Abstract
Belladine N-oxides active against influenza A virus have been synthetized by a novel laccase-catalyzed 1,4-dioxane-mediated oxidation of aromatic and side-chain modified belladine derivatives. Electron paramagnetic resonance (EPR) analysis confirmed the role of 1,4-dioxane as a co-oxidant. The reaction was chemo-selective, showing a high functional-group compatibility. The novel belladine N-oxides were active against influenza A virus, involving the early stage of the virus replication life cycle.
1. Introduction
Belladine 1 and norbelladine 2 (firstly extracted from the Amaryllidaceae family [1]) are bioactive precursors in the synthesis of drugs acting on the central nervous system, such as galantamine 3, lycorine 4, and haemanthamine 5 (Figure 1, Panel a) [2,3]. They are natural substances emerging in therapy, showing a cholinesterase inhibitory activity comparable to that of 3 in the treatment of Alzheimer’s disease [4,5]. In addition, a computational study suggested that quaternary belladine derivatives can interact with the neuroaminidase (NA) protein of influenza A virus, inhibiting viral release from host cell [6].
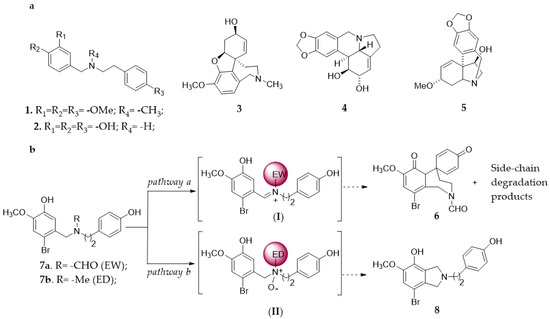
Figure 1.
Panel (a): chemical structure of belladine 1, norbelladine 2, galantamine 3, lycorine 4, and haemanthamine 5. Panel (b): side-reactions observed during the oxidation of N-substituted 2-bromo-O-methylnorbelladines 7a–b with laccase from Trametes versicolor. Pathway a: formation of the iminium ion intermediate (I) in the presence of electron-withdrawing (EW) substituent and successive transformation to spyrocyclohexadienone 6. Pathway b: formation of the N-oxide intermediate (II) in the presence of electron-donating (ED) substituent followed by phenolic intramolecular cycloaddition to isoindoline 8.
Recently, the use of N-formyl-2-bromo-O-methynorbelladine 7a in the total synthesis of 3 by a laccase (benzenediol: oxygen oxidoreductases, EC 1.10.3.2) [7]-mediator system has been reported, focusing on the formation of the spyrocyclohexadienone 6 as a tri-cyclic intermediate (Figure 1, Panel b) [8]. In this latter case, undesired side-products were produced depending on the nature of the N-substituent. Electron withdrawing group EW (R = CHO, 7a) favored the formation of phenoxy radicals and successive oxidative coupling, and the hydrolysis of the iminium ion (I) to side-chain degradation products was the only observed side-process [8,9] (Figure 1; Panel b, pathway a). Conversely, the oxidative coupling was not operative with electron-donating group ED (R = CH3, 7b), in which case the isoindoline 8 was produced by an iminium-ion Polonovski transformation of the N-oxide intermediate (II) (not isolated) [10,11] (Figure 1; Panel b, pathway b).
Amine N-oxides are widely diffused in nature [12,13], and they play an important role as chiral ligands, organo-catalysts, and synthons [14,15]. These compounds are synthetized using hazardous stoichiometric oxidants, or in the alternative, heavy metal catalysts and hydrogen peroxide (H2O2), which leads to the formation of toxic wastes and undesired by-products [16]. As an alternative, dioxygen (O2) is an effective green oxidant [17,18,19] when associated with laccases, favoring the formation of reactive singlet-state species in energy barrierless and green conditions. Laccases are low-cost enzymes with high catalytic activity, broad substrate specificity, and beneficial chemical and physical properties [20,21,22,23,24,25,26,27,28,29]. To the best of our knowledge, only one example of oxidation of tertiary amines by laccase has been reported; however, the yield and selectivity were low [30].
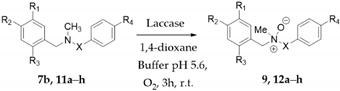
Here we describe the efficient synthesis of belladine N-oxides 9 and 12a–h (Table 2) by use of laccase from Trametes versicolor. The control of the pH of the reaction and the use of 1,4-dioxane as co-oxidant favored the stabilization of intermediate (II) by inhibition of the Polonovski transformation (Figure 1; Panel b, pathway b), affording the desired N-oxides in high yield and regio-selectivity. A modest stereo-selectivity was also observed by the use of chiral shift reagent europium tris-[3-(heptafluoropropylhydroxymethylene)-(+)-camphorate] (Eu(hfc)3) salt. Belladine N-oxides were active against Influenza A virus, and compound 12h showed the highest activity.
2. Results and Discussion
2.1. Optimization of the Reaction Conditions
The Polonovski transformation [11] of amine N-oxides occurs by two successive steps: (a) the protonation of the quaternary N-oxide moiety [31,32]; and (b) the cleavage of the iminium ion to corresponding aminium radical cation, followed by α-hydrogen elimination and skeletal rearrangement [33,34]. In order to avoid the occurrence of the Polonovski reaction in the laccase catalyzed synthesis of belladine N-oxides, the critical reaction step was expected to be the protonation of the N-oxide moiety. We started our investigation using 7b (Scheme 1) as a model substrate (general procedures are in Supplementary Materials (SM) #1, and the synthesis of 7b in SM #2).
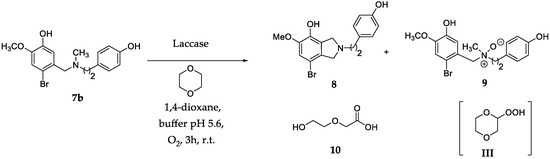
Scheme 1.
Synthesis of belladine N-oxide derivative 9 from 7b. Isoindoline 8 and 2-(2-hydroxyethoxy)acetic acid 10 were isolated as by-products.
The treatment of 7b (0.1 mmol) with laccase (1000 U·mmol−1) and TEMPO (2,2,6,6-tetra methyl-1-piperidinyloxy free radical, 0.06 mmol.) [35,36] at 25 °C for 3.0 h under O2 atmosphere in 1,4-dioxane (0.5 mL) and sodium acetate buffer (2.0 mL; 0.5 M; pH 4.5) afforded 8 as the only recovered product, besides the unreacted substrate (Scheme 1; Table 1, entry 1) [8]. Examples of the retained activity of laccase in organic solvents are reported, and their advantages for the selectivity of the transformation are adequately discussed [37,38]. The oxidation with a lower amount of laccase (100 U·mmol−1) in the absence of TEMPO afforded a tiny amount of N-oxide 9, alongside 8 (Scheme 1; Table 1, entry 2). Better results were obtained at pH 5.6; in this case, 9 was isolated in 28% yield (Table 1, entry 3) (NMR data of 9 are in SM #3). The reaction showed a similar behavior at higher pH. The yield of 9 was further increased by increasing the amount of 1,4-dioxane (1,4-dioxane: buffer 10:1). In this latter case, 2-(2-hydroxyethoxy)acetic acid 10 was isolated as a by-product (Scheme 1, Table 1, entry 4; NMR data of 10 are in SM #3). In addition, 9 was obtained in 56% yield using 1,4-dioxane deprived of the commercial radical scavenger butyl hydroxytoluene (BHT) (Table 1, entry 5). The use of tetrahydrofuran (THF) and acetonitrile (CH3CN) as alternative solvents was not effective (Table 1, entry 6 and 7, respectively).

