Abstract
Herpesviruses are one of the most contagious DNA viruses that threaten human health, causing severe diseases, including, but not limited to, certain types of cancer and neurological complications. The overuse and misuse of anti-herpesvirus drugs are key factors leading to drug resistance. Therefore, targeting human herpesviruses with natural products is an attractive form of therapy, as it might improve treatment efficacy in therapy-resistant herpesviruses. Plant polyphenols are major players in the health arena as they possess diverse bioactivities. Hence, in this article, we comprehensively summarize the recent advances that have been attained in employing plant non-flavonoid polyphenols, such as phenolic acids, tannins and their derivatives, stilbenes and their derivatives, lignans, neolignans, xanthones, anthraquinones and their derivatives, curcuminoids, coumarins, furanocoumarins, and other polyphenols (phloroglucinol) as promising anti-herpesvirus drugs against various types of herpesvirus such as alpha-herpesviruses (herpes simplex virus type 1 and 2 and varicella-zoster virus), beta-herpesviruses (human cytomegalovirus), and gamma-herpesviruses (Epstein–Barr virus and Kaposi sarcoma-associated herpesvirus). The molecular mechanisms of non-flavonoid polyphenols against the reviewed herpesviruses are also documented.
1. Introduction
Human herpesviruses (HHVs) are infectious DNA viruses that belong to the family of Herpesviridae with the capacity to establish lifelong latent infections, which undergo periodic reactivation []. These viruses induce a broad spectrum of ailments, ranging from frequent cold sores to cancer, and remain a major cause of morbidity and mortality, particularly in immunocompromised patients []. HHVs are categorized into three subfamilies, Alphaherpesvirinae, which includes herpes simplex virus type 1 and type 2 (HSV-1 and HSV-2) and varicella-zoster virus (VZV or HHV-3) [,] and Betaherpesvirinae includes human cytomegalovirus (HCMV or HHV-5), HHV-6, and HHV-7 []. Gammaherpesvirinae subfamily consists of two tumor viruses, Epstein–Barr virus (EBV or HHV-4) and Kaposi sarcoma-associated herpesvirus (KSHV or HHV-8) []. Over millions of years, HHVs have developed various mechanisms to infect their hosts and modulate their genetic materials []. The infection begins by attaching the virus to the host cell, specifically, to the binding receptors via several viral glycoproteins (the heterodimer gH–gL and the viral fusion protein gB), which are placed on the surface of the virion [,]. The life cycle of HHVs comprises two critical phases: lytic infection and latent infection. During lytic infection, the virus replicates and produces several progeny virions, and then travels to the latent site (in this stage, the virus is inactive) to form latent infection [,]. Subsequently, the virus reactivates once the host’s immune system is weakened by diverse physiological and environmental factors that adversely affect the immune system. The reactivation process depends on the nature of the latently infected cell [,]. HHV infections are usually asymptomatic, and the symptoms occur once the immune system is compromised []. An overview of the herpesvirus life cycle is shown in Figure 1.
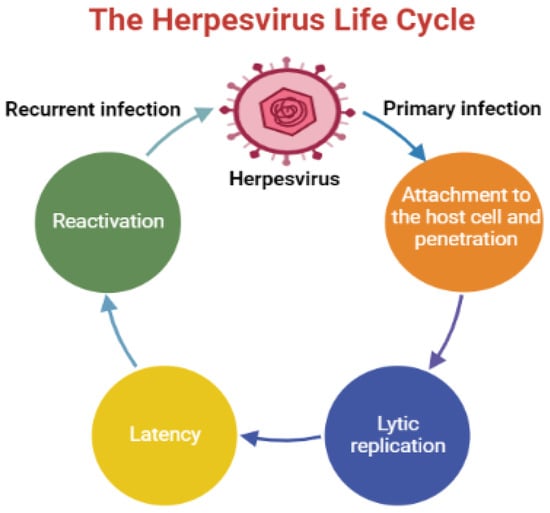
Figure 1.
An illustration displays an overview of the herpesvirus life cycle.
The current effective medical treatment of HHVs is based on acyclovir and other related antiviral medications that target viral DNA polymerases. The overuse of these drugs has led to the developing of the problem of drug resistance, leading to unsuccessful treatment efficacy [,]. Moreover, acyclovir and related nucleoside or nucleotide analogs do not cure herpes infections but reduce the severity and frequency of symptoms []. Therefore, the search for new sources such as natural products that provide effective drugs with the ability to inhibit the herpesvirus at diverse stages of the life cycle with no toxicity and resistance is urgently needed [,].
Consequently, in this article, we comprehensively review the recent investigations that have been performed on the use of plant non-flavonoid polyphenols as promising antiviral agents against HHVs such as alpha-herpesviruses, beta-herpesviruses, and gamma-herpesviruses. To understand how these compounds work as antiviral drugs, we showcase their mechanisms of action at molecular and cellular levels against the reviewed herpesviruses. The effective concentrations or doses are also highlighted.
Several online databases such as Web of Science Core Collection, PubMed, Scopus, SciFinder, Google Scholar, Embase, and ScienceDirect were used during the literature search utilizing proper keywords that characterize antiviral activities of plant polyphenols (excluding flavonoids) against HHVs. Studies that have been published (in the English language) in the years from 2017 to July 2022 were selected to collect the required data. However, limited studies published before 2017 have been included to support or criticize the validity of the data obtained from recent investigations.
