Abstract
5-aminotetrazole is one of the most marked high-nitrogen tetrazole compounds. However, the structural modification of 5-aminotetrazole with nitro groups often leads to dramatically decreased molecular stability, while the N-bridging functionalization does not efficiently improve the density and performance. In this paper, we report on a straightforward approach for improving the density of 5-aminotetrazole by introducing 4-amino-3,5-dinitropyrazole. The following experimental and calculated properties show that nitropyrazole functionalization competes well with energetic performance and mechanic sensitivity. All compounds were thoroughly characterized using IR and NMR spectroscopy, elemental analysis, and differential scanning calorimetry (DSC). Two energetic compounds (DMPT-1 and DMPT-2) were further confirmed by implementing single-crystal X-ray diffraction studies. Compound DMPT-1 featured a high crystal density of 1.806 g cm−3, excellent detonation velocity (vD = 8610 m s−1), detonation pressure (P = 30.2 GPa), and impact sensitivity of 30 J.
1. Introduction
High energy density materials (HEDMs) remain a significant class of materials chemistry because energetic material has been widely applied in the military and civilian fields [1]. However, with the development of science and technology, higher requirements are put forward for new energetic materials, including high energy levels, low sensitivities, excellent thermal stability, and facile preparation. High positive heats of formation and densities constitute two significant parameters for the energetic properties of energetic compounds, where detonation pressures and velocities are proportional to the square of the densities [2]. Thus, developing effective strategies for increasing the densities of energetic materials is highly desirable.
In recent years, nitrogen-rich heterocycles have emerged as a new class of high energy density materials (HEDMs), which have been developed to meet the needs of national defense and environmental protection. Owing to their high thermal stability, high heat of formation, and enormous ring tension of azoles with C-N and N-N bonds, they have been the main building blocks for the design and preparation of nitrogen-rich materials [3,4,5,6]. Compared with other azole units, tetrazole is a highly stable heterocyclic structure with an extremely high nitrogen content and heat of formation, which may endow tetrazole-based energetic compounds with high performance and environmentally friendly properties [7,8,9,10,11].
Furthermore, 5-aminotetrazole (AT) provides a platform for a variety of functionalized tetrazoles, which have broad applications in almost all areas of energetic materials [12]. The functionalization of AT was mainly focused on modifying the tetrazolyl backbone and the substituted group, respectively. As can be seen in Scheme 1, the introduction of explosophores, e.g., azido, nitro, and nitramino groups, enhances the energetic performance remarkably. Nevertheless, balancing high energy with stability is still a highly challenging task. Based on the literature, very few outstanding compounds possess superior overall performance compared to the benchmark energetic materials [13,14,15]. For most high-energy derivatives, further practical applications are impeded by relatively poor thermal stability and sensitivity.
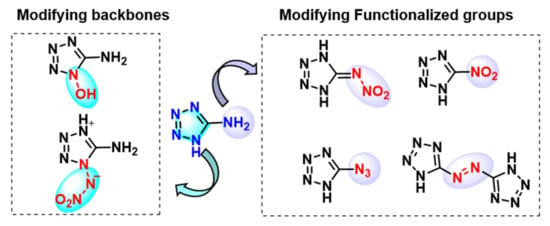
Scheme 1.
Energetic derivatives of AT from modifying the backbone and functionalized group.
Inspired by the attractive chemistry of high-nitrogen materials, a synthetic method for efficiently preparing 1-substituted 5-aminotetrazoles from cyanogen azide and amine was developed (Scheme 2a) [16]. The excellent reaction scope enables this approach to access various monocyclic and bicyclic AT derivatives [17]. In 2016, a new approach for various pyrazole derivatives with 2-haloethylamines, followed by a reaction with cyanogen azide, resulted in ethylene-bridged 5-aminotetrazole and nitropyrazole (Scheme 2b) [18]. Most of these compounds have good thermal stability and high heat of formation, however, the density of aminotetrazole-based derivatives could meet the standard of high-density compounds. As shown in Scheme 2d, most AT-based compounds display a density below 1.70 g cm−3.
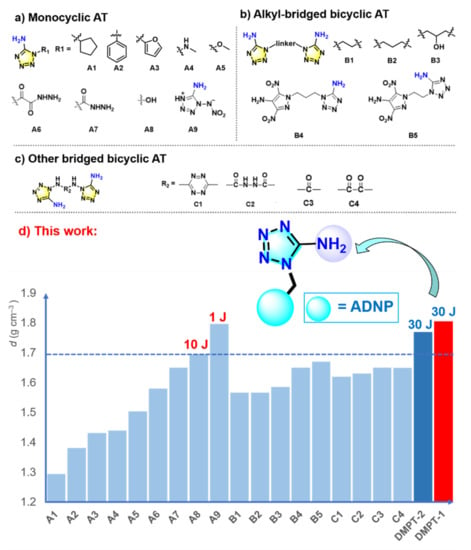
Scheme 2.