Table 1.
Laccase-catalyzed 1,4-dioxane-mediated synthesis of belladine N-oxide derivative 9 starting from 7b. 1
2.2. EPR Studies
Compound 10 is reported to be the ring-opening product of 2-hydroperoxy-1,4-dioxane III (not isolated, Scheme 1) [39,40,41]. EPR studies with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) confirmed the presence of III in the reaction mixture. As reported in Figure 2 (line a), 1,4-dioxane alone showed a tiny signal in the magnetic field range of 348–353 mT compatible with the formation of the III/DMPO-adduct. The intensity of this signal increased after the addition of laccase (line b). In addition, the same signal was detected during the oxidation of 7b with laccase (line c). The III/DMPO-adduct was common to all cases studied with increasing intensity after the addition of laccase. From a spectroscopic point of view, the spin-trapping approach allows to trap the first radical species formed in the reaction, and the increase of intensity of the signal at 348–353 mT confirms the role of 1,4-dioxane as co-oxidant in the oxidation.
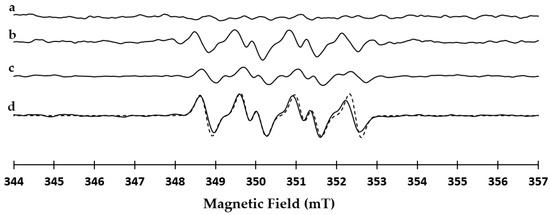
Figure 2.
Line (a): CW (continuous wave) X-band (9 GHz) EPR spectrum at room temperature of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and dioxane; line (b): CW X-band (9 GHz) EPR spectrum at room temperature of DMPO, dioxane, and laccase; line (c): CW X-band (9 GHz) EPR spectrum at room temperature of DMPO, dioxane, laccase, and 7b; line (d): CW X-band (9 GHz) EPR spectrum at room temperature of DMPO, dioxane, laccase and 11h (bold line) paired to it simulation (dotted line). The spectra reported in line a and b were acquired at t = 0 min and those in line c and d at t = 180 min.
2.3. Synthesis and Characterization of Belladine N-Oxide Derivatives 12a–h
The procedure was generalized to derivatives 11a–h, covering a large panel of substituents in the aromatic ring and side chain (the synthesis of compounds 11a–h is in SM #2; NMR data of 11a–h are in SM #3). Compounds 11a–h (0.1 mmol) were treated with laccase (100 U·mmol−1) under O2 atmosphere in 10:1 ratio 1,4-dioxane/sodium acetate buffer (2.50 mL; pH 5.6) at 25 °C for 3.0 h to afford N-oxides 12a–h from good to high yield (53–78%), besides to unreacted substrate (Table 2, entries 1–9) (NMR data of 12a–h are in SM #3). Isoindolines were not detected in the reaction mixture. All type of substituent patterns and side-chain length were allowed, highlighting the high chemo-selectivity and functional-group compatibility of the procedure. A further evidence of the III/DMPO-adduct is reported in Figure 2 (line d) where the EPR signal recorded during the oxidation of 11h is paired to its simulation. The intensity of the signal is higher than in the previous case. The magnetic parameters obtained from the fitting are: g = 2.0061 ± 0.0001, AN = 1.36 mT, AH = 1.01 mT and AH = 0.117 mT. These parameters are typical of peroxyl radical adduct with the DMPO in organic solvents [42]. The intensity of this signal was higher than that previously observed in the oxidation of 7b, in accordance with the higher yield of 12h with respect to 9.

Table 2.
Synthesis of belladine N-oxide derivatives 9 and 12a–h 1.
X-ray data confirmed the structure of 12b, which was the only product isolated as a crystal (Figure 3). The compound crystallizes in a centric space group (C2/c) containing both the enantiomers (for details of the X-ray analysis and crystallization procedure see the Section Materials and Methods).
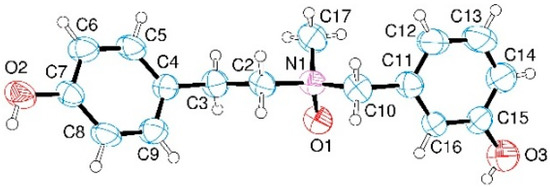
Figure 3.
Crystal structure of 12b. Ellipsoids enclose 50% probability.
As a selected case of study, the 1H-NMR of 12b with chiral lanthanide shift reagent Eu(hfc)3 (700 µL MeOD, 13.9 mM Eu(hfc)3) [43] showed the expected asymmetric shift pattern of the AB quartet system (4.60–4.20 ppm) for the resolution of the two enantiomers, with an enantiomeric excess (ee) of 10% (Figure 4). Polarimetric analyses of 9, 12a–e and 12g–h are reported in Table 2.
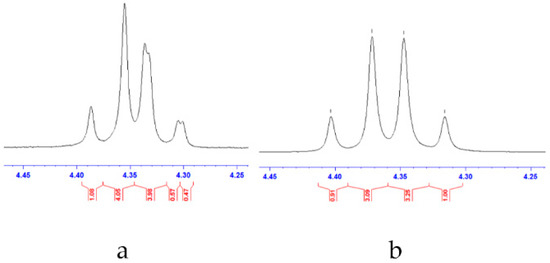
Figure 4.
Panel (a): 1H-NMR spectra of compound 12b in the presence of Eu(hfc)3; panel (b): 1H-NMR spectra of compound 12b in the absence of Eu(hfc)3.
2.4. Antiviral Activity of Compound 7b and 12a–h against Influenza A Virus
Compounds 9 and 12a–h were tested against influenza A/Puerto Rico/8/34 H1N1 (PR8) virus in order to evaluate previously reported computational hypothesis about the inhibition of viral NA [6]. Influenza is responsible for large epidemics and pandemics causing severe health problems [44]. The influenza A virus (Orthomyxoviridae family) is characterized by the release of eight viral RNA segments associated with the nucleoprotein (NP) and the viral RNA-dependent RNA polymerase (RdRp) complex responsible for replication and transcription cycles [45]. Among the inhibitors of the influenza A virus, the compounds active against NA received great attention being involved in the release of viral particles from infected cells [46,47]. In the first set of experiments, A549 cells infected with 0.001 MOI of PR8 were treated with different concentrations (range 10–40 mg/mL) of compounds 9 and 12a–h for 24 h. The expression of Hemagglutinin (HA) was analyzed by means of In Cell Western (ICW) assay (as described in the Materials and Methods section) on cell monolayers. As control of cytotoxicity, cell monolayers were also treated with the same concentrations of compounds 9 and 12a–h and stained with a Cell tag (as described in the Materials and Methods section). The supernatants of the infected A549 cells were recovered and used to newly infect a fresh monolayer of MDCK (Madin-Darby canine epithelial kidney) cells, in order to evaluate whether viral particles released from the infected cells were still infective. Table 3 shows the values of IC50, CC50, and relative SI obtained on A549 and MDCK cells. Compound 12h was the most effective against viral replication in both cell lines (IC50 range 70–73 μg/mL) with the highest SI.

