Abstract
The N-terminal of Myc-like basic helix-loop-helix transcription factors (bHLH TFs) contains an interaction domain, namely the MYB-interacting region (MIR), which interacts with the R2R3-MYB proteins to regulate genes involved in the anthocyanin biosynthetic pathway. However, the functions of MIR-domain bHLHs in this pathway are not fully understood. In this study, PbbHLH2 containing the MIR-domain was identified and its function investigated. The overexpression of PbbHLH2 in ”Zaosu” pear peel increased the anthocyanin content and the expression levels of late biosynthetic genes. Bimolecular fluorescence complementation showed that PbbHLH2 interacted with R2R3-MYB TFs PbMYB9, 10, and 10b in onion epidermal cells and confirmed that MIR-domain plays important roles in the interaction between the MIR-domain bHLH and R2R3-MYB TFs. Moreover, PbbHLH2 bound and activated the dihydroflavonol reductase promoter in yeast one-hybrid (Y1H) and dual-luciferase assays. Taken together these results suggested that the MIR domain of PbbHLH2 regulated anthocyanin biosynthesis in pear fruit peel.
1. Introduction
Anthocyanins represent a major category of secondary metabolites found in many horticultural products [1,2,3]. Anthocyanin accumulation results in plant tissues presenting different colors [3]. In addition, anthocyanins play important roles in plant growth and development, as well as resistance to stresses, such as oxidation [4], light [4,5], and cold [6,7,8]. Anthocyanin is also involved in defending against pathogens [9] and attracting pollinators and seed dispersers [10]. They are also beneficial to human health [11,12,13]. Consuming anthocyanins may improve the body’s metabolism and energy balance, which aids in weight control, thereby reducing obesity risk [14]. Anthocyanins also have roles in fighting other diseases with their antibacterial [15] and antitumor [11,16] activities. Previous studies have indicated that anthocyanin could enhance eye and brain health and functions [11], effectively regulate blood pressure, blood lipids and blood glucose levels [17,18,19]. Additionally, anthocyanins play important roles in the prevention of cardiovascular and nervous system diseases [3,20].
Earlier studies have revealed that the anthocyanin biosynthetic pathway is composed by multiple enzymes classified as early biosynthetic genes and late biosynthetic genes (LBGs). The early biosynthetic genes include chalcone synthase and chalcone isomerase. The LBGs include dihydroflavonol reductase (DFR), anthocyanidin synthase (ANS)/leucoanthocyanidin dioxygenase, and UDP-glucoside: flavonoid glucosyltransferase (UFGT) [21,22,23,24].
Anthocyanins’ biosynthesis is regulated by transcription factors (TFs), such as MYBs and basic helix-loop-helixes (bHLHs). In Arabidopsis thaliana, PAP1 (AtMYB75), PAP2 (AtMYB90), MYB113, and MYB114 are involved in anthocyanin accumulation [23,25,26]. In apple (Malus × domestica), MYBA, MYB1, and MYB10 regulate the anthocyanin biosynthetic pathway [27,28]. In pear (Pyrus bretschneideri Rehd.), MYB10 and MYB10b are involved in the anthocyanin biosynthetic pathway [29,30,31].
The bHLH proteins are a TF class in which each member contains a basic helix-loop-helix structural domain [32] that is important for the formation of the homodimers or heterodimers [33,34]. In plants, the more than 500 known bHLHs are divided into 26 subgroups [35]. Some bHLH proteins associated with the anthocyanin biosynthetic pathway have been identified in fruits, such as grapevine (Vitis vinifera), apple, strawberry (Fragaria × vesca), and pear [7,36,37,38,39]. The N-terminal interaction domain of IIIf bHLH TFs, also known as the MYB-interacting region (MIR) domain, was identified to interact with the R2R3-MYB domain proteins to regulate the anthocyanin biosynthetic pathway [40,41,42,43,44]. The bHLHs, as a subgroup of IIIf, plays important roles in regulating in anthocyanin biosynthesis in plants [21]. The IIIf bHLH TFs can interact with R2R3-MYB TFs and TTG1 (WD40) to form the MYB-bHLH-WD40 (MBW) ternary protein complex [45]. The MBW complex plays important roles in the regulation of LBGs in the anthocyanin biosynthetic pathway [21]. In the MBW complex, bHLH TFs determine the specificities of the recognized target gene promoter and the specificity of the activated transcriptional binding site [46]. Therefore, it is particularly important to explore the roles of bHLH TFs in plant anthocyanin biosynthetic pathways.
In this study, two bHLHs containing MIR-domain were identified as the positive regulators of anthocyanin biosynthesis in pear fruit. The functions of PbbHLH2 in anthocyanin biosynthesis were investigated in pear fruit peel. The MIR-domain played essential roles in the interactions between PbbHLH2 and PbMYB9, 10, and 10b. The MIR-domain PbbHLHs interacted with PbMYBs to form complexes that accelerated anthocyanin biosynthesis by promoting the expression of PbDFR, PbANS, and PbUFGT in pear fruit. In addition, the MIR-domain PbbHLH2 independently induced anthocyanin accumulation and regulated anthocyanin biosynthetic genes expression. Thus, we found that a bHLH TF belonging to the IIIf subgroup, MIR-domain PbbHLH2, is involved in anthocyanin synthesis in pear fruit.
2. Results
2.1. Phylogenetic Analysis and Sequence Analysis of the Anthocyanin Related IIIf bHLH TFs in the Pear
Some bHLH proteins in the IIIf subgroup, such as Arabidopsis thaliana AtTT8, AtGL3, AtEGL3, AtMYC1, AtMYC-146, Chrysanthemum morifolium bHLH2, Myrica rubra bHLH1 and Nicotiana tabacum An1a, participate in the anthocyanin biosynthetic pathway [21,47]. By comparing the protein sequences of AtTT8, AtGL3, AtEGL3, AtMYC-146, CmbHLH2, MrbHLH1, NtAn1a and AtMYC1 of IIIf bHLHs, the MIR domain sequence was revealed (Figure 1a). The MIR-domain is essential for binding the R2R3-MYB to a transcription complexes [41,44]. The HMM model of the MIR-domain was constructed to screen the pear database (Pyrus bretschneideri Rehd.), and five bHLH proteins were identified and selected for further studies (Figure 1b). A phylogenetic tree containing the five candidate PbbHLH proteins and 14 IIIf subgroup bHLHs from different plants was constructed (Figure 1b). The multiple sequence alignment of IIIf proteins was presented in Supplementary Figure S1 (Supplementary Figure S1). We found that the PbbHLH1 and PbbHLH2 proteins, which are on a different branch from MdbHLH3 and MdbHLH33 proteins, regulated anthocyanin synthesis (Figure 1b). Therefore, we focused this study on the characterization of PbbHLH1 and PbbHLH2 function in anthocyanin biosynthesis in this study.

Figure 1.
