Abstract
Parasitic angiosperms, comprising a diverse group of flowering plants, are partially or fully dependent on their hosts to acquire water, mineral nutrients and organic compounds. Some have detrimental effects on agriculturally important crop plants. They are also intriguing model systems to study adaptive mechanisms required for the transition from an autotrophic to a heterotrophic metabolism. No less than any other plant, parasitic plants are affected by abiotic stress factors such as drought and changes in temperature, saline soils or contamination with metals or herbicides. These effects may be attributed to the direct influence of the stress, but also to diminished host availability and suitability. Although several studies on abiotic stress response of parasitic plants are available, still little is known about how abiotic factors affect host preferences, defense mechanisms of both hosts and parasites and the effects of combinations of abiotic and biotic stress experienced by the host plants. The latter effects are of specific interest as parasitic plants pose additional pressure on contemporary agriculture in times of climate change. This review summarizes the existing literature on abiotic stress response of parasitic plants, highlighting knowledge gaps and discussing perspectives for future research and potential agricultural applications.
1. Introduction to Parasitic Flowering Plants
Parasitic flowering plants comprise a group of an estimated 4000 species in more than 20 plant families, or approximately 1.5% of the known vascular plant species [1]. These highly specialized plants are characterized by partial or complete loss of photosynthetic ability and depend on their hosts for photosynthates, mineral nutrients and water [2]. Parasitic plants are classified into two major categories. Hemiparasites contain chlorophyll and are able to photosynthesize. They obtain water and mineral nutrients from the host. Holoparasites are non-photosynthetic. They are obligate parasites, depending completely on the host [3,4]. Of all parasitic plants, relatively few species—less than 400—are holoparasites [5]. Parasitic plants attach to the host plants and absorb nutrients through haustoria, well-defined structural and physiological links with the host. Haustoria may vary in structure among parasitic plant families but remain a common feature of all of them (see [6] for a comprehensive overview of different haustoria types). Despite several anatomical and developmental differences, haustoria establish direct connection between the xylem and/or the phloem of the host and the parasite, to provide bidirectional flow of water, minerals and macromolecules including proteins, mRNAs [7,8] and genetic material, enabling horizontal gene transfer [9,10]. To facilitate this flow, parasitic plants tend to maintain lower water potential and higher transpiration rates in comparison to their hosts. As discussed below, the haustorial connection may also contribute to the exchange of stress-responsive molecules and potentially harmful compounds such as heavy metals. Depending on the attachment site, parasitic plants are categorized into root and stem parasites [6]. Throughout the literature, the term shoot parasites is synonymously used for stem parasitic plants [11]. Stem parasites are plants that attach and form haustoria into aerial host organs—either stem, leaf petiole or leaf surface.
Plant-plant parasitism evolved independently at least 12 or 13 times [1], facilitated by the formation of haustoria. Simultaneously, the transition from hemiparasitism to holoparasitism resulted in significant gene loss, especially in the plastid genome [2]. The mitochondrial genome was also subject to gene loss in at least some parasitic plants [12]. Compared to around 160 kbp of the fully functional tobacco plastome, some parasitic plants may have as little as 11–15 kbp [13] or even none [14].
Some parasitic plants are significant agricultural pests. Several species of dodders (Cuscuta spp.), witchweed (Striga spp.) and broomrapes (Orobanche spp.) cause serious damage to crop plants worldwide [15]. Dodders parasitize and cause yield losses mainly of alfalfa (Medicago sativa L.) and sugar beet (Beta vulgaris L.) but also other eudicot crop plants such as carrot (Daucus carota (Hoffm.) Schübl. & G. Martens), pepper (Capsicum annuum L.) or potato (Solanum tuberosum L.), etc. Orobanche spp. infect a variety of eudicot crops including carrot, sunflower, legumes and several species in the Solanaceae. Several Orobanche species cause significant agricultural losses and can be responsible for over 50% yield reduction, especially in combination with drought [15]. Another member of the Orobanchaceae, Striga spp., and especially Striga hermonthica (Delile) Benth., are major parasites of cereal crops and could account for annual losses of over $1 billion globally [16]. Research efforts are largely focused on identifying host resistance mechanisms [17], selection of parasite-resistant varieties of existing crop plants [18,19] and development of efficient control strategies [20]. However, parasitic plants are also important members of natural plant communities, which they can shape to a significant degree [21]. By selectively foraging on heterogeneous plant communities, parasitic plants suppress the growth of certain plant species, which benefits others and impacts on biodiversity [22,23].
The parasitic plant-host interactions, both in natural habitats and agricultural lands, is likely affected by abiotic stress. Abiotic stress factors, i.e., stress factors resulting from non-living factors [24], generally affect plant life, shape ecosystems and decrease agricultural production globally. Major abiotic stress factors are water stress, including drought and flooding, fluctuations or extremes of temperature or irradiation, salinity, mineral deficiency and toxic substances such as heavy metals, air pollutants or pesticides. Worldwide, approximately 96.5% of land area is affected by individual or multiple abiotic stress factors to a different extent [25], restricting or modifying plant growth and development. Parasitic plants are no less affected by abiotic stress factors, either directly or through the host. The aim of the present review is to summarize the scarce existing reports on the effects of abiotic stress on parasitic plants and the interaction with their hosts, highlighting the need for further research to (1) increase basic knowledge of host-parasite interactions under abiotic stress and (2) to better understand the potentially combined effects of abiotic stress in conjunction with parasite pressure on agriculture.
2. Possible Effects of Abiotic Stress on Parasitic Plants
Parasitic plants may be affected by abiotic stress factors in a similar way as their hosts, e.g., by constraints of seed germination and seedling development due to drought and/or salinity, or indirectly, i.e., due to host-related constraints. The latter is especially true for holoparasites, most of which have no or only limited soil contact and lack photosynthesis. The possible effects of abiotic stress on host-parasitic plant interactions are summarized in Figure 1.
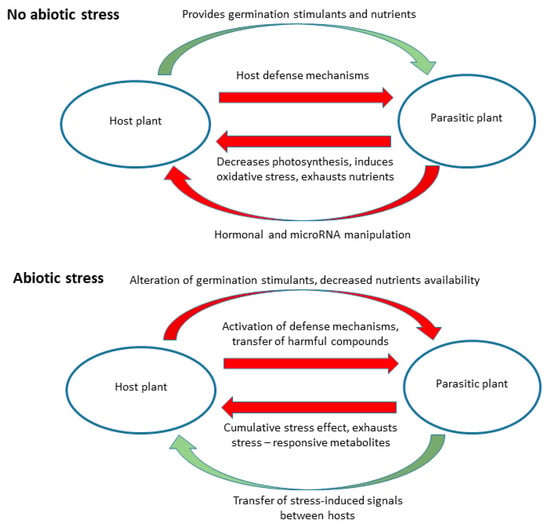
Figure 1.
Possible effects of abiotic stress factors on the interactions between parasitic plants and their hosts. Green arrows represent beneficial interaction and red arrows represent harmful effects.
2.1. Effects through Alteration of Germination Stimulants, Released by the Host
An important adaptive strategy of root holoparasites is seed dormancy, preventing germination and seedling emergence in the absence of a suitable host. Germination occurs only in the presence of specific chemical compounds released by a potential host plant [26]. The best-studied germination stimulants are the strigolactones, confirmed to be essential for germination of members of the Orobanchaceae family, Striga spp. and Orobanche spp. [27]. For comprehensive review of the roles of strigolactones on host-parasitic plant interactions see Yoneyama et al. [28] and Teofanova et al. [29]. In brief, strigolactones, which are derived from carotenoids, are released by host roots to attract arbuscular mycorrhizal fungi but are also perceived by seeds and seedlings of parasites. In some cases, the level of recognition is so specialized that only a particular strigolactone is able to induce germination. For example, whereas several strigolactones, released by a wide variety of hosts, stimulate germination of multiple Orobanche spp., O. cumana is insensitive to them and requires dehydrocostus lactone, released by sunflower roots to germinate [30].
Strigolactone synthesis and release is needed for and regulated by arbuscular mycorrhiza symbiosis but also by different abiotic stress factors quantitatively and qualitatively. Numerous reports indicated negative effects of both salt and drought stress on strigolactone biosynthesis and release into root exudates, although strigolactone synthesis increased under stress in the presence of the fungal symbiotic partner [31,32]. Mycorrhizal symbiosis decreased strigolactone production under optimal conditions in tomato roots [33], impacting negatively on Orobanche ramosa seed germination. Negative effects of mycorrhizal symbiosis were also found in various Orobanche spp. parasitizing pea [34]. By contrast, the combination of salt stress and mycorrhizal symbiosis of the potential host improved germination of parasite seeds. This was clearly shown for the interaction between Lactuca sativa L. (host)–O. ramosa (parasitic plant)–Glomus intraradices Schenk and Smith (mycorrhizal fungus) [31]. Therefore, the release of signaling molecules, and particularly strigolactones, appears to depend on both abiotic and biotic factors and strongly affects at least some parasitic plants.
