An Insight into Microbial Inoculants for Bioconversion of Waste Biomass into Sustainable “Bio-Organic” Fertilizers: A Bibliometric Analysis and Systematic Literature Review
Abstract
1. Introduction
1.1. The Need for Bio- and Bio-Organic Fertilizers
1.2. The Integration of Circular Economy (CE) in Bio-Organic Fertilizer Production
1.2.1. Waste Valorization
1.2.2. Bioconversion
1.2.3. Microbial Inoculants
2. Methods
2.1. Objectives
2.2. Search Analysis in Scopus
2.3. Bibliometric Analysis by VOSviewer
2.4. Systematic Literature Review
2.4.1. Scope and Definitions
2.4.2. Data Retrieval for SLR
3. Results
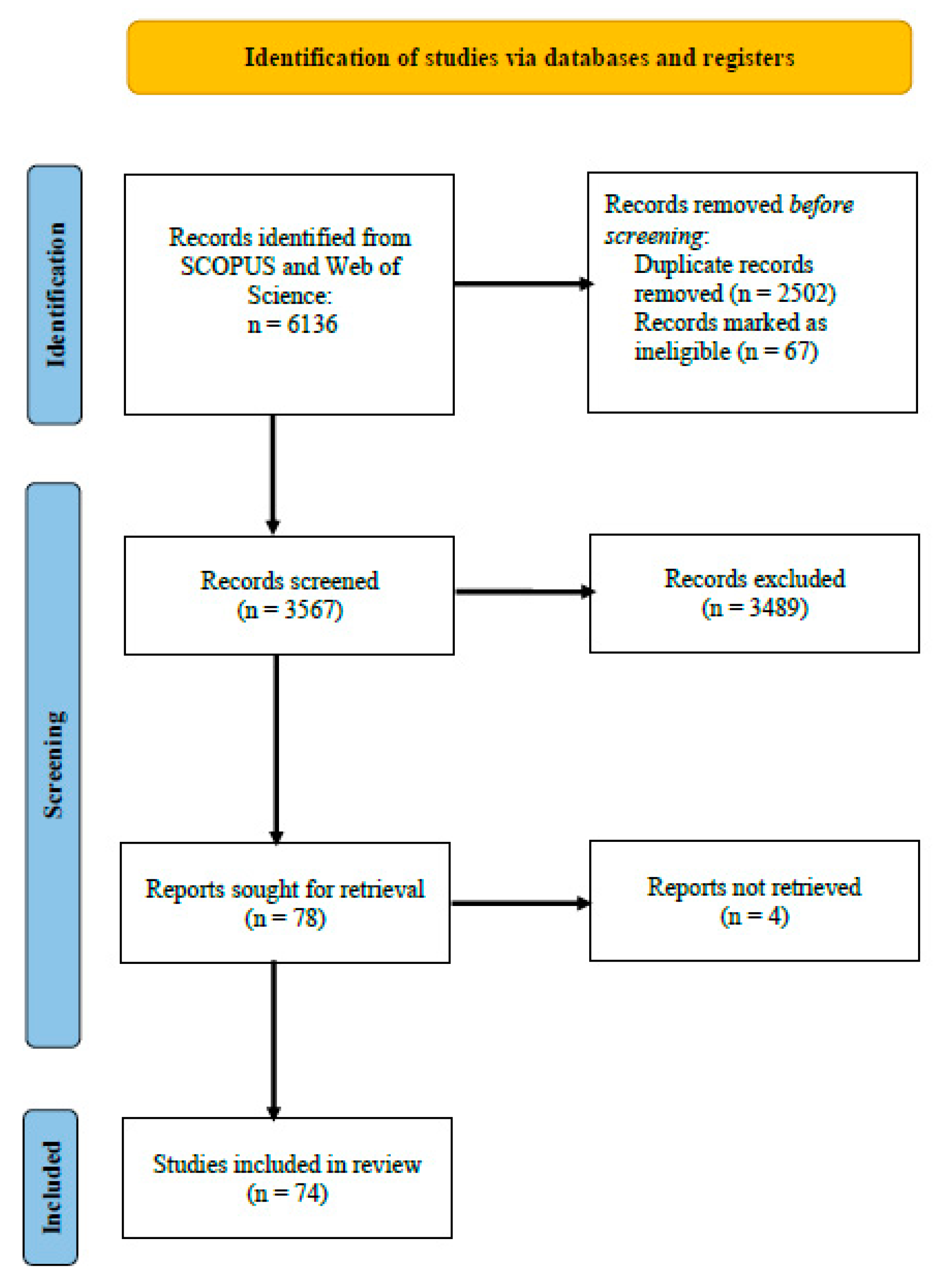
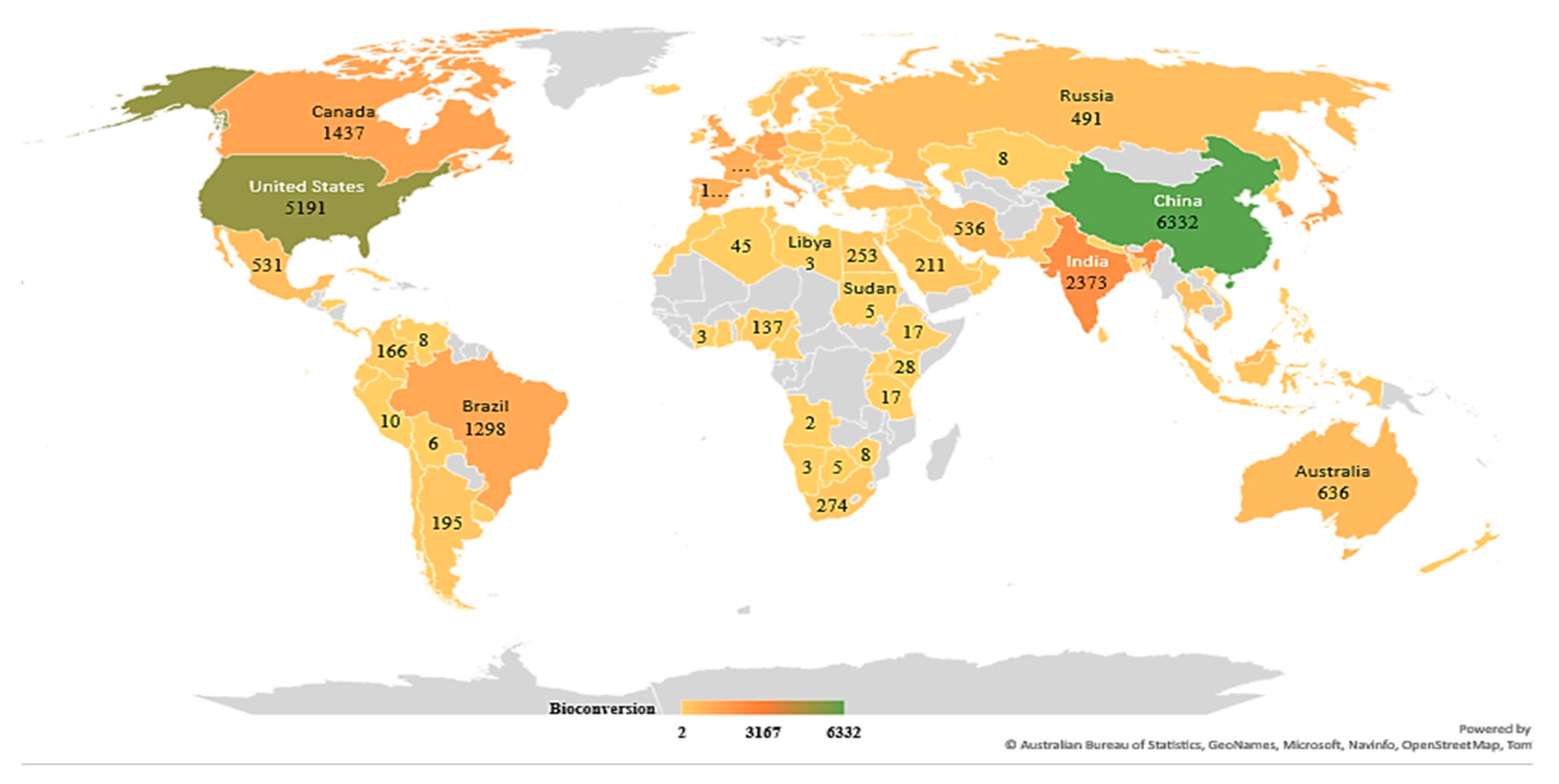
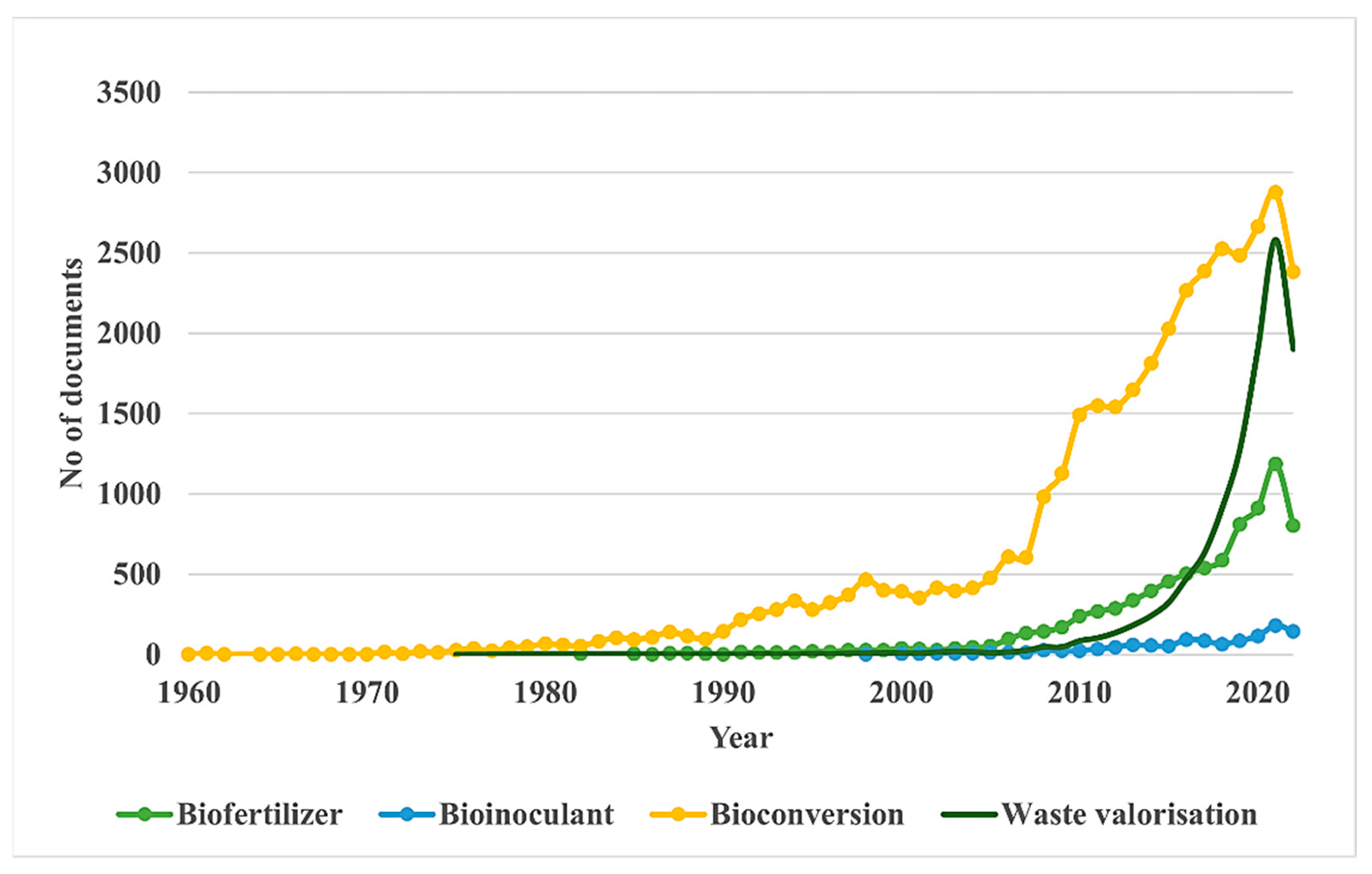
3.1. Analysis of Search Results from Scopus and Web of Science
3.2. Bibliometric Analysis by VOSviewer
3.3. Systematic Literature Review
3.3.1. Microbial Inoculants Used for Bioconversion
3.3.2. Bioconversion Raw Materials and Strategies
3.3.3. Plant Growth Tests and Mode of Application
4. Discussion
4.1. Current Strategies of Fertilizer Production Using Bioconversion
4.1.1. Composting and Vermicomposting
4.1.2. Anaerobic Co-Digestion (AcoD) of Waste
4.1.3. Nutrient Recovery and Wastewater Treatment by Microalgae
4.1.4. Bio-Organic Fertilizers
4.2. Mechanism of Microbial Bioconversion
4.2.1. Microbial Fermentation
4.2.2. Microbial Lignocellulolysis, Keratinolysis, and Chitinolysis
4.2.3. Microbial Micronutrient Solubilization—Phosphorus
4.2.4. EPS Production
4.2.5. PAE and LCT Biodegradation
4.3. Microbial Community Analysis
4.4. Nomenclature
- (a)
- Microbial fertilizers such as bio-inoculation of plant-growth-promoting bacteria (PGPB) and arbuscular mycorrhiza (AM), biocontrol microorganisms (bioorganic fertilizers);
- (b)
- Organic fertilizers which are made from composting, AD, manure, and other waste digestates;
- (c)
- Biofertilizers made from agro-industrial, organic wastes (BFW) by the application of phosphate- and potassium- solubilizing, keratinase-digested, bacteria and fungi;
- (d)
- Combination biofertilizers.
