Abstract
Factor XIII (FXIII) is a transglutaminase enzyme that catalyses the formation of ε-(γ-glutamyl)lysyl isopeptide bonds into protein substrates. The plasma form, FXIIIA2B2, has an established function in haemostasis, with fibrin being its principal substrate. A deficiency in FXIII manifests as a severe bleeding diathesis emphasising its crucial role in this pathway. The FXIII-A gene (F13A1) is expressed in cells of bone marrow and mesenchymal lineage. The cellular form, a homodimer of the A subunits denoted FXIII-A, was perceived to remain intracellular, due to the lack of a classical signal peptide for its release. It is now apparent that FXIII-A can be externalised from cells, by an as yet unknown mechanism. Thus, three pools of FXIII-A exist within the circulation: plasma where it circulates in complex with the inhibitory FXIII-B subunits, and the cellular form encased within platelets and monocytes/macrophages. The abundance of this transglutaminase in different forms and locations in the vasculature reflect the complex and crucial roles of this enzyme in physiological processes. Herein, we examine the significance of these pools of FXIII-A in different settings and the evidence to date to support their function in haemostasis and wound healing.
1. Background
FXIII belongs to the transglutaminase family of enzymes which is named according to its crucial role in blood coagulation. FXIII is a zymogen that must be activated to reveal its transglutaminase function [1]. Plasma FXIII (pFXIII) circulates as a heterotetramer, termed FXIII-A2B2, which is comprised of two catalytic A subunits and two carrier B subunits which envelope the catalytic subunits [2,3]. pFXIII circulates at an average concentration of 68 nM [4]. The A subunits in plasma exist only in complex with the carrier B subunits, while an excess (43–62 nM) of B homodimers is evident in the circulation [4,5]. pFXIII is largely found in a non-covalent complex with one of its dominant substrates, fibrinogen (KD = ~10 nM) [6]. Thrombin cleaves the Arg37-Gly38 peptide bond in the activation peptide (AP-FXIII) which flank the amino terminus of the A2 subunits thereby destabilising the complex (Figure 1A). Subsequent binding of Ca2+ promotes dissociation of the inhibitory FXIII-B2 subunits to release a functional transglutaminase enzyme, FXIII-A2*. Other serine proteases, such as the endogenous platelet acid protease [7] and calpain [8] can also reportedly activate FXIII. Fibrin acts a s a cofactor in activation of FXIII by forming a tertiary complex with thrombin and FXIII thereby promoting cleavage [6,9,10,11,12]. Once activated FXIII (FXIIIa) elicits transamidase activity that introduces ε-(γ-glutamyl)lysyl isopeptide cross-links into protein substrates. Crosslinks can form within a single substrate, such as fibrin, or between different proteins which can impact on their biological function [13].
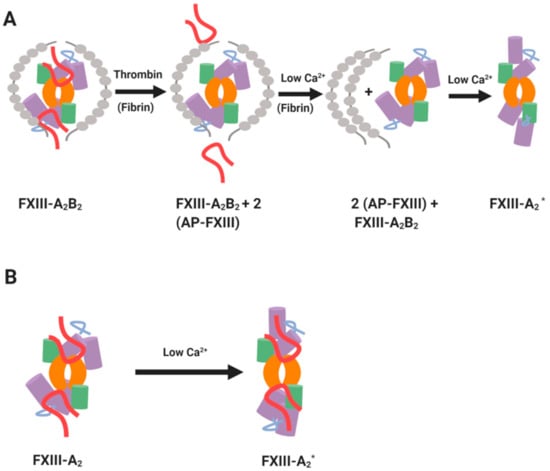
Figure 1.
Mechanisms of FXIII activation. (A) Thrombin- and Ca2+-driven cleavage of FXIIIA2B2. (B) Non-proteolytic activation of cellular FXIII-A by low Ca²⁺. The green and purple cylinders represent β-barrel and β-sandwich domains of FXIII-A subunit, respectively. The core domains in FXIII-A subunit are shown in orange and the activation peptides are shown in red. The inhibitory B subunits are shown in grey. Adapted from the work in [1].
The cellular form of FXIII is a homodimer of the A subunits, termed FXIII-A throughout this review [14]. Cellular FXIII-A is non-proteolytically activated to FXIII-A* by modest increases in intracellular Ca2+ concentrations (Figure 1B) [15,16]. FXIII-A exists in cells of bone marrow and mesenchymal lineage, notably platelets [17,18,19], megakaryocytes [20] monocytes [21,22], circulating [21,23] and tissue macrophages [23], dendritic cells [24], chondrocytes [25,26,27], osteoblasts [28] and preadipocytes [29]. Regulation of FXIII-A* is a complex area. It is clear that the regulatory “B” subunits function to attenuate FXIIIA2B2 in plasma and stabilise the complex in this milieu [30]. However, once activated, the story is more complex. Elegant studies have delineated a role for plasmin regulation of FXIIIa [31], but the FXIIIA2B2 complex is protected against degradation. There is also a suggested role of thrombin [32] and proteolytic enzymes secreted from granulocytes [33] in regulation of FXIII* function. The focus of this review will be on the localization and function of the FXIII-A subunit and examine its crucial function in regulation of haemostasis and wound healing.
2. Structural Considerations
Identification and cloning of the F13A1 and F13B genes led to recombinant expression of the FXIIIA2 and FXIIIB2 subunits [34]. This led to a description of the zymogenic form of the A2 homodimer [35,36] and subsequently a Ca2+ activated and inhibitor stabilized FXIIII-A subunit [37]. The structure of the plasma and cellular forms is identical, which provided an early clue that the plasma pool of FXIII-A was of haematopoietic origin. FXIII-A (Figure 2; 83 kDa) is comprised of several domains including the activation peptide (AP-FXIII (1–37), β-sandwich domain (38–184), the catalytic core domain (185–515), β-barrel-1 domain (516–628) and β-barrel-2 domain (629–731) [38]. The catalytic core domain is largely comprised of helical structures; however, the rest of the domains contain β-sheets with limited helical elements [38] (Figure 2). The catalytic cores of two FXIII-A subunits align to form the FXIII-A2 dimer which is encased by the six β-sheet domains [39]. The active site cysteine residue (Cys314) is completely encased by the AP-FXIII, to impede interaction with target substrates [35]. Dissociation of AP-FXIII promotes structural rearrangement of the catalytic triad (Cys314; His373; Asp396) to allow docking of substrate to the active site. There is also a single Ca2+ binding site per FXIII-A subunit that is crucial for activation of the transglutaminase [40,41].
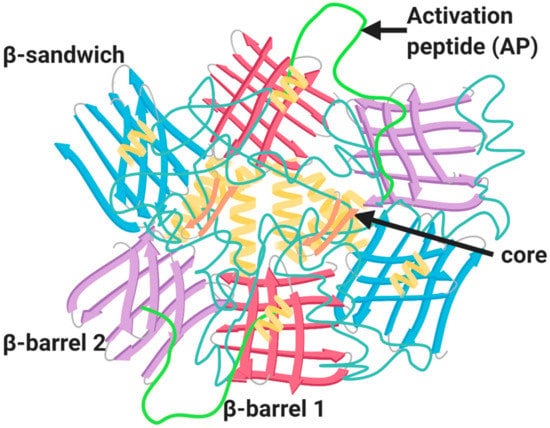
Figure 2.
Structure of FXIII-A zymogenic homodimer. The structure of FXIII-A homodimer is composed of four major domains: catalytic core domain (orange), β-sandwich domain (blue), β-barrel-1 domain (red) and the β-barrel-2 domain (purple); alpha helices strands (pale yellow); and the activation peptide (AP) (green).
FXIIIa uses a double displacement mechanism for cross-linking proteins. In the initial stage, Cys314 attacks the glutamine γ-carboxyamide group of a glutamine acceptor protein, displacing an ammonia molecule to form a thioester intermediate. In the second stage, the reactive thioester intermediate is attacked by the lysine ϵ-amino group of the amine donor protein, thereby displacing Cys314 and generating an isopeptide bond between the two substrate proteins and the concomitant release of FXIIIa [42]. In the absence of lysine residues, water reacts with the thioester intermediate converting glutamine into glutamic acid [42]. Glutamine sites that participate in FXIIIa-catalysed reactions have been identified through incorporation of primary amines, such as dansylcadaverine [43,44,45,46,47] and 5-(biotinamido)pentylamine [48,49]. Alternatively, labelled synthetic peptides have been designed to incorporate into the lysine residues of FXIIIa substrates [44]. In several cases, reactive glutamine and lysine residues have been characterised by mass spectrometry and Edman sequencing analysis [48,49,50].
3. Pools of FXIII-A within the Vasculature
The presence of the FXIII-A subunit in plasma has posed a conundrum: (i) it is not released from hepatocytes alongside its regulatory subunit FXIII-B, (ii) the concentration of FXIII-A is relatively high within the circulation and (iii) it lacks an identifiable endoplasmic reticulum signal sequence beseeching the question of how it can be released within the plasma environment. The most abundant source of FXIII-A is within the cytoplasm of cells of bone marrow and mesenchymal lineage. Bone marrow transplantation studies in humans implicated platelets, macrophages and unidentified circulatory haematopoietic cells as the source of plasma FXIII-A [51,52,53,54]. Cordell et al. [55] demonstrated that platelets were not the source of plasma FXIII-A, as thrombocytopenic mice exhibit normal levels of plasma FXIII-A [55]. Elegant studies from the same group used a complex series of lineage specific Cre mice to demonstrate that resident macrophages maintain plasma FXIII-A and excluded the megakaryocytic lineage as a major contributor. Externalisation of the closely related family member transglutaminase 2 has been shown on the surface of macrophages, but FXIII-A was not evident [56]. The enigma of how FXIII-A is released from these cells remains; nonetheless, current evidence underscores the importance of tissue specific macrophages in the release of this key protein to the blood stream.
3.1. Platelet-Derived FXIII-A
Platelet FXIII-A is synthesised in the precursor megakaryocyte cell during thrombopoiesis and packed during pro-platelet production. Unlike the majority of platelet-derived coagulation factors, FXIII-A is not deposited within the α-granules but is instead found in cytoplasm, most likely due to the lack of ER signal to direct it to the granule cargo. FXIII-A is an abundant protein within the cytoplasm, with levels as high as 60 ± 10 fg/platelet, accounting for approximately 3% of total protein [57]. Platelets therefore harbour approximately150-fold higher concentrations of FXIII-A than plasma, thereby insinuating this pool may be important in certain physiological functions [58]. Importantly, the Muszbek laboratory revealed that platelet FXIII-A can be activated within the cytoplasm following elevation of intracellular Ca2+ during platelet activation [16]. This thrombin-independent process occurs without concomitant release of the activation peptide [16] (Figure 1). Endocytosis of pFXIII into platelet α-granules during their circulation has been reported [59,60,61], but negligible amounts are detectable within the platelet releasate [62,63,64]. In line with these observations, FXIII-A levels are normal in platelets derived from patients with Grey Platelet Syndrome [65] and levels are unchanged in thrombocytopenic mice [55].
The absence of FXIII-A in the secretome of platelets led to the assumption that this pool did not contribute to haemostasis [66]. Our laboratory has revealed that FXIII-A is translocated from the cytoplasm to the outer leaflet of the membrane in stimulated platelets [64]. This pool of FXIII-A, while adhered to the membrane, is functional in conferring resistance against fibrinolytic degradation via the cross-linking of α2-antiplamin (α2AP) to fibrin [64]. The localisation of FXIII-A on the surface of phosphatidylserine-positive (PS-positive) platelets or procoagulant platelets occurs in the platelet “cap” or platelet body (Figure 3) [64]. These procoagulant platelets, as their name indicates, support assembly of coagulation factors which promote thrombin generation and subsequent fibrin formation [67]. Indeed, many other coagulation factors including factor Va, factor VIII, factor IXa, factor X/Xa, and prothrombin are concentrated within the “cap” region alongside FXIII-A [68]. The primary substrate of FXIII-A, fibrin(ogen), and other substrates including thrombospondin [69,70], and factor Va [43] are also abundant within the “cap” region of procoagulant platelets [71,72]. It has been suggested that fibrin and the integrin αIIbβ3 are critical for FXIII-A binding to the “cap” [68,73]. This localises FXIII-A in a prime location in which to promote crosslinking of fibrin and substrates into the fibrin matrix. However, αIIbβ3 is proposed to be inactive on procoagulant platelets, due to high Ca2+ and calpain levels [74]. The “cap” region of procoagulant platelets has been proposed as a point of attachment to aggregates and the forming clot [68]. Cross-linking of adhesive proteins, fibrin and thrombospondin in the “cap” could be a vital element in consolidating the attachment of procoagulant platelets under shear stress.
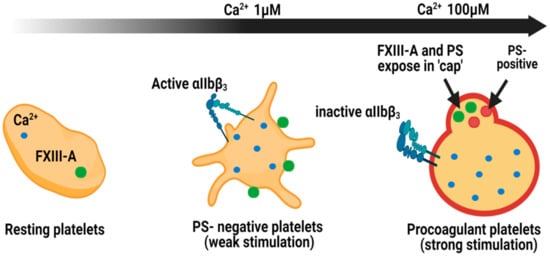
Figure 3.
