Abstract
Vitamin D, formerly known for its role in calcium-phosphorus homeostasis, was shown to exert a broad influence on immunity and on differentiation and proliferation processes in the last few years. In the field of endocrinology, there is proof of the potential role of vitamin D and vitamin D-related genes in the pathogenesis of thyroid cancer—the most prevalent endocrine malignancy. Therefore, the study aimed to systematically review the publications on the association between vitamin D-related gene variants (polymorphisms, mutations, etc.) and thyroid cancer. PubMed, EMBASE, Scopus, and Web of Science electronic databases were searched for relevant studies. A total of ten studies were found that met the inclusion criteria. Six vitamin D-related genes were analyzed (VDR—vitamin D receptor, CYP2R1—cytochrome P450 family 2 subfamily R member 1, CYP24A1—cytochrome P450 family 24 subfamily A member 1, CYP27B1—cytochrome P450 family 27 subfamily B member 1, DHCR7—7-dehydrocholesterol reductase and CUBN—cubilin). Moreover, a meta-analysis was conducted to summarize the data from the studies on VDR polymorphisms (rs2228570/FokI, rs1544410/BsmI, rs7975232/ApaI and rs731236/TaqI). Some associations between thyroid cancer risk (VDR, CYP24A1, DHCR7) or the clinical course of the disease (VDR) and vitamin D-related gene polymorphisms were described in the literature. However, these results seem inconclusive and need validation. A meta-analysis of the five studies of common VDR polymorphisms did not confirm their association with increased susceptibility to differentiated thyroid cancer. Further efforts are necessary to improve our understanding of thyroid cancer pathogenesis and implement targeted therapies for refractory cases.
1. Introduction
Thyroid nodules are commonly found with a prevalence as high as 65–70%, although most are benign [1,2]. Among thyroid gland malignancies (7–15% of all thyroid nodules), thyroid cancer predominates [1]. Others, such as primary thyroid lymphoma or sarcoma, are extremely rare [3]. Differentiated thyroid cancer (DTC), with its main subtypes, papillary (PTC) and follicular (FTC), is the most frequent type and accounts for over 95% of cases. Other thyroid cancer types include medullary thyroid cancer (MTC; 1–2%) and anaplastic thyroid cancer (ATC; less than 1%) [4]. Although not the top of the list of all malignancies, thyroid cancer is the most prevalent endocrine malignant neoplasm, and its incidence is observed to be rising [5]. In the case of DTC, there is generally a good prognosis with a low mortality. However, prevention, optimal management, and identification of cases with an unsatisfactory outcome risk are still challenging [6]. Instead, MTC and especially ATC are associated with poor prognosis, despite advances in medical therapies [3,6]. Therefore, further effort is needed to understand thyroid cancer pathogenesis.
