Abstract
Climate change has been predicted to influence the marine phytoplankton community and its carbon acquisition strategy. Extracellular carbonic anhydrase (eCA) is a zinc metalloenzyme that catalyses the relatively slow interconversion between HCO3− and CO2. Early results indicated that sub-nanomolar levels of eCA at the sea surface were sufficient to enhance the oceanic uptake rate of CO2 on a global scale by 15%, an addition of 0.37 Pg C year−1. Despite its central role in the marine carbon cycle, only in recent years have new analytical techniques allowed the first quantifications of eCA and its activity in the oceans. This opens up new research areas in the field of marine biogeochemistry and climate change. Light and suitable pH conditions, as well as growth stage, are crucial factors in eCA expression. Previous studies showed that phytoplankton eCA activity and concentrations are affected by environmental stressors such as ocean acidification and UV radiation as well as changing light conditions. For this reason, eCA is suggested as a biochemical indicator in biomonitoring programmes and could be used for future response prediction studies in changing oceans. This review aims to identify the current knowledge and gaps where new research efforts should be focused to better determine the potential feedback of phytoplankton via eCA in the marine carbon cycle in changing oceans.
1. Introduction
Extracellular carbonic anhydrase (eCA) is a zinc metalloenzyme that accelerates the slow interconversion between bicarbonate ions (HCO3−) and carbon dioxide (CO2) to the equilibrium concentration at the cell surface []. eCA has been widely found in mammals [], plants and phytoplankton [], and prokaryotes []. In general, there are seven CA gene classes that have been recognized in photosynthetic organisms, identified as α-, β-, ϒ-, δ-, ζ-, θ- [,] as well as a recently discovered ι-CA gene class []. Meanwhile, the η-CA gene class has been found within the malaria pathogen Plasmodium sp. []. The first five gene classes (α, β, ϒ, θ and η) are different in terms of their primary structure [] but share a common feature of bound zinc (Zn2+) on their activation site []. The δ-CA (TWCA1) [,] and ζ-CA (CDCA) [] classes with the capability to bind with alternative metal cofactors as well as Zn2+, such as cobalt (Co2+) and cadmium (Cd2+), respectively, have been identified in the diatom Thalassiosira weisflogii (T. weisflogii). The δ- and ζ- classes are likely to be the major CA classes that facilitate CO2 supply in centric diatoms [] as a carbon-concentrating mechanism (CCM). More recently, Jensen, et al. [] discovered a new ι-CA class in Thalassiosira pseudonona (T. pseudonona), which unusually prefers manganese (Mn2+) to Zn2+ as a cofactor. Overall, the gene distributions of CA in microalgae cells vary between species even if they belong to the same family []. For additional information on the function, physiological relevance, and diverse CA expression in microalgae, we refer to a recent review [].
eCA expression is highly responsive to environmental changes, particularly at low aqueous CO2 concentrations []. Previous studies have shown that the levels of eCA expression differ significantly between phytoplankton species based on laboratory experiments [,,]. The availability of inorganic carbon, light levels and pH [,], as well as the phytoplankton growth stage [], are important factors in the regulation of eCA activity. The taxonomic composition and cell size of a phytoplankton community also influence the level of eCA expression [,]. The different levels of eCA among species provide evidence that the mechanism of inorganic carbon (Ci) acquisition in phytoplankton is species-dependent and eCA is produced when demand for CO2 exceeds the rate of uncatalyzed HCO3− to CO2 conversion [,,].
Under a future of climate change, marine photoautotrophs will undergo complex changes in their physiology, driven by increasing sea-surface temperatures, continuing ocean acidification, and changing light conditions [,]. Numerous laboratory studies have already described the effect of ocean acidification on Ci acquisition of microalgae, specifically diatom and their eCA activity [,,]. Thus, it is not surprising that these changes include the expression of eCA in phytoplankton, particularly those residing in the near-surface layer. In this review, we focus on the available studies on eCA in the marine environment, including biological function and current approaches to understanding the eCA in changing oceans.
