Abstract
The oral cavity is one of the environments on the human body with the highest concentrations of microorganisms that coexist harmoniously and maintain homeostasis related to oral health. Several local factors can shift the microbiome to a pathogenic state of dysbiosis. Existing treatments for infections caused by changes in the oral cavity aim to control biofilm dysbiosis and restore microbial balance. Studies have used probiotics as treatments for oral diseases, due to their ability to reduce the pathogenicity of the microbiota and immunoinflammatory changes. This review investigates the role of the probiotic Bifidobacterium animalis subsp. lactis (B. lactis) HN019 in oral health, and its mechanism of action in pre-clinical and clinical studies. This probiotic strain is a lactic acid bacterium that is safe for human consumption. It mediates bacterial co-aggregation with pathogens and modulates the immune response. Studies using B. lactis HN019 in periodontitis and peri-implant mucositis have shown it to be a potential adjuvant treatment with beneficial microbiological and immunological effects. Studies evaluating its oral effects and mechanism of action show that this probiotic strain has the potential to be used in several dental applications because of its benefit to the host.
1. Introduction
Of all the microbiomes in the human body, the gut microbiome contains the greatest number of microbes [1]. In a state of health, the interactions of the microorganisms with epithelial cells, nerve cells, the immune system, and endocrine cells of the intestine mediate the homeostasis of this site [2,3,4]. The healthy gut microbiome has the ability to produce vitamins, absorb nutrients and ferment fibers, in addition to improving immunity and bringing energy to the body, thus demonstrating its fundamental role in the overall health of the individual [5].
The intestine is one of the main targets for the use of probiotics [3,4], defined as live microorganisms that, when administered in adequate amounts, improve the health of the host [6,7]. Thus, probiotics are used as preventive or therapeutic strategies in various conditions associated with disease, such as obesity, pre-diabetes and diabetes, gastrointestinal diseases, functional abdominal pain, atopic dermatitis, and for oral applications) [7,8,9,10,11,12,13,14].
Consumed for more than 1000 years by humans, probiotics are present in fermented products, such as cheeses, breads, yogurts, and non-fermented products, such as meats and fruits, as well as in industrialized supplements. The actions of probiotics are mediated primarily through their ability to inhibit pathogens through the production of bactericidal bioactive peptides, called bacteriocins, lactic acid, hydrogen peroxide, and bacteriocin-like inhibitory substances, or competing with pathogens for nutrients and binding sites, or killing pathogens [15,16,17]. In addition, probiotics improve epithelial barrier function by modulating signaling pathways and the local and systemic host immune-inflammatory response [18,19].
Lactic acid bacteria (LAB) are generally Gram-positive bacteria that are characterized by their ability to produce bacteriocins, alter the pH, and modulate the immunoinflammatory system [18,20,21,22]. LAB can be used as probiotics in clinical settings so long as there is adherence to strict selection guidelines [23]. One of the main actions of LAB is mediated by the effects of their products and by-products inside other bacterial cells [18,24]. Organic acids, such as acetic acid and lactic acid, produced by these bacteria are responsible for their inhibitory activity on pathogens. These acids enter bacterial cells and dissociate within the cytoplasm, leading to a decrease in pH and intracellular accumulation, which, in turn, kills the pathogenic bacteria. Another product of LAB are bacteriocins, which act against closely related bacteria and can lead to the destruction of target cells via pore formation and/or cell wall inhibition [18,25,26].
Bifidobacterium animalis subsp. lactis (B. lactis) HN019 is a LAB that was isolated from yogurt. Its complete genomic sequence was published in 2018, which allowed for strict control, quality, safety and purity of the B. lactis HN019 strain [3,27,28]. B. lactis HN019 has several gut health benefits, such as improving intestinal mobility and relieving constipation, providing defense against intestinal pathogens, and improving macronutrient absorption [3,29]. It also has benefits in regulating the immune response, such as modulating inflammatory and oxidative biomarkers in healthy subjects and also in patients with systemic diseases, including metabolic syndrome [30,31].
The therapeutic use of probiotics in healthcare and in dentistry has grown significantly in recent years [4,32,33,34,35,36]. The oral cavity contains the second largest microbiome in the human body [37,38,39]. It is colonized by a great diversity of microorganisms, which interact dynamically with each other or with other microbiota from other sites, such as the microbiota of the gut [39]. In a single day, an individual can swallow approximately 600 mL of saliva containing microorganisms from the mouth, which reach the intestine through the gastrointestinal tract [40]. Thus, it is believed that pathogenic bacteria originating in the oral cavity as a result of poor hygiene or alteration of the oral microbiota, will promote a disturbance in the intestinal microbiota, which may lead to inflammation and systemic changes. This highlights the importance of having a balanced and homeostatic microbiota that promotes the health of the patient [41,42]
From the 2000s, studies started emerging on the preventive and therapeutic effects of probiotics in the context of the oral cavity for the management of caries, periodontal diseases, halitosis and periapical lesions [35,43,44,45]. However, a focused review of the role of the probiotic B. lactis HN019 in oral health has not been presented. Thus, the objective of this literature review is to describe the mechanism of action and the effects of the probiotic B. lactis HN019 in the treatment of different oral diseases, drawing from preclinical and clinical studies.
