Abstract
The bioactive lipid lysophosphatidylcholine (LPC), a major phospholipid component of oxidized low-density lipoprotein (Ox-LDL), originates from the cleavage of phosphatidylcholine by phospholipase A2 (PLA2) and is catabolized to other substances by different enzymatic pathways. LPC exerts pleiotropic effects mediated by its receptors, G protein-coupled signaling receptors, Toll-like receptors, and ion channels to activate several second messengers. Lysophosphatidylcholine (LPC) is increasingly considered a key marker/factor positively in pathological states, especially inflammation and atherosclerosis development. Current studies have indicated that the injury of nervous tissues promotes oxidative stress and lipid peroxidation, as well as excessive accumulation of LPC, enhancing the membrane hyperexcitability to induce chronic pain, which may be recognized as one of the hallmarks of chronic pain. However, findings from lipidomic studies of LPC have been lacking in the context of chronic pain. In this review, we focus in some detail on LPC sources, biochemical pathways, and the signal-transduction system. Moreover, we outline the detection methods of LPC for accurate analysis of each individual LPC species and reveal the pathophysiological implication of LPC in chronic pain, which makes it an interesting target for biomarkers and the development of medicine regarding chronic pain.
1. Introduction
Chronic pain is a common, complex, and distressing problem [1], which is characterized by persistent pain even after the initial irritating injury/event has subsided [2], and it has significant societal and personal implications [1]. It affects more than 20% of adults in developed nations. In the U.S. alone, the direct and indirect costs exceed $600 billion annually. Additionally, the experience of chronic pain begins early; as many as 38% of children and adolescents in the community sample have reported chronic pain [3]. It is usually caused by injury or disease; however, it is a separate condition in its own right, not just a symptom accompanying other diseases [1]. Poor management of severe chronic pain, possibly due to an imbalance between analgesics and tolerability, is a burden for patients, with side effects that often lead to discontinuation of treatment [4]. In recent years, interventions for chronic pain are still not completely satisfactory, probably due to the variety of persistent pain conditions with different pathological processes, such as musculoskeletal [5], neuropathic [6], visceral [7], and cancer-related [8] pain, whose pathophysiological mechanisms have not been completely explored.
One mechanism underlying the development and maintenance of chronic pain is oxidative stress [9]. Reactive oxygen species (ROS) have been identified as key factors in nearly all human diseases, including chronic and acute diseases such as atherosclerosis, chronic pain, and acute lung/liver/kidney injuries [10]. The initiation of lipid peroxidation begins with the interaction between polyunsaturated fatty acids and reactive oxygen species [10]. Increased ROS and especially lipid peroxidation are implicated in the pathogenesis of several chronic pain diseases. In the fibromyalgia model, animals were accompanied by increased oxidative stress and lipid peroxidation [11]. Oxidative stress damage was shown to be one of the important factors that induced neuropathic pain [12]. Rats with osteoarthritis had a high level of malonaldehyde (MDA) (lipid peroxidation marker) production [13]. Reactive oxygen species and lipid peroxidation inhibitors reduced mechanical sensitivity in chronic pain models [14]. Compounds such as Apocynin, an NADPH oxidase inhibitor, limited the production of ROS precursor superoxide to reduce ROS, which inhibited inflammation in animal models of nerve tissue damage. The efficacy of 4-oxo-tempo may be related to the effects of the direct scavenging of oxidative radicals in animals with a chronic neuropathic pain model [15]. Antioxidants (N-acetylcysteine and Tempol) significantly reduced oxidative stress in the serum (assessed by MDA and H2O2 levels) of mice with stress-related chronic pain disorders [16]. Importantly, lysophosphatidylcholine (LPC) is an endogenous product derived from peroxidation during oxidative stress [17]. In response to lipid peroxidation from inflammation and tissue injury, phospholipids undergo lipid peroxidation to LPC [18]. Exposure to endogenous and exogenous LPC has emerged as a key contributor to cellular and tissue biology, such as inflammatory cascades [19] in chronic disease states—for example, diabetes, cancer, cardiovascular diseases, or neurodegeneration [20,21,22,23]. The development and maintenance of human chronic pain diseases have possibly established a causal link with specific LPC [24]. Although the clinical and pathological manifestations of chronic pain are broad, inflammation covers all stages of the disease, and various bioactive lipids have been implicated in such inflammation in various cells, highlighting their involvement in the pain transduction process [25]. Following inflammation, the excitatory neurotransmitter substance P and glutamate are released from primary afferent neurons, promoting the synthesis of lysophosphatidylcholine (LPC) [26]. In addition, injury to nervous tissue leads to an increase in reactive oxygen species (ROS) and promotes the synthesis of LPC, which enhances the membrane hyperexcitability to induce chronic pain. Antioxidants also effectively prevent the synthesis of lipid LPC and alleviate the symptoms of chronic hyperalgesia in animal models [16]. The purpose of this article is to summarize what is known about LPC, including its function and related signal regulation pathways in chronic pain diseases.
2. Lysophosphatidylcholine (LPC)
2.1. The Metabolism and Species of LPC
LPC, an important lipid molecule in mammalian tissues, belongs to a group of bioactive lysophospholipids [27]. Molecular species of LPC are identified by the lengths and saturation of their acyl chains. LPCs are produced from cell-membrane-derived phosphatidylcholine (PC) as a result of hydrolysis by phospholipases [28,29] (Figure 1). Two phospholipases have been studied, namely secretory PLA2 (sPLA2) and lipoprotein-associated PLA2 (Lp-PLA2) [30]. sPLA2 is Ca2+-dependent and hydrolyzes the sn-2 acyl group of the glycerophospholipids in lipoproteins and cell membranes to yield LPC and free fatty acids. In contrast to sPLA2, Lp-PLA2, also known as platelet-activating factor (PAF)-acetylhydrolase (PAF-AH) is Ca2+-independent, and it is specifically for short acyl groups at the sn-2 position of the phospholipid substrate. Lp-PLA2 can also hydrolyze oxidized phospholipids to generate LPC and oxidized fatty acids. LPC is usually present in very small concentrations because of LPC catabolism through different pathways mediated by separate enzymes: (1) after synthesized, LPC is secreted outside the cell and hydrolyzed to lysophosphatidic acid (LPA) and choline by autotaxin (ATX) [31]; (2) LPC is converted back to PCs by the enzyme lysophosphatidylcholine acyltransferase (LPCAT) in the presence of Acyl-CoA [32]; (3) LPC molecules catalyzed by cytosolic lysophospholipase-transacylase (LPTA) to form PC and glycerophosphorylcholine (GPC) [33] (Figure 1). The accumulation of LPC reflects increased PLA2-catalyzed PC hydrolysis or decreased LPC catabolism or a combination of both processes [34].
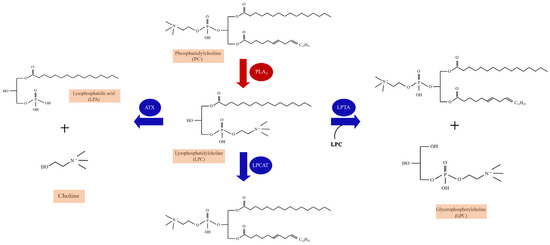
Figure 1.
The enzymatic pathways of lysophosphatidylcholine (LPC) synthesis and catabolism. The production of LPC is the result of the fragmentation of the sn-2 residues of phosphatidylcholine (PC) hydrolyzed by PLA2. Three catabolism pathways of LPC are listed. LPC catabolism occurs through a disproportionation reaction involving two LPC molecules catalyzed by cytosolic lysophospholipase-transacylase (LPTA) to form PC and glycerophosphorylcholine (GPC). A hydrolytic pathway is catalyzed by autotaxin (ATX) to yield lysophosphatidic acid (LPA) and choline, and a reacylation pathway to form PC is catalyzed by lysophosphatidylcholine acyltransferase (LPCAT).
In recent years, research has begun to focus on the accurate analysis of each individual LPC species. Various LPC species have been identified by specific detection methods according to carbon chain length and number of double bonds [35], including LPC(14:0), LPC(15:0), LPC(16:0), LPC(16:1), LPC(17:0), LPC(18:0), LPC(18:1), LPC(18:2), LPC(18:3), LPC(20:0), LPC(20:2), LPC(20:3), LPC(20:4), LPC (22:6), LPC(26:0), LPC(28:1), and so on [36,37,38,39,40]. As a pro-inflammatory lipid, abnormal levels of LPC in body fluids such as blood, urine, synovial fluid, cerebrospinal fluid, and tissues are closely related to pathological states.
2.2. Detection Methods of LPC
The detection of LPC relies on the rise of lipidomics [16,41]. Lipidomics is a branch of metabolomics, and it is generally believed that lipidomics is a discipline that focuses on the qualitative and quantitative screening of metabolites in an organism and their roles in protein expression and gene regulation [42]. Lipidomic analyses have emerged based on existing omics disciplines and have developed rapidly in recent years [43] (Figure 2).
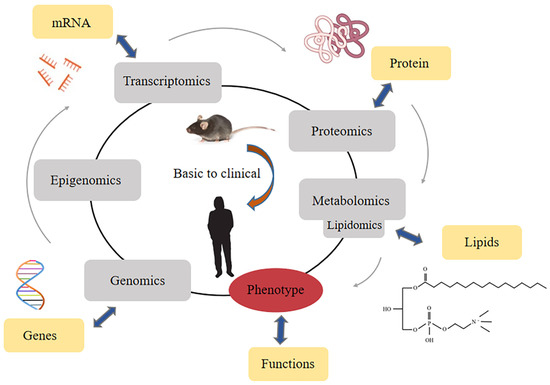
Figure 2.
Classification of lipidomics in the area of all omics methods.
Lipidomics can assay metabolite compositions through various targeted and non-targeted techniques [44,45]. Prevailing technological advances have made accurate profiling of LPC in biological samples, such as nuclear magnetic resonance (NMR) spectroscopy [46,47,48,49], liquid chromatography coupled with mass spectrometry (LC-MS) [50,51,52], gas chromatography coupled to mass spectrometry (GC-MS) [51,53], high- or ultra-high-performance liquid chromatography coupled to UV or fluorescent detection (HPLC/UPLC) [54,55], and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) [56]. Each analytical platform has its own advantages and disadvantages (Table 1).