Localisation of FXIII-A in platelet subpopulations. Within resting platelets, Ca2+ ions and cellular FXIII-A reside within the cytoplasm. Following weak stimulation, with agonists such as ADP, PS-negative platelets expose FXIII-A on the external membrane. The integrin αIIbβ3 is exposed in this subpopulation of platelets. Stimulation with dual agonists, such as thrombin and collagen, generate procoagulant platelets which expose FXIII-A within the phosphatidylserine (PS)-rich cap. In this subpopulation αIIbβ3 is inactive [70]. The cap is also rich in other coagulation factors, such as factor X, factor IX and prothrombin, and substrates for FXIII-A including fibrinogen, thrombospondin and factor Va [64].
PS-negative, spread platelets, also expose FXIII-A where it is diffusely localised across the membrane (Figure 3) [64]. The discrete role played by these platelets in thrombus initiation, propagation and in particular the expression of the active integrin αIIbβ3 and binding of fibrinogen most likely account for this differential staining pattern of FXIII-A. Of interest to consider is the difference in intracellular Ca2+ spikes associated with these platelet subpopulations following stimulation. Procoagulant platelets undergo a massive increase in cytosolic Ca2+ [68,75,76], with a recent report suggesting it is around 100-fold higher than spread, PS-negative platelets [77]. It is therefore feasible that the activity of FXIII-A on the surface of different subpopulations of platelets is hugely different and requires further investigation.
Clot retraction is platelet-mediated process that serves to constrain clot volume [78,79], thereby reducing blood loss [80] and permitting blood flow past otherwise obstructive thrombi [81]. The integrin αIIbβ3 acts as a molecular bridge between extracellular fibrinogen and the intracellular actin cytoskeleton via sphingomyelin-rich lipid rafts [82]. The cytoskeleton interacts with the α3 subunit tails via the adapter proteins talin and vinculin [83]. During clot retraction, fibrin bound to αIIbβ3 triggers outside-in signalling [84], resulting in the contraction of the actin cytoskeleton. FXIIIA2B2 contributes to the strength and rigidity of the condensed clot by cross-linking fibrin, and enhancing platelet spreading [85]. Conflicting evidence exists on the effects of platelet FXIII-A on clot retraction. Early reports found that clot retraction was normal in FXIII-deficient patients [86,87,88]. However, Kasahara et al. [82,89] demonstrated that clot retraction was significantly impaired in the absence of platelet FXIII-A transglutaminase activity in platelet-rich plasma from FXIII-A knockout mice [82,89]. Nevertheless, platelet FXIII-A has been shown not to contribute to retention of red blood cells [90]. It is apparent that further studies are required into the role of platelet FXIII-A in clot retraction in vivo. In procoagulant platelets, FXIII-A and calpain act in concert to downregulate integrin αIIbβ3 adhesive function, thereby limiting platelet recruitment in to the forming aggregate [91]. This FXIII-A-dependent mechanism attenuates thrombus size and may be important in preventing haemostatic clots from becoming obstructive in the vasculature.
3.2. Monocyte/Macrophage-Derived FXIII-A
FXIII-A is expressed on the cell surface of monocytes and macrophages [92] in response to stimulation with certain immune modulators, which is akin to the situation in platelets [64]. Indeed, monocytes isolated from patients with a congenital deficiency in F13A1 show a lack of FXIII-A and transglutaminase activity [93]. Within monocytes/macrophages, FXIII-A is localised to the cytoplasm but several studies have indicated it can be shuttled to the surface [94,95,96], and it is secreted by dendritic cells into the culture medium [97]. Secretion by an alternative secretory pathway has been proposed given the lack of ER signal in the FXIII-A protein [98]. Translocation of FXIII-A to the nucleus of differentiating human macrophages has been reported but the exact function of this transglutaminase enzyme in this locale is unclear [99].
The expression of FXIII-A in macrophages is dynamic in nature and is modulated in response to external stimuli and the phenotype of the activated macrophage. Macrophages can be “alternatively” or “classically” activated depending on the activating stimulus. “Classically activated”, or M1, macrophages are generated in response to stimulation with the immune mediators, interferon-γ, lipopolysaccharide or tumour necrosis factor [100]. These proinflammatory “type 1” macrophages [101] tend to exhibit down-regulation of FXIII-A [102,103]. “Alternatively activated”, or M2 macrophages, are stimulated in response to anti-inflammatory mediators, such as interleukin-4 and -13 [101]. M2 macrophages are reported to function in matrix remodelling, wound healing, allergy and parasite killing [100] and it is this subtype of macrophages that reveal upregulation of FXIII-A [103,104,105]. The selective expression of FXIII-A in M2 macrophages is in line with the capacity of this transglutaminase to act as an anti-inflammatory and pro-wound healing molecule.
Phagocytosis is the active ingestion and breakdown of microbes or other foreign particles by cells such as monocytes and macrophages. Phagocytic processes are driven by a finely controlled rearrangement of the actin cytoskeleton [106]. Considering the key role of FXIII-A in regulating cytoskeletal proteins, it is perhaps not surprising that it is directly linked to this process [107,108,109]. Studies have indicated that FXIII-A activity may play a role in increasing phagocytosis in monocytes and macrophages [110]. Phagocytosis is positively correlated with FXIII-A expression in myelomonocytic cells [111]. In accordance with this, Fcγ and complement receptor-mediated phagocytosis is impaired in monocytes and macrophages following inhibition of FXIII-A in FXIII-A-deficient mice [110]. Similarly, phagocytosis is significantly attenuated in monocytes isolated from FXIII-A deficient patients [110]. FXIII-A is upregulated during monocyte-derived dendritic cell differentiation and supports migration of mature cells [112]. The role of monocyte/macrophage FXIII-A in haemostasis has not been widely explored; however, these cells can promote cross-linking of fibrin [92,95], suggesting a potential role in thrombus stabilisation. Interestingly, thrombin treatment of monocytes does not augment exposure of FXIII-A [92], suggesting these cells may contribute to haemostasis in a situation where there is also an increase in the type 2 immune response, for example, in a wound healing capacity. Together, these data implicate FXIII-A in the phagocytic and/or migration capacity of these cells suggesting an important function of this pool of FXIII-A in innate immunity, inflammation and wound healing. However, there are many unaddressed questions in relation to the externalisation of FXIII-A on these cells. The dominant bleeding phenotype in congenital FXIII-A deficiency has perhaps masked the auxiliary roles of this transglutaminase in innate immunity, inflammation and wound healing. Nonetheless, it is apparent that there is an association with depleted levels of circulatory FXIII-A and delayed wound healing in different settings, such as venous leg ulcers, and in chronic inflammatory conditions, including inflammatory bowel disease [113,114,115].
4. FXIII Deficiency and Associated Complications
4.1. Congenital Deficiency
Congenital FXIII deficiency is a rare haemorrhagic disorder with an estimated prevalence of one per two million [116]. Umbilical stump bleeding in infants within the first few days of life is emblematic and frequently leads to a positive diagnosis. Patients also exhibit soft tissue haematoma, recurrent miscarriage and prolonged wound healing and most acutely intracranial bleeding [3]. Diagnosis of FXIII deficiency is challenging as routine coagulation tests, such as prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT) and platelet count, are normal [117]. Laboratory tests employed to diagnose FXIII deficiency include clot solubility assays; quantitative FXIIIa activity assays; ELISA for the A and B subunits and A2B2 complex; and genetic testing [117,118,119]. More recently, a whole blood clot retraction assay has been reported to be valuable in diagnosis of FXIII deficiency and monitoring of treatment [120]. The International Society on Thrombosis and Haemostasis Scientific and Standardization Committee on Fibrinogen and Factor XIII recommended an algorithm to guide laboratory diagnosis of FXIII deficiency and classify the form of the disease [121]. Challenges remain in that many of these specialised tests are not universally available.
FXIII deficiency is classified into three groups: type I FXIII-A subunit deficiency, type II FXIII-A subunit deficiency and FXIII-B subunit deficiency [118]. Type I is a quantitative defect resulting from decreased synthesis of the protein, whereas type II deficiency is associated with normal levels of FXIII that is functionally deficient. FXIII-B subunit deficiency is exceptionally rare and is associated with a milder bleeding phenotype [3]. The milder penetrance of B subunit deficiency is perhaps not surprising given the fact that the active A subunits still circulate, albeit at a reduced half-life, due to the absence of the inhibitor subunits [122]. Over 70 causative mutations have been described in the FXIII-A gene, while only five have been reported in the FXIII-B gene [118].
Congenital FXIII-A deficiency is associated with spontaneous miscarriage within the first trimester [123]. Similarly, homozygous FXIII-A knockout mice die due to massive uterine bleeding events at approximately 10 days of gestation [124]. FXIII-A is located in the histocytes of the uterus [125], in tissue macrophages and in the placenta [126], and enhances the formation of cytotrophoblastic shell and the cross-linking of Nitabuch’s fibrinoid layers [127]. It is found to directly co-localise with fibrinogen and fibronectin at the Nichbuch layer and is likely to have a protective function by downregulating fibrinolysis within this region. Insufficient development of the cytotrophoblastic shell and Nitabuch’s fibrinoid layers leads to placental abruption and foetal loss [128]. Macrophages manifest in the Nitabuch’s layer and surrounding implantation tissue, suggesting FXIII-A in this region is of cellular origin. Nevertheless, supplementation of plasma with FXIII-A concentrate to trough levels of around 10% is sufficient to permit successful full-term pregnancy [3,123,129]. This poses a conundrum as to the origin of FXIII-A in the placental tissue considering the FXIII-B subunit is absent.
4.2. Pregnancy Complications
During normal pregnancy, levels of many coagulation factors, including fibrinogen and coagulation factors VII, VIII, IX and X, steadily increase [130]. Interestingly, the same is true of the FXIII-B subunit while the levels of FXIII-A, and accordingly the tetrameric complex FXIIIA2B2, decrease to approximately 50% during gestation [131,132,133]. Recurrent pregnancy loss is only associated with deficiencies in fibrinogen or FXIII, reflecting the key contribution of these coagulation factors in stabilization of the placenta [116]. There are limited studies analysing the perturbation of FXIII-A in recurrent pregnancy loss with individuals that are otherwise competent for FXIII-A [134,135]. Neither of these studies were able to establish a direct link between consecutive miscarriage and reduced levels of FXIII-A. It is feasible that mild depletion of FXIII-A in plasma is not sufficient to induce complications in placental development, or that other circulatory pools of FXIII-A can compensate. Indeed, the source of FXIII-A in the placenta is unclear. Clearly there is many unanswered questions pertaining to the role and origin of FXIII-A in pregnancy and whether disruptions to these circulatory pools could explain recurrent spontaneous miscarriage in individuals who do not harbour a FXIII-A deficiency.
4.3. Acquired FXIII Deficiency
Acquired FXIII deficiency is a rare condition in which circulating levels of FXIII can drop to 20–70% of normal due to decreased synthesis or increased consumption of FXIII in different disease states [136]. This can be due to conditions such as disseminated intravascular coagulation (DIC), sepsis, pulmonary embolism, stroke, liver cirrhosis, Crohn’s disease and ulcerative colitis or due to major surgery and trauma [121]. Alternatively, autoantibodies which prevent activation of FXIII, impair binding of FXIII to its substrate and cofactor fibrin, or attenuate the half-life of FXIII in plasma may develop [137]. Several cases relate to patients with systemic lupus erythematosus [138]. The most common clinical feature of acquired FXIII deficiency is haemorrhage in soft tissue. Decreased synthesis of FXIII can be observed in patients undergoing chemotherapy [53] and with chronic liver failure [139]. In a case of acquired FXIII deficiency, decreased thrombus stability was associated with defective crosslinking of α2AP [64].
5. The Indispensable Role of FXIII-A Haemostasis and Wound Healing
Proteomic approaches have identified over 147 potential substrates for FXIIIa in plasma [140]. The identified substrates are involved in processes such as haemostasis, complement, extracellular matrix organisation and inflammatory and immune response thereby illustrating the diverse and crucial function of FXIII-A in normal physiology. Of these substrates 48 were cross-linked into the forming fibrin matrix during formation thereby localising them at the site of injury and subsequent wound healing. Historically, it was viewed that as little as 5% FXIII is sufficient for crosslinking function of FXIII; however, this assumption was largely based on coagulation assays. Our own work on the antifibrinolytic function of FXIII reveal that replenishment to around 50% of circulating levels is necessary for normal haemostasis [141,142].
5.1. FXIII in Haemostasis
The role of plasma FXIII-A in haemostasis is well-established; it confers mechanical stability to thrombi by cross-linking the α- and γ-chains of fibrin, and provides protection against fibrinolytic breakdown by cross-linking fibrinolytic inhibitors to fibrin [44,45,143]. Cross-linking of fibrin reduces the association rate of plasmin for fibrin more than 6-fold [144]. In addition, multiple lysine residues within the C-terminal domain of fibrin act as a substrate for FXIIIa [145]. Cross-linking within these areas has the potential to mitigate binding of plasminogen and tPA to the fibrin network, thereby downregulating fibrinolysis. Another proposed mechanism by which FXIIIa confers resistance to fibrinolytic degradation is by compacting the fibrin fibre diameter and increasing fibre density in the clot [146]. Clots comprised of thinner fibres with smaller pores have previously been shown to show enhanced resistance to fibrinolysis [147], which can be ascribed to reduced solute access and to a reduction in binding of tPA [141,148,149].
