Apoptotic Effects of Anthocyanins from Vitis coignetiae Pulliat Are Enhanced by Augmented Enhancer of the Rudimentary Homolog (ERH) in Human Gastric Carcinoma MKN28 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Anthocyanin Preparations
2.3. Construction of the ERH Expression Plasmid and Transfection
2.4. Cell Viability Assay
2.5. Flow Cytometry Analysis for Cell Cycle Analysis and Apoptosis
2.6. Western Blot Analysis
2.7. In Vitro Caspases Activity Assay
2.8. Measurement of Mitochondrial Membrane Potential (MMP, Δψm) and ROS Generation
2.9. Statistical Analysis
3. Results
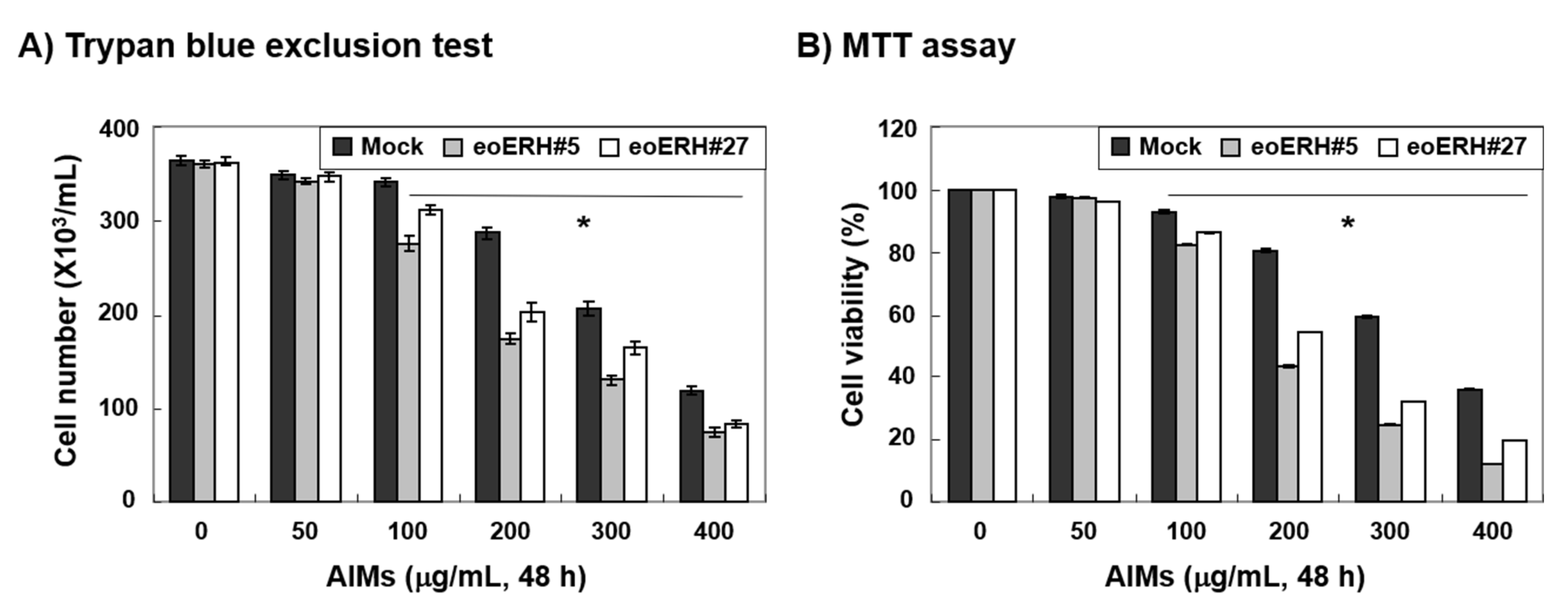
3.1. ERH Augmented The Antiproliferative Effect of Aims on MKN28 Cells
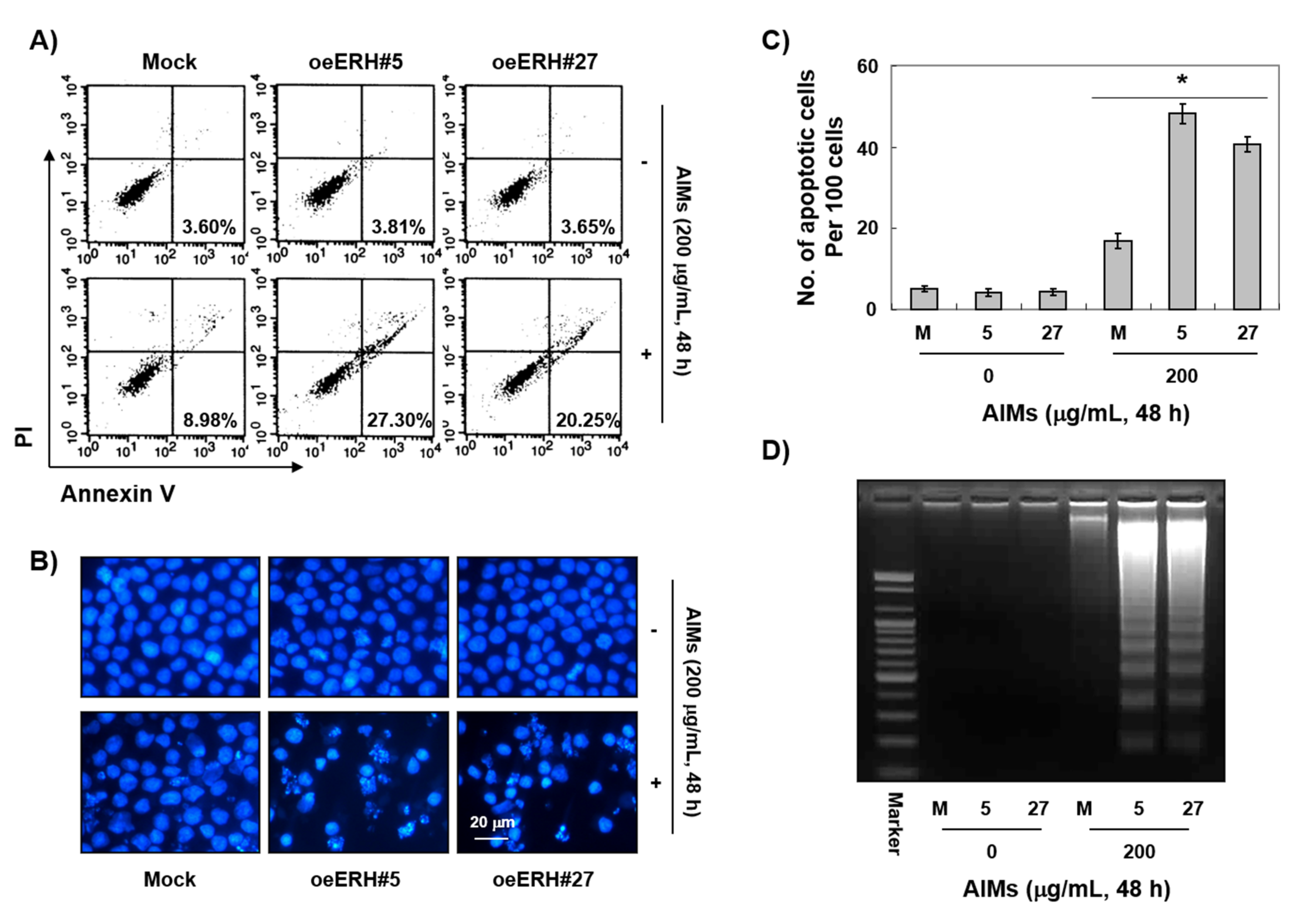
3.2. ERH Augmented AIM-Induced Apoptosis in MKN28 Cells
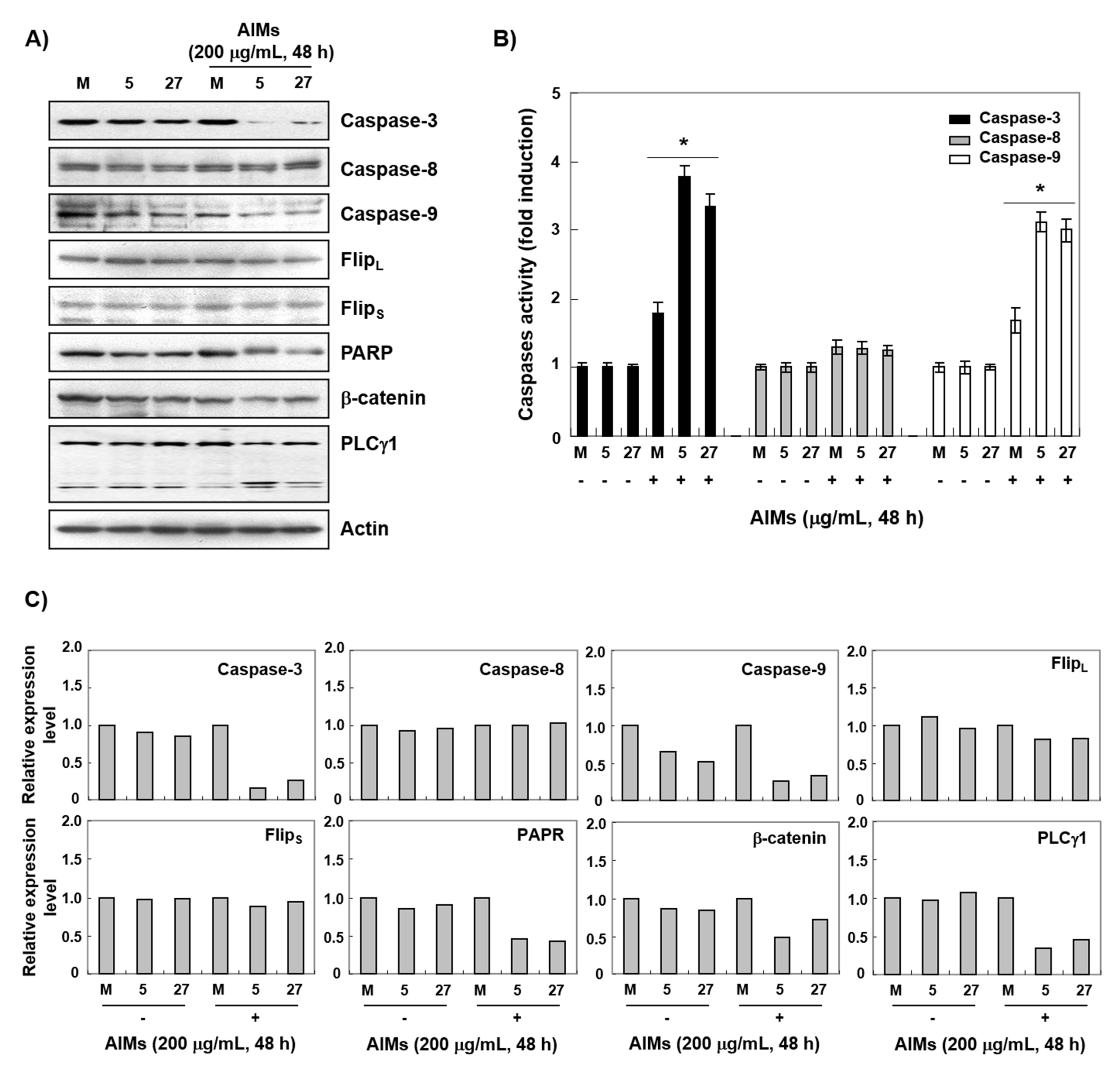
3.3. ERH Augmented AIM-Induced Caspase-Dependent Apoptosis Possibly Through the Intrinsic Apoptotic Pathway in MKN28 Cells
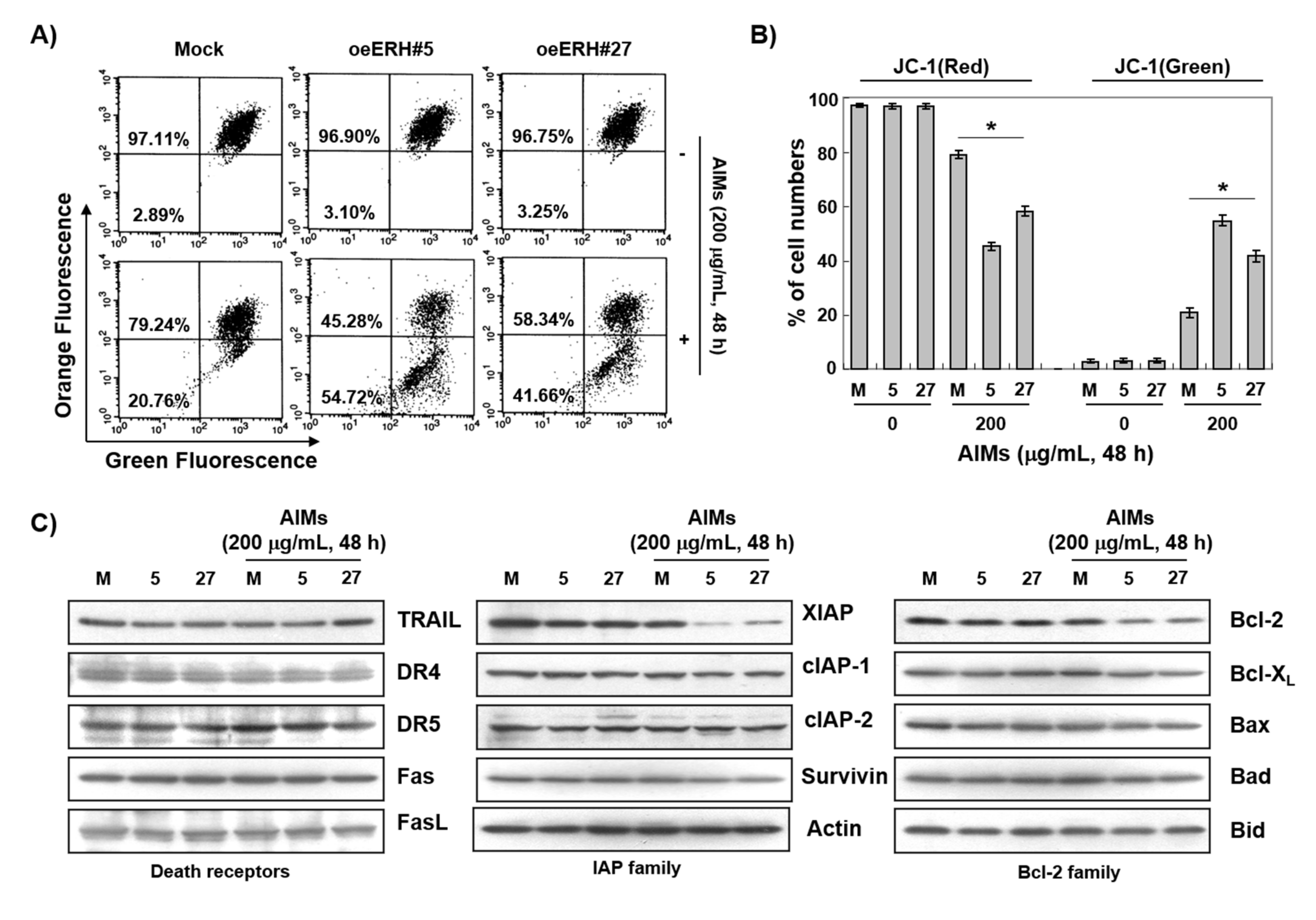
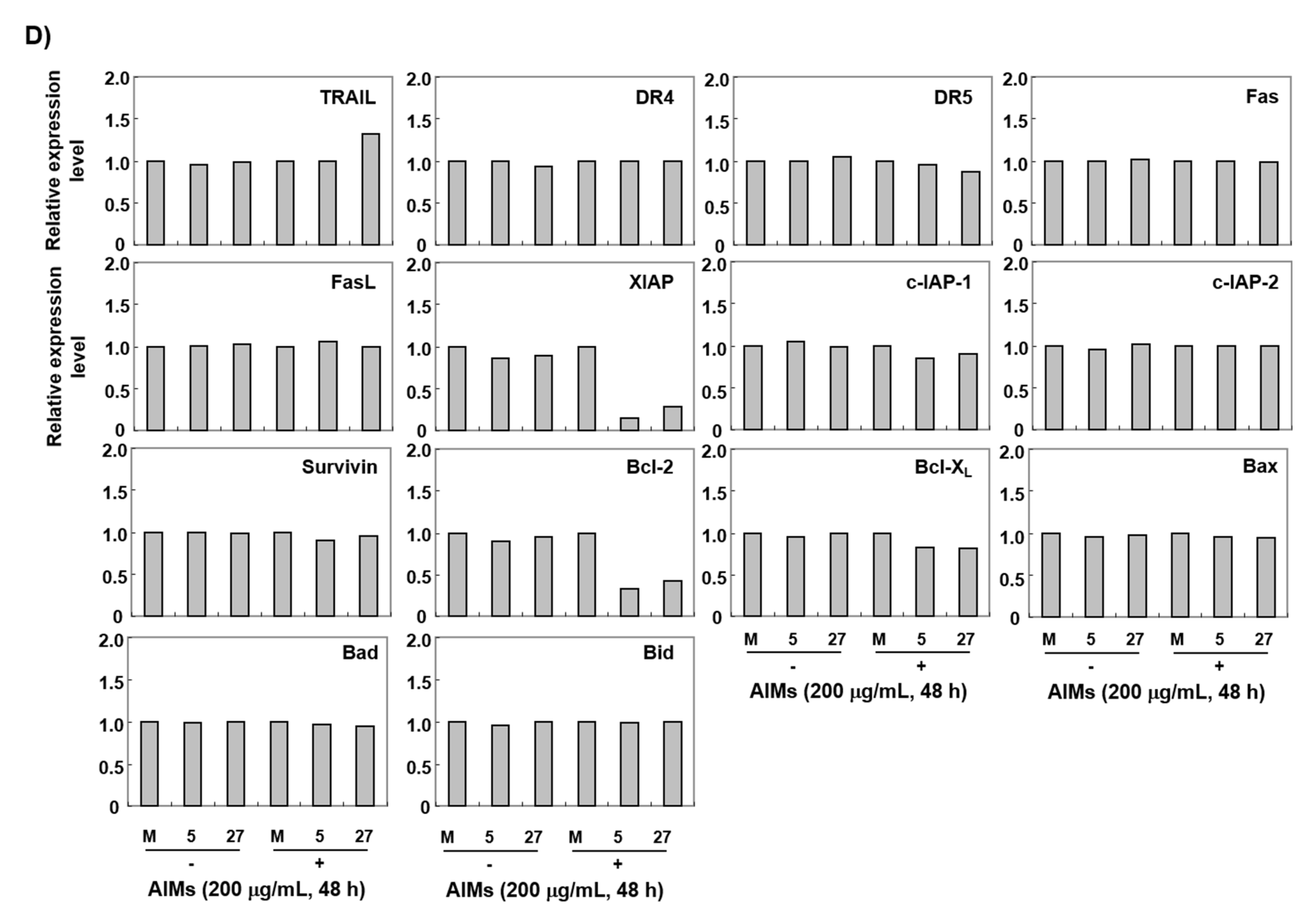
3.4. The ERH-Augmented Apoptotic Effect on AIM-Treated Cells Was Related to Mitochondrial Depolarization and Inhibition of Antiapoptotic Proteins, XIAP and Bcl-2
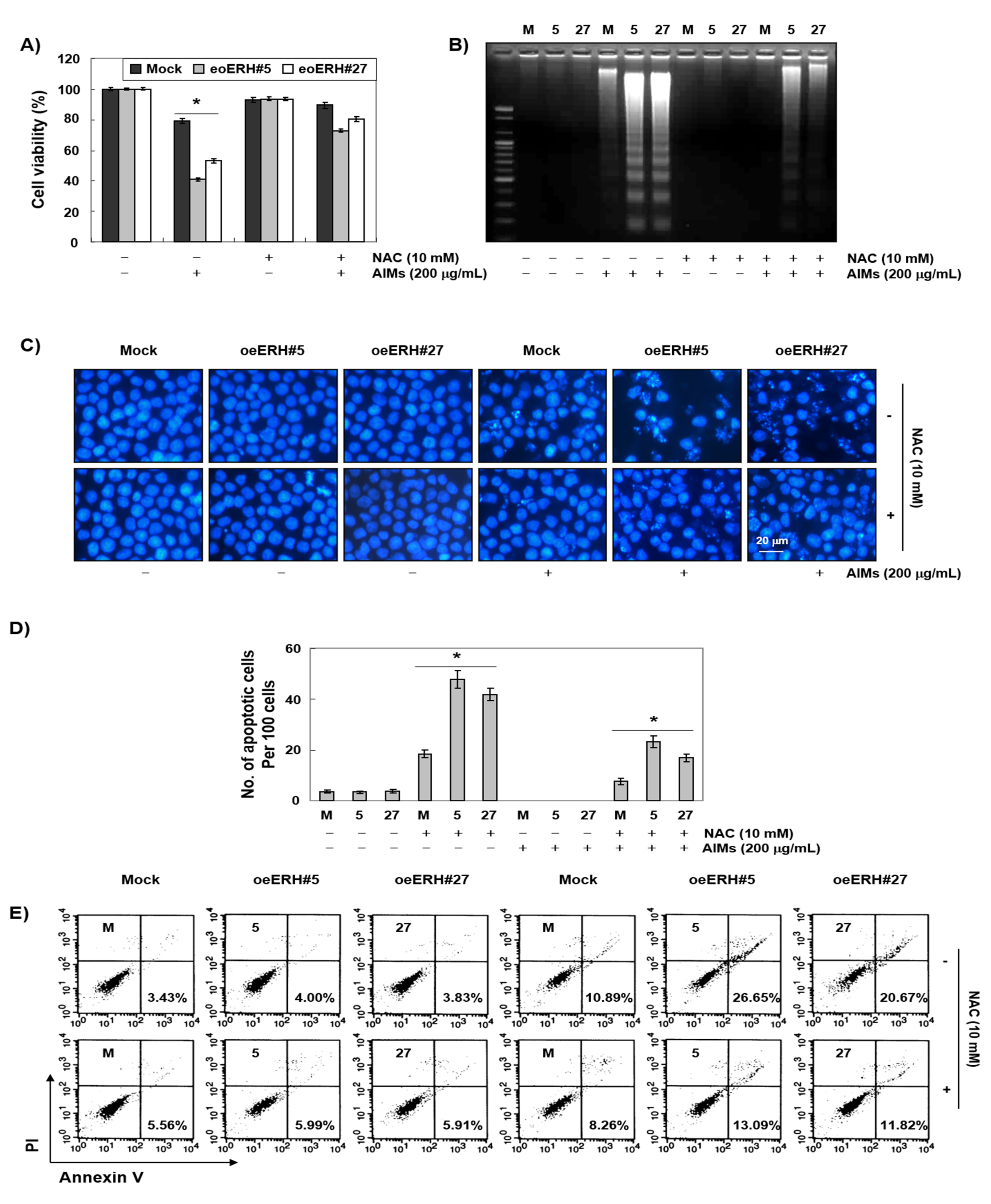
3.5. ERH Augmented ROS Generation Triggered by AIMs