2. Biological Function of eCA in the Marine Environment
For decades, CA has been known to exist in many photosynthetic organisms and to be involved in CCMs, which help the cell to produce biomass via photosynthesis, particularly in a CO2-limited environment []. At the alkaline pH of seawater (pH 7.8–8.4), Ci predominantly exists in ionic forms, whereby approximately 90% is present as HCO3−, 9% as carbonate ions (CO32−), and 1% present as CO2, the substrate for the CO2-fixing enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) [,]. RubisCO has a lower affinity for CO2 and, at the relatively low CO2 concentration found in the marine environment, the activity of this enzyme is less than half-saturated [,]. It was reported that the CO2 concentration at an air-equilibrated water surface is lower (13 µM at 20 °C) than typical values of the half-saturation constant (KC) of RubisCO in diatoms (KC = 23–68 µM) [], cyanobacteria (KC = 100–180 µM) [], and haptophytes (KC = 15–24 µM) []. To overcome the CO2 limitation and slow diffusion rate in seawater, photosynthetic organisms evolved CCMs to increase the concentration of CO2 in the vicinity of the cell internal RubisCO site []. These mechanisms include active uptake of both extracellular HCO3− and CO2 as carbon sources for photosynthesis. RubisCO-mediated carboxylation competes with the oxygenation of ribulose 1,5-biphosphate (RuBP), which reduces carbon fixation and promotes photorespiration []. However, the degree to which these two competitive reactions occur depends on the (O2) and CO2 concentrations at the active site of RubisCO and the relative affinity of the enzyme to these gases.
In phytoplankton and aquatic macrophytes, CA can be located either in periplasmic space (eCA) or attached to the outer cell wall and/or in the chloroplast (internal CA, iCA) (Figure 1) [,,]. There are lines of evidence supporting the role of eCA in some microalgae CCM, particularly diatoms, and eCA expression is induced under low CO2 concentration [,,]. CCM consists of a Ci pump, CA enzyme to equilibrate HCO3− to CO2, and a compartment of RubisCO such as pyrenoid or carboxysome []. The function of eCA in CCMs is mainly to convert available HCO3− to CO2 close to the cell membrane and facilitate CO2 transport through the cell’s membrane by diffusion []. At low partial pressures of CO2 (pCO2) in the surrounding medium, i.e., seawater, the thin diffusion layer around the cell becomes depleted rather quickly compared with the larger bulk phase outside of the diffusion layer. Thus, eCA accelerates the slow dehydration of HCO3− to CO2 within the boundary layer, increasing the surface CO2 concentration for fixation by Rubisco []. Besides, eCA also functions to recover leaked CO2 from the cell and convert it to HCO3− [], implying that the presence or absence of eCA allows more energy-efficient Ci recycling in CO2 and HCO3− users [].
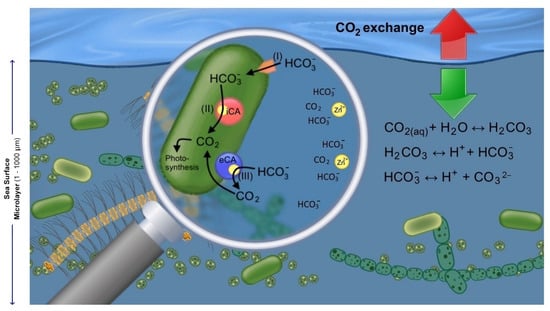
Figure 1.
The role of extracellular (eCA) and internal carbonic anhydrase (iCA) in the sea surface microlayer (SML). Equations are given for the hydration of CO2. (i) Alternative carbon acquisition through direct uptake of HCO3−. (ii) Internal conversion of HCO3− to CO2 by iCA. (iii) Catalytic conversion of HCO3− to CO2 by eCA within the cell’s diffusive layer, and CO2 diffusion through the cell’s membrane.
