Abstract
Systemic lupus erythematosus (SLE) is a chronic, multifactorial autoimmune disease with complex pathogenesis characterized by the imbalance of pro-inflammatory and anti-inflammatory cytokines. Janus kinases (JAKs), intracellular non-receptor tyrosine kinases, are essential for signal pathways of many cytokines. The JAK signal transducers and activators of transcription (STAT) pathways consist of four JAK kinases and seven STATs family members. The dysregulation of JAK-STAT pathways represents an important process in the pathogenesis of SLE. Thus, the use of therapies that target specific signaling pathways would be a challenge in SLE. It is well known that JAK inhibitors have real potential for the treatment of rheumatic diseases, but their efficacy in the treatment of SLE remains to be determined. JAK inhibitors are currently being investigated in phase II and III trials and are considered to become the next stage in SLE therapy. In this review, we report the current data regarding the efficacy of JAK inhibitors in SLE. The development of clinically useful kinase inhibitors might improve upon traditional therapeutic strategies.
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease characterized by complex pathogenesis that includes genetic factors, environmental triggers, and hormone molecules, as well as an overproduction of an array of cytokines [1]. Under these conditions a loss of self-tolerance and an overproduction of autoantibodies took place. Both innate and adaptive immune systems have important roles in SLE pathogenesis [1].
Type 1 interferons (IFN α and ß, mostly released by dendritic cells) and type 2 IFN (IFN γ -mostly secreted by T cells) are present in SLE. Moreover, an elevated expression of type 1 IFN-regulated genes, known as IFN signature, is considered to be the main characteristic of SLE [1]. The relationship between JAK/STAT (Janus kinase and Signal Transducers and Activators of Transcription) and IFNs pathway is based on the discovery of type 1 and type 2 IFNs mechanism of action [1,2]. They use JAK/STAT cascade signaling pathway to exert their biological actions [1]. Analysis of the inhibition of the JAK/STAT pathway has shown that it has a central role in the decrease of SLE inflammation [1].
Existing treatments such as glucocorticoids and immunosuppressive drugs can be associated with various severe side effects and incomplete efficacy [3,4]. Under these circumstances, SLE remains a condition with high morbidity and mortality [3,5]. Biological molecules targeting proinflammatory cytokines have changed the treatment of autoimmune diseases [6]. Thus, an upcoming target for SLE is the JAK/STAT pathway [7]. Many JAK inhibitors have been studied for the treatment of SLE [8]. Regarding the recent information concerning cytokine signaling, the studies analyze if this intracellular signaling can have a safe and efficient role in SLE patients [6]. Therefore, an update on these drugs’ development is essential [9,10].
Cytokines and cell surface molecules which act through the JAK/STAT pathway play a pivotal role in the pathogenesis of inflammatory autoimmune diseases [11,12]. These can transduce a diversity of intracellular signals by binding to receptors, generating cell functions, and the transcription of new cytokines [8]. The evidence of the promising efficacy of the JAK/STAT pathway in type I and II cytokine signaling has increased the research in the field of rheumatic diseases [12,13]. Thus, their targeted inhibition can be associated with disease control [14].
The first JAK inhibitor approved for human use was tofacitinib, a JAK1/3 inhibitor [1]. Baricitinib, which selectively inhibits JAK1 and JAK2, is also under investigation [3,15]. In December 2018, Food and Drug Administration (FDA) granted the “Fast Track” status for baricitinib use in SLE treatment [10]. Next-generation JAK inhibitor agents such as upadacitinib and filgotinib are still being evaluated in clinical trials [16].
The purpose of this paper is to assess the current evidence from case reports and clinical trials regarding JAK inhibitor efficacy in SLE treatment [9].
2. JAK–STAT Pathway
The JAK/STAT pathways are represented by four JAK kinases (JAK1, JAK2, JAK3, and non-receptor tyrosine-protein kinase 2-TYK2) and seven STAT family members (STAT1, STAT2, STAT3, STAT4, STAT 5a, STAT 5b, STAT6) [11,14,17,18]. Their different combinations induce the transcription of various genes via STATs [8]. The enzymes whose role is to phosphorylate signaling molecules are called kinases. Of 518 kinases, JAK is classified as a typical tyrosine kinase [8] that transfer phosphates from adenosine triphosphate (ATP) to tyrosine residues on other proteins such as cytokine receptors or even JAKs [12,19]. Protein kinases are essential regulators of cellular functions. Intracellular signal transduction is realized through the connection between JAK, TYK2 isoforms, and STAT members [8]. The activity of every JAK depends on selective interactions with cytokine receptors. Moreover, each cytokine receptor provides a specific combination with JAK kinases, which has a crucial implication in therapeutic maneuvers [11,20].
JAKs receive signals from various cytokine receptors of interleukin (IL) and IFN members [13,21]. Particularly, the role of JAKs in signaling is focused on a subset of cytokines that use type I and II cytokine receptors. There are described more than 50 soluble molecules, including IL-2, IL-3, IL-4, IL-5, IL-6, IL-12, IFNs, endocrine factors (including growth hormone, prolactin and leptin). colony-stimulating factors (erythropoietin, thrombopoietin and granulocyte–macrophage colony-stimulating factor (GM-CSF)). Their effects are produced after the combination with a specific JAK [14]. TYK2 is activated after the receptor is bound by IL-23, IL-12 and type I IFNs. Specific combinations of STAT family members with their receptors will be phosphorylated by JAKs, leading to STAT dimerization, nuclear translocation and gene regulation [21].