4.5. Mode of Valorized Biomass Supplementation
4.6. Lacunae in Biofertilizer Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Ge, Y. Excessive application of fertilizer, agricultural non-point source pollution, and farmers’ policy choice. Sustainability 2019, 11, 1165. [Google Scholar]
- Srivastav, A.L. Chemical fertilizers and pesticides: Role in groundwater contamination. In Agrochemicals Detection, Treatment and Remediation; Butterworth-Heinemann: Oxford, UK, 2020; pp. 143–159. [Google Scholar]
- Jiang, Y.; Chen, S.; Hu, B.; Zhou, Y.; Liang, Z.; Jia, X.; Shi, Z. A comprehensive framework for assessing the impact of potential agricultural pollution on grain security and human health in economically developed areas. Environ. Pollut. 2020, 263, 114653. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Ni, J.; Xie, D. Adoption behavior of cleaner production techniques to control agricultural non-point source pollution: A case study in the Three Gorges Reservoir Area. J. Clean. Prod. 2019, 223, 897–906. [Google Scholar] [CrossRef]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking crop nutrition in times of modern microbiology: Innovative biofertilizer technologies. Front. Sustain. Food Syst. 2021, 5, 606815. [Google Scholar] [CrossRef]
- Raimi, A.; Roopnarain, A.; Adeleke, R. Biofertilizer production in Africa: Current status, factors impeding adoption and strategies for success. Sci. Afr. 2021, 11, e00694. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, Y.; Liu, Y. Food Waste to Biofertilizer: A Potential Game Changer of Global Circular Agricultural Economy. J. Agric. Food Chem. 2020, 68, 5021–5023. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Dar, G.H.; Mehmood, M.A.; Bhat, R.A. (Eds.) Microbiota and Biofertilizers: A Sustainable Continuum for Plant and Soil Health; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Bhattacharyya, C.; Roy, R.; Tribedi, P.; Ghosh, A.; Ghosh, A. Biofertilizers as substitute to commercial agrochemicals. In Agrochemicals Detection, Treatment and Remediation; Butterworth-Heinemann: Oxford, UK, 2020; pp. 263–290. [Google Scholar]
- Lesueur, D.; Deaker, R.; Herrmann, L.; Bräu, L.; Jansa, J. The production and potential of biofertilizers to improve crop yields. In Bioformulations: For Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 71–92. [Google Scholar]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The opportunity of valorizing agricultural waste, through its conversion into biostimulants, biofertilizers, and biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Du, C.; Abdullah, J.J.; Greetham, D.; Fu, D.; Yu, M.; Ren, L.; Lu, D. Valorization of food waste into biofertiliser and its field application. J. Clean. Prod. 2018, 187, 273–284. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Lam, C.H.; Subramanian, K.; Qin, Z.H.; Mou, J.H.; Lin, C.S.K. Emerging waste valorisation techniques to moderate the hazardous impacts, and their path towards sustainability. J. Hazard. Mater. 2022, 423, 127023. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Arancon, R.A.D.; Lin, C.S.K.; Chan, K.M.; Kwan, T.H.; Luque, R. Advances on waste valorization: New horizons for a more sustainable society. Energy Sci. Eng. 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef]
- Antunes, L.P.; Martins, L.F.; Pereira, R.V.; Thomas, A.M.; Barbosa, D.; Lemos, L.N.; Setubal, J.C. Microbial community structure and dynamics in thermophilic composting viewed through metagenomics and metatranscriptomics. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, X.; Luo, L.; Ni, Y.; Yao, L.; Ni, W. Biostimulants in bioconversion compost of organic waste: A novel booster in sustainable agriculture. J. Clean. Prod. 2021, 319, 128704. [Google Scholar] [CrossRef]
- Canfora, L.; Costa, C.; Pallottino, F.; Mocali, S. Trends in soil microbial inoculants research: A science mapping approach to unravel strengths and weaknesses of their application. Agriculture 2021, 11, 158. [Google Scholar] [CrossRef]
- Van Eck, N.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. Acp J Club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 1–11. [Google Scholar] [CrossRef]
- Biswas, T.; Chatterjee, D.; Barman, S.; Chakraborty, A.; Halder, N.; Banerjee, S.; Chaudhuri, S.R. Cultivable bacterial community analysis of dairy activated sludge for value addition to dairy wastewater. Microbiol. Biotechnol. Lett. 2019, 47, 585–595. [Google Scholar] [CrossRef]
- Jastrzębska, M.; Saeid, A.; Kostrzewska, M.K.; Baśladyńska, S. New phosphorus biofertilizers from renewable raw materials in the aspect of cadmium and lead contents in soil and plants. Open Chem. 2018, 16, 35–49. [Google Scholar] [CrossRef]
- Xiao, X.; Mazza, L.; Yu, Y.; Cai, M.; Zheng, L.; Tomberlin, J.K.; Zhang, J. Efficient co-conversion process of chicken manure into protein feed and organic fertilizer by Hermetia illucens L. (Diptera: Stratiomyidae) larvae and functional bacteria. J. Environ. Manag. 2018, 217, 668–676. [Google Scholar] [CrossRef]
- Stamford, N.P.; Silva, E.V.N.D.; Oliveira, W.D.S.; Silva, M.C.F.D.; Martins, M.D.S.; Silva, V.S.G.D. Organic matter inoculated with diazotrophic bacterium Beijerinckia indica and Cunninghamella elegans fungus containing chitosan on banana” Williams” in field. Acta Scientiarum. Agronomy 2017, 39, 33–41. [Google Scholar]
- Piskaeva, A.I.; Babich, O.O.; Dolganyuk, V.F.; Garmashov, S.Y. Analysis of influence of biohumus on the basis of consortium of effective microorganisms on the productivity of winter wheat. Foods Raw Mater. 2017, 5, 90–99. [Google Scholar] [CrossRef]
- Jastrzuska, M.; Kostrzewska, M.K.; Saeid, A.; Treder, K.; Makowski, P.; Jastrzwski, W.P.; Okorski, A. Granulated phosphorus fertilizer made of ash from biomass combustion and bones with addition of Bacillus megaterium in the field assessment. Part 1. Impact on yielding and sanitary condition of winter wheat. Przem. Chem. 2017, 96, 2168–2174. [Google Scholar]
- Wyciszkiewicz, M.; Saeid, A.; Samoraj, M.; Chojnacka, K. Solid-state solubilization of bones by B. megaterium in spent mushroom substrate as a medium for a phosphate enriched substrate. J. Chem. Technol. Biotechnol. 2017, 92, 1397–1405. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Naik, K.; Mishra, S.; Srichandan, H.; Singh, P.K.; Sarangi, P.K. Plant growth promoting microbes: Potential link to sustainable agriculture and environment. Biocatal. Agric. Biotechnol. 2019, 21, 101326. [Google Scholar] [CrossRef]
- Win, T.T.; Barone, G.D.; Secundo, F.; Fu, P. Algal biofertilizers and plant growth stimulants for sustainable agriculture. Ind. Biotechnol. 2018, 14, 203–211. [Google Scholar] [CrossRef]
- Asadu, C.O.; Aneke, N.G.; Egbuna, S.O.; Agulanna, A.C. Comparative studies on the impact of bio-fertilizer produced from agro-wastes using thermo-tolerant actinomycetes on the growth performance of Maize (Zea mays) and Okro (Abelmoschus esculentus). Environ. Technol. Innov. 2018, 12, 55–71. [Google Scholar] [CrossRef]
- Dini, I.R.; Salbiah, D.; Tryana, S. Application of biofertilizer consortium formulation of cellulolytic bacteria based on organic liquid waste on yield of upland rice (Oryza sativa L.). IOP Conf. Ser. Earth Environ. Sci. 2020, 454, 012142. [Google Scholar]
- Peña García, P.; Querevalú Ortiz, J.; Ochoa Mogollón, G.; Sánchez Suárez, H. Biological silage of shrimp waste fermented with lactic acid bacteria: Use as a biofertilizer in pasture crops and as feed for backyard pigs. Sci. Agropecu. 2020, 11, 459–471. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Kumar, S.J.; Krishna, M.S.; Addanki, M.; Kumar, N.S.S. Studies on bioconversion of agri-waste to biomanure. Indian J. Ecol. 2020, 47, 116–121. [Google Scholar]
- Das, P.; Quadir, M.A.; Thaher, M.I.; Alghasal, G.S.H.S.; Aljabri, H.M.S.J. Microalgal nutrients recycling from the primary effluent of municipal wastewater and use of the produced biomass as bio-fertilizer. Int. J. Environ. Sci. Technol. 2019, 16, 3355–3364. [Google Scholar] [CrossRef]
- Thiyageshwari, S.; Gayathri, P.; Krishnamoorthy, R.; Anandham, R.; Paul, D. Exploration of rice husk compost as an alternate organic manure to enhance the productivity of black gram in typic haplustalf and typic rhodustalf. Int. J. Environ. Res. Public Health 2018, 15, 358. [Google Scholar] [CrossRef]
- Ahmad, J. Bioremediation of petroleum sludge using effective microorganism (EM) technology. Pet. Sci. Technol. 2017, 35, 1515–1522. [Google Scholar] [CrossRef]
- Abirami, S.; Ragavi, R.; Antony, V.S. Utilization of keratinolytic Lichtheimia corymbifera AS1 for degradation of cattle hoove—A slaughter house waste to use in plant growth. Biointerface Res. Appl. Chem. 2020, 10, 6417–6426. [Google Scholar]
- Abou-Zeid, H.; El-Darier, S.; Ghanem, K.; Salah, A. Fertilizing Potentiality of Fungal-Treated Olive Mill Solid Waste to Improve Some Growth and Physiological Parameters of Vicia faba L. Egypt. J. Bot. 2020, 60, 691–705. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Mohammed Ghilan, A.K.; Valan Arasu, M. Composting of vegetable waste using microbial consortium and biocontrol efficacy of Streptomyces Sp. Al-Dhabi 30 isolated from the Saudi Arabian environment for sustainable agriculture. Sustainability 2019, 11, 6845. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Homaidan, A.A. Compost inoculated with fungi from a mangrove habitat improved the growth and disease defense of vegetable plants. Sustainability 2020, 13, 124. [Google Scholar] [CrossRef]