Table 3.
IC50, CC50 values and selective index (SI) of belladine N-oxides derivatives 9 and 12a–h 1
As an example, 12h significantly reduced the HA protein expression on A549 cells (Figure 5, panel a). The released viral particles in the supernatants of A549 cells were then used to infect new monolayers of MDCK cells. After 24 h infection, the ICW assay confirmed a dose-dependent reduction of HA protein expression on MDCK cell monolayers (Figure 5, panel b), suggesting the occurrence of a block in the release of viral particles from the infected cells.
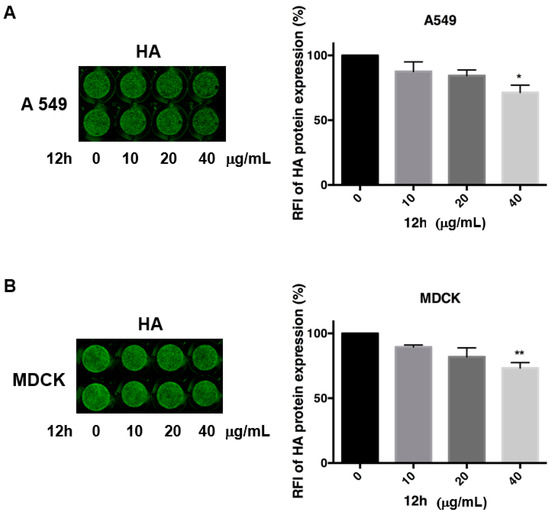
Figure 5.
The expression of Hemagglutinin (HA) by means of In Cell Western (ICW) assay in the presence of most active compound 12h; panel (A): A549 cells were infected with PR8 and treated or not with different concentrations (0–40 μg/mL) of compound 12h. After 24 infection, cells were fixed and stained for HA protein, as described in the Materials and Methods section; panel (B): Supernatants were recovered and used to infect a fresh monolayer of MDCK (Madin-Darby canine epithelial kidney) cells. The expression of viral HA was analyzed by ICW assay, using LI-COR Image Studio Software. The percentage of relative fluorescence intensity (RFI) was calculated in comparison to untreated infected cells (considered 100%). Values are the mean ± S.D. of two replicates of one experiment of two performed (n = 2). Statistical significance of the data vs untreated infected cells was defined as * p < 0.05 and ** p < 0.001.
To evaluate whether the compound 12h was able to impair the cell-to-cell virus spread, higher concentrations (40 and 80 mg/mL) of 12h were added to A549 cell monolayers after viral challenge, and HA protein expression was analyzed directly on these monolayers after 24 h. The ICW assay showed a significant reduction of relative fluorescence intensity of HA protein (~50% inhibition with 80 mg/mL). Furthermore, the reduction of foci of infection in cell monolayers treated with the compound 12h compared to DMSO-treated cells [48], suggested a block in the release of viral particles probably due to an interference with the viral NA (Figure 6).
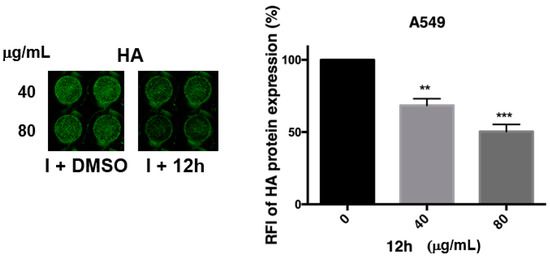
Figure 6.
The expression of Hemagglutinin (HA) by means of ICW assay at late steps of viral replication. A549 cells were infected with PR8 and treated or not with 40 or 80 μg/mL of compound 12h. After 24 infection, cells were fixed and stained for HA protein, as described in the Materials and Methods section. The expression of viral HA was analyzed by ICW assay, using LI-COR Image Studio Software. The percentage of relative fluorescence intensity (RFI) was calculated in comparison to untreated infected cells (considered 100%). Values are the mean ± S.D. of two experiments, each performed in duplicate (n = 4). Statistical significance of the data vs untreated infected cells was defined as ** p < 0.001 and *** p < 0.0001.
3. Conclusions
Laccase was able to activate 1,4-dioxane as co-oxidant in the selective synthesis of belladine N-oxides 9 and 12a–h, as confirmed by the EPR detection of the corresponding DMPO/III adduct. Other organic solvents were not effective in the transformation, highlighting the specific role of the formation of III in the oxygen atom transfer process. This reaction is an alternative to the widespread reported laccase/mediator procedure for the oxidation of amines [9,49,50]. Irrespective of the experimental conditions, the oxidation proceeded from good to high yield, showing high functional-group compatibility and chemo-selectivity avoiding the undesired formation of reactive quinone species and oligomeric products [51,52,53]. In addition, an appreciable stereoselectivity was observed, probably due to partial inhibition of the pyramidal inversion at the nitrogen center as a consequence of steric hindrance of the substituents. Compounds 12a–c and 12h were the most active derivatives against influenza A virus. The highest values of IC50 and SI were observed in the case of 12h, which is characterized by three carbon atoms in the side-chain and only one hydroxy moiety on the aromatic rings. As a general trend, the presence of at least one hydroxy moiety on the aromatic rings and two or three carbon atoms in the side-chain were required to obtain significant antiviral activity. Finally, the presence of an electron-withdrawing substituent on the aromatic ring (12d) deprived the molecule of antiviral activity.
4. Materials and Methods
4.1. Materials
Reagents and laccase from Trametes versicolor were obtained from commercial suppliers (Sigma-Aldrich Srl, Milan, Italy).
4.2. Enzyme Activity Assay
The enzyme activity was assayed by using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)diammonium salt (ABTS) procedure. ABTS (5.0 mM), sodium acetate buffer (2.0 mL, pH 5.0), and the enzyme solution (200 µL) were used as a standard solution. The formation of the cation radical was detected by measuring the increase of absorbance at 420 nm (ε420 = 36,000 M−1 cm−1). One unit of laccase activity has been defined as the amount of enzyme that catalyzed the oxidation of 1.0 µmol of ABTS in a 200 µL reaction mixture at 25 °C during 1.0 min.
4.3. EPR Analysis
The reaction solution was prepared adding 7b and 11h (40 mM), DMPO (60 mM), and laccase (0.12 mM) in 1,4-dioxane/sodium acetate buffer (9:1 ratio). To perform the EPR experiments, capillaries of 1.2 mm diameter were filled in and inserted in a quartz tube of 3 × 3.5 I.D. × O.D. CW (continuous wave) X-band (9 GHz). Experimental condition: 9.86 GHz 123 microwave frequency, 0.1 mT modulation amplitude, and 0.2 mW microwave power. EPR spectra were recorded at room temperature with a Bruker E580 Elexsys Series, using the Bruker ER4122 SHQE cavity. A simulation was carried out with the Easyspin simulation program 5.2.28 version, using the “garlic function”.