The role of eCA in marine biogeochemical cycling is highlighted by the fact that eCA is ubiquitous and requires the binding of trace elements on its activation site, such as Zn, Cd, and Co [,], and, more recently discovered, Mn []. In 1994, Morel and co-workers proposed the “zinc hypothesis” where the low levels of Zn in surface water may limit CO2 uptake and the growth rate of T. weissflogii through eCA. Based on this finding, the low level of Zn in seawater has been suggested to reflect the distribution of eCA in seawater, which was proposed to be at nanomolar levels [], but only recently confirmed with the development of an analytical technique to quantify eCA in seawater []. Analysis with the same diatom, T. weissflogii, showed that Cd [,] and Co [] could partially replace Zn in CA by 50%, depending on the species, when the metals were present at concentrations typical of surface seawater. However, further analyses with chlorophytes and prymnesiophytes [] indicated that Cd only acted as a nutrient in a narrow species-specific concentration range. For this reason, the replacement of Zn with Cd or Co has been suggested to be species-specific []. An activation of CA by Mn was proposed more recently as a ubiquitous sub-class of CA [], and eCA could potentially be important in the understanding of Mn distribution in the oceans. A recent field study by Morel, et al. [] in the eastern tropical South Pacific revealed that the substitution of Cd and Co for Zn occurred when dissolved Zn levels were extremely low and not necessarily with the lowest pCO2 conditions. This further suggests that diatoms in the marine environment may be co-limited by Zn-Cd-Co and CO2 []. To date, the cellular quotas of Zn attached to CA in marine phytoplankton remain an open question. Subhas, et al. [] estimated the use of Zn quota by marine phytoplankton assemblages from the North Pacific Ocean to be in the range of 10–40% using Zn/phosphate and CA/particulate organic carbon (CA/POC) ratios. The estimated values are 10 times lower than our previous estimation from a laboratory experiment using monoculture solutions []. Some species are likely to utilize Cd, Co or even newly found Mn as a cofactor, and such estimations are likely to be very uncertain within natural assemblages. Trace metal quotas in marine phytoplankton also depend on cell size [,]. A holistic approach is needed to resolve the coupling of marine trace metal chemistry to total CA expression and activity in natural phytoplankton assemblages.
3. Extracellular Carbonic Anhydrase in a Changing Ocean
Under future climate change, marine photoautotrophs will undergo complex changes in their physiology, driven by increasing sea-surface temperatures, continuing ocean acidification, and changing light conditions []. As outlined above, changes could include the expression of eCA and changes within CCMs of phytoplankton communities. Between 1994 and 2007, it was reported that the amount of oceanic carbon increased by 34 ± 4 Pg C, which represents over 31 ± 4% of anthropogenic CO2 emissions []. Future concentrations of CO2 in the atmosphere are projected to reach ∼1000 µatm by 2100 if anthropogenic emissions are ongoing at the current rate [] and thus will be taken up by the ocean through the sea surface microlayer (SML). The increased CO2 uptake by the ocean will influence the seawater chemistry, increase acidity, and shift the dissolved inorganic carbon system from carbonate (CO32−) towards HCO3− and CO2 [,]. This phenomenon is termed ocean acidification []. The air-sea CO2 exchange depends not only on temperature, salinity, and physical mixing of water, but also on the photosynthesis and respiration of plankton communities to maintain an air–sea CO2 gradient as a driving force for the exchange. Organisms in the euphotic zone will be exposed to a higher CO2 environment with the consequence of lower pH. Consequently, their physiologies will respond to these changes in marine carbonate chemistry. Ocean acidification generally affects the species composition of phytoplankton assemblages [], changes the cellular mechanisms involved in the acquisition of inorganic carbon, and negatively affects the physiology of calcifying organisms such as coccolithophores []. Laufkötter, et al. [] estimated a decrease in global average phytoplankton net primary production of 6.5% within 50 years of observation (1960–2006) due to changes in climate-relevant factors, with a consequence of reduced efficiency of the biological pump and thus the ocean’s capability to capture anthropogenic CO2 in the deep ocean.