(a) Monocyclic derivatives of 5-aminotetrazoles (A9: determined by X-ray diffraction and converted into room-temperature values); (b) alkyl-bridged bicyclic 5-aminotetrazoles; (c) other bridged bicyclic 5-aminotetrazoles; (d) bar graph of density and impact sensitivity for AT-based energetic compounds.
Five-membered azoles are commonly used as the framework for the construction of nitrogen-rich heterocyclic energetic materials, such as imidazole, pyrazole, triazole, and tetrazole [19,20,21,22]. Pyrazole energetic compounds have good thermal stabilities and low sensitivities, which have been considered a beneficial building block for energetic materials [23,24]. For example, 4-amino-3,5-dinitropyrazole (ADNP) has been widely used in developing energetic materials due to their good density, low friction and impact sensitivities [25,26,27]. Herein, we reported our latest progress on the energetic derivatives of AT. By incorporating ADNP, the bicyclic AT-based compounds DMPT-1 and DMPT-2 are successfully accessed. Compared to previously reported AT derivatives, these compounds exhibit higher densities and detonation properties while retaining thermal stability and sensitivity.
2. Results and Discussion
2.1. Synthesis
The synthesis routes of compounds DMPT are shown in Scheme 3. Compound 1-(chloromethyl)-3,5-dinitro-1H-pyrazol-4-amine (CDPA) was prepared according to similar procedures in the literature [28]. The reaction of intermediate CDPA, 5-aminotetrazoles, KOH and KI at 80 °C in acetonitrile for 12 h, subjected to silica gel column chromatography, gave rise to two methylene-bridged isomers 1-((4-amino-3,5-dinitro-1H-pyrazol-1-yl)methyl)-1H-tetrazol-5-amine (DMPT-1) and 2-((4-amino-3,5-dinitro-1H-pyrazol-1-yl)methyl)-2H-tetrazol-5-amine (DMPT-2). While increasing the loading of 5-aminotetrazoles enabled an increased yield of DMPT-2, the yield of DMPT-1 was significantly reduced. We then increased the loading of 5-aminotetrazole and lowered the reaction temperature to 60 °C; this led to the production of DMPT-1 with a higher yield. Compounds DMPT-1 and DMPT-2 were all fully characterized using infrared (IR) spectroscopy, elemental analysis, and nuclear magnetic resonance (1H and 13C NMR) spectroscopy (see Supporting Information). In addition to the straightforward synthetic route, the preparation conditions and product purification are mild and simple, which contributes to the potential practical application.
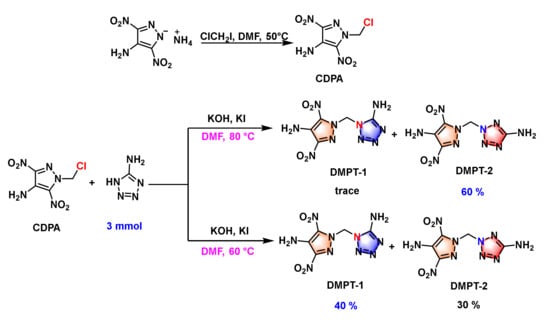
Scheme 3.
Synthesis of compounds DMPT-1 and DMPT-2.
2.2. Single-Crystal X-ray Analysis
The suitable crystals of compounds DMPT-1 and DMPT-2 were obtained by slow evaporation from a mixture of ethyl acetate and petroleum ether. Compound DMPT-1 crystallizes in the monoclinic space group P21/c with a crystal density of 1.806 g cm−3 at 296 K (Z = 4). The crystal structure of compound DMPT-1 is given in Figure 1a. The C-NO2 groups with C-N bonds lengths are 1.394(3) Å (C12-N2), 1.434(3) Å (C7-N1); compared to the other C-N bonds of N,N’-methylene bridge C4-N6, 1.441(3) Å and C4-N5, 1.452(2) Å, the C-N bonds lengths are shorter. Crystal packing of DMPT-1 exhibits a face-to-face stacking with a packing index of 74.8%. The distance between the pyrazoles (purple part) and pyrazoles (purple part) faces is 2.991 Å. The distance between the tetrazoles (blue part) and tetrazoles (blue part) faces is 3.145 Å. The N-methylene-N bridge dihedral angle between tetrazoles (blue part) and pyrazoles (purple part) ring is 87.47° for compound DMPT-1.