Our laboratory illustrated that flow or shear stress is necessary to visualize the impact of FXIII-A on fibrinolysis in a plasma environment [150]. Several fibrinolytic inhibitors can be cross-linked into the forming clot including α2AP [151], thrombin-activatable fibrinolysis inhibitor (TAFI) [44] and plasminogen activator inhibitor-2 (PAI-2) [152]. The principal inhibitor of plasmin, α2AP, is synthesized in the liver and secreted as methionine (Met1-α2AP). In plasma, the N-terminal 12 amino acid residues are rapidly cleaved by an antiplasmin cleaving enzyme (APCE) [153] transforming it to Asn1-α2AP [154]. Only the Asn1-α2-PI isoform is a good substrate for FXIIIa [155]. Our laboratory has shown that the dominant antifibrinolytic action of FXIIIa is mediated exclusively by cross-linking α2AP to fibrin [142] with negligible contribution of the other inhibitors. Rijken and colleagues subsequently reported that compaction or retraction of fibrin clots reveals the strong antifibrinolytic effect of FXIII-A [156]. The authors also confirm our observations that cross-linking of α2AP is required for the antifibrinolytic effect of FXIII to be visualised rather than by fibrin–fibrin cross-links [156]. It is likely that the dominant effect of FXIIIa on fibrinolysis is mediated via α2AP with cross-linking of fibres playing a minor contribution.
Platelet FXIII-A was previously shown to stabilise clots, by inducing the formation of high molecular weight γ-dimer and α-polymer [157,158,159,160,161] and cross-linking α2AP to fibrin [157,160]. The conundrum is that FXIII-A is not found within the secretome of platelets. We have shown that strong agonist stimulation of platelets induces translocation of FXIII-A from the cytoplasm to the platelet membrane where it is actively retained and can participate in extracellular cross-linking reactions [64]. The intensity of FXIII-A staining on the surface of activated platelets increases as a function of time, particularly in those platelets directly associated with collagen fibres. Our work clearly highlights a role for externalised platelet FXIII-A in stabilizing thrombi via cross-linking of α2AP to fibrin [64]. Intriguingly, plasma FXIII-A, but not platelet FXIII-A, aids the retention of red blood cells in clots via fibrin α-chain cross-linking which has a direct impact on the overall size of clots [90,162,163]. The relative contribution of plasma FXIIIA2B2 versus platelet-derived FXIII-A to thrombus stability requires clarification, but it is unlikely to be uniform throughout the thrombus, with the balance tipping toward FXIII-A in platelet-rich areas of the haemostatic plug, where solute transport of the large plasma FXIIIA2B2 tetramer is low. Together with the fact that levels of FXIII-A are around 150-fold higher in the platelet cytoplasm, this advocates a role for these anucleate cells in thrombus stabilisation in certain environments.
5.2. FXIII in Wound Healing
Normal wound healing occurs in response to a haemostatic challenge or necrosis with infection [164] and involves formation of a provisional matrix which is the basis for invasion of cells involved in tissue regeneration. Impaired wound healing occurs in around 15–30% of FXIII-deficient patients [122,165,166]. Elegant studies with FXIII-A-deficient mice show prolonged healing of excisional wounds and delayed tissue repair which could be rectified by infusion of FXIII concentrate in the mice [167]. A rat model of experimental colitis showed a significant improvement of existing and established lesion severity following intravenous infusion of recombinant FXIII-A [168]. These lines of evidence highlight the crucial function of this transglutaminase in wound healing and remodelling.
The contribution of FXIII-A to wound healing and tissue repair is pleiotropic (as reviewed in [137,169]) and commences with its crucial function in the haemostatic cascade in terms of platelet adhesion to the sub-endothelium, which is mediated by the integrins αIIbβ3 and αvβ3 on the platelet surface, but occurs in a transglutaminase independent manner [170] (Figure 4). It subsequently stabilises the forming fibrin matrix and consolidates the clot by participating in clot retraction. Consequently, FXIII-A reduces vascular permeability at the wound and traps invading pathogens by crosslinking them to the provisional matrix. Finally, it promotes repair by supporting cellular invasion and stimulates angiogenic signalling. The many substrates of FXIIIa include adhesive, extracellular matrix proteins, such as fibronectin, vitronectin, thrombospondin, collagen and von Willebrand factor, which are cross-linked into the clot [169] and enhance cell migration and attachment [171]. Indeed, early studies showed that cross-linking of fibronectin into the fibrin clot via FXIII-A enhances fibroblast adherence and migration [172]. FXIII-A binds to endothelial cells via the integrin αVβ3 enhancing platelet adhesion at the site of injury [173]. Binding occurs via a tripeptide motif Leu-Asp-Val in FXIII-A independent of transglutaminase activity thereby permitting ongoing cross-linking of proteins involved in repair to occur in a localised manner [170].
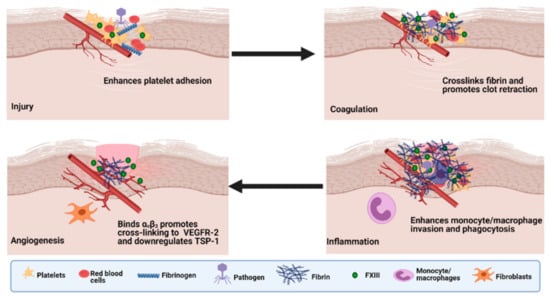
Figure 4.
The roles of Factor XIII in wound healing. During injury, FXIII-A enhances platelet adhesion to the injured endothelium through an integrin-dependent mechanism. Activated FXIII (FXIIIa) mediates the cross-linking of fibrin and promotes clot retraction. FXIII-A enhances invasion of monocytes/macrophages and phagocytosis of cell debris and pathogens. FXIII-A promotes crosslinking of extracellular matrix (ECM) and reduces vascular permeability. FXIIIa binds αvβ3 and promotes cross-linking to vascular endothelial growth factor receptor-2 (VEGFR-2) and downregulates thrombospondin-1 (TSP-1). Adapted from the work in [133].
Macrophages are key to tissue repair and play essential roles in removal of cell debris, invading organisms, neutrophils and other apoptotic cells from the injured site. FXIII-A participates in phagocytic processes including Fcγ and complement-induced uptake of sensitized erythrocytes and complement-coated yeast particles [174]. However, it is clear that macrophages play a complex and intricate role in tissue repair supplying many chemokines, matrix metalloproteinases and other inflammatory mediators that drive the cellular response to injury [175]. Indeed, activated FXIII-A has been shown to generate complement C5-derived monocyte chemotactic factor [176]. A protein that is indistinguishable from ribosomal protein S19 has been shown to be converted to an active form via crosslinking of FXIII-A to the surface of activated platelets [177]. These chemotactic factors formed via cross-linking dependent processes may function to actively recruit monocytes and inflammatory macrophages to the site of injury.
FXIII-A reduces endothelial permeability in transglutaminase dependent manner in in vitro monolayers and saline-perfused rat hearts [178]. Similarly, a reduction in vascular permeability was noted in an in vivo guinea pig model of antiserum-induced vascular damage [179]. In patients undergoing cardiac surgery, infusion of FXIII concentrate reduces vascular leakage [180,181]. Together these findings indicate that FXIII-A protects endothelial barrier function most likely via cross-linking of adhesive proteins to the site of injury.
Angiogenesis is an important part of tissue repair and wound healing [137]. FXIII-A exerts a direct proangiogenic effect on endothelial cells in vitro, promoting migration and proliferation while constraining apoptosis [182]. FXIII-A transamidase activity is a requirement to illicit these proangiogenic effects which were associated with downregulation of the antiangiogenic factor thrombospondin-1. The process is multifaceted with binding of FXIII-A to endothelial cells inducing complex formation between vascular endothelial growth factor (VEGF) and the integrin αVβ3 (vitronectin receptor). Association of FXIII-A with αVβ3 results in partial cross-linking between the β3 subunit and the VEGFR-2 [183]. This promotes a cascade of events including tyrosine phosphorylation and activation of VEGFR-2 accompanied by upregulation of intracellular signalling molecules, such as c-Jun and Egr-1 and a subsequent downregulation of thrombospondin-1 [183]. These actions are entirely dependent on the transamidase activity of FXIII-A. In addition, the interplay between the extracellular matrix (ECM) and integrins is important for the migration of reparative cells into the wound bed and the transmission of intracellular signals caused by extracellular changes [184]. These observations provide a clear link between FXIII-A of plasma and cellular origin in the wound healing and tissue repair process, although evidently this is an intricate process of which there are still several missing links that require further insight and elucidation. There is potential for application of FXIII-A to promote wound healing which may prove beneficial in some context, such as during inflammatory conditions, post-operative bleeding and trauma.
6. FXIII-A Replacement Therapy and Utility as a Drug Target
The management of FXIII deficiency includes regular prophylaxis [185] with replacement therapy to increase the amount of FXIII in plasma by administering cryoprecipitate, fresh frozen plasma (FFP) [186], heat inactivated concentrate or recombinant FXIII-A2 (rFXIII-A2) therapy [187]. FXIII has relatively long half-life (~9 days), making it suitable for routine prophylactic treatment given around every 4 weeks [188]. The use of FFP and cryoprecipitate carries a risk of bloodborne virus transmission, so FXIII concentrates are viewed a safer alternative as they undergo rigorous screening and viral inactivation [189].
Prophylactic treatment aims to have a trough level greater than 5% FXIII obtained with doses of 35 to 40 U/kg [190]. A target level of 8–9% of plasma FXIII was thought to be sufficient to maintain normal haemostasis [117,191,192]. However, to maintain thrombus stability against fibrinolysis, around 50% normal plasma concentrations are required [150]. Fibrogammin P® is a purified heat treated FXIII concentrate used to treat congenital FXIII deficiency and is well tolerated but requires regular intravenous doses. Fibrogammin P® was approved for use in Europe since the early 1990s and subsequently in USA under the name Corifact™. The plasma-derived product is approved for use with both FXIII-A subunit and the rarer FXIII-B subunit deficiency. Preoperative prophylaxis with the concentrate is effective in preventing postoperative bleeding [193]. The recombinant FXIII-A (rFXIII-A) subunit product, termed Tretten, is produced in yeast and therefore benefits from not involving any human or mammalian products in its manufacture. Upon infusion it complexes with the excess of endogenous FXIII-B in plasma generating a heterotetramer with a similar half-life to native FXIII [194]. Long-term safety and efficacy of rFXIII-A were evaluated in the Mentor™2 extension trial and demonstrated a low incidence of bleeding, no reports of development of non-neutralising or neutralising antibodies and that pre-surgery prophylaxis was effective [195].
Congenital deficiency of FXIII-A in pregnancy is generally managed with more regular, low dose (10 IU/kg) prophylaxis [196], ideally aiming for higher than 10–12 IU/d with above 30% plasma FXIII being ideal during labour [123,197]. A bolus of 1000 IU is recommended prior to onset of labour to prevent postpartum haemorrhage [123]. Recently, rFXIII-A2 has been shown to be effective at successfully facilitating a healthy pregnancy [197]. Prompt prophylaxis in neonates with FXIII concentrate is effective at both low (10–26 IU/kg) and high (60/80 IU/kg) doses with those on the higher dose having less bleeding episodes and no incidences of thrombotic events [196].
Acquired FXIII deficiency can result from a decreased production, increased consumption or, more rarely, due to the development of neutralising or non-neutralising autoantibodies [198]. Autoantibodies can be categorised depending how they interfere with FXIII: type Aa both block formation of the tetramer and steal FXIII-A, while Ab blocks the active transglutaminase and B rapidly clears the FXIII–antibody complex [199]. Treatment therefore requires immunosuppression, in addition to FXIII concentrate. Treatments are varied, but the steroid prednisolone is commonly prescribed combined with an immunosuppressant such as cyclophosphamide or the anti-CD20 monoclonal antibody Rituximab [198,200]. Other treatments include plasmapheresis and targeting fibrinolysis with tranexamic acid and epsilon aminocaproic acid [198,200].
FXIII replacement therapy has recently assimilated interest in the field of trauma. Trauma-induced coagulopathy (TIC) is associated with a dramatic decline in fibrinogen over other coagulation factors [201,202,203]. Consequently, there is a concomitant decrease in FXIIIA2B2 which circulates in complex with fibrinogen [6]. Early replacement of fibrinogen is associated with improved outcomes in TIC [204,205,206]. In vitro models of TIC have suggested that there is benefit to replacing not only fibrinogen, but also FXIII to enhance clot stability [207,208]. Patient studies and clinical trials of available sources of FXIII, including concentrate, fresh frozen plasma and cryoprecipitate, will be necessary to extrapolate on its potential benefit in this setting. Similarly, low levels of FXIII-A have been implicated in intraoperative unexplained bleeding episodes [209] indicating the potential of FXIII-A supplementation for perioperative bleeding. Bleeding volume after cardiac surgery shows a strong correlation with FXIII-A activity [210,211]. Pre-operative supplementation with FXIII-A has been shown to be effective in improve clot stiffness and is associated with reduced bleeding [212].