6. Mechanisms of Action of Non-Flavonoid Polyphenols: A Focus Insight
Targeting different stages of the herpesvirus life cycle is an effective way to eliminate the virus and its related complications. Specifically, DNA replication, a multi-step process, is an essential step of the virus life cycle and represents an appealing target for antiviral treatments [,,]. Therefore, and based on the data extracted from the reviewed articles introduced in this paper, we summarize in Table 1 all mechanisms and pathways by which non-flavonoid polyphenols cause antiviral actions by interfering with multiple stages during the herpesvirus life cycle. As seen in the table, the documented compounds were detected to affect the herpesvirus DNA replication by diverse mechanisms at molecular and cellular levels via targeting viral glycoproteins, viral gene expression, viral protein synthesis, viral enzymes, NF-κB activity, and B cells activation. In addition to the impact on viral DNA replication, they were found to interfere with other steps in the viral life cycle, including attachment, entry, latency, and reactivation. The mechanisms of action of ginkgolic acid, tannic acid, mangiferin, emodin, and curcumin have been validated in animal experiments and, therefore, deserve special attention to entering clinical studies (Figure 2). The chemical structures of all reviewed compounds are shown in Figure 3 except 1,2,3,4,5-penta-O-digalloyl-β-D-glucopyranose and 1,2,3,4,5-penta-O-digalloyl-α-D-glucopyranose, where both compounds share the molecular formula C76H52O46. The anti-HSV-1 activity of curcumin via targeting TK was found to be linked with functional groups such as hydroxyl and carbonyl groups and phenyl rings.

Table 1.
A focus insight into the reviewed non-flavonoid polyphenols, summarizing their mechanisms of action against human alpha-, beta-, and gamma-herpesviruses.
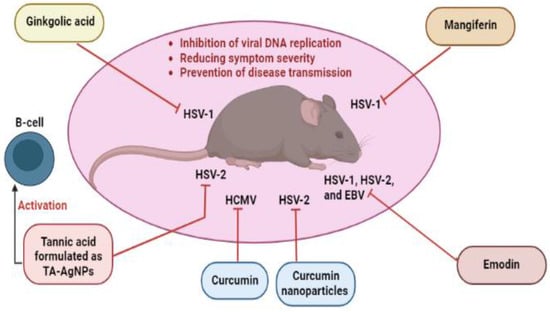
Figure 2.
Non-flavonoid polyphenols with anti-herpesvirus properties validated by animal experiments. EBV, Epstein–Barr virus; HCMV, human cytomegalovirus; HSV-1, herpes simplex virus type 1; HSV-2; herpes simplex virus type 2; TA-AgNPs, tannic acid-modified silver nanoparticles.
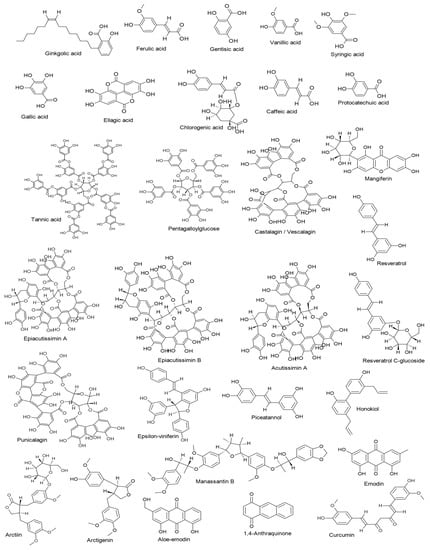
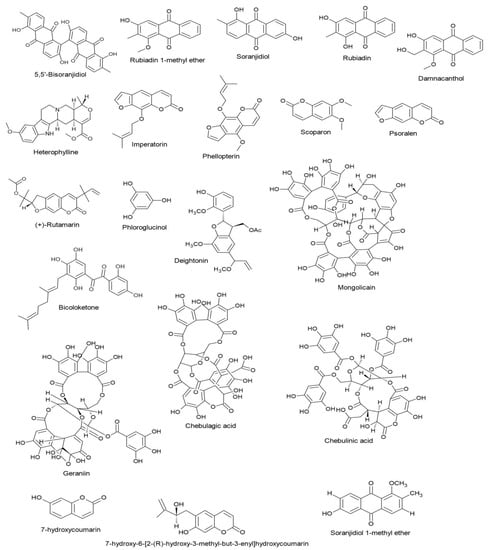
Figure 3.
Chemical structures of the reviewed non-flavonoid polyphenols.
7. Conclusions and Take-Home Message
There is no doubt that treatment of HHV infection and its complications is challenging, and new antiviral medications are urgently required. Polyphenols are one of the best naturally occurring molecules exhibiting numerous health benefits and play a crucial role as a source of natural antivirals. In this article, we showcased various chemical classes of non-flavonoid polyphenols as possible antiviral agents for treating HHV infections with diverse mechanisms at different molecular and cellular levels along with effective concentrations or doses. The reviewed compounds were evaluated by various biochemical, virological, and computational methods performed on multiple cells (animal and human cells) and animal models (mice). Compounds such as castalagin, vescalagin, and honokiol demonstrated excellent ability to interact synergistically with acyclovir, showing improved anti-HSV properties and hence enhanced treatment effectiveness. Additionally, the application of the nano-drug delivery system has shown significant efficacy in improving the anti-HSV properties of tannic acid and ellagic acid formulated in Ag-NPs and ZnO-NPs, respectively. The anti-infectivity of curcumin against HSV-1 and HSV-2 infections has also been improved by curcumin-loaded proniosomes and curcumin nanoparticles, respectively. However, more studies on nano-drug delivery combined with pharmacokinetic and pharmacodynamic assessments should be developed to enhance HHV therapy. Another therapeutic approach was achieved by employing a chelation strategy in which metal chelates of caffeic acid (addition of cations such as Fe3+ and anionic molecules such as molybdate and phosphate) showed significant improvement in antiviral activity against HSV infections. Besides, co-administration of zinc ions with punicalagin was observed to potentiate viricidal activity against HSV infections. The chemical synthesis of polyphenols to produce esters consisting of ferulic and gallic acids has potently increased the anti-infectivity properties against EBV. Although the safety profile of dietary polyphenols (resveratrol and curcumin) is high, their intake should be included in special diet plans, and further animal and clinical investigations should be conducted regarding their antiviral effects on HHVs.