2. Mechanism of Action of Bifidobacterium animalis subsp. lactis (B. lactis) HN019
There is a current focus on trying to understand the intricate mechanisms of action of bacteria on the host, for the purposes of discovering new therapeutic approaches and new materials for promoting health [4,27,46]. Although the mechanism of action of probiotics in oral health is not yet fully understood, it is known that an imbalance in the composition of the microbiota, known as dysbiosis, can cause changes to and negatively impact the oral cavity [47]; whereby probiotics might offer a solution. This dysbiosis can be caused by a loss of beneficial microorganisms and an increase in pathogens, as well as by the loss of microbial diversity [20,47]. In addition, this dysbiosis is associated with increased permeability and disruption of the epithelial barrier, leading to inflammation and chronic inflammatory pathologies [48]. However, it is not known whether the dysbiosis is a cause or a consequence of this change [20] (Figure 1).
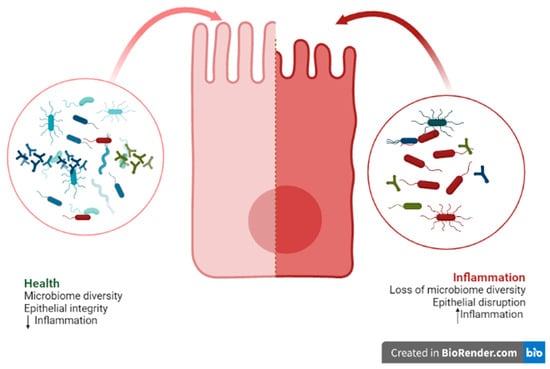
Figure 1.
Representative figure of an epithelial cell and its interactions with the microbiome.
The strain B. lactis HN019 has been documented to be part of human food consumption since 1980, but it is believed that it has been used for this application even before this date [49]. The safe and effective daily dose of this strain ranges from 107 to 1011 colony forming units (CFU) per day, and it can be consumed for at least 7 days and for up to 2 years [4]. It has an excellent capacity to adhere to epithelial cells, a high tolerance for and ability to survive in a low pH environment, resistance to bile salts [4,27,50,51], and ability to modulate the immune response [4,27].
Studies show that this strain acts on intercellular junctions, especially tight junctions [4,52]. Tight junctions are located in the most apical region of the cell, and are comprised of two proteins (claudin and occludin), which are responsible for establishing the epithelial barrier that prevents entry of macromolecules (lipids and proteins) [20,47,53]. These junctions are essential for regulating the permeability of the epithelium, which when altered promotes inflammation and, consequently, the development of disease [20,53]. B. lactis HN019 can increase the strength of these junctions by modulating transepithelial electrical resistance, thereby preventing increases in epithelial cell permeability, although these changes were not statistically significant in a cell-free supernatant assay [4,52].
There are multiple mechanisms that are involved in mediating probiotic effects in the gut-bone axis. Modifications to the epithelial barrier in the gastrointestinal tract may control bone health [36]. In addition to modulating and promoting intestinal health, hormones and immune cells present in the gastrointestinal tract act to maintain bone health by regulating the balance between resorption by osteoclasts and bone neoformation by osteoblasts. The epithelial barrier plays a key role in the absorption of components involved in bone mineralization, such as calcium, phosphorus and magnesium, and they produce endocrine cell factors that carry signals to bone cells, such as incretin and serotonin [14,20]. Therefore, due to these different mechanisms, the manipulation of the microbiota can assist in bone development [36]. The receptor activator of NF-kappaβ (RANK), receptor activator of NF-kappaβ ligand (RANKL) and osteoprotegerin (OPG) system responsible for osteoclastogenesis can be regulated by modifications in the microbiota [14]. These potential mechanisms may also be at play in the context of bone changes in periodontal disease, since B. lactis HN019 can decrease several bone loss parameters in experimental periodontitis in rats [54].
The mouth harbors a diverse and distinct microbiome, as a result of different hard non-shedding surfaces and shedding cellular surfaces [38,39]. Because the oral cavity interacts significantly with the external environment, its microbiome diversity is of extreme importance for the maintenance of both oral and systemic health [39]. The process of colonization of the oral cavity is dynamic, starting at birth and it continues until the completion of the permanent dentition [55,56]. When factors such as poor oral hygiene, diet and immunodeficiency alter the existing microbial balance of the microbiome, infectious diseases of a polymicrobial nature set in [35,57,58]. Microorganisms and toxins that destroy tissues are present together and organized into biofilms, which produce substances known as extracellular polysaccharides that assist in microbial aggregation and co-aggregation and adhesion to surfaces of the oral cavity: enamel, cementum and dental implant surfaces [35,59,60,61,62,63]. These biofilms can change depending on the disease entity and depending on their location, such that each altered biofilm is associated with each disease entity, including caries, periodontitis and endodontic infections [35,63]. In this context, B. lactis HN019 is currently being studied for the purpose of altering the dysbiosis of biofilms present in oral diseases, with beneficial results noted in periodontitis and peri-implant mucositis.