2.2.1. NMR Spectroscopy
Nuclear magnetic resonance (NMR) spectroscopy, a technique for detecting the chemical environment of an atomic nucleus by absorbing radio frequency electromagnetic radiation [57], is an unbiased, nondestructive, and easily quantifiable form of sample processing, requiring little or no chromatographic separation and allowing for the routine identification of novel compounds. In addition, NMR is highly automatable, has extremely high reproducibility, and is feasible for high throughput [58]. NMR covers a wide range of applications, not limited to the analysis of biological fluids or tissue extracts. The nuclei best suited for NMR spectroscopy in biological systems include 1H, 19F, 31P, 13C, and 15N [59]. (1H) NMR spectroscopy (1H NMR) is commonly used in the profiling of LPC [60]. However, NMR spectroscopy has lower sensitivity and is suitable for the quantification of metabolites present in relatively high concentrations [57].
2.2.2. LC-MS
Liquid chromatography coupled with mass spectrometry (LC-MS) is a combination of liquid chromatography and mass spectrometry [61]. LC-MS combines the separation capabilities of LC with the mass analysis power of MS [62]. The LC-MS detection method has the advantages of excellent resolution and sensitivity, small sample volumes, and relatively low costs, making it the most powerful analytical tool for metabolites today [63,64,65,66,67].
2.2.3. GC-MS
Gas chromatography coupled with mass spectrometry (GC-MS) is also a commonly used platform for metabolomic research [68]. GC-MS was the first instrument used for metabolite profiling of human blood and urine by Horning in 1971 [69]. Apart from the high sensitivity and throughput [70,71], due to its longer use in clinical chemistry practice, GC-MS also possesses a higher chromatographic resolution and larger databases of identified peaks compared to the LC-MS. To some extent, GC–MS avoids the common problems of LC–MS, such as matrix effects and ion suppression by co-eluting compounds [72,73].
2.2.4. HPLC/UPLC
The history of high-performance liquid chromatography (HPLC) can be traced back to the early 20th century [74]. Over the years, HPLC has made great progress in terms of speed, convenience, high sensitivity, choice of column stationary phase, suitability for various sample matrices, and the combination of chromatographic methods with spectral detectors [75,76]. It suffers from limitations such as low throughput, lack of high efficiency, inability to observe non-electrochemically active species, and difficulties associated with metabolite identification [77,78]. Ultra-performance liquid chromatography (UPLC) makes full use of chromatographic principles for separation, using short columns packed with smaller particles (sub-2 lm). Reduced analysis time, increased peak efficiency (peak width), better resolution, and reduced solvent usage are observed compared to conventional HPLC [79].

Table 1.
Advantages and disadvantages of metabolomics techniques.
Table 1.
Advantages and disadvantages of metabolomics techniques.
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| NMR spectroscopy | Great range of detectable molecular species; Simple sample preparation; Excellent reproducibility; High automation | Low sensitivity; Quantification of relatively high concentrations of metabolites/extensive | [57,58] |
| LC-MS | High sensitivity; Small sample volumes; Relatively low costs; Superior resolution | Matrix effects and ion suppression by co-eluting compounds; Limitation of detectable metabolites | [63,64,65,66,67] |
| GC-MS | High chromatographic resolution; Large databases of identified peaks; High sensitive; High throughput | A large number of unidentified peaks; Require additional analytical steps; Separate and identify low molecular weight | [70,71,72,73] |
| HPLC | Robustness; Convenience; Good selectivity; High sensitivity | Low throughput; Inability to observe non-electrochemically active species; Difficulties of metabolite identification; Lack of high efficiency | [75,76,77,78] |
| UPLC | Short analysis time; Improved peak efficiency; Better resolution; Decreased use of solvents | Less time life of columns | [79] |
| MALDI-MS | Suitability for solid samples; High sensitivity; Easy sample handling; Salt tolerance; High speed | Limitation of detectable metabolites | [43] |
2.2.5. MALDI Mass Spectrometry
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) is a powerful method for the simultaneous detection and identification of many molecules directly from biological samples of animals or humans. MALDI-MS can detect a variety of biomolecules, from small to large. Due to the broad applicability of this method, MALDI-MS is widely used in lipidomics or metabolomics studies [80]. The advantages of MALDI mass spectrometry include high sensitivity, easy sample handling, salt tolerance, rapid speed, and suitability for solid samples. However, it is selective for the detected lipid metabolites [43].
3. Lysophosphatidylcholine and Chronic Pain Diseases
From numerous reports, it has been clarified that the level and metabolism process of LPC in the body fluid or tissues of animals or humans are elevated in various chronic pain states, such as chronic inflammatory pain [81], chronic joint pain [82,83] neuropathic pain [29,39,84], fibromyalgia [16], and multisite musculoskeletal pain [38] (Table 2). In this section, we summarize these LPC-induced responses and cellular mechanisms in detail.
3.1. Inflammatory Pain
Inflammatory pain is the most important clinical symptom of inflammatory diseases. The skin and joints are systems that are particularly susceptible to the formation of inflammatory pain. The common pathogenesis is that pro-inflammatory mediators such as chemokines, cytokines, growth factors, neuropeptides, and proteases are released at sites of inflammation and subsequently sensitize peripheral pain-sensing neurons [85]. In addition to the above molecules, lipids can also act as inflammatory modulators to induce inflammatory pain. Katelyn E Sadler et al. used LC-MS techniques to confirm that LPC was markedly elevated in mice with CFA-induced inflammatory pain in the skin; the content of LPC in the paw tissue of CFA-injected mice reached 130 μM, twice that of the vehicle-treated group. However, circulating LPC concentrations were unchanged in CFA-injected animals, suggesting that the excess lipid was derived from cells localized to the injured tissue. Furthermore, wild-type mice developed mechanical allodynia after dural injection of LPC [81], which reflected the correlation between LPC and CFA-induced inflammatory pain.
3.2. Chronic Joint Pain
Chronic joint pain is the main reason for patients to seek medical treatment for chronic pain; it seriously affects the quality of life of patients, resulting in disability and psychological distress [82]. Rheumatoid arthritis (RA) and osteoarthritis (OA) are associated with a risk of developing persistent chronic joint pain [83]. Florian Jacquot et al. demonstrated that LPC correlated with pain outcomes in a cohort of chronic joint pain patients. The synovial fluid levels of LPC in the 50 patients (32 women and 18 men) were evidently elevated, especially the LPC (16:0) species, compared with control subjects via high-definition mass spectrometer (HDMS). Intra-articular injection of LPC (16:0) resulted in persistent pain and anxiety-like behavior in mice, suggesting that LPC (16:0) could be considered a trigger for chronic joint pain in male and female mice [82]. Moreover, it has been demonstrated that mice injected with B02/B09 monoclonal antibodies (mAbs) isolated from B cells of patients with RA developed a long-term mechanical hypersensitivity accompanied by bone erosion and elevated LPC (16:0). In addition, elevated levels of LPC and sPLA2, a family of enzymes required for LPC synthesis, have been verified in the plasma and synovial fluid of patients with RA and OA, as well as those with joint pain. Consequently, it was possible that LPC was regarded as a biological target for predicting chronic joint pain in rodents or humans, especially LPC (16:0) (Figure 3) [83].
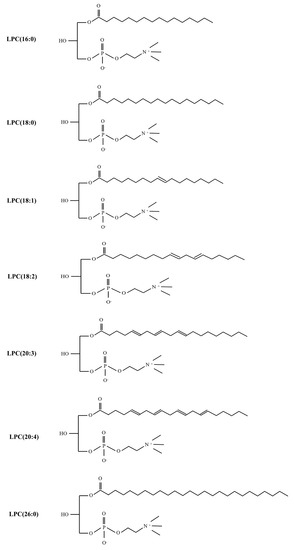
Figure 3.
The structure of different LPC subtypes associated with chronic pain.
3.3. Fibromyalgia and Multisite Musculoskeletal Pain (MSMP)
Fibromyalgia (FM) is characterized by chronic widespread musculoskeletal pain and associated fatigue, memory problems, and sleep disturbances [86,87]. Most of the lipidomics studies identified by our search were on this type of chronic pain. Chih-Hsien Hung et al. utilized untargeted lipidomic analysis and QqQ MS, respectively, to identify the serum and plasma of C57BL/6J mice and 31 fibromyalgia patients and 30 healthy controls at different time points. The identified lipids were mainly LPCs. LPCs (16:0) in the fibromyalgia mouse model were upregulated by 1.37-fold of the basal status in mice. It has also been proposed that central sensitization occurs after repeated intramuscular injections of LPC (16:0) in mice, which resulted in the activation of c-fos and pERK in spinal dorsal horn neurons [16]. Increased LPC (16:0) expression in FM patients also correlated with pain symptoms [16]. In addition to LPC (16:0), LPC (18:1) was also increased in the fibromyalgia mouse model. This may partly explain the increasing prevalence of fibromyalgia in the female population [88]. Wei-Hsiang Hsu et al. revealed several potential biomarkers of FM mice, some not previously described, such as LPC (20:3) in serum via 1HNMR-and LC-MS-based metabolomics profiling [50]. In addition, LPC (16:0) in the serum was also upregulated, which was the same result as in the study of Chih-Hsien Hung. Pierluigi Caboni et al. showed, using a metabolomics approach combining liquid chromatography-quadrupole-time of flight/mass spectrometry (LC-Q-TOF/MS) with multivariate statistical analysis, that lipid compound LPCs were elevated in the plasma of 22 females affected by FM and 21 controls [89]. In addition, in a large targeted metabolic profiling study, the metabolites were measured in the plasma of 122 non-multisite musculoskeletal pain (MSMP) and 83 MSMP patients. This study demonstrated that two lysophosphatidylcholines, LPC (26:0) and LPC (28:1), were significantly upregulated and positively associated with MSMP [38].

Table 2.
The Application of LPC in Chronic pain.
Table 2.