Analysis of IIIf bHLHs. (a) Multiple sequence alignment of the MYB-interacting region (MIR) domain of the IIIf bHLH transcription factors. Identical residues and conservative residues are marked in black and gray, respectively. The black line indicates the MIR. (b) Phylogenetic analysis of IIIf bHLHs from different species. The bHLH protein sequences of PbbHLHs were obtained from the NCBI. The gene accession numbers used are listed in Supplementary Table S1. At, Arabidopsis thaliana; Cm, Chrysanthemum morifolium; Cp, Chimonanthus praecox; Md, Malus domestica; Mp, Marchantia polymorpha; Mr, Myrica rubra; Nt, Nicotiana tabacum; Pb, Pyrus bretschneideri; Vv, Vitis vinifera.
2.2. Expression Patterns of PbbHLH1 and PbbHLH2 Genes in Pear
In order to explore the expression patterns of PbbHLH1 and PbbHLH2 genes in pear, the expression levels of PbbHLH1 and PbbHLH2 genes during three developmental stages of pear fruit and in three different tissues at 0 days after flower bloom (DAFB) were analyzed. The anthocyanin content in ”Red Zaosu” pear peel was higher than in “Zaosu” peel during each of the three stages analysed (Figure 2a), and in all the tested pear tissues at 0 days after flower bloom (Figure 2b). The expression level of PbbHLH2 gene in “Red Zaosu” peel was significantly higher than in “Zaosu” peel in each of the three developmental stages (Figure 2c). Moreover, the expression levels of PbbHLH1 and PbbHLH2 genes in the sepals and petals of “Red Zaosu” were higher than in “Zaosu”. Additionally, the expression levels of PbMYB9, 10, and 10b genes in “Red Zaosu” peel were higher than in “Zaosu” peel (Supplementary Figure S2). Furthermore, PbbHLH2 gene expression was positively correlated with the anthocyanin contents in different “Red Zaosu” pear fruit tissues and during different developmental periods (Supplementary Figure S3). Thus, PbbHLH2 gene expression was correlated with anthocyanin accumulation in pear fruit. Therefore, we chose to further study PbbHLH2 as an active regulator of anthocyanin biosynthesis.
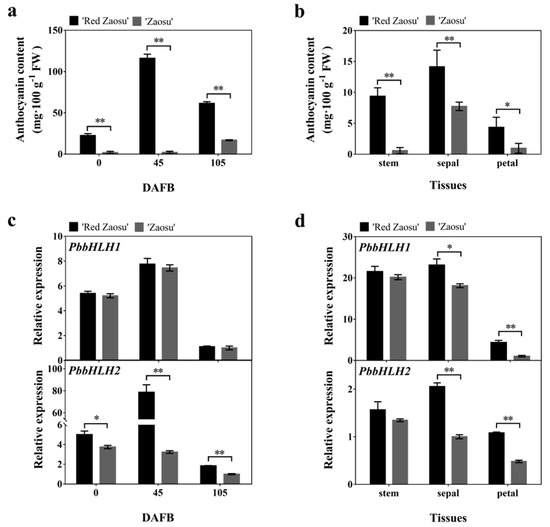
Figure 2.
Expression patterns of bHLH transcription factors in different pear fruit tissues and at different developmental stages (a,b) The anthocyanin contents in pear fruit at different developmental stages (a) and in different pear fruit tissues (b); (c,d) PbbHLH1 and PbbHLH2 expression levels during different developmental stages (c) and in different tissues (d) of “Red Zaosu” and “Zaosu” pear. The significance levels of the differences were analyzed by t-test (* p < 0.05; ** p < 0.01). All data are from three biological replicates and are expressed as means ± SEs (n = 3). All tests were computed using SPSS (ver.20.0).
2.3. PbbHLH2 up-Regulated Anthocyanin Accumulation in the Peel of Pear
In order to determine whether PbbHLH2 gene is involved in anthocyanin biosynthesis, we overexpressed the PbbHLH2 in “Zaosu” pear fruitlets peel. The effectiveness of the infection of the “Zaosu” fruitlets peel was verified by monitoring the GUS signal. The GUS reporter was used to monitor the gene expression patterns in the infected fruitlets peel (Supplementary Figure S4a). The peel of fruitlets overexpressing the PbbHLH2 gene (PbbHLH2-OE) (Figure 3b) was redder than the one of fruitlets overexpressing the empty vector (Figure 3a). Moreover, the expression level in PbbHLH2-OE “Zaosu” pear fruitlets peel increased (Figure 3b), as did the anthocyanin content (Figure 3c). In addition, the transcript levels of PbDFR, PbANS, and PbUFGT genes were significantly increased in PbbHLH2-OE pear fruitlets peel (Figure 3d). The transcript levels of PbMYB9, 10, and 10b genes were increased in PbbHLH2-OE pear fruitlets peel (Figure 3e), as did the expression level of PbGSTF12 gene (Supplementary Figure S4b). Thus, PbbHLH2 gene promoted anthocyanin accumulation in pear fruitlets peel.
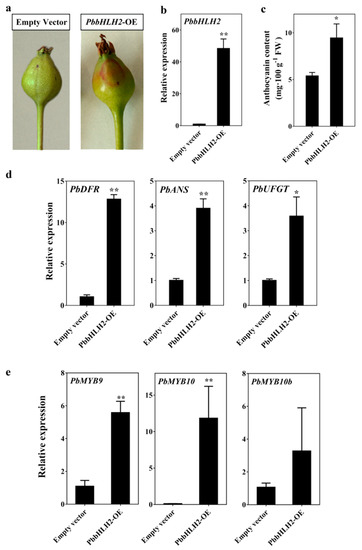
Figure 3.
Anthocyanin patterns in pear fruitlets peel transiently overexpressing PbbHLH2 (PbbHLH2-OE). (a) Overexpression assay of PbbHLH2 in “Zaosu” fruitlets peel; (b) The PbbHLH2 gene expression levels in PbbHLH2-OE fruitlets peel; (c) The anthocyanin contents in PbbHLH2-OE fruitlets peel; (d,e) The expression levels of PbDFR, PbANS and PbUFGT (d) and of PbMYB9, 10, and 10b genes (e) in PbbHLH2-OE fruitlets peel. The significance levels of difference were determined by t-test (* = p < 0.05; ** = p < 0.01). All the data are from three biological replicates and are expressed as means ± SEs (n = 3). All the tests were computed using SPSS (ver.20.0).
2.4. PbbHLH2 Gene was an Essential Part of the Anthocyanin Biosynthesis Pathway in the Pear Peel
To further verify the biological function of PbbHLH2 gene in the anthocyanin biosynthetic pathway, the virus-induced gene silencing (VIGS) system was used to silence PbbHLH2 gene in the peel of ”Palacer” pear fruitlets. The transient assay indicated that the PbbHLH-TRV fruitlets peel did not recover the red pigmentation around the injection holes (Figure 4a). The anthocyanin concentration in PbbHLH-TRV fruitlets peel significantly decreased (Figure 4c). In addition, the expression levels of PbDFR, PbANS and PbUFGT genes as well as those of PbMYB9, 10, and 10b, decreased compared with the empty vector (Figure 4d,e). Furthermore, the expression level of PbGSTF12 in PbbHLH-TRV fruitlets peel decreased (Supplementary Figure S4c). Thus, PbbHLH2 gene appears to play important roles in the anthocyanin biosynthetic pathway of pear fruit.