Vitamin D has been the focus of interest in a broad range of disciplines in recent years. Its role is known to be far beyond the earlier perception, as vitamin D serves as an immune system regulator and alters cell differentiation and proliferation [7]. Therefore, vitamin D seems to be an excellent candidate as an environmental contributor to various autoimmune, inflammatory, and neoplastic diseases [8,9]. An inverse relationship between vitamin D concentration and cancer risk has been confirmed in the case of colon, breast, prostate, gastric, and other cancer types [10]. Moreover, there are indications of a causal relationship between decreased vitamin D level and poor cancer prognosis [11]. Active vitamin D metabolites promote cell differentiation, suppresses proliferation, and alters apoptosis and autophagy. Therefore, different stages of tumorigenesis can be affected [10].
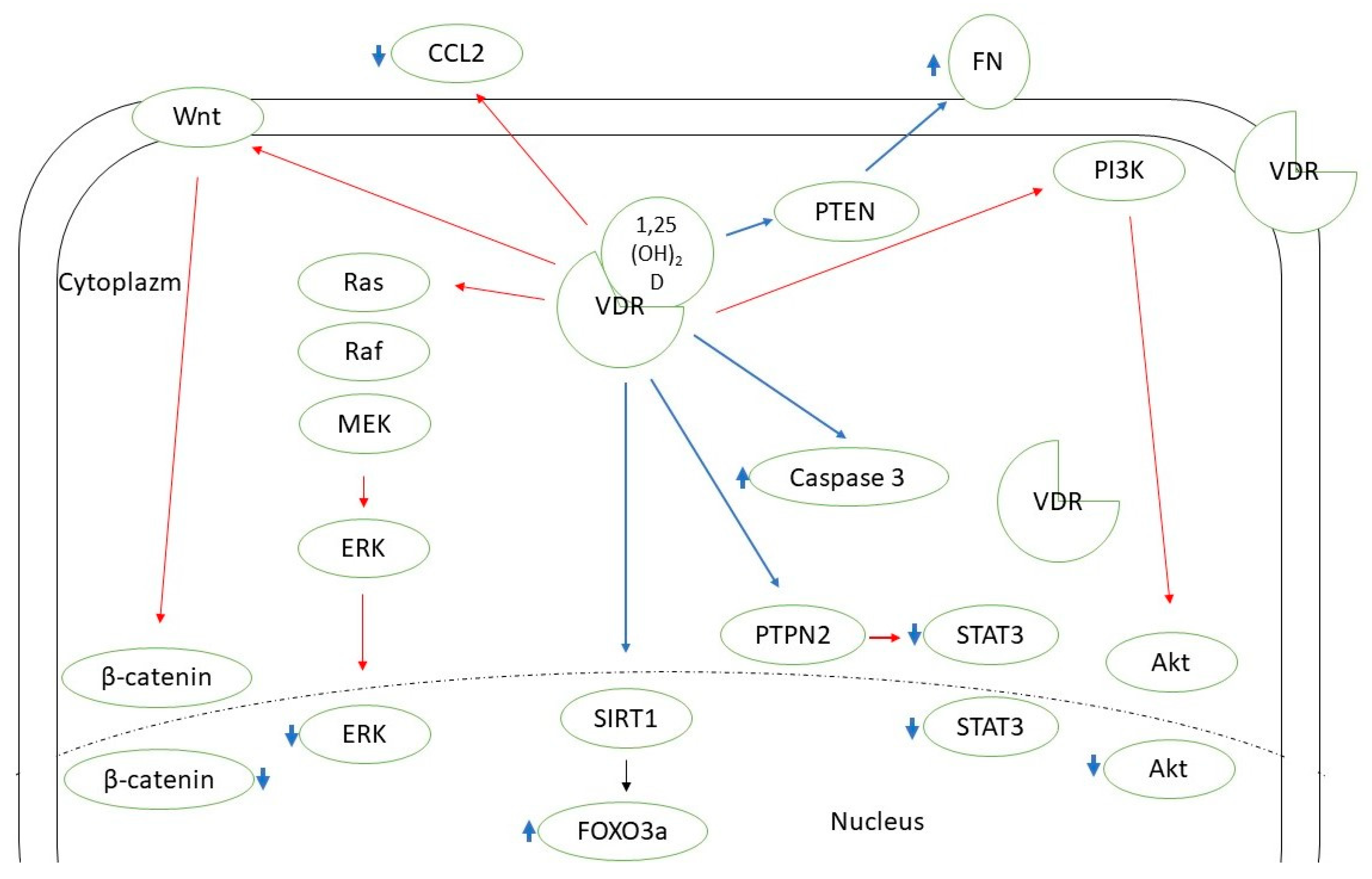
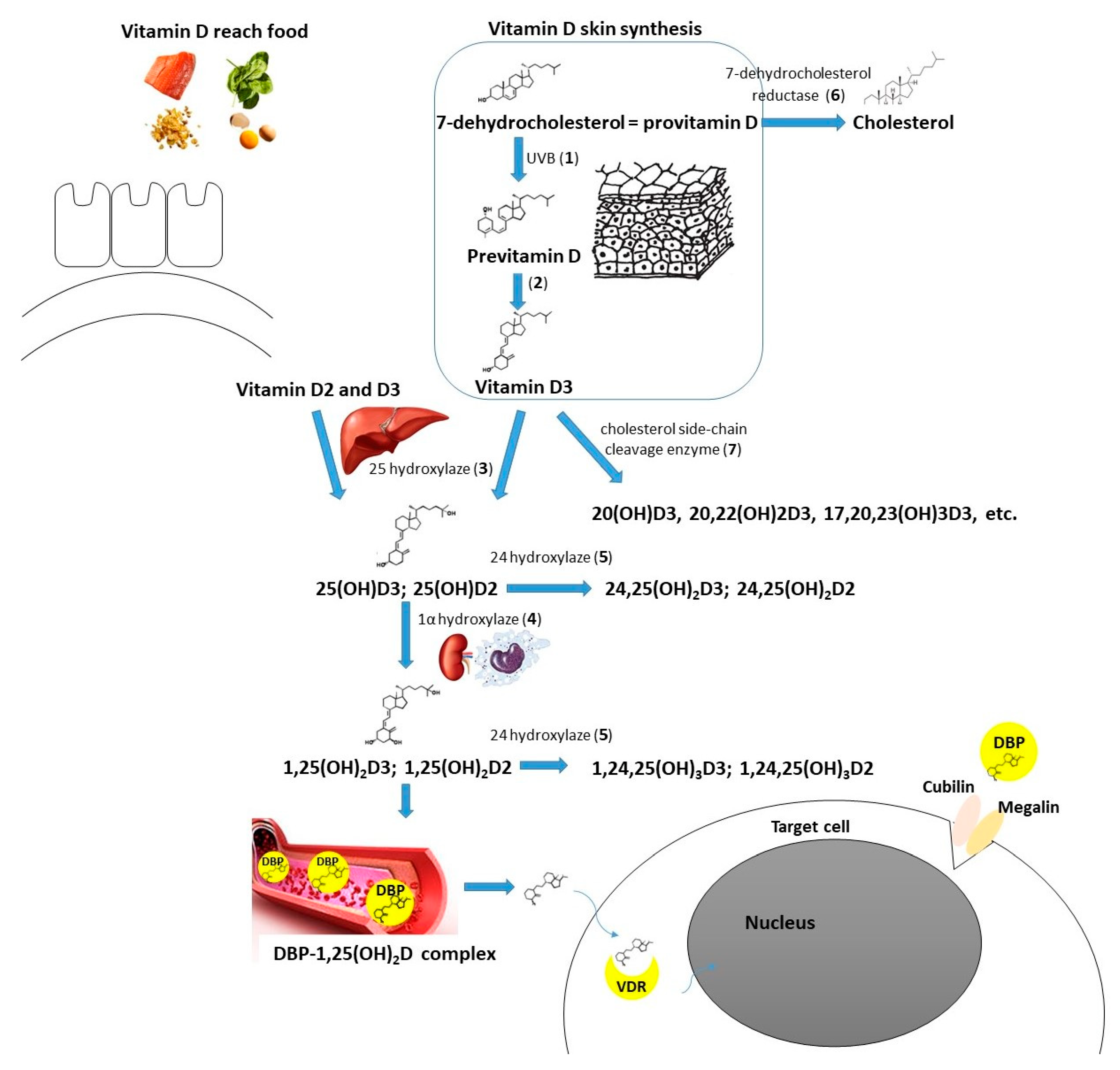
Vitamin D supplementation seems beneficial in the case of autoimmune thyroid diseases [12]. There is also a growing amount of evidence supporting the need for vitamin D intake in the context of thyroid neoplasms. Two recent meta-analyses by Hu et al. and Zhao et al. concluded that 25(OH)D deficiency is associated with a higher thyroid cancer risk, and patients with such a diagnosis are observed to have a significantly lower 25(OH)D concentration [13,14]. Some researchers confirmed the correlation between the vitamin D level and selected clinical features of thyroid cancer, the stage of the disease, or prognosis [15,16,17]. Zhang et al. found that active vitamin D metabolites reduce proliferation and induce apoptosis of PTC cells [18]. In another in vitro study, Peng et al. showed that calcitriol is able to induce differentiation of anaplastic thyroid cancer cells [19]. Calcitriol pretreatment was suggested to improve the effectiveness of chemotherapy (adriamycin) in the course of anaplastic thyroid cancer [20]. In the course of iodine refractory PTC, vitamin D metabolites in combination with other antitumorigenic agents have been used (e.g., sorafenib, doxorubicin, paclitaxel, SAHA) to increase the effectiveness or reduce the dose of the drug needed [21,22,23]. Among the possible pathways mediating vitamin D action on DTC cells there are SIRT1-FOXO3a, Ras-MEK-ERK, PI3K/Akt, PTPN2/p-STAT3, Wnt/β-catenin axes, and some other mechanisms (Figure 1) [23,24,25,26,27,28,29]. Effective vitamin D signaling is dependent on its metabolization to the active form and further interaction with vitamin D receptor (VDR). There are also mechanisms of vitamin D enzymatic deactivation. These processes require the contribution of different molecules (including cytochrome P450 enzymes or vitamin D binding protein) encoded by genes that together can be classified as vitamin D-related genes (Figure 2) [30].
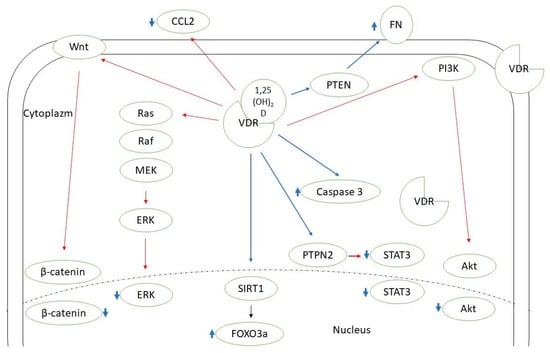
Figure 1.
Known pathways mediating vitamin D action in thyroid cancer cells. CCL2—C-C motif chemokine ligand 2; ERK—extracellular signal-regulated kinase; FN—fibronectin; FOXO3a—forkhead box protein O3a; MEK—mitogen-activated protein kinase kinase 1; PI3K—phosphoinositide 3-kinase; PTEN—phosphatase and tensin homolog; PTPN2—protein tyrosine phosphatase N 2; SIRT1—sirtuin 1 histon deacethylase; STAT3—signal transducer and activator of transcription 3; VDR—vitamin D receptor. Red lines—inhibition; blue lines—stimulation.
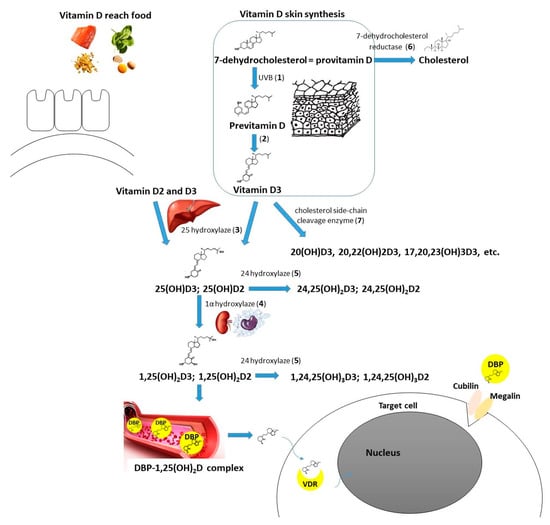
Figure 2.