The Application of LPC in Chronic pain.
| Year | Author | Disease | Samples | Method | Observations | References |
|---|---|---|---|---|---|---|
| 2021 | Katelyn E Sadler et al. | CFA-induced inflammatory pain; skin incision-induced pain; chemotherapy-induced peripheral neuropathic pain | Mice hindpaw skin | LC-MS | CFA induced inflammatory pain, skin incision, and chemotherapy-induced peripheral neuropathy, all of which were characterized by elevated concentrations of LPC. | [81] |
| 2022 | Florian Jacquot et al. | Chronic joint pain | Synovial fluids from 50 patients (32 women and 18 men) | HDMS | The synovial fluid levels of LPC were significantly elevated, especially the LPC (16:0) species, compared with postmortem control subjects. | [82] |
| 2021 | Alexandra Jurczak et al. | B02/B09-induced pain | Bone marrow extracts of B02/B09-treated mice | HDMS | LPC (16:0) was the most abundant and significantly increased in the B02/B09 group compared with control. | [83] |
| 2020 | Chih-Hsien Hung et al. | Fibromyalgia | Serum from RISS mice; plasma from 31 fibromyalgia patients and 30 healthy controls | Untargeted lipidomic analysis/QqQ MS | LPC (16:0) in fibromyalgia mouse and patients were upregulated. | [16] |
| 2019 | Wei-Hsiang Hsu et al. | Fibromyalgia | Mice serum | 1H NMR and LC-MS | Impactful metabolites in the FM model including LPC (16:0), LPC (20:3) in serum. | [50] |
| 2014 | Pierluigi Caboni et al. | Fibromyalgia | Plasma from 22 females FM patients and 21 controls | LC-MS | Plasma of FM patients identified many lipid compounds, mainly including LPC. | [89] |
| 2021 | Ming Liu et al. | Multisite musculoskeletal pain (MSMP) | Plasma of 122 non-MSMP and 83 MSMP patients | Biocrates AbsoluteIDQ p180 kit | LPC (26:0) and LPC (28:1) are associated with MSMP. | [38] |
| 2021 | Baasanjav Uranbileg et al. | Cauda equina compression | CSF and plasma from CEC rats; CSF from lumbar spinal canal stenosis patients and controls | LC-MS/MS; UHPLC-MS/MS | Lots of LPC species were significantly increased, especially LPC (16:0), LPC (18:2), LPC (20:4). | [39] |
| 2020 | Vittoria Rimola et al. | Oxaliplatin-induced Peripheral Pain | Mice sciatic nerve, DRG, dorsal spinal cord | LC-MS/MS | LPC (18:1) and LPC (16:0) were significantly increased after oxaliplatin treatment. | [29] |
| 2011 | Jun Nagai et al. | Partial sciatic nerve injury (SCNI) | Mice spinal cord and dorsal root | NALDI-MS | The levels of LPC (16:0), LPC (18:0) and LPC (18:1) were increased after SCNI. | [84] |
HDMS: High-Definition mass spectrometer: LC-MS: Liquid chromatography mass spectrometry; 1H NMR: 1H-nuclear magnetic resonance; NALDI-MS: Matrix-assisted laser desorption/ionization mass spectrometry.
3.4. Neuropathic Pain
Neuropathic pain caused by a lesion or disease of the somatosensory nervous system is a common chronic pain condition and brings a lot of problems to humans [90]. The efficacy of current therapeutic drugs is limited, and it is essential to develop novel targets that permanently reduce or eliminate neuropathic pain [91]. In recent years, studies have demonstrated that metabolites are involved in the occurrence and development of neuropathic pain [92], and lipid LPCs are screened out. This is due to the fact that, following a nerve injury, the excitatory neurotransmitters substance P and glutamate are released from primary afferent neurons, or the increase in reactive oxygen species (ROS) leads to the upregulated synthesis of LPC [26]. Currently, pain induced by LPC injected into the median nerve has been regarded as a neuropathic pain model in many articles owing to pathological mechanisms of LPC-induced demyelination of the nervous system, which is inconsistent with the mechanism of LPC-mediated chronic joint pain [93,94,95]. Local LPC application results in the focal demyelination of afferent A fibers without axonal damage or loss of neurons in the dorsal root ganglia (DRG) [96]. In the central nervous system (CNS), LPCs also trigger a rapid demyelination without damage to adjacent cells and axons. This was thought to be a key role of immune cells in LPC-induced demyelination [97]. Peripheral macrophage and central microglia, as resident macrophages, contribute to maintaining homeostasis in the nervous system. Macrophages or microglia are activated in response to noxious stimuli such as nerve injury. Activated macrophages or microglia result in the production and release of pro-inflammatory mediators, which lead to the development of chronic pain [98,99]. LPC induced macrophage and microglia recruitment and activation in the mouse spinal cord [100,101,102]. In the LPC-induced model of demyelination, macrophages and microglia were detected at 48 h, when clear evidence of demyelination was observed [101,103]. Interestingly, the application of LPCs in the early presented a rapid but brief influx of T cells, and neutrophils, T cells, and neutrophils were seen in the spinal cord for 6–12 h [103]. This is because LPC caused rapid and extensive disruption of the blood–brain barrier, which induced early and transient T cell and neutrophil responses in the spinal cord. These cells likely promote a rapid influx of monocytes, followed by the activation of macrophages from monocytes and microglia to mediate demyelination [101,103]. In addition, LPC-induced demyelination induces mechanical allodynia and thermal hyperalgesia, which persists for at least 7 days (Table 3) [104,105]. LPC injection increased the levels of pain-related proteins, including neuropeptide Y (NPY), Nav 1.3, Nav 1.8, chemokines, and their receptors, in the DRG or spinal cord [93]. In the mice model of chemotherapy-induced peripheral pain, LPC (16:0) and LPC (18:1) were significantly increased in the sciatic nerve and DRG tissue, as revealed by untargeted and targeted lipidomics. Importantly, pain-like performance induced by LPC (16:0) and (18:1) was dependent on Ca2+ transients in primary sensory neurons [29]. Jun Nagai et al. developed a quantitative mass spectrometry assay to simultaneously analyze several species of LPCs in the SCNI. They found that the levels of LPC (16:0), LPC (18:0), and LPC (18:1) in the spinal cord and DRG were maximally increased [84]. Cauda equina compression (CEC) is a major cause of neurogenic claudication and progresses to neuropathic pain [39]. A study utilizing LC-MS/MS and UHPLC-MS/MS in rats and patients demonstrated that many LPC species were significantly elevated in the CSF and plasma of CEC model rat or CSF of patients with lumbar spinal canal stenosis (LSS), especially LPC (16:0), LPC (18:2), and LPC (20:4) (Figure 3). However, LPC levels in the spinal cord tissue samples of rats did not change dramatically [39].

Table 3.
The application of LPC in the construction of neuropathic pain models.
3.5. The Enzymatic Pathways of Lysophosphatidylcholine (LPC) and Chronic Pain
In addition to the accumulation of LPC causing pain symptoms, molecules in the enzymatic pathways of LPC synthesis and catabolism, such as lysophosphatidic acid (LPA), autotaxin (ATX), and lysophosphatidylcholine acyltransferase (LPCAT), also play an important role in chronic pain. Accumulating evidence has revealed that LPC regulates the participation of platelet-activating factor (PAF)/PAF receptor (PAFr) in pain signal transduction [106]. LPC is hydrolyzed by autotaxin into LPA and acts through LPA receptors present on nociceptors. LPA, a potent bioactive lipid mediator, induces neuropathic pain as well as demyelination and pain-related protein expression changes via LPA receptor signaling [104]. Direct intrathecal administration of LPA was able to induce chronic pain responses in rodents [107,108]. LPA altered the density and activity of Ca11, K1, and TRP ion channels in microglia and neurons, causing allodynia and hyperalgesia, which played a central role in the initiation and maintenance of neuropathic pain [38]. Autotaxin mediated LPC to produce LPA, a bioactive lipid mediator that signals the activation of six GPCRs (LPA receptors 1-6). Autotaxin levels in synovial fluid and plasma correlated with disease severity in patients with knee OA [108]. Intrathecal LPC-induced mechanical allodynia and thermal hyperalgesia were significantly reduced in autotaxin heterozygous animals, indicating reduced conversion of LPC to LPA [104]. Moreover, ATX inhibition could ameliorate neuropathic pain symptoms by using ATX inhibitor (ONO-8430506) [109]. In addition, a recent study demonstrated that nerve injuries induced the production of LPA by converting LPC to LPA under the action of ATX, which was observed only in the spinal dorsal horn, but not in the spinal nerve, sciatic nerve, or DRG, for several hours. Furthermore, injury-induced synthesis of LPC and subsequent conversion to LPA were both involved in the development of neuropathic pain. However, injury-induced neuropathic pain and LPA production were attenuated to approximately 50% in atx+/− mice and abolished in Lpar1−/− mice, which was also observed in LPC-induced demyelination [110]. Therefore, the conversion of LPC to LPA may also be an important target for the treatment of chronic pain [111]. Of course, not all LPC will eventually be converted into LPA. The indications of the increased expression of the LPC to LPA-converting enzyme autotaxin or LPA receptors were not found in several chronic pain models [83]. This is because pathological pain is a complex state that may be related to both the model and the time of onset. Apart from the LPA, cyclic phosphatidic acid (cPA), produced from LPC using ATX, has a structure similar to that of LPA [112,113]. Unlike the biological function of LPA, cPA has the potential for use in the treatment of acute and chronic pain diseases because of its biological properties of anti-inflammatory and neuroprotective activities [112]. The cPA and its stable analog 2-carba-cPA (2ccPA) inhibited chronic and acute inflammation-induced C-fiber stimulation. The administration of 2ccPA significantly attenuated mechanical allodynia and thermal hyperalgesia following the partial ligation of the sciatic nerve, whether pretreatment or repeated post-treatments [114]. Intra-articular injection of 2ccPA also reduced the pain response to OA and articular swelling [115]. LPC is hydrolyzed by autotaxin into LPA and cPA, but the effects are completely opposite, suggesting that it may be related to the period and condition of synthesis. It has been suggested that LPC could be converted into cPA by HCl in a dose-dependent manner [116]. In addition, LPCAT is also promising as a novel therapeutic target for newly classified analgesic drugs. Hideo Shindou et al. confirmed that pain-like behaviors induced by partial sciatic nerve ligation (PSNL) were largely relieved by the deficiency of LPCAT [117].
4. LPC-Related Receptor and Chronic Pain
As mentioned above, the biological effects of LPC have been studied in mice and humans and are important in chronic pain. LPC acts as the ligand and can activate G protein-coupled receptors (GPCRs), Toll-like receptors (TLRs), and several ion channels, implicating the possible molecular mechanisms in the observed effects of LPC (Figure 4).
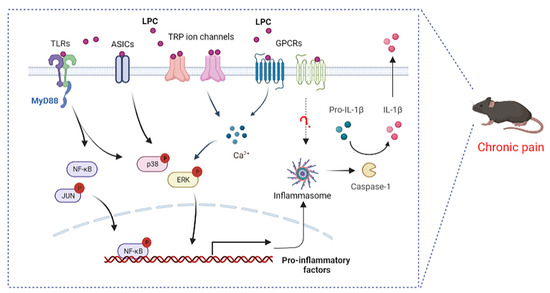
Figure 4.
