2019 have caused three large-scale pandemics in human history during the past 20 years. With the ongoing outbreak of SARS-CoV-2, scientists and researchers around the world are facing finding effective antiviral drugs or vaccines with different strategies in a short time [35]. One of the quickest and relatively safest ways to find therapeutic drugs is to repurpose the clinical drug. Remdesivir, the viral polymerase inhibitor, shows the greatest treatment effect and is being evaluated in several clinical trials [36], and it also obtained emergency-use authorization from the FDA. Lopinavir and Ritonavir, which are HIV drugs, were also reported in some research. However, the combination of the two HIV drugs failed in a clinical trial for the SARS-CoV-2 infection, as no significant therapeutic efficacy was observed [37].
To address this urgent medical need, we designed and initiated a drug repurposing screening to identify effective inhibitors against SARS-CoV-2 3CLpro from the structurally improved protease inhibitors that were approved by the FDA. The Mpro has been shown to be a validated antiviral drug target for SARS-CoV and MERS-CoV [38]. As SARS-CoV-2 3CLpro (also referred to as Mpro) shares high sequence similarity with SARS-CoV and, to a lesser extent, with MERS-CoV, studies have also reasoned that inhibiting the enzymatic activity of SARS-CoV-2 3CLpro will similarly prevent viral replication [6]. Herein, we designed and synthesized a series of compounds improved from several kinds of HIV-1 protease inhibitors that had been used in clinical. Through testing with a high-throughput screening system with a recombinational 3CLpro, we found that compounds 29# and 50# exhibited favorable anti-SARS-CoV-2 3CLpro potency, with IC50 values of 6.30 and 3.50 μM and EC50 values of 8.90 and 13.50 μM. Therefore, we successfully designed two hits that are worthy of being functionally improved for the treatment of COVID-19.
The known SARS-CoV-2 3CLpro inhibitors in the existing studies mainly include two types: peptide inhibitors and small molecule compounds. The reported small molecule inhibitors targeting the 3CLpro are significantly less than the peptide inhibitors, possibly because the latter are designed on the peptidomimetic substrates with known and various chemical warheads, such as α-ketoamide, aldehyde, aldehyde prodrug bisulfite, chloromethyl ketones, and others [6]. Herein, we designed compounds based on these chemical warheads. The 29# and 50# compounds have similar activity to the recently reported SARS-CoV-2 3CLpro inhibitors (ebselen, N3, and 13b), which also represent the potent and selective peptidomimetic hits designed and renovated based on the chemical warheads.
In summary, this study identified two potent SARS-CoV-2 3CLpro inhibitors with convincing enzymatic inhibition in vitro, as well as cellular antiviral activity. Further improvement based on the structure of these hits might produce clinically therapeutic drugs for SARS-CoV-2 infection.
4. Materials and Methods
4.1. Cell Culture and Transfection
HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) and a 1% penicillin-streptomycin solution (Invitrogen, Carlsbad, CA, USA) at 37 °C and 5% CO2. For cell transfection, cells were plated at a density of 2 × 105 cells/mL. After 24 h incubation, cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions.
4.2. Plasmid, Antibodies, and Reagents
The pEYFP-linker-Rluc expression construct was generated by PCR using pEYFP-N1 (Clontech, San Jose, CA, USA) as a template, with the designed primers harboring the 3CLpro recognition sequence (linker: ITSAVLQSGFRK). The PCR-amplified EYFP-linker fragment was then inserted into a pRLuc-N2 expression construct (PerkinElmer, Waltham, MA, USA). The full-length gene encoding SARS-CoV-2 3CLpro was codon-optimized and synthesized (Tsingke Biotechnology Co., Ltd., Beijing, China) and subcloned into a pcDNA3.1 vector with a C-terminal FLAG-tag to produce the pcDNA3.1-3CLpro-FLAG expression plasmid.
Coelenterazine-h was purchased from Promega. Compounds were designed and synthesized in our laboratory and prepared in DMSO. The DYKDDDDK (Flag) Tag antibody (8146) was purchased from Cell Signaling Technology, Inc (CST, Danvers, MA, USA), and the β-actin antibody (ab8224, Cambridge, UK) was purchased from Abcam.
4.3. In Vitro Enzymatic Inhibition Assay
The enzyme activity and inhibition assays have been described previously [9]. The recombinant SARS-CoV-2 3CLpro (1 μM at the final concentration) was mixed with serial dilutions of each compound in a 50 µL assay buffer (50 mM Tris-HCl, pH 7.3, 1 mM EDTA) and incubated for 5 min. The reaction was initiated by adding a 50 µL fluorogenic substrate with a final concentration of 20 µM and then incubated for 30 min with gentle shaking at room temperature. After that, the fluorescence signal at 320 nm (excitation)/405 nm (emission) was measured every 5 min and 10 times with a PerkinElmer plate reader. The Vmax of the reactions added with the compounds at various concentrations compared to the reaction added with the DMSO was calculated and used to generate IC50 curves. For each compound, IC50 values against SARS-CoV-2 3CLpro were measured at nine concentrations, and three independent experiments were performed. All experimental data were analyzed using GraphPad Prism software(San Diego, CA, USA).