Current anticoagulant therapies are targeted indirectly or directly to downregulate thrombin production, thereby attenuating platelet activation and fibrin formation. However, the common anticoagulants, which are vitamin K-mediated or target-specific coagulation factors, generally FXa or thrombin, are associated with bleeding complications. FXIII-A has been considered a promising therapeutic strategy, as it is downstream of thrombin, and therefore permits clot formation but promotes instability, thereby enhancing susceptibility to clearance. In a canine coronary thrombosis model, pretreatment with the FXIIIa inhibitor L-722,151 (2-[l-acetonylthio]-5-methylthiazolo[2,3-b] 1,3,4-thiadiazolium, effectively enhanced tPA-induced reperfusion and reduced thrombus mass but no benefit was observed administering the inhibitor post-thrombus formation [213]. The authors suggested L-722,151 could be a pharmacological tool and as prototype for the development of future therapeutic FXIIIa inhibitors [213]. Tridegin, a small peptide inhibitor purified from the salivary gland extract of the giant Amazon leech Haementeria ghilianii, inhibits both plasma and platelet FXIIIa without interfering with the enzymatic activity of thrombin or Factor Xa [214]. Analogues of this peptidic inhibitor offer insight into the mechanism of action and have potential as lead structures for development [215]. The novel inhibitors with a cis-bisamido epoxides pharmacore were shown to have an improved potency compared to a natural product inhibitor, cerulenin, although still lacked selectivity for FXIII over transglutaminase 2 [216]. ZED3197 has a Michael acceptor warhead which irreversibly blocks the active site cysteine and has recently been shown to restore blood flow in an in vivo rabbit model of venous stasis without affecting clotting time [217]. An alternative strategy to the peptidic inhibitors is siRNA targeting of FXIII-B which causes a depletion of plasma FXIII-A, without altering platelet FXIII-A and has been shown to enhance fibrinolysis [218]. Clearly, the crucial role that FXIII-A plays in haemostasis spotlights this enzyme as a potential target for antithrombotic strategies. However, caution must be applied considering the bleeding phenotype and wound healing complications associated with congenital FXIII-A deficiency even with the mild deficiency of FXIII-B and acquired FXIII-A deficiency.
7. Discussion and Future Perspectives
FXIII-A is crucial to normal physiology as indicated by the bleeding diathesis of deficient patients, which can range from relatively mild to devastatingly fatal in terms of the intracranial haemorrhage associated with congenital FXIII-A deficiency in the absence of FXIII supplementation. This is amalgamated with the vital role of FXIII-A in wound healing, that was perhaps difficult to tease out considering the overbearing impact on the haemostatic cascade. The key roles of FXIII-A in normal biological processes are of course embodied within corresponding pathophysiological processes. These have not been discussed herein, as are expertly discussed in another review in this series on FXIII-A in diseases [219]. A growing body of literature now propels the function of cellular pools of FXIII-A into the limelight. These cells are known to externalise FXIII-A and therefore are capable of delivering extracellular functions of this complex transglutaminase. It is clear we have many unanswered questions to tackle, starting with the mechanisms by which FXIII-A escapes the cells despite its lack of signal sequence. This knowledge will impart us with a clearer understanding of the processes that drive externalisation and the relative contribution of cellular sources in different settings and biological processes. Of interest is the influence of different circulatory pools of FXIII-A to haemostasis in varying locales of the vasculature. The role of FXIII-A in modulating wound healing and tissue repair is now unequivocal, but the mechanisms underpinning this are still very much in their infancy. It appears that the field of FXIII-A is ripe for development particularly with the wealth of potential therapeutic options exploding into the market.
Author Contributions
F.S.M.A. wrote the manuscript; C.S.W. supervised the research and wrote the manuscript; N.J.M. supervised the research and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Fahad S.M. Alshehri is supported by Royal Embassy of Saudi Arabia Cultural Bureau (KFMCS74). Claire S. Whyte and Nicola J. Mutch were supported by the British Heart Foundation project grants (PG/15/82/31721 and PG/20/17/35050).
Acknowledgments
All figures were prepared with https://biorender.com/ (accessed on 16 March 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bagoly, Z.; Koncz, Z.; Hársfalvi, J.; Muszbek, L. Factor XIII, clot structure, thrombosis. Thromb. Res. 2012, 129, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Lorand, L. Factor XIII: Structure, Activation, and Interactions with Fibrinogen and Fibrin. Ann. N. Y. Acad. Sci. 2006, 936, 291–311. [Google Scholar] [CrossRef]
- Karimi, M.; Bereczky, Z.; Cohan, N.; Muszbek, L. Factor XIII Deficiency. Semin. Thromb. Hemost. 2009, 35, 426–438. [Google Scholar] [CrossRef]
- Yorifuji, H.; Anderson, K.; Lynch, G.W.; Van De Water, L.; McDonagh, J. B protein of Factor XIII: Differentiation between free B and complexed B. Blood 1988, 72, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Katona, É.; Pénzes, D.K.; Csapó, A.; Fazakas, F.; Udvardy, M.L.; Bagoly, Z.; Orosz, Z.Z.; Muszbek, L. Interaction of Factor XIII subunits. Blood 2014, 123, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, C.S.; Miraglia, C.C.; Rickles, F.R.; Shuman, M.A. Cleavage of blood coagulation Factor XIII and fibrinogen by thrombin during in vitro clotting. J. Clin. Investig. 1985, 75, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.W.; Pfueller, S.L. Thrombin-independent activation of platelet Factor XIII by endogenous platelet acid protease. Thromb. Haemost. 1988, 59, 372–377. [Google Scholar] [CrossRef]
- Ando, Y.; Imamura, S.; Yamagata, Y.; Kitahara, A.; Saji, H.; Murachi, T.; Kannagi, R. Platelet Factor XIII is activated by calpain. Biochem. Biophys. Res. Commun. 1987, 144, 484–490. [Google Scholar] [CrossRef]
- Lewis, S.D.; Janus, T.J.; Lorand, L.; Shafer, J.A. Regulation of formation of Factor XIIIa by its fibrin substrates. Biochemistry 1985, 24, 6772–6777. [Google Scholar] [CrossRef] [PubMed]
- Hornyak, T.J.; Shafer, J.A. Interactions of Factor XIII with fibrin as substrate and cofactor. Biochemistry 1992, 31, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Naski, M.C.; Shafer, J.A. A kinetic model for the alpha-thrombin-catalyzed conversion of plasma levels of fibrinogen to fibrin in the presence of antithrombin III. J. Biol. Chem. 1991, 266, 13003–13010. [Google Scholar] [CrossRef]
- Janus, T.J.; Lewis, S.D.; Lorand, L.; Shafer, J.A. Promotion of thrombin-catalyzed activation of Factor XIII by fibrinogen. Biochemistry 1983, 22, 6269–6272. [Google Scholar] [CrossRef]
- Folk, J.E.; Finlayson, J.S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv. Protein Chem. 1977, 31, 1–133. [Google Scholar] [PubMed]
- Ádány, R.; Bárdos, H. Factor XIII subunit A as an intracellular transglutaminase. Cell. Mol. Life Sci. 2003, 60, 1049–1060. [Google Scholar] [CrossRef]
- Polgar, J.; Hidasi, V.; Muszbek, L. Non-proteolytic activation of cellular protransglutaminase (placenta macrophage Factor XIII). Biochem. J. 1990, 267, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Polgar, J.; Boda, Z. Platelet Factor XIII becomes active without the release of activation peptide during platelet acti-vation. Thromb. Haemost. 1993, 69, 282–285. [Google Scholar]
- Buluk, K. An unknown action of blood platelets; preliminary communication. Polski Tyg. Lek. 1955, 10, 191. [Google Scholar] [PubMed]
- Kiesselbach, T.H.; Wagner, R.H. Fibrin-stabilizing factor: A thrombin-labile platelet protein. Am. J. Physiol. Content 1966, 211, 1472–1476. [Google Scholar] [CrossRef]
- Luscher, E.F. Fibrin-stabilizing factor from thrombocytes. Schweiz. Med. Wochenschr. 1957, 87, 1220–1221. [Google Scholar]
- Kiesselbach, T.H.; Wagner, R.H. Demonstration of Factor XIII in human megakaryocytes by a fluorescent antibody technique. Ann. N. Y. Acad. Sci. 1972, 202, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, P.; Becker, S.; Lynch, G.; McDonagh, J. Identification of intracellular Factor XIII in human monocytes and macro-phages. J. Clin. Investig. 1985, 76, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Adány, R.; Szegedi, G.; Polgár, J.; Kávai, M. Factor XIII of blood coagulation in human monocytes. Thromb. Res. 1985, 37, 401–410. [Google Scholar] [CrossRef]
- Adany, R.; Belkin, A.; Vasilevskaya, T.; Muszbek, L. Identification of blood coagulation Factor XIII in human peritoneal macro-phages. Eur. J. Cell Biol. 1985, 38, 171–173. [Google Scholar] [PubMed]
- Nestle, F.O.; Zheng, X.G.; Thompson, C.B.; Turka, L.A.; Nickoloff, B.J. Characterization of dermal dendritic cells obtained from nor-mal human skin reveals phenotypic and functionally distinctive subsets. J. Immunol. 1993, 151, 6535–6545. [Google Scholar] [PubMed]
- Nurminskaya, M.; Linsenmayer, T.F. Identification and characterization of up-regulated genes during chondrocyte hypertrophy. Dev. Dyn. 1996, 206, 260–271. [Google Scholar] [CrossRef]
- Nurminskaya, M.; Magee, C.; Nurminsky, D.; Linsenmayer, T.F. Plasma transglutaminase in hypertrophic chondrocytes: Expres-sion and cell-specific intracellular activation produce cell death and externalization. J. Cell Biol. 1998, 142, 1135–1144. [Google Scholar] [CrossRef]
- Rosenthal, A.K.; Masuda, I.; Gohr, C.M.; Derfus, B.A.; Le, M. The transglutaminase, Factor XIIIA, is present in articular chondro-cytes. Osteoarthritis Cartil. 2001, 9, 578–581. [Google Scholar] [CrossRef][Green Version]
- Nurminskaya, M.; Kaartinen, M.T. Transglutaminases in mineralized tissues. Front. Biosci. 2006, 11, 1591–1606. [Google Scholar] [CrossRef][Green Version]
- Myneni, V.D.; Hitomi, K.; Kaartinen, M.T. Factor XIII-A transglutaminase acts as a switch between preadipocyte proliferation and differentiation. Blood 2014, 124, 1344–1353. [Google Scholar] [CrossRef]
- Souri, M.; Osaki, T.; Ichinose, A.; Hsieh, L.-S.; Tsukasa, O.; Han, G.-S.; Carman, G.M. The Non-catalytic B Subunit of Coagulation Factor XIII Accelerates Fibrin Cross-linking. J. Biol. Chem. 2015, 290, 12027–12039. [Google Scholar] [CrossRef]
- Hur, W.S.; Mazinani, N.; Lu, X.J.D.; Britton, H.M.; Byrnes, J.R.; Wolberg, A.S.; Kastrup, C.J. Coagulation Factor XIIIa is inactivated by plasmin. Blood 2015, 126, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Putnam, F.W.; Takahashi, Y. Primary structure of blood coagulation Factor XIIIa (fibrinoligase, transglutaminase) from human placenta. Proc. Natl. Acad. Sci. USA 1986, 83, 8019–8023. [Google Scholar] [CrossRef]
- Bagoly, Z.; Haramura, G.; Muszbek, L. Down-regulation of activated Factor XIII by polymorphonuclear granulocyte proteases within fibrin clot. Thromb. Haemost. 2007, 98, 359–367. [Google Scholar] [CrossRef]