Proposed signaling pathways by which LPC mediates pain. LPC, lysophosphatidylcholine; TLRs, Toll-like receptors; GPCRs, G protein–coupled receptors; ASICs, acid-sensing ion channels; TRP, transient receptor potential; MyD88, myeloid differentiation factor; NF-κB, nuclear factor kappa-B; ERK, extracellular-signal-regulated kinase; Caspase-1, cysteinyl aspartate specific proteinase-1; IL-1β, interleukin-1 beta.
4.1. LPC and G Protein Coupled Receptors
LPC is considered the ligand for GPCR, G2A (GPR132), and GPR4, with a significantly higher affinity for G2A than that for GPR4 [118,119]. LPC plays a key role in the development of chronic inflammatory diseases through the role of the G2A receptor. T cells overexpressing G2A exhibit chemotaxis to LPC; siRNA silencing in mouse T cell hybridomas and retroviral overexpression of G2A demonstrated the requirement for G2A in LPC-induced T cell migration [120]; G2A was also required for LPC-induced chemotaxis of macrophages [121], both demonstrating the interaction between LPC and the G2A effect. LPC led to an increase in intracellular calcium levels by acting on receptor G2A, resulting in increased neuronal excitability and activation of ERK mitogen-activated protein kinase (Figure 4). The signaling lipid receptor G2A and ERK mitogen-activated protein kinase were upregulated in a spared nerve injury (SNI)-induced neuropathic pain model [122,123]. There are few studies focusing on both LPC and GPR4, and the involvement of this mechanism in chronic pain has not been confirmed. Previous research has shown that LPC is associated with NLRP3 inflammasome and the release of IL-1β by GPR4 [124]. In the neurodegeneration and demyelination states, LPC activates NLRP3 inflammasomes in astrocytes and microglia [23], and NLRP3 inflammasome is involved in inflammatory pain [125] (Figure 4). In fact, the direct or indirect effect of LPC and G2A or GPR4 is controversial. In addition, the LPC derivative also targeted four other G protein-coupled receptors, namely GPR40, GPR55, GPR119, and GPR120 [126,127]. Studies have indicated that the stimulatory effect of isoprenoid derivatives of LPC on Ca2+ signaling in MIN6 cells was GPR40-, GPR55-, GPR119-, and GPR120-dependent [127]. GPR40/GPR55 has been implicated in inflammatory pain and neuropathic pain [128,129], but studies on LPC and these receptors in chronic pain are lacking.
4.2. LPC and Toll-like Receptors
TLRs are an important family of receptors involved in complex intercellular signaling networks that develop in the context of chronic pain [130,131]. TLRs can induce an innate immune response by recognizing various pathogen-associated molecular patterns (PAMPs), and the receptors that recognize these molecular structures were named as pattern recognition receptors (PRRs) [132,133]. To date, there are ten known functional TLRs (TLR1-10) in humans and twelve TLRs (TLR1-9, TLR11-13) in mice. TLR1-2, TLR4-6, and TLR10 are located on the cell surface, and TLR3, TLR7-9, and TLR11-13 are observed in intracellular compartments [134]. In addition to innate immunity, TLRs are also expressed in the periphery and in CNS cells, and are coupled with the activation of various non-neuronal cells (microglia, schwann cells and astrocytes) and neurons, thus causing the release of pro-inflammatory cytokines and thereby leading to the generation and maintenance of chronic pain [135]. TLRs play a key role in OA [136], neuropathic pain [137], chronic pelvic pain [138], opioid-induced hyperalgesia [139], and cancer pain [140]. TLR2, TLR4, TLR5, TLR3, TLR7, TLR9, etc., have been reported to contribute to persistent pain [131]. Among them, TLR2 and TLR4 have been proven to be major Toll-like receptors that to mediate LPC function. LPC activated pain markers, such as NF-κB, p38 MAPK, and JUN and cytokine production (IL-6, TNF-α) by combining the TLR2 and TLR4 receptors (Figure 4) [118]. Previous studies have reported that LPC (18:0) and LPC (18:1) were more potent than LPC (16:0) and LPC (14:0) in promoting cytokine secretion from TLR-primed cells [141].
4.3. LPC and Ion Channels
Multiple ion channels are involved in sensing and transmitting nociceptive information in the neurons of the peripheral and central nervous system [142]. LPC can also exert its biological functions by binding to acid-sensing ion channels (ASICs) and transient receptor potential (TRP) ion channels (Figure 4). Acid-sensing ion channels (ASICs) are proton-activated cation channels that are expressed in a variety of neuronal and non-neuronal tissues, encoding several subunits (ASIC1, ASIC2, ASIC3, and ASIC4). ASIC3, an important pain transducer, can be activated by LPC and potentiated by many pro-inflammatory mediators [143,144]. A recent study showed that certain LPCs, especially LPC (16:0), were able to directly activate ASIC3 channels to mechanical stimuli, resulting in altered mechanoneuronal responses of primary afferent neurons [16]. In the fibromyalgia model, LPC-induced chronic hypersensitivity was obviously inhibited in APETx2 (a selective ASIC3 antagonist)-treated mice. Similarly, chronic hyperalgesic changes in WT animals were also robustly improved in Asic3-/- mice after repeated LPC injections [16]. LPC (16:0) drove sufficient peripheral inputs to generate spinal sensitization process via ASIC3 channels in the mouse model of OA-induced inflammatory pain [82,83].
In addition, LPCs, such as LPC (18:1), activated the ligand-gated calcium channels’ transient receptor potential V1 and M8 (TRPV1 and TRPM8) in primary sensory neurons to induce mechanical hypersensitivity in mice, which stimulated chemotherapy-induced peripheral pain [29]. Lipid mass spectrometry indicated tissue-specific increases in LPC in pain models, accompanying mechanical allodynia, neuronal mechanical hypersensitivity, and spontaneous pain, which could be inhibited with transient receptor potential canonical 5 (TRPC5) inhibitors. TRPC5 is also a target of LPC-induced chronic pain [81]. TRPC5 inhibitors have demonstrated analgesic effects in all of the following conditions with elevated LPC: fibromyalgia [89], rheumatoid arthritis [145], osteoarthritis [146], lumbar spinal stenosis [89,147], diabetes [148], and migraine [149]. In addition, TRPV4 in DRG sensory neurons was essential for intrathecally LPC-induced chronic pain [105]. The above findings provide new molecular insights into the mechanism by which LPC may affect the activation of cellular signaling pathways in chronic pain. G protein-coupled receptors (GPCRs), ion channels, and Toll-like receptors are involved in nociceptive signaling and are considered important pharmacological targets for existing or potential drugs. Apart from GPCRs, TLRs, and several known ion channels, there are other receptors that may directly bind to LPC or some molecules that indirectly interact with LPC. Therefore, future research should continue to focus on physiological and therapeutic approaches to inhibit the LPC signaling cascade.
5. Conclusions
By and large, the studies on LPC and chronic pain have been scant as compared to studies on other pathological states. The current findings have highlighted the critical contribution of LPC in CFA induced-inflammatory pain, chronic joint pain, neuropathic pain, fibromyalgia, and multisite musculoskeletal pain, including total LPC and LPC species and rodents and humans (Table 2). We found that LPC (16:0), LPC (18:0), and LPC (18:1) were currently the three most detected LPC species in chronic pain, among which LPC (16:0) was involved in the chronic pain caused by osteoarthritis and fibromyalgia, while LPC (18:1) was more studied in nerve-injury-induced neuropathic pain. This suggests that specific LPC species may reflect some chronic pain diseases; after all, the mechanisms of chronic pain are complex and different. At present, the accurate detection of LPC relies on metabolomics or lipidomics technology, which provides assays for exploiting the role of LPC in chronic pain. Apart from chronic pain, LPC or LPC species in body fluids such as blood, urine, cerebrospinal fluid, and tissues are uniquely or collectively related to cancer [150,151,152], diabetes [153,154,155,156], coronary atherosclerosis [157], Alzheimer’s disease [158,159], rheumatoid arthritis [83], COVID-19 [160], liver and kidney damage [161,162], etc. Whether LPC is necessary for other chronic pain conditions, such as cancer pain, has not been confirmed. In addition, LPC-related metabolites, such as ATX, PLA2, cPA, and LPA, also serve as therapeutic targets of chronic pain. As an inflammatory lipid, LPC can activate downstream signaling pathways by binding to G protein-coupled receptors, Toll-like receptors, and several ion channels. It is also necessary to further explore new receptors for LPC in the future.
