Abstract
Nuclear factor erythroid-2 related factor 2 (Nrf2) is a transcription factor that controls cellular defense responses against toxic and oxidative stress by modulating the expression of genes involved in antioxidant response and drug detoxification. In addition to maintaining redox homeostasis, Nrf2 is also involved in various cellular processes including metabolism and inflammation. Nrf2 activity is tightly regulated at the transcriptional, post-transcriptional and post-translational levels, which allows cells to quickly respond to pathological stress. In the present review, we describe the molecular mechanisms underlying the transcriptional regulation of Nrf2. We also focus on the impact of Nrf2 in cardiac ischemia–reperfusion injury, a condition that stimulates the overproduction of reactive oxygen species. Finally, we analyze the protective effect of several natural and synthetic compounds that induce Nrf2 activation and protect against ischemia–reperfusion injury in the heart and other organs, and their potential clinical application.
1. Introduction
In addition to producing cellular energy in the form of ATP from fuel oxidation, mitochondria are also an important source of reactive oxygen species (ROS), which play a central role in redox signaling and contribute to oxidative damage across a range of pathologies [1,2,3]. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is a critical regulator of the cellular stress response. Nrf2 belongs to the cap’n’collar (CNC) family of basic leucine zipper (bZIP) transcription factors [4,5] together with Nrf1 [6], Nrf3 [7], NF-E2 p45 subunit [8], and the more distant related factors BTB domain and CNC homolog 1 and 2 (Bach1 and Bach2) [9]. Nrf2 is widely expressed in mammalian tissues including kidney, liver, heart, lung, brain and skeletal muscle [8,10]. In response to different activation stimuli, Nrf2 translocates from the cytoplasm to the nucleus, where it activates the transcription of its downstream targets by binding to a cis-acting enhancer with a core nucleotide sequence of 5′-TGACNNNGC-3′ where N is any nucleotide, termed the antioxidant response element (ARE) or the electrophile response element (EpRE) [11,12]. Nrf2 is believed to control the basal and inducible expression of over 1000 genes involved in antioxidant defense, detoxification, inflammatory response, proteasomal and autophagic degradation, and metabolism [13,14,15], reflecting its multiple cellular functions––from antioxidant defense to protein quality control and metabolism regulation. In line with the focus of the present review, there is substantial evidence for a protective role of Nrf2 in cardiovascular diseases, including atherosclerosis, ischemia–reperfusion (IR) injury, cardiac hypertrophy, heart failure and diabetes (reviewed in [16]), although some harmful effects have also been reported [17,18,19]. For example, Nrf2 activation protects the heart along with other organs from the damage that ensues upon restoration of blood flow to an ischemic tissue, which is known as IR injury. In addition, a number of small molecules, mostly derived from natural products such as sulforaphane, carnosic acid and curcumin, have been identified as Nrf2 activators with demonstrated protection against IR injury in several preclinical models. Herein, we examine the role of Nrf2 in cellular redox homeostasis and the regulation of its transcriptional activity. We also review studies on the protection offered by the aforementioned compounds against cardiac IR injury.
2. Nrf2 Signaling and Cellular Redox Homeostasis
Nrf2 activates the expression of genes containing ARE sequences in their promoters, including those coding for glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, malic enzyme 1 and isocitrate dehydrogenase 1, which are all involved in the generation of NADPH, an essential cofactor for antioxidant reactions [20,21,22]. NADH is further used by a myriad of redox reactions, many of which are also regulated by Nrf2. For instance, Nrf2 modulates the expression of critical enzymes involved in the production and use of glutathione (GSH), such as glutamate–cysteine ligase catalytic and modulatory subunits (Gclc, Gclm), glutathione reductase, glutathione peroxidase and several glutathione-S-transferases (GST) [23]. Moreover, proteins of the redoxin family (thioredoxin, thioredoxin reductase, peroxiredoxin and sulfiredoxin), which catalyze redox reactions in different compartments, are controlled by Nrf2 [24,25,26]. It also regulates the expression of genes encoding NAD(P)H:quinone oxidoreductase 1 (NQO1), a flavoprotein that inhibits redox cycling of xenobiotics and maintains the endogenous antioxidants α-tocopherol-hydroquinone and coenzyme Q in their reduced (active) forms, along with heme oxygenase-1 (HO-1), which is involved in the production of the potent physiological antioxidant bilirubin [27]. Additionally, Nrf2 influences cellular elimination of xenobiotics mediated by many phase I/II drug-metabolizing enzymes [13], as well as the multi-drug-resistance-associated transporters (Mrps) [28]. Nrf2 knockout mice provided in vivo evidence that Nrf2 drives the expression of these antioxidant/cytoprotective genes [20,29]. Finally, Nrf2 affects intermediary metabolism, through crosstalk with the pentose phosphate pathway and glycolysis, in addition to increasing the availability of substrates and reducing equivalents for the mitochondrial respiratory chain [30] as well as for mitochondrial DNA (mtDNA) integrity [31]. The finding of an ARE-like element in the proximal promoter of Nrf2 gene (Nfe2l2) indicates that it can autoregulate its own transcription [32], leading to positive feedback and amplification of the Nrf2 transcriptional network once it is activated. In summary, Nrf2 increases the cellular defense mechanisms against xenobiotic and oxidative stress through the coordinated expression of numerous antioxidant and detoxification genes.
3. Regulation of Nrf2 Transcriptional Activity
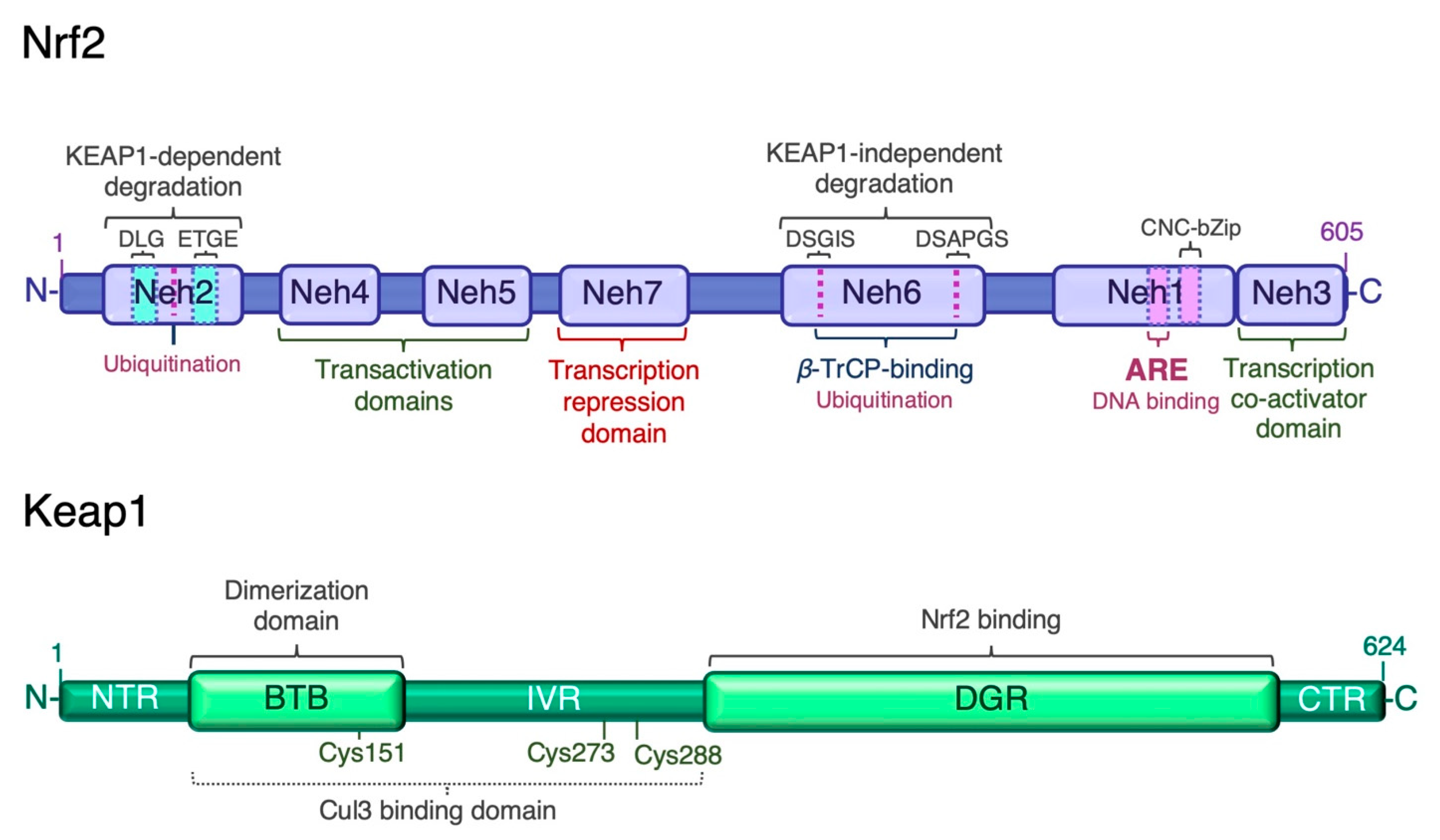
Given the wide range of cellular processes controlled by Nrf2, its activity is tightly regulated at multiple levels. Nrf2 has seven Nrf2-ECH homology domains (Neh1–7), which are critical for its activity and repression [13] (Figure 1). The amino-terminal Neh2 domain contains two binding motifs, DLG and ETGE, which mediate binding to the double glycine repeat (DGR) domain of Kelch-like ECH associated protein 1 (Keap1), a negative regulator of Nrf2. A nuclear localization signal (NLS) sequence is localized in this domain. The Neh6 domain is a serine-rich region involved in the negative regulation of Nrf2 stability independently of Keap1. It contains two conserved peptide motifs, DSGIS and DSAPGS, which are recognized by β-transducing repeat-containing protein (β-TrCP) [33]. β-TrCP binds more efficiently to the Neh6 domain after glycogen synthase kinase-3β (Gsk-3β)-mediated phosphorylation of the DSGIS motif, and promotes the recruitment of the S-phase kinase 1 (Skp1)-Cullin 1 (Cul1)-F-box protein 1 (SCF) ubiquitin ligase complex, leading to proteasomal degradation of Nrf2 [34,35,36,37]. The carboxyl-terminal Neh3 is necessary for transcriptional activation of Nrf2 by recruiting the coactivator chromo-ATPase/helicase DNA-binding protein (CDH) 6. The Neh3 domain contains a second NLS sequence. The Neh1 domain adjacent to Neh3 contains the basic CNC-bZIP region, which is necessary for DNA binding and association with Nrf2 dimerization partners, the small musculoaponeurotic fibrosarcoma (sMaf) proteins [38]. The Neh1 domain contains a nuclear export signal (NES) sequence, and the amino acid sequence of the basic region is highly conserved across a wide range of species [39]. The Neh4 and Neh5 domains are two independent transactivation domains that interact with cAMP response element-binding protein (CREB)-binding protein (CBP) and/or receptor-associated coactivator 3 (RAC3) [40]. The Neh7 domain mediates the repression of Nrf2 transcriptional activity by physical interaction with retinoid X receptor α (RXRα) [41]. The multiple levels of Nrf2 regulation have been recently reviewed [15,42,43]. Transcription mediated by Nrf2 is activated by several transcription factors, which expands the types of stressors that induce Nrf2-target genes. These factors include the aryl hydrocarbon receptor (AhR) [44,45], peroxisome proliferator-activated receptor (PPAR)γ [46,47], nuclear factor-κB (NF-κB) [48,49], specificity protein 1 (Sp-1) [50], p53 [51], c-Jun, c-Myc [52,53], and breast cancer 1 (BRCA1) [54]. As mentioned earlier, Nrf2 also enhances its own transcription [32].
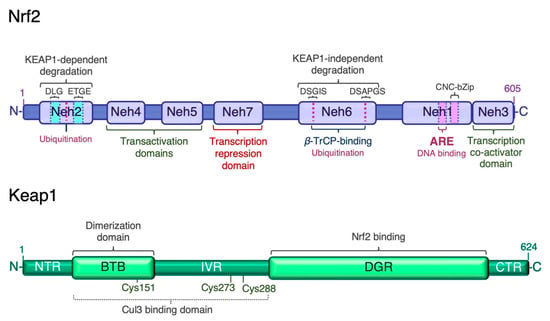
Figure 1.
Structure of human Nrf2 and Keap1. The high-affinity ETGE and low-affinity DLG motifs in the Neh2 domain of Nrf2 are bound by the Kelch domain of Keap1 for Nrf2 ubiquitination and degradation. ARE, antioxidant response element; BTB, broad-complex, tramtrack and bric-à-brac domain; CTR, C-terminal region; DGR or Kelch, double glycine repeat domain; IVR, intervening region; Neh, Nrf2-erythroid-derived CNC homology (ECH) domain; NTR, N-terminal region.