- Ichinose, A.; Takio, K.; Fujikawa, K. Localization of the binding site of tissue-type plasminogen activator to fibrin. J. Clin. Investig. 1986, 78, 163–169. [Google Scholar] [CrossRef]
- Yee, V.C.; Pedersen, L.C.; Le Trong, I.; Bishop, P.D.; Stenkamp, R.E.; Teller, D.C. Three-dimensional structure of a transglutaminase: Human blood coagulation Factor XIII. Proc. Natl. Acad. Sci. USA 1994, 91, 7296–7300. [Google Scholar] [CrossRef]
- Fox, B.A.; Yee, V.C.; Pedersen, L.C.; Le Trong, I.; Bishop, P.D.; Stenkamp, R.E.; Teller, D.C. Identification of the Calcium Binding Site and a Novel Ytterbium Site in Blood Coagulation Factor XIII by X-ray Crystallography. J. Biol. Chem. 1999, 274, 4917–4923. [Google Scholar] [CrossRef]
- Stieler, M.; Weber, J.; Hils, M.; Kolb, P.; Heine, A.; Büchold, C.; Pasternack, R.; Klebe, G. Structure of Active Coagulation Factor XIII Triggered by Calcium Binding: Basis for the Design of Next-Generation Anticoagulants. Angew. Chem. Int. Ed. 2013, 52, 11930–11934. [Google Scholar] [CrossRef] [PubMed]
- Komáromi, I.; Bagoly, Z.; Muszbek, L. Factor XIII: Novel structural and functional aspects. J. Thromb. Haemost. 2011, 9, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Adany, R.; Mikkola, H. Novel Aspects of Blood Coagulation Factor XIII. I. Structure, Distribution, Activation, and Function. Crit. Rev. Clin. Lab. Sci. 1996, 33, 357–421. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.A.; Freyssinet, J.M.; Holbrook, J.J. An equilibrium study of metal ion binding to human plasma coagulation Factor XIII. Biochem. J. 1978, 169, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, A.; Bányai, I.; Weiss, M.S.; Hilgenfeld, R.; Keresztessy, Z.; Muszbek, L.; Fésüs, L. Calcium Binding of Transglutaminases: A43Ca NMR Study Combined with Surface Polarity Analysis. J. Biomol. Struct. Dyn. 2001, 19, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Ariëns, R.A.; Lai, T.-S.; Weisel, J.W.; Greenberg, C.S.; Grant, P.J. Role of Factor XIII in fibrin clot formation and effects of genetic poly-morphisms. Blood 2002, 100, 743–754. [Google Scholar] [CrossRef]
- Francis, R.T.; McDonagh, J.; Mann, K.G. Factor V is a substrate for the transamidase Factor XIIIa. J. Biol. Chem. 1986, 261, 9787–9792. [Google Scholar] [CrossRef]
- Valnickova, Z.; Enghild, J.J. Human procarboxypeptidase U, or thrombin-activable fibrinolysis inhibitor, is a substrate for transglutaminases: Evidence for transglutaminase-catalyzed cross-linking to fibrin. J. Biol. Chem. 1998, 273, 27220–27224. [Google Scholar] [CrossRef]
- Sakata, Y.; Aoki, N. Cross-linking of alpha 2-plasmin inhibitor to fibrin by fibrin-stabilizing factor. J. Clin. Investig. 1980, 65, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Mosher, D.F. Action of fibrin-stabilizing factor on cold-insoluble globulin and alpha2-macroglobulin in clotting plasma. J. Biol. Chem. 1976, 251, 1639–1645. [Google Scholar] [CrossRef]
- Sottrup-Jensen, L.; Stepanik, T.M.; Wierzbicki, D.M.; Jones, C.M.; Lønblad, P.B.; Kristensen, T.N.; Mortensen, S.B.; Petersen, T.E.; Magnusson, S. The primary structure of alpha 2-macroglobulin and localization of a Factor XIIIa cross-linking site. Ann. N. Y. Acad. Sci. 1983, 421, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.N.; Lee, C.S.; Tae, W.-C.; Jackson, K.W.; Christiansen, V.J.; McKee, P.A. Cross-linking of Wild-type and Mutant α2-Antiplasmins to Fibrin by Activated Factor XIII and by a Tissue Transglutaminase. J. Biol. Chem. 2000, 275, 37382–37389. [Google Scholar] [CrossRef]
- Lee, K.N.; Lee, C.S.; Tae, W.C.; Jackson, K.W.; Christiansen, V.J.; McKee, P.A. Crosslinking of alpha 2-antiplasmin to fibrin. Ann. N. Y. Acad. Sci. 2001, 936, 335–339. [Google Scholar] [CrossRef]
- Skorstengaard, K.; Halkier, T.; Hojrup, P.; Mosher, D. Sequence location of a putative transglutaminase cross-linking site in hu-man vitronectin. FEBS Lett. 1990, 262, 269–274. [Google Scholar] [CrossRef]
- Wölpl, A.; Lattke, H.; Board, P.G.; Arnold, R.; Schmeiser, T.; Kubanek, B.; Robin-Winn, M.; Pichelmayr, R.; Goldmann, S.F. Coagulation Factor XIII A and B subunits in bone marrow and liver transplantation. Transplantation 1987, 43, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Pihusch, R.; Salat, C.; Schmidt, E.; Göhring, P.; Pihusch, M.; Hiller, E.; Holler, E.; Kolb, H.-J. Hemostatic complications in bone marrow transplantation: A retrospective analysis of 447 patients. Transplantation 2002, 74, 1303–1309. [Google Scholar] [CrossRef]
- Inbal, A.; Muszbek, L.; Lubetsky, A.; Katona, É.; Levi, I.; Kárpáti, L.; Nagler, A. Platelets but not monocytes contribute to the plasma levels of Factor XIII subunit A in patients undergoing autologous peripheral blood stem cell transplantation. Blood Coagul. Fibrinolysis 2004, 15, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Poon, M.C.; Russell, J.A.; Low, S.; Sinclair, G.D.; Jones, A.R.; Blahey, W.; Ruether, B.A.; Hoar, D.I. Hemopoietic origin of Factor XIII A subunits in platelets, monocytes, and plasma. Evidence from bone marrow transplantation studies. J. Clin. Investig. 1989, 84, 787–792. [Google Scholar] [CrossRef]
- Cordell, P.A.; Kile, B.T.; Standeven, K.F.; Josefsson, E.C.; Pease, R.J.; Grant, P.J. Association of coagulation Factor XIII-A with Golgi pro-teins within monocyte-macrophages: Implications for subcellular trafficking and secretion. Blood 2010, 115, 2674–2681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beckers, C.M.; Simpson, K.R.; Griffin, K.J.; Brown, J.M.; Cheah, L.T.; Smith, K.A.; Vacher, J.; Cordell, P.A.; Kearney, M.T.; Grant, P.J.; et al. Cre/lox Studies Identify Resident Macrophages as the Major Source of Circulating Coagulation Factor XIII-A. Arter. Thromb. Vasc. Biol. 2017, 37, 1494–1502. [Google Scholar] [CrossRef]
- Katona, É.; Ajzner, É.; Tóth, K.; Kárpáti, L.; Muszbek, L. Enzyme-linked immunosorbent assay for the determination of blood co-agulation Factor XIII A-subunit in plasma and in cell lysates. J. Immunol. Methods 2001, 258, 127–135. [Google Scholar] [CrossRef]
- Muszbek, L.; Yee, V.C.; Hevessy, Z. Blood coagulation Factor XIII: Structure and function. Thromb. Res. 1999, 94, 271–305. [Google Scholar] [CrossRef]
- Holme, P.A.; Brosstad, F.; Solum, N.O. The difference between platelet and plasma FXIII used to study the mechanism of platelet microvesicle formation. Thromb. Haemost. 1993, 70, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.; Korner, G.; Mou, X.; Gorodetsky, R. Packaging zinc, fibrinogen, and Factor XIII in platelet α-granules. J. Cell. Physiol. 1993, 156, 437–442. [Google Scholar] [CrossRef]
- Kreutz, R.P.; Bitar, A.; Owens, J.; Desta, Z.; Breall, J.A.; Von Der Lohe, E.; Sinha, A.; Vatta, M.; Nystrom, P.; Jin, Y.; et al. Factor XIII Val34Leu polymorphism and recurrent myocardial infarction in patients with coronary artery disease. J. Thromb. Thromb. 2014, 38, 380–387. [Google Scholar] [CrossRef]
- Lopaciuk, S.; Lovette, K.; McDonagh, J.; Chuang, H.; McDonagh, R. Subcellular distribution of fibrinogen and Factor XIII in hu-man blood platelets. Thromb. Res. 1976, 8, 453–465. [Google Scholar] [CrossRef]
- Sixma, J.J.; Berg, A.V.D.; Schiphorst, M.; Geuze, H.J.; McDonagh, J. Immunocytochemical localization of albumin and Factor XIII in thin cryo sections of human blood platelets. Thromb. Haemost. 1984, 51, 388–391. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Lionikiene, A.S.; Fraser, S.R.; Whyte, C.S.; Booth, N.A.; Mutch, N.J. Functional Factor XIII-A is exposed on the stimulated platelet surface. Blood 2014, 124, 3982–3990. [Google Scholar] [CrossRef]
- Nurden, A.T.; Kunicki, T.J.; Dupuis, D.; Soria, C.; Caen, J.P. Specific protein and glycoprotein deficiencies in platelets isolated from two patients with the gray platelet syndrome. Blood 1982, 59, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Joist, J.H.; Niewiarowski, S. Retention of platelet fibrin stabilizing factor during the platelet release reaction and clot retraction. Thromb. Diath. Haemorrh. 1973, 29, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Agbani, E.O.; Hers, I.; Poole, A.W. Temporal contribution of the platelet body and balloon to thrombin generation. Haematologica 2017, 102, e379–e381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Podoplelova, N.A.; Sveshnikova, A.N.; Kotova, Y.N.; Eckly, A.; Receveur, N.; Nechipurenko, D.Y.; Obydennyi, S.I.; Kireev, I.I.; Gachet, C.; Ataullakhanov, F.I.; et al. Coagulation factors bound to procoagulant platelets concentrate in cap structures to promote clotting. Blood 2016, 128, 1745–1755. [Google Scholar] [CrossRef]
- Bale, M.D.; Mosher, D.F. Effects of thrombospondin on fibrin polymerization and structure. J. Biol. Chem. 1986, 261, 862–868. [Google Scholar] [CrossRef]
- Lynch, G.W.; Slayter, H.; Miller, B.; McDonagh, J. Characterization of thrombospondin as a substrate for Factor XIII transglutaminase. J. Biol. Chem. 1987, 262, 1772–1778. [Google Scholar] [CrossRef]
- Whyte, C.S.; Swieringa, F.; Mastenbroek, T.G.; Lionikiene, A.S.; Lance, M.D.; van der Meijden, P.E.; Heemskerk, J.W.; Mutch, N.J. Plas-minogen associates with phosphatidylserine-exposing platelets and contributes to thrombus lysis under flow. Blood 2015, 125, 2568–2578. [Google Scholar] [CrossRef] [PubMed]
- Abaeva, A.A.; Canault, M.; Kotova, Y.N.; Obydennyy, S.I.; Yakimenko, A.O.; Podoplelova, N.A.; Kolyadko, V.N.; Chambost, H.; Mazurov, A.V.; Ataullakhanov, F.I.; et al. Procoagulant Platelets Form an α-Granule Protein-covered “Cap” on Their Surface That Promotes Their Attachment to Aggregates. J. Biol. Chem. 2013, 288, 29621–29632. [Google Scholar] [CrossRef]
- Kotova, Y.N.; Podoplelova, N.A.; Obydennyy, S.I.; Kostanova, E.A.; Ryabykh, A.A.; Demyanova, A.S.; Biriukova, M.I.; Rosenfeld, M.A.; Sokolov, A.V.; Chambost, H.; et al. Binding of Coagulation Factor XIII Zymogen to Activated Platelet Subpopulations: Roles of Integrin αIIbβ3 and Fibrinogen. Thromb. Haemost. 2019, 119, 906–915. [Google Scholar] [CrossRef]
- Mattheij, N.J.; Swieringa, F.; Mastenbroek, T.G.; Berny-Lang, M.A.; May, F.; Baaten, C.C.; van der Meijden, P.E.; Henskens, Y.M.; Beckers, E.A.; Suylen, D.P. Coated platelets function in platelet-dependent fibrin formation via integrin αIIbβ3 and transglutaminase Factor XIII. Haematologica 2016, 101, 427. [Google Scholar] [CrossRef]
- Heemskerk, J.W.M.; Mattheij, N.J.A.; Cosemans, J.M.E.M. Platelet-based coagulation: Different populations, different functions. J. Thromb. Haemost. 2013, 11, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Arachiche, A.; Kerbiriou-Nabias, D.; Garcin, I.; Letellier, T.; Dachary-Prigent, J. Rapid Procoagulant Phosphatidylserine Exposure Relies on High Cytosolic Calcium Rather Than on Mitochondrial Depolarization. Arter. Thromb. Vasc. Biol. 2009, 29, 1883–1889. [Google Scholar] [CrossRef]
- Abbasian, N.; Millington-Burgess, S.L.; Chabra, S.; Malcor, J.-D.; Harper, M.T. Supramaximal calcium signaling triggers procoagu-lant platelet formation. Blood Adv. 2020, 4, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Stalker, T.J.; Welsh, J.D.; Tomaiuolo, M.; Wu, J.; Colace, T.V.; Diamond, S.L.; Brass, L.F. A systems approach to hemostasis: Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood 2014, 124, 1824–1831. [Google Scholar] [CrossRef]