2.2. Host Biomass and Health Status
Parasitic plants are highly dependent on host availability, and abiotic stress factors potentially reduce this availability. Therefore, host selection is of crucial importance to the success of parasitic plants under non-optimal conditions. The impressive seed dormancy and longevity of root holoparasites [35], in which the lack of seed storage compounds and its own photosynthates does not allow seedling growth in the absence of a suitable host, might be of crucial importance to cope with the issue of host selection. Apart from germination stimulants, some parasitic plants, such as dodders, appear to have highly specialized mechanisms of host localization and selectivity. It was suggested that members of the Cuscuta genus use both chemical signals, such as the terpenoids α-pinene and β-myrcene [36] and light signals [37] to sense their potential hosts. Cuscuta campestris seedlings were shown to selectively infest hosts with high chlorophyll contents, which they detect via the low-red to far-red ratio of transmitted light [37]. In the salt-sensitive model plant Arabidopsis thaliana (L.) Heynh, chlorophyll contents decreased when the plant was grown under saline conditions as compared to its salt-tolerant relative Eutrema halophilum (C.A. Mey.) Al-Shehbaz & S.I. Warwick [38]. Consequently, it could be assumed that a parasite would preferentially attach to a stress-tolerant host, which would produce more photosynthates, and thus more biomass. Some reports confirm this assumption, e.g., dodders appear to be able to sense host quality, possibly via multiple mechanisms, and avoid unsuitable ones even before the formation of haustoria [39].
However, host “quality” may not simply depend on biomass availability. When exposed to abiotic stress factors such as drought and salinity, hosts can accumulate higher concentrations of compatible solutes, potentially resource-rich substrates for parasites, which may even enhance host quality compared to non-stressed hosts [40]. According to the case study of the Arabidopsis–Eutrema pair under salt stress, the salt-tolerant Eutrema showed much better relative growth rates (e.g., more biomass available to a potential parasite) under saline conditions [41]. However, treatment with up to 200 mM NaCl led to much higher concentrations of leaf proline in Arabidopsis. Higher NaCl concentrations proved detrimental to Arabidopsis, whereas Eutrema still grew on substrate containing up to 500 mM NaCl, with a gradual increase in proline contents. Therefore, it appears that a stress-sensitive host may be of better “quality” at lower stress levels and becomes a “worse” host or does not grow (e.g., is not available) when stress levels increase with higher salt concentrations.
2.3. Effects of the Host Defense System
As do pathogens and herbivores, parasitic plants also induce defense mechanisms in the parasitized host. The best-studied responses involve jasmonic acid (JA) and salicylic acid (SA)-induced systemic acquired resistance (SAR) responses, also involving the expression of pathogenesis related (PR) genes [42]. In addition, abscisic acid (ABA)-mediated responses [17] were also established. The same hormones are also important players response to abiotic stress factors, thus accounting for several response mechanisms common to both abiotic and biotic stresses [43]. Evidence is accumulating that plants, weakened by abiotic stress factors become more vulnerable to biotic stress factors [44,45], yet to be confirmed for the interaction between hosts and parasitic plants. Conversely, under conditions of abiotic stress, parasitic plants could face potential hosts with already activated SAR. Several reports support this view that abiotic stress-induced responses could render potential host plants less susceptible or even insensitive to parasitic plant infection. Treatment of Beta vulgaris with NaCl had a dose-dependent effect on Cuscuta salina fecundity [40], suggesting that under moderate salinity the defense mechanisms of the host were harmful to the parasite, whereas at more saline conditions the parasite was able to be more successful despite its lower germination rate and lower host biomass availability. However, the abiotic stress resistance of certain cultivars may also be related to resistance to parasitic plants. This was shown in salt-tolerant Vicia faba L. cultivars, whose tolerance correlated with their resistance to Orobanche [46]. Resistance to parasitic plants is often related to decreased cell wall permeability, induced by protein cross-linking and callose and suberin deposition [47], factors that are also induced by abiotic stress factors. Therefore, at least in some cases, greater abiotic stress tolerance may result in enhanced resistance to parasitic plants due to the upregulation of defense mechanisms shared between abiotic and biotic stress responses. This assumption is supported by the abiotic stress-induced pathogen resistance reported for barley [48]. Cross-tolerance was mediated by a variety of SA, JA, ethylene, ABA and auxin-mediated signaling pathways and/or redox signaling, which, induced by a single stress factor, lead to enhanced resistance to multiple biotic and abiotic factors [49]. Therefore, for an assessment of the effects of the host’s defense mechanisms, host and parasite species and the specific circumstances need to be carefully considered.
2.4. Transmission of Harmful Compounds from Host to Parasite
The haustorial connection between parasitic plants and their hosts is a site of extensive, bidirectional exchange of water, mineral nutrients, organic compounds and macromolecules [8], although a certain degree of selectivity exists [6,50]. The capability to tap into the nutrient supply of the host strongly depends on the lower water potential of the parasite, which in turn depends on the concentrations of particular compounds in the host tissue [6]. Together with the translocation of nutrients from host to parasite, potentially harmful substances, or even pathogens, may also move through haustoria and will cause stress in the parasite. For example, herbicides were found to be transmitted by the host to Cuscuta campestris [51]. Other reports on potentially harmful compounds transmitted from the host to the parasite include those on heavy metals [52,53] as well as Na and Cl ions in the case of salt stress [54]. Recently, selectivity for transport of mineral elements through the haustoria was demonstrated in Cuscuta reflexa Roxb. [50], which suggests that some parasites might be able to exclude harmful elements. The accumulation of harmful elements in the parasite seems to be dependent on host species and environmental conditions (Table 1). In summary, a parasitic plant can experience abiotic stress factors indirectly, through the host.

Table 1.
Examples of parasitic plants and their respective hosts growing in environments characterized by abiotic stress pressure such as low temperatures, water deficiency and high soil salinity.
3. Response to and Tolerance of Abiotic Stress Factors in Parasitic Plants
Assuming that the distribution of parasitic plants is defined by the availability of suitable hosts [64], it could be expected that parasitic plants are present mainly under favorable conditions. Indeed, the diversity of parasitic plant species is far greater in tropical and temperate climates and decreases significantly towards the sub-polar regions and in arid lands (for comprehensive overview see [65]). However, many parasitic plants are found in saline coastal lands, deserts and alpine or arctic climates, which are largely defined by the presence of stress tolerant hosts, adapted to grow and reproduce under suboptimal conditions. Table 1 provides an overview of parasitic plants occurring in extreme environments.
3.1. Drought Stress
Xerophytic root and stem parasites occur in numerous arid areas. The Sonoran Desert in Mexico and South-Western USA is especially rich in unique parasites [65]. Cistanche phelypaea (L.) Cout. is found in sand dunes in the Arabian Peninsula and Amyema fitzgeraldii (Blakely) Danser is known from arid areas of Western Australia. There are many examples of parasites occurring on xerophytic hosts including cacti and euphorbias, e.g., Plicosepalus acaciae (Zucc.) Wiens & Polhill parasitizing on Euphorbia cactus Ehrenb. ex Boiss, but only a few parasites were tested for xerophytic features themselves [65]. The mistletoe Tristerix aphyllus (DC.) Barlow & Wiens [66] is a cacti specialist. These examples are of rare, specifically adapted parasites without substantial agricultural impact, but evidence of economically important weeds such as Orobanche cernua, parasitizing on xerophytes, also exists [57].
When considering the effects of drought stress on parasitic plants, it is important to note that especially holoparasites acquire water mainly from the host. Therefore, with the exception of seed germination and early seedling growth towards the host, the effects of drought on parasitic plants are mainly indirect, through the host. As expected, decreasing water potential affects both germination and early seedling growth negatively (Figure 2), shown for the root parasites Orobanche crenata [67], Striga hermonthica and Alectra vogelii Benth. [68]. However, Gibot-Leclerc [69] reported low sensitivity of Orobanche ramosa seeds to low water potential, similar to Orobanche aegyptiaca [70]. These apparent contradictions may be due to the different experimental conditions used in the above studies and care should be taken in the interpretation of the results. However, if these contradictory results could be confirmed, one might speculate that root parasites can germinate under limited water availability in the presence of a suitable host, allowing them to grow readily after infection. This possibility is supported by work reporting that the release of strigolactones from the host, needed for parasite seed germination, also altered by drought stress and can increase in mycorrhized and decrease in non-mycorrhized plants [32]. Furthermore, a recent report identified the OaMAX2 gene in Orobanche aegyptiaca as a potential candidate to confer drought tolerance to the parasite [71]. Considering that the product of OaMAX2 is part of the strigolactone signaling pathway, it seems that the host availability simultaneously triggers germination and drought response in this parasite, common in arid lands.