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Song, H.N.; Go, S.I.; Lee, W.S.; Kim, Y.; Choi, H.J.; Lee, U.S.; Kang, M.H.; Lee, G.W.; Kim, H.G.; Kang, J.H.; et al. Population-Based Regional Cancer Incidence in Korea: Comparison between Urban and Rural Areas. Cancer Res. Treat. 2016, 48, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Won, Y.J.; Jung, K.W.; Kong, H.J.; Cho, H.; Lee, J.K.; Lee, D.H.; Lee, K.H. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2013. Cancer Res. Treat. 2016, 48, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef]
- Noh, S.H.; Park, S.R.; Yang, H.K.; Chung, H.C.; Chung, I.J.; Kim, S.W.; Kim, H.H.; Choi, J.H.; Kim, H.K.; Yu, W.; et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1389–1396. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Oh, C.M.; Lee, D.H.; Lee, J.S. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2011. Cancer Res. Treat. 2014, 46, 109–123. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Oh, C.M.; Lee, D.H.; Lee, J.S. Prediction of cancer incidence and mortality in Korea, 2014. Cancer Res. Treat. 2014, 46, 124–130. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Prahallad, A.; Sun, C.; Huang, S.; Di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012, 483, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Lu, J.N.; Go, S.I.; Jung, J.H.; Yi, S.M.; Jeong, J.H.; Hah, Y.S.; Han, M.S.; Park, J.W.; Lee, W.S.; et al. p53 restoration can overcome cisplatin resistance through inhibition of Akt as well as induction of Bax. Int. J. Oncol. 2013, 43, 1495–1502. [Google Scholar] [CrossRef][Green Version]