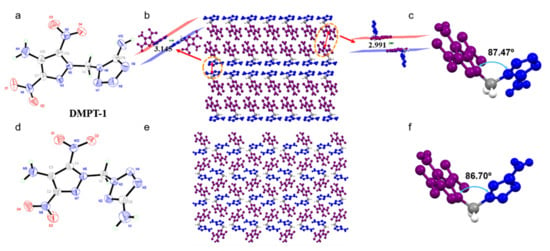
Figure 1.
Single-crystal X-ray structures of compounds (a) DMPT-1, (d) DMPT-2; packing diagrams of (b) DMPT-1, (c) DMPT-1, (e) DMPT-2, (f) DMPT-2.
Compound DMPT-2 belongs to the monoclinic P21/n space group with a crystal density of 1.770 g cm−3 at 296 K. The crystal structure of compound DMPT-2 is given in Figure 2d. The C-N bonds lengths of C-NO2 groups are 1.400(2) Å (C4-N10), 1.430(2) Å (C2-N8), which are shorter than the other C-N bonds of N,N’-methylene bridge C1-N6, 1.442(3) Å, and C1-N3, 1.455(3) Å. Crystal packing of DMPT-2 exhibits a mixing stacking with a packing index of 73.4%. The dihedral angle of methylene bridge tetrazoles (blue part) and pyrazoles (purple part) ring is 86.70° for compound DMPT-2.
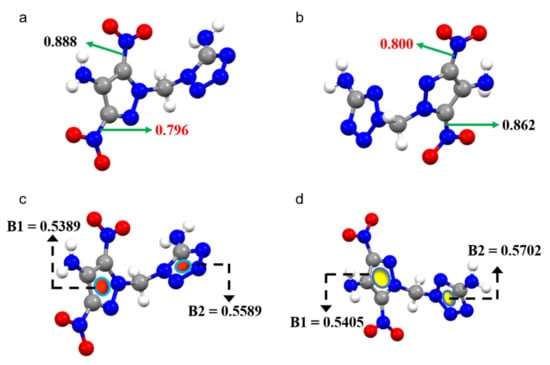
Figure 2.
(a,c) Mayer bond order and the multicenter bond order analysis for DMPT-1; (b,d) Mayer bond order and the multicenter bond orders analysis for DMPT-2.
2.3. Results and Discussion
The thermostability of energetic materials is one of the most significant parameters in assessing their safety level under severe environments. The decomposition temperature was determined using differential scanning calorimetric (DSC) measurements at a heating rate of 10 °C min−1 under a dry nitrogen atmosphere. Compound DMPT-1 exhibits the lowest decomposition temperature of 191 °C, whereas DMPT-2 has a higher thermal stability of 206 °C. In general, for polynitro energetic compounds, the C-NO2 bonds are always regarded as the weakest bonds, which can trigger the decomposition of the compound [29]. The Mayer bond orders of C-NO2 bonds for DMPT-1 and DMPT-2 were also calculated to evaluate their molecular stabilities, as shown in Figure 2a,b. The weakest Mayer bond order of C-NO2 bond for compound DMPT-1 (BN = 0.794) is shorter than that of DMPT-2 (BN = 0.797), showing that the bond strength of C-NO2 bonds in DMPT-1 is stronger than that of DMPT-2, which could explain in part the difference in thermal stability. Generally, aromaticity is considered an important parameter for the determination of molecular stability [30,31]. Therefore, the aromaticity of each heterocycle atmosphere could also be quantitatively described through the multicenter bond orders analysis using Multiwfn3.5 [32]. As shown in Figure 2c,d, the multicenter bond order of the tetrazole ring in DMPT-1 (B1 = 0.5589) is shorter than that of DMPT-2 (B1 = 0.5702), which indicates that the tetrazole ring of DMPT-2 exhibits better aromaticity than that of DMPT-1, which is consistent with their thermal behaviors.
To better explain the bond strength differences between compounds DMPT-1 and DMPT-2, their electrostatic potential (ESP) surfaces were analyzed. As shown in Figure 3, the red and blue regions represent the positive and negative potentials, respectively. In comparison to DMPT-1, the difference between the maxima and minima of ESP for the branching DMPT-2 molecule is smaller (DMPT-1: +49.83, −39.23 kcal/mol; DMPT-2: +44.96, −30.59 kcal/mol), so that DMPT-2 exhibits a more uniform charge distribution. The C-NO2 and tetrazole ring on DMPT-2 moiety exhibit minimum potential parts, which are much lower than the C-NO2 and tetrazole ring on DMPT-1, thus indicating the distinct stability of regioisomers as may be related to the ESP values (DMPT-1, Td, 191 °C; FS, 108 N; DMPT-2, Td, 209 °C; FS, 192N) [33]. In principle, the lower negative ESP values and more significant electronegative regions (blue areas) on the molecular surface often give rise to lower sensitivities, which rationalize the different mechanical sensitivities.