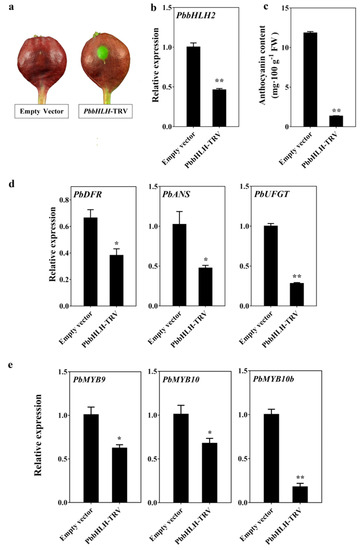
Figure 4.
Anthocyanin patterns in transient PbbHLH-RNAi “Palacer” pear fruitlets peel. (a–e) VIGS assay (a); PbbHLH2 expression levels (b); anthocyanin contents (c); PbDFR, PbANS, and PbUFGT expression levels (d); and PbMYB9, 10, and 10b genes expression levels (e) in transient PbbHLH-RNAi “Palacer” fruitlets peel. The significance levels of difference was analyzed by t-test (* = p < 0.05; ** = p < 0.01). All the data are from three biological replicates and are expressed as means ± SEs (n = 3). All the tests were computed using SPSS (ver.20.0).
2.5. PbbHLH2 Interacts with PbMYB9, PbMYB10 and PbMYB10b via MIR-Domain
To determine whether PbbHLH2 interacts with PbMYBs, a bimolecular fluorescence complementation (BiFC) analysis was performed using onion epidermal cells. PbbHLH2 protein interacted with PbMYB9, 10, and 10b proteins in the onion epidermal cell nucleus (Figure 5a). However, there was no fluorescence detected when PbbHLH2-△NE, having a delete MIR-domain, and PbMYBs were co-infiltrated in onion epidermal cells. These results indicated that the MIR-domain of PbbHLH2 was essential for interactions with PbMYB9, 10, and 10b (Figure 5b).
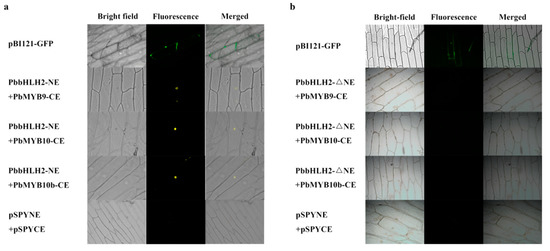
Figure 5.
The interaction of PbbHLH2 and PbMYBs in onion epidermal cell. (a,b) The interaction of PbbHLH2 (a) and PbbHLH2-△NE, having a delete MIR-domain, (b) with PbMYB9, 10, and 10b in onion epidermal cells. The BiFC was observed using a fluorescence microscope (Axio Observer D1, Carl Zeiss Jena, Oberkochen, Germany).
2.6. PbbHLH2 can Activate the Promoters of PbANS, PbDFR and PbUFGT in Pear Fruit Peel
To investigate whether PbbHLH2 gene binds the promoter regions of PbANS, PbDFR, and PbUFGT genes in pear, yeast one-hybrid assay (Y1H) was performed. The results showed that PbbHLH2 directly bound the promoter of PbDFR gene (Figure 6a). When infiltrated into Nicotiana benthamiana leaves, PbbHLH2 gene activated the PbDFR promoter but not the PbANS promoter (Figure 6b). When PbbHLH2 was co-infiltrated with PbMYB10, the promoter of PbDFR was significantly activated (Figure 6b). When infiltrated with PbMYB9 into Nicotiana benthamiana leaves, PbbHLH2 gene can activate the PbANS and PbUFGT promoters (Figure 6b). Thus, PbDFR, PbANS, and PbUFGT were up-regulated when PbbHLH2 was co-infiltrated with PbMYBs.
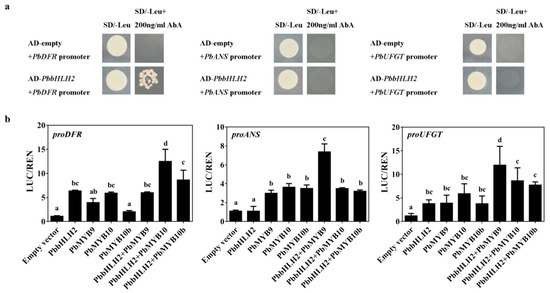
Figure 6.
PbbHLH2 interacted with PbMYBs to activate PbDFR and PbUFGT. (a) The interactions between the PbbHLH2 protein and the PbDFR, PbANS, and PbUFGT promoters as revealed by yeast one-hybrid assays. The yeast transformed with plasmid AbAi-promoters and plasmid AD-PbbHLH2 which grew on SD/-leu plate were used as positive controls; the yeast transformed with plasmid AbAi-promoters and empty plasmid AD which grew on SD/-leu + 200 ng/mL AbA plate were used as negative controls. (b) Transient dual-luciferase detections of PbDFR, PbANS, and PbUFGT promoters in Nicotiana benthamiana leaves. Different letters denote statistical significance (one-way ANOVA, p < 0.05). Values are means ± SDs, n = 3.
3. Discussion
Anthocyanins are important protective substances in plants that aid in resisting biotic and abiotic stresses [1,4,5,6,16]. The bHLH and MYB TFs play important roles in the anthocyanin biosynthetic pathway [48,49,50,51,52]. In Arabidopsis thaliana, AtGL3, AtEGL3, and AtTT8 are classified into the bHLH subgroup IIIf. Moreover, these three IIIf bHLH proteins contain a MIR-domain region and are involved in the anthocyanin biosynthetic pathway [53,54]. Until now, in bHLH TFs no definite MIR-domain had been identified as being involved in the anthocyanin biosynthetic pathway of pear fruit. In the present study, we identified two MIR-domain bHLH TFs, PbbHLH1, and PbbHLH2, in pear fruit. Both PbbHLH1 and PbbHLH2 are highly homologous with AtGL3/EGL3, which is known to regulate anthocyanin biosynthesis [53,55,56,57,58]. In some plants, such as tomato (Solanum lycopersicum) [55] and Arabidopsis thaliana [56,57,58], GL3 and EGL3 also play essential roles in anthocyanin biosynthesis. The expression patterns of genes may be used to infer their biological functions. Here, we detected high PbbHLH2 gene expression levels in “Red Zaosu” pear fruit peel. This result was consistent with the anthocyanin contents of pear fruits (Figure 2). Therefore, we concluded that the biological function of PbbHLH2 is related to the anthocyanin biosynthetic pathway. The overexpression PbbHLH2 increased anthocyanin accumulations and anthocyanin structural gene expression levels in pear fruitlet peel (Figure 3). In agreement with this study, the transient overexpression of AtGL3 or AtMYC-146 can restore the production of anthocyanin production in a white-flowered Matthiola incana mutant [53]. Thus, PbbHLH2 promoted anthocyanin biosynthesis in pear fruit peel.