4.4. X-Ray Crystallography Data for Compound 12b
Compound 12b was crystallized in an NMR tube, adding 5 mg of the compound in 300µL of deuterated methanol (CD3OD). Compound 12b was completely dissolved heating the system, and the solution was slowly cooled overnight. A single crystal of 12b was submitted to X-ray data collection on an Oxford-Diffraction Xcalibur Sapphire 3 diffractometer with a graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) at 293 K. The structure was solved by direct methods implemented in the SHELXS program (Version 2013/1) [54]. The refinement was carried out by full-matrix anisotropic least-squares on F2 for all reflections for non-H atoms by means of the SHELXL program [55]. The structure crystallizes in the monoclinic crystal system, space group C2/c. Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2,045,206. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; (fax: +44-(0)-1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk).
4.5. Procedure for the Synthesis of Amine N-Oxides 9 and 12a-h
Compounds 7b and 11a–h (1.0 eq., 0.1 mmol) were dissolved in a solvent mixture of 1,4-dioxane (2.25 mL) and sodium acetate buffer 0.1 M, pH = 5.6 (0.25 mL). Laccase (100 U·mmol−1) was added and the mixture was gently stirred at 25 °C under O2 atmosphere (balloon) for 3 h. After this period, the mixture was filtered and the solvent was evaporated under reduced pressure. The crude mixture was purified by silica gel column chromatography (ethyl acetate/methanol 7:1) to afford the desired products 9 and 12a–h. Chemical reactions were monitored using thin-layer chromatography on precoated aluminum silica gel Merck 60 F254 plates and a UV lamp was used for visualization. Merck silica gel 60 (230–400 mesh) was used for chromatography. All products were dried in a high vacuum (10–3 mbar). 1H NMR and 13C-NMR and, DEPT−135 NMR were recorded on a Bruker Avance DRX400 (400 MHz/100 MHz) spectrometer. Chemical shifts for protons are reported in parts per million (δ scale) and internally referenced to the CD3OD and DMSO-d6 signal at δ 3.33 ppm and 2.50 ppm respectively. Coupling constants (J) are reported in Hz. Multiplicities are reported in the conventional form: s = singlet, d = doublet, t = triplet, td = triplet of doublets, q = quartet, ABq = AB quartet, m = multiplet, br = broad. Mass spectra (MS) data were obtained using an Agilent 1100 LC/MSD VL system (G1946C) with a 0.4 mL/min flow rate using a binary solvent system of 95:5/methyl alcohol:water. Optical rotations were recorded on a JASCO P−1000 series at 589 nm and reported as follows: ± value (concentration in g/100 mL, solvent).
4.5.1. N-(2-Bromo-5-Hydroxy-4-Methoxybenzyl)-2-(4-Hydroxyphenyl)-N-Methylethan-1-Amine Oxide (9)
1H-NMR (400 MHz, MeOD): 7.30 (s, 1H, ArH), 7.18 (s, 1H, ArH), 7.12 (d, 2H, ArH, J = 8.4 Hz), 6.75–6.72 (m, 2H, ArH), 4.65–4.48 (ABq, 2H, ArCH2N-, JAB = 12.8 Hz), 3.88 (s, 3H, -OCH3), 3.63–3.52 (m, 1H, -NCH2CH2Ar), 3.47–3.40 (td, 1H, -NCH2CH2Ar, J = 12.0, 4.4 Hz), 3.24–3.17 (td, 1H, -NCH2CH2Ar, J = 12.4, 4.4 Hz), 3.10–3.03 (m, 4H, -NCH2CH2Ar, -CH3) ppm.13C-NMR (100 MHz, MeOD): 156.0, 149.9, 145.9, 129.6, 127.4, 121.3, 120.9, 115.4, 115.3, 115.1, 71.7, 69.9, 55.2, 52.4, 28.0 ppm. DEPT-135-NMR (100 MHz, MeOD): 129.6 (-CH), 120.9 (-CH), 115.3 (-CH), 115.1 (-CH), 71.7 (-CH2), 69.9 (-CH2), 55.2 (-CH3), 52.4 (-CH3), 28.0 (-CH2) ppm. Non-racemic mixture = +12.8 (c 1.00, DMSO). MS (ESI) m/z: (C17H20BrNO4)−: 380.08.
4.5.2. N-(3-Hydroxy-4-Methoxybenzyl)-2-(4-Hydroxyphenyl)-N-Methylethan-1-Amine Oxide (12a)
1H-NMR (400 MHz, MeOD): 7.10–7.06 (m, 3H, ArH), 7.03–6.96 (m, 2H, ArH), 6.75–6.71 (m, 2H, ArH), 4.37–4.28 (ABq, 2H, ArCH2N-, JAB = 12.4 Hz), 3.88 (s, 3H, -OCH3), 3.41–3.16 (m, 3H, -NCH2CH2Ar, -NCH2CH2Ar) 3.09–3.02 (m, 4H, -NCH2CH2Ar, -CH3) ppm.13C-NMR (100 MHz, MeOD): 156.0, 149.0, 146.2, 129.5, 127.6, 123.9, 122.5, 118.9, 115.1, 110.9, 73.0, 68.9, 54.9, 53.2, 28.0 ppm. DEPT-135-NMR (100 MHz, MeOD): 129.5 (-CH), 123.9 (-CH), 118.9 (-CH), 115.1 (-CH), 110.9 (-CH), 73.0 (-CH2), 68.9 (-CH2), 54.9 (-CH3), 53.1 (-CH3), 28.0 (-CH2) ppm. Non-racemic mixture = +17.4 (c 1.00, DMSO). MS (ESI) m/z: (C17H21NO4)−: 302.09.
4.5.3. N-(3-hydroxybenzyl)-2-(4-hydroxyphenyl)-N-methylethan-1-amine oxide (12b)
1H-NMR (400 MHz, MeOD): 7.26 (t, 1H, ArH, J = 8.0 Hz), 7.11 (d, 2H, ArH, J = 8.4 Hz), 7.05–7.03 (m, 2H, ArH), 6.90–6.88 (dd, 1H, ArH, J = 8.4, 6.8 Hz), 6.75 (d, 2H, ArH, J = 8.8 Hz), 4.42–4.33 (ABq, 2H, ArCH2N-, JAB = 12.8 Hz), 3.45–3.28 (m, 2H, -NCH2CH2Ar), 3.24–3.17 (td, 1H, -NCH2CH2Ar, J = 12.4, 4.4 Hz), 3.11–3.08 (m, 4H, -NCH2CH2Ar, -CH3) ppm.13C-NMR (100 MHz, MeOD): 157.3, 156.0, 131.2, 129.5, 129.2, 127.5, 123.2, 119.0, 116.4, 115.1, 73.1, 69.3, 53.4, 28.1 ppm. DEPT-135-NMR (100 MHz, MeOD): 129.5 (-CH), 129.2 (-CH), 123.2 (-CH), 119.0 (-CH), 116.4 (-CH), 115.1 (-CH), 73.0 (-CH2), 69.3 (-CH2), 53.3 (-CH3), 28.0 (-CH2) ppm. Non-racemic mixture = +11.5 (c 1.00, DMSO). MS (ESI) m/z: (C16H19NO3)−: 272.19.