- Fang, X.; Yin, H.; Zhang, H.; Wu, F.; Liu, Y.; Fu, Y.; Yu, D.; Zong, L. p53 mediates hydroxyurea resistance in aneuploid cells of colon cancer. Exp. Cell Res. 2019, 376, 39–48. [Google Scholar] [CrossRef]
- Lee, J.E.; Mannisto, S.; Spiegelman, D.; Hunter, D.J.; Bernstein, L.; van den Brandt, P.A.; Buring, J.E.; Cho, E.; English, D.R.; Flood, A.; et al. Intakes of fruit, vegetables, and carotenoids and renal cell cancer risk: A pooled analysis of 13 prospective studies. Cancer Epidemiol. Prev. Biomark. 2009, 18, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Merzenich, H.; Robertson, C.; Boyle, P. Meta-analysis of studies on breast cancer risk and diet: The role of fruit and vegetable consumption and the intake of associated micronutrients. Eur. J. Cancer 2000, 36, 636–646. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.B.; Siddiqui, I.A.; Asim, M.; Malik, A.; Afaq, F.; Adhami, V.M.; Saleem, M.; Din, M.; Mukhtar, H. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: Involvement of nuclear factor-kappaB signaling. Cancer Res. 2008, 68, 8564–8572. [Google Scholar] [CrossRef]
- Haseeb, A.; Chen, D.; Haqqi, T.M. Delphinidin inhibits IL-1beta-induced activation of NF-kappaB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology 2013, 52, 998–1008. [Google Scholar] [CrossRef]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Afaq, F.; Sarfaraz, S.; Khan, N.; Kedlaya, R.; Setaluri, V.; Mukhtar, H. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol. Appl. Pharm. 2008, 231, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Favot, L.; Martin, S.; Keravis, T.; Andriantsitohaina, R.; Lugnier, C. Involvement of cyclin-dependent pathway in the inhibitory effect of delphinidin on angiogenesis. Cardiovasc. Res. 2003, 59, 479–487. [Google Scholar] [CrossRef]