Both PbMYB9 and PbMYB10b are involved in the anthocyanin biosynthetic pathway [31]. In Arabidopsis thaliana, the MYB TFs PAP1, PAP2, AtMYB113, and AtMYB114 act as positive regulators of anthocyanin accumulation [23,25,26]. Previous studies have shown that the MdMYB10 is autoregulated in red apple [59,60]. MdbHLH3 may not regulate the activation of MdMYB10 promoter [60], but MdbHLH3 may interact with MYB9, MYB10 and MYB110 to activate the MYB10 promoter in apple [59,60,61]. Therefore, the expression of MYBs is not completely consistent with the expression of bHLHs in pear fruit (Supplementary Figure S2). In Arabidopsis thaliana, the anthocyanin biosynthesis is regulated by the MBW protein complex through the transcriptional regulation of structural genes [25,62,63,64]. In our study, the expression levels of PbMYB9, 10, and 10b gene were affected by PbbHLH2 gene in transient assays in pear fruit (Figure 3 and Figure 4). This suggests that PbbHLH2 may form a dimeric structure with MYB. In our study, the MIR-domain was identified in the N-terminal of PbbHLH2 protein (Figure 1). We showed that the MIR-domain of PbbHLH2 protein interacts with PbMYB9, 10, and 10b, which are activators in the anthocyanin biosynthetic pathway. In addition, PbMYB9, 10, and 10b cannot interact with the PbbHLH2 when it lacks the MIR-domain. This indicates that the MIR-domain is essential for the interactions between bHLH2 and MYBs. Our study is in substantial agreement with the previous reports [44,45].
The earlier studies indicated that the MIR domains of IIIf bHLH members are indispensable for the interactions with R2R3-MYB TFs [43,44,65]. Both the bHLH domain and ACT-like domains form specific dimers that regulate the flavonoid biosynthetic genes [66,67]. The WD40/AD is an interaction site for WD40 and/or the RNA polymerase II through the acidic domains in bHLH proteins [68]. And MYB factors are involved in the anthocyanin biosynthetic pathways of some plants [49,50,62,69]. According to this study, the MIR domains in bHLHs interacted with MYBs to form dimers and affected LBGs expression levels in pear. Taken together, our results showed that the PbbHLH2 interacts with PbMYB9, 10, and 10b through the MIR-domain to form a transcription complex in pear fruit peel. Moreover, our study indicated that the MIR-domain is essential for the formation of bHLH-MYB protein complexes.
Previous studies have shown that the LBGs in anthocyanin biosynthesis are regulated by an MBW complex that consists of an R2R3-MYB, a subgroup IIIf bHLH TF and a WD40 repeat protein. For example, in Arabidopsis, R2R3-MYB TFs (PAP1, PAP2, MYB113, or MYB114), bHLH TFs (TT8, GL3 or EGL3) and the WD40 protein TTG1 can form the MBW transcriptional activator complex needed to regulate anthocyanin biosynthesis [25]. In Paeonia suffruticosa, PsbHLH1 could increase the transcription expression levels of PsDFR and PsANS by directly binding their promoters [70]. In the present study, we found that the PbbHLH2 directly bound to the promoter of PbDFR and induced the gene’s transcriptional activation (Figure 6).
In cornflower (Centaurea cyanus), CcbHLH1 interacts with CcMYB6–1 to form a complex protein that up-regulates the expression of CcF3H and CcDFR in the anthocyanin biosynthetic pathway [48]. In strawberry fruit, the expression of FvDFR is activated by the formation of heterodimers between FvHY5 and FvbHLH9 [71]. Our results were consistent with these previous studies. When the PbbHLH2 was co-infiltrated with PbMYB10, PbDFR was activated in Nicotiana benthamiana leaves. Although the PbbHLH2 did not bind to the promoter of PbANS, it induced the activation of PbANS promoter when co-infiltrated with PbMYB9 into Nicotiana benthamiana leaves (Figure 6). Both the PbMYB9 and PbMYB10, but not PbMYB10b, bind the PbUFGT promoter region [31]. Here, we found that the co-expression of PbbHLH2 and PbMYB9 induced PbUFGT expression. Therefore, PbbHLH2 overexpression increased PbANS and PbUFGT expression levels in “Zaosu” pear fruit. Taken together, our results suggested that PbbHLH2 forms a bHLH-MYB protein complex through the MIR-domain and plays important roles in the anthocyanin biosynthetic pathway of pear fruit.
4. Materials and Methods
4.1. Plant Treatment and Growth Conditions
The fruit of “Zaosu” (Pyrus bretschneideri Rehd.), “Red Zaosu” (Pyrus bretschneideri Rehd.) and “Palacer” (Pyrus communis L.) from a commercial orchard in Mei County, Baoji, China, were selected as experimental materials in 2017. The “Red Zaosu” pear (P. bretschneideri Rehd.) is a bud sport of “Zaosu” pear. The regulatory mechanism of anthocyanin biosynthesis in “Red Zaosu” and “Zaosu” has been studied [31]. The “Palacer” had been used to transient assays in anthocyanin study [31]. The fruit of ”Palacer” was selected about 40 days after flower blossom (DAFB) and bagged for 30 days until the red pigment completely faded. The fruit of “Zaosu” and “Red Zaosu” were harvested at 0, 45, and 105 DAFB, respectively. The stem, sepal, and petal of “Zaosu” and “Red Zaosu” were harvested at 0 DAFB. The tissues of harvested fruit were frozen in liquid nitrogen and stored at −80 °C for the subsequent measurements of anthocyanin concentrations and RNA extraction.
Nicotiana tabacum plants were grown in a growth room with a photoperiod of 16/8 h (light/dark) at 22 °C. The transformation was performed with Agrobacterium tumefaciens strain EHA105 (Tolo Biotech., Shanghai, China) after the plant had at least six leaves.
4.2. Isolation of bHLH Genes and Their Phylogenetic Analysis
The sequences of bHLH proteins were isolated from the pear database [29]; (https://www.peargenome.njau.edu.cn/, accessed on 13 October 2017). The phylogenetic analysis was performed using the Neighbor-Joining method with a JTT model and a bootstrap test using the MEGA 7.0 program [72]. The GenBank accessions of the functionally labelled bHLH genes were listed in Supplementary Table S1. The complete coding DNA sequences (CDS) of candidate bHLH TFs and MYBs were cloned from “Red Zaosu” genomic DNA using PrimeSTAR Max Premix (TaKaRa, R045A, Dalian, China) with gene-specific primers (Supplementary Table S2).