3.1. Keap1-Dependent Proteasomal Degradation of Nrf2
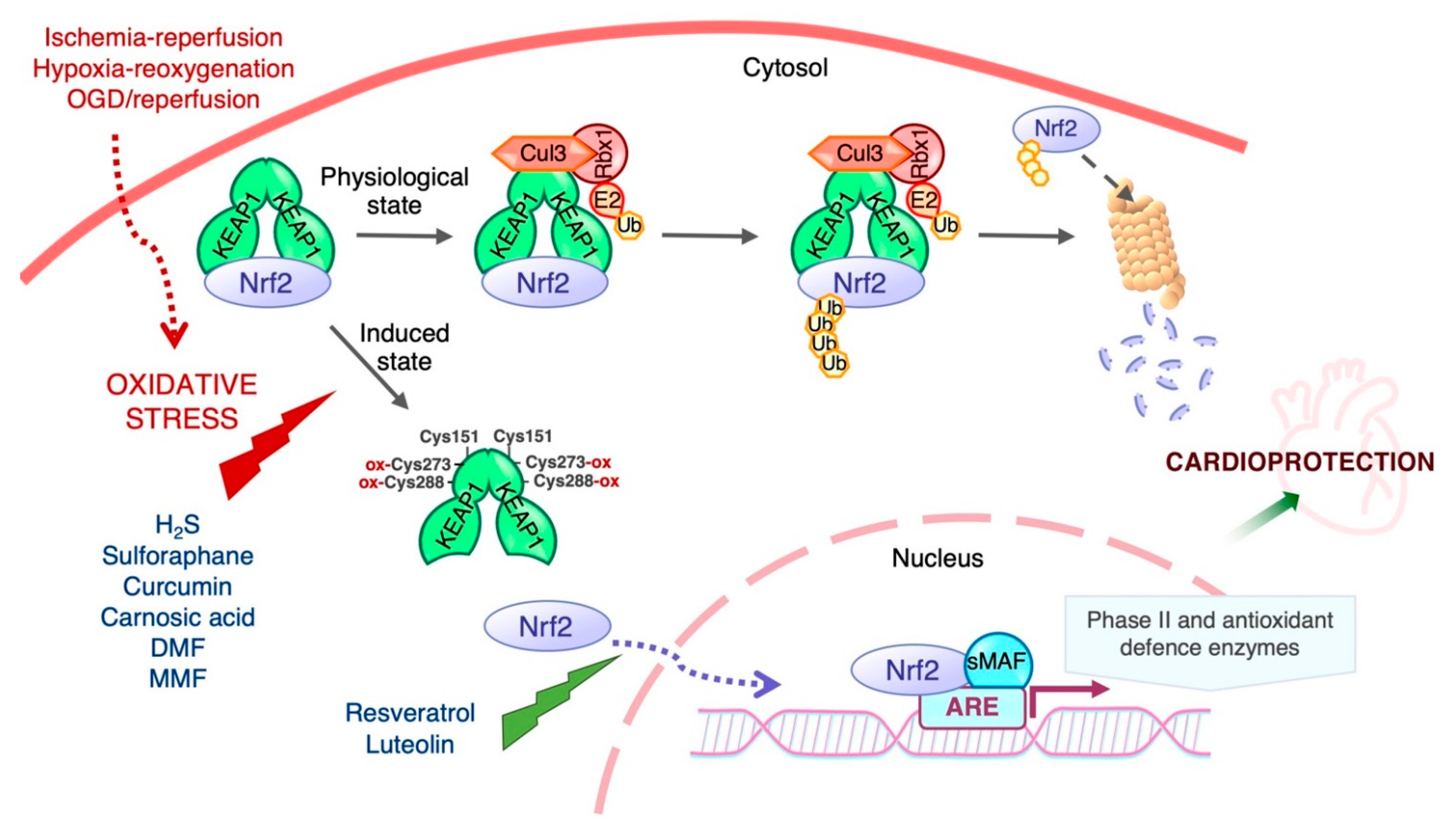
The transcriptional activity and protein stability of Nrf2 is mainly regulated by its cytosolic inhibitor Keap1, a cysteine-rich protein that contains two major domains, BTB and DGR or Kelch, and three additional domains: the N-terminal region (NTR), the intervening region (IVR), and the C-terminal region (CTR). The BTB domain is critical for Keap1 homodimerization and interaction with the Cul3-Rbx1-E3 ligase complex, while the Kelch domain binds to the DLG and ETGE motifs in the Neh2 domain of Nrf2 (Figure 1). Keap1 contains many cysteine residues (27 cysteines of 624 amino acids in the human homologue) in both the BTB and IVR domains that sense oxidative and/or electrophilic molecules [55,56,57]. Under normal conditions, Keap1 targets Nrf2 for degradation in the cytosol. With a half-life of ~20 min [58], the Keap1-mediated high turnover of Nrf2 keeps Nrf2 levels extremely low to avoid unnecessary gene transcription [59,60]. The prevailing mechanistic model for Nrf2 regulation by Keap1 is the “hinge and latch” model [61]: the ETGE motif of Nrf2 acts as the “hinge” while the DIG motif functions as the “latch”. Keap1 binding to Nrf2 leads to its ubiquitination by the Cul3-Rbx1-E3 ligase, thereby targeting it for proteasomal degradation. Under oxidative stress conditions, oxidative or electrophilic molecules (inducers) react with critical cysteine residues of Keap1, triggering conformational changes that release Nrf2, allowing it to evade Keap1-mediated ubiquitination and degradation. Nrf2 translocates to the nucleus, where it enhances the transcription of Nrf2-driven genes (Figure 2). In addition to oxidative and electrophilic reactions, other types of post-translational modifications of Keap1 have been shown to regulate Nrf2 activity, including ubiquitination and phosphorylation [15]. Likewise, protein kinase C (PKC) phosphorylation of Ser40 in the Neh2 domain of Nrf2 disrupts Keap1-Nrf2 association and promotes Nrf2 activation [62].
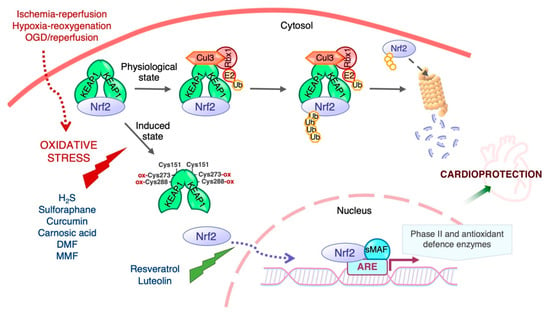
Figure 2.
Regulation of Nrf2 transcriptional activity by Keap1. Nrf2 activity and hence the expression of its target genes is maintained at low levels by Keap1 under normal homeostatic conditions, but increases rapidly in response to redox and electrophilic stressors as well as by stimulation by growth factors. Cys151 is required for triggering Nrf2 signaling by activating agents, and Cys273 and Cys288 are functionally important for the sensing of inducers [56]. The biological effects of Nrf2 are exerted through its ability to mediate the induction of genes containing an antioxidant response element (ARE) in their promoter region upon exposure to a broad spectrum of oxidants and eletrophiles.
3.2. Keap1-Independent Regulation of Nrf2
In addition to Keap1-dependent proteosomal degradation of Nrf2, Keap1-independent mechanisms have also been reported. As described above, the Neh6 domain of Nrf2 contains two highly conserved regions that include the DSGIS and DSAPGS motifs, which are recognized by β-TrCP. Binding of β-TrCP to Nrf2 follows Gsk-3β-mediated phosphorylation of the Neh6 domain of Nrf2. β-TrCP functions as an adaptor for the SCF E3 ubiquitin ligase complex to regulate proteasomal degradation of Nrf2 [34,35]. Gsk-3β action is antagonized by PI3K-PKB/Akt signaling [13,33]. Additionally, Nrf2 activity could potentially be enhanced by some kinases, such as extracellular signal-regulated kinase (ERK), p38 MAP kinase (MAPK), PI3K and PKC, through the inhibition of Gsk-3β [63]. Another ubiquitin-dependent system responsible for Nrf2 degradation involves the E3 ubiquitin ligase synoviolin (Hrd1) during liver cirrhosis [64].
Ubiquitylation of Nrf2 at the N-terminal Neh2 and C-terminal Neh3 domains by CR6-interacting factor (CRIF)1 is yet another mechanism by which Nrf2 activity is suppressed [65], whereas Nrf2 acetylation at multiple Lys residues in the Neh1 domain by p300/CBP in response to arsenite-induced stress is activating [66]. Interestingly, Nrf2 can be polysumoylated by small ubiquitin-like modifier (SUMO)-1 and SUMO-2, which target the protein to promyelocytic leukemia nuclear bodies [67]. Within the nucleus, the polysumoylated Nrf2 can be ubiquitylated by the SUMO-specific RING finger protein 4 (RNF4), thereby providing a means of degrading the transcription factor within the nucleus, thus preventing the induction of Nrf2-target genes.
3.3. Post-Transcriptional Regulation of Nrf2
Recent studies have revealed important roles of microRNAs (miRNAs, miRs) in the regulation of Nrf2 activity through direct targeting of the Nrf2 mRNA and of mRNAs encoding proteins that control the levels and activity of Nrf2 [68,69,70]. miRNAs are short (approximately 23 nucleotides), single-stranded, non-coding RNAs that regulate gene expression by sequence-specific binding with mRNA molecules and consequent inhibition of translation or degradation [71]. Following transcription, the primary miRNA (pri-miRNA) undergoes several steps of maturation to form precursor miRNA (pre-miRNA), which, upon export to the cytoplasm, is processed to form mature miRNA [72]. Complimentary binding of miRNAs to mRNA targets (most commonly within the 3′-UTR) leads to the recruitment of the RNA-induced silencing complex (RISC) to repress the translation of targeted mRNAs or to induce their degradation [73]. Initial in silico analysis predicted 85 Nrf2-miRNA interactions, with 63 miRNAs that can directly or indirectly regulate Nrf2 [74]. Subsequently, using a more stringent criteria to identify miRNAs that can directly target Nrf2, 16 miRNAs were predicted to target Nrf2: miR-374a, miR-28, miR-708, miR-27b, miR-23a, miR-340, miR-153, miR-507, miR-129, miR-93, miR-144, miR-410, miR-140, miR-129, miR-132, miR-212 [68]. Six of these miRNA species have been confirmed to bind to the 3′-UTR of Nrf2 and to regulate translation of the mRNA of a reporter gene: miR-28 [75], miR-153 [76], miR-507 [77], miR-129 [77], miR-93 [78] and miR-144 [79]. In addition, Nrf2 can be targeted by miR-153, miR-27a, miR-142-5p and miR-144 in neuronal SH-SY5Y cells [80]. Expression of miR-144 is upregulated in aged cerebromicrovascular endothelial cells, reducing Nrf2 expression [81]. Overexpression of miR-144 promotes cell death in retinal pigment epithelium (RPE) while its inhibition potentiates Nrf2-dependent antioxidant signaling and protects against oxidative stress-induced outer retinal degeneration [82]. miR-93 also targets Nrf2 inhibiting cell proliferation and promoting apoptosis in RPE cells [83]. Conversely, Nrf2 regulates miRNAs either increasing or decreasing their expression through direct binding of Nrf2 to promoters of genes coding miRNAs and transcriptional activation [84,85,86,87]. Targeting these miRNAs could be a useful strategy to activate specific Nrf2-mediated signaling avoiding the negative consequences of excessive Nrf2 activation.
4. Cardiac Ischemia–Reperfusion Injury
IR injury occurs in many pathological situations including myocardial infarction and stroke, and develops when blood supply is restored to an organ (reperfusion) following a period of poor perfusion (ischemia). Prolonged ischemia triggers a myriad of biochemical and metabolic changes [88]. For instance, the absence of oxygen inhibits oxidative phosphorylation, which causes mitochondrial membrane depolarization, ATP depletion, and inhibition of contractile activity in the myocardium. In this context, cellular metabolism switches to anaerobic glycolysis, resulting in the accumulation of lactate, which leads, in turn, to intracellular calcium overload. While restoration of blood flow to an ischemic organ is essential to prevent irreversible cellular injury, reperfusion per se may augment tissue injury relative to that produced by ischemia alone. During reperfusion, the electron transport chain is reactivated, generating excessive ROS. Other sources of ROS that contribute to reperfusion-induced oxidative stress and represent targets for therapeutic intervention include xanthine oxidase and NADPH oxidase [3,89,90,91,92,93,94,95]. The combined effects of ROS and elevated calcium are believed to lead to the opening of the mitochondrial permeability transition pore (mPTP) in the inner mitochondrial membrane, which plays a critical role in IR injury [96,97,98,99,100]. Regarding the mechanism of mitochondrial ROS production in IR, early reports revealed an increase in succinate in the heart during ischemia and its rapid decrease following reperfusion [101,102]. These observations pointed to mitochondrial superoxide production from complex I driven by reverse electron transfer (RET) from succinate as a key mediator of IR injury. This was confirmed by Chouchani and colleagues using a metabolomics approach and a mitochondria-targeted mass spectrometric hydrogen peroxide probe, MitoB. They showed that succinate-driven RET favors mitochondrial matrix superoxide production from complex I early in reperfusion [103,104,105]. Supporting this analysis, the mitochondria-targeted ubiquinone derivative MitoQ, an established superoxide scavenger [106], has been shown to protect the heart from IR injury [107]. Furthermore, succinate dehydrogenase inhibition by malonate lessens ROS production in both isolated cardiomyocytes and in hearts subjected to IR [103]. However, by monitoring the rapid changes in both NADH/NAD+ ratio and flavoprotein redox state during reperfusion of the heart using real-time surface fluorescence, Andrienko et al. found that these conditions were unfavorable for RET [108,109]. Using a Langendorff perfused heart model, the authors failed to demonstrate that MitoQ was cardioprotectiove, even though it delayed mPTP opening, inhibiting the generation of mitochondrial superoxide during reoxygenation and improving cell survival in isolated cardiomyocytes subjected to hypoxia followed by reoxygenation [110]. The authors also proposed that the rapid loss of succinate upon reperfusion could be due to its efflux rather than to its oxidation by succinate dehydrogenase [109]. Nevertheless, inhibition of succinate dehydrogenase during reperfusion slows the decline in succinate and limits infarct size, supporting the notion that succinate oxidation may contribute to cardiomyocyte death when oxygen becomes available [111].
5. Role of Nrf2 in Ischemia–Reperfusion Injury
Given that oxidative stress-related insults activate Nrf2, which in turn induces the expression of an array of cytoprotective genes, the protective role of Nrf2 against IR injury has been extensively explored in the heart and other organs. Myocardial reperfusion after ischemia induces de novo synthesis of Nrf2, which protects against IR injury [112]. Likewise, reperfusion-specific activation of Nrf2 has been reported in renal epithelial cells exposed to hypoxia/reoxygenation [113]. In this latter study, attenuating ROS overproduction in reoxygenation with the antioxidant N-acetyl cysteine (NAC) suppressed Nrf2 activation, pointing to ROS as signaling mediators of Nrf2. Along the same line, activation of the Nrf2 pathway by miRNAs protects rat and mouse cardiomyocytes against in vitro IR or oxygen and glucose deprivation (OGD) followed by reperfusion [114,115,116]. We have shown that Nrf2 expression is elevated during reperfusion in isolated perfused mouse hearts (Langendorff system) [117]. Data from Xu et al. using a model of left anterior descending (LAD) coronary artery occlusion [112], and our own data in isolated perfused mouse hearts (unpublished), revealed that Nrf2-knockout mice present with increased infarct area as determined by 2,3,5-triphenyltetrazolium chloride (TTC) staining, and enhanced cardiac troponin I [112] or creatine kinase (our data) activity relative to wild-type mice, supporting a protective role for Nrf2 against cardiac IR injury. In addition, administration of sub-lethal concentrations of the lipid peroxidation product 4-hydroxy-2-nonenal (4-HNE) induced Nrf2 activation in mouse cardiomyocytes [118,119], which protected them against glucose-free anoxia followed by reoxygenation [118]. Likewise, intravenous administration of 4-HNE activated Nrf2 in the heart, increasing glutathione content, and improved the functional recovery of the left ventricle following IR in Langendorff-perfused hearts from wild-type but not from Nrf2-knockout mice [118]. Nrf2 activation has also been reported to protect the murine heart against pathological cardiac hypertrophy and heart failure by suppressing oxidative stress [120]. In murine heterotopic heart transplant models, Nrf2 was shown to inhibit NF-κB activation and protect against IR injury, which was mediated by its antioxidant activity, and suppressed the subsequent development of cardiac allograft vasculopathy [121]. Of note, contrary to the great majority of the studies showing beneficial effects of Nrf2 activation against IR injury, Erkens et al. reported that global Nrf2 gene knockout attenuates myocardial IR injury and dysfunction in mice, which the authors suggested was most likely due to cardioprotection by endothelial nitric oxide synthase (eNOS)-derived nitric oxide [17].