- Shin, D.Y.; Ryu, C.H.; Lee, W.S.; Kim, D.C.; Kim, S.H.; Hah, Y.S.; Lee, S.J.; Shin, S.C.; Kang, H.S.; Choi, Y.H. Induction of apoptosis and inhibition of invasion in human hepatoma cells by anthocyanins from meoru. Ann. N. Y. Acad. Sci. 2009, 1171, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lee, W.S.; Kim, G.S.; Park, O.J. Anthocyanins are novel AMPKalpha1 stimulators that suppress tumor growth by inhibiting mTOR phosphorylation. Oncol. Rep. 2010, 24, 1471–1477. [Google Scholar]
- Shin, D.Y.; Lee, W.S.; Kim, S.H.; Kim, M.J.; Yun, J.W.; Lu, J.N.; Lee, S.J.; Tsoy, I.; Kim, H.J.; Ryu, C.H.; et al. Anti-invasive activity of anthocyanins isolated from Vitis coignetiae in human hepatocarcinoma cells. J. Med. Food 2009, 12, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.N.; Lee, W.S.; Yun, J.W.; Kim, M.J.; Kim, H.J.; Kim, D.C.; Jeong, J.H.; Choi, Y.H.; Kim, G.S.; Ryu, C.H.; et al. Anthocyanins from Vitis coignetiae Pulliat Inhibit Cancer Invasion and Epithelial-Mesenchymal Transition, but These Effects Can Be Attenuated by Tumor Necrosis Factor in Human Uterine Cervical Cancer HeLa Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 503043. [Google Scholar] [CrossRef]