4.3. RNA Isolation and Expression Analysis Using qRT-PCR
The total RNA was extracted from pear peel using the RNAprep Pure Plant Kit (Tiangen, DP441, Beijing, China). The RNA concentration and quality were detected by UV spectrophotometry and by running a 0.8% agar gel, respectively. In total, 1 μg of total RNA was reverse transcribed to cDNA using the PrimeScript RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China). The primers used for qRT-PCR were designed with Oligo7 software [73] and synthesized by AuGCT Biotechnology Synthesis Lab (Beijing, China). The primers for actin, anthocyanin biosynthetic genes and candidate bHLHs and MYBs are described in Supplementary Table S2.
4.4. Transient Assays in Pear Fruit
The complete CDS of PbbHLH2 was cloned into the multiple cloning sites (MCS) (BamHI-HindIII) of pGreenII 62-SK vector (PbbHLH2-OE, Supplementary Figure S5a) [1]. The Agrobacterium tumefaciens strain EHA105 containing PbbHLH2-OE was grown in Luria–Bertani solid medium (Oxiod, 81 Wyman Street, Waltham, MA, USA) at 28 °C. After 2 days of culture, the Agrobacterium was collected and re-suspended in infiltration buffer (10 mM MgCl2, 10 mM MES, pH 5.6, 200 mM acetosyringone), and shaken for 3–4 h at room temperature. The OD600 of Agrobacterium was adjusted to 0.8 with infiltration buffer and then injected into pear fruitlets. The fruit was harvested 3 days after injection.
The 400–600 bp fragments of bHLH2 were inserted into the MCS of the pTRV2 VIGS vector (Supplementary Figure S5a). The constructed plasmid was transformed into Agrobacterium strain EHA105. The protocols of Agrobacterium culture and the injection of pear fruit were the same as above.
4.5. Anthocyanin Content Measurements
The content of total anthocyanin in red skin pear fruitlets was measured by pH differential method [74]. In this experiment, we used a previously reported method with slight modifications [74]. The 0.2 g sample was frozen and powdered in liquid nitrogen, and then 1.5 mL of 1% HCL-methanol extract was added. PVP K30 (Sigma, St. Louis, MO, USA) was added to the sample during grinding to prevent browning. After centrifugation at 4 °C and 12,000 rpm for 5 min, the supernatant was transferred separately to two clear tubes for dilution. One was diluted with 0.025 M potassium chloride buffer (pH 1.0), and the other with 0.4 M sodium acetate buffer (pH 4.5). These solutions were placed in the dark at room temperature before the absorbance values were measured synchronously at 520 nm and 700 nm using the Microporous plate spectrophotometer (Multiskan GO; Thermo Scientific, Waltham, MA, USA).
4.6. Dual-Luciferase Assay
The promoters of PbANS, PbDFR, and PbUFGT were amplified using PrimeSTAR Max Premix (TaKaRa, R045A) from “Red Zaosu” genomic DNA and gene-specific primers (Supplementary Table S2). These promoters were cloned into the HindIII and BamHI sites within the dual-LUC plasmid pGreenII 0800-LUC (Supplementary Figure S5b) [75]. The full-length CDS sequences of PbbHLH2 and PbMYBs were cloned into the MCS (BamHI-HindIII) of the pGreenII 62-SK binary vector [75].
Each of these recombinant plasmids and the pSoup helper plasmid [75] were transferred individually into the Agrobacterium strain EHA105. EHA105 containing PbbHLH2-SK or/and PbMYBs-SK were separately mixed with PbDFR promoter-LUC, PbANS promoter-LUC or PbUFGT promoter-LUC at 1:1 ratio before infiltration into 4-week-old N. benthamiana leaves. The ratio of firefly luciferase to Renilla luciferase enzyme activities was analyzed using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) with a Full Wavelength Multifunctional Enzyme Labelling Instrument (Infinite M200pro, TECAN, Männedorf, Switzerland). Proteins were extracted using 1 × PBS. Three independent experiments were carried out with at least four biological replicates per experiment.
4.7. Bimolecular Fluorescence Complementation (BiFC)
The CDS of candidate bHLHs were cloned into pSPYNE (named PbbHLH2-YNE), and the CDS of PbMYBs were fused into pSPYCE (named PbMYB9-YCE, PbMYB10-YCE, and PbMYB10b-YCE). The CDS of PbbHLH2 without MIR-domain was cloned into pSPYNE (named PbbHLH2-ΔNE) (Supplementary Figure S5c). Then, the constructed plasmids were transformed into Agrobacterium strains (EHA105). BiFC assays were performed by the co-transfection of Agrobacterium harboring components of PbbHLH2-YNE and PbMYBs-YCE into onion epidermal cells [76]. The Agrobacterium contained P19 helper plasmid was mixing with the PbbHLH2-YNE and PbMYBs-YCE before infiltration. The pBI121-GFP plasmid was used as a positive control in this experiment. The onion epidermal tissues were cultured on Murashige & Skoog solid plates at 22 °C in darkness. The fluorescence of BiFC was collected using a fluorescence microscope (Axio Observer D1, Carl Zeiss Jena, Oberkochen, Germany).
4.8. Yeast One-Hybrid (Y1H) Assay
The Y1H screening was performed in terms of the Matchmaker Gold Yeast One-Hybrid System Kit (Clontech, Mountain View, CA, USA), as recommended by the manufacturer. The assay used the yeast strain Y1HGold, which is unable to grow in the selective synthetic dextrose medium (SD) absence of uracil. The pAbAi-baits were constructed by inserting the 800 bp fragments of the structural genes’ promoters into the pAbAi vector (Supplementary Figure S5b). The pAbAi-baits were linearized and transformed into Y1HGold cells. Meanwhile, the complete CDS of the PbbHLH2 was cloned into pGADT7 vector to give the AD-prey vectors and then transformed into Y1HGold cells. After 3–4 days, these yeast strains were tested on a selective plate medium.
5. Conclusions
On the basis of our results, a working model describing the function of the MIR-domain PbbHLH2 in the anthocyanin biosynthetic pathway was proposed (Figure 7). The model was established using the known anthocyanin pathway with slight modifications [77,78,79]. The PbbHLH2 gene independently regulates the PbDFR expression to participate in the anthocyanin biosynthetic pathway of pear fruit. Moreover, its encoded protein also forms complexes with PbMYB9 or 10. The protein complexes are involved in the anthocyanin biosynthetic pathway through the transcriptional regulation of PbDFR, PbANS, and PbUFGT in pear fruit.