2.1. Mechanism of JAK-STAT Signaling Pathway
Several extracellular cytokines and growth factors bind to their specific receptors on targeted cells and initiate a cascade of intracellular signals. JAKs attach to the cytoplasmic domains of these cytokine receptors. This results in subsequent rearrangement of the receptor subunits which is called “cytokine receptor engagement”. This mechanism leads to intracellular trans-phosphorylation of receptor-associated JAKs [11,12]. The phosphorylation process enables JAKs activation. Consecutively, they phosphorylate each other, as well as the intracellular components of the receptors, leading to the emergence of docking sites [11,12,14]. This enables the recruitment of transcription factors of the STAT family, allowing them to bind to the receptor and become phosphorylated by JAKs. Then, the phosphorylated STAT homo- or heterodimers enter the nucleus. Here, STAT induces the transcription of targeted genes [11,14,22]. This heterogenicity of STAT dimerization can produce many biological responses [11].
2.2. Inhibition of JAK-STAT Signaling Pathway
JAK inhibitors are the newest class of targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs). These are orally small molecules that are able to enter into cellular cytoplasm and directly modulate intracellular signaling [14]. JAK inhibitors suppress the STAT pathways and inhibit the effects of many proinflammatory cytokines [14]. In particular, tsDMARDs act by blocking the ATP-binding site in the catalyzing region of the JAK enzymes [1,23]. This suppression of downstream signaling pathways is associated with immunomodulatory consequences [14]. In addition, blocking the phosphorylation of cytokine receptors and gene transcription can lead to impaired differentiation of Th1, Th2, and Th17 cells [12,19].
Type I IFN-mediated monogenic autoinflammatory conditions (type I interferonopathies) consist of a heterogeneous group of inflammatory autoimmune diseases characterized by the activation of the type I IFN axis. This multifactorial activation has been also highlighted in SLE. The major signaling pathway activated by IFNs is the JAK/STAT pathway, so its inhibition can be a good therapeutic solution [24,25,26]. Treatments that target JAK/STAT pathways have the potential to show positive effects by minimizing the use of glucocorticoids and immunosuppressant drugs [14]. JAK inhibitors improved clinical symptoms and laboratory parameters, decreased flares, and the expression of IFN-stimulated genes [24]. The use of JAK inhibitors and their potential effectiveness in SLE are currently being investigated.
In the last years, several drugs which inhibit different proteins of the JAK/STAT pathway are under study. Some of them act exclusively on a single site (filgotinib-JAK1) or have effects on multiple sites (tofacitinib-JAK1 and JAK3; ruxolitinib and baricitinib-JAK1 and JAK2). Another classification divided the molecules into first-generation pan-JAK inhibitors (tofacitinib, baricitinib, ruxolitinib, peficitinib) and second-generation selective JAK inhibitors (decernotinib, filgotinib, upadacitinib) [12,24,27,28].
Cytokines that activate the innate and adaptive immune system such as type I IFNs, IL-12, IL-23, and those that activate T–B cell interaction: IL-21, IL-6, and IL-4 are considered to be potential targets of JAK inhibition in SLE [14,16,29]. The disrupted regulation between type I and II IFNs and B cells are two major signatures in SLE, the former being targeted by JAK inhibitors [12]. For instance, the inhibition of JAK1 suppresses both IL-6 and type I IFN signaling [30]. Moreover, cytokines like tumor necrosis factor-alpha (TNF-α), IL-2, IL-10, IL-15, IL-17, and B cell activating factor (BAFF) play prominent roles in SLE pathogenesis. The main intracellular mechanisms of action are represented in Figure 1.
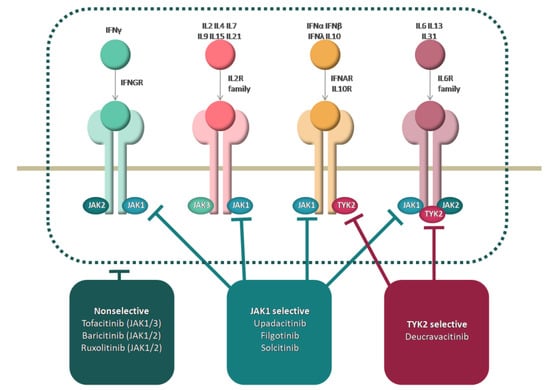
Figure 1.
Mechanism of action of JAK inhibitors.
2.3. Main Inhibitors of JAK/STAT Pathway
2.3.1. Tofacitinib
Tofacitinib, the first tested JAK inhibitor, has a high selectivity for JAK1 and JAK3. It is less effective for the inhibition of JAK2 and has limited action on TYK2 [6,31,32].
Regarding the genetic risk haplotype, results from a pilot phase Ib/IIa, double-blind trial confirmed that the SLE immunological response to tofacitinib depends on STAT4 risk allele rs7574865[T]. This allele is associated with severe SLE symptoms. Furthermore, tofacitinib showed significantly positive outcomes in cardiometabolic markers such as high-density lipoprotein cholesterol (HDL-C) levels [29,33,34,35,36]. In SLE patients positive for STAT4 risk allele, tofacitinib led to a decreased expression of interferon-response genes and low levels of circulating density granulocytes and neutrophil NETosis, as well as to the suppression of pSTAT1 in CD4+ T cells [36].
Evidence from murine SLE models has demonstrated that tofacitinib modulated lupus-associated parameters levels such as antinuclear antibody (ANA), anti-double-stranded deoxyribonucleic acid (anti-dsDNA) antibodies, proteinuria, skin rash, and type I IFN related responses. Regarding the lipoprotein profile in tofacitinib-treated mice, the authors highlighted the reduction of free cholesterol levels. Tofacitinib is considered to be a “vasculoprotective” agent [12,37,38,39,40,41,42].
Similar results were seen in a rhupus patient (coexistence of SLE and rheumatoid arthritis) with class III glomerulonephritis (GN), and joint and skin involvement. The patient treated with tofacitinib 5mg twice daily and a decrease of cortison dose was also reached, in accordance with 2019 EULAR recommendations [43,44].