4.5.4. N-(4-Hydroxybenzyl)-2-(4-Hydroxyphenyl)-N-Methylethan-1-Amine Oxide (12c)
1H-NMR (400 MHz, MeOD): 7.41–7.37 (m, 2H, ArH), 7.09 (d, 2H, ArH, J = 8.4 Hz), 6.85–6.82 (m, 2H, ArH), 6.74–6.72 (m, 2H, ArH), 4.39–4.30 (ABq, 2H, ArCH2N-, JAB = 12.8 Hz), 3.41–3.15 (m, 3H, -NCH2CH2Ar, NCH2CH2Ar), 3.09–3.00 (m, 4H, -NCH2CH2Ar, -CH3) ppm.13C-NMR (100 MHz, MeOD): 158.8, 156.0, 133.6, 129.5, 127.6, 120.5, 115.1, 114.9, 72.8, 68.8, 52.9, 28.0 ppm. DEPT-135-NMR (100 MHz, MeOD): 133.6 (-CH), 129.5 (-CH), 115.1 (-CH), 114.9 (-CH), 72.7 (-CH2), 68.8 (-CH2), 52.8 (-CH3), 28.0 (-CH2) ppm. Non-racemic mixture = +21.5 (c 1.00, DMSO). MS (ESI) m/z: (C16H19NO3)−: 272.21.
4.5.5. N-(3-Hydroxy-4-Nitrobenzyl)-2-(4-Hydroxyphenyl)-N-Methylethan-1-Amine Oxide (12d)
1H-NMR (400 MHz, MeOD): 8.10 (d, 1H, ArH, J = 8.8 Hz), 7.44 (d, 1H, ArH, J = 1,6 Hz), 7.24–7.22 (dd, 1H, ArH, J = 8.8, 1,6 Hz), 7.11 (d, 2H, ArH, J = 8.4 Hz), 6.75–6.72 (m, 2H, ArH), 4.50–4.42 (ABq, 2H, JAB = ArCH2N-, 12.8 Hz), 3.55–3.48 (td, 1H, -NCH2CH2Ar, J = 12.0, 5.6 Hz), 3.40–3.31 (td, 1H, -NCH2CH2Ar, J = 12.0, 4.8 Hz), 3.23–3.16 (td, 1H, -NCH2CH2Ar, J = 12.0, 4.8 Hz), 3.13–3.08 (m, 4H, -NCH2CH2Ar, -CH3) ppm.13C-NMR (100 MHz, MeOD): 155.5, 153.3, 138.1, 134.9, 129.0, 126.9, 124.0, 123.7, 122.7, 114.6, 70.6, 69.9, 53.2, 27.6 ppm. DEPT-135-NMR (100 MHz, MeOD): 129.5 (-CH), 124.6 (-CH), 124.2 (-CH), 123.2 (-CH) 115.1 (-CH), 71.2 (-CH2), 70.5 (-CH2), 53.5 (-CH3), 28.1 (-CH2) ppm. Non-racemic mixture = +6.4 (c 1.00, DMSO). MS (ESI) m/z: (C16H18N2O5)−: 317.09.
4.5.6. N-Benzyl-2-(4-Hydroxyphenyl)-N-Methylethan-1-Amine Oxide (12e)
1H-NMR (400 MHz, MeOD): 7.61–7.59 (m, 2H, ArH,), 7.49–7.42 (m, 3H, ArH), 7.10–7.07 (m, 2H, ArH), 6.75–6.71 (m, 2H, ArH) 4.51–4.43 (ABq, 2H, ArCH2N-, JAB = 12.8 Hz), 3.49–3.41 (td, 1H, -NCH2CH2Ar, J = 12.0, 5.6 Hz), 3.38–3.30 (m, 1H, -NCH2CH2Ar), 3.24–3.16 (td, 1H, -NCH2CH2Ar, J = 12.0, 4.8 Hz), 3.12–3.04 (m, 4H, -NCH2CH2Ar, -CH3) ppm.13C-NMR (100 MHz, MeOD): 156.0, 132.4, 129.8, 129.6, 129.5, 128.2, 127.4, 115.2, 72.7, 69.4, 53.0, 28.0 ppm. DEPT-135-NMR (100 MHz, MeOD): 132.4 (-CH), 129.5 (-CH), 129.5 (-CH), 128.2 (-CH) 115.1 (-CH), 72.7 (-CH2), 69.4 (-CH2), 53.0 (-CH3), 28.0 (-CH2) ppm. Non-racemic mixture = +18.6 (c 1.00, DMSO). MS (ESI) m/z: (C16H19NO2)−: 256.27.
4.5.7. 1-(3-hydroxyphenyl)-N,N-dimethylmethanamine oxide (12f)
1H-NMR (400 MHz, MeOD): 7.28–7.24 (m, 1H, ArH), 7.02–7.00 (m, 2H, ArH), 6.91–6.88 (m, 1H, ArH), 4.35 (s, 2H, ArCH2N-), 3.12 (s, 6H, -N(CH3)2).13C-NMR (100 MHz, MeOD): 157.4, 131.3, 129.2, 123.1, 119.0, 116.4, 74.2, 56.5 ppm. DEPT-135-NMR (100 MHz, MeOD): 129.2 (-CH), 123.1 (-CH), 119.0 (-CH), 116.4 (-CH), 74.1 (-CH2), 56.5 (-CH3) ppm. MS (ESI) m/z: (C9H13NO2)−: 166.12.
4.5.8. N-(3-hydroxybenzyl)-1-(4-hydroxyphenyl)-N-methylmethanamine oxide (12g)
1H-NMR (400 MHz, MeOD): 7.42–7.40 (m, 2H, ArH), 7.25 (t, 1H, ArH, J = 7.6 Hz), 6.89–6.82 (m, 5H, ArH), 4.32–4.30 (m, 4H, ArCH2NCH2Ar), 2.77 (s,3H, -CH3).13C-NMR (100 MHz, MeOD): 158.7, 157.2, 139.4, 133.9, 131.2, 123.5, 120.5, 119.3, 116.2, 114.8, 72.8, 72.5, 51.3 ppm. DEPT-135-NMR (100 MHz, MeOD): 134.9 (-CH), 131.5 (-CH), 120.5 (-CH), 119.3 (-CH), 116.3 (-CH), 114.8 (-CH), 72.5 (-CH2), 72.3 (-CH2), 51.3 (-CH3) ppm. Non-racemic mixture = +8.9 (c 1.00, DMSO). MS (ESI) m/z: (C15H17NO3)−: 258.16.