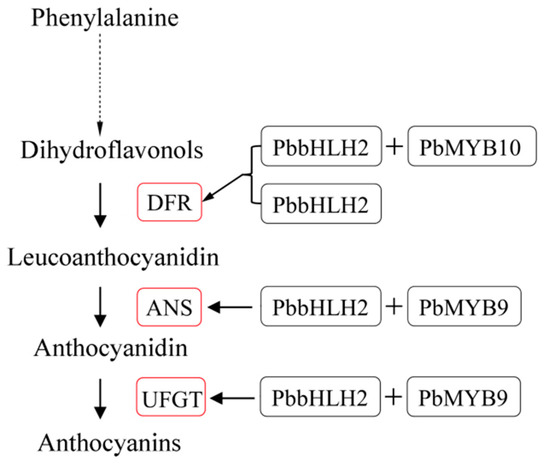
Figure 7.
Proposed model for PbbHLH2 physical interaction, with or without PbMYB9 and PbMYB10, in the regulation of the anthocyanin biosynthetic pathway of pear. PbbHLH2 with or without PbMYB10 binds the promoter of PbDFR and up-regulates the gene’s expression. PbbHLH2 interacts with PbMYB9 to bind the PbANS and PbUFGT promoters and activate the genes’ expression. The red boxes indicate up-regulation.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/6/3026/s1.
Author Contributions
Conceptualization, X.L., R.Z. and L.X.; methodology, X.L., R.Z. and Z.W.; software, X.L., F.X., W.H. and B.Q.; validation, X.L., R.Z., Z.W. and L.X.; data curation, X.L., F.X. and W.H.; writing—original draft, X.L.; writing—review and editing, X.L., R.Z., C.Y. and Z.W.; project administration, Z.W., C.Y. and L.X.; resources, L.X.; supervision, R.Z. and L.X.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (No. 2019YFD1001400) and the National Natural Science Foundation of China (No. 31972372 and 31572086).
Data Availability Statement
The data presented in this study are available in the article or supplementary material.
Conflicts of Interest
All the authors declare that they have no competing interests.
Abbreviations
| ANS | Anthocyanidin synthase |
| bHLH | Basic helix-loop-helix |
| BiFC | Bimolecular fluorescence complementation |
| CDS | Coding DNA sequence |
| DAFB | Days after flower bloom |
| DFR | Dihydroflavonol 4-reductase |
| HAS | Hours after sunrise of day 1 |
| LBGs | Late biosynthetic genes |
| MBW | MYB-bHLH-WD40 ternary protein complex |
| MCS | Multiple cloning sites |
| MIR | MYB-interacting region |
| OE | Overexpression |
| RVE | REVEILLE |
| SD | Selective synthetic dextrose medium |
| TFs | Transcription factors |
| UFGT | UDP-glucoside: flavonoid glucosyltransferase |
| VIGS | Virus-induced gene silencing |
| Y1H | Yeast one-hybrid assay |
References
- Gu, K.D.; Wang, C.K.; Hu, D.G.; Hao, Y.J. How do anthocyanins paint our horticultural products? Sci. Hortic. 2019, 249, 257–262. [Google Scholar] [CrossRef]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Tech. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Sarma, A.D.; Sharma, R. Anthocyanin-DNA copigmentation complex: Mutual protection against oxidative damage. Phytochemistry 1999, 52, 1313–1318. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Do anthocyanins function as osmoregulators in leaf tissues? In Advances in Botanical Research; Gould, K.S., Lee, D.W., Callow, J.A., Eds.; Academic Press Ltd-Elsevier Science Ltd: London, UK, 2002; Volume 37, pp. 103–127. [Google Scholar]
- Xie, Y.; Chen, P.; Yan, Y.; Bao, C.; Li, X.; Wang, L.; Shen, X.; Li, H.; Liu, X.; Niu, C.; et al. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol. 2018, 218, 201–218. [Google Scholar] [CrossRef]
- Xie, X.B.; Li, S.; Zhang, R.F.; Zhao, J.; Chen, Y.C.; Zhao, Q.; Yao, Y.X.; You, C.X.; Zhang, X.S.; Hao, Y.J. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012, 35, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.U.; Park, J.I.; Jung, H.J.; Hur, Y.; Nou, I.S. Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in Brassica rapa. Funct. Integr. Genom. 2015, 15, 383–394. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Feygenberg, O.; Diskin, S.; Wright, B.; Alkan, N. Increased anthocyanin and flavonoids in mango fruit peel are associated with cold and pathogen resistance. Postharvest Biol. Tec. 2016, 111, 132–139. [Google Scholar] [CrossRef]
- Shang, Y.; Venail, J.; Mackay, S.; Bailey, P.C.; Schwinn, K.E.; Jameson, P.E.; Martin, C.R.; Davies, K.M. The molecular basis for venation patterning of pigmentation and its effect on pollinator attraction in flowers of Antirrhinum. New Phytol. 2011, 189, 602–615. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins. Phytotherapy Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef] [PubMed]
- Petroni, K.; Pilu, R.; Tonelli, C. Anthocyanins in corn: A wealth of genes for human health. Planta 2014, 240, 901–911. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.M.; Kondoleon, N.; Gutierrez, E.; Wolforth, J.; Bolling, S. The intake of red raspberry fruit is inversely related to cardiac risk factors associated with metabolic syndrome. J. Funct. Foods 2018, 41, 83–89. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The Influence of Supplementation of Anthocyanins on Obesity-Associated Comorbidities: A Concise Review. Foods 2020, 9, 687. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, B.; Zhong, C.; Guo, J.; Zhang, L.; Mu, T.; Zhang, Q.; Bi, X. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Carcinogenesis 2018, 39, 471–481. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Sorn, S.R.; Park, Y.; Park, H.-K. Anthocyanin Rich-Black Soybean Testa Improved Visceral Fat and Plasma Lipid Profiles in Overweight/Obese Korean Adults: A Randomized Controlled Trial. J. Med. Food 2016, 19, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Draijer, R.; de Graaf, Y.; Slettenaar, M.; de Groot, E.; Wright, C.I. Consumption of a Polyphenol-Rich Grape-Wine Extract Lowers Ambulatory Blood Pressure in Mildly Hypertensive Subjects. Nutrients 2015, 7, 3138–3153. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Sakuta, M. Diversity in plant red pigments: Anthocyanins and betacyanins. Plant Biotechnol. Rep. 2013, 8, 37–48. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, M.; Kim, K.; Bang, G.; Cho, M.; Choi, S.B.; Choi, G.; Park, Y.I. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Lett. 2013, 587, 1543–1547. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.; Zhang, L.; Wang, W.; Hou, H.; Zhao, Y.; Jiang, X.; Yu, J.; Tan, H.; Wang, Y.; et al. Functional demonstration of plant flavonoid carbocations proposed to be involved in the biosynthesis of proanthocyanidins. Plant J. 2020, 101, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Schrader, A.; Kokkelink, L.; Falke, C.; Welter, B.; Iniesto, E.; Rubio, V.; Uhrig, J.F.; Hulskamp, M.; Hoecker, U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013, 74, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef]