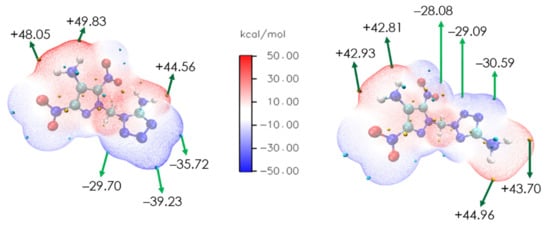
Figure 3.
The electrostatic potential surfaces for DMPT-1 and DMPT-2.
To gain insight into understanding the relationship between weak interactions and the performance in energetic materials, the two-dimensional (2D) fingerprint spectra and Hirshfeld surfaces of DMPT-1 and DMPT-2 were studied using CrystalExplorer [34]. Generally, the blue and red regions on the Hirshfeld surfaces represent low and high close contact populations, respectively. As shown in Figure 4, The Hirshfeld surfaces of DMPT-1 were near “L”-shaped structures, and the Hirshfeld surfaces of DMPT-2 were slightly distorted. In comparison to DMPT-1, the reddest spots are more evenly distributed for the DMPT-2 molecule, showing that compound DMPT-2 can form hydrogen bonds with more surrounding molecules, which suggests that the sensitivity of compound DMPT-2 (FS = 192 N) is better than that of compound DMPT-1 (FS = 108 N). At the same time, the weak interactions in these molecules are given in the 2D fingerprint plots. For DMPT-1 and DMPT-2, the proportion of O…H & H…O and N…H & H…N in the total weak interaction was 50.1% and 52.5%, respectively. They exhibit strong hydrogen bonding interactions, theoretically explaining the lower sensitivity (IS = 30 J).
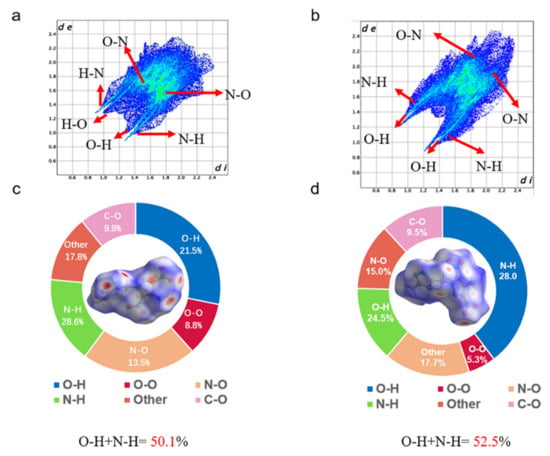
Figure 4.
The 2D fingerprint plots of DMPT-1 (a) and DMPT-2 (b); Hirshfeld surface analyses for DMPT-1 (c) and DMPT-2 (d).
2.4. Physicochemical and Energetic Properties
Detonation performance is a significant parameter of energetic materials for evaluating practical applications. The detonation performances of two compounds are calculated by EXPLO5 (v6.01) software. The values of detonation velocity (vD) and detonation pressure (P) were evaluated according to the crystal densities and calculated heats of formation. The calculated detonation velocities and detonation pressures of DMPT-1 (vD = 8618 m s−1, P = 30.3 GPa) and DMPT-2 (vD = 8450 m s−1, P = 28.6 GPa) can be seen in Table 1. Meanwhile, two compounds both exhibit better detonation velocity and detonation pressure than alkyl-bridged analogues (B5, vD = 7889 m s−1, P = 23.2 GPa, B4, vD = 7768 m s−1, P = 21.8 GPa). Compounds DMPT-1 (Td = 191 °C) and DMPT-2 (Td = 209 °C) exhibit better thermal stability compared to ADNP (Td = 178 °C) (Figure 5).

Table 1.
Physicochemical properties of DMPT.
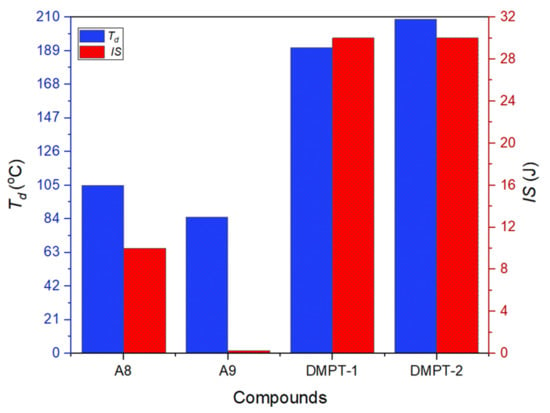
Figure 5.