Vitamin D metabolism (including molecules/enzymes involved, coding genes in brackets): Cubilin (CUBN); DBP—vitamin D binding protein (GC); Megalin (LRP2); VDR—vitamin D receptor (VDR); 1—UVB radiation, 290–315 mm wavelength; 2—nonenzymatic isomerization reaction under the temperature; 3—25-hydroxylase (main enzyme—CYP2R1—cytochrome P450 family 2 subfamily R member 1, minor role of CYP27A1—cytochrome P450 family 27 subfamily A member 1); 4—1α-hydroxylase (CYP27B1—cytochrome P450 family 27 subfamily B member 1); 5—24-hydroxylase (CYP24A1—cytochrome P450 family 24 subfamily A member 1); 6—7-dehydrocholesterol reductase (DHCR7); 7—cholesterol side-chain cleavage enzyme (CYP11A1—cytochrome P450 family 11 subfamily A member 1).
Therefore, it is expected that not only the 25(OH)D level but also vitamin D-related gene variants (polymorphisms or mutations) can modify the risk and outcome of neoplasms, including thyroid cancer. This systematic review would try to give an answer about the role of the vitamin D-related gene variants on thyroid cancer pathogenesis. In the case of a sufficient number of studies available, a meta-analysis of the data would be performed.
2. Methods
2.1. Systematic Review
The authors systematically searched the PubMed, EMBASE, Scopus, and Web of Science databases to identify evidence of an association between VDR or other vitamin D-related genes and thyroid cancer. The search terms were the following: vitamin D, thyroid cancer, and words describing genetic variants (polymorphism, mutation, variant), with the final query: ((vitamin D) OR (25-hydroxyvitamin D)) AND (thyroid cancer) AND ((polymorphism) OR (polymorphisms) OR (mutation) OR (variant)). Articles in English or, optionally, Polish language published prior to September 2022 were considered. The references of selected articles were checked for additional eligible studies. The systematic review was performed following the PRISMA guidelines [31].
Bibliographic research and study selection were performed independently by the two authors. In the event of any doubts or disagreement, the problem was discussed by the authors to reach the final decision. The full text of selected studies was then analyzed independently by both authors for agreement with inclusion criteria: (1) population-based or observational studies, (2) patients diagnosed with thyroid cancer (differentiated, anaplastic, or medullary), (3) control group of healthy volunteers, (4) the assessment of vitamin D-related gene variants (polymorphism, mutation, other variants). Reviews, case reports or case series, and animal or experimental studies were excluded. After eligible studies had been selected, the data of interest were extracted.
2.2. Meta-Analysis
A meta-analysis was performed if at least three different studies assessed the specific vitamin D-related gene variant. Access to raw data on genotype and/or allele frequencies was an additional criterion of inclusion in that case. Statistical analyses were performed using PQStat v.1.8.4.130. To assess the control groups of different studies for deviations from the Hardy–Weinberg equilibrium (HWE), the chi-square test was used. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for three different models: allelic (X vs. x), dominant (XX and Xx vs. xx), and recessive (XX vs. Xx and xx). The random effect or fixed model was used depending on calculated heterogeneity level. Between-study heterogeneity and I2 were assessed by Cochrane’s Q test (significant heterogeneity was defined as I2 > 25%). A sensitivity analysis was performed to estimate the stability of the result. Publication bias was determined by Egger’s test.
3. Results
3.1. Study Selection
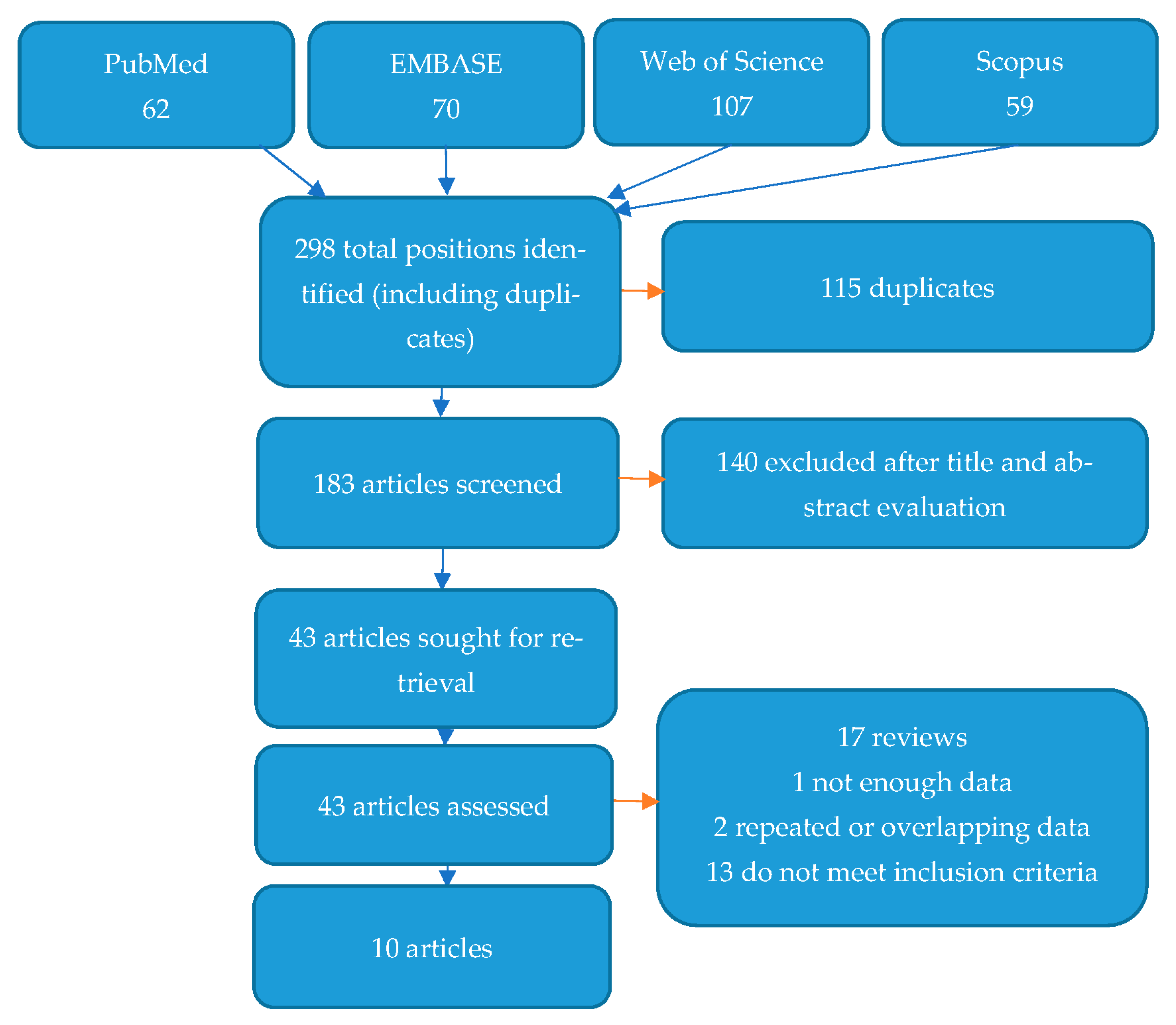
Figure 3 shows the flow diagram depicting the process of selecting studies for systematic review and the search strategy. A total of 298 items were found after a search of four databases. In all, 115 of these items were excluded as duplicates. After assessing their titles and abstracts, a further 140 articles were excluded as inaccurate/unrelated to the subject of the study. Finally, 43 articles were fully reviewed for eligibility. Of these, 33 were excluded for different reasons, as shown in Figure 3. Ten studies met the inclusion criteria and are presented below [32,33,34,35,36,37,38,39,40,41].