3. B. lactis HN019 and Periodontitis
Chronic periodontitis is a polymicrobial disease that is highly prevalent and is characterized by an inflammatory process that affects the supporting tissues of the teeth, but is also associated with other systemic changes/diseases when left untreated [64,65]. Conventional treatment for periodontal disease is often not effective in controlling inflammation, and many patients experience disease recurrence [64,65,66,67].
Cases of recurrent periodontitis are treated with adjuvant antibiotic therapy. However, the possibility of bacterial resistance has been considered a problem after the excessive use of these drugs, leading to the search for new agents to control infectious diseases [64,65,68].The development of therapeutic alternatives that can act as adjuvants to clinical treatment is essential;, thus, probiotics have emerged and have shown satisfactory and effective results [64,67,68,69,70].
Although probiotics are not yet used as alternative treatments in clinical practice, interest in their use for the treatment of periodontal diseases has grown worldwide [71]. Lactobacillus and Bifidobacterium strains have been highlighted for their ability to alter periodontal biofilms and modulate the immunoinflammatory response [70,71,72].Studies testing Bifidobacterium animalis found decreased biofilm virulence, decreased gingival inflammation, and decreased levels of pathogenic bacteria relevant to periodontal diseases [73,74,75,76].
Ricoldi et al. (2017) evaluated the oral administration of the probiotic B. lactis HN019 as an adjunctive treatment to scaling and root planing (SRP) in rats with experimental periodontitis. The animals treated with B. lactis HN019 and SRP showed reduced attachment loss and alveolar bone resorption, when compared with those treated with SRP alone. There were also lower numbers of osteoclasts and pro-inflammatory cytokines, and higher levels of anti-inflammatory cytokines in the probiotic group [68].In addition to being used as an adjuvant treatment, an increasing number of studies have demonstrated the use of subgingival probiotics, that is, as topical treatment in periodontal pockets [77]. Oliveira et al. (2017) evaluated the effects of topical administration of B. lactis HN019 in rats with experimental periodontitis. Histological and micro computed tomography analyses demonstrated that topical administration of this probiotic strain decreased bone resorption, thereby demonstrating a protective effect on the alveolar bone. In addition, there was modulation of the immunoinflammatory response and microbiological profile as assessed by cytokine analyses and microbiological assessment [57]. A study by Oliveira et al. (2022) demonstrated a useful vehicle for the delivery of B. lactis HN019, by showing that this probiotic delivered with milk and in the absence of mechanical treatment potentiated the effects of the probiotic therapy on periodontal lesions [78].
Other preclinical studies using the ligature-induced periodontitis model demonstrated the potential of B. lactis HN019 on systemic diseases associated with periodontitis [79]. Silva et al. (2022) evaluated the effects of this strain on the development of periodontitis associated with metabolic syndrome. The groups with experimental periodontitis +/− metabolic syndrome treated with the probiotic exhibited lower levels of alveolar bone loss compared to the groups not treated with the probiotic. Higher levels of interleukin (IL)-1β and RANKL/OPG were observed in the group with metabolic syndrome that did not receive the probiotic compared to the group which received the probiotic. Furthermore, the serum levels of total cholesterol and triglycerides of the animals with metabolic syndrome and treated with the probiotic were statistically lower than those not treated with the probiotic. Cardoso et al. (2020) analyzed the effects of systemic administration of B. lactis HN019 on ligature-induced periodontitis in rats with experimental rheumatoid arthritis. They showed that the probiotic group exhibited reduced bone loss, tumor necrosis factor-α and IL-6 levels, and increased IL-17 levels. Therefore, these studies demonstrated that B. lactis HN019 modulated immunoinflammation and systemic parameters, reducing the severity of periodontitis associated with systemic diseases [80,81].
Invernici et al. (2018), in a randomized clinical study, revealed that patients with generalized chronic periodontitis who used lozenges containing the probiotic B. lactis HN019 had better clinical results, with a 58% reduction in deep periodontal pockets in contrast with a 22% reduction in patients who used the placebo [82]. The microbiological analysis revealed that only the probiotic group exhibited a lower concentration of pathogenic microorganisms, in addition to having a lower concentration of pro-inflammatory cytokines when compared to the control group. Furthermore, Invernici et al. (2020) showed that improvements in the clinical parameters (gingival bleeding and plaque accumulation) were superior in the group of periodontitis patients that received B. lactis HN019 lozenges compared to the placebo group. An increase in the immunocompetence of the oral epithelial barrier was demonstrated in the patients treated with the probiotic, with increased expression of beta-defensin (BD)-3, toll-like receptor 4 (TLR4), and cluster of differentiation (CD)-4 [83]. Table 1 summarizes the vehicles, dosages and results of studies of this probiotic strain in periodontitis.

Table 1.
Studies using the B. lactis HN019 strain in the context of periodontitis.
In the context of periodontitis, B. lactis HN019 is being studied intensively, showing effective and promising results [54,68,78,79,80,81,82,83], and requiring further clinical studies to demonstrate its full clinical utility for routine care (Figure 2).