Many laboratory studies have already described the effect of ocean acidification on the Ci acquisition of diatoms and their eCA activity (Table 1) [,,]. In most cases, increased CO2 levels inhibit the eCA activity of diatoms. Hence, indirect uptake of HCO3− via the eCA pathway is likely to be reduced under future elevated CO2 levels in the oceans. Nevertheless, diatoms display a high diversity in terms of Ci acquisition strategies, which can take both HCO3− and CO2 [,]. T. weissflogii, where HCO3− is the main Ci species taken up during low pCO2, showed the highest eCA expression under low pCO2 (36 µatm, pH = 9.1) [] and decreased eCA expression by more than 50% after exposure to moderate pCO2 levels (180 µatm and 360 µatm). The eCA expression was close to the detection limit under high pCO2 (1800 µatm). Overall, under Ci limitation, eCA becomes an essential pathway for photosynthetic carbon fixation in T. weissflogii. Decreased eCA expression of T. weissflogii under high pCO2 has been observed in other studies [,]. T. weissflogii exhibited significantly higher photosynthetic oxygen evolution rates at low CO2 or HCO3− levels, suggesting that T. weissflogii has higher affinities for CO2 or HCO3− when their concentrations are not sufficient to support saturated growth and photosynthesis []. Gao and Campbell [] suggested that CCMs in diatoms potentially link to multiple metabolic pathways that differ between species. For instance, under non normal conditions, T. weissflogii employs C4 pathways as additional CCMs before RubisCO-aided carboxylation [], and the δ-CA of T. weissflogii can catalyse the hydration of CO2 and increase the HCO3− concentration intracellularly []. Phaeodactylum tricornutum (P. tricornutum), however, relies solely on biophysical CCMs in which HCO3− is pumped into the cell and converted into CO2 by the eCA in the chloroplast [,]. This explains the low eCA expression with increasing CO2 concentrations in P. tricornutum []. Trimborn, et al. [] observed that the eCA activities of T. pseudonana were not affected by CO2 levels, suggesting that eCA plays a negligible role in its carbon acquisition strategy, but eCA plays an important role in bloom-forming diatom species such as Thalassionema nitzschioides (T. nitzschioides), Eucampia zodiacus (E. zodiacus), and Skeletonema costatum (S. costatum). The absence of eCA activities in T. pseudonana was reported in previous studies using the isotope-disequilibrium [], Membrane inlet mass spectrometry (MIMS) [], and potentiometric methods [,]. Details of the photophysiological responses in terms of growth, respiration, and photoinhibition of 20 species of marine diatoms to ocean acidification were reviewed by Gao and Campbell [], outlining further such complexity.

Table 1.
Studies on the effect of ocean acidification, UV radiation, light, warming, and combination effect on carbon acquisition strategies of phytoplankton through laboratory (diatom species) and incubation experiments on board research vessels (natural phytoplankton assemblages).
The extent of how much the carbon acquisition strategies of natural phytoplankton assemblages are affected by ongoing ocean acidification has been examined in incubation experiments on board research vessels (Table 1). An early study by Tortell and Morel [] demonstrated that HCO3− uptake in the equatorial Pacific Ocean is regulated by the ambient CO2 concentrations, where phytoplankton assemblages did not express eCA under high CO2 concentrations (750 µatm). Several studies observed a reduction in eCA activity as a response to high CO2 concentrations (800 µatm) in diatom assemblages of the West Antarctic Peninsula [,,] and more recently in the Timor Sea phytoplankton assemblages [,]. Contrarily, tolerance of highly variable CO2 levels has been observed for diatom assemblages in the subarctic Pacific, indicating that the direct uptake of HCO3− dominates carbon uptake for these assemblages []. Evidence of direct HCO3− uptake has been seen in southern Bering Sea and Ross Sea diatom assemblages, which is estimated to contribute up to 60–95% of total Ci uptake [,,], suggesting that the HCO3− transport system is probably never completely suppressed under any ocean conditions []. In the Southern Ocean, preferred Ci sources under elevated CO2 are highly variable [,,], whereby phytoplankton assemblages show substantial direct HCO3− uptake. Overall, these findings highlight the fact that the effect of future ocean acidification on phytoplankton Ci acquisition strategies may vary between oceanic provinces due to the changing composition of phytoplankton assemblages and environmental conditions. An increase in seawater acidity increases the hydration rates by eCA []. Meanwhile, future increases in CO2 levels would save about 20% of the energy demand for CCMs [] of diatoms, as less eCA would be required to maintain Ci acquisition. Phytoplankton species that possess direct HCO3− uptake as their preferred Ci may become less CO2 sensitive than those relying solely on CO2 uptake or indirect HCO3− uptake through eCA. More field experiments from different oceanic regions are needed to compile a comprehensive understanding of how marine phytoplankton would acquire Ci in the future oceans.