- Asadu, C.O.; Ike, I.S.; Onu, C.E.; Egbuna, S.O.; Onoh, M.; Mbah, G.O.; Eze, C.N. Investigation of the influence of biofertilizer synthesized using microbial inoculums on the growth performance of two agricultural crops. Biotechnol. Rep. 2020, 27, e00493. [Google Scholar] [CrossRef]
- Bedi, A.; Singh, B.R.; Deshmukh, S.K.; Aggarwal, N.; Barrow, C.J.; Adholeya, A. Development of a novel myconanomining approach for the recovery of agriculturally important elements from jarosite waste. J. Environ. Sci. 2018, 67, 356–367. [Google Scholar] [CrossRef]
- Biswas, I.; Mitra, D.; Senapati, A.; Mitra, D.; Chattaraj, S.; Ali, M.; Das Mohapatra, P.K. Valorization of vermicompost with bacterial fermented chicken feather hydrolysate for the yield improvement of tomato plant: A novel organic combination. Int. J. Recycl. Org. Waste Agric. 2021, 10, 29–42. [Google Scholar]
- Łaba, W.; Żarowska, B.; Chorążyk, D.; Pudło, A.; Piegza, M.; Kancelista, A.; Kopeć, W. New keratinolytic bacteria in valorization of chicken feather waste. AMB Express 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Cárcamo, L.; Sierra, S.; Osorio, M.; Velásquez-Cock, J.; Vélez-Acosta, L.; Gómez-Hoyos, C.; Gañán, P. Bacterial nanocellulose mulch as a potential greener alternative for urban gardening in the small-scale food production of onion plants. Agric. Res. 2021, 10, 66–71. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Z.; Wu, D.; Wang, H.; Li, J.; Bi, M.; Zhang, Y. Development of a novel bio-organic fertilizer for the removal of atrazine in soil. J. Environ. Manag. 2019, 233, 553–560. [Google Scholar] [CrossRef]
- Dimitrijević, S.; Radanović, D.S.; Antić-Mladenović, S.B.; Milutinović, M.; Rajilić-Stojanović, M.; Dimitrijević-Branković, S. Enhanced fertilization effect of a compost obtained from mixed herbs waste inoculated with novel strains of mesophilic bacteria. Hem. Ind. 2017, 71, 503–513. [Google Scholar] [CrossRef]
- Emmanuel, S.A.; Yoo, J.; Kim, E.J.; Chang, J.S.; Park, Y.I.; Koh, S.C. Development of functional composts using spent coffee grounds, poultry manure and biochar through microbial bioaugmentation. J. Environ. Sci. Health Part B 2017, 52, 802–811. [Google Scholar] [CrossRef]
- Feng, N.X.; Liang, Q.F.; Feng, Y.X.; Xiang, L.; Zhao, H.M.; Li, Y.W.; Wong, M.H. Improving yield and quality of vegetable grown in PAEs-contaminated soils by using novel bioorganic fertilizer. Sci. Total Environ. 2020, 739, 139883. [Google Scholar] [CrossRef]
- Ferreira, A.; Ribeiro, B.; Ferreira, A.F.; Tavares, M.L.; Vladic, J.; Vidović, S.; Gouveia, L. Scenedesmus obliquus microalga-based biorefinery–from brewery effluent to bioactive compounds, biofuels and biofertilizers–aiming at a circular bioeconomy. Biofuels Bioprod. Biorefining 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Gao, H.; Lu, C.; Wang, H.; Wang, L.; Yang, Y.; Jiang, T.; Wu, L. Production exopolysaccharide from Kosakonia cowanii LT-1 through solid-state fermentation and its application as a plant growth promoter. Int. J. Biol. Macromol. 2020, 150, 955–964. [Google Scholar] [CrossRef]
- Gaonkar, S.K.; Furtado, I.J. Biorefinery-Fermentation of Agro-Wastes by Haloferax lucentensis GUBF-2 MG076878 to Haloextremozymes for use as Biofertilizer and Biosynthesizer of AgNPs. Waste Biomass Valorization 2022, 13, 1117–1133. [Google Scholar] [CrossRef]
- Gemin, L.G.; Mógor, Á.F.; Mógor, G.; Roder, C.; Szilagyi-Zecchin, V.J. Cambios en el crecimiento y concentración de aminoácidos en las plántulas de col china usando caldo bacteriano fermentado. Idesia 2018, 36, 7–13. [Google Scholar] [CrossRef][Green Version]
- González, I.; Ekelhof, A.; Herrero, N.; Siles, J.Á.; Podola, B.; Chica, A.F.; Gómez, J.M. Wastewater nutrient recovery using twin-layer microalgae technology for biofertilizer production. Water Sci. Technol. 2020, 82, 1044–1061. [Google Scholar] [CrossRef]
- Gunjal, A.B.; Kapadnis, B.P.; Pawar, N.J. Pressmud, a lignocellulosic waste as potential carrier for in-situ production of plant growth promoting substances by Bacillus circulans. J. Solid Waste Technol. Manag. 2018, 44, 281–287. [Google Scholar] [CrossRef]
- Gurav, R.; Nalavade, V.; Aware, C.; Vyavahare, G.; Bhatia, S.K.; Yang, Y.H.; Jadhav, J. Microbial degradation of poultry feather biomass in a constructed bioreactor and application of hydrolysate as bioenhancer to vegetable crops. Environ. Sci. Pollut. Res. 2020, 27, 2027–2035. [Google Scholar] [CrossRef]
- Hadidi, M.; Bahlaouan, B.; Asbai, Z.; Radi Benjelloun, G.; El Antri, S.; Boutaleb, N. Optimizing biogas and biofertilizer production from abundant Moroccan industrial organic wastes by the formulation and the use of a fungal inoculum. Adv. Environ. Technol. 2021, 7, 275–287. [Google Scholar]
- Gandhi, A.; Sivakumar, K. Impact of vermicompost carrier based bioinoculants on the growth, yield and quality of rice (Oryza sativa L.) CV NLR 145. Ecoscan 2010, 4, 83–88. [Google Scholar]
- Hasan, Z.A.E.; Mohd Zainudin, N.A.I.; Aris, A.; Ibrahim, M.H.; Yusof, M.T. Biocontrol efficacy of Trichoderma asperellum-enriched coconut fibre against Fusarium wilts of cherry tomato. J. Appl. Microbiol. 2020, 129, 991–1003. [Google Scholar] [CrossRef]
- Huang, J.; Zhuo, Y.; Lu, J.; Lai, Q.; Zhang, Y. Bacillus cereus liquid fertilizer was produced from Agaricus bisporus industrial wastewater. J. Biotechnol. 2021, 327, 74–85. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hussain, S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 2019, 10, 39. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Stratton, G.; Ravichandran, S.; Shukla, P.S.; Potin, P.; Asiedu, S.; Prithiviraj, B. Microbial degradation of lobster shells to extract chitin derivatives for plant disease management. Front. Microbiol. 2017, 8, 781. [Google Scholar] [CrossRef]
- Irawan, B.; Zulkifli, Z.; Handayani, T.T.; Hadi, S. Effect of induced compost by cellulolitic (Aspergillus fumigatus) and ligninolitic (geotrichum sp.) fungi inoculum application on vegetative growth of red chili (Capsicum annuum L.). J. Pure Appl. Microbiol. 2019, 13, 815–821. [Google Scholar] [CrossRef]
- Jastrzębska, M.; Kostrzewska, M.K.; Treder, K. Phosphorus fertilizers from sewage sludge ash and animal blood have no effect on earthworms. Agronomy 2020, 10, 525. [Google Scholar] [CrossRef]
- Kumar, J.; Kumar, P.; Kushwaha, R.K.S. Recycling of chicken feather protein into compost by Chrysosporium indicum JK14 and their effect on the growth promotion of Zea mays. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 75–80. [Google Scholar]
- Kumari, M.; Kumar, J. Chicken feather waste degradation by Alternaria tenuissima and its application on plant growth. J. Appl. Nat. Sci. 2020, 12, 411–414. [Google Scholar] [CrossRef]
- Li, Y.; Cui, T.; Wang, Y.; Ge, X. Isolation and characterization of a novel bacterium Pseudomonas aeruginosa for biofertilizer production from kitchen waste oil. RSC Adv. 2018, 8, 41966–41975. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Zuo, Z.; Lv, Z.; Yang, Z.; Mao, W.; Song, Z. Evaluation and improvement of phosphate solubilization by an isolated bacterium Pantoea agglomerans ZB. World J. Microbiol. Biotechnol. 2020, 36, 1–14. [Google Scholar] [CrossRef]
- Lopes, C.M.; Silva, A.M.M.; Estrada-Bonilla, G.A.; Ferraz-Almeida, R.; Vieira, J.L.V.; Otto, R.; Cardoso, E.J.B.N. Improving the fertilizer value of sugarcane wastes through phosphate rock amendment and phosphate-solubilizing bacteria inoculation. J. Clean. Prod. 2021, 298, 126821. [Google Scholar] [CrossRef]
- Mahmood, A.; Iguchi, R.; Kataoka, R. Multifunctional food waste fertilizer having the capability of Fusarium-growth inhibition and phosphate solubility: A new horizon of food waste recycle using microorganisms. Waste Manag. 2019, 94, 77–84. [Google Scholar] [CrossRef]
- Naeem, U.; Afzaal, M.; Qazi, A.; Yasar, A.; Mahfooz, Y.; Naz, A.U.; Awan, H. Investigating the effect of Aspergillus niger inoculated press mud (biofertilizer) on the potential of enhancing maize (Zea mays L.) yield, potassium use efficiency and potassium agronomic efficiency. Cereal Res. Commun. 2022, 50, 157–170. [Google Scholar] [CrossRef]
- Nagarajan, S.; Eswaran, P.; Masilamani, R.P.; Natarajan, H. Chicken feather compost to promote the plant growth activity by using keratinolytic bacteria. Waste Biomass Valorization 2018, 9, 531–538. [Google Scholar] [CrossRef]
- Naher, U.A.; Biswas, J.C.; Maniruzzaman, M.; Khan, F.H.; Sarkar, M.I.U.; Jahan, A.; Kabir, M.S. Bio-organic fertilizer: A green technology to reduce synthetic N and P fertilizer for rice production. Front. Plant Sci. 2021, 12, 602052. [Google Scholar] [CrossRef]
- Nasarudin, A.A.; Ngadi, N.; Yusoff, N.A.; Ali, N.; Aziz, M.A.A.; Rahman, R.A. Production of biofertilizer using Lactobacillus inoculants and glycerin pitch from oleochemical industry. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022105. [Google Scholar] [CrossRef]