4.4. BRET Assay
For the BRET assay, transfected cells were seeded in 96-well white plates with a clear bottom. After overnight incubation, the DMSO (Sigma-Aldrich, Burlington, MA, USA) or compounds were added to each well at the designated concentration. After further incubation for 48 h at 37 °C, the supernatant was discarded. A stock of coelenterazine-h was dissolved in absolute ethyl alcohol to a concentration of 1.022 mmol/L. For the luminescence assay, 100 μL of 10 μM coelenterazine-h was injected, and the luminescence was measured using a VICTORTM X5 2030 Multilabel Reader (PerkinElmer, USA).
4.5. Western Blot
HEK293T cells were seeded at a density of 2 × 105 cells per well in a 6-well plate. After overnight incubation, the DMSO (Sigma-Aldrich) or compounds were added to each well at the designated concentration. After further incubation for 48 h at 37 °C, transfected HEK293T cells were collected and lysed in a RIPA buffer containing 50 mM of Tris (pH 7.4), 150 mM of NaCl, 1% NP-40, 0.1% SDS, 1 mM of EDTA, and 0.5% sodium deoxycholate. Cell lysates were mixed with an SDS-loading buffer and boiled for 20 min, followed by electrophoresis in 10% polyacrylamide-SDS gels. Proteins were transferred onto the PVDF membrane. After blocking with 5% skim milk, the membrane was blotted with anti-flag (1:1000) or anti-β-actin (1:5000) antibodies. After incubation with HRP-conjugated secondary antibodies (1:5000), protein signals were detected with enhanced chemiluminescence.
4.6. Cell Viability Assay
Cell viability was performed by a Cell Counting Kit-8 (CCK-8) (Beyotime, China). Typically, HEK293T cells were seeded in the wells of a 96-well plate at 100 μL/well and grown for 24 h. Then, 10 μL of CCk-8 reagent and incubated for 1.5 h at 37 °C with 5% CO2. The absorbance at 450 nm was subsequently measured using an EnSpire 2300 Multilabel Reader (PerkinElmer).
4.7. Bio-Layer Interferometry (BLI) Binding Assay
All binding affinity and kinetic profiles were conducted using a ForteBio Octet RED96 instrument (ForteBio, Inc., CA, USA) equipped with super streptavidin biosensor chips (ForteBio). Purified His-tagged nsp12 (50 μg/mL) were captured via Ni-NTA biosensors (960 s, at 25 °C, with 1000 rpm). Ligand biosensors and reference biosensors were dipped into multiple concentrations of the test compounds for 60 s (kon,1/Ms), then dissociated for 60 s (kdis,1/s). The blank binding using a buffer was used to correct the baseline shift during the analysis. Data analysis on the ForteBio Octet RED instrument was performed using reference well subtraction in the ForteBio data analysis software.
4.8. Docking (or Modeling)
To explore the binding mode of the identified inhibitors, a molecular docking study of simeprevir against SARS-CoV-2 3CLpro (PDB-ID: 6M2N) was performed by AutoDock Vina. Protein structures were prepared for docking using AutoDockTools (ADT, version: 1.5.6) by removing co-crystalized water molecules, adding polar hydrogens, and merging Gasteiger charges. The active site of the 3CLpro was defined as a box of 22 Å × 22 Å × 22 Å, centered near the ligand binding region (x = −32.169 Å, y = −64.8 Å, z = 41.176 Å). The grid step size for each docking volume was set to 1 Å. Ligands of the DRV, 29#, and 50# were prepared using an Open Babel toolkit version. Auto Dock Vina software package was used for docking, as previously described [39].
4.9. Quantification and Statistical Analysis
Data are presented as the mean ± standard deviation (SD) from at least three independent experiments unless otherwise indicated and were analyzed with GraphPad Prism.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232416011/s1, Figure S1: The inhibitory activity of hits against Cathepsin L in vitro; Figure S2: Binding of hits to Norovirus RdRp.