4.5.9. N-(3-Hydroxybenzyl)-N-Methyl-3-Phenylpropan-1-Amine Oxide (12h)
1H-NMR (400 MHz, MeOD): 7.31–7.18 (m, 6H, ArH), 7.00 (t, 1H, ArH, J = 2.0 Hz), 6.95–6.93 (m, 1H, ArH), 6.89–6.87 (m, 1H, ArH), 4.38–4.29 (ABq, 2H, ArCH2N-, JAB = 12.4 Hz), 3.32–3.20 (m, 2H, -NCH2CH2CH2Ar), 3.00 (s, 3H, -CH3), 2.69 (t, 2H, -NCH2CH2CH2Ar, J = 7.6 Hz), 2.34–2.16 (m, 2H, -NCH2CH2CH2Ar) ppm. 13C-NMR (100 MHz, MeOD): 157.3, 140.5, 130.8, 129.2, 128.2, 128.0, 125.9, 123.1, 119.1, 116.4, 72.6, 67.4, 53.2, 32.2, 24.4 ppm. DEPT-135-NMR (100 MHz, MeOD): 129.2 (-CH), 128.1 (-CH), 128.0 (-CH), 125.9 (-CH), 123.1 (-CH) 119.1, (-CH), 116.4 (-CH), 72.6 (-CH2-), 67.4 (-CH2), 53.1 (-CH3), 32.2 (-CH2), 24.4 (-CH2) ppm. Non-racemic mixture = –7.1 (c 1.00, DMSO). MS (ESI) m/z: (C17H21NO2)−: 270.19.
4.6. Cell Cultures
A 549 (human lung epithelial carcinoma) and MDCK (Madin-Darby canine epithelial kidney) cell lines were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS); glutamine 0.3 mg/mL; penicillin 100 U/mL and streptomycin 100 mg/mL. Cell viability was estimated by trypan blue (0.02%) exclusion. All reagents were purchased from Invitrogen (Milan, Italy).
4.7. Virus Production and Infection
Influenza virus A/Puerto Rico/8/34 H1N1 (PR8 virus) was grown in the allantoic cavities of 10-day-old embryonated chicken eggs. After 48 h at 37 °C, the allantoic fluid was harvested, centrifuged at 5000 rpm for 30 min to remove cellular debris, and stored at −80 °C. Virus titration was performed by Tissue Culture Infectious Dose 50% (TCID50%).
Confluent monolayers of A549 epithelial cells were challenged for 1 h at 37 °C with PR8 at a multiplicity of infection (m.o.i.) of 0.001 (TCID50%/cell) incubated for 1 h at 37 °C, washed with PBS, and then incubated with medium supplemented with 2% FCS. Mock infection was performed with the same dilution of allantoic fluid from uninfected eggs. [56].
4.8. In Cell Western (ICW) Assay
The ICW assay was performed using the Odyssey Imaging System (LI-COR, Lincoln, NE, USA) as previously described [47]. Briefly, A549 or MDCK cells grown in 96-well plates (2 × 104 cells/well), either infected or mock-infected (Ctr) with PR8, were fixed with 4% formaldehyde, washed, permeabilized with 0.1% Triton X-100 and incubated with PBS containing Odyssey Blocking buffer (LI-COR Biosciences, Lincoln, NE, USA). The cells were then stained at 4 °C overnight with mouse anti HA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) together with Cell Tag (LI-COR Biosciences, Lincoln, NE, USA) in PBS containing 5% Odyssey Blocking Buffer. Cells were then washed and stained with a mixture of fluorochrome-conjugated secondary antibodies (fluorescence emission at 800 nm) (LI-COR Biosciences, Lincoln, NE, USA) properly diluted in Odyssey blocking buffer and fluorochrome-conjugated Cell Tag (fluorescence emission at 700 nm), for 1 h at room temperature. Cell Tag was used as control of the integrity of the cell monolayer. Subsequently, three washes with PBS plus 0.1% Tween 20 were performed and plates were analyzed by the Odyssey infrared imaging system (LI-COR). Integrated intensities of fluorescence were determined by the LI-COR Image Studio software and the relative fluorescence intensity (RFI) was expressed as a percentage compared to untreated infected cells (100%). The concentration of compounds causing a 50% reduction of viral infection (IC50) and the 50% cytotoxic concentration (CC50), defined as the compound concentration required to reduce cell viability by 50%, were calculated by regression analysis, considering untreated infected cells as control (100%). The Selectivity Index (SI) of each compound was calculated as the ratio CC50/IC50.
Supplementary Materials
Supplementary Materials can be found at https://www.mdpi.com/1422-0067/22/3/1337/s1.
Author Contributions
Conceptualization, C.Z., R.S. and L.B.; methodology and synthesis, C.Z., L.B. and B.M.B.; writing—review and editing, C.Z., L.N. and R.S.; biological test, M.D.A. and V.P.; EPR analyses, M.C.B. and R.P.; X-Ray crystallography data, G.G.; funding acquisition, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by MIUR (Ministero dell’Istruzione, dell’Università della Ricerca Italiano), PRIN 2017 project “ORIGINALE CHEMIAE in Antiviral Strategy—Origin and Modernization of Multi-Component Chemistry as a Source of Innovative Broad Spectrum Antiviral Strategy, cod. 2017BMK8JR (R.S.).