Finally, the presented information in this paper might provide a valuable platform for other researchers to build upon and aid them design and synthesize novel and potent compounds proper for developing anti-herpesvirus drugs that could enter clinical studies.
Author Contributions
Conceptualization, S.T.S.H. and M.Š.; writing—original draft preparation, S.T.S.H., M.Š., A.M. and P.K.; writing—review and critical editing, S.T.S.H., M.Š., A.M. and P.K.; supervision, S.T.S.H. and P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
S.T.S.H. and M.Š. would like to thank their institutions for providing access to the subscribed databases.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Šudomová, M.; Berchová-Bímová, K.; Mazurakova, A.; Šamec, D.; Kubatka, P.; Hassan, S.T.S. Flavonoids Target Human Herpesviruses That Infect the Nervous System: Mechanisms of Action and Therapeutic Insights. Viruses 2022, 14, 592. [Google Scholar] [CrossRef] [PubMed]
- Šudomová, M.; Berchová-Bímová, K.; Marzocco, S.; Liskova, A.; Kubatka, P.; Hassan, S.T.S. Berberine in Human Oncogenic Herpesvirus Infections and Their Linked Cancers. Viruses 2021, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154. [Google Scholar] [CrossRef]
- Adler, B.; Sattler, C.; Adler, H. Herpesviruses and Their Host Cells: A Successful Liaison. Trends Microbiol. 2017, 25, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Šudomová, M.; Hassan, S.T.S. Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms 2021, 9, 292. [Google Scholar] [CrossRef]
- Wołącewicz, M.; Becht, R.; Grywalska, E.; Niedźwiedzka-Rystwej, P. Herpesviruses in Head and Neck Cancers. Viruses 2020, 12, 172. [Google Scholar] [CrossRef]
- Azab, W.; Osterrieder, K. Initial Contact: The First Steps in Herpesvirus Entry. Adv. Anat. Embryol. Cell Biol. 2017, 223, 1–27. [Google Scholar] [CrossRef]
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The Structural Basis of Herpesvirus Entry. Nat. Rev. Microbiol. 2021, 19, 110–121. [Google Scholar] [CrossRef]
- Cohen, J.I. Herpesvirus Latency. J. Clin. Investig. 2020, 130, 3361–3369. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Q.; Wang, M.; Chen, S.; Jia, R.; Yang, Q.; Zhu, D.; Liu, M.; Zhao, X.; Zhang, S.; et al. Multifaceted Roles of ICP22/ORF63 Proteins in the Life Cycle of Human Herpesviruses. Front. Microbiol. 2021, 12, 668461. [Google Scholar] [CrossRef]
- Frappier, L. Regulation of Herpesvirus Reactivation by Host MicroRNAs. J. Virol. 2015, 89, 2456–2458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dochnal, S.A.; Francois, A.K.; Cliffe, A.R. De Novo Polycomb Recruitment: Lessons from Latent Herpesviruses. Viruses 2021, 13, 1470. [Google Scholar] [CrossRef] [PubMed]
- Jarosinski, K.W. Interindividual Spread of Herpesviruses. Adv. Anat. Embryol. Cell Biol. 2017, 223, 195–224. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.L.; James, S.H. Antiviral Therapies for Herpesviruses: Current Agents and New Directions. Clin. Ther. 2018, 40, 1282–1298. [Google Scholar] [CrossRef]
- Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431. [Google Scholar] [CrossRef]
- Kłysik, K.; Pietraszek, A.; Karewicz, A.; Nowakowska, M. Acyclovir in the Treatment of Herpes Viruses—A Review. Curr. Med. Chem. 2020, 27, 4118–4137. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Masarčíková, R.; Berchová, K. Bioactive Natural Products with Anti-Herpes Simplex Virus Properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Švajdlenka, E.; Berchová-Bímová, K. Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-Enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules 2017, 22, 722. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. ISBN 978-3-642-22143-9. [Google Scholar]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of Phenols and Polyphenols in Plant Defense Response to Biotic and Abiotic Stresses. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 419–441. ISBN 978-0-12-822919-4. [Google Scholar]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Z.; Granato, D. Polyphenols in Foods: Classification, Methods of Identification, and Nutritional Aspects in Human Health. Adv. Food Nutr. Res. 2021, 98, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral Properties of Polyphenols from Plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and Their Potential Role to Fight Viral Diseases: An Overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.S.; Šudomová, M.; Berchová-Bímová, K.; Šmejkal, K.; Echeverría, J. Psoromic Acid, a Lichen-Derived Molecule, Inhibits the Replication of HSV-1 and HSV-2, and Inactivates HSV-1 DNA Polymerase: Shedding Light on Antiherpetic Properties. Molecules 2019, 24, 2912. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and Virulence of Herpes Simplex Virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.E.; Oxman, M.N.; et al. Varicella Zoster Virus Infection. Nat. Rev. Dis. Prim. 2015, 1, 15016. [Google Scholar] [CrossRef]