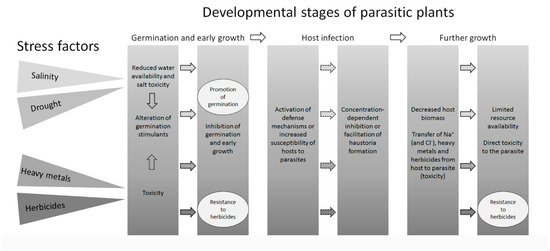
Figure 2.
Summary of the effects of abiotic stress factors on different developmental stages of parasitic plants. Main stress factors and their effects are shown in different shading (not reflecting their severity). Dotted arrows depict suggested effects (to be confirmed experimentally). Ellipses denote exceptions to the rule. Furthermore, no reports were found that confirm that heavy metals or herbicides alter the production of strigolactones, but such an effect is not unlikely. No reports on the effects of abiotic stress factors on haustoria formation were found. Topical literature is cited in the text.
Another parasitic plant, the stem parasite Cuscuta australis R. Br., was reported to be insensitive to abscisic acid-mediated drought stress responses due to lost or non-functional ABA receptors [72]. In response to exogenous ABA, the parasite did not show inhibition of germination or suppression of hypocotyl elongation, defense mechanisms, observed in non-parasitic plants. This might be detrimental under drought but also ensures that even under suboptimal conditions the parasite will be able to grow towards potential hosts. On the other hand, Qin et al. [73] reported a several-fold increase in ABA content of dehydrated stems of Cuscuta reflexa, very similar to an ordinary response of non-parasitic plants. It remains to be clarified whether ABA-related response is species-specific within the Cuscuta genus. Nonetheless, stem parasitic plants may be indirectly affected by drought stress incurred by their hosts, which can decrease a host’s growth rate, thereby limiting the resources available to the parasite, as reported for the stem parasite Cuscuta gronovii Willd. ex Schult.-Verbesina alternifolia (L.) Britton ex Kearney [74] and Amyema miquelii (Lehm. ex Miq.) Tiegh.-Eucalyptus largiflorens F.Muell. [75] parasite-host pairs. The availability of drought-adapted hosts, however, means that parasitic plants could successfully thrive under drought conditions and may not need to evolve their own xerophytic features.
3.2. Salt Stress
Parasitic plants also occur in saline coastal areas. At least four Cuscuta species are known to thrive under saline conditions: C. sandwichiana Choisy in Hawaii, C. tasmanica Engelm. in Tasmania and Southern Australia [76], C. europaea L. spp. halophyta (Fr.) Hartm. in Russia and Southern Scandinavia and C. salina Engelm. in North America. The latter mainly parasitizes on halophytes such as Salicornia virginica L. and Frankenia salina (Molina) I. M. Johnst., although it is not restricted to them [77]. A similar report highlighted the importance of Cuscuta salina for suppression of dominant plant species, benefitting rare species in coastal salt marshes, thus increasing biodiversity [23]. Recently, the North American dodder species Cuscuta campestris was also found on several occasions between 2018 and 2021 in sand dunes in the Bulgarian coastal area, showing an ability to adapt to salinity in its growth environment as well as drought (Figure 3). Hosts included Centaurea arenaria Willd., Peucedanum obtusifolium Sm. and Medicago marina L. This is posing a serious concern as this introduced, invasive species may be significantly harmful to the vulnerable ecosystems of coastal areas. Plicosepalus acaciae was found to infect both halophytes and glycophytes with equal success [54]. Among the root parasites, Cynomorium coccineum L. is an example of a typical halophytic parasitic plant of the Mediterranean [78].

Figure 3.
Cuscuta campestris, parasitizing Centaurea arenaria on a sand dune. Bulgarian Black Sea coast, 42°13′46″ N; 27°46′32″ E, July, 2018. White arrows show (1) the parasite stems, (2) site of infection and (3) inflorescence.
Salt stress leads to a wide range of physiological and biochemical changes, which may differ significantly between salt-sensitive and salt-tolerant species. These include mineral imbalance, mostly altered Na+/K+ ratios, osmotic stress, elevated rates of reactive oxygen (ROS) production and redox changes, alongside the accumulation of compatible solutes as sugars, polyols and free amino acids such as proline [79]. Although parasitic plants have limited (root parasites) or absent (stem parasites) soil contact, they are affected by salt stress indirectly, via the metabolism of the host (Figure 2). Some parasites may prefer stressed hosts, which accumulate low-molecular-weight compounds, making them a rich source of nutrients [40]. Others may not discriminate between salt-tolerant and salt-sensitive hosts and grow on whatever is available, e.g., Plicosepalus acaciae (mistletoe) [54].
Salinity appears to affect parasitic plants on three main levels (Figure 2): firstly, directly in the seed germination phase; secondly, indirectly through changes in host signaling, affecting seed germination of the parasitic plant; and thirdly, indirectly through the effects of salinity on host susceptibility to parasitism. Various authors showed that salt stress significantly inhibited seed germination of parasitic plants, for example in Orobanche cernua [80], Orobanche minor, Orobanche crenata and Striga hermonthica [81]. As outlined above, in parasitic plants seed germinability may also depend strongly on the concentration of germination stimulants. Addition of the synthetic strigolactone analogue GR24 partially alleviated the negative effects of 50 mM and 75 mM NaCl on Orobanche minor germination [81]. Intricate interactions exist between the effects of salt concentration on seed germination of parasitic plants and arbuscular mycorrhizal fungi which suppress the production of strigolactones once the symbiosis is established. Mycorrhized Lactuca sativa plants were able to adapt better to salinity, and in the absence of NaCl, their root exudates were less inductive to Orobanche ramosa seed germination. In turn, in the presence of NaCl, exudates stimulated seed germination of Orobanche ramosa in a similar way as in non-mycorrhized plants [31].
It is also poorly studied if parasitic plants grow well on salt-stressed hosts. Demirbas reported increased susceptibility of Arabidopsis to Orobanche ramosa infection at 50 mM NaCl [82]. In contrast, Al-Khateeb [83] reported significantly reduced infection of Lycopersicon esculentum plants by Orobanche cernua at 50 mM and completely absent infection at 75 mM NaCl. In Vicia faba, several salt-tolerant cultivars were also found to be resistant to Orobanche crenata infection [46]. The growth of Cuscuta campestris, infecting salt stressed Arabidopsis plants was reduced by nearly 50% already at 50 mM NaCl [84]. Still, little is known about how the parasites respond to salinity. In terms of compatible solutes, some members of the Orobanchaceae family were found to synthesize polyols (mainly mannitol) [85], whereas mistletoes were able to actively absorb polyols from the host and develop a host-specific polyol profile [86]. In the case of Cuscuta campestris–an Arabidopsis parasite-host pair—, it was found that at higher salt concentrations the parasite accumulated L-proline, accompanied by decreased concentrations in the host compared to non-infected plants [84]. Despite plant response to salinity being widely studied and numerous mechanisms being well understood [87], these mechanisms have been poorly studied or not studied at all in parasitic plants.
3.3. Heavy Metal Stress
Heavy metals are among the most toxic compounds that affect plant metabolism and are major pollutants in arable lands, especially in areas with industrial manufacturing and mining. As reviewed elsewhere [88], plant response to heavy metals varies greatly; some plants are extremely sensitive, some show a degree of resistance and others can even accumulate or hyperaccumulate certain elements such as cadmium [89], lead [90] and nickel [91]. With regard to parasitic plants important questions are (1) whether parasitic plants acquire heavy metals from their hosts; (2) if so, whether they are able to selectively exclude at least some of them; (3) whether parasitic plants have their own protection mechanisms; and (4) whether parasitic plants comprise a potential threat to bioremediation of soils by suppressing the growth of plant hyperaccumulators.
The scarce publications available are mostly case studies rather than systematic approaches to answer these questions. An additional concern is the medicinal value of numerous parasitic plants, extracts of which are used as herbal remedies. In 2013 in Pakistan, the root parasites Cistanche tubulosa (Schenk) Wight and Orobanche ramosa were tested for their heavy metals load and, compared to several other medicinal plants [92], showed the highest concentrations of Zn, Co and Mn. According to this study, parasitic plants accumulated higher concentrations of heavy metals than all other plants studied. The host range from which the two parasites were collected was not specified, which makes it impossible to assess whether heavy metal accumulation by parasitic plants is host-specific, and it remains unclear whether direct acquisition from soil occurs.