The first report showing the efficacy of tofacitinib in 10 SLE patients was performed by You et al. The results were promising for arthritis and cutaneous manifestations but were limited for serological markers [45].
Another study used the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) score to evaluate skin manifestations. In 3 “recalcitrant” cutaneous lupus patients, the outcomes were encouraging as the CLASI score showed a significant improvement [46]. Additionally, a recent case report confirmed the favorable tofacitinib effects on SLE refractory alopecia. The patient experienced hair regrowth after being treated with a JAK inhibitor [40].
Another case report highlighted a decrease of anti-dsDNA antibody levels to normal values after tofacitinib treatment in a patient with SLE associated with rheumatoid arthritis [47].
Another report presented the cases of two patients with cold-induced finger erythematous lesions. The patients suffered from familial chilblain lupus (FCh-L), which is a monogenic autosomal dominant form of cutaneous lupus erythematosus. This was caused by mutations in the nucleases 3–5′ repair exonuclease 1 (TREX1) or SAMHD1 and is associated with systemic skin and biological involvement due to type I IFN signature. Currently, there is no effective treatment available, but it seems that tofacitinib can induce a strong suppression, leading to lower discomfort and pain in these patients [40,48].
However, tofacitinib’s use in SLE patients still has to be defined and it is now being tested in two phase I/II clinical trials regarding skin manifestation. In the NCT03159936 study, adult individuals with discoid lupus are investigated. The NCT03288324 study is currently recruiting young adults with moderate to severe cutaneous lupus erythematosus (CLE) [49,50,51].
The main ongoing studies on the use of tofacitinib in SLE patients are presented in Table 1 and Table 2. They include the study design, the primary and secondary endpoints, as well as the most important statistically significant results.

Table 1.
Tofacitinib-main case reports and clinical trials.

Table 2.
Tofacitinib-main results of clinical trials in SLE patients.
2.3.2. Baricitinib
Baricitinib is an orally administered, low-weight molecule, which selectively inhibits JAK1 and JAK2 subtypes. Baricitinib received its first global approval in the European Union (EU) on the 13 February 2017 for the treatment of moderate to severe active rheumatoid arthritis patients who did not show a proper response or were intolerant to DMARDs [15,52].
In a phase IIb clinical trial across 11 countries, Wallace et al. presented the role of baricitinib in patients with active non-renal SLE. These individuals had skin and joint manifestations, two of the most common clinical expressions, and were non-responsive to standard treatments. Significantly more subjects in the baricitinib group (in particular, the 4 mg dose) achieved a resolution of arthritis or skin rash (according to SLEDAI-2K criteria) after 24 weeks [3,14,53]. In addition, the patients on baricitinib treatment achieved a higher response according to the SLE Responder Index (SRI) criteria versus placebo [1,3,14]. There were significant improvements in secondary outcomes, including the physician global assessment (PGA), lupus low disease activity state (LLDAS), risk of flares measured by the SSFI, joint tenderness evaluated by 28-joint examination, Worst Joint Pain NRS, and Worst Pain NRS in the baricitinib arm versus placebo. This was the first randomized controlled trial that demonstrated good efficacy of JAK 1/2 inhibition in SLE and an improvement in the quality of patient life. Unfortunately, there were no notable positive changes seen regarding skin lesions [3,54]. Concerning safety issues, side effects were observed in 65% of patients in the placebo group versus 71% of patients in the 2 mg baricitinib group versus 73% of patients in the 4 mg baricitinib group [3,55].
Analyzing the same group of patients, Dörner et al. showed that baricitinib induces modifications in the RNA expression of genes linked to the JAK/STAT pathway [56].
Focusing on specific organ manifestations, two different studies reported improvement of diffuse non-scarring alopecia and refractory papulosquamous rash after baricitinib treatment [57,58]. The same results regarding the positive effects of baricitinib on severe subacute lesions were described by Joos et al. [59].
JAK/STAT signaling is implicated substantially in inflamed CLE skin. Specifically, keratinocytes and dermal immune cells express the activated form of phospho-JAK1. The JAK1/2 and JAK 1/3 inhibitors exert their role by importantly decreasing the expression of CLE-typical chemokines in vitro [60,61,62].
Moreover, a case report showed promising results with JAK1/2 inhibitor. Subacute cutaneous lupus erythematosus (SCLE) with frontal fibrosing alopecia (FFA) presented a complete remission after 2 months of barcitinib therapy [63].
In particular, all patients with FCh-L (familial chilblain lupus) due to TREX1 deficiency from Zimmermann et al. study who were treated with baricitinib accomplished notable improvement in cutaneous manifestations and relief of joint pain. The most important side effects were repeated mild respiratory infections [24,64].
Data of a rhupus subject successfully treated with a JAK1/2 inhibitor was presented by Garufi et al. Baricitinib 4 mg/day induced complete renal remission and controlled joint manifestations in a rhupus patient with class V GN [43].
In a murine model, baricitinib improved renal inflammation, leading to the recovery of the structural proteins in podocytes. This phenomenon was explained through a direct pathogenic effect of INFα whereby the differentiation and maturation of podocytes is inhibited, which consequently induces podocytes loss [43,65,66].
There are also two trials phase III (BRAVE I-NCT03616912 and BRAVE II NCT03616964), in which the efficacy of baricitinib in SLE is under investigation [67,68]. Furthermore, the long-term safety of baricitinib is being studied in a phase III trial SLE-BRAVE-X (NCT03843125) [69].
Table 3 and Table 4 summarize the main case reports and clinical trials using baricitinib in SLE patients. The main endpoints and the most significant results are also described.

Table 3.
Baricitinib-main case reports and clinical trials.

Table 4.