- Lin-Wang, K.; Micheletti, D.; Palmer, J.; Volz, R.; Lozano, L.; Espley, R.; Hellens, R.P.; Chagne, D.; Rowan, D.D.; Troggio, M.; et al. High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant Cell Environ. 2011, 34, 1176–1190. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Shi, Z.; Zhang, S.; Ming, R.; Zhu, S.; Khan, M.A.; Tao, S.; Korban, S.S.; Wang, H.; et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013, 23, 396–408. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Y.; Yang, S.; Xu, Y.; Chen, X. Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta 2010, 232, 245–255. [Google Scholar] [CrossRef]
- Zhai, R.; Wang, Z.; Zhang, S.; Meng, G.; Song, L.; Wang, Z.; Li, P.; Ma, F.; Xu, L. Two MYB transcription factors regulate flavonoid biosynthesis in pear fruit (Pyrus bretschneideri Rehd.). J. Exp. Bot. 2016, 67, 1275–1284. [Google Scholar] [CrossRef]
- Ludwig, S.R.; Habera, L.F.; Dellaporta, S.L.; Wessler, S.R. Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proc. Natl. Acad. Sci. USA 1989, 86, 7092–7096. [Google Scholar] [CrossRef] [PubMed]
- Morohashi, K.; Zhao, M.; Yang, M.; Read, B.; Lloyd, A.; Lamb, R.; Grotewold, E. Participation of the Arabidopsis bHLH factor GL3 in trichome initiation regulatory events. Plant Physiol. 2007, 145, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.L.; Liu, X.F.; Li, X.; Yin, X.R.; Grierson, D.; Li, F.; Chen, K.S. A Novel bHLH Transcription Factor Involved in Regulating Anthocyanin Biosynthesis in Chrysanthemums (Chrysanthemum morifolium Ramat.). PLoS ONE 2015, 10, e0143892. [Google Scholar] [CrossRef]
- Pires, N.; Dolan, L. Origin and Diversification of Basic-Helix-Loop-Helix Proteins in Plants. Mol. Biol. Evol. 2010, 27, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Heppel, S.C.; Pillet, J.; Leon, C.; Czemmel, S.; Delrot, S.; Lauvergeat, V.; Bogs, J. The basic helix-loop-helix transcription factor MYC1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine. Mol. Plant. 2010, 3, 509–523. [Google Scholar] [CrossRef]
- Matus, J.T.; Poupin, M.J.; Canon, P.; Bordeu, E.; Alcalde, J.A.; Arce-Johnson, P. Isolation of WDR and bHLH genes related to flavonoid synthesis in grapevine (Vitis vinifera L.). Plant Mol. Biol. 2010, 72, 607–620. [Google Scholar] [CrossRef]
- Schaart, J.G.; Dubos, C.; Romero De La Fuente, I.; van Houwelingen, A.M.; de Vos, R.C.; Jonker, H.H.; Xu, W.; Routaboul, J.M.; Lepiniec, L.; Bovy, A.G. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria x ananassa) fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Goff, S.A.; Klein, T.M.; Roth, B.A.; Fromm, M.E.; Cone, K.C.; Radicella, J.P.; Chandler, V.L. Transactivation of anthocyanin biosynthetic genes following transfer of B regulatory genes into maize tissues. EMBO J. 1990, 98, 2517–2522. [Google Scholar] [CrossRef]
- Goff, S.A.; Cone, K.C.; Chandler, V.L. Functional analysis of the transcriptional activator encoded by the maize B gene: Evidence for a direct functional interaction between two classes of regulatory proteins. Gene Dev. 2019, 6, 864–875. [Google Scholar] [CrossRef]
- Hernandez, J.M.; Heine, G.F.; Irani, N.G.; Feller, A.; Kim, M.G.; Matulnik, T.; Chandler, V.L.; Grotewold, E. Different mechanisms participate in the R-dependent activity of the R2R3 MYB transcription factor C1. J. Biol. Chem. 2004, 279, 48205–48213. [Google Scholar] [CrossRef]
- Grotewold, E.; Sainz, M.B.; Tagliani, L.; Hernandez, J.M.; Bowen, B.; Chandler, V.L. Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R. Proc. Natl. Acad. Sci. USA 2000, 97, 13579–13584. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, S.; Xie, C.H.; Yuan, L. The interaction domains of the plant Myc-like bHLH transcription factors can regulate the transactivation strength. Planta 2008, 227, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.L.; Shi, M.Z.; Xie, D.Y. Regulation of anthocyanin biosynthesis by nitrogen in TTG1-GL3/TT8-PAP1-programmed red cells of Arabidopsis thaliana. Planta 2012, 236, 825–837. [Google Scholar] [CrossRef]
- Montefiori, M.; Brendolise, C.; Dare, A.P.; Lin-Wang, K.; Davies, K.M.; Hellens, R.P.; Allan, A.C. In the Solanaceae, a hierarchy of bHLHs confer distinct target specificity to the anthocyanin regulatory complex. J. Exp. Bot. 2015, 66, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.A.; Jakoby, M.; Werber, M.; Martin, C.; Weisshaar, B.; Bailey, P.C. The basic helix-loop-helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 2003, 20, 735–747. [Google Scholar] [CrossRef]
- Deng, C.; Wang, J.; Lu, C.; Li, Y.; Kong, D.; Hong, Y.; Huang, H.; Dai, S. CcMYB6–1 and CcbHLH1, two novel transcription factors synergistically involved in regulating anthocyanin biosynthesis in cornflower. Plant Physiol. Biochem. 2020, 151, 271–283. [Google Scholar] [CrossRef]
- Lai, B.; Du, L.N.; Hu, B.; Wang, D.; Huang, X.M.; Zhao, J.T.; Wang, H.C.; Hu, G.B. Characterization of a novel litchi R2R3-MYB transcription factor that involves in anthocyanin biosynthesis and tissue acidification. BMC Plant Biol. 2019, 19, 62. [Google Scholar] [CrossRef]
- Chen, K.; Du, L.; Liu, H.; Liu, Y. A novel R2R3-MYB from grape hyacinth, MaMybA, which is different from MaAN2, confers intense and magenta anthocyanin pigmentation in tobacco. BMC Plant Biol. 2019, 19, 390. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Yan, X.; Yang, Q.; Wu, Z.; Zeng, Y.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef]
- Li, Y.; Shan, X.; Zhou, L.; Gao, R.; Yang, S.; Wang, S.; Wang, L.; Gao, X. The R2R3-MYB Factor FhMYB5 From Freesia hybrida Contributes to the Regulation of Anthocyanin and Proanthocyanidin Biosynthesis. Front. Plant Sci. 2018, 9, 1935. [Google Scholar] [CrossRef]