- Lu, J.N.; Lee, W.S.; Kim, M.J.; Yun, J.W.; Jung, J.H.; Yi, S.M.; Jeong, J.H.; Kim, H.J.; Choi, Y.H.; Kim, G.S.; et al. The inhibitory effect of anthocyanins on Akt on invasion and epithelial-mesenchymal transition is not associated with the anti-EGFR effect of the anthocyanins. Int. J. Oncol. 2014, 44, 1756–1766. [Google Scholar] [CrossRef]
- Lim, B.H.; Cho, B.I.; Kim, Y.N.; Kim, J.W.; Park, S.T.; Lee, C.W. Overexpression of nicotinamide N-methyltransferase in gastric cancer tissues and its potential post-translational modification. Exp. Mol. Med. 2006, 38, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.T.; Lee, J.H.; Wei, S.C.; Li, Q.; Shahamatdar, S.; Hsu, D.; Schetter, A.J.; Swatkoski, S.; Mannan, P.; Garfield, S.; et al. Evolutionarily conserved protein ERH controls CENP-E mRNA splicing and is required for the survival of KRAS mutant cancer cells. Proc. Natl. Acad. Sci. USA 2012, 109, E3659–E3667. [Google Scholar] [CrossRef]
- Pang, K.; Zhang, Z.; Hao, L.; Shi, Z.; Chen, B.; Zang, G.; Dong, Y.; Li, R.; Liu, Y.; Wang, J.; et al. The ERH gene regulates migration and invasion in 5637 and T24 bladder cancer cells. BMC Cancer 2019, 19, 225. [Google Scholar] [CrossRef]
- Yun, J.W.; Lee, W.S.; Kim, M.J.; Lu, J.N.; Kang, M.H.; Kim, H.G.; Kim, D.C.; Choi, E.J.; Choi, J.Y.; Kim, H.G.; et al. Characterization of a profile of the anthocyanins isolated from Vitis coignetiae Pulliat and their anti-invasive activity on HT-29 human colon cancer cells. Food Chem. Toxicol. 