Diversity of Extracellular Vesicles in Human Follicular Fluid: Morphological Analysis and Quantification
Abstract
1. Introduction
2. Results
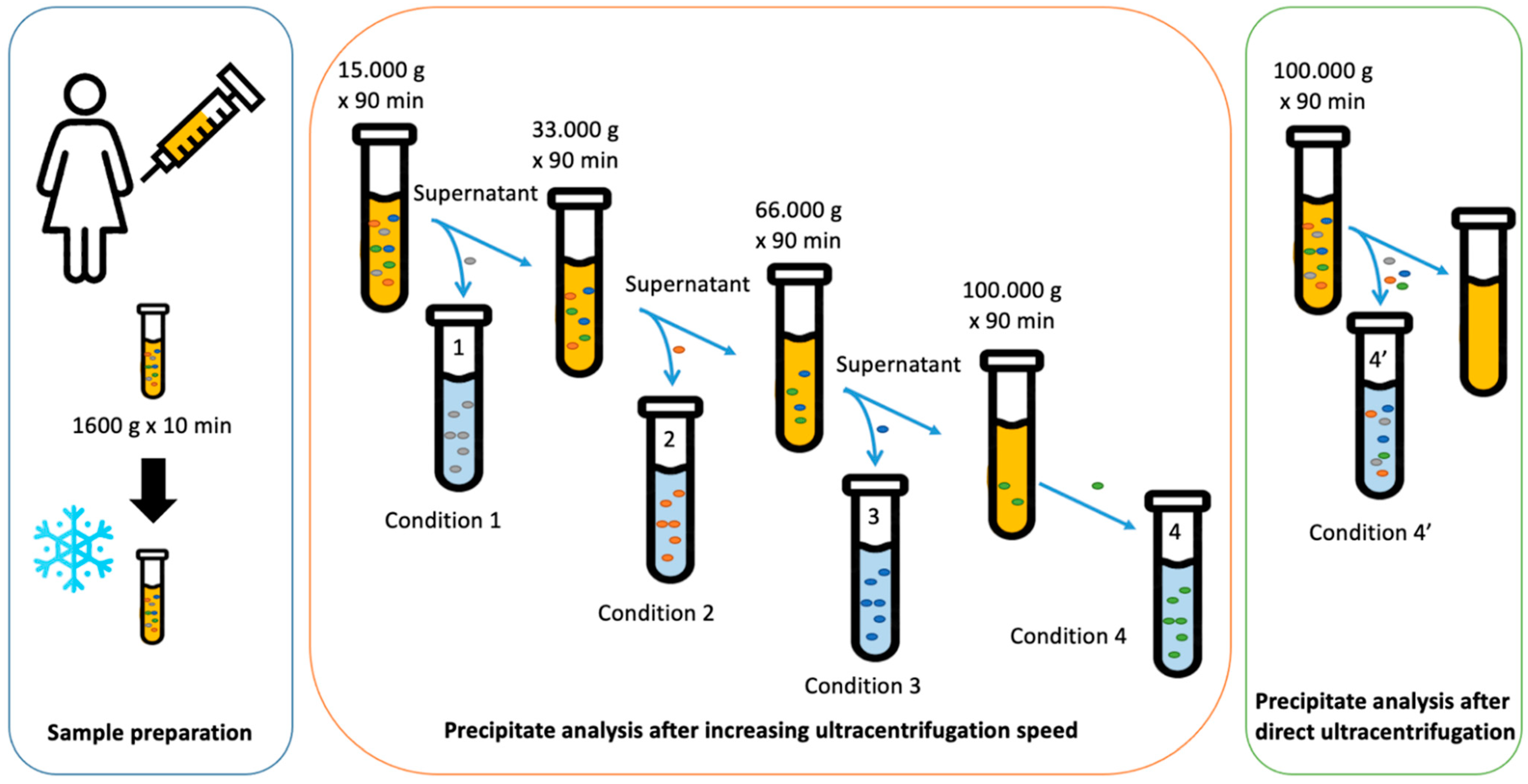
2.1. Size Separation of EVs by Sequential Centrifugation or Direct Ultracentrifugation
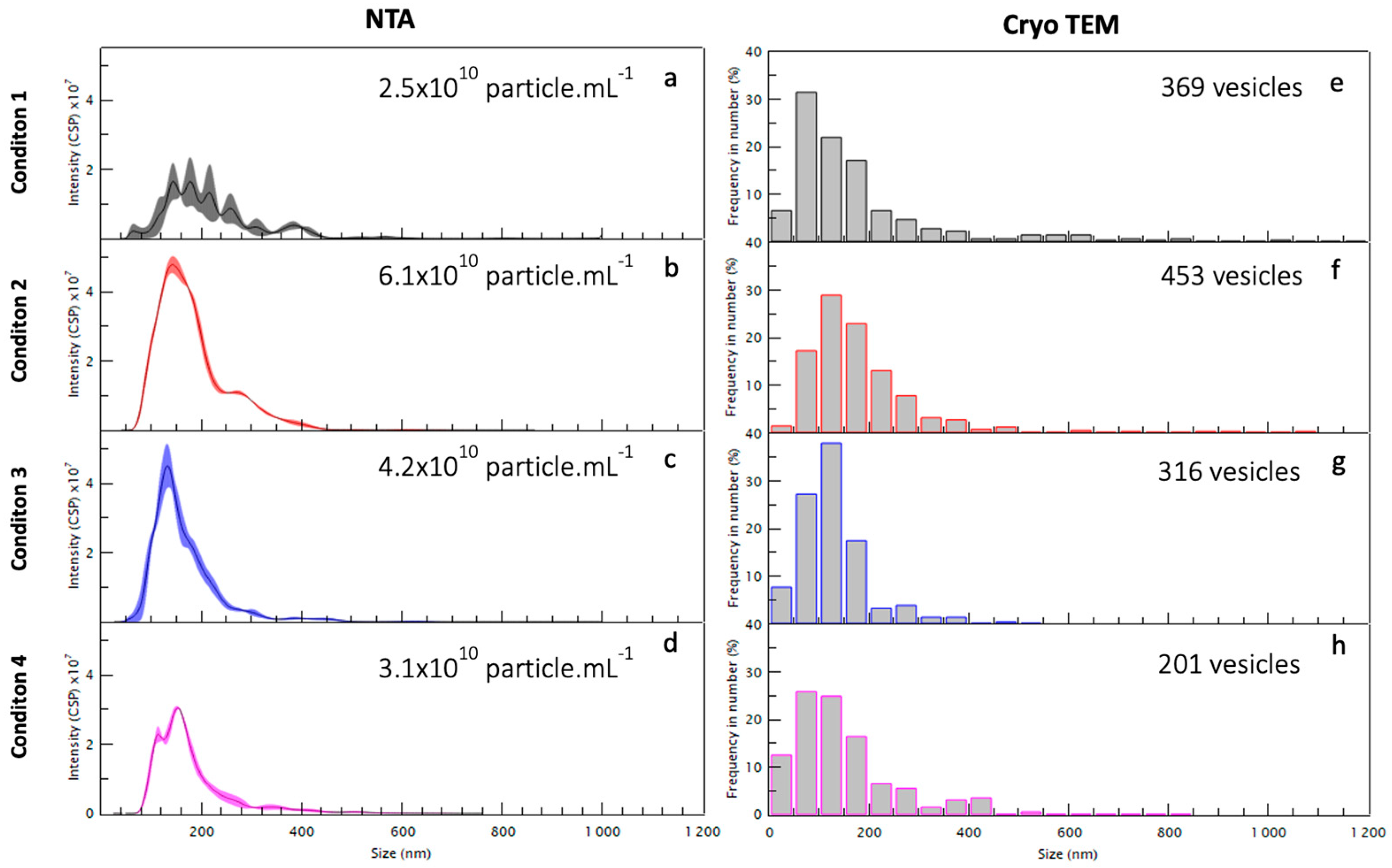
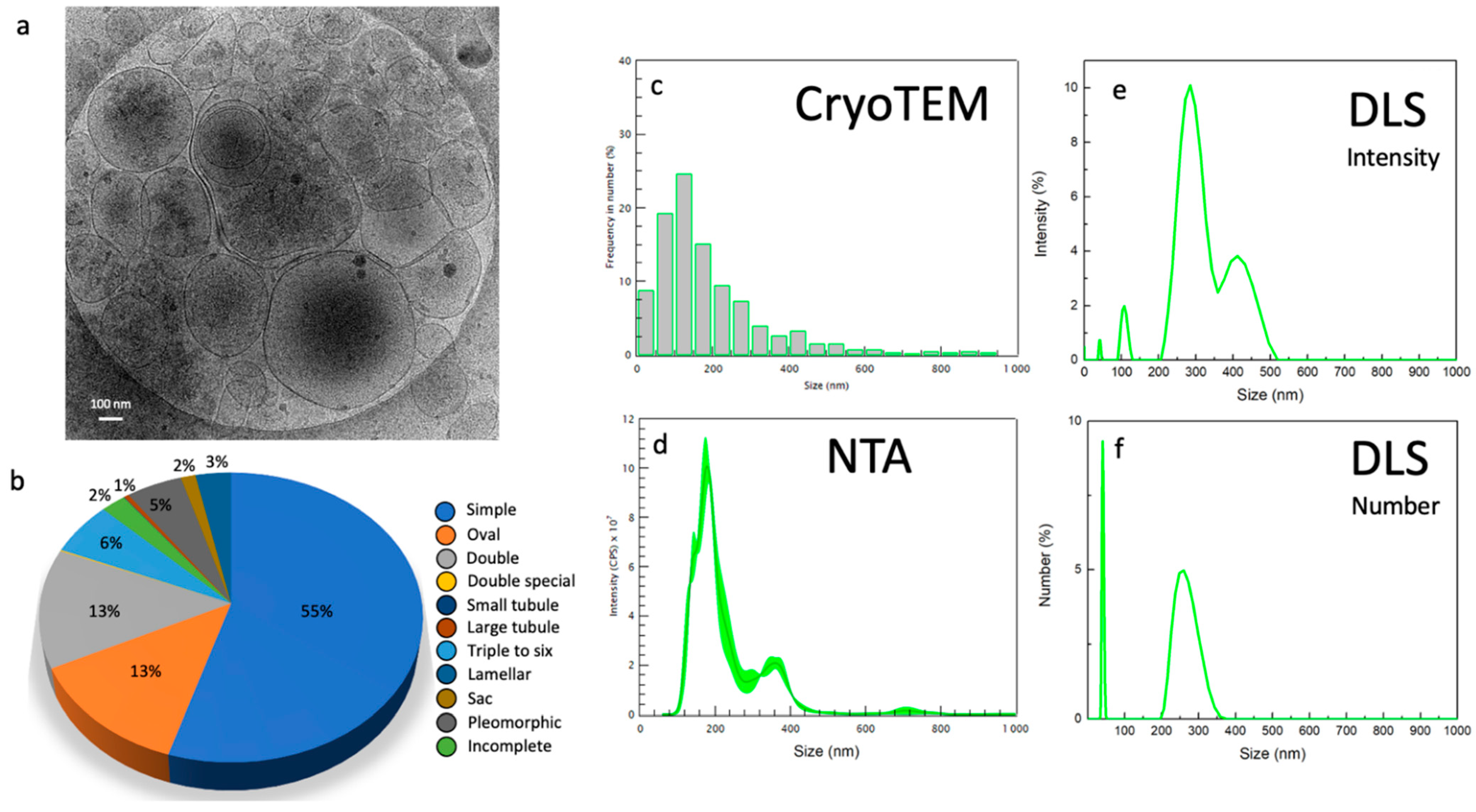
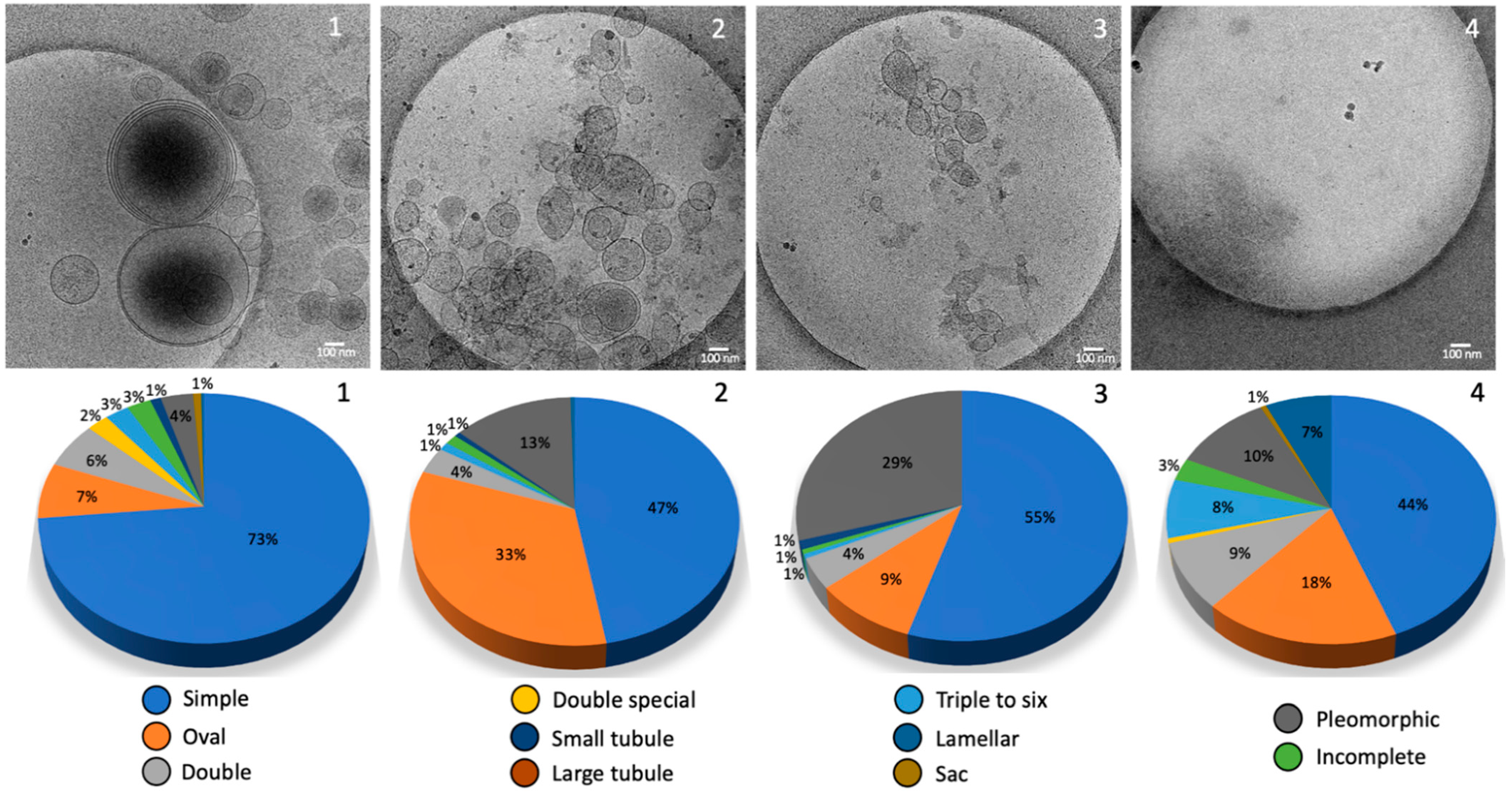
2.2. Morphology and Size Analysis of the EV Subpopulations
Different Subcategories of Extracellular Particles and Vesicles Found in Human FF
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Sample Preparation for Analysis
4.3. Ultracentrifugation
4.4. Cryo-Transmission Electron Microscopy (Cryo-TEM)
4.5. Dynamic Light Scattering (DLS)
4.6. Nanoparticle Tracking Analysis (NTA)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EVs | Extracellular Vesicles |
| Cryo-TEM | Cryo-Transmission Electron Microscopy |
| MVB | MultiVesicular Bodies |
| FF | Follicular Fluid |
| UC | Ultracentrifugation |
| NTA | Nanoparticle Tracking Analysis |
| DLS | Dynamic Light Scattering |
| PBS | Phosphate-Buffered Saline |
References
- Sun, S.; Pimentel, C.; Yefimova, M.; Jaillard, S.; Ravel, C. Neo-oogenesis in the adult ovary: What do we know? Gynecol. Obstet. Fertil. Senol. 2019, 47, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Yefimova, M.G.; Lefevre, C.; Bashamboo, A.; Eozenou, C.; Burel, A.; Lavault, M.T.; Meunier, A.C.; Pimentel, C.; Veau, S.; Neyroud, A.S.; et al. Granulosa Cells Provide Elimination of Apoptotic Oocytes through Unconventional Autophagy-Assisted Phagocytosis. Hum. Reprod. 2020, 35, 1346–1362. [Google Scholar] [CrossRef]