Author Contributions
Conceptualization, S.C., Y.W., and Q.L.; methodology, L.M., Y.X., and M.Z.; software, Q.L. and D.Y.; resources, M.Z. and Y.W.; data curation, J.Z., S.G., and Y.Z.; writing—original draft preparation, J.W.; writing—review and editing, S.C. and Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences [2021-I2M-1-038, 2021-I2M-1-055, and 2022-I2M-JB-014] and the National Natural Science Foundation of China [81903679, 81971950].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the National Infrastructure of Microbial Resources (NIMR-2014-3, China) and the CAMS Collection Center of Pathogenic Micro-organisms (CAMS-CCPM-A, China) for providing valuable reagents.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 588, E6. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nature reviews. Microbiology 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Kankanamalage, A.C.G.; Kim, Y.; Damalanka, V.C.; Rathnayake, A.D.; Fehr, A.R.; Mehzabeen, N.; Battaile, K.P.; Lovell, S.; Lushington, G.H.; Perlman, S.; et al. Structure-guided design of potent and permeable inhibitors of MERS coronavirus 3CL protease that utilize a piperidine moiety as a novel design element. Eur. J. Med. Chem. 2018, 150, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Su, H.; Zhao, W.; Xie, H.; Shao, Q.; Xu, Y. What coronavirus 3C-like protease tells us: From structure, substrate selectivity, to inhibitor design. Med. Res. Rev. 2021, 41, 1965–1998. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, B.; Jiang, X.-M.; Su, H.; Li, J.; Zhao, Y.; Xie, X.; Jin, Z.; Peng, J.; Liu, F.; et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020, 368, 1331–1335. [Google Scholar] [CrossRef]
- Sk, F.; Roy, R.; Jonniya, N.A.; Poddar, S.; Kar, P. Elucidating biophysical basis of binding of inhibitors to SARS-CoV-2 main protease by using molecular dynamics simulations and free energy calculations. J. Biomol. Struct. Dyn. 2021, 39, 3649–3661. [Google Scholar] [CrossRef]
- Lobo-Galo, N.; Terrazas-López, M.; Martínez-Martínez, A.; Díaz-Sánchez, Á.G. FDA-approved thiol-reacting drugs that potentially bind into the SARS-CoV-2 main protease, essential for viral replication. J. Biomol. Struct. Dyn. 2021, 39, 3419–3427. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Zia, K.; Ashraf, S.; Uddin, R.; Ul-Haq, Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2021, 39, 2607–2616. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Parves, R.; Paul, A.S.; Uddin, N.; Rahman, S.; Al Mamun, A.; Hossain, N.; Ali, A.; Halim, M.A. A molecular modeling approach to identify effective antiviral phytochemicals against the main protease of SARS-CoV-2. J. Biomol. Struct. Dyn. 2021, 39, 3213–3224. [Google Scholar] [CrossRef] [PubMed]
- Ton, A.T.; Gentile, F.; Hsing, M.; Ban, F.; Cherkasov, A. Rapid Identification of Potential Inhibitors of SARS-CoV-2 Main Protease by Deep Docking of 1.3 Billion Compounds. Mol. Inform. 2020, 39, e2000028. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, M.; Al-Nazawi, M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020, 251, 117627. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef]
- Ahmad, B.; Batool, M.; Ain, Q.U.; Kim, M.S.; Choi, S. Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations. Int. J. Mol. Sci. 2021, 22, 9124. [Google Scholar] [CrossRef]
- Yang, K.S.; Leeuwon, S.Z.; Xu, S.; Liu, W.R. Evolutionary and Structural Insights about Potential SARS-CoV-2 Evasion of Nirmatrelvir. J. Med. Chem. 2022, 65, 8686–8698. [Google Scholar] [CrossRef]
- Nutho, B.; Mahalapbutr, P.; Hengphasatporn, K.; Pattaranggoon, N.C.; Simanon, N.; Shigeta, Y.; Hannongbua, S.; Rungrotmongkol, T. Why Are Lopinavir and Ritonavir Effective against the Newly Emerged Coronavirus 2019? Atomistic Insights into the Inhibitory Mechanisms. Biochemistry 2020, 59, 1769–1779. [Google Scholar] [CrossRef]