- Nayak, M.; Swain, D.K.; Sen, R. Strategic valorization of de-oiled microalgal biomass waste as biofertilizer for sustainable and improved agriculture of rice (Oryza sativa L.) crop. Sci. Total Environ. 2019, 682, 475–484. [Google Scholar] [CrossRef]
- Ntsobi, N.; Fanadzo, M.; Le Roes-Hill, M.; Nchu, F. Effects of Clonostachys rosea f. Catenula inoculum on the composting of cabbage wastes and the endophytic activities of the composted material on tomatoes and red spider mite infestation. Microorganisms 2021, 9, 1184. [Google Scholar] [CrossRef]
- Nunes Oliveira, F.L.; Silva Oliveira, W.; Pereira Stamford, N.; Nova Silva, E.V.; Santiago Freitas, A.D. Effectiveness of biofertilizer enriched in N by Beijerinckia indica on sugarcane grown on an Ultisol and the interactive effects between biofertilizer and sugarcane filter cake. J. Soil Sci. Plant Nutr. 2017, 17, 1040–1057. [Google Scholar] [CrossRef]
- Pal, K.; Rakshit, S.; Mondal, K.C.; Halder, S.K. Microbial decomposition of crustacean shell for production of bioactive metabolites and study of its fertilizing potential. Environ. Sci. Pollut. Res. 2021, 28, 58915–58928. [Google Scholar] [CrossRef]
- Parastesh, F.; Alikhani, H.A.; Etesami, H. Vermicompost enriched with phosphate–solubilizing bacteria provides plant with enough phosphorus in a sequential cropping under calcareous soil conditions. J. Clean. Prod. 2019, 221, 27–37. [Google Scholar] [CrossRef]
- Pascual, J.A.; Bernal-Vicente, A.; Martinez-Medina, A.; Ros, M.; Sánchez, C. Biostimulant and suppressive effect of Trichoderma harzianum enriched compost for melon cultivation from greenhouse nursery to field production. In Proceedings of the III International Symposium on Organic Greenhouse Horticulture 1164, Izmir, Turkey, 11–14 April 2016; pp. 225–232. [Google Scholar]
- Patil, P.M.; Mahamuni, P.P.; Abdel-Daim, M.M.; Aleya, L.; Chougule, R.A.; Shadija, P.G.; Bohara, R.A. Conversion of organic biomedical waste into potential fertilizer using isolated organisms from cow dung for a cleaner environment. Environ. Sci. Pollut. Res. 2019, 26, 27897–27904. [Google Scholar] [CrossRef]
- Raymond, N.S.; Jensen, L.S.; Stöver, D.M. Enhancing the phosphorus bioavailability of thermally converted sewage sludge by phosphate-solubilising fungi. Ecol. Eng. 2018, 12, 44–53. [Google Scholar] [CrossRef]
- Röder, C.; Mógor, Á.F.; Szilagyi-Zecchin, V.J.; Gemin, L.G.; Mógor, G. Potato yield and metabolic changes by use of biofertilizer containing L-glutamic acid. Comun. Sci. 2018, 9, 211–218. [Google Scholar] [CrossRef]
- Saeid, A.; Patel, A. Valorization of ash and spent mushroom substrate via solid-state solubilization by Acidithiobacillus ferrooxidans. Waste Manag. 2019, 87, 612–620. [Google Scholar] [CrossRef]
- Santhanarajan, A.E.; Han, Y.H.; Koh, S.C. The Efficacy of Functional Composts Manufactured Using Spent Coffee Ground, Rice Bran, Biochar, and Functional Microorganisms. Appl. Sci. 2021, 11, 7703. [Google Scholar] [CrossRef]
- Shen, T.; Lei, Y.; Pu, X.; Zhang, S.; Du, Y. Identification and application of Streptomyces microflavus G33 in compost to suppress tomato bacterial wilt disease. Appl. Soil Ecol. 2021, 157, 103724. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Incharoensakdi, A.; Cornejo, P.; Kamaraj, B.; Chi, N.T.L. Removal of nutrients from domestic wastewater by microalgae coupled to lipid augmentation for biodiesel production and influence of deoiled algal biomass as biofertilizer for Solanum lycopersicum cultivation. Chemosphere 2021, 268, 129323. [Google Scholar] [CrossRef]
- Singh, U.B.; Malviya, D.; Khan, W.; Singh, S.; Karthikeyan, N.; Imran, M.; Oh, J.W. Earthworm grazed-Trichoderma harzianum biofortified spent mushroom substrates modulate accumulation of natural antioxidants and bio-fortification of mineral nutrients in tomato. Front. Plant Sci. 2018, 9, 1017. [Google Scholar] [CrossRef]
- Stanley-Raja, V.; Senthil-Nathan, S.; Chanthini, K.M.P.; Sivanesh, H.; Ramasubramanian, R.; Karthi, S.; Kalaivani, K. Biological activity of chitosan inducing resistance efficiency of rice (Oryza sativa L.) after treatment with fungal based chitosan. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Suleiman, A.K.A.; Lourenço, K.S.; Clark, C.; Luz, R.L.; da Silva, G.H.R.; Vet, L.E.; Kuramae, E.E. From toilet to agriculture: Fertilization with microalgal biomass from wastewater impacts the soil and rhizosphere active microbiomes, greenhouse gas emissions and plant growth. Resour. Conserv. Recycl. 2020, 161, 104924. [Google Scholar] [CrossRef]
- Sun, Z.; Li, X.; Liu, K.; Chi, X.; Liu, L. Optimization for production of a plant growth promoting agent from the degradation of chicken feather using keratinase producing novel isolate Bacillus pumilus JYL. Waste Biomass Valorization 2021, 12, 1943–1954. [Google Scholar] [CrossRef]
- Umamaheswari, J.; Shanthakumar, S. Paddy-soaked rice mill wastewater treatment by phycoremediation and feasibility study on use of algal biomass as biofertilizer. J. Chem. Technol. Biotechnol. 2021, 96, 394–403. [Google Scholar] [CrossRef]
- Unnikrishnan, G.; Vijayaraghavan, R. Evaluation of lignin waste as potential carriers for phosphate solubilizing bio-fertilizers: A zero waste technology. Int. J. Recycl. Org. Waste Agric. 2021, 10, 331–351. [Google Scholar]
- Zeng, Y.H.; Kuo, Y.W.; Chen, H.T. Higher yield of cherry tomato grown in culture medium with microbially inoculated feather compost without fertilizer application. J. Plant Nutr. Soil Sci. 2018, 181, 528–536. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, Z.W.; Liang, W.Z.; Niu, Q.H.; Wang, X. The Prevention of Bio-Organic Fertilizer Fermented from Cow Manure Compost by Bacillus sp. XG-1 on Watermelon Continuous Cropping Barrier. Int. J. Environ. Res. Public Health 2020, 17, 5714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Han, X.; Gou, J.; Li, W.; Zhang, C. A novel bio-fertilizer produced by prickly ash seeds with biochar addition induces soil suppressiveness against black shank disease on tobacco. Appl. Sci. 2021, 11, 7261. [Google Scholar] [CrossRef]
- Jamison, J.; Khanal, S.K.; Nguyen, N.H.; Deenik, J.L. Assessing the effects of digestates and combinations of digestates and fertilizer on yield and nutrient use of Brassica juncea (Kai Choy). Agronomy 2021, 11, 509. [Google Scholar] [CrossRef]
- Pottipati, S.; Chakma, R.; Haq, I.; Kalamdhad, A.S. Composting and vermicomposting: Process optimization for the management of organic waste. In Advanced Organic Waste Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 33–43. [Google Scholar]
- Flores-Félix, J.D.; Menéndez, E.; Rivas, R.; de la Encarnación Velázquez, M. Future perspective in organic farming fertilization: Management and product. In Organic Farming; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 269–315. [Google Scholar]
- Ayilara, M.S.; Olanrewaju, O.S.; Babalola, O.O.; Odeyemi, O. Waste management through composting: Challenges and potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Nguyen, T.K.X.; Thayanukul, P.; Pinyakong, O.; Suttinun, O. Tiamulin removal by wood-rot fungi isolated from swine farms and role of ligninolytic enzymes. Int. Biodeterior. Biodegrad. 2017, 116, 147–154. [Google Scholar] [CrossRef]
- Pan, L.J.; Li, J.; Li, C.X.; Yu, G.W.; Wang, Y. Study of ciprofloxacin biodegradation by a Thermus sp. isolated from pharmaceutical sludge. J. Hazard. Mater. 2018, 343, 59–67. [Google Scholar] [CrossRef]
- Setiawati, M.R.; Suryatmana, P.; Hindersah, R.; Kamaluddin, N.; Efendi, S. The effectiveness of various compositions lignolytic and cellulolytic microbes in composting empty fruit bunch palm oil and sugar cane biomass. IOP Conf. Ser. Earth Environ. Sci. 2019, 393, 012032. [Google Scholar] [CrossRef]
- Nakasaki, K.; Hirai, H.; Mimoto, H.; Quyen, T.N.M.; Koyama, M.; Takeda, K. Succession of microbial community during vigorous organic matter degradation in the primary fermentation stage of food waste composting. Sci. Total Environ. 2019, 671, 1237–1244. [Google Scholar] [CrossRef]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Pandey, A.; Zhang, Z.; Kumar, S.; Awasthi, M.K. Succession of keratin-degrading bacteria and associated health risks during pig manure composting. J. Clean. Prod. 2020, 258, 120624. [Google Scholar] [CrossRef]
- Song, S.; Lim, J.W.; Lee, J.T.; Cheong, J.C.; Hoy, S.H.; Hu, Q.; Tong, Y.W. Food-waste anaerobic digestate as a fertilizer: The agronomic properties of untreated digestate and biochar-filtered digestate residue. Waste Manag. 2021, 136, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Tallou, A.; Salcedo, F.P.; Haouas, A.; Jamali, M.Y.; Atif, K.; Aziz, F.; Amir, S. Assessment of biogas and biofertilizer produced from anaerobic co-digestion of olive mill wastewater with municipal wastewater and cow dung. Environ. Technol. Innov. 2020, 20, 101152. [Google Scholar] [CrossRef]
- Cucina, M.; Castro, L.; Escalante, H.; Ferrer, I.; Garfí, M. Benefits and risks of agricultural reuse of digestates from plastic tubular digesters in Colombia. Waste Manag. 2021, 135, 220–228. [Google Scholar] [CrossRef]
- Yıldırım, E.; Ince, O.; Aydin, S.; Ince, B. Improvement of biogas potential of anaerobic digesters using rumen fungi. Renew. Energy 2017, 109, 346–353. [Google Scholar] [CrossRef]