- Da Silveira, J.; Veeramachaneni, D.R.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-Secreted Vesicles in Equine Ovarian Follicular Fluid Contain MiRNAs and Proteins: A Possible New Form of Cell Communication within the Ovarian Follicle. Biol. Reprod. 2012, 86, 71. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-T.; Navakanitworakul, R.; Khan, T.; Zhang, P.; Davis, J.S.; McGinnis, L.; Christenson, L.K. Stage-Specific Follicular Extracellular Vesicle Uptake and Regulation of Bovine Granulosa Cell Proliferation. Biol. Reprod. 2017, 97, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Da Silveira, J.C.; Andrade, G.M.; del Collado, M.; Sampaio, R.; Sangalli, J.R.; Silva, L.A.; Pinaffi, F.V.L.; Jardim, I.B.; Cesar, M.C.; Nogueira, M.F.G.; et al. Supplementation with Small-Extracellular Vesicles from Ovarian Follicular Fluid during in Vitro Production Modulates Bovine Embryo Development. PLoS ONE 2017, 12, e0179451. [Google Scholar] [CrossRef]
- Navakanitworakul, R.; Hung, W.-T.; Gunewardena, S.; Davis, J.S.; Chotigeat, W.; Christenson, L.K. Characterization and Small RNA Content of Extracellular Vesicles in Follicular Fluid of Developing Bovine Antral Follicles. Sci. Rep. 2016, 6, 25486. [Google Scholar] [CrossRef]