Short-term shifts in phytoplankton species composition with variable CO2 concentrations are expected in future oceans. The effect of CO2 concentrations on species composition also varies between oceanic regimes. For instance, incubations of equatorial Pacific phytoplankton assemblages resulted in the dominance in diatoms over the prymnesiophyte Phaeocystis antarctica under elevated CO2 (750 µatm) []. Meanwhile, increased CO2 levels (800 µatm) would also favour the growth of larger cells (e.g., Chaetoceros spp.) over smaller cells (e.g., pennate diatom Pseudo-nitzschia) as observed in incubation experiments with Ross Sea phytoplankton communities [], because larger cells are subject to greater reaction-diffusion limitations []. Shifting towards larger diatoms (Thalassiosira sp., T. nitzschioides and Nitzschia longissimi) is also observed in natural phytoplankton assemblages from the Kiel Fjord (Germany) under distinct “greenhouse” conditions (8.5 °C and 990 µatm) []. In very different oceanic conditions, combining high CO2 and surface solar radiation resulted in declines in the diatom abundance from the South China Sea and their primary productivity []. Such taxonomic shifts are likely to be influenced by the physiological mechanisms of Ci use by specific species and, therefore, it is essential to fully describe the CCMs of natural phytoplankton assemblages to predict how they will respond to future changes in CO2 levels []. A shift towards larger cells—as observed for diatoms—could increase the vertical flux of POC and the efficiency of the carbon pump to the deep ocean by forming rapidly sinking aggregates []. Moreover, larger diatoms had higher total CA activity for a given Zn- or Cd-limited growth rate, and thus, the cell could be co-limited by Zn, Cd, and CO2 at low external CO2 concentrations []. This is because larger cells have lower cellular Zn due to their lower cell surface to volume ratio, and a greater restriction of a diffusive flux of biologically available dissolved Zn to their surface due to a thicker diffusive boundary layer around their cells [].
As continuing ocean acidification directly affects the physiology of certain diatoms, it may also indirectly influence their response to other environmental factors including ultraviolet (UV) radiation, light, increasing temperature, or nutrients []. The net effect of ocean acidification on marine producers largely depends on the photo-biological conditions (light or UV radiation) [,], as well as interaction with rising sea-surface temperatures [] and probably other variables such as changes in nutrient availability. These environmental factors may have a synergistic or antagonistic effect on the Ci acquisition of diatom species. A combination of low light and high CO2 reduced the eCA activity of S. costatum by 2.5-fold, implying that besides CO2, the efficiency of CO2 uptake is dependent on the availability of light []. This highlights the importance of light in CCMs efficiency.
4. Enrichment of eCA in the Sea Surface Microlayer
The hypothetical enrichment of eCA within the sea surface microlayer (SML) was proposed by Berger and Libby [] in the late 1960s considering the hydrophobic nature of eCA []. The SML is a boundary layer between the ocean and the atmosphere, covering a significant fraction of the Earth’s surface [], and is characterized as a distinct habitat for plankton communities []. A high abundance of microorganisms such as picophytoplankton accumulating in the SML compared to underlying water at 1-metre depth has been frequently reported [,]. Besides, earlier studies [,] have described that the SML is dominated by diatom, cryptophytes, and dinoflagellates species. Indeed, various species of dinoflagellates and diatom are reported to express eCA [,,].