Thermostability and impact sensitivity of compounds.
3. Materials and Methods
3.1. Safety Precaution
In this work, compounds DMPT-1 and DMPT-2 are potential energetic materials that tend to explode under certain external stimuli. Therefore, the whole experimental process should be carried out using proper safety equipment, such as safety shields, eye protection, and leather gloves.
3.2. General Methods
All of the reactions were carried out in the air. Ammonium 4-amino-3,5-dinitropyrazolate monohydrate [37] was prepared following procedures found in the literature. Other commercial reagents and solvents were obtained from commercial providers and used without further purification. 1H NMR and 13C NMR spectra were recorded at 25 °C on a Bruker 400 MHz and 125 MHz, respectively, and TMS as the internal standard. Chemical shifts were reported in parts per million (ppm). The onset decomposition temperature was measured using a TA Instruments DSC25 differential scanning calorimeter at a heating rate of 10 °C min−1 under a dry nitrogen atmosphere. Infrared spectra (IR) were obtained on a PerkinElmer Spectrum BX FT-IR instrument equipped with an ATR unit at 25 °C. Elemental analyses of C/H/N were investigated on a Thermo Scientific Flash 2000 Elemental Analyzer. A BAM fallhammer and friction tester tested impact and friction sensitivities. Densities were determined at room temperature by employing a Micromeritics AccuPyc 1340 gas pycnometer. The crystal structures were produced employing Mercury 2021.1.0 software.
3.3. Computational Details
The heats of formation of compounds DMPT-1 and DMPT-2 were performed by using the Gaussian 09 suite of programs [38,39]. Gas phase heats of formation of the title compounds were computed based on an isodesmic reaction (Scheme S1). The enthalpy of reaction was carried out by combining the M062X/6-311++G** energy dif-ference for the reactions, the scaled zero-point energies (ZPE), values of thermal cor-rection (HT), and other thermal factors. The solid state heats of formation were further obtained by employing Trouton’s rule according to Equation (1) (T represents either melting point or decomposition temperature when no melting occurs prior to decom-position) [40] (Scheme S1).
ΔHsub = 188/J mol−1 K−1 × T
3.4. Synthesis of Compound 1-(Chloromethyl)-3,5-dinitro-1H-pyrazol-4-amine (CDPA)
ClCH2I (2 mL) was added to a 100 mL round-bottom flask equipped with a condenser and ammonium 4-amino-3,5-dinitropyrazolate monohydrate (1 mmol) in DMF (2 mL) was dropped into ClCH2I. Then the reaction was stirred at 50 °C for 30 min. The reaction was monitored by TLC. After the reaction was complete, the reaction mixture was allowed to cool down to rt and H2O (10 mL) and ether (10 mL) were added to the vessel. The resulting suspension was extracted with ether (3 × 30 mL). The organic phases were combined and dried over Na2SO4. After filtration, the solvent was removed from the filtrate under reduced pressure. The acquired residue was subjected to silica gel column chromatography (Rf = 0.33; PE:EA = 4:1) to give 1-(chloromethyl)-3,5-dinitro-1H-pyrazol-4-amine (50 mg, 25%). 1H NMR (d6-DMSO): δ 7.25 (s, 2H), 6.46 (s, 2H) ppm. 13C NMR (d6-DMSO): δ 142.0, 130.9, 129.9, 59.8 ppm. Elemental analysis of C4H4ClN5O4 (270.06): calcd C 21.68, H 1.82, N 28.88%; found: C 21.51, H 1.79, N 28.92%. HRMS (ESI) m/z calcd for C4H3ClN5O4 (M − H)− 219.98790, found 219.98890. (Scheme S2; Table S1; Figures S1 and S2)
3.5. Synthesis of Compound 1-((4-Amino-3,5-dinitro-1H-pyrazol-1-yl)methyl)-1H-tetrazol-5-amine (DMPT-1)