- Orkin, C.; DeJesus, E.; Khanlou, H.; Stoehr, A.; Supparatpinyo, K.; Lathouwers, E.; Lefebvre, E.; Opsomer, M.; Van de Casteele, T.; Tomaka, F. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naïve patients in the ARTEMIS trial. HIV Med. 2013, 14, 49–59. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Kristine, E.; Jillian Hardi, C.; Kurniawan, A. Efficacy of Lopinavir/Ritonavir Compared with Standard Care for Treatment of Coronavirus Disease 2019 (COVID-19): A Systematic Review. Infect. Disord. Drug Targets 2021, 21, e270421187364. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; De Clercq, E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nature reviews. Drug Discov. 2020, 19, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Abian, O.; Ortega-Alarcon, D.; Jimenez-Alesanco, A.; Ceballos-Laita, L.; Vega, S.; Reyburn, H.T.; Rizzuti, B.; Velazquez-Campoy, A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020, 164, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Zhu, M.; Du, X.N.; Li, Y.G.; Zhang, G.N.; Wang, J.X.; Wang, Y.C. Design, synthesis and biological evaluation of novel HIV-1 protease inhibitors with pentacyclic triterpenoids as P2-ligands. Bioorg. Med. Chem. Lett. 2019, 29, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ma, L.; Zhou, H.; Dong, B.; Wang, Y.; Wang, Z.; Zhou, J.; Zhang, G.; Wang, J.; Liang, C.; et al. Preliminary SAR and biological evaluation of potent HIV-1 protease inhibitors with pyrimidine bases as novel P2 ligands to enhance activity against DRV-resistant HIV-1 variants. Eur. J. Med. Chem. 2020, 185, 111866. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Dong, B.; Zhang, G.N.; Wang, J.X.; Cen, S.; Wang, Y.C. Synthesis and biological evaluation of new HIV-1 protease inhibitors with purine bases as P2-ligands. Bioorg. Med. Chem. Lett. 2019, 29, 1541–1545. [Google Scholar] [CrossRef]
- Zhu, M.; Dou, Y.; Ma, L.; Dong, B.; Zhang, F.; Zhang, G.; Wang, J.; Zhou, J.; Cen, S.; Wang, Y. Novel HIV-1 Protease Inhibitors with Morpholine as the P2 Ligand to Enhance Activity against DRV-Resistant Variants. ACS Med. Chem. Lett. 2020, 11, 1196–1204. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, L.; Wen, J.; Dong, B.; Wang, Y.; Wang, Z.; Zhou, J.; Zhang, G.; Wang, J.; Guo, Y.; et al. Rational design and Structure-Activity relationship of coumarin derivatives effective on HIV-1 protease and partially on HIV-1 reverse transcriptase. Eur. J. Med. Chem. 2020, 186, 111900. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, L.; Dong, B.; Zhang, G.; Wang, J.; Zhou, J.; Cen, S.; Wang, Y. Synthesis and evaluation of potent human immunodeficiency virus 1 protease inhibitors with epimeric isopropanol as novel P1’ ligands. Future Med. Chem. 2020, 12, 775–794. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, L.; Dong, B.; Zhang, G.; Wang, J.; Zhou, J.; Cen, S.; Wang, Y. Design and biological evaluation of novel HIV-1 protease inhibitors with isopropanol as P1’ ligand to enhance binding with S1’ subsite. Bioorg. Med. Chem. 2020, 28, 115623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-M.; Yang, W.-L.; Yang, F.-Y.; Zhang, L.; Huang, W.-J.; Hou, W.; Fan, C.-F.; Jin, R.-H.; Feng, Y.-M.; Wang, Y.-C.; et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target. Ther. 2021, 6, 134. [Google Scholar] [CrossRef]
- Ma, L.; Li, Q.; Xie, Y.; Zhao, J.; Yi, D.; Guo, S.; Guo, F.; Wang, J.; Yang, L.; Cen, S. Repurposing of HIV/HCV protease inhibitors against SARS-CoV-2 3CLpro. Antivir. Res. 2022, 207, 105419. [Google Scholar] [CrossRef]
- Aoki, M.; Hayashi, H.; Yedidi, R.S.; Martyr, C.D.; Takamatsu, Y.; Aoki-Ogata, H.; Nakamura, T.; Nakata, H.; Das, D.; Yamagata, Y.; et al. C-5-Modified Tetrahydropyrano-Tetrahydofuran-Derived Protease Inhibitors (PIs) Exert Potent Inhibition of the Replication of HIV-1 Variants Highly Resistant to Various PIs, including Darunavir. J. Virol. 2015, 90, 2180–2194. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Goldstein, S.A.; Rasmussen, A.L.; Robertson, D.L.; Crits-Christoph, A.; Wertheim, J.O.; Anthony, S.J.; Barclay, W.S.; Boni, M.F.; Doherty, P.C.; et al. The origins of SARS-CoV-2: A critical review. Cell 2021, 184, 4848–4856. [Google Scholar] [CrossRef] [PubMed]
- Simonis, A.; Theobald, S.J.; Fätkenheuer, G.; Rybniker, J.; Malin, J.J. A comparative analysis of remdesivir and other repurposed antivirals against SARS-CoV-2. EMBO Mol. Med. 2021, 13, e13105. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef]
- Li, Q.; Yi, D.; Lei, X.; Zhao, J.; Zhang, Y.; Cui, X.; Xiao, X.; Jiao, T.; Dong, X.; Zhao, X.; et al. Corilagin inhibits SARS-CoV-2 replication by targeting viral RNA-dependent RNA polymerase. Acta Pharm. Sin. B 2021, 11, 1555–1567. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).