It was suggested that eCA expression in the surface water is associated with surface water ecology [,], and so the SML may contain a sufficient amount of extracellular and membrane-bound eCA to enhance the conversion between HCO3− and CO2 in the boundary layer between the ocean and the atmosphere. Thus, any CO2 produced by eCA at the SML would rapidly be utilized by cells and converted to biomass. Berger’s and Libby’s hypothesis remained unanswered for five decades as existing analytical techniques were too insensitive and impractical for immediate shipboard measurements. Using a fluorescent technique [], we found that the concentrations of eCA in natural seawater are in the nanomolar range (0.10 nM–0.76 nM) and enriched in the SML by a mean of 1.5 ± 0.7 compared to underlying water from 1-metre depth []. This finding is supported by Subhas, et al. [], whereby CA in natural seawater was externally bound and accounted for up to 80% of total CA. Nevertheless, the eCA concentrations observed in Mustaffa, et al. [] were considerably low based on an estimated value of 1.8–4.8 nM considering that for eCA about 0.3% of its molecular weight consists of Zn [], and Zn is enriched in the SML by an enrichment factor (EF) of 1.5–4.0 []. A short residence time of Zn in the SML [] and a short lifetime of eCA could explain the low levels in the SML. Meanwhile, a complex enrichment process in the SML [,] including wind speed, intense UV radiation, and temperature fluctuation [], excludes a simple explanation of eCA enrichment and opens up a new research field.
Recently, Watson, et al. [] pointed out that most computer models underestimate oceanic carbon uptake, partially due to constraints in the measurements of sea-surface temperature. However, using a conservative laminar film model [], we concluded that the existing nanomolar level of eCA in the SML can enhance CO2 exchange by up to 15% [], which represents 0.37 petagrams (Pg) carbon year−1 considering a global estimate of oceanic carbon uptake of 2.5 Pg C yr−1 []. Based on the EF of eCA per chlorophyll-a from our study [] and a global concentration of chlorophyll-a (0.1–2.1 mg/m3; source: http://oceancolor.gsfc.nasa.gov/, accessed on 5 March 2021) during the cruise, we estimate here that the concentration of eCA in the SML could be in the range of 0.12–1.20 nM (EF = 0.3–3.4) and could contribute up to a 23% enhancement of CO2 exchange based on Keller’s model. With this estimation, we suggest that ignoring the enrichment of eCA at the SML further explains why computer models underestimate global carbon uptake rates. However, the enhancement could be less than 23% considering the complexity of the SML and uncertainty in the measurement of air–sea CO2 exchange in natural conditions. Further validation is needed as the eCA expression in natural communities is dependent on pCO2 conditions, light, and nutrient availability. Besides, the eCA levels may vary between oceanic provinces with different phytoplankton communities and sizes, as diatoms commonly express eCA when demand for CO2 outstrips the rate of supply by uncatalyzed bicarbonate to CO2 conversion, whereas cyanobacteria do not [,].
Life in the SML is challenging as the communities are exposed to intense light, UV radiation, and temperature fluctuations [], which limit the activity and abundance of photosynthetic organisms []. Thus, the efficiency of CO2 uptake by phytoplankton in the SML is likely to be affected by UV radiation. In a laboratory experiment, Wu and Gao [] observed that the eCA activity of S. costatum was enhanced by 28% and 24% under UV-A and UV-B radiation, respectively. This was observed at relatively low irradiance (PAR = 161 Wm−2) after 1-hour exposure and contributed up to 6% of the photosynthetic carbon fixation rate. However, exposure to higher levels of UV radiation (UV-A + UV-B) for 2 h degraded the eCA by 78%, implying that UV radiation contributes to greater photoinhibition of photosynthesis []. Degradation of RubisCO has been observed under similar high UV conditions [,]. The light conditions and warming of the ocean (i.e., 200 µmol photons m−2 s−1 and 25 °C)—predicted future climate conditions []—substantially declined the expression of eCA and RubisCO activity in T. weisflogii but not in P. tricornutum []. This suggests that climate-related feedbacks are species-specific and T. weisflogii may have benefits in terms of its growth in the future ocean.