Baricitinib-main results of clinical trials in SLE patient.
2.3.3. Other New Therapies under Study
Ruxolitinib
Data from murine lupus models indicated a significant improvement in skin modifications in MRL/lpr mice treated with the selective JAK1/2 inhibitor ruxolitinib [70].
Wenzel et al. provided further support for the therapeutic implications of ruxolitinib, presenting a successfully controlled Chilblain Lupus Erythematosus (ChLE) [7,71].
Consistent results with those highlighted in patients with ChLE treated with JAK1/2 inhibitors were shown by Briand et al. focusing on skin vasculopathy. Thus, a fast clinical improvement with almost complete resolution of skin lesions was described in a patient with FChL due to TREX1 deficiency associated with Aicardi-Goutières syndrome [7,60,72].
In addition, ruxolitinib has been revealed to be effective in blocking the anti-extractable nuclear antigen (ENA) and anti-dsDNA antibody production in SLE patients [73].
Solcitinib
A selective JAK1 inhibitor (GSK2586184) had been proposed as a novel therapeutic agent in a phase IIb study that investigated patients with moderate-to-severe active SLE without renal or cerebral involvement who have failed standard therapy. However, the results were not satisfying, and it was prematurely stopped. Notably, the study on solcitinib pointed out important side effects such as drug reaction with eosinophilia and systemic symptoms (DRESS) and hepatic function modifications, as well as a lack of predicted response [74,75,76,77].
R333
R333 (R932333), a topical JAK1/3/SYK (Janus kinase and spleen tyrosine kinase) inhibitor, was studied regarding SLE skin involvement. It also failed to achieve its intended aim of demonstrating positive results in discoid lupus with erythema and scaling possibly due to poor penetration [1,45,78,79].
Brepocitinib
A large phase IIb trial of brepocitinib, an inhibitor of JAK1 and TYK2, is ongoing for active SLE [80].
Filgotinib
Filgotinib, a highly selective JAK1 inhibitor, is the subject of a phase II clinical trial for active CLE (NCT03134222), being evaluated in comparison with lanraplenib (a Syk kinase inhibitor) [33,81]. Filgotinib is also being evaluated in lupus membranous nephropathy (NCT03285711) [82].
Upadacitinib
In addition to the above, upadacitinib’s role in SLE has to be defined. A phase II trial is underway to investigate the safety and efficacy of the JAK1 selective inhibitor in SLE in monotherapy or in combination with a Bruton’s tyrosine kinase (BTK) inhibitor-elsubrutinib (NCT03978520) [83].
Deucravacitinib
Deucravacitinib is a highly selective TYK2 inhibitor having minimal or no activity against JAK1-3. Two clinical trials referring to the use of deucravacitinib to treat SLE patients were identified: PAISLEY LN NCT03943147 which evaluated participants with lupus nephritis stopped due to an insufficient number of subjects [84] and PAISLEY SLE NCT03252587 [85] a long-term trial on safety and efficacy NCT03920267 [86].
The main clinical trials, as well as their preliminary results regarding new JAK inhibitors used in SLE treatment, are described in Table 5.

Table 5.
New JAK inhibitors for SLE patients and the main results of clinical trials.
3. Conclusions
Despite promising results from clinical trials, none of these therapies have managed to receive approval to be used in clinical practice. Thus far, the best results have been obtained from the use of tofacitinib and baricitinib.
Due to its complicated pathogenesis, the management of SLE is still changeling. While the therapeutic use of JAK inhibitors has been demonstrated, their role in SLE remains to be determined. Despite the safety and efficacy profiles showing encouraging results, future clinical trials are necessary. All these data published so far represent a cornerstone for future studies that we hope will bring new useful information regarding the therapy of SLE patients.
Author Contributions
Conceptualization, P.R. and A.C.; methodology, P.R. and I.B.; software, A.M.B. and A.C.; validation, E.R., A.C. and A.M.B.; formal analysis, L.A.M. and O.N.B.-F.; investigation, P.R.; resources, P.R., A.M.B. and L.A.M.; data curation, P.R. and I.B.; writing—original draft preparation, P.R.; writing—review and editing, A.C.; visualization, A.M.B. and E.R.; supervision, E.R.; project administration, E.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al Khalili, A.; Dutz, J.P. Janus Kinase Inhibition and SLE: Is this a Plausible Treatment Option for SLE? Curr. Treat. Options Rheumatol. 2020, 6, 406–417. [Google Scholar] [CrossRef]
- Bengtsson, A.A.; Ronnblom, L. Role of interferons in SLE. Best Pr. Res. Clin. Rheumatol. 2017, 31, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.J.; Furie, R.A.; Tanaka, Y.; Kalunian, K.C.; Mosca, M.; Petri, M.A.; Dörner, T.; Cardiel, M.H.; Bruce, I.N.; Gomez, E. Baricitinib for systemic lupus erythematosus: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2018, 392, 222–231. [Google Scholar] [CrossRef]
- Thong, B.; Olsen, N.J. Systemic lupus erythematosus diagnosis and management. Rheumatology 2017, 56, i3–i13. [Google Scholar] [CrossRef]
- Yurkovich, M.; Vostretsova, K.; Chen, W.; Avina-Zubieta, J.A. Overall and cause-specific mortality in patients with systemic lupus erythematosus: A meta-analysis of observational studies. Arthritis Care Res. 2014, 66, 608–616. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Kontzias, A.; Yamaoka, K.; Tanaka, Y.; Laurence, A. Janus kinase inhibitors in autoimmune diseases. Ann. Rheum. Dis. 2013, 72, ii111–ii115. [Google Scholar] [CrossRef]
- Wenzel, J.; van Holt, N.; Maier, J.; Vonnahme, M.; Bieber, T.; Wolf, D. JAK1/2 inhibitor ruxolitinib controls a case of chilblain lupus erythematosus. J. Investig. Dermatol. 2016, 136, 1281–1283. [Google Scholar] [CrossRef]