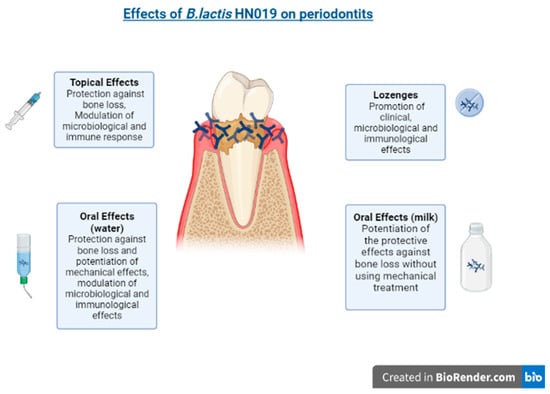
Figure 2.
The effects of B. lactis HN19 on periodontitis.
The effects of probiotics on bone led to the investigation of their use in orthodontic tooth movement. Duffles et al. (2022) investigated the impact of B. lactis HN019 on bone remodeling induced by orthodontic tooth movement in mice. Although microtomography analysis showed that probiotic treatment did not modify the alveolar bone, the therapy did restrain the tooth movement. Groups treated with the probiotic had a decreased number of osteoclast cells and a higher concentration of short-chain fatty acid in their feces [84].
4. B. lactis HN019 and Peri-Implant Mucositis
Currently, the use of dental implants in oral rehabilitation is increasing in popularity, because of their ability to address aesthetic and functional needs and due to their durability and high success rates [85]. However, there are frequent complications with dental implants, such as peri-implant mucositis and peri-implantitis [86,87]. Healthy peri-implant mucosa is composed of keratinized epithelium and its basal lamina faces the surface of the dental implant or abutment [86,88]. When there is an accumulation of biofilm at the abutment-mucosal interface, an inflammatory lesion may develop. However, it is a reversible change, without radiographic bone loss, thus differentiating it from peri-implantitis [86,89].
Conventional non-surgical mechanical therapy is still a standard treatment used to treat peri-implant diseases [90]. However, since this treatment does not completely eliminate the bacterial biofilm, several biological and chemical agents and methods have been used as alternative or adjuvant treatments, such as antibiotics, laser therapy, chlorhexidine, enamel matrix derivatives, and probiotics [90,91].
Several probiotic strains have been used to treat peri-implant diseases [91]. In a randomized clinical trial, B. lactis HN019 was tested along with two other probiotic strains (L. rhamnosus HN001 and L. paracasei Lpc-37) as adjuvants to mechanical debridement in patients presenting with peri-implant mucositis. The probiotics were administered topically as well as ingested by the patients. After 24 weeks, the group using the probiotics showed no bleeding in 72.2% of the patients, demonstrating that there was a better clinical effect with the probiotics than the control group, which received debridement alone. In addition to the clinical benefits, a lower concentration of pro-inflammatory cytokines was also observed [92]. However, this was the first study using this probiotic strain in a dental implant setting. Thus, further preclinical and clinical studies are needed to validate these results and to confirm the beneficial role of B. lactis HN019 in addressing peri-implant mucositis.
5. Conclusions
The probiotic strain B.lactis HN019 has been shown to play a role in modulating the immunoinflammatory response of the human organism, including promoting oral health benefits, when administered in adequate doses. Furthermore, the use of this strain in periodontitis is supported by data from pre-clinical studies and clinical trials. However, due to the significant potential of this strain to maintain oral health, further studies on several oral diseases are needed, to expand the current findings and to determine the application of B. lactis HN019 in other oral diseases, like peri-implant mucositis.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, roles and dynamics in the expanded human microbiome design. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Laitila, A.; Ouwehand, A.C. Bifidobacterium animalis subsp. lactis HN019 Effects on Gut Health: A Review. Front. Nutr. 2021, 8, 790561. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. The gut microbiota and gut disease. Intern. Med. J. 2021, 51, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Stamatova, I. Probiotics: Contributions to oral health. Oral. Dis. 2007, 13, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Stamatova, I.V. Probiotics: Evidence of Oral Health Implications. Folia Med. 2018, 60, 21–29. [Google Scholar] [CrossRef]
- Ritchie, M.L.; Romanuk, T.N. A Meta-Analysis of Probiotic Efficacy for Gastrointestinal Diseases. PLoS ONE 2012, 7, e34938. [Google Scholar] [CrossRef]