With future changes of UV flux to the ocean [] as well as an increase in sea-surface temperature, it is reasonable to expect a lower eCA expression in SML communities with a consequence of decreased CO2 uptake by the ocean and thus decreased air-sea CO2 exchange. Despite the relevance of the SML in air–sea CO2 exchange [,] and the fact that phytoplankton at the near-surface layer have been suggested to control the air-sea CO2 equilibrium [], the sensitivity of the SML communities to ocean acidification and combination effects including UV radiation and temperature are still largely unexplored []. Because of the unique location of the SML between the ocean and atmosphere, the communities in this layer are likely to be the first to be exposed to climate-related changes. For instance, previous studies have shown that light limitation affects growth rates and biomass in SML communities [], and high nutrient loads changed the density and composition of SML communities []. Incomplete understanding of the Ci acquisition strategy in the SML community’s response to future climate change leads to difficultly in predicting the global chemical enhancement of CO2 and biogeochemical cycling by eCA. Overall, futures investigations are necessary to get a mechanistic understanding of phytoplankton and its carbon acquisition strategies in the dynamic SML and upper ocean layer.
5. Conclusions
Our review highlighted the current knowledge and gaps in the knowledge about the role of eCA in the changing ocean. eCA activity and concentrations are affected by environmental stressors such as ocean acidification and UV radiation as well as changing light conditions. Thus, eCA potentially serves as a biochemical indicator in biomonitoring programmes and could be used for future response prediction studies in changing oceans. As most of the studies were carried out in the short term (i.e., days), we propose that studies aiming for a long-term response of diatoms to environmental changes should be conducted in the future. We also suggest including the near-surface layer (including the SML) communities in a research effort to study physiological responses towards ocean acidification, UV radiation, temperature fluctuations, as well as nutrient limitations. Such studies will provide further insights into the global chemical enhancement of CO2 and biogeochemical cycling in future oceans. Furthermore, advancing technology such as analytical methods, molecular tools, and bioinformatics are needed to resolve the metabolic roles of eCA in photosynthetic organisms. The application of such tools will be crucial to widening the perspective of eCA studies in natural seawater and predicting changes for the future oceans, including the interaction of multiple concurrent changes such as pH and light conditions. In this context, the SML—covering 71% of the Earth’s surface—seems to be a good candidate with more drastic changes likely to occur. Overall, eCA from different oceanic provinces remains to be explored to further improve computer models of marine carbon cycling, including the oceanic CO2 uptake as well as their response in a future ocean.
Author Contributions
N.I.H.M.: Conceptualization, Writing-Original draft preparation, visualization. M.T.L.: Supervision, Reviewing and Editing. O.W.: Conceptualization, Writing- Reviewing and Editing, Supervision, Funding. All authors read and approved the final manuscript before submission.