- Carr, M.E.; Angchaisuksiri, P.; Carr, S.L.; Martin, E.J. Effect of Non-Heparin Thrombin Antagonists on Thrombin Generation, Platelet Function, and Clot Structure in Whole Blood. Cell Biophys. 2003, 39, 89–100. [Google Scholar] [CrossRef]
- Lam, W.A.; Chaudhuri, O.; Crow, A.; Webster, K.D.; Li, T.-D.; Kita, A.; Huang, J.; Fletcher, D.A. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat. Mater. 2010, 10, 61–66. [Google Scholar] [CrossRef]
- Muthard, R.W.; Diamond, S.L. Blood clots are rapidly assembled hemodynamic sensors: Flow arrest triggers intraluminal throm-bus contraction. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2938–2945. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Kaneda, M.; Miki, T.; Iida, K.; Sekino-Suzuki, N.; Kawashima, I.; Suzuki, H.; Shimonaka, M.; Arai, M.; Ohno-Iwashita, Y.; et al. Clot retraction is mediated by Factor XIII-dependent fibrin-alphaIIbbeta3-myosin axis in platelet sphingomyelin-rich membrane rafts. Blood 2013, 122, 3340–3348. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, I.; Leisner, T.M.; Lam, S.C. Direct binding of the platelet integrin alphaIIbbeta3 (GPIIb-IIIa) to talin. Evidence that in-teraction is mediated through the cytoplasmic domains of both alphaIIb and beta3. J. Biol. Chem. 1996, 271, 16416–16421. [Google Scholar] [CrossRef]
- Shattil, S.J. Signaling through platelet integrin alpha IIb beta 3: Inside-out, outside-in, and sideways. Thromb. Haemost. 1999, 82, 318–325. [Google Scholar]
- Cohen, I.; Gerrard, J.M.; White, J.G. Ultrastructure of clots during isometric contraction. J. Cell Biol. 1982, 93, 775–787. [Google Scholar] [CrossRef]
- Jelenska, M.; Kopeć, M.; Breddin, K. On the Retraction of Collagen and Fibrin Induced by Normal, Defective and Modified Platelets. Pathophysiol. Haemost. Thromb. 1985, 15, 169–175. [Google Scholar] [CrossRef]
- Niewiarowski, S.; Markiewicz, M.; Nath, N. Inhibition of the platelet-dependent fibrin retraction by the fibrin stabilizing factor (FSF, factor 13). J. Lab. Clin. Med. 1973, 81, 641–650. [Google Scholar]
- Rao, K.M.K.; Newcomb, T.F. Clot Retraction in a Factor XIII Free System. Scand. J. Haematol. 2009, 24, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Souri, M.; Kaneda, M.; Miki, T.; Yamamoto, N.; Ichinose, A. Impaired clot retraction in Factor XIII A subunit–deficient mice. Blood 2010, 115, 1277–1279. [Google Scholar] [CrossRef] [PubMed]
- Kattula, S.; Byrnes, J.R.; Martin, S.M.; Holle, L.A.; Cooley, B.C.; Flick, M.J.; Wolberg, A.S. Factor XIII in plasma, but not in platelets, me-diates red blood cell retention in clots and venous thrombus size in mice. Blood Adv. 2018, 2, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Jackson, S.P. Platelet Factor XIII and Calpain Negatively Regulate Integrin αIIbβ3 Adhesive Function and Thrombus Growth. J. Biol. Chem. 2004, 279, 30697–30706. [Google Scholar] [CrossRef] [PubMed]
- Kradin, R.L.; Lynch, G.W.; Kurnick, J.T.; Erikson, M.; Colvin, R.B.; McDonagh, J. Factor XIII A is synthesized and expressed on the surface of U937 cells and alveolar macrophages. Blood 1987, 69, 778–785. [Google Scholar] [CrossRef]
- Muszbek, L.; Adány, R.; Kávai, M.; Boda, Z.; Lopaciuk, S. Monocytes of patients congenitally deficient in plasma Factor XIII lack Factor XIII subunit a antigen and transglutaminase activity. Thromb. Haemost. 1988, 59, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Kradin, R.L.; Boyle, L.A.; Preffer, F.I.; Callahan, R.J.; Barlai-Kovach, M.; Strauss, H.W.; Dubinett, S.; Kurnick, J.T. Tumor-derived inter-leukin-2-dependent lymphocytes in adoptive immunotherapy of lung cancer. Cancer Immunol. Immunother. 1987, 24, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Conkling, P.R.; Achyuthan, K.E.; Greenberg, C.S.; Newcomb, T.F.; Weinberg, J.B. Human mononuclear phagocyte transglutaminase activity cross-links fibrin. Thromb. Res. 1989, 55, 57–68. [Google Scholar] [CrossRef]
- Akimov, S.S.; Belkin, A.M. Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fi-bronectin. Blood 2001, 98, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Akagi, A.; Tajima, S.; Ishibashi, A.; Matsubara, Y.; Takehana, M.; Kobayashi, S.; Yamaguchi, N. Type XVI Collagen is Expressed in Factor XIIIa+ Monocyte-Derived Dermal Dendrocytes and Constitutes a Potential Substrate for Factor XIIIa. J. Investig. Dermatol. 2002, 118, 267–274. [Google Scholar] [CrossRef]
- Piercy-Kotb, S.A.; Mousa, A.; Al-Jallad, H.F.; Myneni, V.D.; Chicatun, F.; Nazhat, S.N.; Kaartinen, M.T. Factor XIIIA transglutaminase expression and secretion by osteoblasts is regulated by extracellular matrix collagen and the MAP kinase signaling pathway. J. Cell. Physiol. 2012, 227, 2936–2946. [Google Scholar] [CrossRef]
- Ádány, R.; Bárdos, H.; Antal, M.; Módis, L.; Sárváry, A.; Szücs, S.; Balogh, I. Factor XIII of blood coagulation as a nuclear crosslink-ing enzyme. Thromb. Haemost. 2001, 85, 845–851. [Google Scholar] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Spellberg, B.; Edwards, J.E., Jr. Type 1/Type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef]
- Pabst, M.J.; Pabst, K.M.; Handsman, D.B.; Beranova-Giorgianni, S.; Giorgianni, F. Proteome of monocyte priming by lipopolysaccharide, including changes in interleukin-1beta and leukocyte elastase inhibitor. Proteome Sci. 2008, 6, 13. [Google Scholar] [CrossRef]
- Töröcsik, D.; Szeles, L.; Paragh, G.; Rákosy, Z.; Bárdos, H.; Nagy, L.; Balázs, M.; Inbal, A.; Ádány, R. Factor XIII-A is involved in the regulation of gene expression in alternatively activated human macrophages. Thromb. Haemost. 2010, 104, 709–717. [Google Scholar] [CrossRef]
- Chaitidis, P.; O’Donnell, V.; Kuban, R.J.; Bermudez-Fajardo, A.; Ungethuem, U.; Kuhn, H. Gene expression alterations of human peripheral blood monocytes induced by medium-term treatment with the TH2-cytokines interleukin-4 and -13. Cytokine 2005, 30, 366–377. [Google Scholar] [CrossRef]
- Gratchev, A.; Kzhyshkowska, J.; Utikal, J.; Goerdt, S. Interleukin-4 and dexamethasone counterregulate extracellular matrix re-modelling and phagocytosis in type-2 macrophages. Scand. J. Immunol. 2005, 61, 10–17. [Google Scholar] [CrossRef]
- May, R.C.; Machesky, L.M. Phagocytosis and the actin cytoskeleton. J. Cell Sci. 2001, 114, 1061–1077. [Google Scholar] [PubMed]
- Cohen, I.; Blankenberg, T.A.; Borden, D.; Kahn, D.R.; Veis, A. Factor XIIIa-catalyzed cross-linking of platelet and muscle actin. Regulation by nucleotides. Biochim. Biophys. Acta 1980, 628, 365–375. [Google Scholar] [CrossRef]
- Cohen, I.; Glaser, T.; Veis, A.; Bruner-Lorand, J. Ca2+-dependent cross-linking processes in human platelets. Biochim. Biophys. Acta 1981, 676, 137–147. [Google Scholar] [CrossRef]
- Serrano, K.; Devine, D.V. Intracellular Factor XIII crosslinks platelet cytoskeletal elements upon platelet activation. Thromb. Haemost. 2002, 88, 315–320. [Google Scholar] [CrossRef]
- Sarvary, A.; Szucs, S.; Balogh, I.; Becsky, A.; Bardos, H.; Kavai, M.; Seligsohn, U.; Egbring, R.; Lopaciuk, S.; Muszbek, L.; et al. Possible role of Factor XIII subunit A in Fcgamma and complement receptor-mediated phagocytosis. Cell Immunol. 2004, 228, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kavai, M.; Adany, R.; Pasti, G.; Suranyi, P.; Szucs, G.; Muszbek, L.; Bojan, F.; Szegedi, G. Marker profile, enzyme activity, and func-tion of a human myelomonocytic leukemia cell line. Cell Immunol. 1992, 139, 531–540. [Google Scholar] [CrossRef]
- Jayo, A.; Conde, I.; Lastres, P.; Jimenez-Yuste, V.; Gonzalez-Manchon, C. Possible role for cellular FXIII in monocyte-derived den-dritic cell motility. Eur. J. Cell Biol. 2009, 88, 423–431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Higaki, S.; Nakano, K.; Onaka, S.; Amano, A.; Tanioka, Y.; Harada, K.; Hashimoto, S.; Sakaida, I.; Okita, K. Clinical significance of measuring blood coagulation Factor XIIIA regularly and continuously in patients with Crohn’s disease. J. Gastroenterol. Hepatol. 2006, 21, 1407–1411. [Google Scholar] [CrossRef]
- Chamouard, P.; Grunebaum, L.; Wiesel, M.L.; Sibilia, J.; Coumaros, G.; Wittersheim, C.; Baumann, R.; Cazenave, J.P. Significance of diminished Factor XIII in Crohn’s disease. Am. J. Gastroenterol. 1998, 93, 610–614. [Google Scholar] [CrossRef]
- Hudson, M.; Wakefield, A.J.; Hutton, R.A.; Sankey, E.A.; Dhillon, A.P.; More, L.; Sim, R.; Pounder, R.E. Factor XIIIA subunit and Crohn’s disease. Gut 1993, 34, 75–79. [Google Scholar] [CrossRef][Green Version]
- Inbal, A.; Muszbek, L. Coagulation Factor Deficiencies and Pregnancy Loss. Semin. Thromb. Hemost. 2003, 29, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Miloszewski, K.J.A. FACTOR XIII DEFICIENCY. Br. J. Haematol. 1999, 107, 468–484. [Google Scholar] [CrossRef] [PubMed]
- Kohler, H.; Ichinose, A.; Seitz, R.; Ariens, R.; Muszbek, L.; Factor XIII and Fibrinogen SSC Subcommittee of the ISTH. Diagnosis and classification of Factor XIII deficiencies. J. Thromb. Haemost. 2011, 9, 1404–1406. [Google Scholar] [CrossRef]
- Durda, M.A.; Wolberg, A.S.; Kerlin, B.A. State of the art in Factor XIII laboratory assessment. Transfus. Apher. Sci. 2018, 57, 700–704. [Google Scholar] [CrossRef]
- Beckman, J.D.; Kasthuri, R.S.; Wolberg, A.S.; Ma, A.D. Challenges in diagnosis and management of acquired Factor XIII (FXIII) in-hibitors. Haemoph. Off. J. World Fed. Hemoph. 2018, 24, e417. [Google Scholar] [CrossRef] [PubMed]
- Kohler, H.P. Novel treatment for congenital FXIII deficiency. Blood 2012, 119, 5060–5061. [Google Scholar] [CrossRef][Green Version]
- Board, P.; Lososky, M.; Miloszewski, K. Factor XIII: Inherited and acquired deficiency. Blood Rev. 1993, 7, 229–242. [Google Scholar] [CrossRef]
- Asahina, T.; Kobayashi, T.; Takeuchi, K.; Kanayama, N. Congenital blood coagulation Factor XIII deficiency and successful deliv-eries: A review of the literature. Obstetr. Gynecol. Surv. 2007, 62, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Koseki-Kuno, S.; Yamakawa, M.; Dickneite, G.; Ichinose, A. Factor XIII A subunit-deficient mice developed severe uterine bleed-ing events and subsequent spontaneous miscarriages. Blood 2003, 102, 4410–4412. [Google Scholar] [CrossRef]
- Adány, R.; Muszbek, L. Immunohistochemical detection of Factor XIII subunit a in histiocytes of human uterus. Histochem. Cell Biol. 1989, 91, 169–174. [Google Scholar]
- Adány, R.; Glukhova, M.A.; Kabakov, A.Y.; Muszbek, L. Characterisation of connective tissue cells containing Factor XIII subunit a. J. Clin. Pathol. 1988, 41, 49–56. [Google Scholar] [CrossRef]
- Kobayashi, T.; Asahina, T.; Okada, Y.; Terao, T. Studies on the localization of adhesive proteins associated with the development of extravillous cytotrophoblast. Placenta 1999, 20, 35–53. [Google Scholar] [CrossRef]
- Asahina, T.; Kobayashi, T.; Okada, Y.; Goto, J.; Terao, T. Maternal Blood Coagulation Factor XIII is Associated with the Development of Cytotrophoblastic Shell. Placenta 2000, 21, 388–393. [Google Scholar] [CrossRef]