- Golovko, O.; Ahrens, L.; Schelin, J.; Sörengård, M.; Bergstrand, K.J.; Asp, H.; Wiberg, K. Organic micropollutants, heavy metals and pathogens in anaerobic digestate based on food waste. J. Environ. Manag. 2022, 313, 114997. [Google Scholar] [CrossRef]
- Södergren, J.; Larsson, C.U.; Wadsö, L.; Bergstrand, K.J.; Asp, H.; Hultberg, M.; Schelin, J. Food waste to new food: Risk assessment and microbial community analysis of anaerobic digestate as a nutrient source in hydroponic production of vegetables. J. Clean. Prod. 2022, 333, 130239. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Liang, S. Nutrient recovery from wastewater: From technology to economy. Bioresour. Technol. Rep. 2020, 11, 100425. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of nutrients from wastewaters using microalgae. Front. Sustain. Food Syst. 2018, 2, 59. [Google Scholar] [CrossRef]
- Khan, S.A.; Sharma, G.K.; Malla, F.A.; Kumar, A.; Gupta, N. Microalgae based biofertilizers: A biorefinery approach to phycoremediate wastewater and harvest biodiesel and manure. J. Clean. Prod. 2019, 211, 1412–1419. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Chanakya, H.N.; Joshi, N.V.; Ramachandra, T.V.; Murthy, G.S. Algae-based biofertilizers: A biorefinery approach. In Microorganisms for Green Revolution; Springer: Singapore, 2018; pp. 177–196. [Google Scholar]
- Ogata-Gutiérrez, K.; Zúñiga-Dávila, D. Bacteria-Plant interactions: An added value of microbial inoculation. Rev. Peru. De Biol. 2020, 27, 021–025. [Google Scholar] [CrossRef]
- Choudhury, D.; Tarafdar, S.; Dutta, S. Plant growth promoting rhizobacteria (PGPR) and their eco-friendly strategies for plant growth regulation: A review. Plant Sci. Today 2022, 9, 3. [Google Scholar] [CrossRef]
- Murali, M.; Naziya, B.; Ansari, M.A.; Alomary, M.N.; Al Yahya, S.; Almatroudi, A.; Amruthesh, K.N. Bioprospecting of rhizosphere-resident fungi: Their role and importance in sustainable agriculture. J. Fungi 2021, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Dey, G.; Banerjee, P.; Sharma, R.K.; Maity, J.P.; Etesami, H.; Shaw, A.K.; Chen, C.Y. Management of phosphorus in salinity-stressed agriculture for sustainable crop production by salt-tolerant phosphate-solubilizing bacteria—A review. Agronomy 2021, 11, 1552. [Google Scholar] [CrossRef]
- Basak, B.B.; Maity, A.; Ray, P.; Biswas, D.R.; Roy, S. Potassium supply in agriculture through biological potassium fertilizer: A promising and sustainable option for developing countries. Arch. Agron. Soil Sci. 2022, 68, 101–114. [Google Scholar] [CrossRef]
- Kuila, D.; Ghosh, S. Aspects, problems and utilization of Arbuscular Mycorrhizal (AM) application as bio-fertilizer in sustainable agriculture. Curr. Res. Microb. Sci. 2022, 3, 100107. [Google Scholar] [CrossRef]
- Kumaar, S.A.; Babu, R.P.; Vivek, P.; Saravanan, D. Role of Nitrogen Fixers as Biofertilizers in Future Perspective: A Review. Res. J. Pharm. Technol. 2020, 13, 2459–2467. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Biotechnological overview of agriculturally important endophytic fungi. Hortic. Environ. Biotechnol. 2021, 62, 507–520. [Google Scholar] [CrossRef]
- Willey, J.M.; Sandman, K.; Wood, D. Prescott’s Microbiolog, 11th ed.; McGraw-Hill: New York, NY, USA, 2022. [Google Scholar]
- Kapoor, M.; Panwar, D.; Kaira, G.S. Bioprocesses for enzyme production using agro-industrial wastes: Technical challenges and commercialization potential. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Academic Press: Cambridge, MA, USA, 2016; pp. 61–93. [Google Scholar]
- Chilakamarry, C.R.; Sakinah, A.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef] [PubMed]
- Buntić, A.V.; Milić, M.D.; Stajković-Srbinović, O.S.; Rasulić, N.V.; Delić, D.I.; Mihajlovski, K.R. Cellulase production by Sinorhizobium meliloti strain 224 using waste tobacco as substrate. Int. J. Environ. Sci. Technol. 2019, 16, 5881–5890. [Google Scholar] [CrossRef]
- Riddech, N.; Sarin, P.; Phibunwatthanawong, T. Effect of bio-liquid organic fertilizer on the growth of Dipterocarpus alatus Roxb seedlings in the pot experiment. Malays. J. Microbiol. 2019, 15, 213–219. [Google Scholar] [CrossRef]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A review on bioconversion of agro-industrial wastes to industrially important enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, M.; Gramegna, G.; Benedetti, M.; Mattei, B. Industrial use of cell wall degrading enzymes: The fine line between production strategy and economic feasibility. Front. Bioeng. Biotechnol. 2020, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.P.; Prabhu, M.; Mutnuri, S. A novel and sustainable approach for biotransformation of phosphogypsum to calcium carbonate using urease producing Lysinibacillus sphaericus strain GUMP2. Environ. Technol. 2021, 5, 1–14. [Google Scholar] [CrossRef]
- Chakravarty, I.; Mandavgane, S.A. Valorization of fruit and vegetable waste for biofertilizer and biogas. J. Food Process Eng. 2021, 44, e13512. [Google Scholar] [CrossRef]
- Mishra, V.; Jana, A.K. Sweet sorghum bagasse pretreatment by Coriolus versicolor in mesh tray bioreactor for selective delignification and improved saccharification. Waste Biomass Valorization 2019, 10, 2689–2702. [Google Scholar] [CrossRef]
- Moreno-Bayona, D.A.; Gómez-Méndez, L.D.; Blanco-Vargas, A.; Castillo-Toro, A.; Herrera-Carlosama, L.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M. Simultaneous bioconversion of lignocellulosic residues and oxodegradable polyethylene by Pleurotus ostreatus for biochar production, enriched with phosphate solubilizing bacteria for agricultural use. PLoS ONE 2019, 14, e0217100. [Google Scholar]
- Silva, C.O.; Vaz, R.P.; Filho, E.X. Bringing plant cell wall-degrading enzymes into the lignocellulosic biorefinery concept. Biofuels Bioprod. Biorefining 2018, 12, 277–289. [Google Scholar]
- Fu, X.; Guo, Y.; Jin, Y.; Ma, M. Bioconversion of chitin waste using a cold-adapted chitinase to produce chitin oligosaccharides. Lwt 2020, 133, 109863. [Google Scholar] [CrossRef]
- Jayakumar, A.; Nair, I.C.; Radhakrishnan, E.K. Environmental adaptations of an extremely plant beneficial Bacillus subtilis Dcl1 identified through the genomic and metabolomic analysis. Microb. Ecol. 2021, 81, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mandal, N.C. Diversity of chitinase-producing bacteria and their possible role in plant pest control. In Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications; Springer: Singapore, 2019; pp. 457–491. [Google Scholar]
- Calin, M.; Raut, I.; Arsene, M.L.; Capra, L.; Gurban, A.M.; Doni, M.; Jecu, L. Applications of fungal strains with keratin-degrading and plant growth promoting characteristics. Agronomy 2019, 9, 543. [Google Scholar] [CrossRef]
- Tamreihao, K.; Mukherjee, S.; Khunjamayum, R.; Devi, L.J.; Asem, R.S.; Ningthoujam, D.S. Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J. Basic Microbiol. 2019, 59, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Saeid, A. Phosphorus Microbial Solubilization as a Key for Phosphorus Recycling in Agriculture. In Phosphorus—Recovery and Recycling; Zhang, T., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Saeid, A.; Labuda, M.; Chojnacka, K.; Górecki, H. Valorization of bones to liquid phosphorus fertilizer by microbial solubilization. Waste Biomass Valorization 2014, 5, 265–272. [Google Scholar] [CrossRef]
- Nandimath, A.P.; Karad, D.D.; Gupta, S.G.; Kharat, A.S. Consortium inoculum of five thermo-tolerant phosphate solubilizing Actinomycetes for multipurpose biofertilizer preparation. Iran. J. Microbiol. 2017, 9, 295. [Google Scholar] [PubMed]
- Santana, C.A.; Piccirillo, C.; Pereira, S.I.A.; Pullar, R.C.; Lima, S.M.; Castro, P.M.L. Employment of phosphate solubilising bacteria on fish scales–Turning food waste into an available phosphorus source. J. Environ. Chem. Eng. 2019, 7, 103403. [Google Scholar] [CrossRef]
- Busato, J.G.; Zandonadi, D.B.; Mól, A.R.; Souza, R.S.; Aguiar, K.P.; Júnior, F.B.R.; Olivares, F.L. Compost biofortification with diazotrophic and P-solubilizing bacteria improves maturation process and P availability. J. Sci. Food Agric. 2017, 97, 949–955. [Google Scholar] [CrossRef]
- Ortega-Torres, A.E.; Rico-García, E.; Guzmán-Cruz, R.; Torres-Pacheco, I.; Tovar-Pérez, E.G.; Guevara-González, R.G. Addition of Phosphatases and Phytases to Mature Compost to Increase Available Phosphorus: A Short Study. Agronomy 2021, 11, 2555. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Yadav, A.; Yadav, A.N.; Suman, A.; Saxena, A.K. Minerals solubilizing and mobilizing microbiomes: A sustainable approach for managing minerals deficiency in agricultural soil. J. Appl. Microbiol. 2022, 133, 1245–1272. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, G.; Kecskés, M.L.; Kennedy, I.R. Biofilmed biofertilisers: Novel inoculants for efficient nutrient use in plants. ACIAR Proc. 2008, 130, 126–130. [Google Scholar]