A more detailed study of the host-root hemiparasite pair Cistus spp.–Odontites lutea Clairv. [52] contradicted earlier reports [93] that Odontites spp. are sensitive to heavy metals and do not occur on metalliferous soils. In polluted areas, this hemiparasite accumulated significantly lower Fe and Zn concentrations, but equal or slightly higher concentrations of Cu and Pb (Table 2). This apparent selectivity to heavy metal uptake was also reported in the stem parasite Cuscuta campestris on Daucus carota, where similar or higher concentrations of Zn and Cu and exclusion of Cd were found [53]. A striking host-specific heavy metal accumulation was reported for Cuscuta californica Hook. & Arn., parasitizing Ni hyperaccumulator Streptanthus polygaloides A. Gray and the non-accumulating host Lessingia nemaclada Greene [94]. The Ni concentration in the parasitic plant was considerably higher when the parasite grew on the hyperaccumulating host (compared to a non-hyperaccumulating host), but lower than that of the host itself (Table 2). Copper and Cr concentrations were equal to the respective value in the hosts. However, Co and Pb accumulated to higher values than in the host when parasitizing the non-accumulating host and lower than in the host when parasitizing the Ni hyperaccumulator. Clearly, the lack of extensive studies does not allow a conclusive overview. So far, it can be concluded that the transfer of heavy metals from the host to the parasite seems to be host- and element-specific, also depending on the parasitic plant species.

Table 2.
Transfer of various elements from hosts to parasitic plants. No simple relationship exists for the proportion of chemical elements found in hosts and parasites: according to the scarce data available in the literature, a certain element in the same parasite can apparently range from less than 10% of the host concentration up to almost 1000-fold, corresponding to a ratio of concentration in the parasite divided by the concentration in the host of 0.1 to almost 10.
The response of parasitic plants to heavy metals is poorly understood. One of the first papers on heavy metal toxicity in parasitic plants reported that six heavy metals tested on Cuscuta reflexa had detrimental effects above 0.5 µg mL−1 [95]. Other papers showed effective heavy metal detoxification in the parasitic Euphrasia spp., but high sensitivity of Odontites spp. and Rhinanthus spp. [93]. More recently, Cd was shown to be toxic to in vitro cultures of Cuscuta reflexa, with significant inhibition of growth, shoot length and seed germination [96].
In plants, phytochelatins (PC) confer protection from heavy metal toxicity [97], and their synthesis was reported in Cuscuta spp. on several occasions. When Cuscuta reflexa was exposed to Cd, the activities of catalase, peroxidase and glutathione reductase increased up to a concentration of 300 µM and decreased at 500 µM Cd [96]. Phytochelatin synthesis occurred only in Cd-treated callus cultures and seedlings and increased dramatically at higher concentrations, up to 7-fold at 500 µM Cd, in seedlings. Furthermore, constitutive expression of PC synthase and increase in PC concentrations upon exposure to low Cd concentrations (36 µM) was reported in Cuscuta campestris on Daucus carota [53]. The authors suggested a substantial role for phytochelatins not only in heavy metal detoxification, but also in the homeostasis of essential metals in Cuscuta spp.—a challenging task for a parasite—, which is entirely dependent on the host for the acquisition of mineral nutrients. As there is extensive molecular trafficking between hosts and parasites [8,98] it is not unlikely that phytochelatins or other thiol compounds are transferred from the host to the parasite, but we did not find any reports on this topic.
Finally, the potential role of heavy metals to confer resistance to hyperaccumulating hosts against parasitic plants was also studied. The “elemental defense hypothesis”, reviewed by Poschenrieder [99], suggests that heavy metals protect plants against herbivores, fungal and bacterial pathogens, a potential evolutionary advantage for hyperaccumulators. Apparently, at least some parasitic plants are not affected by heavy metals in their hyperaccumulating hosts. As mentioned above, Cuscuta californica successfully infested the Ni hyperaccumulator Streptanthus polygaloides [94]. Similarly, the root parasite Orobanche nowackiana Markgr. was found to be a major pest on another Ni hyperaccumulator, Alyssum murale Waldst. & Kit. [100]. Moreover, some parasitic plants like Orobanche lutea may even benefit from heavy metal pollution and thrive better in such areas, simultaneously providing beneficial unload of toxic elements from the host [101], as such areas are characterized by lower competition and heavy metal accumulation provides herbivore defence [102]. More research is needed before informed conclusions can be drawn regarding the role of heavy metals in the interaction between hosts and parasitic plants, and the knowledge gained could benefit bioremediation programs.
3.4. Herbicide Resistance
Herbicide treatment is one of the oldest approaches to control parasitic plants in arable lands, unfortunately with questionable effectiveness. Attempts to control parasitic plants include the application of systemic herbicides at doses that are non-lethal to crops, treatment with substances that specifically target the parasitic plant species and the use of herbicide-resistant transgenic crops. The herbicide might be preferably applied immediately after germination or the initial attachment of the parasite and/or it must be transferred from the host to the parasite before being detoxified [103]. The efforts to combat parasitic plants are enormous [20], considering that the economic losses, caused by Striga spp. only account for $7 billion per year in Sub-Saharan Africa alone [104].
Commonly used herbicides can be effectively applied to control the root parasites Striga spp. and Orobanche spp. [105], but the stem parasites Cuscuta spp. are more challenging. Nadler-Hassar [51] showed that Cuscuta campestris is even more resistant to herbicides that inhibit amino acids biosynthesis than transgenic herbicide-resistant crop plants. Notably, the I50 value (defined as the rate in g ha−1, causing 50% reduction in tissue elongation) of glyphosate-treated Cuscuta was eight-fold higher than that of glyphosate-resistant cotton (Gossypium hirsutum L., cv. DP5415RR). Similar results were obtained for seven other herbicides. In another study, three Cuscuta species were shown to be more resistant to glyphosate and imazamox and equally resistant to glufosinate compared to either wild type or transgenic crop plants, with the exception of glufosinate-resistant oilseed rape, which showed several-fold higher I50 values when exposed to glufosinate than all Cuscuta species tested [106]. However, Cuscuta campestris was not substantially affected by glufosinate when parasitizing on glufosinate-resistant oilseed rape. A possible answer to this phenomenon may lie in the protein trafficking between hosts and parasites, as shown in the case of Cuscuta pentagona, parasitizing on transgenic soybean, where the glufosinate detoxifying phosphinothricin acetyl transferase enzyme appeared also in the parasite [107]. Thus, the parasitic plant acquired resistance from the host. It should be noted that such resistance could also be acquired through mRNA transfer into the parasite, not detected in the above study, but conceivable considering the extensive trafficking of RNAs [108] and through horizontal gene transfer, which is also common in parasitic plants [109].
4. Agricultural Aspects of Host-Parasite Interactions under Abiotic Stress
Parasitic plants exert major effects on the host by tapping into the host’s nutrients and photosynthates, thus restricting host growth and development. In addition, they may alter the photosynthetic performance of their hosts. Some reports showed that the host’s photosynthetic capacity is increased in order to compensate for the organic compounds sink from the host to the parasite [74]. By contrast, stomatal conductance, photosynthetic rates and carboxylation efficiency severely decreased in Mikania micrantha Kunth infected with Cuscuta campestris [110] and this effect was exacerbated by drought in the case of Cuscuta australis infection [111]. The negative effect of Orobanche ramosa infection on Lycopersicon esculentum was also largely attributed to the inhibition of photosynthesis rather than simple exhaustion of nutrients [112].
In a recent report we showed that Cuscuta campestris infection caused differential effects on different organs of the parasitized host plant [84]. The effects of infection on antioxidant enzymes were most pronounced at the infection site (direct effect), but also substantial in non-infected aerial parts and roots (indirect effects). Parasitism by Cuscuta further interfered with the host’s ability to accumulate osmoprotectant L-proline and to properly respond to salt stress. Similarly, the root parasite Orobanche aegyptiaca showed a negative effect on the salt stress response of the host, Lycopersicon esculentum [113].
The influence of parasitic plants on their hosts is not limited to simple exhaustion of nutrients. It was reported that parasitic plants could also manipulate host metabolism to serve their own needs. For examples, the root hemiparasite Phtheirospermum japonicum was found to transfer cytokinins into the host to cause root hypertrophy [114]. Moreover, Cuscuta spp. can actively transfer microRNAs into theirs hosts, targeting mRNAs in order to improve nutrient uptake [115,116,117]. So far, it remains unknown whether the pattern of host manipulation is affected by abiotic stress factors. However, evidence was provided that Cuscuta spp. may be responsible for transfer of stress signals between simultaneously infected hosts, thus affecting positively response to salt stress [118], which was also previously shown for herbivore-induced signals [119].