- Hsieh, L.; Nugent, D. Factor XIII deficiency. Haemophilia 2008, 14, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Stirling, Y.; Woolf, L.; North, W.R.; Seghatchian, M.J.; Meade, T.W. Haemostasis in normal pregnancy. Thromb. Haemost. 1984, 52, 176–182. [Google Scholar] [CrossRef]
- Coopland, A.; Alkjaersig, N.; Fletcher, A.P. Reduction in plasma Factor XIII (fibrin stabilizing factor) concentration during preg-nancy. J. Lab. Clin. Med. 1969, 73, 144–153. [Google Scholar] [PubMed]
- Nossel, H.L.; Lanzkowsky, P.; Levy, S.; Mibashan, R.S.; Hansen, J.D. A study of coagulation factor levels in women during labour and in their newborn infants. Thromb. Diath. Haemorrh. 1966, 16, 185–197. [Google Scholar] [CrossRef]
- Mercelina-Roumans, P.; Ubachs, J.; van Wersch, J. Smoking and pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 75, 113–114. [Google Scholar]
- Ogasawara, M.S.; Aoki, K.; Katano, K.; Ozaki, Y.; Suzumori, K. Factor XII but not protein C, protein S, antithrombin III, or Factor XIII is a predictor of recurrent miscarriage. Fertil. Steril. 2001, 75, 916–919. [Google Scholar] [CrossRef]
- Pasquier, E.; Martin, L.D.S.; Bohec, C.; Chauleur, C.; Bretelle, F.; Marhic, G.; Le Gal, G.; Debarge, V.; LeComte, F.; Denoual-Ziad, C.; et al. Enoxaparin for prevention of unexplained recurrent miscarriage: A multicenter randomized double-blind placebo-controlled trial. Blood 2015, 125, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Bagoly, Z.; Cairo, A.; Peyvandi, F. Novel aspects of Factor XIII deficiency. Curr. Opin. Hematol. 2011, 18, 366–372. [Google Scholar] [CrossRef]
- Soendergaard, C.; Kvist, P.H.; Seidelin, J.B.; Nielsen, O.H. Tissue-regenerating functions of coagulation Factor XIII. J. Thromb. Haemost. 2013, 11, 806–816. [Google Scholar] [CrossRef]
- Ajzner, É.; Schlammadinger, Á.; Kerényi, A.; Bereczky, Z.; Katona, É.; Haramura, G.; Boda, Z.; Muszbek, L. Severe bleeding complica-tions caused by an autoantibody against the B subunit of plasma Factor XIII: A novel form of acquired Factor XIII deficiency. Blood J. Am. Soc. Hematol. 2009, 113, 723–725. [Google Scholar]
- Ballerini, G.; Gemmati, D.; Moratelli, S.; Morelli, P.; Serino, M.L. A photometric method for the dosage of Factor XIII applied to the study of chronic hepatopathies. Thromb. Res. 1995, 78, 451–456. [Google Scholar] [CrossRef]
- Nikolajsen, C.L.; Dyrlund, T.F.; Poulsen, E.T.; Enghild, J.J.; Scavenius, C. Coagulation Factor XIIIa substrates in human plasma: Identification and incorporation into the clot. J. Chem. 2014, 289, 6526–6534. [Google Scholar] [CrossRef] [PubMed]
- Mutch, N.J.; Engel, R.; De Willige, S.U.; Philippou, H.; Ariëns, R.A.S. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood 2010, 115, 3980–3988. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.R.; Booth, N.A.; Mutch, N.J. The antifibrinolytic function of Factor XIII is exclusively expressed through alpha-antiplasmin cross-linking. Blood 2011, 117, 6371–6374. [Google Scholar] [CrossRef] [PubMed]
- Robbie, L.A.; Kinghorn, S.; Exley, R.; Booth, N.A.; Ritchie, H. Monocyte Plasminogen Activator Inhibitor 2 (PAI-2) Inhibits u-PA-mediated Fibrin Clot Lysis and Is Cross-linked to Fibrin. Thromb. Haemost. 1999, 81, 96–103. [Google Scholar] [CrossRef]
- Kolev, K.; Tenekedjiev, K.; Komorowicz, E.; Machovich, R. Functional Evaluation of the Structural Features of Proteases and Their Substrate in Fibrin Surface Degradation. J. Biol. Chem. 1997, 272, 13666–13675. [Google Scholar] [CrossRef]
- Sobel, J.H.; Gawinowicz, M.A. Identification of the α Chain Lysine Donor Sites Involved in Factor XIIIa Fibrin Cross-linking. J. Biol. Chem. 1996, 271, 19288–19297. [Google Scholar] [CrossRef]
- Hethershaw, E.L.; La Corte, A.L.C.; Duval, C.; Ali, M.; Grant, P.J.; Ariëns, R.A.S.; Philippou, H. The effect of blood coagulation Factor XIII on fibrin clot structure and fibrinolysis. J. Thromb. Haemost. 2014, 12, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Park, D.; Lesty, C.; Soria, J.; Soria, C.; Montalescot, G.; Weisel, J.W. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: Dynamic and structural approaches by confocal microscopy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1354–1361. [Google Scholar] [CrossRef]
- Dunn, E.J.; Philippou, H.; Ariëns, R.A.S.; Grant, P.J. Molecular mechanisms involved in the resistance of fibrin to clot lysis by plasmin in subjects with type 2 diabetes mellitus. Diabetoloia 2006, 49, 1071–1080. [Google Scholar] [CrossRef]
- Longstaff, C.; Thelwell, C.; Williams, S.C.; Silva, M.M.; Szabo, L.; Kolev, K. The interplay between tissue plasminogen activator do-mains and fibrin structures in the regulation of fibrinolysis: Kinetic and microscopic studies. Blood 2011, 117, 661–668. [Google Scholar] [CrossRef]
- Mutch, N.J.; Koikkalainen, J.S.; Fraser, S.R.; Duthie, K.M.; Griffin, M.; Mitchell, J.; Watson, H.G.; Booth, N.A. Model thrombi formed un-der flow reveal the role of Factor XIII-mediated cross-linking in resistance to fibrinolysis. J. Thromb. Haemost. JTH 2010, 8, 2017–2024. [Google Scholar] [CrossRef]
- Minkema, J.; Bouma, B.; Jansen, J. Cross-linking of alpha 2-antiplasmin to fibrin is a key factor in regulating blood clot lysis: Species differences. Blood Coagul. Fibrinol. Int. J. Haemost. Thromb. 1993, 4, 869–875. [Google Scholar]
- Jensen, P.H.; Lorand, L.; Ebbesen, P.; Gliemann, J. Type-2 plasminogen-activator inhibitor is a substrate for trophoblast transglu-taminase and Factor XIIIa: Transglutaminase-catalyzed cross-linking to cellular and extracellular structures. Eur. J. Biochem. 1993, 214, 141–146. [Google Scholar] [CrossRef]
- Lee, K.N.; Jackson, K.W.; Christiansen, V.J.; Chung, K.H.; McKee, P.A. A novel plasma proteinase potentiates α2-antiplasmin inhibi-tion of fibrin digestion. Blood 2004, 103, 3783–3788. [Google Scholar] [CrossRef]
- Bangert, K.; Johnsen, A.H.; Christensen, U.; Thorsen, S. Different N-terminal forms of α2-plasmin inhibitor in human plasma. Biochem. J. 1993, 291, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Ichikawa, Y.; Nakamura, Y.; Miura, O.; Aoki, N. Expression and Characterization of Pro α2-Plasmin Inhibitor. J. Biochem. 1989, 106, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Rijken, D.; Abdul, S.; Malfliet, J.J.M.C.; Leebeek, F.W.G.; De Willige, S.U. Compaction of fibrin clots reveals the antifibrinolytic effect of Factor XIII. J. Thromb. Haemost. 2016, 14, 1453–1461. [Google Scholar] [CrossRef][Green Version]
- Reed, G.L.; Matsueda, G.R.; Haber, E. Fibrin-fibrin and alpha 2-antiplasmin-fibrin cross-linking by platelet Factor XIII increases the resistance of platelet clots to fibrinolysis. Trans. Assoc. Am. Physicians 1991, 104, 21–28. [Google Scholar] [PubMed]
- Reed, G.L.; Matsueda, G.R.; Haber, E. Platelet Factor XIII increases the fibrinolytic resistance of platelet-rich clots by accelerating the crosslinking of alpha 2-antiplasmin to fibrin. Thromb. Haemost. 1992, 68, 315–320. [Google Scholar] [PubMed]
- Francis, C.W.; Marder, V.J. Rapid formation of large molecular weight alpha-polymers in cross-linked fibrin induced by high Factor XIII concentrations. Role of platelet Factor XIII. J. Clin. Investig. 1987, 80, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Hevessy, Z.; Haramura, G.; Boda, Z.; Udvardy, M.; Muszbek, L. Promotion of the crosslinking of fibrin and alpha 2-antiplasmin by platelets. Thromb. Haemost. 1996, 75, 161–167. [Google Scholar] [PubMed]
- Rubens, F.D.; Perry, D.W.; Hatton, M.W.; Bishop, P.D.; Packham, M.A.; Kinlough-Rathbone, R.L. Platelet accumulation on fibrin-coated polyethylene: Role of platelet activation and Factor XIII. Thromb. Haemost. 1995, 73, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Aleman, M.M.; Byrnes, J.R.; Wang, J.G.; Tran, R.; Lam, W.A.; Di Paola, J.; Mackman, N.; Degen, J.L.; Flick, M.J.; Wolberg, A.S. Factor XIII activity mediates red blood cell retention in venous thrombi. J. Clin. Investig. 2014, 124, 3590–3600. [Google Scholar] [CrossRef]
- Byrnes, J.R.; Duval, C.; Wang, Y.; Hansen, C.E.; Ahn, B.; Mooberry, M.J.; Clark, M.A.; Johnsen, J.M.; Lord, S.T.; Lam, W.A.; et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin alpha-chain cross-linking. Blood 2015, 126, 1940–1948. [Google Scholar] [CrossRef]
- Paragh, L.; Törőcsik, D. Factor XIII Subunit A in the Skin: Applications in Diagnosis and Treatment. BioMed Res. Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Seitz, R.; Kohler, H.P.; Schroeder, V.; Muszbek, L.; Ariens, R.A.S.; Seifried, E.; Oldenburg, J.; Ivaskevicius, V. International Registry on Factor XIII Deficiency: A basis formed mostly on European data. Thromb. Haemost. 2007, 97, 914–921. [Google Scholar] [CrossRef]
- Seitz, R.; Duckert, F.; Lopaciuk, S.; Muszbek, L.; Rodeghiero, F.; Seligsohn, U. ETRO Working Party on Factor XIII Questionnaire on Congenital Factor XIII Deficiency in Europe: Status and Perspectives. Semin. Thromb. Hemost. 1996, 22, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Inbal, A.; Lubetsky, A.; Krapp, T.; Castel, D.; Shaish, A.; Dickneitte, G.; Módis, L.; Muszbek, L.; Inbal, A. Impaired wound healing in Factor XIII deficient mice. Thromb. Haemost. 2005, 94, 432–437. [Google Scholar] [CrossRef]
- D’Argenio, G.; Grossman, A.; Cosenza, V.; Della Valle, N.; Mazzacca, G.; Bishop, P.D. Recombinant Factor XIII Improves Established Experimental Colitis in Rats. Dig. Dis. Sci. 2000, 45, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Bereczky, Z.; Bagoly, Z.; Komáromi, I.; Katona, É. Factor XIII: A coagulation factor with multiple plasmatic and cellu-lar functions. Physiol. Rev. 2011, 91, 931–972. [Google Scholar] [CrossRef]
- Magwenzi, S.G.; Ajjan, R.A.; Standeven, K.F.; Parapia, L.A.; Naseem, K.M. Factor XIII supports platelet activation and enhances thrombus formation by matrix proteins under flow conditions. J. Thromb. Haemost. 2011, 9, 820–833. [Google Scholar] [CrossRef]
- Lanir, N.; Ciano, P.S.; Van De Water, L.; McDonagh, J.; Dvorak, A.M.; Dvorak, H.F. Macrophage migration in fibrin gel matrices. II. Effects of clotting Factor XIII, fibronectin, and glycosaminoglycan content on cell migration. J. Immunol. 1988, 140, 2340–2349. [Google Scholar] [PubMed]
- Grinnell, F.; Feld, M.; Minter, D. Fibroblast adhesion to fibrinogen and fibrin substrata: Requirement for cold-insoluble globulin (plasma fibronectin). Cell 1980, 19, 517–525. [Google Scholar] [CrossRef]
- Dardik, R.; Shenkman, B.; Tamarin, I.; Eskaraev, R.; Hársfalvi, J.; Varon, D.; Inbal, A. Factor XIII mediates adhesion of platelets to endothelial cells through αvβ3 and glycoprotein IIb/IIIa integrins. Thromb. Res. 2002, 105, 317–323. [Google Scholar] [CrossRef]
- Törőcsik, D.; Bárdos, H.; Nagy, L.; Ádány, R. Identification of Factor XIII-A as a marker of alternative macrophage activation. Cell. Mol. Life Sci. 2005, 62, 2132–2139. [Google Scholar] [CrossRef]
- Wynn, T.A.; Barron, L. Macrophages: Master Regulators of Inflammation and Fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Yamamoto, T.; Matsubara, S.; Kukita, I.; Takeya, M.; Miyauchi, Y.; Kambara, T. Factor XIII-dependent generation of 5th complement component(C5)-derived monocyte chemotactic factor coinciding with plasma clotting. Biochim. Biophys. Acta BBA Mol. Basis Dis. 1992, 1138, 53–61. [Google Scholar] [CrossRef]