Author Contributions
Conceptualization, J.R. and M.Y.; writing—original draft preparation, J.R. and J.L.; writing—review and editing, J.R. and L.Y.; supervision, M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Clinical Key Specialty Construction Project of China (2021); and the National Natural Science Foundation of China (82071227) to M.Y.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
LPC, lysophosphatidylcholine; Ox-LDL, oxidized low-density lipoprotein; PLA2, phospholipase A2; ROS, reactive oxygen species; PC, phosphatidylcholine; sPLA2, secretory PLA2; Lp-PLA2, lipoprotein-associated PLA2; PAF-AH, platelet activating factor-acetylhydrolase; LPA, lysophosphatidic acid; ATX, autotaxin; LPCAT, lysophosphatidylcholine acyltransferase; LPTA, lysophospholipase-transacylase; GPC, glycerophosphorylcholine; NMR, nuclear magnetic resonance; LC-MS, liquid chromatography coupled with mass spectrometry; GC-MS, gas chromatography coupled to mass spectrometry; HPLC, high performance liquid chromatography; UPLC, ultra-performance liquid chromatography; MALDI-MS, matrix-assisted laser desorption/ionization mass spectrometry; RA, rheumatoid arthritis; OA, osteoarthritis; HDMS, high-definition mass spectrometer; MSMP, multisite musculoskeletal pain; FM, fibromyalgia; LC-Q-TOF/MS, liquid chromatography-quadrupole-time of flight/mass spectrometry; DRG, dorsal root ganglia; NPY, neuropeptide Y; CEC, cauda equina compression; LSS, lumbar spinal canal stenosis; PSNL, partial sciatic nerve ligation; GPCRs, G protein coupled receptors; TLRs, Toll-like receptors; SNI, spared nerve injury; TRP, transient receptor potential; TRPV1, transient receptor potential V1; TRPM8, transient receptor potential M8; TRPC5, transient receptor potential canonical 5; cPA, cyclic phosphatidic acid; PAMPs, pathogen-associated molecular patterns; PRRs, pattern recognition receptors
References
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef] [PubMed]
- Reckziegel, D.; Vachon-Presseau, E.; Petre, B.; Schnitzer, T.J.; Baliki, M.N.; Apkarian, A.V. Deconstructing biomarkers for chronic pain: Context- and hypothesis-dependent biomarker types in relation to chronic pain. Pain 2019, 160 (Suppl. 1), S37–S48. [Google Scholar] [CrossRef]
- Edwards, R.R.; Dworkin, R.H.; Sullivan, M.D.; Turk, D.C.; Wasan, A.D. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J. Pain 2016, 17, T70–T92. [Google Scholar] [CrossRef]
- Barroso, J.; Branco, P.; Apkarian, A.V. Brain mechanisms of chronic pain: Critical role of translational approach. Transl. Res. 2021, 238, 76–89. [Google Scholar] [CrossRef]
- McWilliams, D.F.; Walsh, D.A. Pain mechanisms in rheumatoid arthritis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 107), 94–101. [Google Scholar]
- Alles, S.R.A.; Smith, P.A. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Rev. 2018, 70, 315–347. [Google Scholar] [CrossRef]
- Gebhart, G.F.; Bielefeldt, K. Physiology of Visceral Pain. Compr. Physiol. 2016, 6, 1609–1633. [Google Scholar] [PubMed]
- Christo, P.J.; Mazloomdoost, D. Cancer pain and analgesia. Ann. N. Y. Acad. Sci. 2008, 1138, 278–298. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Averitt, D.L.; Maier, C.; Basu, A. The Effects of Nuclear Factor Erythroid 2 (NFE2)-Related Factor 2 (Nrf2) Activation in Preclinical Models of Peripheral Neuropathic Pain. Antioxidants 2022, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Cristescu, S.M.; Risby, T.H.; Marczin, N. Lipid peroxidation in cardiac surgery: Towards consensus on biomonitoring, diagnostic tools and therapeutic implementation. J. Breath Res. 2018, 12, 027109. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, S.; Zhang, C.; Sun, J.; Zhang, D.; Chang, S.; Lin, Y.; Zhao, G. Higenamine Attenuates Neuropathic Pain by Inhibition of NOX2/ROS/TRP/P38 Mitogen-Activated Protein Kinase/NF-kB Signaling Pathway. Front. Pharmacol. 2021, 12, 716684. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Sudirman, S.; Yen, Y.W.; Mao, C.F.; Ong, A.D.; Kong, Z.L. Blue Mussel (Mytilus edulis) Water Extract Ameliorates Inflammatory Responses and Oxidative Stress on Osteoarthritis in Obese Rats. J. Pain Res. 2020, 13, 1109–1119. [Google Scholar] [CrossRef]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human Schwann cells. Exp. Neurol. 2012, 233, 154–162. [Google Scholar] [CrossRef]
- Hassler, S.N.; Johnson, K.M.; Hulsebosch, C.E. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J. Neurochem. 2014, 131, 413–417. [Google Scholar] [CrossRef]
- Hung, C.H.; Lee, C.H.; Tsai, M.H.; Chen, C.H.; Lin, H.F.; Hsu, C.Y.; Lai, C.L.; Chen, C.C. Activation of acid-sensing ion channel 3 by lysophosphatidylcholine 16:0 mediates psychological stress-induced fibromyalgia-like pain. Ann. Rheum. Dis. 2020, 79, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.X.; Zhu, H.Y.; Hu, Y.H. Effects of lysophosphatidylcholine on beta-amyloid-induced neuronal apoptosis. Acta Pharmacol. Sin. 2009, 30, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Hellenthal, K.E.M.; Brabenec, L.; Gross, E.R.; Wagner, N.M. TRP Channels as Sensors of Aldehyde and Oxidative Stress. Biomolecules 2021, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kokotou, M.G.; Vasilakaki, S.; Kokotos, G. Small-molecule inhibitors as potential therapeutics and as tools to understand the role of phospholipases A2. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 941–956. [Google Scholar] [CrossRef]
- Jianyong, Z.; Yanruo, H.; Xiaoju, T.; Yiping, W.; Fengming, L. Roles of Lipid Profiles in Human Non-Small Cell Lung Cancer. Technol. Cancer Res. Treat. 2021, 20, 15330338211041472. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Maity, S.; Patel, J.; Lupo, P.J.; Nembhard, W.N. Metabolomics Signatures and Subsequent Maternal Health among Mothers with a Congenital Heart Defect-Affected Pregnancy. Metabolites 2022, 12, 100. [Google Scholar] [CrossRef]
- Chiarini, A.; Armato, U.; Hu, P.; Dal Pra, I. Danger-Sensing/Patten Recognition Receptors and Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 9036. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Majchrzak-Hong, S.; Faurot, K.R.; Broste, S.K.; Frantz, R.P.; Davis, J.M.; Ringel, A.; Suchindran, C.M.; Hibbeln, J.R. Re-evaluation of the traditional diet-heart hypothesis: Analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ 2016, 353, i1246. [Google Scholar] [CrossRef]
- Sasso, O.; Wagner, K.; Morisseau, C.; Inceoglu, B.; Hammock, B.D.; Piomelli, D. Peripheral FAAH and soluble epoxide hydrolase inhibitors are synergistically antinociceptive. Pharmacol. Res. 2015, 97, 7–15. [Google Scholar] [CrossRef]
- Velasco, M.; O’Sullivan, C.; Sheridan, G.K. Lysophosphatidic acid receptors (LPARs): Potential targets for the treatment of neuropathic pain. Neuropharmacology 2017, 113, 608–617. [Google Scholar] [CrossRef]
- Huang, F.; Subbaiah, P.V.; Holian, O.; Zhang, J.; Johnson, A.; Gertzberg, N.; Lum, H. Lysophosphatidylcholine increases endothelial permeability: Role of PKCalpha and RhoA cross talk. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L176–L185. [Google Scholar] [CrossRef]
- Kabarowski, J.H.; Xu, Y.; Witte, O.N. Lysophosphatidylcholine as a ligand for immunoregulation. Biochem. Pharmacol. 2002, 64, 161–167. [Google Scholar] [CrossRef]
- Rimola, V.; Hahnefeld, L.; Zhao, J.; Jiang, C.; Angioni, C.; Schreiber, Y.; Osthues, T.; Pierre, S.; Geisslinger, G.; Ji, R.R.; et al. Lysophospholipids Contribute to Oxaliplatin-Induced Acute Peripheral Pain. J. Neurosci. 2020, 40, 9519–9532. [Google Scholar] [CrossRef]
- Vickers, K.C.; Castro-Chavez, F.; Morrisett, J.D. Lyso-phosphatidylcholine induces osteogenic gene expression and phenotype in vascular smooth muscle cells. Atherosclerosis 2010, 211, 122–129. [Google Scholar] [CrossRef][Green Version]
- Barbayianni, E.; Magrioti, V.; Moutevelis-Minakakis, P.; Kokotos, G. Autotaxin inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.M.; Schiller, J.; Galuska, C.E.; Fuchs, B. Phospholipases and Reactive Oxygen Species Derived Lipid Biomarkers in Healthy and Diseased Humans and Animals—A Focus on Lysophosphatidylcholine. Front. Physiol. 2021, 12, 732319. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Saw, W.Y.; Tantoso, E.; Begum, H.; Zhou, L.; Zou, R.; He, C.; Chan, S.L.; Tan, L.W.; Wong, L.P.; Xu, W.; et al. Establishing multiple omics baselines for three Southeast Asian populations in the Singapore Integrative Omics Study. Nat. Commun. 2017, 8, 653. [Google Scholar] [CrossRef]
- Cao, B.; Wang, D.; Pan, Z.; McIntyre, R.S.; Brietzke, E.; Subramanieapillai, M.; Nozari, Y.; Wang, J. Metabolic profiling for water-soluble metabolites in patients with schizophrenia and healthy controls in a Chinese population: A case-control study. World J. Biol. Psychiatry 2020, 21, 357–367. [Google Scholar] [CrossRef]
- Bergqvist, F.; Ossipova, E.; Idborg, H.; Raouf, J.; Checa, A.; Englund, K.; Englund, P.; Khoonsari, P.E.; Kultima, K.; Wheelock, C.E.; et al. Inhibition of mPGES-1 or COX-2 Results in Different Proteomic and Lipidomic Profiles in A549 Lung Cancer Cells. Front. Pharmacol. 2019, 10, 636. [Google Scholar]
- Liu, M.; Xie, Z.; Costello, C.A.; Zhang, W.; Chen, L.; Qi, D.; Furey, A.; Randell, E.W.; Rahman, P.; Zhai, G. Metabolomic analysis coupled with extreme phenotype sampling identified that lysophosphatidylcholines are associated with multisite musculoskeletal pain. Pain 2021, 162, 600–608. [Google Scholar] [CrossRef]
- Uranbileg, B.; Ito, N.; Kurano, M.; Saigusa, D.; Saito, R.; Uruno, A.; Kano, K.; Ikeda, H.; Yamada, Y.; Sumitani, M.; et al. Alteration of the lysophosphatidic acid and its precursor lysophosphatidylcholine levels in spinal cord stenosis: A study using a rat cauda equina compression model. Sci. Rep. 2019, 9, 16578. [Google Scholar]