2010, 48, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, A.; Nakashima, J.; Yoshioka, K.; Tachibana, M.; Tazaki, H.; Murai, M. Role of reactive oxygen species in cis-dichlorodiammineplatinum-induced cytotoxicity on bladder cancer cells. Br. J. Cancer 1997, 76, 206–210. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Du, J.; Liu, J.; Ritchie, J.M.; Oberley, L.W.; Cullen, J.J. Metastatic progression of pancreatic cancer: Changes in antioxidant enzymes and cell growth. Clin. Exp. Metastasis 2005, 22, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Irani, K.; Xia, Y.; Zweier, J.L.; Sollott, S.J.; Der, C.J.; Fearon, E.R.; Sundaresan, M.; Finkel, T.; Goldschmidt-Clermont, P.J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 1997, 275, 1649–1652. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Leibowitz, B.; Yu, J. Mitochondrial signaling in cell death via the Bcl-2 family. Cancer Biol. Ther. 2010, 9, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Sasaki, C.Y.; Hardwick, J.M.; Longo, D.L. Bcl-2-mediated drug resistance: Inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription. J. Exp. Med. 1999, 190, 253–265. [Google Scholar] [CrossRef]
- Yang, T.; Xu, F.; Sheng, Y.; Zhang, W.; Chen, Y. A targeted proteomics approach to the quantitative analysis of ERK/Bcl-2-mediated anti-apoptosis and multi-drug resistance in breast cancer. Anal. Bioanal. Chem. 2016, 408, 7491–7503. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Nakajima, T.; Moriguchi, M.; Jo, M.; Sekoguchi, S.; Ishii, M.; Takashima, H.; Katagishi, T.; Kimura, H.; Minami, M.; et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J. Hepatol. 