Several miRNAs modulate IR injury by regulating the Nrf2 pathway (reviewed in [122,123,124]). Thus, upregulation of miRNA-153 inhibits Nrf2/heme oxygenase-1 and induces ROS production and apoptosis in cardiomyocytes after OGD followed by reoxygenation [115] and in a rat model of IR [116]. Overexpression of miR-210 in cardiomyocytes reduces ROS production and cell death, while downregulation of miR-210 increases ROS levels after hypoxia/reoxygenation [125]. Although not investigated in this study, it seems likely that the effects of miR-210 are mediated by Nrf2. The dual blockade of miR-24-3p and miR-145-5p leads to synergistic upregulation of shared target protective antioxidant genes and inhibition of ROS production in human umbilical vein endothelial cells (HUVECs) after hypoxia/reoxygenation [126]. Interestingly, miR-24-3p [114] and miR-200a [127] target Keap-1, consequently activating Nrf2, thus protecting cardiomyocytes from IR injury [114] or hypoxia-induced apoptosis [127].
In addition to heart protection, Nrf2 also protects other organs against IR injury. For instance, in a murine model of middle cerebral artery occlusion (MCAO) and reperfusion, Nrf2-knockout mice showed a larger hemisphere infarct volume than wild-type mice along with a greater neurological deficit [128]. Consistent with a protective role of Nrf2, IR-mediated cortical damage and sensorimotor deficit in rats was ameliorated by treatment with tert-butylhydroquinone, an Nrf2 activator [129]. Nrf2-knockout mice have also been reported to show more severe retinal damage after IR (high intraocular pressure) than wild-type mice, with Nrf2 believed to mediate the neuroprotective effects of histone deacetylase inhibitors in retinal IR injury [130]. Likewise, a higher number of apoptotic cells was reported in the skeletal muscle of Nrf2-knockout mice when compared with wild-type counterparts after hind-limb IR injury [131]. In the context of liver IR injury, Nrf2 deficiency in mice enhanced tissue damage, impaired ARE-regulated gene signaling, disturbed the redox state and aggravated tumor necrosis factor α mRNA expression [132]. Similarly, in a murine model of warm hepatic IR injury, Nrf2 deficiency exacerbated hepatic injury, whereas hepatocyte-specific Nrf2 overactivation provided protection [133]. Nrf2 activation has been shown to limit macrophage and neutrophil trafficking, proinflammatory cytokine programs and hepatocellular necrosis or apoptosis in IR-stressed mouse liver while increasing antiapoptotic functions, whereas ablation of Nrf2 signaling exacerbated IR-induced liver inflammation and damage [134]. At the molecular level, Nrf2 activation augmented HO-1 expression and promoted PI3K-Akt signaling while suppressing forkhead box O (FoxO)1 signaling, leading to diminished expression of TLR4 proinflammatory mediators [134]. Nrf2 also prevented oxidative injury in a murine model of IR-stressed orthotopic liver transplantation, limiting hepatic inflammatory responses and hepatocellular apoptosis in a PI3K-dependent manner [135]. Nrf2 deficiency is known to enhance susceptibility to both ischemic and nephrotoxic acute kidney injury, as reflected in worsened renal function, histology, vascular permeability and survival in Nrf2-knockout mice compared with wild-type mice after IR, in addition to increasing proinflammatory cytokine and chemokine expression [136]. The knockout mice were also more susceptible to cisplatin-induced nephrotoxicity, which was blunted by NAC pre-treatment, partly mitigating Nrf2 deficiency. These results agree with the finding that global and tubular-specific Nrf2 activation enhances gene expression of antioxidant and NADPH synthesis enzymes, including glucose-6-phosphate dehydrogenase, and ameliorates both the initiation of injury in the outer medulla and the progression of tubular damage in the cortex [137]. Taken together, the data obtained in different organs identify Nrf2 as a potential therapeutic target in IR injury.
6. Involvement of Nrf2 in Protective Ischemic Conditioning
Ischemic conditioning is a phenomenon where brief episodes of ischemia confer protection against injury from subsequent prolonged ischemia. In patients, this cardioprotective strategy can be applied to the heart directly before surgical intervention (ischemic preconditioning, IPC) or after the injury (ischemic postconditioning); it can also be applied to other parts of the body, such as a limb (remote pre- or postconditioning) [138]. Although the phenomenon was first described 35 years ago [139], the mechanism of protection is still not fully understood. Recent studies have investigated the role of Nrf2 in IPC, as the activation of the Nrf2/ARE pathway could be responsible for the induction of antioxidant enzymes caused by IPC, contributing to the late cardioprotection. For example, in H9c2 cells, a cell line derived from embryonic rat heart, IPC induced the expression of Nrf2-dependent antioxidant enzymes, which conferred late protection against the resultant oxidative stress from hypoxia/reoxygenation [140]. Similarly, in an isolated rabbit heart model, IPC significantly induced activation of Nrf2 and downstream antioxidant genes HO-1 and manganese superoxide dismutase (MnSOD), and led to a better recovery of the isolated rabbit heart after IR [141]. These latter effects were mediated by the activation of protein kinase C (PKC), which induces nuclear translocation of Nrf2 and enhances endogenous antioxidant defenses. PKC was also reported to mediate Nrf2 phosphorylation and activation in an in vivo rat model of IR during post-conditioning, which resulted in cardioprotection [142]. As might be expected, Nrf2-knockout mice lose IPC-mediated cardioprotection and exhibit increased infarct size in response to IR [112]. Indeed, several studies have shown that Nrf2 is activated in IPC and induces antioxidant enzymes and antiapoptotic proteins to protect against subsequent IR [112,143,144]. In addition, Nrf2 was found to mediate hepatoprotection afforded by remote ischemic conditioning in a murine model of hemorrhagic shock/resuscitation [145]. Similarly, in a rat model of renal ischemia, IPC and the phytochemical sulforaphane (see below), or a combination of both, activated Nrf2 and increased the expression of its target genes, improving renal function and reducing the expression of inflammatory cytokines [146]. Hepatic IR in rats and mice induces the kidney expression of HO-1, an enzyme with antioxidant and anti-inflammatory functions, via Nrf2, which protects the kidney from remote organ damage after liver IR [147].
In a rat brain model of ischemic stroke, sulforaphane preconditioning prevented neurological dysfunction by activating the Nrf2/ARE pathway [148]. In addition, Nrf2 mediated the IPC-induced protection in astrocytes in an OGD model of cerebral ischemia and in cultured astrocytes derived from wild-type and Nrf2 knockout mice, which can impact the ischemic tolerance of neurons [149]. Similarly, mild oxidative stress insults, including subtoxic H2O2, strongly activated Nrf2/ARE-dependent gene expression in murine astrocytes, contributing to neuroprotective IPC [150]. IPC was shown to protect the murine blood–brain barrier against ischemic injury by generation of endogenous electrophiles and activation of the Nrf2 pathway through inhibition of Keap1 and Gsk-3β-dependent Nrf2 degradation [151]. Finally, IPC provided long-lasting neuroprotection against ischemic brain injury and post-stroke cognitive dysfunction both in primary rodent cortical neurons subjected to OGD preconditioning and in a model of transient MCAO in wild-type but not in Nrf2-knockout mice [152].
7. Activators of Nrf2 and Their Protective Role against Ischemia–Reperfusion Injury
Many natural and synthetic compounds have been identified as Nrf2 activators and have been tested for pharmacological potential, with several showing protective effects against IR injury mainly in the heart, brain and kidney (Table 1). Pharmacological activation of Nrf2 is a promising therapeutic approach for several chronic diseases including neurodegenerative, cardiovascular and metabolic diseases [153,154,155]. Some of these protective compounds induce chemical modification of the sensor cysteines of Keap1, blocking the Keap1-dependent Nrf2 degradation, and allowing de novo synthesized Nrf2 accumulate, translocate to the nucleus and initiate transcription of its downstream target genes [16].

Table 1.
Natural and synthetic activators of Nrf2 and their effects on IR injury. The Table shows the compound administered and the models used, and describes the outcomes of the study. ARE, antioxidant response element; CCA, common carotid artery; CGL, cystathionine γ-lyase; Fh1, fumarate hydratase; H/R, hypoxia/reoxygenation; IR, ischemia–reperfusion; ISO, isoproterenol; LAD, left anterior descending; LCA, left coronary artery; MCAO, middle cerebral artery occlusion; OGD, oxygen and glucose deprivation; ROS, reactive oxygen species.
Hydrogen sulfide (H2S) is a gaseous signaling molecule produced endogenously in different cells and tissues by the enzymes cystathionine γ-lyase (CGL or CSE) and cystathionine β-synthase (CBS) [157,185,186]. Physiological concentrations are cytoprotective through mechanisms related to ROS scavenging, regulation of cell growth, relaxation of blood vessels and inhibition of leukocyte–endothelial cell interaction [157,186]. Exogenous administration and endogenous overexpression of H2S have been shown to protect the heart against IR injury by limiting infarct size and cardiomyocytes apoptosis [187,188,189] through neutralizing ROS overproduction in the peri-infarct zone (the area most affected by reperfusion). The mechanism involved was mediated by the upregulation of protective enzymes by Nrf2. In this context, Calvert and colleagues reported that H2S induced the nuclear localization of Nrf2 and increased the protein expression of phase II enzymes, whereas it offered no protection against myocardial IR in Nrf2-deficient mice [156,157]. Similar results have been observed to occur in the brain of mice treated with H2S before cerebral IR and in kidney. In this case, sodium hydrosulfide (NaHS), an exogenous H2S donor, was found to alleviate tissue injury and inflammation by activating the Nrf2 pathway [158,159]. Using mouse embryonic fibroblasts isolated from CSE-knockout mice, Yang et al. showed that H2S inhibits Keap1 by S-sulfhydration of Cys151, resulting in a conformational change in Keap1 that releases Nrf2 [185].
The isothiocyanate sulforaphane is a natural antioxidant compound found in cruciferous vegetables, and is a strong inducer of phase II (biotransforming) enzymes [190,191]. Talalay and Zhang were the first to isolate sulforaphane from broccoli (Brassica oleracea) [191] and to demonstrate its cancer protective properties [192]. Sulforaphane has been found to have protective effects against IR injury, degenerative diseases and cancer in preclinical studies [154,193,194]. For instance, sulforaphane has cardioprotective effects in the setting of cardiac injury by preserving cardiac function, decreasing infarct size, oxidative stress and inflammation and activating several kinases and phase II enzymes (glutathione, glutathione reductase, GST, thioredoxin reductase, NQO1) in in vivo and in vitro IR models [160,195,196]. This protective effect has also been investigated in other tissues, particularly in the brain, where sulforaphane pretreatment is neuroprotective [197]. Indeed, sulforaphane has been shown to reduce cerebral infarct volume in a neonatal hypoxia-ischemia brain rat model [161] and in a focal ischemia rat model with common carotid or middle cerebral artery occlusion and reperfusion, and to reduce cytotoxic oxygen radicals in cultured mouse brain microvascular endothelial cells [148,162,163,164]. Of note, the hybrid compound of melatonin and sulforaphane ITH12674 shows improved neuroprotective effects in brain ischemia [198]. Renal damage by IR has also been investigated as a potential target for sulforaphane treatment [197]. Yoon et al. found that sulforaphane protected HK2 cells against hypoxia/reoxygenation damage and improved the renal dysfunction generated by IR in rat kidneys [165]. Importantly, sulforaphane was found to protect both rat kidney [199] and heart [200] after experimental transplantation. These protective properties of sulforaphane are due to nuclear Nrf2 translocation and activation of endogenous oxidative defenses. Mechanistically, it has been proposed that sulforaphane acts similar to H2S, oxidizing Cys151 of Keap1 and releasing Nrf2 [16,160,161,165,190,194]. Sulforaphane can also activate the MAPK and PI3K/Akt pathway to phosphorylate Nrf2 and induce its nuclear inclusion [201,202,203]. Clinical trials of sulforaphane (broccoli sprout extract) administration have shown reduced fasting blood glucose and glycated hemoglobin [204], in addition to reduced oxidative stress [205] and favorable effects on lipid profiles and oxidized-LDL/LDL ratio as risk factors for cardiovascular disease [205] in type 2 diabetic patients.
The polyphenolic diterpene carnosic acid, derived from the rosemary plant, has various protective biological properties, including antimicrobial, neuroprotective, anti-inflammatory, anti-cancer and free radical scavenging [167,168]. Carnosic acid was found to be cardioprotective in a H9c2 cardiomyocyte in vitro IR model by diminishing ROS overproduction and improving mitochondrial dysfunction [206]. Likewise, in rodent models of isoproterenol-induced myocardial injury, carnosic acid ameliorated heart dysfunction by activating antioxidant defense system through the Nrf2 pathway [166,167]. According to other studies, carnosic acid has also neuroprotective properties, decreasing infarct size in the brain of mice after MCAO/reperfusion injury and activating Nrf2 in rat PC12h cells [168]. Similar to the aforementioned compounds, carnosic acid stabilizes Nrf2 by S-alkylation of targeted cysteines on Keap1, blocking Nrf2 ubiquitination and degradation, and allowing Nrf2 nuclear accumulation [168,207].