- Rohnisch, H.E.; Kyro, C.; Olsen, A.; Thysell, E.; Hallmans, G.; Moazzami, A.A. Identification of metabolites associated with prostate cancer risk: A nested case-control study with long follow-up in the Northern Sweden Health and Disease Study. BMC Med. 2020, 18, 187. [Google Scholar] [CrossRef]
- Gessner, D.K.; Winkler, A.; Koch, C.; Dusel, G.; Liebisch, G.; Ringseis, R.; Eder, K. Analysis of hepatic transcript profile and plasma lipid profile in early lactating dairy cows fed grape seed and grape marc meal extract. BMC Genom. 2017, 18, 253. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, M.; Fu, T.; Li, Z.; Chen, Y.; He, T.; Feng, D.; Wang, Z.; Fan, Q.; Chen, M.; et al. Lipidomics Indicates the Hepatotoxicity Effects of EtOAc Extract of Rhizoma Paridis. Front. Pharmacol. 2022, 13, 799512. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bagarolo, G.I.; Thoroe-Boveleth, S.; Jankowski, J. “Lipidomics”: Mass spectrometric and chemometric analyses of lipids. Adv. Drug Deliv. Rev. 2020, 159, 294–307. [Google Scholar] [CrossRef]
- Shah, S.H.; Kraus, W.E.; Newgard, C.B. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: Form and function. Circulation 2012, 126, 1110–1120. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern analytical techniques in metabolomics analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef]
- Malatji, B.G.; Meyer, H.; Mason, S.; Engelke, U.F.H.; Wevers, R.A.; van Reenen, M.; Reinecke, C.J. A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurol. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Mickiewicz, B.; Kelly, J.J.; Ludwig, T.E.; Weljie, A.M.; Wiley, J.P.; Schmidt, T.A.; Vogel, H.J. Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. J. Orthop. Res. 2015, 33, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A.; van der Plas, A.A.; van Dasselaar, N.T.; Deelder, A.M.; van Hilten, J.J.; Mayboroda, O.A. 1H-NMR metabolic profiling of cerebrospinal fluid in patients with complex regional pain syndrome-related dystonia. Pain 2014, 155, 190–196. [Google Scholar] [CrossRef]
- Kim, J.W.; Ryu, S.H.; Kim, S.; Lee, H.W.; Lim, M.S.; Seong, S.J.; Kim, S.; Yoon, Y.R.; Kim, K.B. Pattern recognition analysis for hepatotoxicity induced by acetaminophen using plasma and urinary 1H NMR-based metabolomics in humans. Anal. Chem. 2013, 85, 11326–11334. [Google Scholar] [CrossRef]
- Hsu, W.H.; Lee, C.H.; Chao, Y.M.; Kuo, C.H.; Ku, W.C.; Chen, C.C.; Lin, Y.L. ASIC3-dependent metabolomics profiling of serum and urine in a mouse model of fibromyalgia. Sci. Rep. 2019, 9, 12123. [Google Scholar] [CrossRef]
- Ciborowski, M.; Lipska, A.; Godzien, J.; Ferrarini, A.; Korsak, J.; Radziwon, P.; Tomasiak, M.; Barbas, C. Combination of LC-MS- and GC-MS-based metabolomics to study the effect of ozonated autohemotherapy on human blood. J. Proteome. Res. 2012, 11, 6231–6241. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Malatji, B.G.; Mason, S.; Mienie, L.J.; Wevers, R.A.; Meyer, H.; van Reenen, M.; Reinecke, C.J. The GC-MS metabolomics signature in patients with fibromyalgia syndrome directs to dysbiosis as an aspect contributing factor of FMS pathophysiology. Metabolomics 2019, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Wang, M.; Shi, W.; Du, X.; Sun, C. The toxicity of 3-chloropropane-1,2-dipalmitate in Wistar rats and a metabonomics analysis of rat urine by ultra-performance liquid chromatography-mass spectrometry. Chem. Biol. Interact. 2013, 206, 337–345. [Google Scholar] [CrossRef]
- Lan, K.; Zhang, Y.; Yang, J.; Xu, L. Simple quality assessment approach for herbal extracts using high performance liquid chromatography-UV based metabolomics platform. J. Chromatogr. A 2010, 1217, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, Y.; Takahashi, M.; Sugiura, Y.; Izumi, Y.; Nishiyama, K.; Nishida, M.; Suematsu, M.; Bamba, T.; Yamada, K.I. Structural library and visualization of endogenously oxidized phosphatidylcholines using mass spectrometry-based techniques. Nat. Commun. 2021, 12, 6339. [Google Scholar] [CrossRef]
- Goodarzi, P.; Alavi-Moghadam, S.; Payab, M.; Larijani, B.; Rahim, F.; Gilany, K.; Bana, N.; Tayanloo-Beik, A.; Foroughi Heravani, N.; Hadavandkhani, M.; et al. Metabolomics Analysis of Mesenchymal Stem Cells. Int. J. Mol. Cell. Med. 2019, 8, 30–40. [Google Scholar] [PubMed]
- Guennec, A.L.; Giraudeau, P.; Caldarelli, S. Evaluation of fast 2D NMR for metabolomics. Anal. Chem. 2014, 86, 5946–5954. [Google Scholar] [CrossRef] [PubMed]
- Wagen, C.C.; Ingoglia, B.T.; Buchwald, S.L. Unexpected Formation of Hexasubstituted Arenes through a 2-fold Palladium-Mediated Ligand Arylation. J. Org. Chem. 2019, 84, 12672–12679. [Google Scholar] [CrossRef] [PubMed]
- Kostara, C.E.; Ferrannini, E.; Bairaktari, E.T.; Papathanasiou, A.; Elisaf, M.; Tsimihodimos, V. Early Signs of Atherogenic Features in the HDL Lipidomes of Normolipidemic Patients Newly Diagnosed with Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 8835. [Google Scholar] [CrossRef]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS-based metabolomics. Mol. Biosyst. 2012, 8, 470–481. [Google Scholar] [CrossRef]
- Loos, G.; Van Schepdael, A.; Cabooter, D. Quantitative mass spectrometry methods for pharmaceutical analysis. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2016, 374, 20150366. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.S.; Shearer, J. Metabolomics and Type 2 Diabetes: Translating Basic Research into Clinical Application. J. Diabetes Res. 2016, 2016, 3898502. [Google Scholar] [CrossRef]
- Feng, H.; Wu, Y.Q.; Xu, Y.S.; Wang, K.X.; Qin, X.M.; Lu, Y.F. LC-MS-Based Metabolomic Study of Oleanolic Acid-Induced Hepatotoxicity in Mice. Front. Pharmacol. 2020, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Gadepalli, S.G.; Deme, P.; Kuncha, M.; Sistla, R. Simultaneous determination of amlodipine, valsartan and hydrochlorothiazide by LC-ESI-MS/MS and its application to pharmacokinetics in rats. J. Pharm. Anal. 2014, 4, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kostic, N.; Dotsikas, Y.; Jovic, N.; Stevanovic, G.; Malenovic, A.; Medenica, M. Quantitation of pregabalin in dried blood spots and dried plasma spots by validated LC-MS/MS methods. J. Pharm. Biomed. Anal. 2015, 109, 79–84. [Google Scholar] [CrossRef] [PubMed]
- de Meulder, M.; Waldron, M.P.; Li, L.; Peay, M.G.; Tingler, M.J.; Hidy, B.J.; Verhaeghe, T.; Jenkins, R.G. Development and validation of HILIC-ESI/MS/MS methods for simultaneous quantitation of several antipsychotics in human plasma and blood. Bioanalysis 2016, 8, 765–794. [Google Scholar] [CrossRef] [PubMed]
- Gakuubi, M.M.; Wagacha, J.M.; Dossaji, S.F.; Wanzala, W. Chemical Composition and Antibacterial Activity of Essential Oils of Tagetes minuta (Asteraceae) against Selected Plant Pathogenic Bacteria. Int. J. Microbiol. 2016, 2016, 7352509. [Google Scholar] [CrossRef]
- Zarate, E.; Boyle, V.; Rupprecht, U.; Green, S.; Villas-Boas, S.G.; Baker, P.; Pinu, F.R. Fully Automated Trimethylsilyl (TMS) Derivatisation Protocol for Metabolite Profiling by GC-MS. Metabolites 2016, 7, 1. [Google Scholar] [CrossRef]
- Kanani, H.; Chrysanthopoulos, P.K.; Klapa, M.I. Standardizing GC-MS metabolomics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 871, 191–201. [Google Scholar] [CrossRef]
- Spagou, K.; Theodoridis, G.; Wilson, I.; Raikos, N.; Greaves, P.; Edwards, R.; Nolan, B.; Klapa, M.I. A GC-MS metabolic profiling study of plasma samples from mice on low- and high-fat diets. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.A.; Djukovic, D. Overview of mass spectrometry-based metabolomics: Opportunities and challenges. Methods Mol. Biol. 2014, 1198, 3–12. [Google Scholar] [PubMed]
- Mastrangelo, A.; Ferrarini, A.; Rey-Stolle, F.; Garcia, A.; Barbas, C. From sample treatment to biomarker discovery: A tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta 2015, 900, 21–35. [Google Scholar] [CrossRef]
- Lynch, K.B.; Chen, A.; Liu, S. Miniaturized high-performance liquid chromatography instrumentation. Talanta 2018, 177, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L. HPLC in natural product analysis: The detection issue. Planta Med. 2009, 75, 719–734. [Google Scholar] [CrossRef]
- Nahar, L.; Onder, A.; Sarker, S.D. A review on the recent advances in HPLC, UHPLC and UPLC analyses of naturally occurring cannabinoids (2010–2019). Phytochem. Anal. 2020, 31, 413–457. [Google Scholar] [CrossRef]
- Vigneau-Callahan, K.E.; Shestopalov, A.I.; Milbury, P.E.; Matson, W.R.; Kristal, B.S. Characterization of diet-dependent metabolic serotypes: Analytical and biological variability issues in rats. J. Nutr. 2001, 131, 924S–932S. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, A.; Lestremau, F.; Szucs, R.; Gelebart, S.; David, F.; Sandra, P. Evaluation of ultra performance liquid chromatography. Part, I. Possibilities and limitations. J. Chromatogr. A 2006, 1127, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, I.; Gliszczynska-Swiglo, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef]
- Zaima, N.; Hayasaka, T.; Goto-Inoue, N.; Setou, M. Matrix-assisted laser desorption/ionization imaging mass spectrometry. Int. J. Mol. Sci. 2010, 11, 5040–5055. [Google Scholar] [CrossRef]