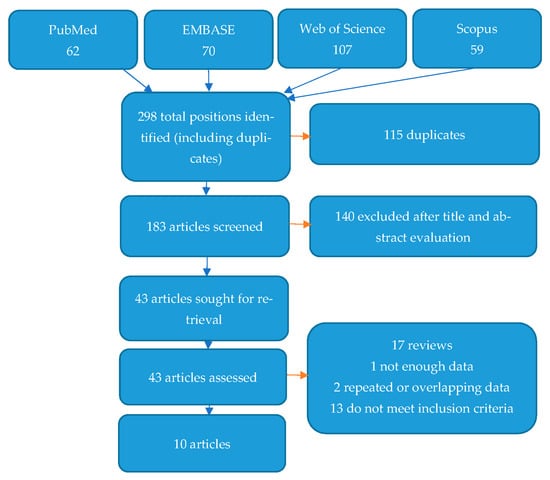
Figure 3.
Flow diagram demonstrating the search and selection process for articles.
3.2. Study Characteristic
Of the ten studies included, seven assessed the VDR gene, while other genes studied were CYP2R1—cytochrome P450 family 2 subfamily R member 1 (two studies), CYP24A1—cytochrome P450 family 24 subfamily A member 1 (two studies), CYP27B1—cytochrome P450 family 27 subfamily B member 1, DHCR7—7-dehydrocholesterol reductase, and CUBN—cubilin (each gene assessed by one study). As expected, most of these studies (all but one) analyzed patients with a diagnosis of DTC. Only in one study were MTC patients enrolled, while there are no data about the anaplastic thyroid cancer [32,33,34,35,36,37,38,39,40,41]. Detailed characteristics of the studies included are presented in Table 1.

Table 1.
Characteristics of the studies included.
3.3. Study Results
3.3.1. VDR
VDR gene SNPs have been studied for the first time in DTC patients by Haghpanah et al. in 2007 (five SNPs: rs2228570/FokI, rs1544410/BsmI, rs7975232/ApaI, rs731236/TaqI, and rs757343/Tru9I). No significant differences in genotype distributions were observed between patients and controls (only a difference in rs757343 allele frequencies between groups) [32]. In a study from 2009, Penna-Martinez et al. found that AA and Aa genotypes of the rs7975232 VDR SNP, FF of the rs2228570 SNP, and tABF haplotype are associated with a decreased risk of FTC. Instead, the Tabf haplotype may increase the risk of FTC. No associations were observed for PTC in this study (no differences found in both genotype and haplotype distributions). There were also no significant associations between vitamin D level or lymphocytic infiltration of thyroid gland and the SNPs studied [33]. On the contrary, Beysel et al. reported that TT and CT (ff and Ff) genotypes of rs2228570 VDR SNP may confer an increased risk of PTC. Moreover, these variants of rs2228570 SNP correlated with a more aggressive course of the disease including a more advanced stage, larger tumor size, more frequent lymph node involvement, extrathyroidal invasion, multifocality, and need for radioiodine treatment [37]. The authors did not find any associations for other studied VDR polymorphisms (rs7975232, rs1544410 and rs731236) [37]. Another Turkish study from 2021 did not confirm these results as there were no significant associations between any of the four VDR SNPs and DTC. The only significant association was between FF (CC) genotype and nodular goiter in comparison to healthy controls [40]. Lushchyk et al. studied additionally another VDR SNP—rs11568820/Cdx2. On a small DTC patients’ group, they showed its association with increased disease risk. No association was found with other SNPs studied (rs7975232, rs1544410 and rs731236) [36]. In the latest study of the role of VDR polymorphism in the development of DTC, again a set of four SNPs was analyzed: rs2228570, rs7975232, rs1544410, and rs731236. The authors showed a potential predisposing role of Ff and FF genotypes of rs2228570 SNP and Tt heterozygote of rs731236 SNP in thyroid cancer pathogenesis [41].
In only one study, VDR gene SNPs were assessed in MTC patients. Among the three SNPs studied (rs2228570, rs1544410 and rs757343), a significant association with cancer risk was found only for rs757343 SNP. Tt and tt genotypes were not only a risk factor for developing MTC but were also associated with a more aggressive course of the disease (more advanced stage, presence of metastases). Tt and tt genotypes of rs757343 also correlated with a higher 25(OH)D concentration, but only in the patients’ group [39]. All study results are also presented in Table 2.

Table 2.
Association between vitamin D-related gene polymorphisms and thyroid cancer—results of the studies.
3.3.2. CYP2R1
Penna-Martinez et al. studied two CYP2R1 SNPs in DTC (rs12794714, rs10741657), although no significant differences in allele or genotype frequencies were observed between the patients and controls [34]. In another study by Carvalho et al., rs2060793 SNP of the CYP2R1 gene was tested for its association with the risk of DTC. However, again no significant association was found [38]. There were also no significant associations between the studied SNPs and other parameters in both papers.
3.3.3. CYP24A1
In a study by Penna-Martinez et al., none of the polymorphisms of CYP24A1 studied (rs927650, rs2248137 and rs2296241) were found to confer an increased risk of thyroid cancer development. There was only a small difference (not significant after adjustment) in allele distribution of rs2296241 SNP between FTC and healthy controls. However, further haplotype distribution analyses revealed some significant differences, mainly between the FTC group and controls [34]. The authors also observed that some haplotype combinations of CYP24A1 may be associated with a lower level of circulating 1,25(OH)2D3 [34]. Carvalho et al. assessed another CYP24A1 SNP-rs6013897, although they did not observe significant difference between DTC patients and controls [38].
3.3.4. CYP27B1
In 2012, Penna-Martinez et al. tested the role of two CYP27B1 polymorphisms (rs10877012, rs4646536) in the pathogenesis of DTC. They failed to find any significant differences between patients (PTC or FTC) and controls in genotype and allele distribution. Haplotype analysis also did not show any evident influence on disease risk or vitamin D level [34].
3.3.5. DHCR7
In a study by Carvalho et al., it was found that G allele and GG/TG genotypes of DHCR7 rs12785878 SNP may be associated with an increased overall risk of DTC (both PTC and FTC). No influence on clinicopathologic parameters was found [38]. No other studies have tested rs12785878 polymorphism in thyroid cancer.
3.3.6. CUBN
Cubilin encoded by CUBN gene was found to be expressed on thyrocytes as well. One of the SNPs of CUBN—rs1801222 was studied for its association with DTC risk (PTC and FTC cases). However, no significant differences in genotype distribution were found between patients and controls. There was only some increase in PTC risk in the case of a combination of G allele and vitamin D deficiency (35).
3.4. Meta-Analysis
A total of seven studies assessing VDR SNPs in thyroid cancer were found. Six different SNPs were involved: rs2228570, rs7975232, rs1544410, rs731236, rs757343, and rs11568820 [32,33,36,37,39,40,41]. In the case of one study, genotype and allele frequencies were not available [36]. In a paper by Ramezani et al., MTC was analyzed, contrary to other studies assessing DTC patients (PTC, FTC, or DTC without distinguished subtypes) [39]. Moreover, in the case of two SNPs, rs757343 and rs11568820, the number of studies presenting results was too low (two and one, respectively) [32,36,39]. Therefore, finally five different papers were included in the meta-analysis, with data on four different VDR SNPs (rs2228570, rs7975232, rs1544410, rs731236) [32,33,37,40,41].
The results of meta-analysis are presented in Table 3. None of the SNPs studied showed any significant associations. Sensitivity analysis was performed, showing that overall ORs did not change significantly after single study elimination. To assess the publication bias, Egger’s test was used, and in two analyses p was less than 0.05 (rs7975232 recessive, rs1544410 recessive model). Heterogeneity analysis results are shown in Table 3.

Table 3.
Meta-analysis results—association between four vitamin D receptor gene polymorphisms (rs2228570, rs1544410, rs7975232 and rs731236) and differentiated thyroid cancer risk.