Crop infestation by parasitic plants is highly detrimental to agricultural plant communities, leading to severe yield reduction or even complete crop loss. It may exacerbate the effects of abiotic stress (and vice versa). For example, Vicia faba (fava bean), a major grain legume that is often cultivated on saline soils and irrigated with diluted sea water (e.g., in Egypt), is susceptible to Orobanche crenata infestation, which may lead to complete loss of yield [120]. Broomrapes also cause crop losses in areas characterized by arid and saline soils in Sub-Saharan Africa and Israel [121]. Fernández-Aparicio reported a case of acquired susceptibility of Lupinus albus L. (white lupin) to infection with Orobanche crenata when the crop plant was cultivated on alkaline soils [122]. In several cases, however, abiotic stress tolerance of selected cultivars coincided with insusceptibility to parasitic plant. Notable examples are salt- and broomrape-tolerant Vicia faba cultivars [46] and drought-tolerant and witchweed-resistant Zea mays L. cultivars [123]. Again, more research is needed to better understand the effects of abiotic stress factors on the interactions between host and parasitic plants, especially with a view to climate change, the ever-increasing threats of which to agricultural production can be exacerbated by parasitic plants.
5. Challenges and Outlook
The interpretation of data on parasitic plants generally, and with regard to their response to abiotic stress factors specifically, is challenging due to the wide spectrum of hosts studied. The situation is further complicated by the distinct features of root and stem parasites [1] and the apparent influence of symbiotic microorganisms in the case of root parasites [31]. In order to acquire large sets of comparable data on the response of parasitic plants to abiotic stress factors, a well-established model system needs to be developed. Evidence is emerging that Arabidopsis thaliana may be a suitable, albeit not common, host plant to study plant-plant parasitism. Arabidopsis is susceptible to many Orobanche spp. [82,124,125,126,127,128] and Cuscuta spp. [129,130] and could be used to study interactions between parasitic plants and their hosts regarding germination stimulants, haustoria-inducing factors and host response to parasitism [131]. The ease of acquiring Arabidopsis mutants and the possibility to identify parasite-resistant genotypes is a further bonus of this system. With regard to salt tolerance, Arabidopsis thaliana and Eutrema halophilum represent a promising glycophyte/halophyte pair of closely related host species for future research [41], but it is still to be confirmed if Eutrema halophilum is susceptible to parasitic plants.
Most research into parasitic plants has been directed towards economically significant parasites, but knowledge of stress-tolerant parasitic plants is lacking. The latter are interesting evolutionary cases [1] but may represent a major threat to agricultural production in times of climate change and also to natural ecosystems [22]. The study of stress-tolerant parasitic plants may be compromised by their low biomass production, but this could be overcome by the development of in vitro cultures of parasitic plants [132,133]. Another future focus should be on stress-induced changes in hormones involved in response to environmental stress factors, such as abscisic acid, jasmonic acid, salicylic acid and ethylene [134] in parasitic plants.
In conclusion, parasitic plants represent a fascinating line of evolution, but can compromise agricultural production. Despite the studies available on the molecular mechanisms of plant parasitism and the ecological and agricultural impacts of this highly specialized group of angiosperms, knowledge gaps exist regarding their response to abiotic stress factors. Parasitic plants are not uncommon in challenging environments, either saline, arid, polluted or cold. Suboptimal conditions may alter their host preference, and stress effect and response may be host-dependent or -independent. To better understand their potential impacts on contemporary agriculture in times of climate change, a more systematic approach is needed, requiring the development of suitable models of stress-tolerant and stress-sensitive pairs of hosts and parasitic plants. More data on the plastid, mitochondrial and nuclear genomes sequences would also be helpful. We hope that this review helps to raise awareness for and stimulate more research on parasitic plants and their multiple facets from representing agricultural pests to being important members of plant communities and intriguing models to study plant-plant interactions.
Author Contributions
L.Z., W.S., D.T., J.L. and I.K. conceptualized the idea and prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This review was supported by the National Science Fund of the Bulgarian, Ministry of Education and Science (KP-06-N31/10) and a grant from of the Talented Young Scientist Programme of the Ministry of Science and Technology of the People’s Republic of China, given to Lyuben Zagorchev.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Westwood, J.H.; Yoder, J.I.; Timko, M.P. The evolution of parasitism in plants. Trends Plant Sci. 2010, 15, 227–235. [Google Scholar] [CrossRef]
- Bungard, R.A. Photosynthetic evolution in parasitic plants: Insight from the chloroplast genome. Bioessays 2004, 26, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Hegenauer, V.; Körner, M.; Albert, M. Plants under stress by parasitic plants. Curr. Opin. Plant Biol. 2017, 38, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Nickrent, D.L.; Musselman, L.J. Introduction to parasitic flowering plants. Plant Health Instr. 2004, 13, 300–315. [Google Scholar] [CrossRef]
- Heide-Jørgensen, H.S. Introduction: The parasitic syndrome in higher plants. In Parasitic Orobanchaceae; Springer: Berlin, Germany, 2013; pp. 1–18. [Google Scholar]
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The haustorium, a specialized invasive organ in parasitic plants. Annu. Rev. Plant Biol. 2016, 67, 643–667. [Google Scholar] [CrossRef] [PubMed]
- Aly, R.; Hamamouch, N.; Abu-Nassar, J.; Wolf, S.; Joel, D.M.; Eizenberg, H.; Kaisler, E.; Cramer, C.; Gal-On, A.; Westwood, J.H. Movement of protein and macromolecules between host plants and the parasitic weed Phelipanche aegyptiaca Pers. Plant Cell Rep. 2011, 30, 2233–2241. [Google Scholar] [CrossRef]
- Kim, G.; Westwood, J.H. Macromolecule exchange in Cuscuta-host plant interactions. Curr. Opin. Plant Biol. 2015, 26, 20–25. [Google Scholar] [CrossRef]
- Davis, C.C.; Xi, Z. Horizontal gene transfer in parasitic plants. Curr. Opin. Plant Biol. 2015, 26, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Mower, J.P.; Stefanović, S.; Young, G.J.; Palmer, J.D. Plant genetics: Gene transfer from parasitic to host plants. Nature 2004, 432, 165. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, J.; Yue, J.; Huang, J.; Sun, T.; Li, S.; Wen, J.-F.; Hettenhausen, C.; Wu, J.; Wang, L. Root parasitic plant Orobanche aegyptiaca and shoot parasitic plant Cuscuta australis obtained Brassicaceae-specific strictosidine synthase-like genes by horizontal gene transfer. BMC Plant Biol. 2014, 14, 19. [Google Scholar] [CrossRef]
- Skippington, E.; Barkman, T.J.; Rice, D.W.; Palmer, J.D. Miniaturized mitogenome of the parasitic plant Viscum scurruloideum is extremely divergent and dynamic and has lost all nad genes. Proc. Natl. Acad. Sci. USA 2015, 112, E3515–E3524. [Google Scholar] [CrossRef] [PubMed]
- Bellot, S.; Renner, S.S. The plastomes of two species in the endoparasite genus Pilostyles (Apodanthaceae) each retain just five or six possibly functional genes. Genome Biol. Evol. 2015, 8, 189–201. [Google Scholar] [CrossRef]