- Ramsay, N.A.; Walker, A.R.; Mooney, M.; Gray, J.C. Two basic-helix-loop-helix genes (MYC-146 and GL3) from Arabidopsis can activate anthocyanin biosynthesis in a white-flowered Matthiola incana mutant. Plant Mol. Biol. 2003, 52, 679–688. [Google Scholar] [CrossRef]
- Baudry, A.; Heim, M.A.; Dubreucq, B.; Caboche, M.; Weisshaar, B.; Lepiniec, L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 2004, 39, 366–380. [Google Scholar] [CrossRef]
- Wada, T.; Kunihiro, A.; Tominaga-Wada, R. Arabidopsis CAPRICE (MYB) and GLABRA3 (bHLH) control tomato (Solanum lycopersicum) anthocyanin biosynthesis. PLoS ONE 2014, 9, e109093. [Google Scholar] [CrossRef] [PubMed]
- Feyissa, D.N.; Lovdal, T.; Olsen, K.M.; Slimestad, R.; Lillo, C. The endogenous GL3, but not EGL3, gene is necessary for anthocyanin accumulation as induced by nitrogen depletion in Arabidopsis rosette stage leaves. Planta 2009, 230, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, D.; Jin, C.; Duan, S.; Qi, S.; Liu, K.; Wang, H.; Ma, H.; Hai, J.; Chen, M. Genome-wide identification of GLABRA3 downstream genes for anthocyanin biosynthesis and trichome formation in Arabidopsis. Biochem. Biophys. Res. Commun. 2017, 485, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Nemie-Feyissa, D.; Heidari, B.; Blaise, M.; Lillo, C. Analysis of interactions between heterologously produced bHLH and MYB proteins that regulate anthocyanin biosynthesis: Quantitative interaction kinetics by Microscale Thermophoresis. Phytochemistry 2015, 111, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Brendolise, C.; Chagne, D.; Kutty-Amma, S.; Green, S.; Volz, R.; Putterill, J.; Schouten, H.J.; Gardiner, S.E.; Hellens, R.P.; et al. Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 2009, 21, 168–183. [Google Scholar] [CrossRef]
- Brendolise, C.; Espley, R.V.; Lin-Wang, K.; Laing, W.; Peng, Y.; McGhie, T.; Dejnoprat, S.; Tomes, S.; Hellens, R.P.; Allan, A.C. Multiple Copies of a Simple MYB-Binding Site Confers Trans-regulation by Specific Flavonoid-Related R2R3 MYBs in Diverse Species. Front. Plant Sci. 2017, 8, 1864. [Google Scholar] [CrossRef]
- Boase, M.R.; Brendolise, C.; Wang, L.; Ngo, H.; Espley, R.V.; Hellens, R.P.; Schwinn, K.E.; Davies, K.M.; Albert, N.W. Failure to launch: The self-regulating Md-MYB10 R6 gene from apple is active in flowers but not leaves of Petunia. Plant Cell Rep. 2015, 34, 1817–1823. [Google Scholar] [CrossRef]
- Shi, M.-Z.; Xie, D.-Y. Engineering of red cells of Arabidopsis thaliana and comparative genome-wide gene expression analysis of red cells versus wild-type cells. Planta 2011, 233, 787–805. [Google Scholar] [CrossRef]
- Gonzalez, A.; Mendenhall, J.; Huo, Y.; Lloyd, A. TTG1 complex MYBs, MYB5 and TT2, control outer seed coat differentiation. Dev Biol. 2009, 325, 412–421. [Google Scholar] [CrossRef]
- Ramsay, N.A.; Glover, B.J. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005, 10, 63–70. [Google Scholar] [CrossRef]
- Li, Y.; Shan, X.; Gao, R.; Yang, S.; Wang, S.; Gao, X.; Wang, L. Two IIIf Clade-bHLHs from Freesia hybrida Play Divergent Roles in Flavonoid Biosynthesis and Trichome Formation when Ectopically Expressed in Arabidopsis. Sci. Rep. 2016, 6, 30514. [Google Scholar] [CrossRef] [PubMed]
- Feller, A.; Hernandez, J.M.; Grotewold, E. An ACT-like domain participates in the dimerization of several plant basic-helix-loop-helix transcription factors. J. Biol. Chem. 2006, 281, 28964–28974. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Pattanaik, S.; Feller, A.; Werkman, J.R.; Chai, C.; Wang, Y.; Grotewold, E.; Yuan, L. Regulatory switch enforced by basic helix-loop-helix and ACT-domain mediated dimerizations of the maize transcription factor R. Proc. Natl. Acad. Sci. USA 2012, 109, E2091–E2097. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Lee, H. MYB-related transcription factors function as regulators of the circadian clock and anthocyanin biosynthesis in Arabidopsis. Plant Signal Behav. 2016, 11, e1139278. [Google Scholar] [CrossRef]
- Qi, Y.; Zhou, L.; Han, L.; Zou, H.; Miao, K.; Wang, Y. PsbHLH1, a novel transcription factor involved in regulating anthocyanin biosynthesis in tree peony (Paeonia suffruticosa). Plant Physiol. Biochem. 2020, 154, 396–408. [Google Scholar] [CrossRef]
- Li, Y.; Xu, P.; Chen, G.; Wu, J.; Liu, Z.; Lian, H. FvbHLH9 Functions as a Positive Regulator of Anthocyanin Biosynthesis by Forming a HY5-bHLH9 Transcription Complex in Strawberry Fruits. Plant Cell Physiol. 2020, 61, 826–837. [Google Scholar] [CrossRef]
- MEGA 7.0; University of Kent: Canterbury, UK, 2020.
- Oligo 7; Molecular Biology Insights, Inc.: Colorado Springs, CO, USA, 2007.
- Wang, Z.; Du, H.; Zhai, R.; Song, L.; Ma, F.; Xu, L. Transcriptome Analysis Reveals Candidate Genes Related to Color Fading of ‘Red Bartlett’ (Pyrus communis L.). Front. Plant Sci. 2017, 8, 455. [Google Scholar] [CrossRef]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Chaban, C.; Schutze, K.; Batistic, O.; Weckermann, K.; Nake, C.; Blazevic, D.; Grefen, C.; Schumacher, K.; Oecking, C.; et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 2004, 40, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.C.; Gosch, C.; Pfeiffer, J.; Halbwirth, H.; Halle, C.; Stich, K.; Forkmann, G. Flavonoid genes of pear (Pyrus communis). Trees 2007, 21, 521–529. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, X.T.; Feng, W.T.; Chen, S.S.; Xu, L.F.; Wang, Z.G.; Zhang, J.L.; Li, P.M.; Ma, F.W. Different biosynthesis patterns among flavonoid 3-glycosides with distinct effects on accumulation of other flavonoid metabolites in pears (Pyrus bretschneideri Rehd.). PLoS ONE 2014, 9, e91945. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhao, Y.; Tao, R.; Yin, L.; Gao, L.; Strid, A.; Qian, M.; Li, J.; Li, Y.; Shen, J.; et al. Ethylene mediates the branching of the jasmonate-induced flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits. Plant Biotechnol J. 2020, 18, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).