Curcumin is a phenolic compound extracted from the rhizome of Curcuma longa and is commonly used in Asia as a spice and food additive [208,209]. Curcumin has several antioxidant anti-inflammatory and anti-cancer properties by virtue of its ROS scavenging capacity, and also induces the up-regulation of cytoprotective and antioxidant proteins [169,172,201,208,209]. Curcumin has beneficial effects on infarct size and inflammation in several rat brain global and focal ischemia models, thus demonstrating its neuroprotective role [170,171,172,209,210,211]. Likewise, a curcumin-supplemented diet generated neuroprotection in a gerbil ischemia model [212]. A downside to the use of curcumin as a potential cardioprotectant, however, is that it is poorly absorbed by the digestive system and is quickly metabolized [213]. In primary cultures of rat cortical neurons with OGD/reperfusion and in an MCAO rat model, pre- and post-treatment with curcumin protected against the consequences of IR by increasing the abundance of Nrf2 in the nucleus [169,170,171,172]. Other studies have demonstrated a hepatoprotective role of curcumin against IR damage in rats [173]. There is also evidence of cardioprotection after acute myocardial ischemia in rats [214]. Curcumin has been further studied using analogues (14p) synthesized for greater stability and bioavailability, which act as antioxidants in cardiac H9c2 cells treated with H2O2 and limit IR-mediated injury in mice with LAD coronary artery occlusion by activating Nrf2. Mechanistically, Keap1 thiol groups are modified by curcumin, which releases Nrf2 [171,201]. A number of clinical trials have shown beneficial effects of dietary supplementation with curcumin in patients with type 2 diabetes [215,216,217,218,219], including positive effects on fasting blood glucose, weight, atherogenic risk and severity of sensorimotor polyneuropathy.
Luteolin is a flavonoid found in many vegetables and fruits commonly used in traditional Chinese medicine because of its anti-oxidative, anti-inflammatory and anti-carcinogenic activities [220,221]. This compound has been tested against myocardial IR injury in diabetic rats. Luteolin was found to act directly as a ROS scavenger and to enhance eNOS-mediated S-nitrosylation of Keap1 [174,175]. Plant extracts with high luteolin content also produce neuroprotection against cerebral IR injury [220]. Luteolin-mediated protection against other tissue damage situations, such as traumatic brain injury or hepatotoxicity, is promoted at least partly by Nrf2 translocation to the nucleus and induction of antioxidant genes [220,221]. Luteolin has also been reported to induce Nrf2-driven HO-1 expression through the activation of the ERK signaling pathway [222,223], and to protect the heart from mercuric chloride (HgCl2)-induced cardiac damage via PI3K/Akt activation [221].
Another natural Nrf2 activator is resveratrol, a polyphenolic phytochemical biosynthesized by some plants including grapes, peanut skins and berries. Resveratrol has anti-inflammatory, anti-apoptotic, anti-cancer and oxidative stress-reducing activities. Research on resveratrol began with the “French Paradox”, which describes improved cardiovascular outcomes in French people despite a high dietary intake of fats [177,201,224]. The cardioprotective effect of resveratrol has been studied using in vivo (LAD coronary artery occlusion rat model), ex vivo (isolated perfused rat hearts in Langendorff system) and in vitro (H9c2 cells) IR models, where it has been shown to decrease ROS production and ameliorate infarct size and cardiac dysfunction [176,225]. Cheng and colleagues reported that Nrf2 was key for resveratrol-mediated cardiac protection as rats administered with resveratrol before reperfusion showed increased activity of antioxidant enzymes and enhanced levels of Nrf2 and HO-1 in a model of LAD coronary artery occlusion [176]. In addition, it has been shown that resveratrol protects kidney against IR injury through Nrf2/Keap1 up-regulation, alleviating renal dysfunction [177]. Unlike other Nrf2 activator compounds, resveratrol does not act on Keap1, but instead activates the ERK kinase [226,227] and PI3K/Akt [228,229,230] pathways to phosphorylate Nrf2 and protect against oxidative stress. Although no antioxidant or anti-inflammatory effect of resveratrol was found in non-dialyzed chronic kidney disease patients [231], other clinical trials have demonstrated decreased cardiovascular risk [232] and antidiabetic and antioxidant effects [233] after resveratrol supplementation.
In addition to natural compounds, synthetic compounds have also been studied in IR injury models, including fumaric acid derivatives (FADs), specifically dimethyl fumarate (DMF) and its primary metabolite monomethyl fumarate (MMF). Both are fumaric acid diesters clinically used to treat psoriasis and multiple sclerosis, and both have several biological properties (anti-oxidative stress, anti-apoptotic and immunomodulatory) and are well tolerated with minimal adverse effects [180,234]. DMF has been recognized as a potent agent against oxidative injury in cardiomyocytes cultured in an anaerobic chamber or exposed to OGD followed by reoxygenation through Nrf2 activation [180,234]. Furthermore, Ashrafian et al. showed that both endogenous fumarate accumulation and treatment with exogenous fumarate could protect the heart in a perfused model of myocardial infarction in wild-type mice but not in Nrf2-knockout mice [179]. Both MMF and DMF have also been reported to protect the brain against IR damage by reducing infarct volume, attenuating intracellular oxidative stress and improving the neurological deficits in subacute stages and promoting the Nrf2 expression in MCAO/reperfusion and cerebral HI/reperfusion in mouse wild-type models but not in Nrf2-knockout models [181,182]. The protective role of DMF has also been described in hepatic and intestinal IR lesions [173,183]. Fumaric acids activate Nrf2 by modifying the Cys151 of Keap1, as in the case of H2S and sulforaphane, releasing Nrf2 to activate the phase II enzyme transcription in the nucleus [179,181,234]. Although MMF has been used in some studies in brain and retina neuroprotection against IR [181,184] as it is the main bioactive metabolite of DMF, it has been shown that the two compounds have different effects, with DMF being a stronger Nrf2 activator [184].
In summary, many phytochemicals and related compounds activate Nrf2 to increase phase II detoxifying enzymes and other cytoprotective proteins that play significant roles not only in cardioprotection but also in the prevention of IR injury in other organs.
8. Concluding Remarks and Future Perspectives
The Nrf2/Keap1 pathway plays a key role in the defense against oxidative stress and the maintenance of redox homeostasis. The damage associated with reperfusion of ischemic tissues (IR injury) is mainly mediated by the excessive production of ROS. The protective capacity of the activation of the Nrf2/Keap1 pathway against IR injury in the heart and other organs has been demonstrated in a number of cell lines and animal models. The most compelling evidence of the protective effect of Nrf2 activation against IR injury is the loss of protection in Nrf2-knockout mice. Many natural and synthetic compounds have demonstrated significant efficacy on activating Nrf2 and, consequently, on inducing genes that collectively regulate much of the endogenous defense system, enhancing cell survival. Some of these compounds are commercially available as dietary supplements. By virtue of their capacity to activate Nrf2, they protect against IR damage. However, the clinical potential of these compounds in protecting of the heart and other organs during reperfusion needs careful evaluation in future clinical trials.
Funding
Work in my laboratory is funded by the Instituto de Salud Carlos III (FIS PI19/01030) cofounded by Fondos de Desarrollo Regional (FEDER). Institutional grants from the Fundación Ramón Areces and Banco de Santander to the CBMSO are also acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murphy, M.P. Understanding and Preventing Mitochondrial Oxidative Damage. Biochem. Soc. Trans. 2016, 44, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Cadenas, S. ROS and Redox Signaling in Myocardial Ischemia-Reperfusion Injury and Cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Itoh, K.; Igarashi, K.; Hayashi, N.; Nishizawa, M.; Yamamoto, M. Cloning and Characterization of a Novel Erythroid Cell-Derived CNC Family Transcription Factor Heterodimerizing with the Small Maf Family Proteins. Mol. Cell Biol. 1995, 15, 4184–4193. [Google Scholar] [CrossRef] [Green Version]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-Related Factor 2 (Nrf2), a NF-E2-like Basic Leucine Zipper Transcriptional Activator That Binds to the Tandem NF-E2/AP1 Repeat of the Beta-Globin Locus Control Region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.Y.; Han, X.L.; Kan, Y.W. Cloning of Nrf1, an NF-E2-Related Transcription Factor, by Genetic Selection in Yeast. Proc. Natl. Acad. Sci. USA 1993, 90, 11371–11375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, A.; Ito, E.; Toki, T.; Kogame, K.; Takahashi, S.; Igarashi, K.; Hayashi, N.; Yamamoto, M. Molecular Cloning and Functional Characterization of a New Cap’n’ Collar Family Transcription Factor Nrf3. J. Biol. Chem. 1999, 274, 6443–6452. [Google Scholar] [CrossRef] [Green Version]
- Andrews, N.C.; Erdjument-Bromage, H.; Davidson, M.B.; Tempst, P.; Orkin, S.H. Erythroid Transcription Factor NF-E2 Is a Haematopoietic-Specific Basic-Leucine Zipper Protein. Nature 1993, 362, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Oyake, T.; Itoh, K.; Motohashi, H.; Hayashi, N.; Hoshino, H.; Nishizawa, M.; Yamamoto, M.; Igarashi, K. Bach Proteins Belong to a Novel Family of BTB-Basic Leucine Zipper Transcription Factors That Interact with MafK and Regulate Transcription through the NF-E2 Site. Mol. Cell Biol. 1996, 16, 6083–6095. [Google Scholar] [CrossRef] [Green Version]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Chanas, S.A.; Henderson, C.J.; McLellan, L.I.; Wolf, C.R.; Cavin, C.; Hayes, J.D. The Cap’n’Collar Basic Leucine Zipper Transcription Factor Nrf2 (NF-E2 P45-Related Factor 2) Controls Both Constitutive and Inducible Expression of Intestinal Detoxification and Glutathione Biosynthetic Enzymes. Cancer Res. 2001, 61, 3299–3307. [Google Scholar]
- Rushmore, T.H.; Morton, M.R.; Pickett, C.B. The Antioxidant Responsive Element. Activation by Oxidative Stress and Identification of the DNA Consensus Sequence Required for Functional Activity. J. Biol. Chem. 1991, 266, 11632–11639. [Google Scholar] [CrossRef]
- Nioi, P.; McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Identification of a Novel Nrf2-Regulated Antioxidant Response Element (ARE) in the Mouse NAD(P)H:Quinone Oxidoreductase 1 Gene: Reassessment of the ARE Consensus Sequence. Biochem. J. 2003, 374, 337–348. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 Regulatory Network Provides an Interface between Redox and Intermediary Metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Cui, T.; Lai, Y.; Janicki, J.S.; Wang, X. Nuclear Factor Erythroid-2 Related Factor 2 (Nrf2)-Mediated Protein Quality Control in Cardiomyocytes. Front. Biosci. 2016, 21, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond Repression of Nrf2: An Update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the Heart of Oxidative Stress and Cardiac Protection. Physiol. Genom. 2018, 50, 77–97. [Google Scholar] [CrossRef]
- Erkens, R.; Suvorava, T.; Sutton, T.R.; Fernandez, B.O.; Mikus-Lelinska, M.; Barbarino, F.; Flögel, U.; Kelm, M.; Feelisch, M.; Cortese-Krott, M.M. Nrf2 Deficiency Unmasks the Significance of Nitric Oxide Synthase Activity for Cardioprotection. Oxid. Med. Cell. Longev. 2018, 2018, 8309698. [Google Scholar] [CrossRef] [Green Version]
- Barajas, B.; Che, N.; Yin, F.; Rowshanrad, A.; Orozco, L.D.; Gong, K.W.; Wang, X.; Castellani, L.W.; Reue, K.; Lusis, A.J.; et al. NF-E2-Related Factor 2 Promotes Atherosclerosis by Effects on Plasma Lipoproteins and Cholesterol Transport That Overshadow Antioxidant Protection. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, H.; Mathew, R.O.; Cui, T. The Dark Side of Nrf2 in the Heart. Front. Physiol. 2020, 11, 722. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mai, K.H.; Srisuma, S.; Kensler, T.W.; Yamamoto, M.; Biswal, S. Identification of Nrf2-Regulated Genes Induced by the Chemopreventive Agent Sulforaphane by Oligonucleotide Microarray. Cancer Res. 2002, 62, 5196–5203. [Google Scholar] [PubMed]