- Nole, K.L.B.; Yim, E.; Keri, J.E. Probiotics and prebiotics in dermatology. J. Am. Acad. Dermatol. 2014, 71, 814–821. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R.; Recto, M.S.T.; Castor, M.A.R.; Casis-Hao, R.J.; Nano, A.L.M. Comparative effectiveness of probiotic strains on the prevention of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr. Allergy Immunol. 2021, 32, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Trivić, I.; Niseteo, T.; Jadrešin, O.; Hojsak, I. Use of probiotics in the treatment of functional abdominal pain in children—systematic review and meta-analysis. Eur. J. Pediatr. 2020, 180, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Qiu, X.; Wen, Q.; Liu, M.; Zhou, D.; Chen, Q. Probiotics, Pre-biotics and Synbiotics in the Treatment of Pre-diabetes: A Systematic Review of Randomized Controlled Trials. Front. Public Health 2021, 9, 645035. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; de Sire, R.; Curci, C.; Castiglione, F.; Wahli, W. Role of Dietary Supplements and Probiotics in Modulating Microbiota and Bone Health: The Gut-Bone Axis. Cells 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Gillor, O.; Etzion, A.; Riley, M.A. The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 2008, 81, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M. The potential of bacteriocin-producing probiotics and associated caveats. Future Microbiol. 2009, 4, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; De Foy, J.-M.P.; Dequenne, I.; De Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef]
- Martens, K.; Pugin, B.; De Boeck, I.; Spacova, I.; Steelant, B.; Seys, S.F.; Lebeer, S.; Hellings, P.W. Probiotics for the airways: Potential to improve epithelial and immune homeostasis. Allergy 2018, 73, 1954–1963. [Google Scholar] [CrossRef]
- Essayas, A.; Pandit, S. Anti-microbial activity of potential probiotic lactic acid bacteria against methicillin-resistant Staphylococcus aureus (MRSA). Ann. Rom. Soc. Cell Biol. 2021, 25, 7772–7785. [Google Scholar]
- Yaacob, S.N.; Wahab, R.A.; Misson, M.; Sabullah, M.K.; Huyop, F.; Zin, N.M. Lactic acid bacteria and their bacteriocins: New potential weapons in the fight against methicillin-resistant Staphylococcus aureus. Future Microbiol. 2022, 17, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Skytta, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [PubMed]
- Makras, L.; Triantafyllou, V.; Fayol-Messaoudi, D.; Adriany, T.; Zoumpopoulou, G.; Tsakalidou, E.; Servin, A.; DeVuyst, L. Análise cinética da atividade antibacteriana de lactobacilos probióticos contra Salmonella enterica sorovar typhimurium revela um papel para o ácido lático e outros compostos inibitórios. Res. Microbiol. 2006, 157, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.S.; Cho, G.S.; Hanak, A.; Huch, M.; Franz, C.M.; Arneborg, N. The effect of bacteriocin-producing Lactobacillus plantarum strains on the intracellular pH of sessile and planktonic Listeria monocytogenes single cells. Int. J. Food Microbiol. 2010, 141, S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E. Summary of Probiotic Activities of Bifidobacterium lactis HN019. J. Clin. Gastroenterol. 2006, 40, 776–783. [Google Scholar] [CrossRef]
- Morovic, W.; Roos, P.; Zabel, B.; Hidalgo-Cantabrana, C.; Kiefer, A.; Barrangou, R. Transcriptional and Functional Analysis of Bifidobacterium animalis subsp. lactis Exposure to Tetracycline. Appl. Environ. Microbiol. 2018, 84, e01999-18. [Google Scholar] [CrossRef]
- Dalziel, J.E.; Anderson, R.; Peters, J.S.; Lynch, A.T.; Spencer, N.; Dekker, J.; Roy, N.C. Promotility Action of the Probiotic Bifidobacterium lactis HN019 Extract Compared with Prucalopride in Isolated Rat Large Intestine. Front. Neurosci. 2017, 11, 20. [Google Scholar] [CrossRef]
- Gill, H.S.; Rutherfurd, K.J.; Prasad, J.; Gopal, P.K. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br. J. Nutr. 2000, 83, 167–176. [Google Scholar] [CrossRef]
- Bernini, L.J.; Simão, A.N.C.; de Souza, C.H.B.; Alfieri, D.F.; Segura, L.G.; Costa, G.N.; Dichi, I. Effect of Bifidobacterium lactis HN019 on inflammatory markers and oxidative stress in subjects with and without the metabolic syndrome. Br. J. Nutr. 2018, 120, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Ahola, A.J.; Yli-Knuuttila, H.; Suomalainen, T.; Poussa, T.; Ahlström, A.; Meurman, J.H.; Korpela, R. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch. Oral Biol. 2002, 47, 799–804. [Google Scholar] [CrossRef]
- Keller, M.K.; Hasslöf, P.; Stecksén-Blicks, C.; Twetman, S. Co-aggregation and growth inhibition of probiotic lactobacilli and clinical isolates of mutans streptococci: An in vitro study. Acta Odontol. Scand. 2011, 5, 263–268. [Google Scholar] [CrossRef]