- Molina, J.; Hazzouri, K.M.; Nickrent, D.; Geisler, M.; Meyer, R.S.; Pentony, M.M.; Flowers, J.M.; Pelser, P.; Barcelona, J.; Inovejas, S.A. Possible loss of the chloroplast genome in the parasitic flowering plant Rafflesia lagascae (Rafflesiaceae). Mol. Biol. Evol. 2014, 31, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Parker, C. Parasitic weeds: A world challenge. Weed Sci. 2012, 60, 269–276. [Google Scholar] [CrossRef]
- Parker, C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Rispail, N.; Dita, M.A.; González-Verdejo, C.; Pérez-de-Luque, A.; Castillejo, M.A.; Prats, E.; Román, B.; Jorrín, J.; Rubiales, D. Plant resistance to parasitic plants: Molecular approaches to an old foe. New Phytol. 2007, 173, 703–712. [Google Scholar] [CrossRef]
- Krause, K.; Johnsen, H.R.; Pielach, A.; Lund, L.; Fischer, K.; Rose, J.K. Identification of tomato introgression lines with enhanced susceptibility or resistance to infection by parasitic giant dodder (Cuscuta reflexa). Physiol. Plant. 2018, 162, 205–218. [Google Scholar] [CrossRef]
- Mohemed, N.; Charnikhova, T.; Fradin, E.F.; Rienstra, J.; Babiker, A.G.; Bouwmeester, H.J. Genetic variation in Sorghum bicolor strigolactones and their role in resistance against Striga hermonthica. J. Exp. Bot. 2018, 69, 2415–2430. [Google Scholar] [CrossRef]
- Joel, D.M. The long-term approach to parasitic weeds control: Manipulation of specific developmental mechanisms of the parasite. Crop. Prot. 2000, 19, 753–758. [Google Scholar] [CrossRef]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef]
- Koch, A.M.; Binder, C.; Sanders, I.R. Does the generalist parasitic plant Cuscuta campestris selectively forage in heterogeneous plant communities? New Phytol. 2004, 162, 147–155. [Google Scholar] [CrossRef]
- Grewell, B.J. Parasite facilitates plant species coexistence in a coastal wetland. Ecology 2008, 89, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Ruyter-Spira, C.; Al-Babili, S.; van der Krol, S.; Bouwmeester, H. The biology of strigolactones. Trends Plant Sci. 2013, 18, 72–83. [Google Scholar] [CrossRef]
- Matusova, R.; Rani, K.; Verstappen, F.W.; Franssen, M.C.; Beale, M.H.; Bouwmeester, H.J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef]
- Yoneyama, K.; Awad, A.A.; Xie, X.; Yoneyama, K.; Takeuchi, Y. Strigolactones as germination stimulants for root parasitic plants. Plant and Cell Physiology 2010, 51, 1095–1103. [Google Scholar] [CrossRef]
- Teofanova, D.; Odjakova, M.; Abumhadi, N.; Zagorchev, L. Strigolactones: Mediators of Abiotic Stress Response and Weakness in Parasite Attraction. In Plant Tolerance to Environmental Stress; CRC Press: Boca Raton, FL, USA, 2019; pp. 115–128. [Google Scholar]
- Joel, D.M.; Chaudhuri, S.K.; Plakhine, D.; Ziadna, H.; Steffens, J.C. Dehydrocostus lactone is exuded from sunflower roots and stimulates germination of the root parasite Orobanche cumana. Phytochemistry 2011, 72, 624–634. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Charnikhova, T.; Fernández, I.; Bouwmeester, H.; Pozo, M.J. Arbuscular mycorrhizal symbiosis decreases strigolactone production in tomato. J. Plant Physiol. 2011, 168, 294–297. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; García-Garrido, J.; Ocampo, J.; Rubiales, D. Colonisation of field pea roots by arbuscular mycorrhizal fungi reduces Orobanche and Phelipanche species seed germination. Weed Res. 2010, 50, 262–268. [Google Scholar] [CrossRef]
- López-Granados, F.; García-Torres, L. Longevity of crenate broomrape (Orobanche crenata) seed under soil and laboratory conditions. Weed Sci. 1999, 47, 161–166. [Google Scholar] [CrossRef]
- Runyon, J.B.; Mescher, M.C.; De Moraes, C.M. Volatile chemical cues guide host location and host selection by parasitic plants. Science 2006, 313, 1964–1967. [Google Scholar] [CrossRef]
- Benvenuti, S.; Dinelli, G.; Bonetti, A.; Catizone, P. Germination ecology, emergence and host detection in Cuscuta campestris. Weed Res. 2005, 45, 270–278. [Google Scholar] [CrossRef]
- Stepien, P.; Johnson, G.N. Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: Role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef]
- Kelly, C.K. Resource choice in Cuscuta europaea. Proc. Natl. Acad. Sci. USA 1992, 89, 12194–12197. [Google Scholar] [CrossRef]
- Frost, A.; Lopez-Gutierrez, J.C.; Purrington, C.B. Fitness of Cuscuta salina (Convolvulaceae) parasitizing Beta vulgaris (Chenopodiaceae) grown under different salinity regimes. Am. J. Bot. 2003, 90, 1032–1037. [Google Scholar] [CrossRef]
- Ghars, M.A.; Parre, E.; Debez, A.; Bordenave, M.; Richard, L.; Leport, L.; Bouchereau, A.; Savouré, A.; Abdelly, C. Comparative salt tolerance analysis between Arabidopsis thaliana and Thellungiella halophila, with special emphasis on K+/Na+ selectivity and proline accumulation. J. Plant Physiol. 2008, 165, 588–599. [Google Scholar] [CrossRef]
- Smith, J.L.; de Moraes, C.M.; Mescher, M.C. Jasmonate- and salicylate-mediated plant defense responses to insect herbivores, pathogens and parasitic plants. Pest Manag. Sci. 2009, 65, 497–503. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.T.; Shokr, M.; Bekheta, M. Effects of induced salinity on four Vicia faba cultivars differing in their broomrape tolerance. In Sustainable Management of Saline Waters and Salt-Affected Soils for Agriculture, Proceedings of the Second Bridging Workshop, Aleppo, Syria, 15–18 November 2009.; ICARDA, Aleppo, Syria and IWMI: Colombo, Sri Lanka, 2009; p. 58. [Google Scholar]
- Pérez-de-Luque, A.; Moreno, M.; Rubiales, D. Host plant resistance against broomrapes (Orobanche spp.): Defence reactions and mechanisms of resistance. Ann. Appl. Biol. 2008, 152, 131–141. [Google Scholar] [CrossRef]
- Wiese, J.; Kranz, T.; Schubert, S. Induction of pathogen resistance in barley by abiotic stress. Plant Biol. 2004, 6, 529–536. [Google Scholar] [CrossRef]
- Foyer, C.H.; Rasool, B.; Davey, J.W.; Hancock, R.D. Cross-tolerance to biotic and abiotic stresses in plants: A focus on resistance to aphid infestation. J. Exp. Bot. 2016, 67, 2025–2037. [Google Scholar] [CrossRef]
- Förste, F.; Mantouvalou, I.; Kanngießer, B.; Stosnach, H.; Lachner, L.A.M.; Fischer, K.; Krause, K. Selective mineral transport barriers at Cuscuta-host infection sites. Physiol. Plant. 2020, 168, 934–947. [Google Scholar] [CrossRef]
- Nadler-Hassar, T.; Rubin, B. Natural tolerance of Cuscuta campestris to herbicides inhibiting amino acid biosynthesis. Weed Res. 2003, 43, 341–347. [Google Scholar] [CrossRef]
- Llugany, M.; Lombini, A.; Dinelli, E.; Poschenrieder, C.; Barceló, J. Transfer of selected mineral nutrients and trace elements in the host–hemiparasite association, Cistus–Odontites lutea, growing on and off metal-polluted sites. Plant Biol. 2009, 11, 170–178. [Google Scholar] [CrossRef]
- Vurro, E.; Ruotolo, R.; Ottonello, S.; Elviri, L.; Maffini, M.; Falasca, G.; Zanella, L.; Altamura, M.M.; di Toppi, L.S. Phytochelatins govern zinc/copper homeostasis and cadmium detoxification in Cuscuta campestris parasitizing Daucus carota. Environ. Exp. Bot. 2011, 72, 26–33. [Google Scholar] [CrossRef]
- Veste, M.; Todt, H.; Breckle, S.-W. Influence of halophytic hosts on their parasites—The case of Plicosepalus acaciae. AoB Plants 2015, 7, plu084. [Google Scholar] [CrossRef]
- Watson, D.M. Parasitic plants as facilitators: More Dryad than Dracula? J. Ecol. 2009, 97, 1151–1159. [Google Scholar] [CrossRef]
- Huangy, Y.; Liu, X.; Luo, X.; Zhai, Z.; Guo, Y. Effects of Cistanche deserticola on biomass and carbohydrates content of Haloxylon ammodendron. J. China Agric. Univ. 2009, 14, 76–79. [Google Scholar]
- Fahmy, G.; El-Tantawy, H.; El-Ghani, M.A. Distribution, host range and biomass of two species of Cistanche and Orobanche cernua parasitizing the roots of some Egyptian xerophytes. J. Arid. Environ. 1996, 34, 263–276. [Google Scholar] [CrossRef]