4. Discussion
Vitamin D may directly affect the thyroid gland, as thyrocytes express VDR. Numerous studies have shown that the VDR level is increased in the case of DTC (mainly PTC was assessed) in comparison to the normal thyroid [18,42,43,44,45,46]. Moreover, VDR was observed among genes overexpressed in the follicular variant of PTC comparing to follicular adenoma [47]. Choi et al. found that high VDR level was associated with a more advanced stage of the disease, an increased risk of lateral neck lymph node metastases, and shorter recurrence-free survival [42]. In contrast, Clinckspoor et al. observed that VDR expression was lower in the case of lymph node metastases [45]. Unlike DTC, in many anaplastic thyroid cancer cases, loss of VDR expression was reported [45]. Observations of other neoplasms also show that loss of cell differentiation is often associated with reduced VDR expression. In the development of cancer, both genetic (mutations, polymorphisms) and epigenetic modifications occur that can lead to changes in the VDR protein level or its function [48,49]. The association between VDR SNPs and cancer risk was shown among others in the case of breast, keratinocyte, colorectal ovarian, or lung malignancies [50,51,52]. Unfortunately, in the case of thyroid cancer, the results of papers published to date seem heterogeneous and largely inconclusive (as mentioned above). Rs2228570 VDR SNP, a polymorphism with a proven functional role, is suggested by some researchers as modulating the risk of disease development [33,37,41]. However, a meta-analysis of available data did not confirm a significant association between rs2228570 VDR SNP or the other three most frequently studied VDR SNPs (rs1544410, rs7975232, rs731236) and DTC risk. Further studies and larger groups of patients are needed.
Less is known about the potential role of the CYP2R1 gene, encoding a major enzyme with vitamin D 25-hydroxylase activity, in neoplasm development. However, some studies have shown an association between polymorphisms of CYP2R1 and cancer risk (e.g., breast) [53] or suggested altered gene expression in tumor cells (e.g., renal cell carcinoma) [54]. CYP2R1 mRNA was detected in both normal thyrocytes and various thyroid cancer subtypes (including anaplastic, papillary, and follicular thyroid cancer). Gene expression was observed to be higher in thyroid cancer cell lines, although these differences were only modest [55]. Among the three different SNPs of CYP2R1 studied so far in DTC patients, there were no significant associations with disease risk [34,38].
25-hydroxyvitamin D-1 alpha hydroxylase and its gene-CYP27B1 have been broadly studied for association with different tumors susceptibility. Significant associations between CYP27B1 expression or gene polymorphisms and cancer risk were found, for example, for colorectal, ovarian, and non-small cell lung cancer [56,57,58,59]. Both normal thyroid follicular cells and DTC cells express CYP27B1. Some authors postulate that its level is increased in the case of DTC cells [44,45,55]. In contrast, Yavropoulou et al. and Balla et al. did not observe significant differences in CYP27B1 expression between PTC and non-neoplastic thyroid cells [43,60]. In anaplastic thyroid cancer, there was a tendency to more frequent negative results for CYP27B1 with increasing Ki67 or in cases with distant metastases [45]. However, the role of two studied CYP27B1 polymorphisms (rs10877012, rs4646536) in the pathogenesis of DTC was not confirmed [34].
CYP24A1 expression has been observed to be increased in the course of different malignancies e.g., colon, ovarian, lung, liver, or esophageal cancer [60]. Increased activity of 24-hydroxylase in the tumor tissues could be a mechanism for the neutralization of vitamin D antitumor activity. Most of the studies of DTC also confirmed that CYP24A1 expression is increased in cancerous compared to normal cells [43,45,60,61,62]. There was also an association between higher CYP24A1 expression and resistance to active vitamin D analogs [63]. Yavropoulou et al. observed that there is an association between CYP24A1 expression and the risk of lymph node metastases or extrathyroidal extension of the tumor [43]. In other studies, a positive correlation between CYP24A1 expression and the risk of vascular invasion, lymph node metastasis, tumor size [60], or more advanced stage of the disease [62] was observed. A study on anaplastic thyroid cancer cell lines showed that CYP24A1 knockdown led to the enhancement of vitamin D antitumor activity [64]. Another approach is to search for polymorphisms that may impair gene function and, in consequence, facilitate the disease development. There are suggestions about the association between CYP24A1 SNPs and some cancer types, including prostate, breast, or pancreas cancer [65,66]. However, in the case of thyroid, only a weak association between rs2296241 and FTC has been observed [34]. Rs2296241 is a synonymous SNP, although it could influence gene expression or protein function in other mechanisms or be in linkage disequilibrium with different, functional, SNPs [67].
Among the other vitamin D-related genes, there are also some limited data about DHCR7 role in DTC. 7-dehydrocholesterol reductase is postulated to regulate the balance between cholesterol and vitamin D synthesis [68]. Carvalho et al. found that the G allele and GG/TG genotypes of rs12785878 SNP may be associated with an increased overall risk of DTC (both PTC and FTC). No influence on clinicopathologic parameters was found [38]. Unfortunately, no other studies have yet searched for the role of this gene in thyroid gland neoplasms. DHCR7 is one of a few genes found to influence 25(OH)D plasma level in GWAS [69]. Rs12785878 SNP was also found to increase a general cancer risk in a meta-analysis of five studies including patients with breast, hepatocellular, and thyroid cancer [69].
Cubilin encoded by the CUBN gene plays variable roles, including the process of renal 1,25(OH)2D synthesis (25(OH)D—vitamin D binding protein (DBP) complexes absorption from glomerular ultrafiltrate) [70]. It was found to be expressed on thyrocytes too. One of the SNPs of CUBN-rs1801222- was studied for its association with DTC, although no significant differences were found between patients and controls (35).
There are also other candidates in the group of vitamin D-related genes that have not yet been studied in thyroid cancer patients. Among them, there is CYP27A1 (cytochrome P450 family 27 subfamily A member 1) encoding sterol 26-hydroxylase, an enzyme playing a minor role as vitamin D 25-hydroxylase. CYP27A1 was found to be expressed in both thyroid cancer cells and normal thyrocytes [55]. In addition to classic vitamin D metabolism pathways, also alternative routes have been reported. One is mediated by cholesterol side-chain cleavage enzyme encoded by CYP11A1 (cytochrome P450 family 11 subfamily A member 1; enzyme expressed, i.e., in the gastrointestinal tract or skin, catalyzes hydroxylation predominantly at C-20 and C-22) [71]. The main product of its action-17,20,23(OH)3D, promotes differentiation and inhibits proliferation and inflammatory processes. It was found to work as a biased noncalcemic agonist of VDR with possible anticancer activity [71,72]. Similarly, the GC gene that encodes DBP requires further exploration. This protein, which is responsible among other things for vitamin D and its metabolites transportation, was significantly under-expressed in the samples of papillary thyroid cancer comparing to normal thyroid cells [73,74]. In a recent study by Mull et al., an inverse correlation was observed between DBP expression in thyroid cancer tissue samples and PTC staging [75].
5. Conclusions
There are relatively large and comprehensive reports on the role of vitamin D in the pathogenesis of thyroid neoplasms. Some in vitro studies using vitamin D or its analogs also seem promising. It is expected that vitamin D-related gene variants could also have an important modulating role in thyroid tumorigenesis. To date, polymorphic variants of VDR have been studied most extensively, although results are inconclusive. In the case of other vitamin D-related genes, only single studies are available or even no specific results. Therefore, further effort is needed to explain the complex interaction between vitamin D concentration, vitamin D-related gene variants modifying its metabolism, and thyroid cancer pathogenesis.