- Tanaka, Y. State-of-the-art treatment of systemic lupus erythematosus. Int. J. Rheum. Dis. 2020, 23, 465–471. [Google Scholar] [CrossRef]
- Sharabi, A. Updates on Clinical Trials in Systemic Lupus Erythematosus. Curr. Rheumatol. Rep. 2021, 23, 1–8. [Google Scholar] [CrossRef]
- Luo, S.; Long, H.; Lu, Q. Recent advances in understanding pathogenesis and therapeutic strategies of Systemic Lupus Erythematosus. Int. Immunopharmacol. 2020, 89, 107028. [Google Scholar] [CrossRef]
- Virtanen, A.T.; Haikarainen, T.; Raivola, J.; Silvennoinen, O. Selective JAKinibs: Prospects in inflammatory and autoimmune diseases. BioDrugs 2019, 33, 15–32. [Google Scholar] [CrossRef]
- You, H.; Xu, D.; Zhao, J.; Li, J.; Wang, Q.; Tian, X.; Li, M.; Zeng, X. JAK inhibitors: Prospects in connective tissue diseases. Clin. Rev. Allergy Immunol. 2020, 59, 334–351. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef]
- Tanaka, Y.; Luo, Y.; O’Shea, J.J.; Nakayamada, S. Janus kinase-targeting therapies in rheumatology: A mechanisms-based approach. Nat. Rev. Rheumatol. 2022, 5, 1–3. [Google Scholar] [CrossRef]
- Markham, A. Baricitinib: First global approval. Drugs 2017, 77, 697–704. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Tanaka, Y. Baricitinib for the treatment of rheumatoid arthritis and systemic lupus erythematosus: A 2019 update. Expert Rev. Clin. Immunol. 2019, 15, 693–700. [Google Scholar] [CrossRef]
- Tanaka, Y. The JAK inhibitors: Do they bring a paradigm shift for the management of rheumatic diseases? Rheumatology 2019, 58, i1–i3. [Google Scholar] [CrossRef]
- Choy, E.H. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology 2019, 58, 953–962. [Google Scholar] [CrossRef]
- Gadina, M.; Johnson, C.; Schwartz, D.; Bonelli, M.; Hasni, S.; Kanno, Y.; Changelian, P.; Laurence, A.; O’Shea, J.J. Translational and clinical advances in JAK-STAT biology: The present and future of jakinibs. J. Leukoc. Biol. 2018, 104, 499–514. [Google Scholar] [CrossRef]
- Hammarén, H.M.; Virtanen, A.T.; Raivola, J.; Silvennoinen, O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine 2019, 118, 48–63. [Google Scholar] [CrossRef]
- Zarrin, A.A.; Bao, K.; Lupardus, P.; Vucic, D. Kinase inhibition in autoimmunity and inflammation. Nat. Rev. Drug Discov. 2021, 20, 39–63. [Google Scholar] [CrossRef]
- Alunno, A.; Padjen, I.; Fanouriakis, A.; Boumpas, D.T. Pathogenic and therapeutic relevance of JAK/STAT signaling in systemic lupus erythematosus: Integration of distinct inflammatory pathways and the prospect of their inhibition with an oral agent. Cells 2019, 8, 898. [Google Scholar] [CrossRef]
- Clark, J.D.; Flanagan, M.E.; Telliez, J.B. Discovery and development of Janus Kinase (JAK) inhibitors for inflammatory diseases: Miniperspective. J. Med. Chem. 2014, 57, 5023–5038. [Google Scholar] [CrossRef]
- Gómez-Arias, P.J.; Gómez-García, F.; Hernández-Parada, J.; Montilla-López, A.M.; Ruano, J.; Parra-Peralbo, E. Efficacy and Safety of Janus Kinase Inhibitors in Type I Interferon-Mediated Monogenic Autoinflammatory Disorders: A Scoping Review. Dermatol. Ther. 2021, 11, 733–750. [Google Scholar] [CrossRef]
- Lee-Kirsch, M.A. The type I interferonopathies. Annu. Rev. Med. 2017, 68, 297–315. [Google Scholar] [CrossRef]
- Frémond, M.L.; Crow, Y.J. Mendelian disorders of immunity related to an upregulation of type I interferon. In Stiehm’s Immune Deficiencies; Academic Press: Cambridge, MA, USA, 2020; pp. 751–772. [Google Scholar]
- Schwartz, D.M.; Bonelli, M.; Gadina, M.; O’shea, J.J. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 2016, 12, 25–36. [Google Scholar] [CrossRef]
- Baker, K.F.; Isaacs, J.D. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann. Rheum. Dis. 2018, 77, 175–187. [Google Scholar] [CrossRef]
- Hagberg, N.; Joelsson, M.; Leonard, D.; Reid, S.; Eloranta, M.L.; Mo, J.; Nilsson, M.K.; Syvänen, A.C.; Bryceson, Y.T.; Rönnblom, L. The STAT4 SLE risk allele rs7574865 [T] is associated with increased IL-12-induced IFN-γ production in T cells from patients with SLE. Ann. Rheum. Dis. 2018, 77, 1070–1077. [Google Scholar] [CrossRef]
- Petitdemange, A.; Blaess, J.; Sibilia, J.; Felten, R.; Arnaud, L. Shared development of targeted therapies among autoimmune and inflammatory diseases: A systematic repurposing analysis. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20969261. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Jesson, M.I.; Li, X.; Lee, J.L.; Ghosh, S.; Alsup, J.W.; Warner, J.D.; Tanaka, M.; Steward-Tharp, S.M.; Gadina, M. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J. Immunol. 2011, 186, 4234–4243. [Google Scholar] [CrossRef]
- Chasset, F.; Dayer, J.M.; Chizzolini, C. Type I interferons in systemic autoimmune diseases: Distinguishing between afferent and efferent functions for precision medicine and individualized treatment. Front. Pharmacol. 2021, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Klavdianou, K.; Lazarini, A.; Fanouriakis, A. Targeted biologic therapy for systemic lupus erythematosus: Emerging pathways and drug pipeline. BioDrugs 2020, 34, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Hasni, S.; Gupta, S.; Davis, M.A.; Poncio, E.; Temesgen-Oyelakin, Y.; Biehl, A.; Carlucci, P.; Wang, X.; Ochoa-Navas, I.; Manna, Z.G. 183 A phase 1B/2A trial of tofacitinib, an oral janus kinase inhibitor, in systemic lupus erythematosus. Lupus. Sci. Med. 2019, 6, A139–A140. [Google Scholar] [CrossRef]
- Safety of Tofacitinib, an Oral Janus Kinase Inhibitor, in Systemic Lupus Erythematosus. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02535689 (accessed on 22 August 2022).