- Rodosthenous, R.S.; Baccarelli, A.A.; Mansour, A.; Adir, M.; Israel, A.; Racowsky, C.; Hauser, R.; Bollati, V.; Machtinger, R. Supraphysiological Concentrations of Bisphenol A Alter the Expression of Extracellular Vesicle-Enriched miRNAs From Human Primary Granulosa Cells. Toxicol. Sci. 2019, 169, 5–13. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques Used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Gee, M.M.M. A Comparison of Methods for the Isolation and Separation of Extracellular Vesicles from Protein and Lipid Particles in Human Serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef]
- Hung, W.-T.; Hong, X.; Christenson, L.K.; McGinnis, L.K. Extracellular Vesicles from Bovine Follicular Fluid Support Cumulus Expansion1. Biol. Reprod. 2015, 93, 117. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, A.D.; Gilbert, I.; Caballero, J.; Barreto, R.; Fournier, E.; Tossou, P.; Sirard, M.-A.; Clarke, H.J.; Khandjian, W.; Richard, F.J.; et al. The Gametic Synapse: RNA Transfer to the Bovine Oocyte. Biol. Reprod. 2014, 91, 90. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.H.; Hoelker, M.; Noferesti, S.S.; Wondim, D.S.; Tholen, E.; Looft, C.; Rings, F.; Uddin, M.J.; Spencer, T.; Schellander, K.; et al. Exosomal and Non-Exosomal Transport of Extra-Cellular MicroRNAs in Follicular Fluid: Implications for Bovine Oocyte Developmental Competence. PLoS ONE 2013, 8, e78505. [Google Scholar] [CrossRef] [PubMed]
- Asaadi, A.; Dolatabad, N.A.; Atashi, H.; Raes, A.; Van Damme, P.; Hoelker, M.; Hendrix, A.; Pascottini, O.B.; Van Soom, A.; Kafi, M.; et al. Extracellular Vesicles from Follicular and Ampullary Fluid Isolated by Density Gradient Ultracentrifugation Improve Bovine Embryo Development and Quality. Int. J. Mol. Sci. 2021, 22, 578. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.; Morille, M.; Piffoux, M.; Arumugam, S.; Mauduit, P.; Larghero, J.; Bianchi, A.; Aubertin, K.; Blanc-Brude, O.; Noël, D.; et al. Development of Extracellular Vesicle-Based Medicinal Products: A Position Paper of the Group “Extracellular Vesicle TranslatiOn to ClinicaL PerspectiVEs-EVOLVE France”. Adv. Drug Deliv. Rev. 2021, 179, 114001. [Google Scholar] [CrossRef] [PubMed]
- Yefimova, M.; Bere, E.; Neyroud, A.; Jegou, B.; Bourmeyster, N.; Ravel, C. Myelinosome-like Vesicles in Human Seminal Plasma: A Cryo-Electron Microscopy Study. Cryobiology 2020, 92, 15–20. [Google Scholar] [CrossRef]
- Höög, J.L.; Lötvall, J. Diversity of Extracellular Vesicles in Human Ejaculates Revealed by Cryo-Electron Microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef] [PubMed]
- Kenigsberg, S.; Wyse, B.A.; Librach, C.L.; da Silveira, J.C. Protocol for Exosome Isolation from Small Volume of Ovarian Follicular Fluid: Evaluation of Ultracentrifugation and Commercial Kits. In Extracellular Vesicles; Kuo, W.P., Jia, S., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 1660; pp. 321–341. [Google Scholar] [CrossRef]
- Pavani, K.C.; Lin, X.; Hamacher, J.; Broeck, W.V.D.; Couck, L.; Peelman, L.; Hendrix, A.; Van Soom, A. The Separation and Characterization of Extracellular Vesicles from Medium Conditioned by Bovine Embryos. Int. J. Mol. Sci. 2020, 21, 2942. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.; Lazarev, V.N.; Kulemin, N.; Semina, S.E.; Generozov, E.; Govorun, V.M. Isolation of Exosomes by Differential Centrifugation: Theoretical Analysis of a Commonly Used Protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Keidel, A.; Bartsch, T.F.; Florin, E.-L. Direct Observation of Intermediate States in Model Membrane Fusion. Sci. Rep. 2016, 6, 23691. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Shah, S. Methods of Isolating Extracellular Vesicles Impact Down-Stream Analyses of Their Cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.-M.; Mornet, S.; Brisson, A.R. Extracellular Vesicles from Blood Plasma: Determination of Their Morphology, Size, Phenotype and Concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, A.-S.; Chiechio, R.; Yefimova, M.; Faro, M.J.L.; Dejucq-Rainsford, N.; Jaillard, S.; Even-Hernandez, P.; Marchi, V.; Ravel, C. Extra-Cellular Vesicles of the Male Genital Tract: New Actors in Male Fertility? Basic Clin. Androl. 2021, 31, 25. [Google Scholar] [CrossRef]
- Diaz-Rohrer, B.; Levental, K.R.; Levental, I. Rafting through Traffic: Membrane Domains in Cellular Logistics. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2014, 1838, 3003–3013. [Google Scholar] [CrossRef]
- Santonocito, M.; Vento, M.; Guglielmino, M.R.; Battaglia, R.; Wahlgren, J.; Ragusa, M.; Barbagallo, D.; Borzì, P.; Rizzari, S.; Maugeri, M.; et al. Molecular Characterization of Exosomes and Their MicroRNA Cargo in Human Follicular Fluid: Bioinformatic Analysis Reveals That Exosomal MicroRNAs Control Pathways Involved in Follicular Maturation. Fertil. Steril. 2014, 102, 1751–1761. [Google Scholar] [CrossRef]