Author Contributions
Conceptualization, K.L. and A.M.; methodology, A.M. and K.L.; resources, A.M. and K.L.; data curation, A.M.; writing—original draft preparation, A.M.; writing—review and editing, K.L. and A.M.; supervision, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wong, R.; Farrell, S.G.; Grossmann, M. Thyroid nodules: Diagnosis and management. Med. J. Aust. 2018, 209, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Grani, G.; Lamartina, L.; Filetti, S.; Mandel, S.J.; Cooper, D.S. The Diagnosis and Management of Thyroid Nodules: A Review. JAMA 2018, 319, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Spielman, D.B.; Badhey, A.; Kadakia, S.; Inman, J.C.; Ducic, Y. Rare Thyroid Malignancies: An Overview for the Oncologist. Clin. Oncol. 2017, 29, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Morand, G.B.; da Silva, S.D.; Hier, M.P.; Alaoui-Jamali, M.A. Insights into genetic and epigenetic determinants with impact on vitamin d signaling and cancer association studies: The case of thyroid cancer. Front. Oncol. 2014, 4, 309. [Google Scholar] [CrossRef]
- Jarzab, B.; Dedecjus, M.; Lewiński, A.; Adamczewski, Z.; Bakuła-Zalewska, E.; Bałdys-Waligórska, A.; Barczyński, M.; Biskup-Frużyńska, M.; Bobek-Billewicz, B.; Bossowski, A.; et al. Diagnosis and treatment of thyroid cancer in adult patients—Recommendations of Polish Scientific Societies and the National Oncological Strategy. 2022 Update. Endokrynol. Pol. 2022, 73, 173–300. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Mazur, A.; Frączek, P.; Tabarkiewicz, J. Vitamin D as a Nutri-Epigenetic Factor in Autoimmunity-A Review of Current Research and Reports on Vitamin D Deficiency in Autoimmune Diseases. Nutrients 2022, 14, 4286. [Google Scholar] [CrossRef]
- Płazińska, M.T.; Czarnywojtek, A.; Sawicka-Gutaj, N.; Zgorzalewicz-Stachowiak, M.; Czarnocka, B.; Gut, P.; Karlinska, M.; Fichna, M.; Stachowski, A.; Ruchała, M.; et al. Vitamin D deficiency and thyroid autoantibody fluctuations in patients with Graves’ disease—A mere coincidence or a real relationship? Adv. Med. Sci. 2020, 65, 39–45. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 20. [Google Scholar] [CrossRef]
- Robsahm, T.E.; Tretli, S.; Torjesen, P.A.; Babigumira, R.; Schwartz, G.G. Serum 25-hydroxyvitamin D levels predict cancer survival: A prospective cohort with measurements prior to and at the time of cancer diagnosis. Clin. Epidemiol. 2019, 11, 695–705. [Google Scholar] [CrossRef]
- Mikulska, A.A.; Karaźniewicz-Łada, M.; Filipowicz, D.; Ruchała, M.; Główka, F.K. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview. Int. J. Mol. Sci. 2022, 23, 6580. [Google Scholar] [CrossRef]
- Hu, M.J.; Zhang, Q.; Liang, L.; Wang, S.Y.; Zheng, X.C.; Zhou, M.M.; Yang, Y.W.; Zhong, Q.; Huang, F. Association between vitamin D deficiency and risk of thyroid cancer: A case-control study and a meta-analysis. J. Endocrinol. Investig. 2018, 41, 1199–1210. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Zhang, Z.; Zhou, X.; Yao, J.; Zhang, R.; Liao, L.; Dong, J. Vitamin D deficiency as a risk factor for thyroid cancer: A meta-analysis of case-control studies. Nutrition 2019, 57, 5–11. [Google Scholar] [CrossRef]
- Kim, J.R.; Kim, B.H.; Kim, S.M.; Oh, M.Y.; Kim, W.J.; Jeon, Y.K.; Kim, S.S.; Lee, B.J.; Kim, Y.K.; Kim, I.J. Low serum 25 hydroxyvitamin D is associated with poor clinicopathologic characteristics in female patients with papillary thyroid cancer. Thyroid 2014, 24, 1618–1624. [Google Scholar] [CrossRef]
- Abuduwaili, M.; Xing, Z.; Xia, B.; Fei, Y.; Zhu, J.; Su, A. Correlation between Pre-Operative 25-Hydroxyvitamin D Levels and Poor Prognostic Factors for Papillary Thyroid Cancer. J. Investig. Surg. 2021, 35, 1076–1082. [Google Scholar] [CrossRef]
- Sulibhavi, A.; Rohlfing, M.L.; Jalisi, S.M.; McAneny, D.B.; Doherty, G.M.; Holick, M.F.; Noordzij, J.P. Vitamin D deficiency and its relationship to cancer stage in patients who underwent thyroidectomy for papillary thyroid carcinoma. Am. J. Otolaryngol. 2019, 40, 536–541. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H.; He, L.; Wang, Z.; Dong, W.; Sun, W.; Zhang, P. Potential Use of 1-25-dihydroxyvitamin D in the Diagnosis and Treatment of Papillary Thyroid Cancer. Med. Sci. Monit. 2018, 24, 1614–1623. [Google Scholar] [CrossRef]
- Peng, W.; Wang, K.; Zheng, R.; Derwahl, M. 1,25 dihydroxyvitamin D3 inhibits the proliferation of thyroid cancer stem-like cells via cell cycle arrest. Endocr. Res. 2016, 41, 71–80. [Google Scholar] [CrossRef]
- Zhang, T.; He, L.; Sun, W.; Qin, Y.; Zhang, P.; Zhang, H. 1,25-Dihydroxyvitamin D3 enhances the susceptibility of anaplastic thyroid cancer cells to adriamycin-induced apoptosis by increasing the generation of reactive oxygen species. Mol. Med. Rep. 2019, 20, 2641–2648. [Google Scholar] [CrossRef]
- Clinckspoor, I.; Verlinden, L.; Overbergh, L.; Korch, C.; Bouillon, R.; Mathieu, C.; Verstuyf, A.; Decallonne, B. 1,25-dihydroxyvitamin D3 and a superagonistic analog in combination with paclitaxel or suberoylanilide hydroxamic acid have potent antiproliferative effects on anaplastic thyroid cancer. J. Steroid Biochem. Mol. Biol. 2011, 124, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Izkhakov, E.; Sharon, O.; Knoll, E.; Aizic, A.; Fliss, D.M.; Kohen, F.; Stern, N.; Somjen, D. A sorafenib-sparing effect in the treatment of thyroid carcinoma cells attained by co-treatment with a novel isoflavone derivative and 1,25 dihydroxyvitamin D3. J. Steroid Biochem. Mol. Biol. 2018, 182, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, L.; Wang, Z.; Dong, W.; Sun, W.; Qin, Y.; Zhang, P.; Zhang, H. Calcitriol enhances Doxorubicin-induced apoptosis in papillary thyroid carcinoma cells via regulating VDR/PTPN2/p-STAT3 pathway. J. Cell Mol. Med. 2020, 24, 5629–5639. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Doering, C.; Hansmann, M.L.; Gruenwald, F.; Vorlaender, C.; Bechstein, W.O.; Holzer, K.; Badenhoop, K.; Penna-Martinez, M. Vitamin D, FOXO3a, and Sirtuin1 in Hashimoto’s Thyroiditis and Differentiated Thyroid Cancer. Front. Endocrinol. 2018, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lv, L.; Li, W. 1,25-Dihydroxy vitamin D3 inhibits the Ras-MEK-ERK pathway and regulates proliferation and apoptosis of papillary thyroid carcinoma. Steroids 2020, 159, 108585. [Google Scholar] [CrossRef]
- Kemmochi, S.; Fujimoto, H.; Woo, G.H.; Hirose, M.; Nishikawa, A.; Mitsumori, K.; Shibutani, M. Preventive effects of calcitriol on the development of capsular invasive carcinomas in a rat two-stage thyroid carcinogenesis model. J. Vet. Med. Sci. 2011, 73, 655–664. [Google Scholar] [CrossRef]