1-(Chloromethyl)-3,5-dinitro-1H-pyrazol-4-amine CDPA (1 mmol), 5-aminotetrazoles (3 mmol), KOH (3 mmol), KI (1 mmol) and DMF (5 mL) were added to a 100 mL round-bottom flask equipped with a condenser. Then the reaction was stirred at 60 °C for 12 h. The reaction mixture was allowed to cool down to rt and H2O (15 mL) was added to the vessel. The resulting suspension was extracted with ethyl acetate (3 × 30 mL). The organic phases were combined and dried over Na2SO4. After filtration, the solvent was removed from the solution under reduced pressure. The acquired residue was subjected to silica gel column chromatography (Rf = 0.22; PE:EA = 1:1) to give 1-((4-amino-3,5-dinitro-1H-pyrazol-1-yl)methyl)-1H-tetrazol-5-amine (DMPT-1, 108 mg ). Yellow solid, 40% yield. Tm = 164 °C, Td (onset) = 191 °C. 1H NMR (400 MHz, d6-DMSO) δ 7.41 (s, 2H), 7.16 (s, 2H), 6.91 (s, 2H) ppm. 13C NMR (101 MHz, d6-DMSO) δ 156.0, 141.1, 131.2, 130.6, 60.3 ppm. IR (KBr): ṽ 3499, 3388, 2163, 2050, 1980, 1628, 1580, 1527, 1509, 1474, 1429, 1372, 1329, 1315, 1296, 1237, 1087, 1017, 965, 884, 825, 791, 760, 746, 735, 706, 674, 613, 541, 501 cm−1. Elemental analysis of C5H6N10O4 (270.06): calcd C 22.23, H 2.24, N 51.85%; found: C 22.36, H 2.31, N 51.72%. (Tables S1 and S2, Figures S3, S4 and S7)
3.6. Synthesis of Compound 2-((4-Amino-3,5-dinitro-1H-pyrazol-1-yl)methyl)-2H-tetrazol-5-amine (DMPT-2)
1-(Chloromethyl)-3,5-dinitro-1H-pyrazol-4-amine CDPA (1 mmol), 5-aminotetrazoles (3 mmol), KOH (3 mmol), KI (1 mmol), and DMF (5 mL) were added to a 100 mL round-bottom flask equipped with a condenser. Then the reaction was stirred at 80 °C for 12 h. The reaction mixture was allowed to cool down to rt and H2O (15 mL) was added to the vessel. The resulting suspension was extracted with ethyl acetate (3 × 30 mL). The organic phases were combined and dried over Na2SO4. After filtration, the solvent was removed from the solution under reduced pressure. The acquired residue was subjected to silica gel column chromatography (Rf = 0.28; PE:EA = 4:1)to give 2-((4-amino-3,5-dinitro-1H-pyrazol-1-yl)methyl)-2H-tetrazol-5-amine (DMPT-2, 162 mg). Yellow solid, 60% yield. Tm = 205 °C, Td (onset) = 209 °C. 1H NMR (400 MHz, d6-DMSO) δ 7.44 (s, 2H), 7.12 (s, 2H), 6.33 (s, 2H) ppm. 13C NMR (101 MHz, d6-DMSO) δ 167.6, 141.9, 130.6, 130.6, 65.4 ppm. IR (KBr): 3432, 3374, 2323, 2176, 2163, 2050, 2037, 1645, 1553, 1516, 1478, 1446, 1432,1315, 1289, 1200, 1180, 1082, 1010, 995, 893, 824, 797, 746, 712, 678, 663, 537, 513, 490, 411, 403 cm−1. Elemental analysis of C5H6N10O4 (270.06): calcd C 22.23, H 2.24, N 51.85%; found: C 22.31, H 2.35, N 51.68%. (Tables S1 and S3, Figures S5, S6 and S8)
4. Conclusions
In summary, N,N-methylene bridged 5-aminotetrazole and pyrazole were synthesized according to the methylene bridge strategy, using 5-aminotetrazole as a skeleton. A simple synthetic route, including the reaction of ammonium 4-amino-3,5-dinitropyrazolate with chloroiodomethane, followed by a reaction with 5-aminotetrazoles, was proposed to form two high-density compounds, DMPT-1 and DMPT-2. The two compounds were fully characterized using IR and NMR spectroscopic data and elemental analysis. Compared to similar N,N′-ethylene-bridged asymmetric compounds, the two methylene-bridged asymmetric compounds possess higher density and enhanced detonation performance. Among them, DMPT-1 possesses a promising overall performance (d = 1.806 g cm−3, vD = 8610 m s−1; P = 30.2 GPa). The detailed comparative properties of methylene bridged isomers indicate the pivotal role the bridging strategy plays in the energetic molecular design of novel energetic materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232415841/s1. References [38,39,40] are cited in the supplementary materials.