- Qasemi, M.; Amidi, F. Extracellular microRNA profiling in human follicular fluid: New biomarkers in female reproductive potential. J. Assist. Reprod. Genet. 2020, 37, 1769–1780. [Google Scholar] [CrossRef]
- Rooda, I.; Hasan, M.M.; Roos, K.; Viil, J.; Andronowska, A.; Smolander, O.-P.; Jaakma, Ü.; Salumets, A.; Fazeli, A.; Velthut-Meikas, A. Cellular, Extracellular and Extracellular Vesicular MiRNA Profiles of Pre-Ovulatory Follicles Indicate Signaling Disturbances in Polycystic Ovaries. Int. J. Mol. Sci. 2020, 21, 9550. [Google Scholar] [CrossRef]
- Montjean, D.; Neyroud, A.-S.; Yefimova, M.G.; Benkhalifa, M.; Cabry, R.; Ravel, C. Impact of Endocrine Disruptors upon Non-Genetic Inheritance. Int. J. Mol. Sci. 2022, 23, 3350. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy Delivery Tools and Biomarkers of Diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- Dubochet, J.; Adrian, M.; Chang, J.-J.; Homo, J.-C.; Lepault, J.; McDowall, A.W.; Schultz, P. Cryo-Electron Microscopy of Vitrified Specimens. Q. Rev. Biophys. 1988, 21, 129–228. [Google Scholar] [CrossRef] [PubMed]



| Condition Centrifugation Speed | 1 15,000 g | 2 33,000 g | 3 66,000 g | 4 100,000 g | 4′ 100,000 g |
|---|---|---|---|---|---|
| NTA (particle·mL−1) mean size (nm) | 2.5 × 1010 | 6.1 × 1010 | 4.2 × 1010 | 3.1 × 1010 | 1.11 × 1011 |