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Acknowledgments
We thank Dante Rotili (Sapienza university of Rome) and Alessia Ciogli (Sa-pienza university of Rome) for providing access to the JASCO P-1000 series polarimeter and for their constructive support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, M.; Qu, C.; Gao, O.; Hu, X.; Hong, X. Biological and pharmacological activities of amaryllidaceae alkaloids. RSC Adv. 2015, 5, 16562–16574. [Google Scholar] [CrossRef]
- Hostettmann, K.; Borloz, A.; Urbain, A.; Marston, A. Natural Product Inhibitors of Acetylcholinesterase. Curr. Org. Chem. 2006, 10, 825–847. [Google Scholar] [CrossRef]
- Patil, D.N.; Patil, S.A.; Sistla, S.; Jadhav, J.P. Comparative biophysical characterization: A screening tool for acetylcholinesterase inhibitors. PLoS ONE 2019, 14, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Maříková, J.; Hulcová, D.; Janoušek, J.; Šafratová, M.; Nováková, L.; Kučera, T.; Hrabinová, M.; Kuneš, J.; Korábečný, J.; et al. Amaryllidaceae Alkaloids of Belladine-Type from Narcissus pseudonarcissus cv. Carlton as New Selective Inhibitors of Butyrylcholinesterase. Biomolecules 2020, 10, 800. [Google Scholar] [CrossRef]
- Carmona-Viglianco, F.; Zaragoza-Puchol, D.; Parravicini, O.; Garro, A.; Enriz, R.D.; Feresin, G.E.; Kurina-Sanz, M.; Orden, A.A. Synthesis, biological evaluation and molecular modeling studies of substituted N-benzyl-2-phenylethanamines as cholinesterase inhibitors. New J. Chem. 2020, 44, 9466–9476. [Google Scholar] [CrossRef]
- Shawky, E. In-silico profiling of the biological activities of Amaryllidaceae alkaloids. J. Pharm. Pharmacol. 2017, 69, 1592–1605. [Google Scholar] [CrossRef]
- Solomon, E.I.; Baldwin, M.J.; Lowery, M.D. Electronic structures of active sites in copper proteins: Contributions to reactivity. Chem. Rev. 1992, 92, 521–542. [Google Scholar] [CrossRef]
- Zippilli, C.; Botta, L.; Bizzarri, B.M.; Baratto, M.C.; Pogni, R.; Saladino, R. Biomimetic synthesis of galantamine: Via laccase/TEMPO mediated oxidative coupling. RSC Adv. 2020, 10, 10897–10903. [Google Scholar] [CrossRef]
- Galletti, P.; Funiciello, F.; Soldati, R.; Giacomini, D. Selective Oxidation of Amines to Aldehydes or Imines using Laccase-Mediated Bio-Oxidation. Adv. Synth. Catal. 2015, 357, 1840–1848. [Google Scholar] [CrossRef]
- Polonovski, M.; Polonovski, M. Sur les aminoxydes des alcaloïdes. III. Action des anhydrides et chlorures d’acides organiques. Préparation des bases nor. Bull. Soc. Chim. Fr. 1927, 41, 1190–1208. [Google Scholar]
- Grierson, D. The Polonovski Reaction. In Organic Reactions; American Cancer Society: Atlanta, GA, USA, 2004; pp. 85–295. ISBN 9780471264187. [Google Scholar]
- Zhan, G.; Zhou, J.; Liu, R.; Liu, T.; Guo, G.; Wang, J.; Xiang, M.; Xue, Y.; Luo, Z.; Zhang, Y.; et al. Galanthamine, Plicamine, and Secoplicamine Alkaloids from Zephyranthes candida and Their Anti-acetylcholinesterase and Anti-inflammatory Activities. J. Nat. Prod. 2016, 79, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Katoch, D.; Kumar, D.; Padwad, Y.S.; Singh, B.; Sharma, U. Pseudolycorine N-oxide, a new N-oxide from Narcissus tazetta. Nat. Prod. Res. 2020, 34, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, P.; Sun, N.; Lu, Y.-J.; Wong, W.-L.; Fang, Z.; Zhang, K. The Diversity of Heterocyclic N-oxides Molecules: Highlights on their Potential in Organic Synthesis, Catalysis and Drug Applications. Curr. Org. Chem. 2019, 23, 616–627. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Z.; Wang, Y. Construction of N-Heterocycles through Cyclization of Tertiary Amines. Chem. A Eur. J. 2019, 25, 2423–2441. [Google Scholar] [CrossRef]
- Yanai, K.; Togo, H. Novel preparation of N-arylmethyl-N-arylmethyleneamine N-oxides from benzylic bromides with zinc and isobutyl nitrite. Tetrahedron 2019, 75, 3523–3529. [Google Scholar] [CrossRef]
- Liu, K.-J.; Deng, J.-H.; Yang, J.; Gong, S.-F.; Lin, Y.-W.; He, J.-Y.; Cao, Z.; He, W.-M. Selective oxidation of (hetero)sulfides with molecular oxygen under clean conditions. Green Chem. 2020, 22, 433–438. [Google Scholar] [CrossRef]
- Pibiri, I.; Buscemi, S.; Palumbo Piccionello, A.; Pace, A. Photochemically Produced Singlet Oxygen: Applications and Perspectives. ChemPhotoChem 2018, 2, 535–547. [Google Scholar] [CrossRef]
- Liu, K.-J.; Duan, Z.-H.; Zeng, X.-L.; Sun, M.; Tang, Z.; Jiang, S.; Cao, Z.; He, W.-M. Clean Oxidation of (Hetero)benzylic Csp3–H Bonds with Molecular Oxygen. ACS Sustain. Chem. Eng. 2019, 7, 10293–10298. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase: A multi-purpose biocatalyst at the forefront of biotechnology. Microb. Biotechnol. 2017, 10, 1457–1467. [Google Scholar] [CrossRef]
- Agrawal, K.; Chaturvedi, V.; Verma, P. Fungal laccase discovered but yet undiscovered. Bioresour. Bioprocess. 2018, 5, 4. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, G.; Ngo, H.H.; Guo, W.; Zhang, S. Advances in thermostable laccase and its current application in lignin-first biorefinery: A review. Bioresour. Technol. 2020, 298, 122511. [Google Scholar] [CrossRef]
- Perna, V.; Meyer, A.S.; Holck, J.; Eltis, L.D.; Eijsink, V.G.H.; Wittrup Agger, J. Laccase-Catalyzed Oxidation of Lignin Induces Production of H2O2. ACS Sustain. Chem. Eng. 2020, 8, 831–841. [Google Scholar] [CrossRef]
- Kudanga, T.; Nemadziva, B.; Le Roes-Hill, M. Laccase catalysis for the synthesis of bioactive compounds. Appl. Microbiol. Biotechnol. 2017, 101, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.; Wlizło, K.; Pogni, R.; Petricci, E.; Grąz, M.; Szałapata, K.; Osińska-Jaroszuk, M.; Kapral-Piotrowska, J.; Pawlikowska-Pawlęga, B.; Jarosz-Wilkołazka, A. Structure and Bioactive Properties of Novel Textile Dyes Synthesised by Fungal Laccase. Int. J. Mol. Sci. 2020, 21, 2052. [Google Scholar] [CrossRef] [PubMed]
- Meschini, R.; D’Eliseo, D.; Filippi, S.; Bertini, L.; Bizzarri, B.M.; Botta, L.; Saladino, R.; Velotti, F. Tyrosinase-Treated Hydroxytyrosol-Enriched Olive Vegetation Waste with Increased Antioxidant Activity Promotes Autophagy and Inhibits the Inflammatory Response in Human THP-1 Monocytes. J. Agric. Food Chem. 2018, 66, 12274–12284. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Martini, A.; Serafini, F.; Aversa, D.; Piccinino, D.; Botta, L.; Berretta, N.; Guatteo, E.; Saladino, R. Tyrosinase mediated oxidative functionalization in the synthesis of DOPA-derived peptidomimetics with anti-Parkinson activity. RSC Adv. 2017, 7, 20502–20509. [Google Scholar] [CrossRef]