- Hasni, S.A.; Gupta, S.; Davis, M.; Poncio, E.; Temesgen-Oyelakin, Y.; Carlucci, P.M.; Wang, X.; Naqi, M.; Playford, M.P.; Goel, R.R. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat. Commun. 2021, 12, 3391. [Google Scholar] [CrossRef]
- Furumoto, Y.; Smith, C.K.; Blanco, L.; Zhao, W.; Brooks, S.R.; Thacker, S.G.; Zarzour, A.; Sciumè, G.; Tsai, W.L.; Trier, A.M. Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheumatol. 2017, 69, 148–160. [Google Scholar] [CrossRef]
- Ikeda, K.; Hayakawa, K.; Fujishiro, M.; Kawasaki, M.; Hirai, T.; Tsushima, H.; Miyashita, T.; Suzuki, S.; Morimoto, S.; Tamura, N. JAK inhibitor has the amelioration effect in lupus-prone mice: The involvement of IFN signature gene downregulation. BMC Immunol. 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Ripoll, È.; de Ramon, L.; Draibe, J.; Merino, A.; Bolaños, N.; Goma, M.; Cruzado, J.M.; Grinyó, J.M.; Torras, J. JAK3-STAT pathway blocking benefits in experimental lupus nephritis. Arthritis Res. Ther. 2016, 18, 1–12. [Google Scholar]
- Chen, Y.L.; Liu, L.X.; Huang, Q.; Li, X.Y.; Hong, X.P.; Liu, D.Z. Case Report: Reversal of Long-Standing Refractory Diffuse Non-Scarring Alopecia Due to Systemic Lupus Erythematosus Following Treatment with Tofacitinib. Front. Immunol. 2021, 12, 654376. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, C.; Li, X.; Huang, Y.; Li, M.; Zhang, T.; Zhao, S.; Wang, S.; Zhang, H.; Yang, N. JAK/STAT signaling controls the fate of CD8+ CD103+ tissue-resident memory T cell in lupus nephritis. J. Autoimmun. 2020, 109, 102424. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, W.; Song, H.; Long, X.; Zhang, Z.; Tang, X.; Chen, H.; Lin, H.; Sun, L. Tofacitinib Ameliorates Lupus Through Suppression of T Cell Activation Mediated by TGF-Beta Type I Receptor. Front. Immunol. 2021, 12, 675542. [Google Scholar] [CrossRef]
- Garufi, C.; Mancuso, S.; Spinelli, F.R.; Truglia, S.; Ceccarelli, F.; Alessandri, C.; Conti, F. Janus kinases inhibitors for treating patients with rhupus. Jt. Bone Spine 2020, 87, 673–674. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef]
- You, H.; Zhang, G.; Wang, Q.; Zhang, S.; Zhao, J.; Tian, X.; Li, H.; Li, M.; Zeng, X. Successful treatment of arthritis and rash with tofacitinib in systemic lupus erythematosus: The experience from a single centre. Ann. Rheum. Dis. 2019, 78, 1441–1443. [Google Scholar] [CrossRef]
- Bonnardeaux, E.; Dutz, J.P. Oral tofacitinib citrate for recalcitrant cutaneous lupus. JAAD Case Rep. 2022, 20, 61–64. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yokoyama, Y.; Shimizu, Y.; Yajima, H.; Sakurai, N.; Suzuki, C.; Naishiro, Y.; Takahashi, H. Tofacitinib can decrease anti-DNA antibody titers in inactive systemic lupus erythematosus complicated by rheumatoid arthritis. Mod. Rheumatol. 2016, 26, 633–634. [Google Scholar] [CrossRef]
- König, N.; Fiehn, C.; Wolf, C.; Schuster, M.; Costa, E.C.; Tüngler, V.; Alvarez, H.A.; Chara, O.; Engel, K.; Goldbach-Mansky, R.; et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann. Rheum. Dis. 2017, 76, 468–472. [Google Scholar] [CrossRef]
- Open-label Study of Tofacitinib for Moderate to Severe Skin Involvement in Young Adults with Lupus. Available online: https://clinicaltrials.gov/ct2/show/NCT03288324 (accessed on 10 May 2022).
- Oral Tofacitinib in Adult Subjects with Discoid Lupus Erythematosus (DLE) and Systemic Lupus Erythematosus (SLE). Available online: https://clinicaltrials.gov/ct2/show/NCT03159936 (accessed on 20 August 2022).