- Kennedy, P.G.E.; Gershon, A.A. Clinical Features of Varicella-Zoster Virus Infection. Viruses 2018, 10, E609. [Google Scholar] [CrossRef]
- Azab, W.; Dayaram, A.; Greenwood, A.D.; Osterrieder, N. How Host Specific Are Herpesviruses? Lessons from Herpesviruses Infecting Wild and Endangered Mammals. Annu. Rev. Virol 2018, 5, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Lum, K.K.; Cristea, I.M. Host Innate Immune Response and Viral Immune Evasion During Alphaherpesvirus Infection. Curr. Issues Mol. Biol. 2021, 42, 635–686. [Google Scholar] [CrossRef]
- Borenstein, R.; Hanson, B.A.; Markosyan, R.M.; Gallo, E.S.; Narasipura, S.D.; Bhutta, M.; Shechter, O.; Lurain, N.S.; Cohen, F.S.; Al-Harthi, L.; et al. Ginkgolic Acid Inhibits Fusion of Enveloped Viruses. Sci. Rep. 2020, 10, 4746. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Sobczyński, M.; Ochnik, M.; Zwolińska, K.; Leszek, J. Hampering Herpesviruses HHV-1 and HHV-2 Infection by Extract of Ginkgo Biloba (EGb) and Its Phytochemical Constituents. Front. Microbiol. 2019, 10, 2367. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, M.S.; Shechter, O.; Gallo, E.S.; Martin, S.D.; Jones, E.; Doncel, G.F.; Borenstein, R. Ginkgolic Acid Inhibits Herpes Simplex Virus Type 1 Skin Infection and Prevents Zosteriform Spread in Mice. Viruses 2021, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Todorova, N.; Rangelov, M.; Dincheva, I.; Badjakov, I.; Enchev, V.; Markova, N. Potential of Hydroxybenzoic Acids from Graptopetalum Paraguayense for Inhibiting of Herpes Simplex Virus DNA Polymerase–Metabolome Profiling, Molecular Docking and Quantum-Chemical Analysis. Pharmacia 2022, 69, 113–123. [Google Scholar] [CrossRef]
- AbouAitah, K.; Allayh, A.K.; Wojnarowicz, J.; Shaker, Y.M.; Swiderska-Sroda, A.; Lojkowski, W. Nanoformulation Composed of Ellagic Acid and Functionalized Zinc Oxide Nanoparticles Inactivates DNA and RNA Viruses. Pharmaceutics 2021, 13, 2174. [Google Scholar] [CrossRef]
- Di Sotto, A.; Di Giacomo, S.; Amatore, D.; Locatelli, M.; Vitalone, A.; Toniolo, C.; Rotino, G.L.; Lo Scalzo, R.; Palamara, A.T.; Marcocci, M.E.; et al. A Polyphenol Rich Extract from Solanum Melongena L. DR2 Peel Exhibits Antioxidant Properties and Anti-Herpes Simplex Virus Type 1 Activity In Vitro. Molecules 2018, 23, E2066. [Google Scholar] [CrossRef]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral Activity of Metal Chelates of Caffeic Acid and Similar Compounds towards Herpes Simplex, VSV-Ebola Pseudotyped and Vaccinia Viruses. Antivir. Res. 2018, 160, 143–150. [Google Scholar] [CrossRef]
- Kesharwani, A.; Polachira, S.K.; Nair, R.; Agarwal, A.; Mishra, N.N.; Gupta, S.K. Anti-HSV-2 Activity of Terminalia Chebula Retz Extract and Its Constituents, Chebulagic and Chebulinic Acids. BMC Complement. Altern. Med. 2017, 17, 110. [Google Scholar] [CrossRef]
- Siqueira, E.M.D.S.; Lima, T.L.; Boff, L.; Lima, S.G.; Lourenço, E.M.; Ferreira, É.G.; Barbosa, E.G.; Machado, P.R.; Farias, K.J.; Ferreira, L.D.S.; et al. Antiviral Potential of Spondias Mombin L. Leaves Extract Against Herpes Simplex Virus Type-1 Replication Using In Vitro and In Silico Approaches. Planta Med. 2020, 86, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobelny, J.; Basa, A.; Krzyżowska, M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. IJMS 2018, 19, 387. [Google Scholar] [CrossRef]