2006, 44, 1074–1082. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Eckelman, B.P.; Salvesen, G.S. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J. Biol. Chem. 2006, 281, 3254–3260. [Google Scholar] [CrossRef]
- Desplanques, G.; Giuliani, N.; Delsignore, R.; Rizzoli, V.; Bataille, R.; Barille-Nion, S. Impact of XIAP protein levels on the survival of myeloma cells. Haematologica 2009, 94, 87–93. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.C.; Lefebvre, C.A.; Cherton-Horvat, G.; St-Jean, M.; Kandimalla, E.R.; Agrawal, S.; Morris, S.J.; Durkin, J.P.; Lacasse, E.C. Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene 2004, 23, 8105–8117. [Google Scholar] [CrossRef]
- Evans, M.K.; Sauer, S.J.; Nath, S.; Robinson, T.J.; Morse, M.A.; Devi, G.R. X-linked inhibitor of apoptosis protein mediates tumor cell resistance to antibody-dependent cellular cytotoxicity. Cell Death Dis. 2016, 7, e2073. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, M.; Park, S.Y.; Lee, Y.J.; Hong, S.C.; Jung, E.J.; Ju, Y.T.; Jeong, C.Y.; Kim, J.Y.; Ko, G.H.; et al. ERH overexpression is associated with decreased cell migration and invasion and a good prognosis in gastric cancer. Transl. Cancer Res. 2020, 9, 5281–5291. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Lee, W.S.; Go, S.-I.; Jeong, S.-H.; Yoo, J.; Cha, H.-J.; Lee, Y.-J.; Kim, H.-S.; Leem, S.