- Chaichian, Y.; Strand, V. Interferon-directed therapies for the treatment of systemic lupus erythematosus: A critical update. Clin. Rheumatol. 2021, 40, 3027–3037. [Google Scholar] [CrossRef]
- Eli Lilly and Company, Incyte Corporation. European Commission Approves Once-Daily Olumiant Tablets for Treatment of Adults with Moderate-to-Severe Active Rheumatoid Arthritis [Media Release]; Incyte: Morges, Switzerland, 13 February 2017. [Google Scholar]
- Kerschbaumer, A.; Smolen, J.S.; Nash, P.; Doerner, T.; Dougados, M.; Fleischmann, R.; Geissler, K.; McInnes, I.B.; Takeuchi, T.; Trauner, M.; et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: A systematic literature research. RMD Open 2020, 6, e001374. [Google Scholar] [CrossRef]
- A Study of Baricitinib (LY3009104) in Participants with Systemic Lupus Erythematosus (SLE). Available online: https://clinicaltrials.gov/ct2/show/NCT02708095 (accessed on 20 August 2022).
- Marcu, D.T.M.; Arsenescu-Georgescu, C. Adverse Drug Reactions and Atrioventricular Conduction Disorders-A Female Gender Related Aproach. Intern. Med. 2021, 18, 15–29. [Google Scholar] [CrossRef]
- Dörner, T.; Tanaka, Y.; Petri, M.A.; Smolen, J.S.; Wallace, D.J.; Dow, E.R.; Higgs, R.E.; Rocha, G.; Crowe, B.; Benschop, R.J.; et al. Baricitinib-associated changes in global gene expression during a 24-week phase II clinical systemic lupus erythematosus trial implicates a mechanism of action through multiple immune-related pathways. Lupus Sci. Med. 2020, 7, e000424. [Google Scholar] [CrossRef]
- Maeshima, K.; Shibata, H. Efficacy of JAK 1/2 inhibition in the treatment of diffuse non-scarring alopecia due to systemic lupus erythematosus. Ann. Rheum. Dis. 2020, 79, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, M.; Coladonato, L.; Venerito, V.; Cacciapaglia, F.; Lopalco, G.; Iannone, F. Efficacy of baricitinib on refractory skin papulosquamous rash in a patient with systemic lupus erythematosus. Rheumatology 2020, 59, 1188. [Google Scholar] [CrossRef] [PubMed]
- Joos, L.; Vetterli, F.; Jaeger, T.; Cozzio, A.; von Kempis, J.; Rubbert-Roth, A. Treatment of refractory subacute cuataneous lupus erythematosus with baricitinib. Clin. Exp. Dermatol. 2022, 47, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Fetter, T.; Smith, P.; Guel, T.; Braegelmann, C.; Bieber, T.; Wenzel, J. Selective janus kinase 1 inhibition is a promising therapeutic approach for lupus erythematosus skin lesions. Front. Immunol. 2020, 11, 344. [Google Scholar] [CrossRef]
- Klaeschen, A.S.; Wolf, D.; Brossart, P.; Bieber, T.; Wenzel, J. JAK inhibitor ruxolitinib inhibits the expression of cytokines characteristic of cutaneous lupus erythematosus. Exp. Dermatol. 2017, 26, 728–730. [Google Scholar] [CrossRef]
- Srivastava, A.; Ståhle, M.; Pivarcsi, A.; Sonkoly, E. Tofacitinib represses the Janus kinase-signal transducer and activators of transcription signalling pathway in keratinocytes. Acta Derm. Venereol. 2018, 98, 772–775. [Google Scholar] [CrossRef]
- Kreuter, A.; Licciardi-Fernandez, M.J.; Burmann, S.N.; Paschos, A.; Michalowitz, A.L. Baricitinib for recalcitrant subacute cutaneous lupus erythematosus with concomitant frontal fibrosing alopecia. Clin. Exp. Dermatol. 2022, 47, 787–788. [Google Scholar] [CrossRef]
- Zimmermann, N.; Wolf, C.; Schwenke, R.; Lüth, A.; Schmidt, F.; Engel, K.; Lee-Kirsch, M.A.; Günther, C. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019, 55, 342–346. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.; Jang, S.G.; Hong, S.M.; Song, Y.S.; Kim, M.J.; Baek, S.; Park, S.H.; Kwok, S.K. Baricitinib Attenuates Autoimmune Phenotype and Podocyte Injury in a Murine Model of Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 704526. [Google Scholar] [CrossRef]
- Migliorini, A.; Angelotti, M.L.; Mulay, S.R.; Kulkarni, O.O.; Demleitner, J.; Dietrich, A.; Sagrinati, C.; Ballerini, L.; Peired, A.; Shankland, S.J.; et al. The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: Implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am. J. Pathol. 2013, 183, 431–440. [Google Scholar] [CrossRef]
- A Study of Baricitinib (LY3009104) in Participants with Systemic Lupus Erythematosus (BRAVE I). Available online: https://clinicaltrials.gov/ct2/show/NCT03616912 (accessed on 10 May 2022).
- A Study of Baricitinib in Participants with Systemic Lupus Erythematosus (BRAVE II). Available online: https://clinicaltrials.gov/ct2/show/NCT03616964 (accessed on 20 August 2022).
- A Study of Baricitinib in Participants with Systemic Lupus Erythematosus (SLE) (SLE-BRAVE-X). Available online: https://clinicaltrials.gov/ct2/show/NCT03843125 (accessed on 10 May 2022).