Author Contributions
Conceptualization, J.X.; methodology, J.X. and P.Y.; validation, J.X., J.C. (Jinjie Chang), J.C. (Jinxiong Cai) and P.Y.; formal analysis, J.X.; investigation, J.X., J.C. (Jinjie Chang) and J.C. (Jinxiong Cai); data curation, J.X.; writing—original draft preparation, J.X.; writing—review and editing, S.P., J.X. and P.Y.; supervision, P.Y. and S.P.; project administration, J.X. and P.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was completed with support from the National Natural Science Foundation of China (No. 22075023) and the China Postdoctoral Science Foundation (No. 2020M670168).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Experimental Center of Advanced Materials in BIT for the analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Sullivan, O.T.; Zdilla, M.J. Properties and promise of catenated nitrogen systems as high-energy-density materials. Chem. Rev. 2020, 120, 5682–5744. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Chen, W.; Ding, N.; Zhao, C.; Jiang, Z.; Li, S.; Pang, S. Unraveling the direct effect of hydrogen bonding on density and thermostability of energetic materials through isomerism. Chem. Eng. J. 2022, 444, 136539. [Google Scholar] [CrossRef]
- Benz, M.; Klapötke, T.M.; Krumm, B.; Lommel, M.; Stierstorfer, J. Nitrocarbamoyl azide O2NN(H)C(O)N3: A stable but highly energetic member of the carbonyl azide family. J. Am. Chem. Soc. 2021, 143, 1323–1327. [Google Scholar] [CrossRef]
- Singh, J.; Staples, R.J.; Shreeve, J.M. Pushing the limit of nitro groups on a pyrazole ring with energy-stability balance. ACS Appl. Mater. Interfaces 2021, 13, 61357–61364. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, Y.; Wang, P.; Lin, Q.; Lu, M. Novel metal-organic frameworks assembled from the combination of polynitro-pyrazole and 5-nitroamine-1,2,4-oxadiazole: Synthesis, structure and thermal properties. Dalton Trans. 2021, 50, 12906–12912. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, Y.; Wang, P.; Lin, Q.; Lu, M. Oxygen-enriched metal-organic frameworks based on 1-(Trinitromethyl)-1H-1,2,4-triazole-3-carboxylic acid and their thermal decomposition and effects on the decomposition of ammonium perchlorate. ACS Appl. Mater. Interfaces 2021, 13, 21516–21526. [Google Scholar] [CrossRef]
- Dong, Z.; Ye, Z. Synthesis and properties of salts derived from C4N182-, C4N18H3- and C4N18H3- anions. J. Mater. Chem. A 2020, 8, 25035–25039. [Google Scholar] [CrossRef]
- Yang, J.; Yin, X.; Wu, L.; Wu, J.; Zhang, J.; Gozin, M. Alkaline and earth alkaline energetic materials based on a versatile and multifunctional 1-aminotetrazol-5-one ligand. Inorg. Chem. 2018, 57, 15105–15111. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Khimeche, K. Tetrazole-functionalized microcrystalline cellulose: A promising biopolymer for advanced energetic materials. Chem. Eng. J. 2020, 400, 125960. [Google Scholar] [CrossRef]
- Gettings, M.L.; Thoenen, M.T.; Byrd, E.F.C.; Sabatini, J.J.; Zeller, M.; Piercey, D.G. Tetrazole azasydnone (C2N7O2H) and its salts: High-performing zwitterionic energetic materials containing a unique explosophore. Chem.-Eur. J. 2020, 26, 14530–14535. [Google Scholar] [CrossRef]
- Fischer, D.; Klapötke, T.M.; Stierstorfer, J. 1,5-Di(nitramino)tetrazole: High sensitivity and superior explosive performance. Angew. Chem. Int. Ed. 2015, 54, 10299–10302. [Google Scholar] [CrossRef]
- Bauer, J.; Benz, M.; Klapötke, T.M.; Stierstorfer, J. Chemistry of 2,5-diaminotetrazole. Dalton Trans. 2022, 51, 11806–11813. [Google Scholar] [CrossRef]
- Lei, C.J.; Yang, H.W.; Yang, W.; Zhuang, Q.H.; Cheng, G.B. Synthesis of ideal energetic materials with high density and performance based on 5-aminotetrazole. Cryst. Growth Des. 2022, 22, 2594–2601. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sabaté, C.M.; Stierstorfer, J. Neutral 5-nitrotetrazoles: Easy initiation with low pollution. New J. Chem. 2009, 33, 136–147. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Stierstorfer, J. The CN7− Anion. J. Am. Chem. Soc. 2009, 131, 1122–1134. [Google Scholar] [CrossRef]
- Joo, Y.H.; Shreeve, J.M. 1-substituted 5-aminotetrazoles: Syntheses from CNN3 with primary amines. Org. Lett. 2008, 20, 4665–4667. [Google Scholar] [CrossRef]
- Joo, Y.H.; Twamley, B.; Garg, S.; Shreeve, J.M. Energetic nitrogen-rich derivatives of 1,5- diaminotetrazole. Angew. Chem. Int. Ed. 2008, 47, 6236–6239. [Google Scholar] [CrossRef]