- Orłowski, P.; Kowalczyk, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Węgrzyn, A.; Grzesiak, J.; Celichowski, G.; Grobelny, J.; Eriksson, K.; Krzyzowska, M. Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis. Viruses 2018, 10, 524. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Jacquet, R.; Deffieux, D.; Pouységu, L.; Sylla, T.; Chassaing, S.; Nikolova, I.; Quideau, S.; Galabov, A.S. Anti-Herpes Simplex Virus Type 1 Activity of Specially Selected Groups of Tannins. Drug Res. 2019, 69, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Vilhelmova-Ilieva, N.; Jacquet, R.; Quideau, S.; Galabov, A.S. Ellagitannins as Synergists of ACV on the Replication of ACV-Resistant Strains of HSV 1 and 2. Antivir. Res. 2014, 110, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, J.; Rajarajan, S. Study on Antiviral Activities, Drug-Likeness and Molecular Docking of Bioactive Compounds of Punica Granatum L. to Herpes Simplex Virus-2 (HSV-2). Microb. Pathog. 2018, 118, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Houston, D.M.J.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. Potentiated Virucidal Activity of Pomegranate Rind Extract (PRE) and Punicalagin against Herpes Simplex Virus (HSV) When Co-Administered with Zinc (II) Ions, and Antiviral Activity of PRE against HSV and Aciclovir-Resistant HSV. PLoS ONE 2017, 12, e0179291. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.Y.; Do, M.H.; Lee, C.H.; Song, Y.-J. 1,2,3,4,6-Penta-O-Galloyl-ß-D-Glucose, a Bioactive Compound in Elaeocarpus Sylvestris Extract, Inhibits Varicella-Zoster Virus Replication. Antivir. Res. 2017, 144, 266–272. [Google Scholar] [CrossRef]
- Rechenchoski, D.Z.; Agostinho, K.F.; Faccin-Galhardi, L.C.; Lonni, A.A.S.G.; da Silva, J.V.H.; de Andrade, F.G.; Cunha, A.P.; Ricardo, N.M.P.S.; Nozawa, C.; Linhares, R.E.C. Mangiferin: A Promising Natural Xanthone from Mangifera Indica for the Control of Acyclovir - Resistant Herpes Simplex Virus 1 Infection. Bioorg. Med. Chem. 2020, 28, 115304. [Google Scholar] [CrossRef]
- Rechenchoski, D.Z.; Samensari, N.L.; Faccin-Galhardi, L.C.; de Almeida, R.R.; Cunha, A.P.; Ricardo, N.M.P.S.; Nozawa, C.; Linhares, R.E.C. The Combination of Dimorphandra Gardneriana Galactomannan and Mangiferin Inhibits Herpes Simplex and Poliovirus. Curr. Pharm. Biotechnol. 2019, 20, 215–221. [Google Scholar] [CrossRef]
- Abba, Y.; Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral Activity of Resveratrol against Human and Animal Viruses. Adv. Virol. 2015, 2015, 184241. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, X.; Zhao, X.; Zhang, Y.; Wang, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Insights into the Anti-Inflammatory and Antiviral Mechanisms of Resveratrol. Mediat. Inflamm. 2022, 2022, 7138756. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.J.; Fu, M.M.; Stiffler, B.S.; Limperos, R.J.; Pokabla, C.M.; DeLucia, A.L. Resveratrol Inhibition of Herpes Simplex Virus Replication. Antivir. Res. 1999, 43, 145–155. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a Novel Anti-Herpes Simplex Virus Nutraceutical Agent: An Overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef]
- Ding, L.; Jiang, P.; Xu, X.; Lu, W.; Yang, C.; Zhou, P.; Liu, S. Resveratrol Promotes HSV-2 Replication by Increasing Histone Acetylation and Activating NF-ΚB. Biochem. Pharmacol. 2020, 171, 113691. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, X.; Wu, Y.; Zhao, Q.; Liu, X.; Zhang, G.; Zhao, Z.; Ning, Y.; Wang, K.; Tan, Y.; et al. Synergistic Effect of Resveratrol and HSV-TK/GCV Therapy on Murine Hepatoma Cells. Cancer Biol. Ther. 2019, 20, 183–191. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, X.-W.; Schols, D.; Mori, M.; Botta, B.; Chevigné, A.; Mulinge, M.; Steinmetz, A.; Schmit, J.-C.; Seguin-Devaux, C. Active Components from Cassia Abbreviata Prevent HIV-1 Entry by Distinct Mechanisms of Action. Int. J. Mol. Sci. 2021, 22, 5052. [Google Scholar] [CrossRef]
- Tarbeeva, D.V.; Krylova, N.V.; Iunikhina, O.V.; Likhatskaya, G.N.; Kalinovskiy, A.I.; Grigorchuk, V.P.; Shchelkanov, M.Y.; Fedoreyev, S.A. Biologically Active Polyphenolic Compounds from Lespedeza Bicolor. Fitoterapia 2022, 157, 105121. [Google Scholar] [CrossRef]
- Squillaci, G.; Zannella, C.; Carbone, V.; Minasi, P.; Folliero, V.; Stelitano, D.; Cara, F.L.; Galdiero, M.; Franci, G.; Morana, A. Grape Canes from Typical Cultivars of Campania (Southern Italy) as a Source of High-Value Bioactive Compounds: Phenolic Profile, Antioxidant and Antimicrobial Activities. Molecules 2021, 26, 2746. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Tan, L.; Liang, X. Inhibition of Herpes Simplex Virus-1 Replication by Natural Compound Honokiol. Virol. Sin. 2019, 34, 315–323. [Google Scholar] [CrossRef]