- Liu, W.; Asa, S.L.; Ezzat, S. 1alpha,25-Dihydroxyvitamin D3 targets PTEN-dependent fibronectin expression to restore thyroid cancer cell adhesiveness. Mol. Endocrinol. 2005, 19, 2349–2357. [Google Scholar] [CrossRef]
- Pang, R.; Xu, Y.; Hu, X.; Liu, B.; Yu, J. Vitamin D receptor knockdown attenuates the antiproliferative, pro-apoptotic and anti-invasive effect of vitamin D by activating the Wnt/β-catenin signaling pathway in papillary thyroid cancer. Mol. Med. Rep. 2020, 22, 4135–4142. [Google Scholar] [CrossRef]
- Coperchini, F.; Greco, A.; Croce, L.; Petrosino, E.; Grillini, B.; Magri, F.; Chiovato, L.; Rotondi, M. Vitamin D Reduces Thyroid Cancer Cells Migration Independently From the Modulation of CCL2 and CXCL8 Chemokines Secretion. Front. Endocrinol. 2022, 13, 876397. [Google Scholar] [CrossRef]
- Tomei, S.; Singh, P.; Mathew, R.; Mattei, V.; Garand, M.; Alwakeel, M.; Sharif, E.; Al Khodor, S. The Role of Polymorphisms in Vitamin D-Related Genes in Response to Vitamin D Supplementation. Nutrients 2020, 12, 2608. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Haghpanah, V.; Ghaffari, S.H.; Rahimpour, P.; Abbasi, A.; Saeedi, M.; Pak, H.; Alborzi, F.; Barzegar, S.; Heshmat, R.; Alimoghadam, K.; et al. Vitamin D receptor gene polymorphisms in patients with thyroid cancer. Gene Ther. Mol. Biol. 2007, 11, 299–304. [Google Scholar]
- Penna-Martinez, M.; Ramos-Lopez, E.; Stern, J.; Hinsch, N.; Hansmann, M.L.; Selkinski, I.; Grünwald, F.; Vorländer, C.; Wahl, R.A.; Bechstein, W.O.; et al. Vitamin D receptor polymorphisms in differentiated thyroid carcinoma. Thyroid 2009, 19, 623–628. [Google Scholar] [CrossRef]
- Penna-Martinez, M.; Ramos-Lopez, E.; Stern, J.; Kahles, H.; Hinsch, N.; Hansmann, M.L.; Selkinski, I.; Grünwald, F.; Vorländer, C.; Bechstein, W.O.; et al. Impaired vitamin D activation and association with CYP24A1 haplotypes in differentiated thyroid carcinoma. Thyroid 2012, 22, 709–716. [Google Scholar] [CrossRef]
- Penna-Martinez, M.; Angeletti, F.; Kahles, H.; Badenhoop, K. Cubilin exon polymorphism and its effects on vitamin D levels in differentiated thyroid carcinoma. In Proceedings of the Endocrine Society’s 94th Annual Meeting and Expo, Houston, TX, USA, 23–26 June 2012. [Google Scholar]
- Lushchyk, M.; Drozd, V.; Tuzava, A.; Dudzik, N.; Danilova, L.; Ameliyanovich, M.; Mosse, I.; Kilchevsky, A.; Branovan, I. Vitamin D receptor (VDR) polymorphisms variations in patients with differentiated thyroid cancer. In Proceedings of the 88th Annual Meeting of the American Thyroid Association, Washington, DC, USA, 3–7 October 2018. [Google Scholar]
- Beysel, S.; Eyerci, N.; Pinarli, F.A.; Apaydin, M.; Kizilgul, M.; Caliskan, M.; Ozcelik, O.; Kan, S.; Cakal, E. VDR gene FokI polymorphism as a poor prognostic factor for papillary thyroid cancer. Tumour. Biol. 2018, 40, 1010428318811766. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.S.; Gonçalves, C.I.; Almeida, J.T.; Azevedo, T.; Martins, T.; Rodrigues, F.J.; Lemos, M.C. Association of Vitamin D Pathway Genetic Variation and Thyroid Cancer. Genes 2019, 10, 572. [Google Scholar] [CrossRef]
- Ramezani, M.; Mazani, M.; Tabatabaei, M.; Rahimian, A.; Mosaferi, E.; Hedayati, M. Medullary thyroid cancer is associated with high serum vitamin D level and polymorphism of vitamin D receptors. Physiol. Int. 2020, 107, 120–133. [Google Scholar] [CrossRef]
- Gunes, A.; Yazicioglu, M.B.; Tiryaki, C.; Uren, N.; Ergul, E.; Simsek, T.; Cubukcu, A. Evaluation of vitamin D receptor gene polymorphisms in patients with differentiated thyroid carcinomas and nodular goiter. Minerva Endocrinol. 2021, 46, 317–324. [Google Scholar] [CrossRef]
- Cocolos, A.M.; Muresan, A.; Caragheorgheopol, A.; Ghemigian, M.; Ioachim, D.; Poiana, C. Vitamin D status and VDR polymorphisms as prognostic factors in differentiated thyroid carcinoma. In Vivo 2022, 36, 2434–2441. [Google Scholar] [CrossRef]
- Choi, J.Y.; Yi, J.W.; Lee, J.H.; Song, R.Y.; Yu, H.; Kwon, H.; Chai, Y.J.; Kim, S.J.; Lee, K.E. VDR mRNA overexpression is associated with worse prognostic factors in papillary thyroid carcinoma. Endocr. Connect. 2017, 6, 172–178. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Panagiotou, G.; Topouridou, K.; Karayannopoulou, G.; Koletsa, T.; Zarampoukas, T.; Goropoulos, A.; Chatzaki, E.; Yovos, J.G.; Pazaitou-Panayiotou, K. Vitamin D receptor and progesterone receptor protein and gene expression in papillary thyroidcarcinomas: Associations with histological features. J. Endocrinol. Investig. 2017, 40, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Khadzkou, K.; Buchwald, P.; Westin, G.; Dralle, H.; Akerström, G.; Hellman, P. 25-hydroxyvitamin D3 1alpha-hydroxylase and vitamin D receptor expression in papillary thyroid carcinoma. J. Histochem. Cytochem. 2006, 54, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Clinckspoor, I.; Hauben, E.; Verlinden, L.; Van den Bruel, A.; Vanwalleghem, L.; Vander Poorten, V.; Delaere, P.; Mathieu, C.; Verstuyf, A.; Decallonne, B. Altered expression of key players in vitamin D metabolism and signaling in malignant and benign thyroid tumors. J. Histochem. Cytochem. 2012, 60, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Xu, F.; Xia, X.; Dai, D.; Sun, R.; Xie, Z. Vitamin D receptor regulates proliferation and differentiation of thyroid carcinoma via the E-cadherin-β-catenin complex. J. Mol. Endocrinol. 2022, 68, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Schulten, H.J.; Al-Mansouri, Z.; Baghallab, I.; Bagatian, N.; Subhi, O.; Karim, S.; Al-Aradati, H.; Al-Mutawa, A.; Johary, A.; Meccawy, A.A.; et al. Comparison of microarray expression profiles between follicular variant of papillary thyroid carcinomas and follicular adenomas of the thyroid. BMC Genom. 2015, 16 (Suppl. 1), S7. [Google Scholar] [CrossRef]
- DeSmet, M.L.; Fleet, J.C. Constitutively active RAS signaling reduces 1,25 dihydroxyvitamin D-mediated gene transcription in intestinal epithelial cells by reducing vitamin D receptor expression. J. Steroid Biochem. Mol. Biol. 2017, 173, 194–201. [Google Scholar] [CrossRef]
- Marik, R.; Fackler, M.; Gabrielson, E.; Zeiger, M.A.; Sukumar, S.; Stearns, V.; Umbricht, C.B. DNA methylation-related vitamin D receptor insensitivity in breast cancer. Cancer Biol. Ther. 2010, 10, 44–53. [Google Scholar] [CrossRef]