- Mahasneh, S.A.; Mahasneh, A.D. Probiotics: A Promising Role in Dental Health. Dent. J. 2017, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Seminario-Amez, M.; Lopez-Lopez, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jane-Salas, E. Probiotics and oral health: A systematic review. Med. Oral. Patol. Oral. Cir. Bucal 2017, 22, e282–e288. [Google Scholar] [CrossRef] [PubMed]
- McCabe, L.R.; Parameswaran, N. Advances in Probiotic Regulation of Bone and Mineral Metabolism. Calcif. Tissue Res. 2018, 102, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Hoare, A.; Hong, B.-Y. Subgingival Microbiome Shifts and Community Dynamics in Periodontal Diseases. J. Calif. Dent. Assoc. 2016, 44, 421–435. [Google Scholar]
- Rosier, B.; Marsh, P.; Mira, A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J. Dent. Res. 2017, 97, 371–380. [Google Scholar] [CrossRef]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef]
- Dawes, C. Padrões de fluxo salivar e a saúde dos tecidos orais duros e moles. J. Am. Dent. Assoc. 2008, 139, 18S–24S. [Google Scholar] [CrossRef]
- Iwauchi, M.; Horigome, A.; Ishikawa, K.; Mikuni, A.; Nakano, M.; Xiao, J.; Odamaki, T.; Hironaka, S. Relationship between oral and gut microbiota in elderly people. Immun. Inflamm. Dis. 2019, 7, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.A.; Hoffman, K.; Gurwara, S.; White, D.L.; Kanwal, F.; El-Serag, H.B.; Petrosino, J.F.; Jiao, L. Oral Health and the Altered Colonic Mucosa-Associated Gut Microbiota. Am. J. Dig. Dis. 2020, 66, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Essche, M.; Sliepen, I.; Quirynen, M. Probiotics and oral healthcare. Periodontology 2000 2008, 48, 111–147. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Loozen, G.; Quirynen, M. Do probiotics offer opportunities to manipulate the periodontal oral microbiota? J. Clin. Periodontol. 2011, 38 (Suppl. S11), 159–177. [Google Scholar] [CrossRef] [PubMed]
- Näse, L.; Hatakka, K.; Savilahti, E.; Saxelin, M.; Pönkä, A.; Poussa, T.; Korpela, R.; Meurman, J.H. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus, GG, in milk on dental caries and caries risk in children. Caries Res. 2001, 35, 412–420. [Google Scholar] [CrossRef]
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestin. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Steelant, B.; Farré, R.; Wawrzyniak, P.; Belmans, J.; Dekimpe, E.; Vanheel, H.; Van Gerven, L.; Krohn, I.K.; Bullens, D.M.; Ceuppens, J.L.; et al. Impaired barrier function in patients with house dust mite–induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J. Allergy Clin. Immunol. 2016, 137, 1043–1053.e5. [Google Scholar] [CrossRef]
- Bourdichon, F.; Alper, I.; Bibiloni, R.; Dubois, A.; Laulund, S.; Miks, M.; Morelli, L.; Zuliani, V.; Yao, S. Inventory of microbial food cultures with safety demonstration in fermented food products. Bull. Int. Dairy Federat. 2018, 495, 5–71. [Google Scholar]
- Prasad, J.; Gill, H.; Smart, J.; Gopal, P.K. Selection and Characterisation of Lactobacillus and Bifidobacterium Strains for Use as Probiotics. Int. Dairy J. 1998, 8, 993–1002. [Google Scholar] [CrossRef]
- Gopal, P.K.; Prasad, J.; Smart, J.; Gill, H.S. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 2001, 67, 207–216. [Google Scholar] [CrossRef]
- Putaala, H.; Salusjärvi, T.; Nordström, M.; Saarinen, M.; Ouwehand, A.C.; Hansen, E.B.; Rautonen, N. Effect of four probiotic strains and Escherichia coli O157:H7 on tight junction integrity and cyclo-oxygenase expression. Res. Microbiol. 2008, 159, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight Junction Pore and Leak Pathways: A Dynamic Duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.F.; Salvador, S.L.; Silva, P.H.; Furlaneto, F.A.; Figueiredo, L.; Casarin, R.; Ervolino, E.; Palioto, D.B.; Souza, S.L.; Taba, M., Jr.; et al. Benefits of Bifidobacterium animalis subsp. lactis Probiotic in Experimental Periodontitis. J. Periodontol. 2017, 88, 197–208. [Google Scholar]
- Mason, M.R.; Chambers, S.; Dabdoub, S.M.; Thikkurissy, S.; Kumar, P.S. Characterizing oral microbial communities across dentition states and colonization niches. Microbiome 2018, 6, 67. [Google Scholar] [CrossRef]
- Brookes, Z.L.; Belfield, L.A.; Ashworth, A.; Casas-Agustench, P.; Raja, M.; Pollard, A.J.; Bescos, R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021, 113, 103768. [Google Scholar] [CrossRef]
- Hasslöf, P.; West, C.E.; Videhult, F.K.; Brandelius, C.; Stecksén-Blicks, C. Early Intervention with Probiotic Lactobacillus paracasei F19 Has No Long-Term Effect on Caries Experience. Caries Res. 2013, 47, 559–565. [Google Scholar] [CrossRef]