- Dias, M.M.; Zuza, O.; Riani, L.R.; de Faria Pinto, P.; Pinto, P.L.S.; Silva, M.P.; de Moraes, J.; Ataíde, A.C.Z.; de Oliveira Silva, F.; Cecílio, A.B.; et al. In Vitro Schistosomicidal and Antiviral Activities of Arctium Lappa L. (Asteraceae) against Schistosoma Mansoni and Herpes Simplex Virus-1. Biomed. Pharmacother. 2017, 94, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Saidu, M.B.; Kúsz, N.; Tsai, Y.-C.; Vágvölgyi, M.; Berkecz, R.; Kókai, D.; Burián, K.; Hohmann, J.; Rédei, D. Triterpenes and Phenolic Compounds from Euphorbia Deightonii with Antiviral Activity against Herpes Simplex Virus Type-2. Plants 2022, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.-R.; Luo, J.; Hou, W.; Xiao, H.; Yang, Z.-Q. The Effect of Emodin, an Anthraquinone Derivative Extracted from the Roots of Rheum Tanguticum, against Herpes Simplex Virus in Vitro and in Vivo. J. Ethnopharmacol. 2011, 133, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Pan, C.; Cheng, W.; Wang, X.; Yang, Z.; Zheng, L. The Intervention Mechanism of Emodin on TLR3 Pathway in the Process of Central Nervous System Injury Caused by Herpes Virus Infection. Neurol. Res. 2021, 43, 307–313. [Google Scholar] [CrossRef]
- Mugas, M.L.; Marioni, J.; Martinez, F.; Aguilar, J.J.; Cabrera, J.L.; Contigiani, M.S.; Konigheim, B.S.; Núñez-Montoya, S.C. Inactivation of Herpes Simplex Virus by Photosensitizing Anthraquinones Isolated from Heterophyllaea Pustulata. Planta Med. 2021, 87, 716–723. [Google Scholar] [CrossRef]
- Roa-Linares, V.C.; Miranda-Brand, Y.; Tangarife-Castaño, V.; Ochoa, R.; García, P.A.; Castro, M.Á.; Betancur-Galvis, L.; San Feliciano, A. Anti-Herpetic, Anti-Dengue and Antineoplastic Activities of Simple and Heterocycle-Fused Derivatives of Terpenyl-1,4-Naphthoquinone and 1,4-Anthraquinone. Molecules 2019, 24, 1279. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma Longa) and Its Major Constituent (Curcumin) as Nontoxic and Safe Substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Flores, D.J.; Lee, L.H.; Adams, S.D. Inhibition of Curcumin-Treated Herpes Simplex Virus 1 and 2 in Vero Cells. AiM 2016, 6, 276–287. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin Inhibits Herpes Simplex Virus Immediate-Early Gene Expression by a Mechanism Independent of P300/CBP Histone Acetyltransferase Activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef]
- Vitali, D.; Bagri, P.; Wessels, J.M.; Arora, M.; Ganugula, R.; Parikh, A.; Mandur, T.; Felker, A.; Garg, S.; Kumar, M.N.V.R.; et al. Curcumin Can Decrease Tissue Inflammation and the Severity of HSV-2 Infection in the Female Reproductive Mucosa. IJMS 2020, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. Alpha-Herpesvirus Thymidine Kinase Genes Mediate Viral Virulence and Are Potential Therapeutic Targets. Front. Microbiol. 2019, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- El-Halim, S.M.A.; Mamdouh, M.A.; El-Haddad, A.E.; Soliman, S.M. Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins. Sci. Pharm. 2020, 88, 9. [Google Scholar] [CrossRef]
- Badria, F.A.; Abdelaziz, A.E.; Hassan, A.H.; Elgazar, A.A.; Mazyed, E.A. Development of Provesicular Nanodelivery System of Curcumin as a Safe and Effective Antiviral Agent: Statistical Optimization, In Vitro Characterization, and Antiviral Effectiveness. Molecules 2020, 25, 5668. [Google Scholar] [CrossRef]
- Rajtar, B.; Skalicka-Woźniak, K.; Świątek, Ł.; Stec, A.; Boguszewska, A.; Polz-Dacewicz, M. Antiviral Effect of Compounds Derived from Angelica Archangelica L. on Herpes Simplex Virus-1 and Coxsackievirus B3 Infections. Food Chem. Toxicol. 2017, 109, 1026–1031. [Google Scholar] [CrossRef]
- Okba, M.M.; El Gedaily, R.A.; Ashour, R.M. UPLC-PDA-ESI-QTOF-MS Profiling and Potent Anti-HSV-II Activity of Eucalyptus Sideroxylon Leaves. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1068–1069, 335–342. [Google Scholar] [CrossRef]
- Fulkerson, H.L.; Nogalski, M.T.; Collins-McMillen, D.; Yurochko, A.D. Overview of Human Cytomegalovirus Pathogenesis. Methods Mol. Biol. 2021, 2244, 1–18. [Google Scholar] [CrossRef]
- O’Connor, C.M. Cytomegalovirus (CMV) Infection and Latency. Pathogens 2021, 10, 342. [Google Scholar] [CrossRef]
- Griffiths, P.; Baraniak, I.; Reeves, M. The Pathogenesis of Human Cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef]
- Michaelis, M.; Doerr, H.W.; Cinatl, J. The Story of Human Cytomegalovirus and Cancer: Increasing Evidence and Open Questions. Neoplasia 2009, 11, 1–9. [Google Scholar] [CrossRef]
- Golais, F.; Mrázová, V. Human Alpha and Beta Herpesviruses and Cancer: Passengers or Foes? Folia Microbiol. 2020, 65, 439–449. [Google Scholar] [CrossRef]