- Zhao, Z.; Cai, W.; Xing, J.; Zhao, C. Lower vitamin D levels and VDR variants are risk factors for breast cancer: An updated meta-analysis. Nucleosides Nucleotides Nucleic Acids 2022, 9, 1–21. [Google Scholar] [CrossRef]
- Wan, Y.; Lyu, Y.; Xu, Y.; Huang, P. The relationship between VDR polymorphisms and keratinocyte carcinomas: A systematic review and meta-analysis. Future Oncol. 2022, 18, 2613–2626. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Pasquali, E.; Serrano, D.; Raimondi, S.; Disalvatore, D.; Gandini, S. Vitamin D receptor polymorphism FokI and cancer risk: A comprehensive meta-analysis. Carcinogenesis 2014, 35, 1913–1919. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Sandler, D.P.; Kinyamu, H.K.; Taylor, J.A.; Weinberg, C.R. Single-Nucleotide Polymorphisms in Vitamin D-Related Genes May Modify Vitamin D-Breast Cancer Associations. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1761–1771. [Google Scholar] [CrossRef]
- Urbschat, A.; Paulus, P.; von Quernheim, Q.F.; Brück, P.; Badenhoop, K.; Zeuzem, S.; Ramos-Lopez, E. Vitamin D hydroxylases CYP2R1, CYP27B1 and CYP24A1 in renal cell carcinoma. Eur. J. Clin. Investig. 2013, 43, 1282–1290. [Google Scholar] [CrossRef]
- Bennett, R.G.; Wakeley, S.E.; Hamel, F.G.; High, R.R.; Korch, C.; Goldner, W.S. Gene expression of vitamin D metabolic enzymes at baseline and in response to vitamin D treatment in thyroid cancer cell lines. Oncology 2012, 83, 264–272. [Google Scholar] [CrossRef]
- Latacz, M.; Snarska, J.; Kostyra, E.; Wroński, K.; Fiedorowicz, E.; Savelkoul, H.; Jarmołowska, B.; Płomiński, J.; Grzybowski, R.; Cieślińska, A. CYP27B1 Gene Polymorphism rs10877012 in Patients Diagnosed with Colorectal Cancer. Nutrients 2020, 12, 998. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Jóźwicki, W.; Jochymski, C.; Slominski, A.T. Decreased expression of CYP27B1 correlates with the increased aggressiveness of ovarian carcinomas. Oncol. Rep. 2015, 33, 599–606. [Google Scholar] [CrossRef]
- Ge, N.; Chu, X.M.; Xuan, Y.P.; Ren, D.Q.; Wang, Y.; Ma, K.; Gao, H.J.; Jiao, W.J. Associations between abnormal vitamin D metabolism pathway function and non-small cell lung cancer. Oncol. Lett. 2017, 14, 7538–7544. [Google Scholar] [CrossRef]
- Gong, C.; Long, Z.; Yu, Y.; Zhu, L.; Tian, J.; Li, S.; Li, J.; Yu, H.; Chi, Q.; Piao, D.; et al. Dietary factors and polymorphisms in vitamin D metabolism genes: The risk and prognosis of colorectal cancer in northeast China. Sci. Rep. 2017, 7, 8827. [Google Scholar] [CrossRef]
- Balla, B.; Tobiás, B.; Kósa, J.P.; Podani, J.; Horváth, P.; Nagy, Z.; Horányi, J.; Járay, B.; Székely, E.; Krenács, L.; et al. Vitamin D-neutralizing CYP24A1 expression, oncogenic mutation states and histological findings of human papillary thyroid cancer. J. Endocrinol. Investig. 2015, 38, 313–321. [Google Scholar] [CrossRef]
- Balla, B.; Kósa, J.P.; Tobiás, B.; Halászlaki, C.; Takács, I.; Horváth, H.; Speer, G.; Nagy, Z.; Horányi, J.; Járay, B.; et al. Marked increase in CYP24A1 gene expression in human papillary thyroid cancer. Thyroid 2011, 21, 459–460. [Google Scholar] [CrossRef]
- Zou, M.; BinHumaid, F.S.; Alzahrani, A.S.; Baitei, E.Y.; Al-Mohanna, F.A.; Meyer, B.F.; Shi, Y. Increased CYP24A1 expression is associated with BRAF(V600E) mutation and advanced stages in papillary thyroid carcinoma. Clin. Endocrinol. 2014, 81, 109–116. [Google Scholar] [CrossRef]
- Sharma, V.; Fretwell, D.; Crees, Z.; Kerege, A.; Klopper, J.P. Thyroid cancer resistance to vitamin D receptor activation is associated with 24-hydroxylase levels but not the ff FokI polymorphism. Thyroid 2010, 20, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Zhang, H. CYP24A1 depletion facilitates the antitumor effect of vitamin D3 on thyroid cancer cells. Exp. Ther. Med. 2018, 16, 2821–2830. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Qiu, S.; Zhang, X.; Wang, Y.; Souraka, T.D.M.; Wen, X.; Liang, C.; Tu, J. The associations between CYP24A1 polymorphisms and cancer susceptibility: A meta-analysis and trial sequential analysis. Pathol. Res. Pract. 2018, 214, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jin, M. Genetic associations between CYP24A1 polymorphisms and predisposition of cancer: A meta-analysis. Int. J. Biol. Markers 2020, 35, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, H.; Zhang, Z.; Qin, L.; Li, B. Association of the CYP24A1-rs2296241 polymorphism of the vitamin D catabolism enzyme with hormone-related cancer risk: A meta-analysis. OncoTargets Ther. 2015, 8, 1175–1183. [Google Scholar]
- Prabhu, A.V.; Luu, W.; Li, D.; Sharpe, L.J.; Brown, A.J. DHCR7: A vital enzyme switch between cholesterol and vitamin D production. Prog. Lipid Res. 2016, 64, 138–151. [Google Scholar] [CrossRef]
- Wen, J.; Li, J.; Liang, X.; Wang, A. Association of Polymorphisms in Vitamin D-Metabolizing Enzymes DHCR7 and CYP2R1 with Cancer Susceptibility: A Systematic Review and Meta-Analysis. Dis. Markers 2021, 2021, 6615001. [Google Scholar] [CrossRef]
- Christensen, E.I.; Nielsen, R. Role of megalin and cubilin in renal physiology and pathophysiology. Rev. Physiol. Biochem. Pharmacol. 2007, 158, 1–22. [Google Scholar]
- Slominski, A.T.; Kim, T.K.; Hobrath, J.V.; Oak, A.S.W.; Tang, E.K.Y.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORalpha and RORgamma. J. Steroid Biochem. Mol. Biol. 2017, 173, 42–56. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Tuckey, R.C.; Li, W.; Raman, C.; Panich, U.; Slominski, A.T. CYP11A1-derived vitamin D3 products protect against UVB-induced inflammation and promote keratinocytes differentiation. Free Radic. Biol. Med. 2020, 155, 87–98. [Google Scholar] [CrossRef]
- Brown, L.M.; Helmke, S.M.; Hunsucker, S.W.; Netea-Maier, R.T.; Chiang, S.A.; Heinz, D.E.; Shroyer, K.R.; Duncan, M.W.; Haugen, B.R. Quantitative and qualitative differences in protein expression between papillary thyroid carcinoma and normal thyroid tissue. Mol. Carcinog. 2006, 45, 613–626. [Google Scholar] [CrossRef]
- Farrokhi Yekta, R.; Arefi Oskouie, A.; Rezaei Tavirani, M.; Mohajeri-Tehrani, M.R.; Soroush, A.R. Decreased apolipoprotein A4 and increased complement component 3 as potential markers for papillary thyroid carcinoma: A proteomic study. Int. J. Biol. Markers 2018, 33, 455–462. [Google Scholar] [CrossRef]
- Mull, B.; Davis, R.; Munir, I.; Perez, M.C.; Simental, A.A.; Khan, S. Differential expression of Vitamin D binding protein in thyroid cancer health disparities. Oncotarget 2021, 12, 596–607. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).