- Kumar, D.; He, C.H.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. Connecting energetic nitropyrazole and aminotetrazole moieties with N,N′-ethylene bridges: A promising approach for fine tuning energetic properties. J. Mater. Chem. A 2016, 4, 9220–9228. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Stierstorfer, J.; Wallek, A.U. Nitrogen-rich salts of 1-methyl-5-nitriminotetrazolate: An auspicious class of thermally stable energetic materials. Chem. Mater. 2008, 20, 4519–4530. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, J.; Shreeve, J.M. 3,6-Dinitropyrazolo[4,3-c]pyrazole-based multipurpose energetic materials through versatile n-functionalization strategies. Angew. Chem. Int. Ed. 2016, 55, 12895–12897. [Google Scholar] [CrossRef]
- Piercey, D.G.; Chavez, D.E.; Scott, B.L.; Imler, G.H.; Parrish, D.A. An energetic triazolo-1,2,4-triazine and its N-oxide. Angew. Chem. Int. Ed. 2016, 55, 15315–15318. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Gu, H.; Huang, J.L.; Nie, F.D.; Fan, G.J.; Liao, L.Y.; Yang, W. Formation of trinitromethyl functionalized 1,2,4-triazole-based energetic ionic salts and a zwitterionic salt directed by an intermolecular and intramolecular metathesis strategy. New J. Chem. 2018, 42, 2376–2380. [Google Scholar] [CrossRef]
- He, C.; Zhang, J.; Parrish, D.A.; Shreeve, J.M. 4-chloro-3,5-dinitropyrazole: A precursor for promising insensitive energetic compounds. J. Mater. Chem. A 2013, 1, 2863–2868. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, H.; Parrish, D.A.; Shreeve, J.M. 1,2,4-triazole links and N-azo bridges yield energetic compounds. Chem. Eur. J. 2015, 21, 11401–11407. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.W.A.; Habraken, C.L. Pyrazoles. VIII. Rearrangement of N-nitropyrazoles. Formation of 3-nitropyrazoles. J. Org. Chem. 1971, 36, 3081–3084. [Google Scholar]
- Janssen, J.W.A.; Koeners, H.J.; Kruse, C.G.; Habraken, C.L. Pyrazoles. XII. Preparation of 3(5)-nitropyrazoles by thermal rearrangement of N-nitropyrazoles. J. Org. Chem. 1973, 38, 1777–1782. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Huang, Y.G.; Parrish, D.A.; Shreeve, J.M. 4-Amino-3,5-dinitropyrazolate salts-highly insensitive energetic materials. J. Mater. Chem. 2011, 21, 6891–6897. [Google Scholar] [CrossRef]
- Xiong, J.; Cai, J.X.; Lai, Q.; Yin, P.; Pang, S.P. Asymmetric assembly of pyrazole and 1,2,3-triazole with a methylene bridge: Regioisomerism and energetic properties. Chem. Commun. 2022, 58, 10647–10650. [Google Scholar] [CrossRef]
- Yan, T.; Yang, H.; Yang, C.; Yi, Z.; Zhu, S.; Cheng, G. An advanced and applicable heat- resistant explosive through controllable regiochemical modulation. J. Mater. Chem. A 2020, 8, 23857–23865. [Google Scholar] [CrossRef]
- Veljković, L.S.; Radovanović, J.I.; Veljković, D.Ž. How aromatic system size affects the sensitivities of highly energetic molecules? RSC Adv. 2021, 11, 31933–31940. [Google Scholar] [CrossRef]
- Kretić, D.S.; Radovanović, J.I.; Veljković, D.Ž. Can the sensitivity of energetic materials be tuned by using hydrogen bonds? Another look at the role of hydrogen bonding in the design of high energetic compounds. Phys. Chem. Chem. Phys. 2021, 23, 7472–7479. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. High performance, low sensitivity: Conflicting or compatible? Propellants Explos. Pyrotech. 2016, 41, 414–425. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Fischer, D.; Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Synthesis of 5-aminotetrazole-1N-oxide and its azo derivative: A key step in the development of new energetic materials. Chem. Eur. J. 2013, 19, 4602–4613. [Google Scholar] [CrossRef]
- Zhang, J.C.; Zhang, J.H.; Pattish, D.A.; Shreeve, J.M. Desensitization of the dinitromethyl group: Molecular/crystalline factors that affect the sensitivities of energetic materials. J. Mater. Chem. A 2018, 6, 22705–22712. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, J.H.; Parrish, D.A.; Shreeve, J.M. Energetic N,N′-ethylene-bridged bis(nitropyrazoles): Diversified functionalities and properties. Chem. Eur. J. 2014, 20, 16529. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Phys. Chem. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623. [Google Scholar] [CrossRef]
- Westwell, M.S.; Searle, M.S.; Williams, D.H. Empirical correlations between thermodynamic properties and intermolecular forces. J. Am. Chem. Soc. 1995, 117, 5013. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).