- Lee, J.-M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-Related Factor-2-Dependent Genes Conferring Protection against Oxidative Stress in Primary Cortical Astrocytes Using Oligonucleotide Microarray Analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Beneficial Role of Nrf2 in Regulating NADPH Generation and Consumption. Toxicol. Sci. 2011, 123, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweier, J.L.; Li, Y. Role of Nrf2 Signaling in Regulation of Antioxidants and Phase 2 Enzymes in Cardiac Fibroblasts: Protection against Reactive Oxygen and Nitrogen Species-Induced Cell Injury. FEBS Lett. 2005, 579, 3029–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanito, M.; Agbaga, M.-P.; Anderson, R.E. Upregulation of Thioredoxin System via Nrf2-Antioxidant Responsive Element Pathway in Adaptive-Retinal Neuroprotection in Vivo and in Vitro. Free Radic. Biol. Med. 2007, 42, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, A.K.; McMahon, M.; Plummer, S.M.; Higgins, L.G.; Penning, T.M.; Igarashi, K.; Hayes, J.D. Characterization of the Cancer Chemopreventive NRF2-Dependent Gene Battery in Human Keratinocytes: Demonstration That the KEAP1-NRF2 Pathway, and Not the BACH1-NRF2 Pathway, Controls Cytoprotection against Electrophiles as Well as Redox-Cycling Compounds. Carcinogenesis 2009, 30, 1571–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agyeman, A.S.; Chaerkady, R.; Shaw, P.G.; Davidson, N.E.; Visvanathan, K.; Pandey, A.; Kensler, T.W. Transcriptomic and Proteomic Profiling of KEAP1 Disrupted and Sulforaphane-Treated Human Breast Epithelial Cells Reveals Common Expression Profiles. Breast Cancer Res. Treat. 2012, 132, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and Glutathione Have Complementary Antioxidant and Cytoprotective Roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, J.M.; Dieter, M.Z.; Aleksunes, L.M.; Slitt, A.L.; Guo, G.; Tanaka, Y.; Scheffer, G.L.; Chan, J.Y.; Manautou, J.E.; Chen, Y.; et al. Oxidative and Electrophilic Stress Induces Multidrug Resistance-Associated Protein Transporters via the Nuclear Factor-E2-Related Factor-2 Transcriptional Pathway. Hepatology 2007, 46, 1597–1610. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 Impacts Cellular Bioenergetics by Controlling Substrate Availability for Mitochondrial Respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Ren, J.; Li, G.; Wu, J.; Wu, X.; Wang, G.; Gu, G.; Ren, H.; Hong, Z.; Li, J. The Mitochondrially Targeted Antioxidant MitoQ Protects the Intestinal Barrier by Ameliorating Mitochondrial DNA Damage via the Nrf2/ARE Signaling Pathway. Cell Death Dis. 2018, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.-K.; Itoh, K.; Yamamoto, M.; Kensler, T.W. Enhanced Expression of the Transcription Factor Nrf2 by Cancer Chemopreventive Agents: Role of Antioxidant Response Element-like Sequences in the Nrf2 Promoter. Mol. Cell Biol. 2002, 22, 2883–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 Is Controlled by Two Distinct β-TrCP Recognition Motifs in Its Neh6 Domain, One of Which Can Be Modulated by GSK-3 Activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef] [Green Version]
- Rada, P.; Rojo, A.I.; Chowdhry, S.; McMahon, M.; Hayes, J.D.; Cuadrado, A. SCF/{beta}-TrCP Promotes Glycogen Synthase Kinase 3-Dependent Degradation of the Nrf2 Transcription Factor in a Keap1-Independent Manner. Mol. Cell Biol. 2011, 31, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobón-Velasco, J.C.; Devijver, H.; García-Mayoral, M.F.; Van Leuven, F.; et al. Structural and Functional Characterization of Nrf2 Degradation by the Glycogen Synthase Kinase 3/β-TrCP Axis. Mol. Cell Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Chiba, T.; Suzuki, T.; Fujita, T.; Ikenoue, T.; Omata, M.; Furuichi, K.; Shikama, H.; Tanaka, K. Homodimer of Two F-Box Proteins BetaTrCP1 or BetaTrCP2 Binds to IkappaBalpha for Signal-Dependent Ubiquitination. J. Biol. Chem. 2000, 275, 2877–2884. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Xu, G.; Schulman, B.A.; Jeffrey, P.D.; Harper, J.W.; Pavletich, N.P. Structure of a Beta-TrCP1-Skp1-Beta-Catenin Complex: Destruction Motif Binding and Lysine Specificity of the SCF(Beta-TrCP1) Ubiquitin Ligase. Mol. Cell 2003, 11, 1445–1456. [Google Scholar] [CrossRef]
- Motohashi, H.; Katsuoka, F.; Engel, J.D.; Yamamoto, M. Small Maf Proteins Serve as Transcriptional Cofactors for Keratinocyte Differentiation in the Keap1-Nrf2 Regulatory Pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 6379–6384. [Google Scholar] [CrossRef] [Green Version]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef]
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two Domains of Nrf2 Cooperatively Bind CBP, a CREB Binding Protein, and Synergistically Activate Transcription. Genes Cells 2001, 6, 857–868. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRα Inhibits the NRF2-ARE Signaling Pathway through a Direct Interaction with the Neh7 Domain of NRF2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.D.; Chowdhry, S.; Dinkova-Kostova, A.T.; Sutherland, C. Dual Regulation of Transcription Factor Nrf2 by Keap1 and by the Combined Actions of β-TrCP and GSK-3. Biochem. Soc. Trans. 2015, 43, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [Green Version]
- Miao, W.; Hu, L.; Scrivens, P.J.; Batist, G. Transcriptional Regulation of NF-E2 P45-Related Factor (NRF2) Expression by the Aryl Hydrocarbon Receptor-Xenobiotic Response Element Signaling Pathway: Direct Cross-Talk between Phase I and II Drug-Metabolizing Enzymes. J. Biol. Chem. 2005, 280, 20340–20348. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T.; McMahon, M. Cross-Talk between Transcription Factors AhR and Nrf2: Lessons for Cancer Chemoprevention from Dioxin. Toxicol. Sci. 2009, 111, 199–201. [Google Scholar] [CrossRef]
- Ikeda, Y.; Sugawara, A.; Taniyama, Y.; Uruno, A.; Igarashi, K.; Arima, S.; Ito, S.; Takeuchi, K. Suppression of Rat Thromboxane Synthase Gene Transcription by Peroxisome Proliferator-Activated Receptor Gamma in Macrophages via an Interaction with NRF2. J. Biol. Chem. 2000, 275, 33142–33150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.Y.; Cho, I.J.; Kim, S.G. Transactivation of the PPAR-Responsive Enhancer Module in Chemopreventive Glutathione S-Transferase Gene by the Peroxisome Proliferator-Activated Receptor-Gamma and Retinoid X Receptor Heterodimer. Cancer Res. 2004, 64, 3701–3713. [Google Scholar] [CrossRef] [Green Version]
- Rushworth, S.A.; Zaitseva, L.; Murray, M.Y.; Shah, N.M.; Bowles, K.M.; MacEwan, D.J. The High Nrf2 Expression in Human Acute Myeloid Leukemia Is Driven by NF-ΚB and Underlies Its Chemo-Resistance. Blood 2012, 120, 5188–5198. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription Factors NRF2 and NF-ΚB Are Coordinated Effectors of the Rho Family, GTP-Binding Protein RAC1 during Inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef] [Green Version]
- Siswanto, F.M.; Oguro, A.; Imaoka, S. Sp1 Is a Substrate of Keap1 and Regulates the Activity of CRL4AWDR23 Ubiquitin Ligase toward Nrf2. J. Biol. Chem. 2021, 296, 100704. [Google Scholar] [CrossRef]
- Tung, M.-C.; Lin, P.-L.; Wang, Y.-C.; He, T.-Y.; Lee, M.-C.; Yeh, S.D.; Chen, C.-Y.; Lee, H. Mutant P53 Confers Chemoresistance in Non-Small Cell Lung Cancer by Upregulating Nrf2. Oncotarget 2015, 6, 41692–41705. [Google Scholar] [CrossRef] [PubMed]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-Induced Nrf2 Transcription Promotes ROS Detoxification and Tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Levy, S.; Forman, H.J. C-Myc Is a Nrf2-Interacting Protein That Negatively Regulates Phase II Genes through Their Electrophile Responsive Elements. IUBMB Life 2010, 62, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Baniasadi, P.S.; Harris, I.S.; Silvester, J.; Inoue, S.; Snow, B.; Joshi, P.A.; Wakeham, A.; Molyneux, S.D.; Martin, B.; et al. BRCA1 Interacts with Nrf2 to Regulate Antioxidant Signaling and Cell Survival. J. Exp. Med. 2013, 210, 1529–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sihvola, V.; Levonen, A.-L. Keap1 as the Redox Sensor of the Antioxidant Response. Arch. Biochem. Biophys. 2017, 617, 94–100. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1, the Cysteine-Based Mammalian Intracellular Sensor for Electrophiles and Oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct Evidence That Sulfhydryl Groups of Keap1 Are the Sensors Regulating Induction of Phase 2 Enzymes That Protect against Carcinogens and Oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [Green Version]
- Katoh, Y.; Iida, K.; Kang, M.-I.; Kobayashi, A.; Mizukami, M.; Tong, K.I.; McMahon, M.; Hayes, J.D.; Itoh, K.; Yamamoto, M. Evolutionary Conserved N-Terminal Domain of Nrf2 Is Essential for the Keap1-Mediated Degradation of the Protein by Proteasome. Arch. Biochem. Biophys. 2005, 433, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, D.; Killeen, E.; Naquin, R.; Alam, S.; Alam, J. Degradation of Transcription Factor Nrf2 via the Ubiquitin-Proteasome Pathway and Stabilization by Cadmium. J. Biol. Chem. 2003, 278, 2396–2402. [Google Scholar] [CrossRef] [Green Version]
- Tong, K.I.; Padmanabhan, B.; Kobayashi, A.; Shang, C.; Hirotsu, Y.; Yokoyama, S.; Yamamoto, M. Different Electrostatic Potentials Define ETGE and DLG Motifs as Hinge and Latch in Oxidative Stress Response. Mol. Cell Biol. 2007, 27, 7511–7521. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser-40 by Protein Kinase C Regulates Antioxidant Response Element-Mediated Transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef] [Green Version]
- Kaidanovich-Beilin, O.; Woodgett, J.R. GSK-3: Functional Insights from Cell Biology and Animal Models. Front. Mol. Neurosci. 2011, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zhao, F.; Gao, B.; Tan, C.; Yagishita, N.; Nakajima, T.; Wong, P.K.; Chapman, E.; Fang, D.; Zhang, D.D. Hrd1 Suppresses Nrf2-Mediated Cellular Protection during Liver Cirrhosis. Genes Dev. 2014, 28, 708–722. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Hong, Y.B.; Kim, H.J.; Bae, I. CR6-Interacting Factor 1 (CRIF1) Regulates NF-E2-Related Factor 2 (NRF2) Protein Stability by Proteasome-Mediated Degradation. J. Biol. Chem. 2010, 285, 21258–21268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Chin, Y.E.; Zhang, D.D. Acetylation of Nrf2 by P300/CBP Augments Promoter-Specific DNA Binding of Nrf2 during the Antioxidant Response. Mol. Cell Biol. 2009, 29, 2658–2672. [Google Scholar] [CrossRef] [Green Version]
- Malloy, M.T.; McIntosh, D.J.; Walters, T.S.; Flores, A.; Goodwin, J.S.; Arinze, I.J. Trafficking of the Transcription Factor Nrf2 to Promyelocytic Leukemia-Nuclear Bodies: Implications for Degradation of NRF2 in the Nucleus. J. Biol. Chem. 2013, 288, 14569–14583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurinna, S.; Werner, S. NRF2 and MicroRNAs: New but Awaited Relations. Biochem. Soc. Trans. 2015, 43, 595–601. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Ivanova-Radkevich, V.I.; Chernov, N.N. Role of MicroRNAs in the Regulation of Redox-Dependent Processes. Biochemistry 2019, 84, 1233–1246. [Google Scholar] [CrossRef]
- Włodarski, A.; Strycharz, J.; Wróblewski, A.; Kasznicki, J.; Drzewoski, J.; Śliwińska, A. The Role of MicroRNAs in Metabolic Syndrome-Related Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 6902. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of MicroRNA Biogenesis and Its Crosstalk with Other Cellular Pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Papp, D.; Lenti, K.; Módos, D.; Fazekas, D.; Dúl, Z.; Türei, D.; Földvári-Nagy, L.; Nussinov, R.; Csermely, P.; Korcsmáros, T. The NRF2-Related Interactome and Regulome Contain Multifunctional Proteins and Fine-Tuned Autoregulatory Loops. FEBS Lett. 2012, 586, 1795–1802. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Yao, Y.; Eades, G.; Zhang, Y.; Zhou, Q. MiR-28 Regulates Nrf2 Expression through a Keap1-Independent Mechanism. Breast Cancer Res. Treat. 2011, 129, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Riar, A.K.; Rathinam, M.L.; Vedpathak, D.; Henderson, G.; Mahimainathan, L. Hydrogen Peroxide Responsive MiR153 Targets Nrf2/ARE Cytoprotection in Paraquat Induced Dopaminergic Neurotoxicity. Toxicol. Lett. 2014, 228, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Inoue, J.; Kawano, T.; Kozaki, K.; Omura, K.; Inazawa, J. The Impact of MiRNA-Based Molecular Diagnostics and Treatment of NRF2-Stabilized Tumors. Mol. Cancer Res. 2014, 12, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Ronghe, A.M.; Chatterjee, A.; Bhat, N.K.; Bhat, H.K. MicroRNA-93 Regulates NRF2 Expression and Is Associated with Breast Carcinogenesis. Carcinogenesis 2013, 34, 1165–1172. [Google Scholar] [CrossRef] [Green Version]