- Sadler, K.E.; Moehring, F.; Shiers, S.I.; Laskowski, L.J.; Mikesell, A.R.; Plautz, Z.R.; Brezinski, A.N.; Mecca, C.M.; Dussor, G.; Price, T.J.; et al. Transient receptor potential canonical 5 mediates inflammatory mechanical and spontaneous pain in mice. Sci. Transl. Med. 2021, 13, eabd7702. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, F.; Khoury, S.; Labrum, B.; Delanoe, K.; Pidoux, L.; Barbier, J.; Delay, L.; Bayle, A.; Aissouni, Y.; Barriere, D.A.; et al. Lysophosphatidylcholine 16: 0 mediates chronic joint pain associated to rheumatic diseases through acid-sensing ion channel 3. Pain 2022. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, A.; Delay, L.; Barbier, J.; Simon, N.; Krock, E.; Sandor, K.; Agalave, N.M.; Rudjito, R.; Wigerblad, G.; Rogoz, K.; et al. Antibody-induced pain-like behavior and bone erosion: Links to subclinical inflammation, osteoclast activity, and acid-sensing ion channel 3-dependent sensitization. Pain 2021, 163, 1542–1559. [Google Scholar] [CrossRef]
- Nagai, J.; Ueda, H. Pre-emptive morphine treatment abolishes nerve injury-induced lysophospholipid synthesis in mass spectrometrical analysis. J. Neurochem. 2011, 118, 256–265. [Google Scholar] [CrossRef]
- Muley, M.M.; Krustev, E.; McDougall, J.J. Preclinical Assessment of Inflammatory Pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andres-Marin, N.; Fernandez-Eulate, G.; Abecia, L.; Lavin, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. Ebiomedicine 2019, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Liori, B.; Kumar, A.; Santoru, M.L.; Asthana, S.; Pieroni, E.; Fais, A.; Era, B.; Cacace, E.; Ruggiero, V.; et al. Metabolomics analysis and modeling suggest a lysophosphocholines-PAF receptor interaction in fibromyalgia. PLoS ONE 2014, 9, e107626. [Google Scholar]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Kuffler, D.P. Injury-Induced Effectors of Neuropathic Pain. Mol. Neurobiol. 2020, 57, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Osthues, T.; Sisignano, M. Oxidized Lipids in Persistent Pain States. Front. Pharmacol. 2019, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Tsai, Y.J.; Chen, S.H.; Lin, C.T.; Lue, J.H. Lysophosphatidylcholine causes neuropathic pain via the increase of neuronal nitric oxide synthase in the dorsal root ganglion and cuneate nucleus. Pharmacol. Biochem. Behav. 2013, 106, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Chen, S.H.; Chang, C.F.; Lin, S.C.; Lue, J.H.; Tsai, Y.J. Melatonin reduces neuropathic pain behavior and glial activation through MT2 melatonin receptor modulation in a rat model of lysophosphatidylcholine-induced demyelination neuropathy. Neurochem. Int. 2020, 140, 104827. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Tanaka, H.; Sayanagi, J.; Iwahashi, T.; Suzuki, K.; Nishimoto, S.; Okada, K.; Murase, T.; Yoshikawa, H. Neurotropin((R)) Accelerates the Differentiation of Schwann Cells and Remyelination in a Rat Lysophosphatidylcholine-Induced Demyelination Model. Int. J. Mol. Sci. 2018, 19, 516. [Google Scholar] [CrossRef]
- Wallace, V.C.; Cottrell, D.F.; Brophy, P.J.; Fleetwood-Walker, S.M. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J. Neurosci. 2003, 23, 3221–3233. [Google Scholar] [CrossRef] [PubMed]
- Ousman, S.S.; David, S. MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J. Neurosci. 2001, 21, 4649–4656. [Google Scholar] [CrossRef]
- Serizawa, K.; Tomizawa-Shinohara, H.; Miyake, S.; Yogo, K.; Matsumoto, Y. Interleukin-6: Evolving role in the management of neuropathic pain in neuroimmunological disorders. Inflamm. Regen. 2021, 41, 34. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Li, Z.; Li, Y.; Yu, Y.; Zhang, L. The Involvement of Caspases in Neuroinflammation and Neuronal Apoptosis in Chronic Pain and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 898574. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, Y.H.; Lee, Y.; Jung, S.J.; Oh, S.B. TRPM2 contributes to LPC-induced intracellular Ca(2+) influx and microglial activation. Biochem. Biophys. Res. Commun. 2017, 485, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Ousman, S.S.; David, S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia 2000, 30, 92–104. [Google Scholar] [CrossRef]
- el Waly, B.; Buttigieg, E.; Karakus, C.; Brustlein, S.; Debarbieux, F. Longitudinal Intravital Microscopy Reveals Axon Degeneration Concomitant With Inflammatory Cell Infiltration in an LPC Model of Demyelination. Front. Cell. Neurosci. 2020, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, N.; Jeong, S.Y.; Lacroix, S.; David, S. T cells contribute to lysophosphatidylcholine-induced macrophage activation and demyelination in the CNS. Glia 2007, 55, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Xie, W.; Matsushita, Y.; Chun, J.; Aoki, J.; Ueda, H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience 2008, 152, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.L.; Yeo, M.; Zhang, Q.J.; Lopez-Romero, A.E.; Ding, H.P.; Zhang, X.; Zeng, Q.; Morales-Lazaro, S.L.; Moore, C.; et al. Epithelia-Sensory Neuron Cross Talk Underlies Cholestatic Itch Induced by Lysophosphatidylcholine. Gastroenterology 2021, 161, 301–317 e316. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Morioka, N.; Abdin, J.; Kitayama, S.; Nakata, Y.; Dohi, T. Development of tactile allodynia and thermal hyperalgesia by intrathecally administered platelet-activating factor in mice. Pain 2004, 111, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Rashid, M.H.; Fujita, R.; Contos, J.J.; Chun, J.; Ueda, H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 2004, 10, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, K.; Swearingen, C.A.; Oskins, J.L.; Lin, C.; Bui, H.H.; Jones, S.B.; Pfeifer, L.A.; Norman, B.H.; Mitchell, P.G.; Chambers, M.G. Identification and pharmacological characterization of a novel inhibitor of autotaxin in rodent models of joint pain. Osteoarthr. Cartil. 2017, 25, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Uranbileg, B.; Ito, N.; Kurano, M.; Kano, K.; Uchida, K.; Sumitani, M.; Aoki, J.; Yatomi, Y. Inhibition of autotaxin activity ameliorates neuropathic pain derived from lumbar spinal canal stenosis. Sci. Rep. 2021, 11, 3984. [Google Scholar] [CrossRef]
- Nagai, J.; Uchida, H.; Matsushita, Y.; Yano, R.; Ueda, M.; Niwa, M.; Aoki, J.; Chun, J.; Ueda, H. Autotaxin and lysophosphatidic acid1 receptor-mediated demyelination of dorsal root fibers by sciatic nerve injury and intrathecal lysophosphatidylcholine. Mol. Pain 2010, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Herr, D.R.; Chew, W.S.; Satish, R.L.; Ong, W.Y. Pleotropic Roles of Autotaxin in the Nervous System Present Opportunities for the Development of Novel Therapeutics for Neurological Diseases. Mol. Neurobiol. 2020, 57, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K.; Nakajima, S.; Gotoh, M.; Tanaka, S.; Murofushi, H.; Murakami-Murofushi, K. Qualitative and quantitative comparison of cyclic phosphatidic acid and its related lipid species in rat serum using hydrophilic interaction liquid chromatography with tandem-mass spectrometry. J. Chromatogr. A 2018, 1567, 177–184. [Google Scholar] [CrossRef]
- Tsuda, S.; Okudaira, S.; Moriya-Ito, K.; Shimamoto, C.; Tanaka, M.; Aoki, J.; Arai, H.; Murakami-Murofushi, K.; Kobayashi, T. Cyclic phosphatidic acid is produced by autotaxin in blood. J. Biol. Chem. 2006, 281, 26081–26088. [Google Scholar] [CrossRef]
- Kakiuchi, Y.; Nagai, J.; Gotoh, M.; Hotta, H.; Murofushi, H.; Ogawa, T.; Ueda, H.; Murakami-Murofushi, K. Antinociceptive effect of cyclic phosphatidic acid and its derivative on animal models of acute and chronic pain. Mol. Pain 2011, 7, 33. [Google Scholar] [CrossRef]
- Gotoh, M.; Nagano, A.; Tsukahara, R.; Murofushi, H.; Morohoshi, T.; Otsuka, K.; Murakami-Murofushi, K. Cyclic phosphatidic acid relieves osteoarthritis symptoms. Mol. Pain 2014, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Li, S.; Jaffe, K.; Davis, L. Quantitative determination of cyclic phosphatidic acid in human serum by LC/ESI/MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 862, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Shindou, H.; Shiraishi, S.; Tokuoka, S.M.; Takahashi, Y.; Harayama, T.; Abe, T.; Bando, K.; Miyano, K.; Kita, Y.; Uezono, Y.; et al. Relief from neuropathic pain by blocking of the platelet-activating factor-pain loop. FASEB J. 2017, 31, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Murakami, N.; Yokomizo, T.; Okuno, T.; Shimizu, T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J. Biol. Chem. 2004, 279, 42484–42491. [Google Scholar] [CrossRef] [PubMed]
- Radu, C.G.; Yang, L.V.; Riedinger, M.; Au, M.; Witte, O.N. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.V.; Radu, C.G.; Wang, L.; Riedinger, M.; Witte, O.N. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood 2005, 105, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Osthues, T.; Zimmer, B.; Rimola, V.; Klann, K.; Schilling, K.; Mathoor, P.; Angioni, C.; Weigert, A.; Geisslinger, G.; Munch, C.; et al. The Lipid Receptor G2A (GPR132) Mediates Macrophage Migration in Nerve Injury-Induced Neuropathic Pain. Cells 2020, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Merlo, S.; Drago, F.; Caruso, G.; Parenti, C.; Sortino, M.A. Rescue of Noradrenergic System as a Novel Pharmacological Strategy in the Treatment of Chronic Pain: Focus on Microglia Activation. Front. Pharmacol. 2019, 10, 1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, X.; Guo, F.; Sun, X.; Yuan, K.; Wang, Q.; Lan, C. Lysophosphatidylcholine induces apoptosis and inflammatory damage in brain microvascular endothelial cells via GPR4-mediated NLRP3 inflammasome activation. Toxicol. Vitr. 2021, 77, 105227. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Wang, H.; Fan, X.; An, N.; Li, J.; Song, H.; Kong, E.; Li, Y.; Yuan, H. BRD4 Inhibition Attenuates Inflammatory Pain by Ameliorating NLRP3 Inflammasome-Induced Pyroptosis. Front. Immunol. 2022, 13, 837977. [Google Scholar] [CrossRef] [PubMed]