- Rathnathilaka, T.; Premarathna, M.; Madawala, S.; Pathirana, A.; Karunaratne, K.; Seneviratne, G. Biofilm biofertilizer application rapidly increases soil quality and grain yield in large scale conventional rice cultivation: A case study. J. Plant Nutr. 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Joulak, I.; Concórdio-Reis, P.; Torres, C.A.; Sevrin, C.; Grandfils, C.; Attia, H.; Azabou, S. Sustainable use of agro-industrial wastes as potential feedstocks for exopolysaccharide production by selected Halomonas strains. Environ. Sci. Pollut. Res. 2022, 29, 22043–22055. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Ko, S.H.; Lee, M.E.; Kim, H.M.; Yang, J.E.; Jeong, S.G.; Park, H.W. Production, characterization, and antioxidant activities of an exopolysaccharide extracted from spent media wastewater after Leuconostoc mesenteroides WiKim32 fermentation. ACS Omega 2021, 6, 8171–8178. [Google Scholar] [CrossRef]
- Nazli, F.; Jamil, M.; Hussain, A.; Hussain, T. Exopolysaccharides and indole-3-acetic acid producing Bacillus safensis strain FN13 potential candidate for phytostabilization of heavy metals. Environ. Monit. Assess. 2020, 192, 1–16. [Google Scholar] [CrossRef]
- Marchetti, R.; Vasmara, C.; Fiume, F. Pig slurry improves the anaerobic digestion of waste cooking oil. Appl. Microbiol. Biotechnol. 2019, 103, 8267–8279. [Google Scholar] [CrossRef]
- Bialy, H.E.; Gomaa, O.M.; Azab, K.S. Conversion of oil waste to valuable fatty acids using oleaginous yeast. World J. Microbiol. Biotechnol. 2011, 27, 2791–2798. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Zhou, B.; Zheng, X.; Zhu, Z.; Qin, Q.; Song, K.; Sun, L.; Xue, Y. Effects of fertilizer application on phthalate ester pollution and the soil microbial community in plastic-shed soil on long-term fertilizer experiment. Chemosphere 2022, 2, 136315. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Q.; Gao, R.; Hou, H.; Tan, W.; He, X.; Wang, X. Contamination of phthalate esters (PAEs) in typical wastewater-irrigated agricultural soils in Hebei, North China. PLoS ONE 2015, 10, e0137998. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Cui, K.; Xie, Z.; Wu, L.; Liu, M.; Sun, G.; Zeng, Z. Phthalate esters (PAEs): Emerging organic contaminants in agricultural soils in peri-urban areas around Guangzhou, China. Environ. Pollut. 2008, 156, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Zhong, X.Z.; Wang, T.T.; Sun, Z.Y.; Tang, Y.Q.; Kida, K. Aerobic composting of distilled grain waste eluted from a Chinese spirit-making process: The effects of initial pH adjustment. Bioresour. Technol. 2017, 245, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Sun, Z.Y.; Zhong, X.Z.; Wang, T.T.; Tan, L.; Tang, Y.Q.; Kida, K. Aerobic composting of digested residue eluted from dry methane fermentation to develop a zero-emission process. Waste Manag. 2017, 61, 206–212. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Wang, H.; Xing, R.; Zheng, Z.; Qiu, J.; Yang, L. Bacterial community structure and diversity responses to the direct revegetation of an artisanal zinc smelting slag after 5 years. Environ. Sci. Pollut. Res. 2018, 25, 14773–14788. [Google Scholar] [CrossRef]
- Chi, C.P.; Chu, S.; Wang, B.; Zhang, D.; Zhi, Y.; Yang, X.; Zhou, P. Dynamic bacterial assembly driven by Streptomyces griseorubens JSD-1 inoculants correspond to composting performance in swine manure and rice straw co-composting. Bioresour. Technol. 2020, 313, 123692. [Google Scholar] [CrossRef]
- Jiang, Y.; Dennehy, C.; Lawlor, P.G.; Hu, Z.; McCabe, M.; Cormican, P.; Gardiner, G.E. Exploring the roles of and interactions among microbes in dry co-digestion of food waste and pig manure using high-throughput 16S rRNA gene amplicon sequencing. Biotechnol. Biofuels 2019, 12, 1–16. [Google Scholar] [CrossRef]
- Jin, K.; Pezzuolo, A.; Gouda, S.G.; Jia, S.; Eraky, M.; Ran, Y.; Ai, P. Valorization of bio-fertilizer from anaerobic digestate through ammonia stripping process: A practical and sustainable approach towards circular economy. Environ. Technol. Innov. 2022, 27, 102414. [Google Scholar] [CrossRef]
- Santiago, B.; Moreira, M.T.; Feijoo, G.; González-García, S. Environmental comparison of banana waste valorisation strategies under a biorefinery approach. Waste Manag. 2022, 142, 77–87. [Google Scholar] [CrossRef]
- de Oliveira Gonçalves, M.; Carpanez, T.G.; Silva, J.B.G.; Otenio, M.H.; de Paula, V.R.; de Mendonça, H.V. Biomass Production of the Tropical Forage Grass Pennisetum purpureum (BRS Capiaçu) Following Biofertilizer Application. Waste Biomass Valorization 2022, 13, 2137–2147. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; van Wickeren, N.; Salehi Hosseini, P.; De Neve, S. The impacts of black soldier fly frass on nitrogen availability, microbial activities, C sequestration, and plant growth. Front. Sustain. Food Syst. 2022, 6, 795950. [Google Scholar] [CrossRef]
- Green, B.W. Fertilizer use in aquaculture. In Feed and Feeding Practices in Aquaculture; Woodhead Publishing: Cambridge, MA, USA, 2022; pp. 29–63. [Google Scholar]
- Ibeto, C.N.; Lag-Brotons, A.J.; Marshall, R.; Semple, K.T. The nutritional effects of digested and undigested organic wastes combined with wood ash amendments on carrot plants. J. Soil Sci. Plant Nutr. 2020, 20, 460–472. [Google Scholar] [CrossRef]
- Hidaka, T.; Nakamura, M.; Oritate, F.; Nishimura, F. Utilization of high solid waste activated sludge from small facilities by anaerobic digestion and application as fertilizer. Water Sci. Technol. 2019, 80, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Hassan, T.U.; Tahir, M.I.; Ali, A.; Jeyasundar, P.G.S.A.; Hussain, Q.; Zhang, Z. Tea leaves biochar as a carrier of Bacillus cereus improves the soil function and crop productivity. Appl. Soil Ecol. 2021, 157, 103732. [Google Scholar] [CrossRef]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C.; Ismail, S.; Lau, B.F.; Ong, H.C.; Chang, J.S. Bioformulation of biochar as a potential inoculant carrier for sustainable agriculture. Environ. Technol. Innov. 2020, 20, 101168. [Google Scholar] [CrossRef]
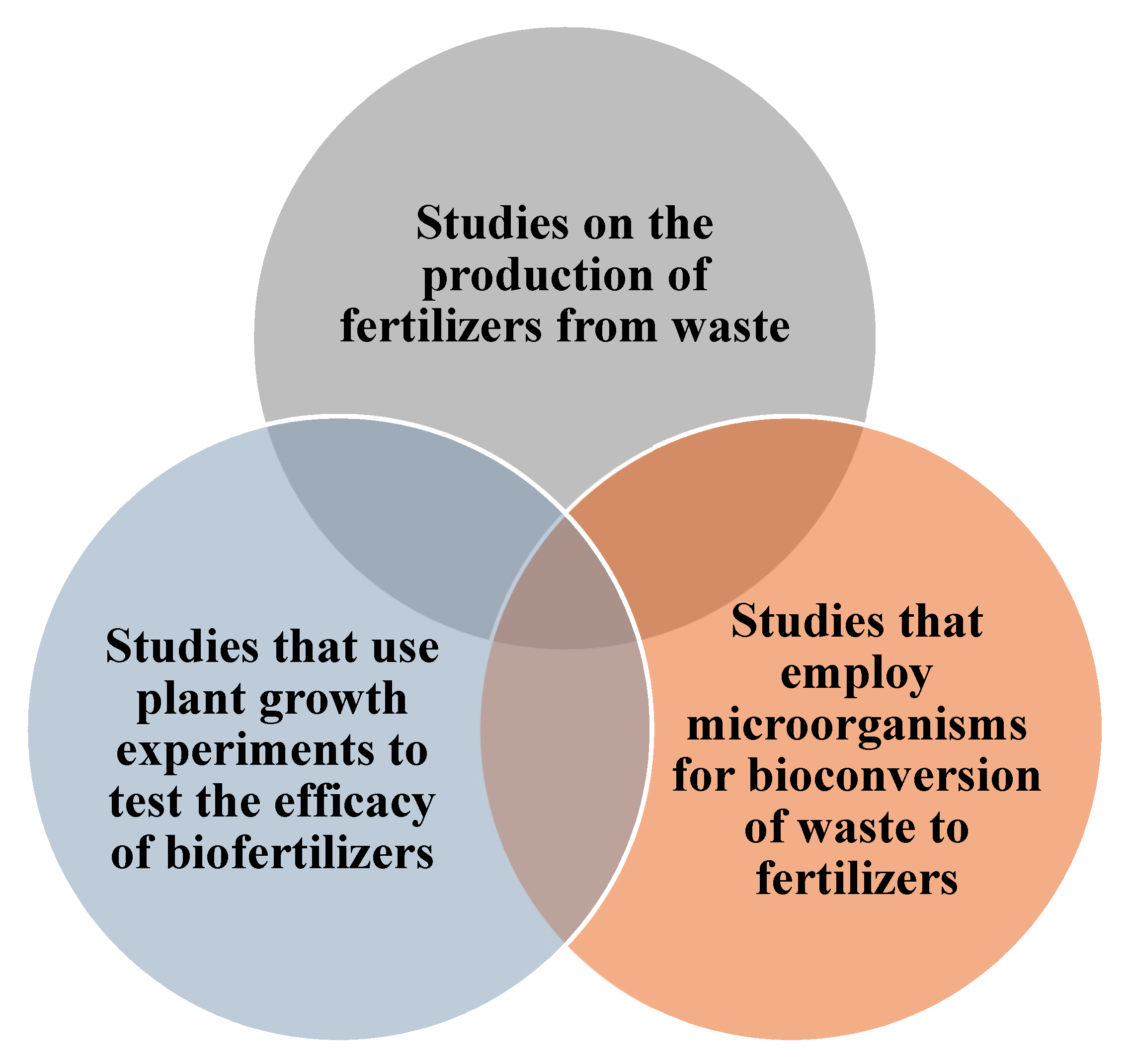
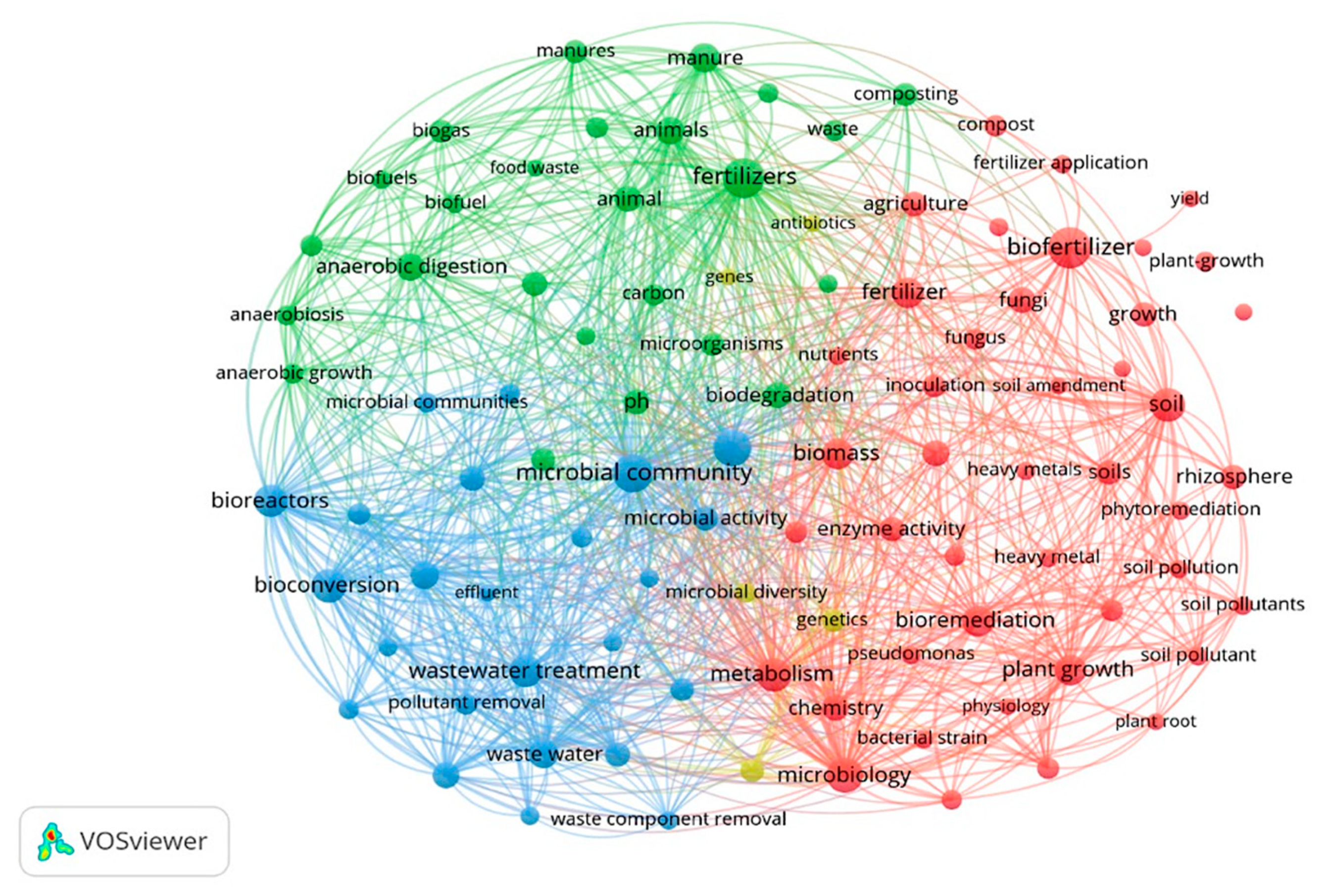
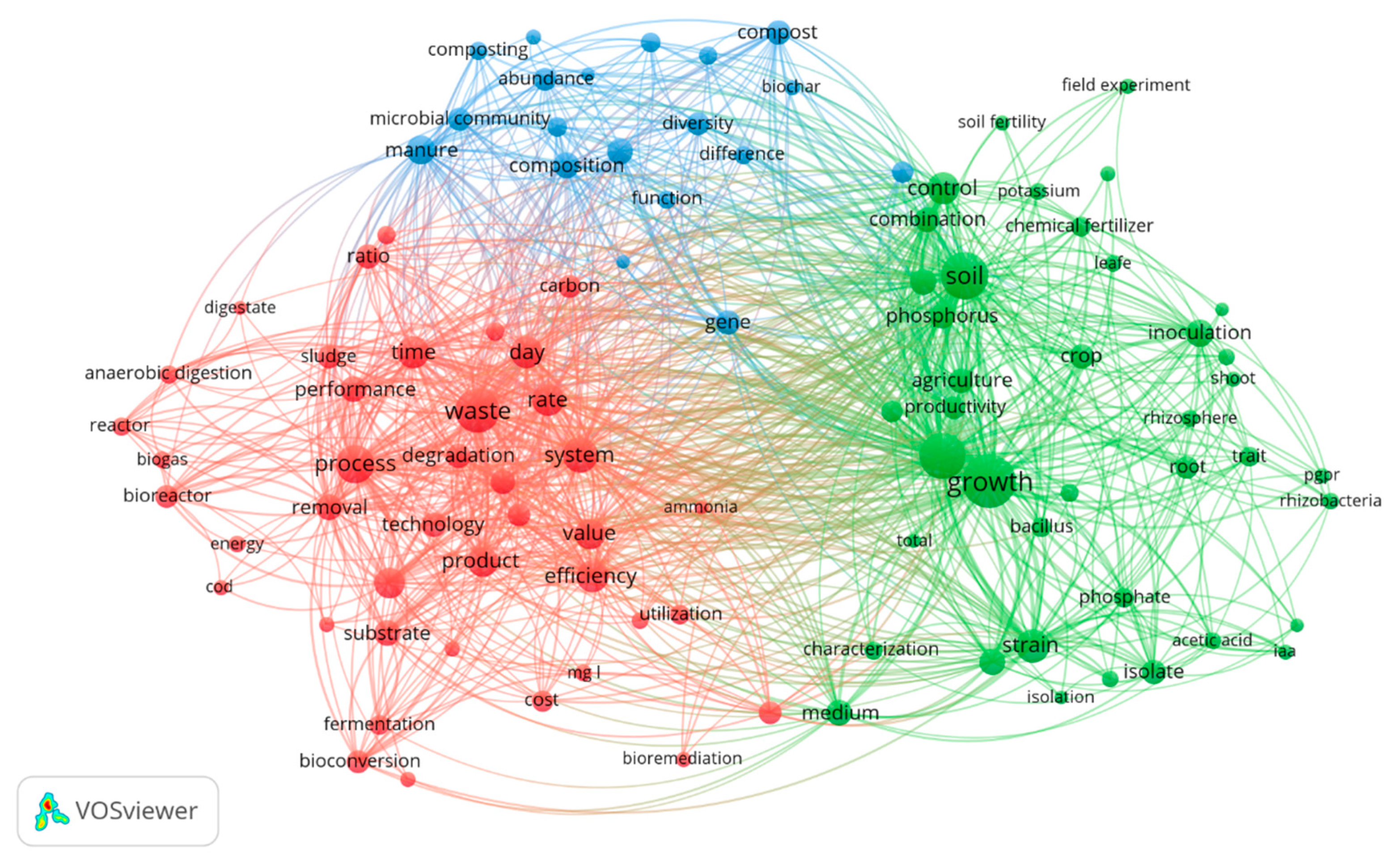

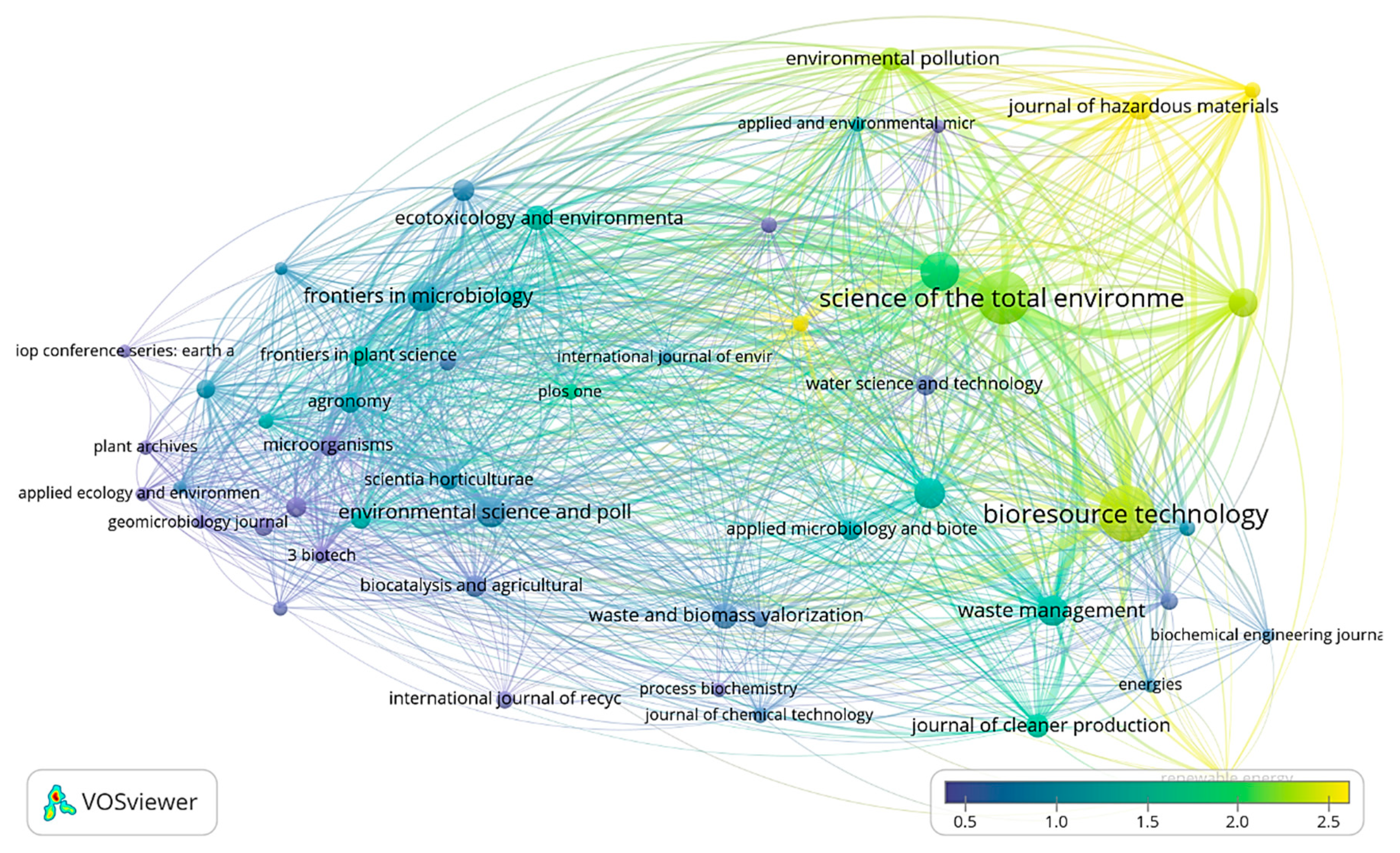
| Search Strategy | Scopus | Web of Science |
|---|---|---|
| biofertilizer * AND waste * AND fung * | 56 | 64 |
| biofertilizer * AND waste * AND bacteria * | 109 | 121 |
| “Plant growth” AND waste * AND bacteria * | 449 | 461 |
| “Plant growth” AND waste * AND fung * | 202 | 217 |
| fertilizer AND waste AND fungi | 148 | 154 |
| fertilizer AND waste AND bacteria | 632 | 412 |
| biofertilizer AND waste | 298 | 252 |
| biofertilizer AND bacteria | 606 | 507 |
| biofertilizer AND fungi | 268 | 252 |
| bioconversion AND waste AND fungi | 127 | 99 |
| bioconversion AND waste AND bacteria | 469 | 203 |
| bioinoculant * AND waste * | 11 | 19 |
| Year range was limited to 2017–2021 (last five years) | ||
| Problem (P) | Extensive Use of Chemical Fertilizers |
|---|---|
| Intervention (I) | application of biofertilizers produced by the waste valorization |
| Comparison (C) | different types of microbial inoculants used for waste valorization for biofertilizer production |
| Outcome (O) | Identification of microbial inoculants with potential and used for wastes bioconversion strategies |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies that used plant growth studies in pot, or plot settings | Review articles associated with anaerobic digestion or composting |
| Articles published during 2017–2021 | Studies that were related to waste pre-treatment techniques, biorefinery, microalgae production |
| Studies that studied microbial inoculants for the bioconversion of wastes into fertilizers only on plate-based or culture-based setups without plant growth assessments | |
| Studies that assessed microbial inoculants for the bioconversion of wastes into fertilizers | Conference proceedings and posters were excluded |
| Parameters of Interest in Fertilizer Studies | Plant Growth Promoting Microorganisms | Keywords in Circular Economy | Parameters of Plant Growth Promotion by Microorganisms |
|---|---|---|---|
| Ash, soil amendment, Biofertilizer treatment, Carrier material, seed inoculation, co-inoculation, chlorophyll content, available p, bacterial community, abundance, field experiment, root, shoot, dry and fresh weight, urea, greenhouse experiment, crop yield, harvest, growth parameters, NPK, nutrient content and uptake, Oryza sativa, Triticum aestivum, soybean | Bacillus subtilis, Burkholderia, Fusarium oxysporum, Enterobacter, Azotobacter chroococcum, Bacillus megaterium, endophytic bacterium, solubilizing bacteria, Trichoderma, Pseudomonas, Rhizobium, rhizobacteria, Serratia | Agricultural waste, anaerobic digestion, biochar, biofuel, biogas, composting, circular economy, feedstock, digestate, electricity, energy, environmental impact, recovery, recycling, reduction, industry, pollution, sustainable development, circular economy, waste valorization, wastewater, food waste, sewage sludge | ACC deaminase, acetic acid, ammonia, biocontrol, biopesticide, catalase, protease, chitinase, IAA production, nitrogen fixation, phosphate solubilization, phytohormone, salt stress, seedling germination, siderophore and gibberellic acid production |
| Inoculum Type | Type of Microorganism | Microbial Inoculant Used | Waste Used | Reference |
|---|---|---|---|---|
| consortia | actinomycetes | Actinomycetes | sawdust plus chicken litter | [34] |