- Sangokoya, C.; Telen, M.J.; Chi, J.-T. MicroRNA MiR-144 Modulates Oxidative Stress Tolerance and Associates with Anemia Severity in Sickle Cell Disease. Blood 2010, 116, 4338–4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narasimhan, M.; Patel, D.; Vedpathak, D.; Rathinam, M.; Henderson, G.; Mahimainathan, L. Identification of Novel MicroRNAs in Post-Transcriptional Control of Nrf2 Expression and Redox Homeostasis in Neuronal, SH-SY5Y Cells. PLoS ONE 2012, 7, e51111. [Google Scholar] [CrossRef] [Green Version]
- Csiszar, A.; Gautam, T.; Sosnowska, D.; Tarantini, S.; Banki, E.; Tucsek, Z.; Toth, P.; Losonczy, G.; Koller, A.; Reglodi, D.; et al. Caloric Restriction Confers Persistent Anti-Oxidative, pro-Angiogenic, and Anti-Inflammatory Effects and Promotes Anti-Aging MiRNA Expression Profile in Cerebromicrovascular Endothelial Cells of Aged Rats. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H292–H306. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Jones, M.A.; Abdelrahman, A.A.; Powell, F.L.; Thounaojam, M.C.; Gutsaeva, D.; Bartoli, M.; Martin, P.M. Inhibiting MicroRNA-144 Potentiates Nrf2-Dependent Antioxidant Signaling in RPE and Protects against Oxidative Stress-Induced Outer Retinal Degeneration. Redox Biol. 2020, 28, 101336. [Google Scholar] [CrossRef]
- Luo, R.; Jin, H.; Li, L.; Hu, Y.-X.; Xiao, F. Long Noncoding RNA MEG3 Inhibits Apoptosis of Retinal Pigment Epithelium Cells Induced by High Glucose via the MiR-93/Nrf2 Axis. Am. J. Pathol. 2020, 190, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Chorley, B.N.; Campbell, M.R.; Wang, X.; Karaca, M.; Sambandan, D.; Bangura, F.; Xue, P.; Pi, J.; Kleeberger, S.R.; Bell, D.A. Identification of Novel NRF2-Regulated Genes by ChIP-Seq: Influence on Retinoid X Receptor Alpha. Nucleic Acids Res. 2012, 40, 7416–7429. [Google Scholar] [CrossRef] [Green Version]
- Kurinna, S.; Schäfer, M.; Ostano, P.; Karouzakis, E.; Chiorino, G.; Bloch, W.; Bachmann, A.; Gay, S.; Garrod, D.; Lefort, K.; et al. A Novel Nrf2-MiR-29-Desmocollin-2 Axis Regulates Desmosome Function in Keratinocytes. Nat. Commun. 2014, 5, 5099. [Google Scholar] [CrossRef] [Green Version]
- Joo, M.S.; Lee, C.G.; Koo, J.H.; Kim, S.G. MiR-125b Transcriptionally Increased by Nrf2 Inhibits AhR Repressor, Which Protects Kidney from Cisplatin-Induced Injury. Cell Death Dis. 2013, 4, e899. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.M.; Zaitseva, L.; Bowles, K.M.; MacEwan, D.J.; Rushworth, S.A. NRF2-Driven MiR-125B1 and MiR-29B1 Transcriptional Regulation Controls a Novel Anti-Apoptotic MiRNA Regulatory Network for AML Survival. Cell Death Differ. 2015, 22, 654–664. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Yellon, D.M. Myocardial Ischemia-Reperfusion Injury: A Neglected Therapeutic Target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Kevin, L.G.; Camara, A.K.S.; Riess, M.L.; Novalija, E.; Stowe, D.F. Ischemic Preconditioning Alters Real-Time Measure of O2 Radicals in Intact Hearts with Ischemia and Reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H566–H574. [Google Scholar] [CrossRef] [Green Version]
- Braunersreuther, V.; Jaquet, V. Reactive Oxygen Species in Myocardial Reperfusion Injury: From Physiopathology to Therapeutic Approaches. Curr. Pharm. Biotechnol. 2012, 13, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Kuppusamy, P.; Williams, R.; Rayburn, B.K.; Smith, D.; Weisfeldt, M.L.; Flaherty, J.T. Measurement and Characterization of Postischemic Free Radical Generation in the Isolated Perfused Heart. J. Biol. Chem. 1989, 264, 18890–18895. [Google Scholar] [CrossRef]
- Henry, T.D.; Archer, S.L.; Nelson, D.; Weir, E.K.; From, A.H. Enhanced Chemiluminescence as a Measure of Oxygen-Derived Free Radical Generation during Ischemia and Reperfusion. Circ. Res. 1990, 67, 1453–1461. [Google Scholar] [CrossRef] [Green Version]
- Zweier, J.L.; Talukder, M.A.H. The Role of Oxidants and Free Radicals in Reperfusion Injury. Cardiovasc. Res. 2006, 70, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Granger, D.N.; Kvietys, P.R. Reperfusion Injury and Reactive Oxygen Species: The Evolution of a Concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Di Lisa, F.; Bernardi, P. Modulation of Mitochondrial Permeability Transition in Ischemia-Reperfusion Injury of the Heart. Advantages and Limitations. Curr. Med. Chem. 2015, 22, 2480–2487. [Google Scholar] [CrossRef]
- Garcia-Dorado, D.; Rodriguez-Sinovas, A.; Ruiz-Meana, M.; Inserte, J.; Agulló, L.; Cabestrero, A. The End-Effectors of Preconditioning Protection against Myocardial Cell Death Secondary to Ischemia-Reperfusion. Cardiovasc. Res. 2006, 70, 274–285. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Clarke, S.J.; Javadov, S.A. Mitochondrial Permeability Transition Pore Opening during Myocardial Reperfusion—A Target for Cardioprotection. Cardiovasc. Res. 2004, 61, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Meana, M.; Abellán, A.; Miró-Casas, E.; Agulló, E.; Garcia-Dorado, D. Role of Sarcoplasmic Reticulum in Mitochondrial Permeability Transition and Cardiomyocyte Death during Reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1281–H1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solaini, G.; Harris, D.A. Biochemical Dysfunction in Heart Mitochondria Exposed to Ischaemia and Reperfusion. Biochem. J. 2005, 390, 377–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisarenko, O.; Studneva, I.; Khlopkov, V.; Solomatina, E.; Ruuge, E. An Assessment of Anaerobic Metabolism during Ischemia and Reperfusion in Isolated Guinea Pig Heart. Biochim. Biophys. Acta 1988, 934, 55–63. [Google Scholar] [CrossRef]
- Peuhkurinen, K.J.; Takala, T.E.; Nuutinen, E.M.; Hassinen, I.E. Tricarboxylic Acid Cycle Metabolites during Ischemia in Isolated Perfused Rat Heart. Am. J. Physiol. 1983, 244, H281–H288. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic Accumulation of Succinate Controls Reperfusion Injury through Mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pell, V.R.; Chouchani, E.T.; Frezza, C.; Murphy, M.P.; Krieg, T. Succinate Metabolism: A New Therapeutic Target for Myocardial Reperfusion Injury. Cardiovasc. Res. 2016, 111, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.A.J.; Murphy, M.P. Animal and Human Studies with the Mitochondria-Targeted Antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef]
- Adlam, V.J.; Harrison, J.C.; Porteous, C.M.; James, A.M.; Smith, R.A.J.; Murphy, M.P.; Sammut, I.A. Targeting an Antioxidant to Mitochondria Decreases Cardiac Ischemia-Reperfusion Injury. FASEB J. 2005, 19, 1088–1095. [Google Scholar] [CrossRef]
- Andrienko, T.; Pasdois, P.; Rossbach, A.; Halestrap, A.P. Real-Time Fluorescence Measurements of ROS and [Ca2+] in Ischemic/Reperfused Rat Hearts: Detectable Increases Occur Only after Mitochondrial Pore Opening and Are Attenuated by Ischemic Preconditioning. PLoS ONE 2016, 11, e0167300. [Google Scholar] [CrossRef] [Green Version]
- Andrienko, T.N.; Pasdois, P.; Pereira, G.C.; Ovens, M.J.; Halestrap, A.P. The Role of Succinate and ROS in Reperfusion Injury—A Critical Appraisal. J. Mol. Cell Cardiol. 2017, 110, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, M.J.; Llwyd, O.; Morin, D.; de Paulis, D.; Arnoux, T.; Gouarné, C.; Koul, S.; Engblom, H.; Bordet, T.; Tissier, R.; et al. Differences in the Profile of Protection Afforded by TRO40303 and Mild Hypothermia in Models of Cardiac Ischemia/Reperfusion Injury. Eur. J. Pharmacol. 2015, 760, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Valls-Lacalle, L.; Barba, I.; Miró-Casas, E.; Alburquerque-Béjar, J.J.; Ruiz-Meana, M.; Fuertes-Agudo, M.; Rodríguez-Sinovas, A.; García-Dorado, D. Succinate Dehydrogenase Inhibition with Malonate during Reperfusion Reduces Infarct Size by Preventing Mitochondrial Permeability Transition. Cardiovasc. Res. 2016, 109, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Zhang, J.; Strom, J.; Lee, S.; Chen, Q.M. Myocardial Ischemic Reperfusion Induces de Novo Nrf2 Protein Translation. Biochim. Biophys. Acta 2014, 1842, 1638–1647. [Google Scholar] [CrossRef] [Green Version]
- Leonard, M.O.; Kieran, N.E.; Howell, K.; Burne, M.J.; Varadarajan, R.; Dhakshinamoorthy, S.; Porter, A.G.; O’Farrelly, C.; Rabb, H.; Taylor, C.T. Reoxygenation-Specific Activation of the Antioxidant Transcription Factor Nrf2 Mediates Cytoprotective Gene Expression in Ischemia-Reperfusion Injury. FASEB J. 2006, 20, 2624–2626. [Google Scholar] [CrossRef]
- Xiao, X.; Lu, Z.; Lin, V.; May, A.; Shaw, D.H.; Wang, Z.; Che, B.; Tran, K.; Du, H.; Shaw, P.X. MicroRNA MiR-24-3p Reduces Apoptosis and Regulates Keap1-Nrf2 Pathway in Mouse Cardiomyocytes Responding to Ischemia/Reperfusion Injury. Oxid. Med. Cell. Longev. 2018, 2018, 7042105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, Y.; Hou, W.; Guo, L. MiR-153 Regulates Cardiomyocyte Apoptosis by Targeting Nrf2/HO-1 Signaling. Chromosome Res. 2019, 27, 167–178. [Google Scholar] [CrossRef]
- Hou, W.; Zhu, X.; Liu, J.; Ma, J. Inhibition of MiR-153 Ameliorates Ischemia/Reperfusion-Induced Cardiomyocytes Apoptosis by Regulating Nrf2/HO-1 Signaling in Rats. Biomed. Eng. Online 2020, 19, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anedda, A.; López-Bernardo, E.; Acosta-Iborra, B.; Saadeh Suleiman, M.; Landázuri, M.O.; Cadenas, S. The Transcription Factor Nrf2 Promotes Survival by Enhancing the Expression of Uncoupling Protein 3 under Conditions of Oxidative Stress. Free Radic. Biol. Med. 2013, 61, 395–407. [Google Scholar] [CrossRef]
- Zhang, Y.; Sano, M.; Shinmura, K.; Tamaki, K.; Katsumata, Y.; Matsuhashi, T.; Morizane, S.; Ito, H.; Hishiki, T.; Endo, J.; et al. 4-Hydroxy-2-Nonenal Protects against Cardiac Ischemia-Reperfusion Injury via the Nrf2-Dependent Pathway. J. Mol. Cell Cardiol. 2010, 49, 576–586. [Google Scholar] [CrossRef]
- López-Bernardo, E.; Anedda, A.; Sánchez-Pérez, P.; Acosta-Iborra, B.; Cadenas, S. 4-Hydroxynonenal Induces Nrf2-Mediated UCP3 Upregulation in Mouse Cardiomyocytes. Free Radic. Biol. Med. 2015, 88, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ichikawa, T.; Villacorta, L.; Janicki, J.S.; Brower, G.L.; Yamamoto, M.; Cui, T. Nrf2 Protects against Maladaptive Cardiac Responses to Hemodynamic Stress. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1843–1850. [Google Scholar] [CrossRef]
- Fukunaga, N.; Kawajiri, H.; Badiwala, M.V.; Butany, J.; Li, R.-K.; Billia, F.; Rao, V. Protective Role of Nrf2 against Ischemia Reperfusion Injury and Cardiac Allograft Vasculopathy. Am. J. Transpl. 2020, 20, 1262–1271. [Google Scholar] [CrossRef]
- Kura, B.; Szeiffova Bacova, B.; Kalocayova, B.; Sykora, M.; Slezak, J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int. J. Mol. Sci. 2020, 21, 358. [Google Scholar] [CrossRef] [Green Version]
- Makkos, A.; Ágg, B.; Petrovich, B.; Varga, Z.V.; Görbe, A.; Ferdinandy, P. Systematic Review and Network Analysis of MicroRNAs Involved in Cardioprotection against Myocardial Ischemia/Reperfusion Injury and Infarction: Involvement of Redox Signalling. Free Radic. Biol. Med. 2021, 172, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, T.; Gomes, A.V. MicroRNAs in the Regulation of Cellular Redox Status and Its Implications in Myocardial Ischemia-Reperfusion Injury. Redox Biol. 2020, 36, 101607. [Google Scholar] [CrossRef]
- Mutharasan, R.K.; Nagpal, V.; Ichikawa, Y.; Ardehali, H. MicroRNA-210 Is Upregulated in Hypoxic Cardiomyocytes through Akt- and P53-Dependent Pathways and Exerts Cytoprotective Effects. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1519–H1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tingle, S.J.; Sewpaul, A.; Bates, L.; Thompson, E.R.; Shuttleworth, V.; Figueiredo, R.; Ibrahim, I.K.; Ali, S.; Wilson, C.; Sheerin, N.S. Dual MicroRNA Blockade Increases Expression of Antioxidant Protective Proteins: Implications for Ischemia-Reperfusion Injury. Transplantation 2020, 104, 1853–1861. [Google Scholar] [CrossRef]
- Sun, X.; Zuo, H.; Liu, C.; Yang, Y. Overexpression of MiR-200a Protects Cardiomyocytes against Hypoxia-Induced Apoptosis by Modulating the Kelch-like ECH-Associated Protein 1-Nuclear Factor Erythroid 2-Related Factor 2 Signaling Axis. Int. J. Mol. Med. 2016, 38, 1303–1311. [Google Scholar] [CrossRef]
- Shah, Z.A.; Li, R.-C.; Thimmulappa, R.K.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. Role of Reactive Oxygen Species in Modulation of Nrf2 Following Ischemic Reperfusion Injury. Neuroscience 2007, 147, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, A.Y.; Li, P.; Murphy, T.H. A Small-Molecule-Inducible Nrf2-Mediated Antioxidant Response Provides Effective Prophylaxis against Cerebral Ischemia in Vivo. J. Neurosci. 2005, 25, 10321–10335. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Luo, D.; Xia, W.; Gu, C.; Lahm, T.; Xu, X.; Qiu, Q.; Zhang, Z. Nuclear Factor (Erythroid-Derived 2)-Like 2 (Nrf2) Contributes to the Neuroprotective Effects of Histone Deacetylase Inhibitors In Retinal Ischemia-Reperfusion Injury. Neuroscience 2019, 418, 25–36. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Fragoulis, A.; Rosen, C.; Kan, Y.W.; Sönmez, T.T.; Pufe, T.; Wruck, C.J. Nrf2 Protects against TWEAK-Mediated Skeletal Muscle Wasting. Sci. Rep. 2014, 4, 3625. [Google Scholar] [CrossRef] [Green Version]