- Griffiths, P. Cytomegalovirus Infection of the Central Nervous System. Herpes 2004, 11 (Suppl. 2), 95A–104A. [Google Scholar] [PubMed]
- Tselis, A.C. Cytomegalovirus Infections of the Adult Human Nervous System. Handb. Clin. Neurol. 2014, 123, 307–318. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Fang, F. Congenital Human Cytomegalovirus Infection and Neurologic Diseases in Newborns. Chin. Med. J. (Engl) 2019, 132, 2109–2118. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Zhang, J.; Xu, X.-G.; Su, H.-L.; Xing, W.-M.; Zhang, Z.-S.; Jin, W.-H.; Dai, J.-H.; Wang, Y.-Z.; He, X.-Y.; et al. Inhibitory Effects of Piceatannol on Human Cytomegalovirus (HCMV) in Vitro. J. Microbiol. 2020, 58, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Alam, Z.; Al-Mahdi, Z.; Zhu, Y.; McKee, Z.; Parris, D.S.; Parikh, H.I.; Kellogg, G.E.; Kuchta, A.; McVoy, M.A. Anti-Cytomegalovirus Activity of the Anthraquinone Atanyl Blue PRL. Antivir. Res. 2015, 114, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; An, Z.; Chen, H.; Wang, Z.; Liu, L. Mechanism of Curcumin Resistance to Human Cytomegalovirus in HELF Cells. BMC Complement. Altern. Med. 2014, 14, 284. [Google Scholar] [CrossRef]
- Lv, Y.; Gong, L.; Wang, Z.; Han, F.; Liu, H.; Lu, X.; Liu, L. Curcumin Inhibits Human Cytomegalovirus by Downregulating Heat Shock Protein 90. Mol. Med. Rep. 2015, 12, 4789–4793. [Google Scholar] [CrossRef]
- Lv, Y.-L.; Jia, Y.; Wan, Z.; An, Z.-L.; Yang, S.; Han, F.-F.; Gong, L.-L.; Xuan, L.-L.; Ren, L.-L.; Zhang, W.; et al. Curcumin Inhibits the Formation of Atherosclerosis in ApoE-/- Mice by Suppressing Cytomegalovirus Activity in Endothelial Cells. Life Sci. 2020, 257, 117658. [Google Scholar] [CrossRef]
- Lv, Y.; Lei, N.; Wang, D.; An, Z.; Li, G.; Han, F.; Liu, H.; Liu, L. Protective Effect of Curcumin against Cytomegalovirus Infection in Balb/c Mice. Environ. Toxicol. Pharmacol. 2014, 37, 1140–1147. [Google Scholar] [CrossRef]
- Möhl, B.S.; Chen, J.; Longnecker, R. Gammaherpesvirus Entry and Fusion: A Tale How Two Human Pathogenic Viruses Enter Their Host Cells. Adv. Virus Res. 2019, 104, 313–343. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.J. Epstein-Barr Virus and Cancer. Annu. Rev. Pathol. 2019, 14, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Yiu, S.P.T.; Dorothea, M.; Hui, K.F.; Chiang, A.K.S. Lytic Induction Therapy against Epstein-Barr Virus-Associated Malignancies: Past, Present, and Future. Cancers 2020, 12, 2142. [Google Scholar] [CrossRef]
- Wen, K.W.; Wang, L.; Menke, J.R.; Damania, B. Cancers Associated with Human Gammaherpesviruses. FEBS J. 2021. [Google Scholar] [CrossRef]
- Goncalves, P.H.; Ziegelbauer, J.; Uldrick, T.S.; Yarchoan, R. Kaposi Sarcoma Herpesvirus-Associated Cancers and Related Diseases. Curr. Opin. HIV AIDS 2017, 12, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M. Pathogenesis of Gammaherpesvirus Infections. Vet. Microbiol. 2006, 113, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Soldan, S.S.; Lieberman, P.M. Epstein-Barr Virus Infection in the Development of Neurological Disorders. Drug Discov. Today Dis. Models 2020, 32, 35–52. [Google Scholar] [CrossRef]
- Jha, H.C.; Mehta, D.; Lu, J.; El-Naccache, D.; Shukla, S.K.; Kovacsics, C.; Kolson, D.; Robertson, E.S. Gammaherpesvirus Infection of Human Neuronal Cells. mBio 2015, 6, e01844-15. [Google Scholar] [CrossRef]
- Nowalk, A.; Green, M. Epstein-Barr Virus. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Ciccarese, G.; Trave, I.; Herzum, A.; Parodi, A.; Drago, F. Dermatological Manifestations of Epstein-Barr Virus Systemic Infection: A Case Report and Literature Review. Int. J. Dermatol. 2020, 59, 1202–1209. [Google Scholar] [CrossRef]
- Li, S.; Bai, L.; Dong, J.; Sun, R.; Lan, K. Kaposi’s Sarcoma-Associated Herpesvirus: Epidemiology and Molecular Biology. In Infectious Agents Associated Cancers: Epidemiology and Molecular Biology; Cai, Q., Yuan, Z., Lan, K., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2017; Volume 1018, pp. 91–127. ISBN 978-981-10-5764-9. [Google Scholar]
- Nomura, E.; Hosoda, A.; Morishita, H.; Murakami, A.; Koshimizu, K.; Ohigashi, H.; Taniguchi, H. Synthesis of Novel Polyphenols Consisted of Ferulic and Gallic Acids, and Their Inhibitory Effects on Phorbol Ester-Induced Epstein-Barr Virus Activation and Superoxide Generation. Bioorg. Med. Chem. 2002, 10, 1069–1075. [Google Scholar] [CrossRef]