- Botta, G.; Bizzarri, B.M.; Garozzo, A.; Timpanaro, R.; Bisignano, B.; Amatore, D.; Palamara, A.T.; Nencioni, L.; Saladino, R. Carbon nanotubes supported tyrosinase in the synthesis of lipophilic hydroxytyrosol and dihydrocaffeoyl catechols with antiviral activity against DNA and RNA viruses. Bioorg. Med. Chem. 2015, 23, 5345–5351. [Google Scholar] [CrossRef]
- Tahmasbi, H.; Khoshayand, M.R.; Bozorgi-Koushalshahi, M.; Heidary, M.; Ghazi-Khansari, M.; Faramarzi, M.A. Biocatalytic conversion and detoxification of imipramine by the laccase-mediated system. Int. Biodeterior. Biodegrad. 2016, 108, 1–8. [Google Scholar] [CrossRef]
- Ferris, J.P.; Gerwe, R.D.; Gapski, G.R. Detoxication mechanisms. II. Iron-catalyzed dealkylation of trimethylamine oxide. J. Am. Chem. Soc. 1967, 89, 5270–5275. [Google Scholar] [CrossRef]
- Ferris, J.P.; Gerwe, R.D.; Gapski, G.R. Detoxication mechanisms. III. Scope and mechanism of the iron-catalyzed dealkylation of tertiary amine oxides. J. Org. Chem. 1968, 33, 3493–3498. [Google Scholar] [CrossRef]
- Aimi, N.; Yamanaka, E.; Endo, J.; Sakai, S.; Haginiwa, J. Transformation of indole alkaloids—I: Conversion of oxindole alkaloids into indole alkaloids. Tetrahedron 1973, 29, 2015–2021. [Google Scholar] [CrossRef]
- Aimi, N.; Yamanaka, E.; Endo, J.; Sakai, S.; Haginiwa, J. Conversion of oxindole alkaloids into indole alkaloids. Tetrahedron Lett. 1972, 13, 1081–1084. [Google Scholar] [CrossRef]
- Galli, C.; Gentili, P. Chemical messengers: Mediated oxidations with the enzyme laccase. J. Phys. Org. Chem. 2004, 17, 973–977. [Google Scholar] [CrossRef]
- Baiocco, P.; Barreca, A.M.; Fabbrini, M.; Galli, C.; Gentili, P. Promoting laccase activity towards non-phenolic substrates: A mechanistic investigation with some laccase–mediator systems. Org. Biomol. Chem. 2003, 1, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Milstein, O.; Nicklas, B.; Hüttermann, A. Oxidation of aromatic compounds in organic solvents with laccase from Trametes versicolor. Appl. Microbiol. Biotechnol. 1989, 31, 70–74. [Google Scholar] [CrossRef]
- Wu, M.-H.; Lin, M.-C.; Lee, C.-C.; Yu, S.-M.; Wang, A.H.-J.; Ho, T.-H.D. Enhancement of laccase activity by pre-incubation with organic solvents. Sci. Rep. 2019, 9, 9754. [Google Scholar] [CrossRef]
- Gerchikov, A.Y.; Akhatova, G.R.; Sharipova, G.M.; Mustafin, A.G.; Sakhibgareeva, M.V.; Spivak, S.I. Investigation of the mechanism of the inhibited oxidation of 1,4-dioxane by mathematical modeling. Kinet. Catal. 2015, 56, 300–303. [Google Scholar] [CrossRef]
- Nasibullina, R.A.; Gimadieva, A.R.; Yakupova, L.R.; Safiullin, R.L. Free-radical chain oxidation of 1,4-dioxane inhibited by 2-thio-6-aminouracil. Kinet. Catal. 2016, 57, 154–158. [Google Scholar] [CrossRef]
- Weinstein, A.B.; Stahl, S.S. Palladium catalyzed aryl C–H amination with O2via in situ formation of peroxide-based oxidant(s) from dioxane. Catal. Sci. Technol. 2014, 4, 4301–4307. [Google Scholar] [CrossRef]
- Clément, J.L.; Ferré, N.; Siri, D.; Karoui, H.; Rockenbauer, A.; Tordo, P. Assignment of the EPR spectrum of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) superoxide spin adduct. J. Org. Chem. 2005, 70, 1198–1203. [Google Scholar] [CrossRef]
- Tori, K.; Yoshimura, Y.; Kainosho, M.; Ajisaka, K. Evidence for the presence of contact term contribution to lanthanide induced shifts in 1H and 13C NMR spectra of pyridine N-oxides. Tetrahedron Lett. 1973, 14, 1573–1576. [Google Scholar] [CrossRef]
- Shaw, M.L.; Palese, P. Orthomyxoviridae: The viruses and their replication. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1647–1689. [Google Scholar]
- Nencioni, L.; Iuvara, A.; Aquilano, K.; Ciriolo, M.R.; Cozzolino, F.; Rotilio, G.; Garaci, E.; Palamara, A.T. Influenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2. FASEB J. 2003, 17, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.; Zhang, L.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza Neuraminidase Inhibitors Possessing a Novel Hydrophobic Interaction in the Enzyme Active Site: Design, Synthesis, and Structural Analysis of Carbocyclic Sialic Acid Analogues with Potent Anti-Influenza Activity. J. Am. Chem. Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Fanelli, A.; Piccinino, D.; De Angelis, M.; Dolfa, C.; Palamara, A.T.; Nencioni, L.; Zippilli, C.; Crucianelli, M.; Saladino, R. Synthesis of Stilbene and Chalcone Inhibitors of Influenza A Virus by SBA-15 Supported Hoveyda-Grubbs Metathesis. Catalysts 2019, 9, 983. [Google Scholar] [CrossRef]
- Marcocci, M.E.; Amatore, D.; Villa, S.; Casciaro, B.; Aimola, P.; Franci, G.; Grieco, P.; Galdiero, M.; Palamara, A.T.; Mangoni, M.L.; et al. The Amphibian Antimicrobial Peptide Temporin B Inhibits In Vitro Herpes Simplex Virus 1 Infection. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Madej, A.; Koszelewski, D.; Paprocki, D.; Brodzka, A.; Ostaszewski, R. The amine as carbonyl precursor in the chemoenzymatic synthesis of Passerini adducts in aqueous medium. Catal. Commun. 2020, 145, 106118. [Google Scholar] [CrossRef]
- Correia Cordeiro, R.S.; Ríos-Lombardía, N.; Morís, F.; Kourist, R.; González-Sabín, J. One-Pot Transformation of Ketoximes into Optically Active Alcohols and Amines by Sequential Action of Laccases and Ketoreductases or ω-Transaminases. ChemCatChem 2019, 11, 1272–1277. [Google Scholar] [CrossRef]
- de Aguiar, V.M.; Esquinelato Silva, R.; Leão, R.A.C.; de Souza, R.O.M.A.; Gonçalves, R.S.B.; de Mariz e Miranda, L.S. Studies on the laccases catalyzed oxidation of norbelladine like acetamides. Mol. Catal. 2020, 485, 110788. [Google Scholar] [CrossRef]
- Feng, Y.; Shen, M.; Wang, Z.; Liu, G. Transformation of atenolol by a laccase-mediator system: Efficiencies, effect of water constituents, and transformation pathways. Ecotoxicol. Environ. Saf. 2019, 183, 109555. [Google Scholar] [CrossRef]
- Wellington, K.W.; Govindjee, V.P.; Steenkamp, P. A laccase-catalysed synthesis of triaminated cyclohexa-2,4-dienones from catechol. J. Catal. 2018, 368, 306–314. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Neri, V.; Farina, A.; Crestini, C.; Nencioni, L.; Palamara, A.T. A Novel and Efficient Synthesis of Tocopheryl Quinones by Homogeneous and Heterogeneous Methyltrioxorhenium/Hydrogen Peroxide Catalytic Systems. Adv. Synth. Catal. 2008, 350, 321–331. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).