- Semba, U.; Chen, J.; Ota, Y.; Jia, N.; Arima, H.; Nishiura, H.; Yamamoto, T. A plasma protein indistinguishable from ribosomal pro-tein S19: Conversion to a monocyte chemotactic factor by a Factor XIIIa-catalyzed reaction on activated platelet membrane phosphatidylserine in association with blood coagulation. Am. J. Pathol. 2010, 176, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Noll, T.; Wozniak, G.; McCarson, K.; Hajimohammad, A.; Metzner, H.J.; Inserte, J.; Kummer, W.; Hehrlein, F.W.; Piper, H.M. Effect of Factor XIII on Endothelial Barrier Function. J. Exp. Med. 1999, 189, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, K.; Shinbo, K.; Takahashi, M.; Matsuishi, T. Suppressive effect of human blood coagulation Factor XIII on the vascular permeability induced by anti-guinea pig endothelial cell antiserum in guinea pigs. Thromb. Res. 1993, 71, 139–148. [Google Scholar] [CrossRef]
- Wozniak, G.; Noll, T.; Akintürk, H.; Thul, J.; Müller, M. Factor XIII prevents development of myocardial edema in children under-going surgery for congenital heart disease. Ann. N. Y. Acad. Sci. 2001, 936, 617–620. [Google Scholar] [CrossRef]
- Schroth, M.; Meissner, U.; Cesnjevar, R.; Weyand, M.; Singer, H.; Rascher, W.; Klinge, J. Plasmatic [corrected] Factor XIII reduces severe pleural effusion in children after open-heart surgery. Pediatr. Cardiol. 2006, 27, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Dardik, R.; Solomon, A.; Loscalzo, J.; Eskaraev, R.; Bialik, A.; Goldberg, I.; Schiby, G.; Inbal, A. Novel Proangiogenic Effect of Factor XIII Associated with Suppression of Thrombospondin 1 Expression. Arter. Thromb. Vasc. Biol. 2003, 23, 1472–1477. [Google Scholar] [CrossRef]
- Dardik, A.; Chen, L.; Frattini, J.; Asada, H.; Aziz, F.; Kudo, F.A.; Sumpio, B.E. Differential effects of orbital and laminar shear stress on endothelial cells. J. Vasc. Surg. 2005, 41, 869–880. [Google Scholar] [CrossRef]
- Auerbach, W.; Auerbach, R. Angiogenesis inhibition: A review. Pharmacol. Ther. 1994, 63, 265–311. [Google Scholar] [CrossRef]
- Nugent, D.; Ashley, C.; Garcia-Talavera, J.; Lo, L.; Mehdi, A.; Mangione, A. Pharmacokinetics and safety of plasma-derived Factor XIII concentrate (human) in patients with congenital Factor XIII deficiency. Haemophilia 2015, 21, 95–101. [Google Scholar] [CrossRef]
- Suzuki, H.; Kaneda, T. Tooth extraction in two patients who had a congenital deficiency of Factor XIII. J. Oral Maxillofac. Surg. 1985, 43, 221–224. [Google Scholar] [CrossRef]
- Key, N.S.; Negrier, C. Coagulation factor concentrates: Past, present, and future. Lancet 2007, 370, 439–448. [Google Scholar] [CrossRef]
- Brackmann, H.H.; Egbring, R.; Ferster, A.; Fondu, P.; Girardel, J.M.; Kreuz, W.; Masure, R.; Miloszewski, K.; Stibbe, J.; Zimmermann, R.; et al. Pharmacokinetics and tolerability of Factor XIII concentrates prepared from human placenta or plasma: A crossover ran-domised study. Thromb. Haemost. 1995, 74, 622–625. [Google Scholar] [CrossRef]
- Lusher, J.; Pipe, S.W.; Alexander, S.; Nugent, D. Prophylactic therapy with Fibrogammin P is associated with a decreased inci-dence of bleeding episodes: A retrospective study. Haemophilia 2010, 16, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Katona, É.; Muszbek, L. Diagnosis and Management of Congenital and Acquired FXIII Deficiencies. Semin. Thromb. Hemost. 2016, 42, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, A. Physiopathology and Regulation of Factor XIII. Thromb. Haemost. 2001, 86, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lorand, L. Sol Sherry Lecture in Thrombosis: Research on clot stabilization provides clues for improving thrombolytic thera-pies. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2–9. [Google Scholar] [CrossRef]
- Nugent, D. Corifact/Fibrogammin(R) P in the prophylactic treatment of hereditary Factor XIII deficiency: Results of a prospec-tive, multicenter, open-label study. Thromb. Res. 2012, 130 (Suppl. S2), S12–S14. [Google Scholar] [CrossRef]
- Lovejoy, A.E.; Reynolds, T.C.; Visich, J.E.; Butine, M.D.; Young, G.; Belvedere, M.A.; Blain, R.C.; Pederson, S.M.; Ishak, L.M.; Nugent, D.J. Safety and pharmacokinetics of recombinant Factor XIII-A2 administration in patients with congenital Factor XIII deficiency. Blood 2006, 108, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Carcao, M.; Altisent, C.; Castaman, G.; Fukutake, K.; Kerlin, B.A.; Kessler, C.; Lassila, R.; Nugent, D.; Oldenburg, J.; Garly, M.-L.; et al. Recombinant FXIII (rFXIII-A2) Prophylaxis Prevents Bleeding and Allows for Surgery in Patients with Congenital FXIII A-Subunit Deficiency. Thromb. Haemost. 2018, 118, 451–460. [Google Scholar] [CrossRef]
- Naderi, M.; Dorgalaleh, A.; Alizadeh, S.; Tabibian, S.; Hosseini, S.; Shamsizadeh, M.; Bamedi, T. Clinical manifestations and man-agement of life-threatening bleeding in the largest group of patients with severe Factor XIII deficiency. Int. J. Hematol. 2014, 100, 443–449. [Google Scholar] [CrossRef]
- Abdel-Samad, N. Treatment with Recombinant Factor XIII (Tretten) in a Pregnant Woman with Factor XIII Deficiency. Am. J. Case Rep. 2017, 18, 436–439. [Google Scholar] [CrossRef]
- Ichinose, A.; Japanese Collaborative Research Group on AH. Autoimmune acquired Factor XIII deficiency due to anti-Factor XIII/13 antibodies: A summary of 93 patients. Blood Rev. 2017, 31, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Souri, M.; Osaki, T.; Ichinose, A. Anti-Factor XIII A subunit (FXIII-A) autoantibodies block FXIII-A2 B2 assembly and steal FXIII-A from native FXIII-A2 B. J. Thromb. Haemost. 2015, 13, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Tone, K.J.; James, T.E.; Fergusson, D.A.; Tinmouth, A.; Tay, J.; Avey, M.T.; Kilty, S.; Lalu, M.M. Acquired Factor XIII Inhibitor in Hospi-talized and Perioperative Patients: A Systematic Review of Case Reports and Case Series. Transfus. Med. Rev. 2016, 30, 123–131. [Google Scholar] [CrossRef]
- Hiippala, S.T.; Myllyla, G.J.; Vahtera, E.M. Hemostatic factors and replacement of major blood loss with plasma-poor red cell con-centrates. Anesth. Analg. 1995, 81, 360–365. [Google Scholar] [PubMed]
- Floccard, B.; Rugeri, L.; Faure, A.; Saint Denis, M.; Boyle, E.M.; Peguet, O.; Levrat, A.; Guillaume, C.; Marcotte, G.; Vulliez, A. Early co-agulopathy in trauma patients: An on-scene and hospital admission study. Injury 2012, 43, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hagemo, J.S.; Stanworth, S.; Juffermans, N.P.; Brohi, K.; Cohen, M.J.; Johansson, P.I.; Røislien, J.; Eken, T.A.; Næss, P.; Gaarder, C. Prevalence, predictors and outcome of hypofibrinogenaemia in trauma: A multicentre observational study. Crit. Care 2014, 18, R52. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.; Stanworth, S.J.; Curry, N. Do we still need cryoprecipitate? Cryoprecipitate and fibrinogen concentrate as treatments for major hemorrhage—How do they compare? Expert Rev. Hematol. 2018, 11, 351–360. [Google Scholar] [CrossRef]
- Rourke, C.; Curry, N.; Khan, S.; Taylor, R.; Raza, I.; Davenport, R.; Stanworth, S.; Brohi, K. Fibrinogen levels during trauma hemor-rhage, response to replacement therapy, and association with patient outcomes. J. Thromb. Haemost. 2012, 10, 1342–1351. [Google Scholar] [CrossRef]
- Morrison, J.J.; Ross, J.D.; Dubose, J.J.; Jansen, J.O.; Midwinter, M.J.; Rasmussen, T.E. Association of cryoprecipitate and tranexamic acid with improved survival following wartime injury: Findings from the MATTERs II Study. JAMA Surg. 2013, 148, 218–225. [Google Scholar] [CrossRef]
- Schlimp, C.J.; Voelckel, W.; Inaba, K.; Maegele, M.; Ponschab, M.; Schöchl, H. Estimation of plasma fibrinogen levels based on hemoglobin, base excess and Injury Severity Score upon emergency room admission. Crit. Care 2013, 17, R137. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.; Fries, D.; Velik-Salchner, C.; Reif, C.; Klingler, A.; Innerhofer, P. The In Vitro Effects of Fibrinogen Concentrate, Factor XIII and Fresh Frozen Plasma on Impaired Clot Formation After 60% Dilution. Anesth. Analg. 2008, 106, 1360–1365. [Google Scholar] [CrossRef]
- Wettstein, P.; Haeberli, A.; Stutz, M.; Rohner, M.; Corbetta, C.; Gabi, K.; Schnider, T.; Korte, W. Decreased Factor XIII Availability for Thrombin and Early Loss of Clot Firmness in Patients with Unexplained Intraoperative Bleeding. Anesth. Analg. 2004, 99, 1564–1569. [Google Scholar] [CrossRef]
- Chandler, W.L.; Patel, M.A.; Gravelle, L.; Soltow, L.O.; Lewis, K.; Bishop, P.D.; Spiess, B.D. Factor XIIIA and clot strength after cardio-pulmonary bypass. Blood Coagul. Fibrinolysis 2001, 12, 101–108. [Google Scholar] [CrossRef]
- Shainoff, J.R.; Estafanous, F.G.; Yared, J.P.; DiBello, P.M.; Kottke-Marchant, K.; Loop, F.D. Low Factor XIIIA levels are associated with increased blood loss after coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 1994, 108, 437–445. [Google Scholar] [CrossRef]
- Korte, W.C.; Szadkowski, C.; Gahler, A.; Gabi, K.; Kownacki, E.; Eder, M.; Degiacomi, P.; Zoller, N.; Devay, J.; Lange, J.; et al. Factor XIII substitution in surgical cancer patients at high risk for intraoperative bleeding. Anesthesiology 2009, 110, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Shebuski, R.J.; Sitko, G.R.; Claremon, D.A.; Baldwin, J.J.; Remy, D.C.; Stern, A.M. Inhibition of Factor XIIIa in a canine model of coro-nary thrombosis: Effect on reperfusion and acute reocclusion after recombinant tissue-type plasminogen activator. Blood 1990, 75, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Finney, S.; Seale, L.; Sawyer, R.T.; Wallis, R.B. Tridegin, a new peptidic inhibitor of Factor XIIIa, from the blood-sucking leech Haementeria ghilianii. Biochem. J. 1997, 324, 797–805. [Google Scholar] [CrossRef][Green Version]
- Schmitz, T.; Paul George, A.A.; Nubbemeyer, B.; Bauml, C.A.; Steinmetzer, T.; Ohlenschlager, O.; Biswas, A.; Imhof, D. NMR-Based Structural Characterization of a Two-Disulfide-Bonded Analogue of the FXIIIa Inhibitor Tridegin: New Insights into Structure-Activity Relationships. Int. J. Mol. Sci. 2021, 22, 880. [Google Scholar] [CrossRef]
- Avery, C.A.; Pease, R.J.; Smith, K.; Boothby, M.; Buckley, H.M.; Grant, P.J.; Fishwick, C.W. (±) cis-bisamido epoxides: A novel series of potent FXIII-A inhibitors. Eur. J. Med. Chem. 2015, 98, 49–53. [Google Scholar] [CrossRef]
- Pasternack, R.; Büchold, C.; Jähnig, R.; Pelzer, C.; Sommer, M.; Heil, A.; Florian, P.; Nowak, G.; Gerlach, U.; Hils, M. Novel inhibitor ZED3197 as potential drug candidate in anticoagulation targeting coagulation FXIIIa (F13a). J. Thromb. Haemost. 2020, 18, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Strilchuk, A.W.; Meixner, S.C.; Leung, J.; Safikhan, N.; Kulkarni, J.A.; Russell, H.M.; Van Der Meel, R.; Sutherland, M.R.; Iii, A.P.O.; Palumbo, J.; et al. Sustained Depletion of FXIII-A by Inducing Acquired FXIII-B Deficiency. Blood 2020, 136, 2946–2954. [Google Scholar] [CrossRef]
- Dull, K.; Fazekas, F.; Törőcsik, D. Factor XIII-A in Diseases: Role Beyond Blood Coagulation. Int. J. Mol. Sci. 2021, 22, 1459. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).