- Bolin, J.F.; Maass, E.; Tennakoon, K.U.; Musselman, L.J. Host-specific germination of the root holoparasite Hydnora triceps (Hydnoraceae). Botany 2009, 87, 1250–1254. [Google Scholar] [CrossRef]
- Silva, A.; del Rio, C.M. Effects of the mistletoe Tristerix aphyllus (Loranthaceae) on the reproduction of its cactus host Echinopsis chilensis. Oikos 1996, 75, 437–442. [Google Scholar] [CrossRef]
- Costea, M.; Wright, M.A.; Stefanović, S. Untangling the systematics of salt marsh dodders: Cuscuta pacifica, a new segregate species from Cuscuta salina (Convolvulaceae). Syst. Bot. 2009, 34, 787–795. [Google Scholar] [CrossRef]
- Prahalad, V. A Guide to the Plants of Tasmanian Saltmarsh Wetlands; University of Tasmania: Hobart, Australia; NRM North: Hobart, Australia, 2014. [Google Scholar]
- Qasem, J.R. Parasitic flowering plants on cultivated plants in Jordan-the present status and management. Pak. J. Weed Sci. Res. 2010, 16, 227–239. [Google Scholar]
- Yang, Y.; Yi, X.; Peng, M.; Zhou, Y. Stable carbon and nitrogen isotope signatures of root-holoparasitic Cynomorium songaricum and its hosts at the Tibetan plateau and the surrounding Gobi desert in China. Isot. Environ. health Stud. 2012, 48, 483–493. [Google Scholar] [CrossRef]
- Watson, D.M. Determinants of parasitic plant distribution: The role of host quality. Botany 2008, 87, 16–21. [Google Scholar] [CrossRef]
- Heide-Jørgensen, H. Parasitic Flowering Plants; Brill: Leiden, The Netherlands, 2008. [Google Scholar]
- Gonzáles, W.L.; Suárez, L.H.; Medel, R. Outcrossing increases infection success in the holoparasitic mistletoe Tristerix aphyllus (Loranthaceae). Evol. Ecol. 2007, 21, 173–183. [Google Scholar] [CrossRef]
- Moral, J.; Lozano-Baena, M.D.; Rubiales, D. Temperature and water stress during conditioning and incubation phase affecting Orobanche crenata seed germination and radicle growth. Front. Plant Sci. 2015, 6, 408. [Google Scholar] [CrossRef]
- Dawoud, D.A.; Sauerborn, J. Impact of drought stress and temperature on the parasitic weeds Striga hermonthica and Alectra vogelii in their early growth stages. Exp. Agric. 1994, 30, 249–257. [Google Scholar] [CrossRef]
- Gibot-Leclerc, S.; Corbineau, F.; Sallé, G.; Côme, D. Responsiveness of Orobanche ramosa L. seeds to GR 24 as related to temperature, oxygen availability and water potential during preconditioning and subsequent germination. Plant Growth Regul. 2004, 43, 63–71. [Google Scholar] [CrossRef]
- Kebreab, E.; Murdoch, A. Modelling the effects of water stress and temperature on germination rate of Orobanche aegyptiaca seeds. J. Exp. Bot. 1999, 50, 655–664. [Google Scholar] [CrossRef]
- Li, W.; Nguyen, K.H.; Watanabe, Y.; Yamaguchi, S.; Tran, L.-S.P. OaMAX2 of Orobanche aegyptiaca and Arabidopsis AtMAX2 share conserved functions in both development and drought responses. Biochem. Biophys. Res. Commun. 2016, 478, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hettenhausen, C.; Sun, G.; Zhuang, H.; Li, J.-H.; Wu, J. The parasitic plant Cuscuta australis is highly insensitive to abscisic acid-induced suppression of hypocotyl elongation and seed germination. PLoS ONE 2015, 10, e0135197. [Google Scholar] [CrossRef]
- Qin, X.; Yang, S.H.; Kepsel, A.C.; Schwartz, S.H.; Zeevaart, J.A. Evidence for abscisic acid biosynthesis in Cuscuta reflexa, a parasitic plant lacking neoxanthin. Plant Physiol. 2008, 147, 816–822. [Google Scholar] [CrossRef]
- Evans, B.A.; Borowicz, V.A. The plant vigor hypothesis applies to a holoparasitic plant on a drought-stressed host. Botany 2015, 93, 685–689. [Google Scholar] [CrossRef]
- Miller, A.C.; Watling, J.R.; Overton, I.C.; Sinclair, R. Does water status of Eucalyptus largiflorens (Myrtaceae) affect infection by the mistletoe Amyema miquelii (Loranthaceae)? Funct. Plant Biol. 2003, 30, 1239–1247. [Google Scholar] [CrossRef]
- Barton, A.; Watson, P. Storm tide to mean high water: Tasmanian salt marshes. Wildl. Aust. 2011, 48, 24–29. [Google Scholar]
- Pennings, S.C.; Callaway, R.M. Impact of a parasitic plant on the structure and dynamics of salt marsh vegetation. Ecology 1996, 77, 1410–1419. [Google Scholar] [CrossRef]
- Fahmy, G. Transpiration and dry matter allocation in the angiosperm root parasite Cynomorium coccineum L. and two of its halophytic hosts. Biol. Plant. 1993, 35, 603–608. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.-K. Understanding and improving salt tolerance in plants. Crop. Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Al-Khateeb, W.; Hameed, K.; Shibli, R. Effect of salinity on Orobanche cernua seed germination. Plant Pathol. J. 2003, 19, 148–151. [Google Scholar] [CrossRef]
- Hassan, M.; Sugmuto, Y.; Babiker, A.; Yamauchi, Y.; Osman, M.; Yagoub, S. Effect of NaCl on Orobanche spp and Striga hermonthica seeds germination during and after conditioning. Biosci. Res. 2010, 7, 26–31. [Google Scholar]
- Demirbas, S.; Vlachonasios, K.; Acar, O.; Kaldis, A. The effect of salt stress on Arabidopsis thaliana and Phelipanche ramosa interaction. Weed Res. 2013, 53, 452–460. [Google Scholar] [CrossRef]
- Al-Khateeb, W.; Hameed, K.; Shibli, R. Influence of soil salinity on the interaction between tomato and broomrape plant (Orobanche cernua). Plant Pathol. J. 2005, 21, 391–394. [Google Scholar] [CrossRef]
- Zagorchev, L.; Albanova, I.; Tosheva, A.; Li, J.; Teofanova, D. Salinity effect on Cuscuta campestris Yunck. Parasitism on Arabidopsis thaliana L. Plant Physiol. Biochem. 2018, 132, 408–414. [Google Scholar] [CrossRef]
- Delavault, P.; Simier, P.; Thoiron, S.; Véronési, C.; Fer, A.; Thalouarn, P. Isolation of mannose 6-phosphate reductase cDNA, changes in enzyme activity and mannitol content in broomrape (Orobanche ramosa) parasitic on tomato roots. Physiol. Plant. 2002, 115, 48–55. [Google Scholar] [CrossRef]
- Noiraud, N.; Maurousset, L.; Lemoine, R. Transport of polyols in higher plants. Plant Physiol. Biochem. 2001, 39, 717–728. [Google Scholar] [CrossRef]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. Plant Stress Physiol. 2012, 59–93. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.; Wu, L.; Liu, A.; Zhao, F.J.; Xu, W. Heavy metal ATPase 3 (HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola. New Phytol. 2017, 215, 687–698. [Google Scholar] [CrossRef]
- Dinh, N.; van der Ent, A.; Mulligan, D.R.; Nguyen, A.V. Zinc and lead accumulation characteristics and in vivo distribution of Zn 2+ in the hyperaccumulator Noccaea caerulescens elucidated with fluorescent probes and laser confocal microscopy. Environ. Exp. Bot. 2018, 147, 1–12. [Google Scholar] [CrossRef]
- Meindl, G.A.; Bain, D.J.; Ashman, T.-L. Variation in nickel accumulation in leaves, reproductive organs and floral rewards in two hyperaccumulating Brassicaceae species. Plant Soil 2014, 383, 349–356. [Google Scholar] [CrossRef]
- EI-Salam, N.M.A.; Ahmad, S.; Murad, W.; Iqbal, T.; Zuman, L.; Bibi, A.; Rehman, A.; Ullah, R.; Mohammad, Z.; Shad, A.A. Profile of heavy metals in medicinal plants collected from different areas of Karak, Khyber Pakhtunkhwa, Pakistan. Life Sci. J. 2013, 10, 914–921. [Google Scholar]
- Ernst, W. Mine vegetation in Europe. In Heavy metal tolerance in plants: Evolutionary aspects; CRC Press: Boca Raton, FL, USA, 1990; Volume 18, pp. 21–38. [Google Scholar]
- Boyd, R.S.; Martens, S.N.; Davis, M.A. The nickel hyperaccumulator Streptanthus polygaloides (Brassicaceae) is attacked by the parasitic plant Cuscuta californica (Cuscutaceae). Madrono 1999, 46, 92–99. [Google Scholar]
- Jana, S.; Dalal, T.; Barua, B. Effects and relative toxicity of heavy metals on Cuscuta reflexa. Water Air Soil Pollut. 1987, 33, 23–27. [Google Scholar] [CrossRef]