- Laleman, I.; Detailleur, V.; Slot, D.E.; Slomka, V.; Quirynen, M.; Teughels, W. Probiotics reduce mutans streptococci counts in humans: A systematic review and meta-analysis. Clin. Oral Investig. 2014, 18, 1539–1552. [Google Scholar] [CrossRef]
- Zijnge, V.; van Leeuwen, M.B.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmür, R. Oral biofilm architecture on natural teeth. PLoS ONE 2010, 5, e9321. [Google Scholar] [CrossRef]
- Faran Ali, S.M.; Tanwir, F. Oral microbial habitat a dynamic entity. J. Oral Biol. Craniofac. Res. 2012, 2, 181–187. [Google Scholar] [CrossRef]
- Devine, D.A.; Marsh, P.D.; Meade, J. Modulation of host responses by oral commensal bacteria. J. Oral Microbiol. 2015, 7, 26941. [Google Scholar] [CrossRef] [PubMed]
- Piwat, S.; Sophatha, B.; Teanpaisan, R. An assessment of adhesion, aggregation and surface charges of Lactobacillus strains derived from the human oral cavity. Lett. Appl. Microbiol. 2015, 61, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Eick, S. Biofilms. Monogr. Oral Sci. 2021, 29, 1–11. [Google Scholar] [PubMed]
- Morales, A.; Gandolfo, A.; Bravo, J.; Carvajal, P.; Silva, N.; Godoy, C.; Garcia-Sesnich, J.; Hoare, A.; Diaz, P.; Gamonal, J. Microbiological and clinical effects of probiotics and antibiotics on nonsurgical treatment of chronic periodontitis: A randomized placebocontrolled trial with 9-month follow-up. J. Appl. Oral Sci. 2018, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leow, N.M.; Moreno, F.; Marletta, D.; Hussain, S.B.; Buti, J.; Almond, N.; Needleman, I. Recurrence and progression of periodontitis and methods of management in long-term care: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 49, 291–313. [Google Scholar] [CrossRef]
- Vivekananda, M.; Vandana, K.; Bhat, K. Effect of the probioticLactobacilli reuteri(Prodentis) in the management of periodontal disease: A preliminary randomized clinical trial. J. Oral Microbiol. 2010, 2, 5344. [Google Scholar] [CrossRef]
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G.; et al. Porphyromonas gingivalis Manipulates Complement and TLR Signaling to Uncouple Bacterial Clearance from Inflammation and Promote Dysbiosis. Cell Host Microbe 2014, 15, 768–778. [Google Scholar] [CrossRef]
- Ricoldi, M.S.T.; Furlaneto, F.; Oliveira, L.F.F.; Teixeira, G.C.; Pischiotini, J.P.; Moreira, A.L.G.; Ervolino, E.; De Oliveira, M.N.; Bogsan, C.S.B.; Salvador, S.L.; et al. Effects of the probiotic Bifidobacterium animalis subsp. lactis on the non-surgical treatment of periodontitis. A histomorphometric, microtomographic and immunohistochemical study in rats. PLoS ONE 2017, 12, e0179946. [Google Scholar] [CrossRef]
- Ruiz Núñez, M.D.R.; da Luz Raulino, M.; Goulart Castro, R.; Schaefer Ferreira de Mello, A.L. Dental plaque control strategies for the elderly population: A scoping review. Int. J. Dent. Hyg. 2022, 20, 167–181. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, W.; Ma, J.; Zhang, M.; Lu, X.; Liu, J.; Kou, Y. The rationale and potential for using Lactobacillus in the management of periodontitis. J. Microbiol. 2022, 60, 355–363. [Google Scholar] [CrossRef]
- Haas, A.N.; Furlaneto, F.; Gaio, E.J.; Gomes, S.C.; Palioto, D.B.; Castilho, R.M.; Sanz, M.; Messora, M.R. New tendencies in non-surgical periodontal therapy. Br. Oral Res. 2021, 35 (Suppl. S2), e095. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Fang, B.; Wuri, G.; Zhao, L.; Liu, F.; Zhang, M. Metagenomic Analysis Reveals a Mitigating Role for Lactobacillus paracasei and Bifidobacterium animalis in Experimental Periodontitis. Nutrients 2022, 14, 2125. [Google Scholar] [CrossRef] [PubMed]
- Toiviainen, A.; Jalasvuori, H.; Lahti, E.; Gursoy, U.; Salminen, S.; Fontana, M.; Flannagan, S.; Eckert, G.; Kokaras, A.; Paster, B.; et al. Impact of orally administered lozenges with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 on the number of salivary mutans streptococci, amount of plaque, gingival inflammation and the oral microbiome in healthy adults. Clin. Oral Investig. 2014, 19, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kuru, B.E.; Laleman, I.; Yalnızoğlu, T.; Kuru, L.; Teughels, W. The Influence of a Bifidobacterium animalis Probiotic on Gingival Health: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Alanzi, A.; Honkala, S.; Honkala, E.; Varghese, A.; Tolvanen, M.; Soderling, E. Effect of Lactobacillus rhamnosus and Bifidobacterium lactis on gingival health, dental plaque, and periodontopathogens in adolescents: A randomised placebo-controlled clinical trial. Benef. Microbes 2018, 9, 593–602. [Google Scholar] [CrossRef]