-H.; Kim, H.J.; et al. Apoptotic Effects of Anthocyanins from Vitis coignetiae Pulliat Are Enhanced by Augmented Enhancer of the Rudimentary Homolog (ERH) in Human Gastric Carcinoma MKN28 Cells. Int. J. Mol. Sci. 2021, 22, 3030. https://doi.org/10.3390/ijms22063030
Park C, Lee WS, Go S-I, Jeong S-H, Yoo J, Cha H-J, Lee Y-J, Kim H-S, Leem S-H, Kim HJ, et al. Apoptotic Effects of Anthocyanins from Vitis coignetiae Pulliat Are Enhanced by Augmented Enhancer of the Rudimentary Homolog (ERH) in Human Gastric Carcinoma MKN28 Cells. International Journal of Molecular Sciences. 2021; 22(6):3030. https://doi.org/10.3390/ijms22063030
Chicago/Turabian StylePark, Cheol, Won Sup Lee, Se-Il Go, Sang-Ho Jeong, Jiyun Yoo, Hee-Jae Cha, Young-Joon Lee, Heui-Soo Kim, Sun-Hee Leem, Hye Jung Kim, and et al. 2021. "Apoptotic Effects of Anthocyanins from Vitis coignetiae Pulliat Are Enhanced by Augmented Enhancer of the Rudimentary Homolog (ERH) in Human Gastric Carcinoma MKN28 Cells" International Journal of Molecular Sciences 22, no. 6: 3030. https://doi.org/10.3390/ijms22063030
APA StylePark, C., Lee, W. S., Go, S.-I., Jeong, S.-H., Yoo, J., Cha, H.-J., Lee, Y.-J., Kim, H.-S., Leem, S.-H., Kim, H. J., Kim, G. S., Hong, S.-C., & Choi, Y. H. (2021). Apoptotic Effects of Anthocyanins from Vitis coignetiae Pulliat Are Enhanced by Augmented Enhancer of the Rudimentary Homolog (ERH) in Human Gastric Carcinoma MKN28 Cells. International Journal of Molecular Sciences, 22(6), 3030. https://doi.org/10.3390/ijms22063030