- Kudoh, K.; Uchinami, H.; Yoshioka, M.; Seki, E.; Yamamoto, Y. Nrf2 Activation Protects the Liver from Ischemia/Reperfusion Injury in Mice. Ann. Surg. 2014, 260, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.-Y.; Harberg, C.; Matkowskyj, K.A.; Cook, S.; Roenneburg, D.; Werner, S.; Johnson, J.; Foley, D.P. Overactivation of the Nuclear Factor (Erythroid-Derived 2)-like 2-Antioxidant Response Element Pathway in Hepatocytes Decreases Hepatic Ischemia/Reperfusion Injury in Mice. Liver Transpl. 2016, 22, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Yue, S.; Ke, B.; Zhu, J.; Shen, X.; Zhai, Y.; Yamamoto, M.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Nuclear Factor Erythroid 2-Related Factor 2 Regulates Toll-like Receptor 4 Innate Responses in Mouse Liver Ischemia-Reperfusion Injury through Akt-Forkhead Box Protein O1 Signaling Network. Transplantation 2014, 98, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, B.; Shen, X.-D.; Zhang, Y.; Ji, H.; Gao, F.; Yue, S.; Kamo, N.; Zhai, Y.; Yamamoto, M.; Busuttil, R.W.; et al. KEAP1-NRF2 Complex in Ischemia-Induced Hepatocellular Damage of Mouse Liver Transplants. J. Hepatol. 2013, 59, 1200–1207. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Grigoryev, D.N.; Crow, M.T.; Haas, M.; Yamamoto, M.; Reddy, S.P.; Rabb, H. Transcription Factor Nrf2 Is Protective during Ischemic and Nephrotoxic Acute Kidney Injury in Mice. Kidney Int. 2009, 76, 277–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nezu, M.; Souma, T.; Yu, L.; Suzuki, T.; Saigusa, D.; Ito, S.; Suzuki, N.; Yamamoto, M. Transcription Factor Nrf2 Hyperactivation in Early-Phase Renal Ischemia-Reperfusion Injury Prevents Tubular Damage Progression. Kidney Int. 2017, 91, 387–401. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Ischaemic Conditioning and Reperfusion Injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with Ischemia: A Delay of Lethal Cell Injury in Ischemic Myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-S.; Chen, H.-P.; Yu, H.-H.; Yan, Y.-F.; Liao, Z.-P.; Huang, Q.-R. Nrf2-Dependent Upregulation of Antioxidative Enzymes: A Novel Pathway for Hypoxic Preconditioning-Mediated Delayed Cardioprotection. Mol. Cell Biochem. 2014, 385, 33–41. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Z.; Yao, J.; Zhao, G.; Fa, X.; Niu, J. Participation of Protein Kinase C in the Activation of Nrf2 Signaling by Ischemic Preconditioning in the Isolated Rabbit Heart. Mol. Cell Biochem. 2013, 372, 169–179. [Google Scholar] [CrossRef]
- Buelna-Chontal, M.; Guevara-Chávez, J.-G.; Silva-Palacios, A.; Medina-Campos, O.-N.; Pedraza-Chaverri, J.; Zazueta, C. Nrf2-Regulated Antioxidant Response Is Activated by Protein Kinase C in Postconditioned Rat Hearts. Free Radic. Biol. Med. 2014, 74, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Motori, E.; Fabbri, D.; Malaguti, M.; Leoncini, E.; Lorenzini, A.; Hrelia, S. H2O2 Preconditioning Modulates Phase II Enzymes through P38 MAPK and PI3K/Akt Activation. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2196–H2205. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.B.; Bolli, R.; Dawn, B.; Sanganalmath, S.K.; Zhu, Y.; Wang, O.-L.; Guo, Y.; Motterlini, R.; Xuan, Y.-T. Carbon Monoxide Induces a Late Preconditioning-Mimetic Cardioprotective and Antiapoptotic Milieu in the Myocardium. J. Mol. Cell Cardiol. 2012, 52, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.H.; Caldarone, C.A.; Guan, R.; Wen, X.-Y.; Ailenberg, M.; Kapus, A.; Szaszi, K.; Rotstein, O.D. Nuclear Factor (Erythroid-Derived 2)-Like 2 Regulates the Hepatoprotective Effects of Remote Ischemic Conditioning in Hemorrhagic Shock. Antioxid. Redox Signal. 2019, 30, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Shokeir, A.A.; Barakat, N.; Hussein, A.M.; Awadalla, A.; Harraz, A.M.; Khater, S.; Hemmaid, K.; Kamal, A.I. Activation of Nrf2 by Ischemic Preconditioning and Sulforaphane in Renal Ischemia/Reperfusion Injury: A Comparative Experimental Study. Physiol. Res. 2015, 64, 313–323. [Google Scholar] [CrossRef]
- Tanaka, Y.; Maher, J.M.; Chen, C.; Klaassen, C.D. Hepatic Ischemia-Reperfusion Induces Renal Heme Oxygenase-1 via NF-E2-Related Factor 2 in Rats and Mice. Mol. Pharmacol. 2007, 71, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, A.; Srivastava, S.; Siow, R.C.M.; Cash, D.; Modo, M.; Duchen, M.R.; Fraser, P.A.; Williams, S.C.R.; Mann, G.E. Sulforaphane Preconditioning of the Nrf2/HO-1 Defense Pathway Protects the Cerebral Vasculature against Blood-Brain Barrier Disruption and Neurological Deficits in Stroke. Free Radic. Biol. Med. 2013, 65, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.V.; Dave, K.R.; Perez-Pinzon, M.A. Ischemic Preconditioning Protects Astrocytes against Oxygen Glucose Deprivation Via the Nuclear Erythroid 2-Related Factor 2 Pathway. Transl. Stroke Res. 2018, 9, 99–109. [Google Scholar] [CrossRef]
- Bell, K.F.; Al-Mubarak, B.; Fowler, J.H.; Baxter, P.S.; Gupta, K.; Tsujita, T.; Chowdhry, S.; Patani, R.; Chandran, S.; Horsburgh, K.; et al. Mild Oxidative Stress Activates Nrf2 in Astrocytes, Which Contributes to Neuroprotective Ischemic Preconditioning. Proc. Natl. Acad. Sci. USA 2011, 108, E1–E2. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Sun, Y.; Mao, L.; Zhang, M.; Li, Q.; Zhang, L.; Shi, Y.; Leak, R.K.; Chen, J.; Zhang, F. Brain Ischemic Preconditioning Protects against Ischemic Injury and Preserves the Blood-Brain Barrier via Oxidative Signaling and Nrf2 Activation. Redox Biol. 2018, 17, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sun, Y.; Li, Q.; Li, S.; Shi, Y.; Leak, R.K.; Chen, J.; Zhang, F. Ischemic Preconditioning Provides Long-Lasting Neuroprotection against Ischemic Stroke: The Role of Nrf2. Exp. Neurol. 2020, 325, 113142. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Yagishita, Y.; Gatbonton-Schwager, T.N.; McCallum, M.L.; Kensler, T.W. Current Landscape of NRF2 Biomarkers in Clinical Trials. Antioxidants 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic Targeting of the NRF2 and KEAP1 Partnership in Chronic Diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Calvert, J.W.; Elston, M.; Nicholson, C.K.; Gundewar, S.; Jha, S.; Elrod, J.W.; Ramachandran, A.; Lefer, D.J. Genetic and Pharmacologic Hydrogen Sulfide Therapy Attenuates Ischemia-Induced Heart Failure in Mice. Circulation 2010, 122, 11–19. [Google Scholar] [CrossRef]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen Sulfide Mediates Cardioprotection Through Nrf2 Signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, K.; Xue, L.; Cheng, J.; Bai, Y. Preconditioning of H2S Inhalation Protects against Cerebral Ischemia/Reperfusion Injury by Induction of HSP70 through PI3K/Akt/Nrf2 Pathway. Brain Res. Bull. 2016, 121, 68–74. [Google Scholar] [CrossRef]
- Su, Y.; Wang, Y.; Liu, M.; Chen, H. Hydrogen Sulfide Attenuates Renal I/R-induced Activation of the Inflammatory Response and Apoptosis via Regulating Nrf2-mediated NLRP3 Signaling Pathway Inhibition. Mol. Med. Rep. 2021, 24, 1–14. [Google Scholar] [CrossRef]
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Sánchez-Garibay, C.; Rojas-Morales, P.; Galván-Arzate, S.; Buelna-Chontal, M.; Pavón, N.; Pedraza-Chaverrí, J.; Königsberg, M.; Zazueta, C. Sulforaphane Protects from Myocardial Ischemia-Reperfusion Damage through the Balanced Activation of Nrf2/AhR. Free Radic. Biol. Med. 2019, 143, 331–340. [Google Scholar] [CrossRef]
- Ping, Z.; Liu, W.; Kang, Z.; Cai, J.; Wang, Q.; Cheng, N.; Wang, S.; Wang, S.; Zhang, J.H.; Sun, X. Sulforaphane Protects Brains against Hypoxic–Ischemic Injury through Induction of Nrf2-Dependent Phase 2 Enzyme. Brain Res. 2010, 1343, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kobori, N.; Aronowski, J.; Dash, P.K. Sulforaphane Reduces Infarct Volume Following Focal Cerebral Ischemia in Rodents. Neurosci. Lett. 2006, 393, 108–112. [Google Scholar] [CrossRef]
- Srivastava, S.; Alfieri, A.; Siow, R.C.M.; Mann, G.E.; Fraser, P.A. Temporal and Spatial Distribution of Nrf2 in Rat Brain Following Stroke: Quantification of Nuclear to Cytoplasmic Nrf2 Content Using a Novel Immunohistochemical Technique. J. Physiol. 2013, 591, 3525–3538. [Google Scholar] [CrossRef]
- Warpsinski, G.; Smith, M.J.; Srivastava, S.; Keeley, T.P.; Siow, R.C.M.; Fraser, P.A.; Mann, G.E. Nrf2-Regulated Redox Signaling in Brain Endothelial Cells Adapted to Physiological Oxygen Levels: Consequences for Sulforaphane Mediated Protection against Hypoxia-Reoxygenation. Redox Biol. 2020, 37, 101708. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-Y.; Kang, N.-I.; Lee, H.-K.; Jang, K.Y.; Park, J.-W.; Park, B.-H. Sulforaphane Protects Kidneys against Ischemia-Reperfusion Injury through Induction of the Nrf2-Dependent Phase 2 Enzyme. Biochem. Pharmacol. 2008, 75, 2214–2223. [Google Scholar] [CrossRef]
- Kocak, C.; Kocak, F.E.; Akcilar, R.; Isiklar, O.O.; Kocak, H.; Bayat, Z.; Simsek, H.; Taser, F.; Altuntas, I. Molecular and Biochemical Evidence on the Protective Effects of Embelin and Carnosic Acid in Isoproterenol-Induced Acute Myocardial Injury in Rats. Life Sci. 2016, 147, 15–23. [Google Scholar] [CrossRef]
- Sahu, B.D.; Putcha, U.K.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Carnosic Acid Promotes Myocardial Antioxidant Response and Prevents Isoproterenol-Induced Myocardial Oxidative Stress and Apoptosis in Mice. Mol. Cell Biochem. 2014, 394, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Kosaka, K.; Itoh, K.; Kobayashi, A.; Yamamoto, M.; Shimojo, Y.; Kitajima, C.; Cui, J.; Kamins, J.; Okamoto, S.; et al. Carnosic Acid, a Catechol-Type Electrophilic Compound, Protects Neurons Both in Vitro and in Vivo through Activation of the Keap1/Nrf2 Pathway via S-Alkylation of Targeted Cysteines on Keap1. J. Neurochem. 2008, 104, 1116–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-X.; Zhang, L.-Y.; Chen, Y.-L.; Yu, S.-S.; Zhao, Y.; Zhao, J. Curcumin Pretreatment and Post-Treatment Both Improve the Antioxidative Ability of Neurons with Oxygen-Glucose Deprivation. Neural Regen. Res. 2015, 10, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin by Down-Regulating NF-KB and Elevating Nrf2, Reduces Brain Edema and Neurological Dysfunction after Cerebral I/R. Microvasc. Res. 2016, 106, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin Upregulates Transcription Factor Nrf2, HO-1 Expression and Protects Rat Brains against Focal Ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by Curcumin in Ischemic Brain Injury Involves the Akt/Nrf2 Pathway. PLoS ONE 2013, 8, e59843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.G.; El-Emam, S.Z.; Mohamed, E.A.; Abd Ellah, M.F. Dimethyl Fumarate and Curcumin Attenuate Hepatic Ischemia/Reperfusion Injury via Nrf2/HO-1 Activation and Anti-Inflammatory Properties. Int. Immunopharmacol. 2020, 80, 106131. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Xia, M.-L.; Wang, J.; Zhou, X.-R.; Lou, Y.-Y.; Tang, L.-H.; Zhang, F.-J.; Yang, J.-T.; Qian, L.-B. Luteolin Attenuates Cardiac Ischemia/Reperfusion Injury in Diabetic Rats by Modulating Nrf2 Antioxidative Function. Oxidative Med. Cell. Longev. 2019, 2019, e2719252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-R.; Ru, X.-C.; Xiao, C.; Pan, J.; Lou, Y.-Y.; Tang, L.-H.; Yang, J.-T.; Qian, L.-B. Sestrin2 Is Involved in the Nrf2-Regulated Antioxidative Signaling Pathway in Luteolin-Induced Prevention of the Diabetic Rat Heart from Ischemia/Reperfusion Injury. Food Funct. 2021, 12, 3562–3571. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, Z.; Zhao, R.; Ren, K.; Deng, C.; Yu, S. Resveratrol Attenuates Inflammation and Oxidative Stress Induced by Myocardial Ischemia-Reperfusion Injury: Role of Nrf2/ARE Pathway. Int. J. Clin. Exp. Med. 2015, 8, 10420–10428. [Google Scholar]
- Li, J.; Li, L.; Wang, S.; Zhang, C.; Zheng, L.; Jia, Y.; Xu, M.; Zhu, T.; Zhang, Y.; Rong, R. Resveratrol Alleviates Inflammatory Responses and Oxidative Stress in Rat Kidney Ischemia-Reperfusion Injury and H2O2-Induced NRK-52E Cells via the Nrf2/TLR4/NF-ΚB Pathway. CPB 2018, 45, 1677–1689. [Google Scholar] [CrossRef]
- Li, W.; Wu, M.; Tang, L.; Pan, Y.; Liu, Z.; Zeng, C.; Wang, J.; Wei, T.; Liang, G. Novel Curcumin Analogue 14p Protects against Myocardial Ischemia Reperfusion Injury through Nrf2-Activating Anti-Oxidative Activity. Toxicol. Appl. Pharmacol. 2015, 282, 175–183. [Google Scholar] [CrossRef]