- Jäsberg, H.; Tervahartiala, T.; Sorsa, T.; Söderling, E.; Haukioja, A. Probiotic intervention influences the salivary levels of Matrix Metalloproteinase (MMP)-9 and Tissue Inhibitor of metalloproteinases (TIMP)-1 in healthy adults. Arch. Oral Biol. 2018, 85, 58–63. [Google Scholar] [CrossRef]
- Nguyen, T.; Brody, H.; Radaic, A.; Kapila, Y. Probiotics for periodontal health—Current molecular findings. Periodontology 2000 2021, 87, 254–267. [Google Scholar] [CrossRef]
- Oliveira, L.F.F.; da Silva, G.A.; Silva, P.H.F.; Moreira, A.L.G.; Salvador, S.L.; da Cruz, A.G.; Messora, M.R.; Furlaneto, F.A.C. Comparison Between Different Delivery Vehicles for the Probiotic Bifidobacterium animalis subsp. lactis HN019 on Experimental Periodontitis in Rats. Probiotics Antimicrob. Proteins 2022, 14, 313–325. [Google Scholar] [CrossRef]
- De Oliveira, L.F.F.; Silva, P.H.F.; Salvador, S.L.; Ervolino, E.; Casarin, R.; Figueiredo, L.; Ricoldi, M.T.; de Souza, S.L.S.; Furlaneto, F.; Messora, M.R. Probiotic consumption can modify the resilience of periodontal tissues in rats under experimental periodontitis. J. Periodontol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Cardoso, R.S.; Messora, M.R.; Silva, P.H.F.; Oliveira, L.F.; Leite-Panissi, C.; Salvador, S.; Casarin, R.; Novaes, A.B., Jr.; Palioto, D.B.; Furlaneto, F.A.C. Effects of Bifidobacterium animalis subsp. lactis HN019 on ligature-induced periodontitis in rats with experimental rheumatoid arthritis. Benef. Microbes 2020, 11, 33–46. [Google Scholar] [CrossRef]
- Silva, G.A.; Moreira, A.L.G.; Silva, P.H.F.; Salvador, S.L.; Casarin, R.C.V.; Vicente, R.M.; Ferreira, G.C.; Tanus-Santos, J.E.; Furlaneto, F.A.C.; Messora, M.R. The use of probiotics can reduce the severity of experimental periodontitis in rats with metabolic syndrome: An immunoenzymatic and microtomographic study. J. Periodontol. 2021, 93, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; Novaes, A.B., Jr.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Invernici, M.M.; Furlaneto, F.A.C.; Salvador, S.L.; Ouwehand, A.C.; Salminen, S.; Mantziari, A.; Vinderola, G.; Ervolino, E.; Santana, S.I.; Silva, P.H.F.; et al. Bifidobacterium animalis subsp. lactis HN019 presents antimicrobial potential against periodontopathogens and modulates the immunological response of oral mucosa in periodontitis patients. PLoS ONE 2020, 15, e0238425. [Google Scholar] [CrossRef] [PubMed]
- Duffles, L.F.; Menino, A.P.; Taira, T.M.; de Oliveira, S.; Salvador, S.L.; Messora, M.R.; Vinolo, M.A.R.; Fukada, S.Y. Probiotic Bifidobacterium animalis subsp. lactis consumption slows down orthodontic tooth movement in mice. Arch. Oral Biol. 2021, 134, 105324. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Spille, J.H.; Wiltfang, J.; Naujokat, H. Systematic review on diabetes mellitus and dental implants: An update. Int. J. Implant Dent. 2022, 8, 1–21. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-implant mucositis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S237–S245. [Google Scholar] [CrossRef]
- Derks, J.; Ichioka, Y.; Dionigi, C.; Trullenque-Eriksson, A.; Berglundh, J.; Tomasi, C.; Graziani, F. Prevention and management of peri-implant mucositis and peri-implantitis: A systematic review of outcome measures used in clinical studies in the last 10 years. J. Clin. Periodontol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Listgarten, M.A.; Lang, N.P.; Schroeder, H.E.; Schroeder, A. Periodontal tissues and their counterparts around endosseous implants. Clin. Oral Implants Res. 1991, 2, 1–19. [Google Scholar] [CrossRef]
- Barootchi, S.; Wang, H.L. Peri-implant diseases: Current understanding and management. Int. J. Oral Implantol. 2021, 14, 263–282. [Google Scholar]
- Barootchi, S.; Ravidà, A.; Tavelli, L.; Wang, H.-L. Nonsurgical treatment for peri-implant mucositis: A systematic review and meta-analysis. Int. J. Oral Implant. 2020, 13, 123–139. [Google Scholar]
- Bianco, L.L.; Montevecchi, M.; Ostanello, M.; Checchi, V. Recognition and treatment of peri-implant mucositis: Do we have the right perception? A structured review. Dent Med Probl. 2021, 58, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Santana, S.I.; Silva, P.H.F.; Salvador, S.L.; Casarin, R.C.V.; Furlaneto, F.A.C.; Messora, M.R. Adjuvant use of multispecies probiotic in the treatment of peri-implant mucositis: A randomized controlled trial. J. Clin. Periodontol. 2022, 49, 828–839. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).