| cryoTEM (particle number) | 369 | 453 | 316 | 201 | 744 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neyroud, A.-S.; Chiechio, R.M.; Moulin, G.; Ducarre, S.; Heichette, C.; Dupont, A.; Budzynski, M.; Even-Hernandez, P.; Faro, M.J.L.; Yefimova, M.; et al. Diversity of Extracellular Vesicles in Human Follicular Fluid: Morphological Analysis and Quantification. Int. J. Mol. Sci. 2022, 23, 11676. https://doi.org/10.3390/ijms231911676
Neyroud A-S, Chiechio RM, Moulin G, Ducarre S, Heichette C, Dupont A, Budzynski M, Even-Hernandez P, Faro MJL, Yefimova M, et al. Diversity of Extracellular Vesicles in Human Follicular Fluid: Morphological Analysis and Quantification. International Journal of Molecular Sciences. 2022; 23(19):11676. https://doi.org/10.3390/ijms231911676
Chicago/Turabian StyleNeyroud, Anne-Sophie, Regina Maria Chiechio, Gregory Moulin, Solène Ducarre, Claire Heichette, Aurélien Dupont, Mathieu Budzynski, Pascale Even-Hernandez, Maria Jose Lo Faro, Marina Yefimova, and et al. 2022. "Diversity of Extracellular Vesicles in Human Follicular Fluid: Morphological Analysis and Quantification" International Journal of Molecular Sciences 23, no. 19: 11676. https://doi.org/10.3390/ijms231911676
APA StyleNeyroud, A.-S., Chiechio, R. M., Moulin, G., Ducarre, S., Heichette, C., Dupont, A., Budzynski, M., Even-Hernandez, P., Faro, M. J. L., Yefimova, M., Marchi, V., & Ravel, C. (2022). Diversity of Extracellular Vesicles in Human Follicular Fluid: Morphological Analysis and Quantification. International Journal of Molecular Sciences, 23(19), 11676. https://doi.org/10.3390/ijms231911676