- Ashrafian, H.; Czibik, G.; Bellahcene, M.; Aksentijević, D.; Smith, A.C.; Mitchell, S.J.; Dodd, M.S.; Kirwan, J.; Byrne, J.J.; Ludwig, C.; et al. Fumarate Is Cardioprotective via Activation of the Nrf2 Antioxidant Pathway. Cell Metab. 2012, 15, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Kuang, Y.; Zhang, Y.; Xiao, Z.; Xu, L.; Wang, P.; Ma, Q. Protective Effect of Dimethyl Fumarate on Oxidative Damage and Signaling in Cardiomyocytes. Mol. Med. Rep. 2020, 22, 2783–2790. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, W.; Liu, Z.; Han, W.; Shi, K.; Shen, Y.; Li, H.; Liu, Q.; Fu, Y.; Huang, D.; et al. Dimethyl Fumarate and Monomethyl Fumarate Promote Post-Ischemic Recovery in Mice. Transl. Stroke Res. 2016, 7, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Vollmer, M.K.; Kelly, M.G.; Fernandez, V.M.; Fernandez, T.G.; Kim, H.; Doré, S. Reactive Gliosis Contributes to Nrf2-Dependent Neuroprotection by Pretreatment with Dimethyl Fumarate or Korean Red Ginseng Against Hypoxic-Ischemia: Focus on Hippocampal Injury. Mol. Neurobiol. 2020, 57, 105–117. [Google Scholar] [CrossRef]
- Gendy, A.; Soubh, A.; Al-Mokaddem, A.; Kotb El-Sayed, M. Dimethyl Fumarate Protects against Intestinal Ischemia/Reperfusion Lesion: Participation of Nrf2/HO-1, GSK-3β and Wnt/β-Catenin Pathway. Biomed. Pharmacother. 2021, 134, 111130. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Hartsock, M.J.; Xu, Z.; He, M.; Duh, E.J. Monomethyl Fumarate Promotes Nrf2-Dependent Neuroprotection in Retinal Ischemia-Reperfusion. J. Neuroinflammation 2015, 12, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen Sulfide Protects against Cellular Senescence via S-Sulfhydration of Keap1 and Activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wu, B.; Cao, Q.; Wu, L.; Yang, G. Hydrogen Sulfide Mediates the Anti-Survival Effect of Sulforaphane on Human Prostate Cancer Cells. Toxicol. Appl. Pharmacol. 2011, 257, 420–428. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen Sulfide Attenuates Myocardial Ischemia-Reperfusion Injury by Preservation of Mitochondrial Function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodha, N.R.; Clements, R.T.; Feng, J.; Liu, Y.; Bianchi, C.; Horvath, E.M.; Szabo, C.; Sellke, F.W. The Effects of Therapeutic Sulfide on Myocardial Apoptosis in Response to Ischemia—Reperfusion Injury. Eur. J. Cardiothorac. Surg. 2008, 33, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, C.K.; Calvert, J.W. Hydrogen Sulfide and Ischemia-Reperfusion Injury. Pharmacol. Res. 2010, 62, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular Basis for Chemoprevention by Sulforaphane: A Comprehensive Review. Cell Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A Major Inducer of Anticarcinogenic Protective Enzymes from Broccoli: Isolation and Elucidation of Structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kensler, T.W.; Cho, C.G.; Posner, G.H.; Talalay, P. Anticarcinogenic Activities of Sulforaphane and Structurally Related Synthetic Norbornyl Isothiocyanates. Proc. Natl. Acad. Sci. USA 1994, 91, 3147–3150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Protective Effect of Sulforaphane against Oxidative Stress: Recent Advances. Exp. Toxicol. Pathol. 2012, 64, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Santín-Márquez, R.; Alarcón-Aguilar, A.; López-Diazguerrero, N.E.; Chondrogianni, N.; Königsberg, M. Sulforaphane—Role in Aging and Neurodegeneration. GeroScience 2019, 41, 655–670. [Google Scholar] [CrossRef]
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Modulation of Phase II Enzymes by Sulforaphane: Implications for Its Cardioprotective Potential. J. Agric. Food Chem. 2009, 57, 5615–5622. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.S.; Gao, S.; Lee, G.-H.; Kim, D.S.; Park, B.-H.; Chae, S.W.; Chae, H.-J.; Kim, S.H. Sulforaphane Protects Ischemic Injury of Hearts through Antioxidant Pathway and Mitochondrial KATP Channels. Pharmacol. Res. 2010, 61, 342–348. [Google Scholar] [CrossRef]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; Rada, P.; Cuadrado, A.; López, M.G.; García, A.G.; León, R. Melatonin-Sulforaphane Hybrid ITH12674 Induces Neuroprotection in Oxidative Stress Conditions by a “drug-Prodrug” Mechanism of Action. Br. J. Pharmacol. 2015, 172, 1807–1821. [Google Scholar] [CrossRef] [Green Version]
- Cekauskas, A.; Bruns, H.; Manikas, M.; Herr, I.; Gross, M.-L.; Zorn, M.; Jankevicius, F.; Strupas, K.; Schemmer, P. Sulforaphane Decreases Kidney Injury after Transplantation in Rats: Role of Mitochondrial Damage. Ann. Transplant. 2013, 18, 488–496. [Google Scholar] [CrossRef]
- Li, Z.; Galli, U.; Becker, L.E.; Bruns, H.; Nickkolgh, A.; Hoffmann, K.; Karck, M.; Schemmer, P. Sulforaphane Protects Hearts from Early Injury after Experimental Transplantation. Ann. Transplant. 2013, 18, 558–566. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. The Emerging Role of Redox-Sensitive Nrf2–Keap1 Pathway in Diabetes. Pharmacol. Res. 2015, 91, 104–114. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxid. Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leoncini, E.; Malaguti, M.; Angeloni, C.; Motori, E.; Fabbri, D.; Hrelia, S. Cruciferous Vegetable Phytochemical Sulforaphane Affects Phase II Enzyme Expression and Activity in Rat Cardiomyocytes through Modulation of Akt Signaling Pathway. J. Food Sci. 2011, 76, H175–H181. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane Reduces Hepatic Glucose Production and Improves Glucose Control in Patients with Type 2 Diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef] [Green Version]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Rajab, A.; Asghari, G.; Azizi, F. Broccoli Sprouts Powder Could Improve Serum Triglyceride and Oxidized LDL/LDL-Cholesterol Ratio in Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Diabetes Res. Clin. Pract. 2012, 96, 348–354. [Google Scholar] [CrossRef]
- Liu, P.; Dong, J. Protective Effects of Carnosic Acid against Mitochondria-mediated Injury in H9c2 Cardiomyocytes Induced by Hypoxia/Reoxygenation. Exp. Ther. Med. 2017, 14, 5629–5634. [Google Scholar] [CrossRef]
- Yoshida, H.; Mimura, J.; Imaizumi, T.; Matsumiya, T.; Ishikawa, A.; Metoki, N.; Tanji, K.; Ota, K.; Hayakari, R.; Kosaka, K.; et al. Edaravone and Carnosic Acid Synergistically Enhance the Expression of Nerve Growth Factor in Human Astrocytes under Hypoxia/Reoxygenation. Neurosci. Res. 2011, 69, 291–298. [Google Scholar] [CrossRef]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective Effect of the Antioxidant Curcumin: Recent Findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef] [Green Version]
- Ghoneim, A.I.; Abdel-naim, A.B.; Khalifa, A.E.; El-denshary, E.S. Protective Effects of Curcumin against Ischaemia/Reperfusion Insult in Rat Forebrain. Pharmacol. Res. 2002, 46, 273–279. [Google Scholar] [CrossRef]
- Thiyagarajan, M.; Sharma, S.S. Neuroprotective Effect of Curcumin in Middle Cerebral Artery Occlusion Induced Focal Cerebral Ischemia in Rats. Life Sci. 2004, 74, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, Y.; Zheng, W.; Lu, Y.; Feng, G.; Yu, S. Neuroprotective Effect of Curcumin on Transient Focal Cerebral Ischemia in Rats. Brain Res. 2008, 1229, 224–232. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, A.Y.; Simonyi, A.; Jensen, M.D.; Shelat, P.B.; Rottinghaus, G.E.; MacDonald, R.S.; Miller, D.K.; Lubahn, D.E.; Weisman, G.A.; et al. Neuroprotective Mechanisms of Curcumin against Cerebral Ischemia-Induced Neuronal Apoptosis and Behavioral Deficits. J. Neurosci. Res. 2005, 82, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qiao, Z.; Xu, Y. Protective Effect of Curcumin against Myocardium Injury in Ischemia Reperfusion Rats. Pharm. Biol. 2017, 55, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Na, L.-X.; Li, Y.; Pan, H.-Z.; Zhou, X.-L.; Sun, D.-J.; Meng, M.; Li, X.-X.; Sun, C.-H. Curcuminoids Exert Glucose-Lowering Effect in Type 2 Diabetes by Decreasing Serum Free Fatty Acids: A Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Ž.; Majeed, M.; Sahebkar, A. Curcuminoids Modify Lipid Profile in Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Complement. Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of Atherogenic Risk in Patients with Type 2 Diabetes by Curcuminoid Extract: A Randomized Controlled Trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano Curcumin Supplementation Reduced the Severity of Diabetic Sensorimotor Polyneuropathy in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo- Controlled Clinical Trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The Effect of Curcumin Supplementation on Anthropometric Indices, Insulin Resistance and Oxidative Stress in Patients with Type 2 Diabetes: A Randomized, Double-Blind Clinical Trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wang, H.; Ding, K.; Zhang, L.; Wang, C.; Li, T.; Wei, W.; Lu, X. Luteolin Provides Neuroprotection in Models of Traumatic Brain Injury via the Nrf2-ARE Pathway. Free Radic. Biol. Med. 2014, 71, 186–195. [Google Scholar] [CrossRef]
- Yang, D.; Tan, X.; Lv, Z.; Liu, B.; Baiyun, R.; Lu, J.; Zhang, Z. Regulation of Sirt1/Nrf2/TNF-α Signaling Pathway by Luteolin Is Critical to Attenuate Acute Mercuric Chloride Exposure Induced Hepatotoxicity. Sci. Rep. 2016, 6, 37157. [Google Scholar] [CrossRef]
- Lin, C.-W.; Wu, M.-J.; Liu, I.Y.-C.; Su, J.-D.; Yen, J.-H. Neurotrophic and Cytoprotective Action of Luteolin in PC12 Cells through ERK-Dependent Induction of Nrf2-Driven HO-1 Expression. J. Agric. Food Chem. 2010, 58, 4477–4486. [Google Scholar] [CrossRef]
- Kitakaze, T.; Makiyama, A.; Samukawa, Y.; Jiang, S.; Yamashita, Y.; Ashida, H. A Physiological Concentration of Luteolin Induces Phase II Drug-Metabolizing Enzymes through the ERK1/2 Signaling Pathway in HepG2 Cells. Arch. Biochem. Biophys. 2019, 663, 151–159. [Google Scholar] [CrossRef]
- Malhotra, A.; Bath, S.; Elbarbry, F. An Organ System Approach to Explore the Antioxidative, Anti-Inflammatory, and Cytoprotective Actions of Resveratrol. Oxidative Med. Cell. Longev. 2015, 2015, e803971. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Zhu, M.; Zhang, Q.; Wang, X.; Wang, Y.; Zhang, J.; Li, J.; Yang, L.; Liu, J.; et al. Resveratrol Attenuates Myocardial Ischemia/Reperfusion Injury through up-Regulation of Vascular Endothelial Growth Factor B. Free Radic. Biol. Med. 2016, 101, 1–9. [Google Scholar] [CrossRef]
- Cheng, A.-S.; Cheng, Y.-H.; Chiou, C.-H.; Chang, T.-L. Resveratrol Upregulates Nrf2 Expression to Attenuate Methylglyoxal-Induced Insulin Resistance in Hep G2 Cells. J. Agric. Food Chem. 2012, 60, 9180–9187. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Jang, J.-H.; Li, M.-H.; Surh, Y.-J. Resveratrol Upregulates Heme Oxygenase-1 Expression via Activation of NF-E2-Related Factor 2 in PC12 Cells. Biochem. Biophys. Res. Commun. 2005, 331, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Song, W.; Wang, Z.; Wang, Z.; Jin, X.; Xu, J.; Bai, L.; Li, Y.; Cui, J.; Cai, L. Resveratrol Attenuates Testicular Apoptosis in Type 1 Diabetic Mice: Role of Akt-Mediated Nrf2 Activation and P62-Dependent Keap1 Degradation. Redox. Biol. 2018, 14, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Chengyong, T.; Cheng, L.; Haixia, H.; Yuanda, Z.; Weihua, Y. Resveratrol Attenuates the Cytotoxicity Induced by Amyloid-Β1-42 in PC12 Cells by Upregulating Heme Oxygenase-1 via the PI3K/Akt/Nrf2 Pathway. Neurochem. Res. 2018, 43, 297–305. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Leal, V.O.; Rizzetto, F.; Grimmer, G.H.; Ribeiro-Alves, M.; Daleprane, J.B.; Carraro-Eduardo, J.C.; Mafra, D. Effects of Resveratrol Supplementation in Nrf2 and NF-ΚB Expressions in Nondialyzed Chronic Kidney Disease Patients: A Randomized, Double-Blind, Placebo-Controlled, Crossover Clinical Trial. J. Ren. Nutr. 2016, 26, 401–406. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; García-Almagro, F.J.; Avilés-Plaza, F.; Parra, S.; Yáñez-Gascón, M.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; et al. Consumption of a Grape Extract Supplement Containing Resveratrol Decreases Oxidized LDL and ApoB in Patients Undergoing Primary Prevention of Cardiovascular Disease: A Triple-Blind, 6-Month Follow-up, Placebo-Controlled, Randomized Trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Raj, P.; Nabipour, I.; Mahmoodi, M.; Ostovar, A.; Kalantarhormozi, M.; Netticadan, T. Efficacy and Safety of Resveratrol in Type 1 Diabetes Patients: A Two-Month Preliminary Exploratory Trial. Nutrients 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Cheng, Z.; Quan, X.; Xie, Z.; Zhang, L.; Ding, Z. Dimethyl Fumarate Protects Cardiomyocytes against Oxygen-Glucose Deprivation/Reperfusion (OGD/R)-Induced Inflammatory Response and Damages via Inhibition of Egr-1. Int. Immunopharmacol. 2020, 86, 106733. [Google Scholar] [CrossRef] [PubMed]
- Linker, R.A.; Lee, D.-H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric Acid Esters Exert Neuroprotective Effects in Neuroinflammation via Activation of the Nrf2 Antioxidant Pathway. Brain 2011, 134, 678–692. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).