- Chan, E.S.; Herlitz, L.C.; Jabbari, A. Ruxolitinib attenuates cutaneous lupus development in a mouse lupus model. J. Investig. Dermatol. 2015, 135, 1912–1915. [Google Scholar] [CrossRef]
- Garcia-Melendo, C.; Cubiro, X.; Puig, L. Janus Kinase Inhibitors in Dermatology: Part 2: Applications in Psoriasis, Atopic Dermatitis, and Other Dermatoses. Actas Dermo-Sifiliográficas (Engl. Ed.) 2021, 112, 586–600. [Google Scholar] [CrossRef]
- Briand, C.; Frémond, M.L.; Bessis, D.; Carbasse, A.; Rice, G.I.; Bondet, V.; Duffy, D.; Chatenoud, L.; Blanche, S.; Crow, Y.J.; et al. Efficacy of JAK1/2 inhibition in the treatment of chilblain lupus due to TREX1 deficiency. Ann. Rheum. Dis. 2019, 78, 431–433. [Google Scholar] [CrossRef]
- de la Varga Martínez, R.; Rodríguez-Bayona, B.; Añez, G.A.; Medina Varo, F.; Pérez Venegas, J.J.; Brieva, J.A.; Rodríguez, C. Clinical relevance of circulating anti-ENA and anti-dsDNA secreting cells from SLE patients and their dependence on STAT-3 activation. Eur. J. Immunol. 2017, 47, 1211–1219. [Google Scholar] [CrossRef]
- Yung, S.; Yap, D.Y.; Chan, T.M. A review of advances in the understanding of lupus nephritis pathogenesis as a basis for emerging therapies. F1000Research 2020, 9, 905. [Google Scholar] [CrossRef]
- Kahl, L.; Patel, J.; Layton, M.; Binks, M.; Hicks, K.; Leon, G.; Hachulla, E.; Machado, D.; Staumont-Sallé, D.; Dickson, M.; et al. Safety, tolerability, efficacy and pharmacodynamics of the selective JAK1 inhibitor GSK2586184 in patients with systemic lupus erythematosus. Lupus 2016, 25, 1420–1430. [Google Scholar] [CrossRef]
- Van Vollenhoven, R.F.; Layton, M.; Kahl, L.; Schifano, L.; Hachulla, E.; Machado, D.; Staumont-Sallé, D.; Patel, J. DRESS syndrome and reversible liver function abnormalities in patients with systemic lupus erythematosus treated with the highly selective JAK-1 inhibitor GSK2586184. Lupus 2015, 24, 648–649. [Google Scholar] [CrossRef]
- An Adaptive Phase II Study to Evaluate the Efficacy, Pharmacodynamics, Safety and Tolerability of GSK2586184. Available online: https://clinicaltrials.gov/ct2/show/NCT01777256 (accessed on 20 August 2022).
- Presto, J.K.; Okon, L.G.; Feng, R.; Wallace, D.J.; Furie, R.; Fiorentino, D.; Werth, V.P. Computerized planimetry to assess clinical responsiveness in a phase II randomized trial of topical R333 for discoid lupus erythematosus. Br. J. Dermatol. 2018, 178, 1308–1314. [Google Scholar] [CrossRef]
- Safety and Efficacy of Topical R333 in Patients with Discoid Lupus Erythematosus (DLE) and Systemic Lupus Erythematosus (SLE) Lesions (SKINDLE). Available online: https://clinicaltrials.gov/ct2/show/NCT01597050 (accessed on 20 August 2022).
- A Dose-Ranging Study to Evaluate Efficacy and Safety of PF-06700841 in Systemic Lupus Erythematosus (SLE). Available online: https://clinicaltrials.gov/ct2/show/NCT03845517 (accessed on 15 July 2022).
- Study to Evaluate Safety and Efficacy of Filgotinib and Lanraplenib in Females with Moderately-to-Severely Active Cutaneous Lupus Erythematosus (CLE). Available online: https://clinicaltrials.gov/ct2/show/NCT03134222 (accessed on 20 August 2022).
- Study to Evaluate the Safety and Efficacy of Filgotinib and Lanraplenib in Adults with Lupus Membranous Nephropathy (LMN). Available online: https://www.clinicaltrials.gov/ct2/show/NCT03285711 (accessed on 20 August 2022).
- A Study to Investigate the Safety and Efficacy of Elsubrutinib and Upadacitinib Given Alone or in Combination in Participants with Moderately to Severely Active Systemic Lupus Erythematosus (SLE) (SLEek). Available online: https://clinicaltrials.gov/ct2/show/NCT03978520 (accessed on 20 August 2022).
- An Investigational Study to Evaluate the Safety and Effectiveness of BMS-986165 With Background Treatment in Participants with Lupus Nephritis. Available online: https://clinicaltrials.gov/ct2/show/NCT03943147 (accessed on 20 August 2022).
- An Investigational Study to Evaluate BMS-986165 in Participants with Systemic Lupus Erythematosus. Available online: https://clinicaltrials.gov/ct2/show/NCT03252587 (accessed on 20 August 2022).
- Long-Term Safety and Efficacy Study of Deucravacitinib in Participants with Systemic Lupus Erythematosus. Available online: https://clinicaltrials.gov/ct2/show/NCT03920267 (accessed on 20 August 2022).
- Werth, V.P.; Fleischmann, R.; Robern, M.; Touma, Z.; Tiamiyu, I.; Gurtovaya, O.; Pechonkina, A.; Mozaffarian, A.; Downie, B.; Matzkies, F.; et al. Filgotinib or lanraplenib in moderate to severe cutaneous lupus erythematosus: A phase 2, randomized, double-blind, placebo-controlled study. Rheumatol. (Oxf.) 2022, 61, 2413–2423. [Google Scholar] [CrossRef]
- Baker, M.; Chaichian, Y.; Genovese, M.; Derebail, V.; Rao, P.; Chatham, W.; Bubb, M.; Lim, S.; Hajian, H.; Gurtovaya, O.; et al. Phase II, randomised, double-blind, multicentre study evaluating the safety and efficacy of filgotinib and lanraplenib in patients with lupus membranous nephropathy. RMD Open 2020, 6, e001490. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).