- Crowley, A.; Connell, J.; Schaffer, K.; Hall, W.; Hassan, J. Is There Diagnostic Value in Detection of Immunoglobulin g Antibodies to the Epstein-Barr Virus Early Antigen? Biores. Open Access 2012, 1, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Boonsopon, S.; Maghsoudlou, A.; Kombo, N.E.; Foster, C.S. A Therapeutic Trial of Valganciclovir in Patients with Uveitis and Positive Epstein-Barr Virus Early Antigen D IgG Titers. Eur. J. Ophthalmol. 2016, 26, 30–35. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, W.-F.; Xu, J.; Kitdamrongtham, W.; Manosroi, A.; Manosroi, J.; Tokuda, H.; Abe, M.; Akihisa, T.; Feng, F. Potential Cancer Chemopreventive and Anticancer Constituents from the Fruits of Ficus Hispida L.f. (Moraceae). J. Ethnopharmacol. 2018, 214, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.-Y.; Chen, C.-Y.; Shyu, H.-W.; Hong, S.; Chen, H.-M.; Chiou, Y.-H.; Lin, K.-H.; Chou, M.-C.; Wang, L.-Y.; Wang, Y.-F. Resveratrol Induces Cell Death and Inhibits Human Herpesvirus 8 Replication in Primary Effusion Lymphoma Cells. Chem. Biol. Interact. 2015, 242, 372–379. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, N.; Hu, J.; Wang, Y.; Xu, J.; Gu, Q.; Lieberman, P.M.; Yuan, Y. The MTOR Inhibitor Manassantin B Reveals a Crucial Role of MTORC2 Signaling in Epstein-Barr Virus Reactivation. J. Biol. Chem. 2020, 295, 7431–7441. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chen, M.-S.; Cheng, Y.-J.; Ko, Y.-C.; Lin, S.-F.; Chiu, I.-M.; Chen, J.-Y. Emodin Inhibits EBV Reactivation and Represses NPC Tumorigenesis. Cancers 2019, 11, 1795. [Google Scholar] [CrossRef]
- Dheekollu, J.; Wiedmer, A.; Ayyanathan, K.; Deakyne, J.S.; Messick, T.E.; Lieberman, P.M. Cell-Cycle-Dependent EBNA1-DNA Crosslinking Promotes Replication Termination at OriP and Viral Episome Maintenance. Cell 2021, 184, 643–654.e13. [Google Scholar] [CrossRef]
- Jakhmola, S.; Jonniya, N.A.; Sk, M.F.; Rani, A.; Kar, P.; Jha, H.C. Identification of Potential Inhibitors against Epstein–Barr Virus Nuclear Antigen 1 (EBNA1): An Insight from Docking and Molecular Dynamic Simulations. ACS Chem. Neurosci. 2021, 12, 3060–3072. [Google Scholar] [CrossRef]
- Yiu, C.-Y.; Chiu, Y.-J.; Lin, T.-P. The Ethyl Acetate Subfraction of Polygonum Cuspidatum Root Containing Emodin Affect EBV Gene Expression and Induce EBV-Positive Cells Apoptosis. Biol. Pharm. Bull. 2021, 44, 1837–1842. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Hohmann, J.; El-Shazly, M.; Chang, L.-K.; Dankó, B.; Kúsz, N.; Hsieh, C.-T.; Hunyadi, A.; Chang, F.-R. Bioactive Constituents of Lindernia Crustacea and Its Anti-EBV Effect via Rta Expression Inhibition in the Viral Lytic Cycle. J. Ethnopharmacol. 2020, 250, 112493. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.; Ji, W.; Wang, C. Curcumin Inhibits Proliferation of Epstein-Barr Virus-Associated Human Nasopharyngeal Carcinoma Cells by Inhibiting EBV Nuclear Antigen 1 Expression. Biomed. Res. Int. 2019, 2019, 8592921. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castillo, M.; Cruz-Robledo, G.; Hernández-Zavala, A.; Córdova, E.J. Curcumin Sensitizes Epstein-Barr-Immortalized Lymphoblastoid Cell Lines to Inorganic Arsenic Toxicity. Exp. Ther. Med. 2021, 22, 872. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhong, C.; Wang, Q.; Chen, W.; Yuan, Y. Curcumin Is an APE1 Redox Inhibitor and Exhibits an Antiviral Activity against KSHV Replication and Pathogenesis. Antivir. Res. 2019, 167, 98–103. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Y.; Yuan, Y. Antiviral Activity of Topoisomerase II Catalytic Inhibitors against Epstein-Barr Virus. Antivir. Res. 2014, 107, 95–101. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Q.; Gu, Q.; Zhang, H.; Jiang, C.; Hu, J.; Wang, Y.; Yan, Y.; Xu, J. Semisynthesis of (-)-Rutamarin Derivatives and Their Inhibitory Activity on Epstein-Barr Virus Lytic Replication. J. Nat. Prod. 2017, 80, 53–60. [Google Scholar] [CrossRef]
- Xu, B.; Wang, L.; González-Molleda, L.; Wang, Y.; Xu, J.; Yuan, Y. Antiviral Activity of (+)-Rutamarin against Kaposi’s Sarcoma-Associated Herpesvirus by Inhibition of the Catalytic Activity of Human Topoisomerase II. Antimicrob. Agents Chemother. 2014, 58, 563–573. [Google Scholar] [CrossRef]
- Coen, D.M.; Lawler, J.L.; Abraham, J. Herpesvirus DNA Polymerase: Structures, Functions, and Mechanisms. Enzymes 2021, 50, 133–178. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Antiviral Drugs Against Herpesviruses. Adv. Exp. Med. Biol. 2021, 1322, 1–30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).