- Drzazga, A.; Okulus, M.; Rychlicka, M.; Biegala, L.; Gliszczynska, A.; Gendaszewska-Darmach, E. Lysophosphatidylcholine Containing Anisic Acid Is Able to Stimulate Insulin Secretion Targeting G Protein Coupled Receptors. Nutrients 2020, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Drzazga, A.; Kaminska, D.; Gliszczynska, A.; Gendaszewska-Darmach, E. Isoprenoid Derivatives of Lysophosphatidylcholines Enhance Insulin and GLP-1 Secretion through Lipid-Binding GPCRs. Int. J. Mol. Sci. 2021, 22, 5748. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; He, L. The role of polyunsaturated fatty acids and GPR40 receptor in brain. Neuropharmacology 2017, 113, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Lingerfelt, M.A.; Zhao, P.; Sharir, H.P.; Hurst, D.P.; Reggio, P.H.; Abood, M.E. Identification of Crucial Amino Acid Residues Involved in Agonist Signaling at the GPR55 Receptor. Biochemistry 2017, 56, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Helley, M.P.; Abate, W.; Jackson, S.K.; Bennett, J.H.; Thompson, S.W. The expression of Toll-like receptor 4, 7 and co-receptors in neurochemical sub-populations of rat trigeminal ganglion sensory neurons. Neuroscience 2015, 310, 686–698. [Google Scholar] [CrossRef]
- Lacagnina, M.J.; Watkins, L.R.; Grace, P.M. Toll-like receptors and their role in persistent pain. Pharmacol. Ther. 2018, 184, 145–158. [Google Scholar] [CrossRef]
- Chen, R.X.; Dai, M.D.; Zhang, Q.Z.; Lu, M.P.; Wang, M.L.; Yin, M.; Zhu, X.J.; Wu, Z.F.; Zhang, Z.D.; Cheng, L. TLR Signaling Pathway Gene Polymorphisms, Gene-Gene and Gene-Environment Interactions in Allergic Rhinitis. J. Inflamm. Res. 2022, 15, 3613–3630. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gao, Y.J.; Ji, R.R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012, 28, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Acioglu, C.; Heary, R.F.; Elkabes, S. Roles of neuronal toll-like receptors in neuropathic pain and central nervous system injuries and diseases. Brain Behav. Immun. 2022, 102, 163–178. [Google Scholar] [CrossRef]
- Thakur, K.K.; Saini, J.; Mahajan, K.; Singh, D.; Jayswal, D.P.; Mishra, S.; Bishayee, A.; Sethi, G.; Kunnumakkara, A.B. Therapeutic implications of toll-like receptors in peripheral neuropathic pain. Pharmacol. Res. 2017, 115, 224–232. [Google Scholar] [CrossRef]
- Miller, R.E.; Scanzello, C.R.; Malfait, A.M. An emerging role for Toll-like receptors at the neuroimmune interface in osteoarthritis. Semin. Immunopathol. 2019, 41, 583–594. [Google Scholar] [CrossRef]
- Stokes, J.A.; Cheung, J.; Eddinger, K.; Corr, M.; Yaksh, T.L. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J. Neuroinflammation 2013, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Schrepf, A.; Bradley, C.S.; O’Donnell, M.; Luo, Y.; Harte, S.E.; Kreder, K.; Lutgendorf, S.; Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network. Toll-like receptor 4 and comorbid pain in Interstitial Cystitis/Bladder Pain Syndrome: A multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav. Immun. 2015, 49, 66–74. [Google Scholar] [CrossRef]
- Grace, P.M.; Strand, K.A.; Galer, E.L.; Urban, D.J.; Wang, X.; Baratta, M.V.; Fabisiak, T.J.; Anderson, N.D.; Cheng, K.; Greene, L.I.; et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA 2016, 113, E3441–E3450. [Google Scholar] [CrossRef]
- Qi, J.; Buzas, K.; Fan, H.; Cohen, J.I.; Wang, K.; Mont, E.; Klinman, D.; Oppenheim, J.J.; Howard, O.M. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J. Immunol. 2011, 186, 6417–6426. [Google Scholar] [CrossRef]
- Sharma, N.; Akhade, A.S.; Ismaeel, S.; Qadri, A. Serum-borne lipids amplify TLR-activated inflammatory responses. J. Leukoc. Biol. 2021, 109, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Socuellamos, P.G.; Olivos-Ore, L.A.; Barahona, M.V.; Cercos, P.; Perez Pascual, M.; Arribas-Blazquez, M.; Naranjo, J.R.; Valenzuela, C.; Gutierrez-Rodriguez, M.; Artalejo, A.R. IQM-PC332, a Novel DREAM Ligand with Antinociceptive Effect on Peripheral Nerve Injury-Induced Pain. Int. J. Mol. Sci. 2022, 23, 2142. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.S.; Brito, R.G.; Fusaro, M.; Sluka, K.A. ASIC3 Is Required for Development of Fatigue-Induced Hyperalgesia. Mol. Neurobiol. 2016, 53, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Marra, S.; Ferru-Clement, R.; Breuil, V.; Delaunay, A.; Christin, M.; Friend, V.; Sebille, S.; Cognard, C.; Ferreira, T.; Roux, C.; et al. Non-acidic activation of pain-related Acid-Sensing Ion Channel 3 by lipids. EMBO J. 2016, 35, 414–428. [Google Scholar] [CrossRef]
- Sevastou, I.; Kaffe, E.; Mouratis, M.A.; Aidinis, V. Lysoglycerophospholipids in chronic inflammatory disorders: The PLA(2)/LPC and ATX/LPA axes. Biochim. Biophys. Acta 2013, 1831, 42–60. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, G.; Aitken, D.; Likhodii, S.; Liu, M.; Martin, G.; Furey, A.; Randell, E.; Rahman, P.; Jones, G.; et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology 2016, 55, 1566–1574. [Google Scholar] [CrossRef]
- Hayakawa, K.; Kurano, M.; Ohya, J.; Oichi, T.; Kano, K.; Nishikawa, M.; Uranbileg, B.; Kuwajima, K.; Sumitani, M.; Tanaka, S.; et al. Lysophosphatidic acids and their substrate lysophospholipids in cerebrospinal fluid as objective biomarkers for evaluating the severity of lumbar spinal stenosis. Sci. Rep. 2019, 9, 9144. [Google Scholar] [CrossRef]
- Rabini, R.A.; Galassi, R.; Fumelli, P.; Dousset, N.; Solera, M.L.; Valdiguie, P.; Curatola, G.; Ferretti, G.; Taus, M.; Mazzanti, L. Reduced Na(+)-K(+)-ATPase activity and plasma lysophosphatidylcholine concentrations in diabetic patients. Diabetes 1994, 43, 915–919. [Google Scholar] [CrossRef]
- Ren, C.; Liu, J.; Zhou, J.; Liang, H.; Wang, Y.; Sun, Y.; Ma, B.; Yin, Y. Lipidomic analysis of serum samples from migraine patients. Lipids Health Dis. 2018, 17, 22. [Google Scholar] [CrossRef]
- Wang, G.; Qiu, M.; Xing, X.; Zhou, J.; Yao, H.; Li, M.; Yin, R.; Hou, Y.; Li, Y.; Pan, S.; et al. Lung cancer scRNA-seq and lipidomics reveal aberrant lipid metabolism for early-stage diagnosis. Sci. Transl. Med. 2022, 14, eabk2756. [Google Scholar] [CrossRef]
- Wolrab, D.; Jirasko, R.; Cifkova, E.; Horing, M.; Mei, D.; Chocholouskova, M.; Peterka, O.; Idkowiak, J.; Hrnciarova, T.; Kuchar, L.; et al. Lipidomic profiling of human serum enables detection of pancreatic cancer. Nat. Commun. 2022, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, X.; Zhang, X.; Han, F.; Lu, X.; Liu, L.; Zhang, J.; Dong, M.; Yang, H.; Li, H. Pharmacometabolomics Identifies 3-Hydroxyadipic Acid, d-Galactose, Lysophosphatidylcholine (P-16:0), and Tetradecenoyl-l-Carnitine as Potential Predictive Indicators of Gemcitabine Efficacy in Pancreatic Cancer Patients. Front. Oncol. 2019, 9, 1524. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, S.M.; Fernandes Silva, L.; Lankinen, M.A.; Schwab, U.; Laakso, M. Metabolite Signature of Physical Activity and the Risk of Type 2 Diabetes in 7271 Men. Metabolites 2022, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, H.; Yang, Y.; Yu, X.; Zhang, X.; Xing, Q.; Zhang, G. Using Metabolomics in Diabetes Management with Traditional Chinese Medicine: A Review. Am. J. Chin. Med. 2021, 49, 1813–1837. [Google Scholar] [CrossRef]
- Chen, S.; Zong, G.; Wu, Q.; Yun, H.; Niu, Z.; Zheng, H.; Zeng, R.; Sun, L.; Lin, X. Associations of plasma glycerophospholipid profile with modifiable lifestyles and incident diabetes in middle-aged and older Chinese. Diabetologia 2022, 65, 315–328. [Google Scholar] [CrossRef]
- Suvitaival, T.; Bondia-Pons, I.; Yetukuri, L.; Poho, P.; Nolan, J.J.; Hyotylainen, T.; Kuusisto, J.; Oresic, M. Lipidome as a predictive tool in progression to type 2 diabetes in Finnish men. Metabolism 2018, 78, 1–12. [Google Scholar] [CrossRef]
- Tan, S.H.; Koh, H.W.L.; Chua, J.Y.; Burla, B.; Ong, C.C.; Teo, L.S.L.; Yang, X.; Benke, P.I.; Choi, H.; Torta, F.; et al. Variability of the Plasma Lipidome and Subclinical Coronary Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 100–112. [Google Scholar] [CrossRef]
- Pena-Bautista, C.; Alvarez-Sanchez, L.; Canada-Martinez, A.J.; Baquero, M.; Chafer-Pericas, C. Epigenomics and Lipidomics Integration in Alzheimer Disease: Pathways Involved in Early Stages. Biomedicines 2021, 9, 1812. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Kim, H.S.; Cho, J.Y. Nicotinamide attenuates the decrease in dendritic spine density in hippocampal primary neurons from 5xFAD mice, an Alzheimer’s disease animal model. Mol. Brain 2020, 13, 17. [Google Scholar] [CrossRef]
- Masuda, R.; Lodge, S.; Whiley, L.; Gray, N.; Lawler, N.; Nitschke, P.; Bong, S.H.; Kimhofer, T.; Loo, R.L.; Boughton, B.; et al. Exploration of Human Serum Lipoprotein Supramolecular Phospholipids Using Statistical Heterospectroscopy in n-Dimensions (SHY-n): Identification of Potential Cardiovascular Risk Biomarkers Related to SARS-CoV-2 Infection. Anal. Chem. 2022, 94, 4426–4436. [Google Scholar] [CrossRef]
- Yoshioka, K.; Hirakawa, Y.; Kurano, M.; Ube, Y.; Ono, Y.; Kojima, K.; Iwama, T.; Kano, K.; Hasegawa, S.; Inoue, T.; et al. Lysophosphatidylcholine mediates fast decline in kidney function in diabetic kidney disease. Kidney Int. 2022, 101, 510–526. [Google Scholar] [CrossRef]
- Ismaiel, A.; Spinu, M.; Socaciu, C.; Budisan, L.; Leucuta, D.C.; Popa, S.L.; Chis, B.A.; Berindan-Neagoe, I.; Olinic, D.M.; Dumitrascu, D.L. Metabolic biomarkers related to cardiac dysfunction in metabolic-dysfunction-associated fatty liver disease: A cross-sectional analysis. Nutr. Diabetes 2022, 12, 4. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).