| consortia | bacteria | Aeromonas, Bacillus, Thaueraamino aromatica, Acinetobacter (a consortium of 7 isolates) | Dairy Wastewater | [24] |
| consortia | bacteria | cellulolytic bacterial consortium | organic liquid waste (rice washing, coconut water liquid, tofu liquid waste, and palm oil mill liquid waste) | [35] |
| consortia | bacteria | consortium of decomposer microorganisms: Bacillus pumilus AL16, Microbacterium terregens AC1180, Aeromonas sp. B5376, Arthrobacter globiformis AC1529, Streptomyces olivocinereus AC1169 and Acinetobacter sp. B390. | feather-downy waste and dung | [28] |
| consortia | bacteria | lactic acid bacteria | silage (BE) of shrimp head, molasses, and milk | [36] |
| consortia | cyanobacteria | cyanobacteria (Fischerella muscicola, Anabaena variabilis, Aulosira fertilissima, Tolypothrix tenuis) | Chilli waste (stems and leaves after harvesting the fruit) | [37] |
| consortia | cyanobacteria | Chlorella sp., Dictyosphaerium sp., Monoraphidium sp., Neochloris sp. and Scenedesmus sp. | municipal wastewater (MWW) with simulated flue gas | [38] |
| consortia | mixed | Enhydrobacter aerosaccus (ACCA2 JX042472), Aspergillus sp. (ALUH KT356201) | raw rice husk (RRH) | [39] |
| consortia | mixed | Acidithiobacillus, Beijerinckia indica and Cunninghamella elegans | rock biofertilizers mixed with sulfur | [27] |
| consortia | unknown | EM bokashi | petroleum sludge | [40] |
| Waste Used | Bioconversion Strategy | Microbial Inoculant Used | Plant Tested | Mode of Application | Type of Test (In Vitro/Pot/Plot) | Role of Microbe | References |
|---|---|---|---|---|---|---|---|
| Cattle hooves (slaughterhouse waste) | submerged fermentation | Lichtheimia corymbifera AS1 | Vigna mungo | irrigation with hydrolysed hooves | germination and pot tests | keratinolysis | [41] |
| Olive mill solid waste (OMSW) | fermentation | Aspergillus tamarii | Vicia faba | soil amendment | germination and pot tests | fermentation | [42] |
| petroleum sludge | biodegradation | EM bokashi | Onion | soil amendment | pot tests | biodegradation | [40] |
| Municipal solid wastes (MSW) | composting | Streptomyces sp. Al-Dhabi 30 | Solanum lycopersicum | soil amendment | germination and pot tests | plant growth promotion (IAA, siderophore, phosphate solubilization), hydrolytic enzyme production (cellulase, pectinase), biocontrol | [43] |
| Municipal organic wastes | composting | Penicillium vinaceum and Eupenicillium hirayama | Capsicum annuum (pepper), Solanum melongena (aubergine) and S. lycopersicum (tomato) | soil supplementation | plot tests | plant growth promotion, biocontrol | [44] |
| Mixture of sawdust, sewage sludge, chicken litter | composting | Actinomycetes (thermo-tolerant) | Okra (Albenus Esculentus) and Maize (Zea-mays) | soil amendment | plot tests | fermentation, nitrogen fixation | [34] |
| composite of sawdust, chicken litter, vegetable waste, sewage sludge | composting | Streptomyces spp and Rothia spp | Okra (Albenus Esculentus) and Maize (Zea-mays) | soil amendment | plot tests | cellulolytic, ligninolytic, nitrogen mineralization | [45] |
| jarosite waste | nanoparticle synthesis | Aspergillus terreus strain J4 | Triticum aestivum | seed priming | germination tests | bioleaching | [46] |
| Dairy Wastewater | biofilm bioreactor | Aeromonas, Bacillus, Thaueraamino aromatica, Acinetobacter (consortium of 7 isolates) | Mung bean (var. Meha) | soil irrigation | plot tests | biofilm production | [24] |
| Chicken feathers | submerged fermentation | Bacillus cereus | tomato | soil amendment | plot tests | keratinolysis | [47] |
| 1% (w/w) keratin wastes (chicken feathers and sheep wool) | preparation of Protein Hydrolysates (PHs) | Trichoderma asperellum | Solanum lycopersicum | weekly application of PHS | germination and pot tests | plant growth promotion (IAA production, siderophore, cellulase activity, phosphate solubilization), biocontrol | [48] |
| fruit pulp from of ripened fruits not suitable for human consumption | submerged fermentation (culture medium) | Komagataeibacter medellinensis | Onion | soil amendment | pot tests | production of Bacterial Nanocellulose Mulch | [49] |
| Mixture of Cattle manure organic fertilizer (composted) and biochar | fermentation | Arthrobacter sp. DNS10 | soybean | soil amendment | germination and pot tests | [50] | |
| Chilli waste (stems and leaves after harvesting the fruit) | microbial enrichment | cyanobacteria (Fischerella muscicola, Anabaena variabilis, Aulosira fertilissima, Tolypothrix tenuis) | Brinjal (Solanum melongena) | soil amendment | pot tests | nutrient recovery | [37] |
| municipal wastewater (MWW) with simulated flue gas | micro-algal biorefinery approach | Chlorella sp., Dictyosphaerium sp., Monoraphidium sp., Neochloris sp. and Scenedesmus sp. | Wheat | soil supplementation | Germination test | nutrient recovery | [38] |
| Mixed medicinal plant waste | composting | consortia of Streptomyces, Paenibacillus, Bacillus and Hymenobacter | Fagopirum esculentum, Thymus vulgaris, Cynara scolimus and Lavandula officinalis | seed priming | germination tests | plant growth promotion | [51] |
| combination of rice washing water waste, tofu liquid waste, coconut water waste, and palm oil liquid waste | Incubation of liquid waste with bacterial consortium for 21 days (fermentation) | Bacillus cereus JP6, Bacillus cereus JP7, Proteus mirabilis TKKS3, Proteus mirabilis TKKS7, Providencia vermicola SA1 and Bacillus cereus SA6 | upland rice (Oryza sativa L.) | soil amendment | plot tests | cellulolysis, fermentation | [35] |
| Spent coffee grounds (SCG), poultry manure, and agricultural waste-derived biochar | composting | Streptomyces albus, Gibellulopsis nigrescens, Bacillus licheniformis, Bacillus smithii, and Alternaria tenuissima. | pepper and leek | soil amendment | germination and pot tests | microbial bioaugmentation | [52] |
| sewage sludge and agricultural waste | composting | Bacillus megaterium | Chinese flowering cabbage | polluted soil supplementation | plot tests | PAEs degradation and phosphate solubilization | [53] |
| brewery wastewater (BWW) | micro-algal biorefinery approach | Scenedesmus obliquus (ACOI 204/07) | barley and wheat seeds | soil supplementation | germination and pot tests | nutrient recovery | [54] |
| cane bagasse and broadbean seed capsule composite | solid-state fermentation (SSF) | Kosakonia cowanii LT-1 | Zea mays L. | soil amendment | pot tests | EPS production | [55] |
| feather waste (FW) and coconut oil cake (COC) | Biorefinery-Fermentation | Haloferax lucentensis GUBF-2 MG076878 | Oryza sativa L. var. Korgut | seed priming, soil amendment | germination and pot tests | production of protease and lipase haloextremozymes, Feather and COC Hydrolysate | [56] |
| biological silage (BE) of shrimp head, molasses and milk | fermentation | lactic acid bacteria | maralfalfa grass | application of leachate | plot tests | production of fermented liquid fertilizer | [36] |
| sugarcane molasses | fermentation | Corynebacterium glutamicum | Brassica campestris var. Pekinensis | foliar applications of fermented broth | pot tests | fermentation | [57] |
| wastewater from a WWTP | solid-state formulations | Scenedesmus sp. | Ryegrass and (b) barley | soil application of lyophilised microalgae with vegetable compost | plot tests | nutrient recovery | [58] |
| pressmud | solid state fermentation | Bacillus circulans | jowar and bajra | soil amendment | plot tests | fermentation and phosphate solubilization | [59] |
| native chicken feathers | fermentation | Chryseobacterium sp. RBT | Solanum melongena L and Capsicum annuum L. | foliar spray (S 5%, v/v) and root drenching (RD) (20%, v/v) | pot and plot tests | Keratinase | [60] |
| sardine waste (SW), potato peels (PP), and poultry waste (PW) | mesophilic bio-digestion | Aspergillus niger and Saccharomyces cerevisiae | bell peppers | soil amendment | seed germination and pot tests | fermentation | [61] |
| Vermicompost and lignite (carrier) | fermentation | Azospirillum lipoferum (Az 204), Bacillus megaterium (PB2) and Pseudomonas fluorescens(Pf1) | upland rice NLR 145 (Oryza sativa L.) | soil supplementation | pot tests | cellulolysis | [62] |
| coconut fibre | medium for mass micropropagule production | Trichoderma asperellum B1092 | Cherry tomato var. Sakura 318 | soil amendment | pot tests | biocontrol against Fusarium oxysporum f. sp. lycopersici B713T | [63] |
| A. bisporus industrial wastewater | submerged fermentation (culture medium) | Bacillus cereus | Brassica chinensis L | Application of treated liquid fermentation broth at proper intervals | plot tests | fermentation | [64] |
| (ZnO)-orange peel waste composite | bio-activation | Bacillus sp. AZ6 | Zea mays L. | soil amendment | plot tests | zinc solubilization | [65] |
| Lobster processing wastes | Submerged fermentation | Streptomyces griseus | Arabidopsis thaliana | foliar applications of extracts | germination tests | chitinolysis, biocontrol | [66] |
| lignocellulosic green waste (GW) from Municipal solid waste (MSW) | composting | Aspergillus fumigatus and Geotrichum sp. | Capsicum annuum L. | soil amendment | pot tests | cellulolytic and ligninolytic decomposer inducer | [67] |