- Srivastava, S.; Tripathi, R.D.; Dwivedi, U.N. Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa–an angiospermic parasite. J. Plant Physiol. 2004, 161, 665–674. [Google Scholar] [CrossRef]
- Yadav, S. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Birschwilks, M.; Haupt, S.; Hofius, D.; Neumann, S. Transfer of phloem-mobile substances from the host plants to the holoparasite Cuscuta sp. J. Exp. Bot. 2006, 57, 911–921. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Tolrà, R.; Barceló, J. Can metals defend plants against biotic stress? Trends Plant Sci. 2006, 11, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Bani, A.; Pavlova, D.; Benizri, E.; Shallari, S.; Miho, L.; Meco, M.; Shahu, E.; Reeves, R.; Echevarria, G. Relationship between the Ni hyperaccumulator Alyssum murale and the parasitic plant Orobanche nowackiana from serpentines in Albania. Ecol. Res. 2018, 33, 549–559. [Google Scholar] [CrossRef]
- Turnau, K.; Jedrzejczyk, R.; Domka, A.; Anielska, T.; Piwowarczyk, R. Expansion of a holoparasitic plant, Orobanche lutea (Orobanchaceae), in post-industrial areas—A possible Zn effect. Sci. Total Environ. 2018, 639, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Gressel, J. Global advances in weed management. J. Agric. Sci. 2011, 149, 47–53. [Google Scholar] [CrossRef]
- Sibhatu, B. Review on Striga weed management. Int. J. Life. Sci. Scienti. Res 2016, 2, 110–120. [Google Scholar]
- Gressel, J. Crops with target-site herbicide resistance for Orobanche and Striga control. Pest Manag. Sci. 2009, 65, 560–565. [Google Scholar] [CrossRef]
- Nadler-Hassar, T.; Shaner, D.L.; Nissen, S.; Westra, P.; Rubin, B. Are herbicide-resistant crops the answer to controlling Cuscuta? Pest Manag. Sci. 2009, 65, 811–816. [Google Scholar] [CrossRef]
- Jiang, L.; Qu, F.; Li, Z.; Doohan, D. Inter-species protein trafficking endows dodder (Cuscuta pentagona) with a host-specific herbicide-tolerant trait. New Phytol. 2013, 198, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Haupt, S.; Oparka, K.J.; Sauer, N.; Neumann, S. Macromolecular trafficking between Nicotiana tabacum and the holoparasite Cuscuta reflexa. J. Exp. Bot. 2001, 52, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.O.; Palmer, J.D. Horizontal gene transfer in plants. J. Exp. Bot. 2006, 58, 1–9. [Google Scholar] [CrossRef]
- Shen, H.; Hong, L.; Ye, W.; Cao, H.; Wang, Z. The influence of the holoparasitic plant Cuscuta campestris on the growth and photosynthesis of its host Mikania micrantha. J. Exp. Bot. 2007, 58, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.V.; Tennakoon, K.U.; Metali, F.; Lim, L.B.; Bolin, J.F. Impact of Cuscuta australis infection on the photosynthesis of the invasive host, Mikania micrantha, under drought condition. Weed Biol. Manag. 2015, 15, 138–146. [Google Scholar] [CrossRef]
- Mauromicale, G.; Monaco, A.L.; Longo, A.M. Effect of branched broomrape (Orobanche ramosa) infection on the growth and photosynthesis of tomato. Weed Sci. 2008, 56, 574–581. [Google Scholar] [CrossRef]
- Cochavi, A.; Ephrath, J.; Eizenberg, H.; Rachmilevitch, S. Phelipanche aegyptiaca parasitism impairs salinity tolerance in young leaves of tomato. Physiol. Plant. 2018, 164, 191–2013. [Google Scholar] [CrossRef]
- Abdelhamid, M.T.; Shokr, M.M.; Bekheta, M. Growth, root characteristics, and leaf nutrients accumulation of four faba bean (Vicia faba L.) cultivars differing in their broomrape tolerance and the soil properties in relation to salinity. Commun. Soil Sci. Plant Anal. 2010, 41, 2713–2728. [Google Scholar] [CrossRef]
- Mohamed, K.I.; Papes, M.; Williams, R.; Benz, B.W.; Peterson, A.T. Global invasive potential of 10 parasitic witchweeds and related Orobanchaceae. Ambio 2006, 35, 281–288. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Emeran, A.; Moral, A.; Rubiales, D. First report of crenate broomrape (Orobanche crenata) on white lupine (Lupinus albus) growing in alkaline soils in Spain and Egypt. Plant Dis. 2009, 93, 970. [Google Scholar] [CrossRef]
- Ewansiha, S.U.; Menkir, A.; Tofa, A.I. Agronomic response of drought-tolerant and Striga-resistant maize cultivars to nitrogen fertilization in the Nigerian Guinea savannahs. Maydica 2012, 57, 114–120. [Google Scholar]
- Westwood, J.H. Characterization of the Orobanche-Arabidopsis system for studying parasite-host interactions. Weed Sci. 2000, 48, 742–748. [Google Scholar] [CrossRef]
- Goldwasser, Y.; Yoder, J.I. Differential induction of Orobanche seed germination by Arabidopsis thaliana. Plant Sci. 2001, 160, 951–959. [Google Scholar] [CrossRef]
- Dos Santos, C.V.; Letousey, P.; Delavault, P.; Thalouarn, P. Defense gene expression analysis of Arabidopsis thaliana parasitized by Orobanche ramosa. Phytopathology 2003, 93, 451–457. [Google Scholar] [CrossRef]
- Bar-Nun, N.; Sachs, T.; Mayer, A.M. A role for IAA in the infection of Arabidopsis thaliana by Orobanche aegyptiaca. Ann. Bot. 2008, 101, 261–265. [Google Scholar] [CrossRef]
- Spallek, T.; Melnyk, C.W.; Wakatake, T.; Zhang, J.; Sakamoto, Y.; Kiba, T.; Yoshida, S.; Matsunaga, S.; Sakakibara, H.; Shirasu, K. Interspecies hormonal control of host root morphology by parasitic plants. Proc. Natl. Acad. Sci. USA 2017, 114, 5283–5288. [Google Scholar] [CrossRef]
- Shahid, S.; Kim, G.; Johnson, N.R.; Wafula, E.; Wang, F.; Coruh, C.; Bernal-Galeano, V.; Phifer, T.; Depamphilis, C.W.; Westwood, J.H. MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 2018, 553, 82–85. [Google Scholar] [CrossRef]
- Song, J.; Bian, J.; Xue, N.; Xu, Y.; Wu, J. Inter-species mRNA transfer among green peach aphids, dodder parasites, and cucumber host plants. Plant Diversity 2021. [Google Scholar] [CrossRef]
- Johnson, N.R.; Axtell, M.J. Small RNA warfare: exploring origins and function of trans-species microRNAs from the parasitic plant Cuscuta. Curr. Opin. Plant Biol. 2019, 50, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Liu, H.; Liu, N.; Shen, G.; Zhuang, H.; Wu, J. Dodder-transmitted mobile signals prime host plants for enhanced salt tolerance. J. Exp. Bot. 2020, 71, 1171–1184. [Google Scholar] [CrossRef]
- Hettenhausen, C.; Li, J.; Zhuang, H.; Sun, H.; Xu, Y.; Qi, J.; Zhang, J.; Lei, Y.; Qin, Y.; Sun, G. Stem parasitic plant Cuscuta australis (dodder) transfers herbivory-induced signals among plants. Proc. Natl. Acad. Sci. USA 2017, 114, E6703–E6709. [Google Scholar] [CrossRef]
- Goldwasser, Y.; Yoneyama, K.; Xie, X.; Yoneyama, K. Production of strigolactones by Arabidopsis thaliana responsible for Orobanche aegyptiaca seed germination. Plant Growth Regul. 2008, 55, 21–28. [Google Scholar] [CrossRef]
- Birschwilks, M.; Sauer, N.; Scheel, D.; Neumann, S. Arabidopsis thaliana is a susceptible host plant for the holoparasite Cuscuta spec. Planta 2007, 226, 1231–1241. [Google Scholar] [CrossRef]
- LeBlanc, M.; Kim, G.; Patel, B.; Stromberg, V.; Westwood, J. Quantification of tomato and Arabidopsis mobile RNAs trafficking into the parasitic plant Cuscuta pentagona. New Phytol. 2013, 200, 1225–1233. [Google Scholar] [CrossRef]
- Goldwasser, Y.; Westwood, J.; Yoder, J. The use of Arabidopsis to study interactions between parasitic angiosperms and their plant hosts. Arab. Book 2002, 1, e0035. [Google Scholar] [CrossRef]
- Furuhashi, K. Establishment of a successive culture of an obligatory parasitic flowering plant, Cuscuta japonica, in vitro. Plant Sci. 1991, 79, 241–246. [Google Scholar] [CrossRef]
- Zhou, W.; Yoneyama, K.; Takeuchi, Y.; Iso, S.; Rungmekarat, S.; Chae, S.; Sato, D.; Joel, D. In vitro infection of host roots by differentiated calli of the parasitic plant Orobanche. J. Exp. Bot. 2004, 55, 899–907. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yu, J.-Q. (Eds.) Plant Hormones under Challenging Environmental Factors; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).