| WWTP ash and poultry bone wastes | granulation and microbiological activation | Bacillus megaterium and Acidithiobacillus ferrooxidans | Wheat | soil supplementation | Phytotoxicity seed germination test | phosphate solubilization | [25] |
| sewage sludge ash and dried animal (porcine) blood | biological activation and granulation | Bacillus megaterium | wheat (Triticum aestivum ssp. vulgare MacKey) | soil amendment | pot tests | phosphate solubilization | [68] |
| sewage sludge ash and animal bones | enrichment and granulation | Bacillus megaterium and Acidithiobacillus ferrooxidans | winter wheat | soil supplementation | plot tests | phosphate solubilization | [29] |
| chicken feathers | composting | Chrysosporium indicum JK14 | Zea mays L. | soil amendment | pot tests | keratinolysis | [69] |
| Chicken feather waste | submerged state fermentation | Alternaria tenuissima | Chickpea (Cicer arietinum) | soil supplementation | plot tests | keratinolysis | [70] |
| kitchen waste oil | fermentation | Pseudomonas aeruginosa ATC 15442 | Cabbage | drip irrigation | pot tests | long chain triglycerides (LCTs) degradation | [71] |
| spent mushroom substrate (SMS) compost | semi-solid fermentation | Pantoea agglomerans ZB | Chili pepper seedlings | soil supplementation | plot tests | phosphate solubilization | [72] |
| sugarcane filter cake and boiler ash | composting | consortia of Pseudomonas aeruginosa PSBR12, Bacillus sp. BACBR04, Bacillus sp. BACBR06, Bacillus sp. BACBR01 and Rhizobium sp. RIZBR01 | sugarcane | soil amendment | plot tests | phosphate solubilization | [73] |
| low-moisture food waste material | composting | Aspergillus niger UY2015_11 | Lactuca sativa var. crispa (lettuce), and Brassica rapa var. perviridis | soil supplementation | phytotoxicity and plot tests | nitrogen release, phosphate solubilization | [74] |
| raw press mud | composting | Aspergillus niger (RHS/M492-NAIMCC-F-02890) | Zea mays L. | soil amendment | plot tests | phosphate and zinc solubilization | [75] |
| chicken feathers | composting | Bacillus subtilis FW12 | green gram | soil amendment | pot tests | keratinolysis | [76] |
| kitchen waste (79%), chita-dhan (unfilled rice grain) biochar (15%), rock phosphate (5%) | co-composting | a consortium of 10 PGPB (1%) (Bacillus mycoides, Proteus sp., Bacillus cereus, Bacillus subtilis, Bacillus pumilus, Paenibacillus polymyxa, and Paenibacillus spp.) | Oryza sativa L | fertilizer amendment | plot tests | plant growth promotion (IAA production, phosphate solubilization, N2 fixation) | [77] |
| Glycerin pitch | fermentation | Lactobacillus spp. | cucumber | soil supplementation | pot tests | glycerin biodegradation | [78] |
| domestic wastewater from wastewater sewage pump with coal-fired flue gas (2.5% CO2) | microalgae cultivation and lipid extraction | Scenedesmus sp. | Oryza sativa | soil amendment | pot tests | nutrient recovery | [79] |
| organic vegetable heaps | composting | Clonostachys rosea f. catenula | Solanum lycopersicum | soil amendment | pot tests | biocontrol, plant growth promotion | [80] |
| natural phosphate and potash rock | phosphate solubilization piles | Acidithiobacillus thiooxidans, Beijerinckia indica | Sugarcane | soil supplementation | plot tests | phosphate solubilization | [81] |
| Crustacean shell waste (shrimp and crab shell powder (SCSP)) | Submerged fermentation | Alcaligenes faecalis SK10 | Pisum sativum and Cicer arietinum | soil amendment | pot tests | chitinolysis and proteolysis | [82] |
| vermicompost | microbial enrichment | consortia of Serratia marcescens, Pseudomonas aeruginosa, and Bacillus cereus | tomato and wheat | soil amendment | pot tests | phosphate solubilization | [83] |
| vineyard waste | composting | Trichoderma harzianum T-78 | muskmelon | soil supplementation | pot tests | plant growth promotion, biocontrol | [84] |
| organic biomedical waste | anerobic fermentation followed by incineration | Pasterulla canis circinelloides | Solanum lycopersicum | soil amendment | pot tests | plant nutrient dynamics, biodegradation | [85] |
| mixture of feather-downy waste and dung (8 : 2) | biohumus production through decomposition | consortium of decomposer microorganisms: Bacillus pumilus AL16, Microbacterium terregens AC1180, Aeromonas sp. B5376, Arthrobacter globiformis AC1529, Streptomyces olivocinereus AC1169 and Acinetobacter sp. B390 | winter wheat crops | soil amendment | plot tests | decomposition | [28] |
| sewage sludge ashes | fungal spore inoculation for 8 days | Penicillium bilaiae DBS-5 and Aspergillus niger ATCC 9142 | spring wheat (Triticum aestivum L. cv. Dacke) | soil application | plot tests | phosphate solubilization | [86] |
| sugarcane molasses | fermentation | Corynebacterium glutamicum | Potato | foliar application | plot tests | glutamic acid production | [87] |
| sewage sludge ash and spent mushroom substrate | solid-state solubilization and composting | Acidithiobacillus ferrooxidans | sunflower seeds | soil amendment | germination tests | phosphate solubilization | [88] |
| Spent Coffee Grounds, Rice Bran, and Biochar | composting | Bacillus sp. and Actinomyces sp. | pepper and leek | soil amendment | pot tests | plant growth, biocontrol | [89] |
| biogas residue, rice straw and cattle manure | composting | Streptomyces microflavus G33 | Solanum lycopersicum | soil amendment | plot tests | biocontrol | [90] |
| domestic wastewater | microalgae cultivation and lipid extraction | Chlorella sp. (KP972095) and Scenedesmus sp. (KR025877) | Solanum lycopersicum | soil amendment | pot tests | nutrient recovery | [91] |
| spent mushroom substrate (SMS) | fungal digestion | Trichoderma harzianum | Solanum lycopersicum | soil amendment | pot tests | mycodegradation | [92] |
| rock biofertilizers mixed with sulfur | composting | Acidithiobacillus, Beijerinckia indica and Cunninghamella elegans | banana “Williams” | soil supplementation | plot tests | phosphate solubilization | [27] |
| rice straw | solid-state fermentation (SSF) | Aspergillus niger (RHS/M492-NAIMCC-F-02890) | Oryza sativa L | seedling spray | germination and pot tests | production of fungal-based chitosan | [93] |
| black water (toilet wastewater) | micro-algal biorefinery approach | Chlorella sorokiniana | spring barley (Hordeum vulgare L.) | soil supplementation | Phytotoxicity seed germination test and plot tests | nutrient recovery | [94] |
| Chicken feathers powder | Liquid Fermentation | Bacillus pumilus JYL | wheat | soil supplementation | pot tests | keratinolysis | [95] |
| rice husk (RH) | composting | Aspergillus sp | black gram | soil amendment | pot tests | cellulolytic activity | [39] |
| paddy-soaked rice mill wastewater (PSRMW) | micro-algal biorefinery approach | Chlorella pyrenoidosa | Indian okra (Abelmoschus angulosus) | soil amendment | pot tests | nutrient recovery | [96] |
| lignin waste | fermentation | Meyerozyma guilliermondii and Providencia rettgeri | Cowpea | foliar application | Seed germination pot tests | ligninolysis | [97] |
| chicken manure | fermentation | Bacillus subtilis | Chinese cabbage and rape seeds | seed immersion | germination and pot tests | decomposition | [26] |
| duck feathers | composting | consortia of Arthrobacter ureafaciens K10 and Streptomyces sp. CP3 | cherry tomato | seed priming | germination and pot tests | feather degradation, phosphatesolubilization, and IAA formation | [98] |
| Cow Manure and rapeseed meal | Solid State Fermentation | Bacillus sp. XG-1 | Citrullus lanatus Thumb. | soil amendment | plot tests | plant growth promotion (IAA, gibberellins, phytase), biocontrol | [99] |
| prickly ash seeds (PAS) and biochar from rice husks | solid state fermentation | Bacillus subtilis Tpb55 | rape seeds | seed immersion and soil amendment | germination and pot tests | biocontrol | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiruba N, J.M.; Saeid, A. An Insight into Microbial Inoculants for Bioconversion of Waste Biomass into Sustainable “Bio-Organic” Fertilizers: A Bibliometric Analysis and Systematic Literature Review. Int. J. Mol. Sci. 2022, 23, 13049. https://doi.org/10.3390/ijms232113049
Kiruba N JM, Saeid A. An Insight into Microbial Inoculants for Bioconversion of Waste Biomass into Sustainable “Bio-Organic” Fertilizers: A Bibliometric Analysis and Systematic Literature Review. International Journal of Molecular Sciences. 2022; 23(21):13049. https://doi.org/10.3390/ijms232113049
Chicago/Turabian StyleKiruba N, Jennifer Michellin, and Agnieszka Saeid. 2022. "An Insight into Microbial Inoculants for Bioconversion of Waste Biomass into Sustainable “Bio-Organic” Fertilizers: A Bibliometric Analysis and Systematic Literature Review" International Journal of Molecular Sciences 23, no. 21: 13049. https://doi.org/10.3390/ijms232113049
APA StyleKiruba N, J. M., & Saeid, A. (2022). An Insight into Microbial Inoculants for Bioconversion of Waste Biomass into Sustainable “Bio-Organic” Fertilizers: A Bibliometric Analysis and Systematic Literature Review. International Journal of Molecular Sciences, 23(21), 13049. https://doi.org/10.3390/ijms232113049