Funding
Our previous work cited here was funded by the European Research Council (ERC grant GA336408) through a PASSME project. We would like to acknowledge Universiti Kebangsaan Malaysia for DIP-2019-006 funding. We also thank the support by the Open Access Publication Fund from University of Oldenburg.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
NIH. Mustaffa would like to acknowledge Universiti Kebangsaan Malaysia for supporting her postdoctoral research. We thank Rose Norman for proofreading this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aizawa, K.; Miyachi, S. Carbonic anhydrase and CO2 concentrating mechanisms in microalgae and cyanobacteria. FEMS Microbiol. Lett. 1986, 39, 215–233. [Google Scholar] [CrossRef]
- Whittington, D.A.; Waheed, A.; Ulmasov, B.; Shah, G.N.; Grubb, J.H.; Sly, W.S.; Christianson, D.W. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc. Natl. Acad. Sci. USA 2001, 98, 9545–9550. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D. The role of carbonic anhydrase in photosynthesis. Annu. Rev. Plant Biol. 1994, 45, 369–392. [Google Scholar] [CrossRef]
- Smith, K.S.; Ferry, J.G. Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 2000, 24, 335–366. [Google Scholar] [CrossRef] [PubMed]
- Aspatwar, A.; Haapanen, S.; Parkkila, S. An update on the metabolic roles of carbonic anhydrases in the model alga Chlamydomonas reinhardtii. Metabolites 2018, 8, 22. [Google Scholar] [CrossRef]
- Jensen, E.L.; Clement, R.; Kosta, A.; Maberly, S.C.; Gontero, B. A new widespread subclass of carbonic anhydrase in marine phytoplankton. ISME J. 2019, 13, 2094–2106. [Google Scholar] [CrossRef]
- Del Prete, S.; Vullo, D.; Fisher, G.M.; Andrews, K.T.; Poulsen, S.-A.; Capasso, C.; Supuran, C.T. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum—The η-carbonic anhydrases. Bioorg. Med. Chem. Lett. 2014, 24, 4389–4396. [Google Scholar] [CrossRef] [PubMed]
- Tripp, B.C.; Smith, K.; Ferry, J.G. Carbonic anhydrase: New insights for an ancient enzyme. J. Biol. Chem. 2001, 276, 48615–48618. [Google Scholar] [CrossRef] [PubMed]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Roberts, S.B.; Lane, T.W.; Morel, F.M.M. Carbonic anhydrase in the marine diatom Thalassiosira weissflogii (bacillariophyceae). J. Phycol. 1997, 33, 845–850. [Google Scholar] [CrossRef]
- Yee, D.; Morel, F.M. In vivo substitution of zinc by cobalt in carbonic anhydrase of a marine diatom. Limnol. Oceanogr. 1996, 41, 573–577. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, L.; Jeffrey, P.D.; Shi, Y.; Morel, F.M. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 2008, 452, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Hopkinson, B.M. Size scaling of extracellular carbonic anhydrase activity in centric marine diatoms. J. Phycol. 2015, 51, 255–263. [Google Scholar] [CrossRef]
- Jensen, E.L.; Maberly, S.C.; Gontero, B. Insights on the Functions and Ecophysiological Relevance of the Diverse Carbonic Anhydrases in Microalgae. Int. J. Mol. Sci. 2020, 21, 2922. [Google Scholar] [CrossRef] [PubMed]
- Tortell, P.D. Evolutionary and ecological perspectives on carbon acquisition in phytoplankton. Limnol. Oceanogr. 2000, 45, 744–750. [Google Scholar] [CrossRef]
- Rost, B.; Riebesell, U.; Burkhardt, S.; Sültemeyer, D. Carbon acquisition of bloom-forming marine phytoplankton. Limnol. Oceanogr. 2003, 48, 55–67. [Google Scholar] [CrossRef]
- Nimer, N.A.; Iglesias-Rodriguez, M.D.; Merrett, M.J. Bicarbonate utilization by marine phytoplankton species. J. Phycol. 1997, 33, 625–631. [Google Scholar] [CrossRef]
- Mustaffa, N.I.H.; Striebel, M.; Wurl, O. Extracellular carbonic anhydrase: Method development and its application to natural seawater. Limnol. Oceanogr. Methods 2017, 15, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Rigobello-Masini, M.; Aidar, E.; Masini, J.C. Extra and intracelular activities of carbonic anhydrase of the marine microalga Tetraselmis gracilis (Chlorophyta). Braz. J. Microbiol. 2003, 34, 267–272. [Google Scholar] [CrossRef]
- Moroney, J.V.; Husic, H.D.; Tolbert, N. Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol. 1985, 79, 177–183. [Google Scholar] [CrossRef]
- Martin, C.L.; Tortell, P.D. Bicarbonate transport and extracellular carbonic anhydrase activity in Bering Sea phytoplankton assemblages: Results from isotope disequilibrium experiments. Limnol. Oceanogr. 2006, 51, 2111–2121. [Google Scholar] [CrossRef][Green Version]
- Martin, C.L.; Tortell, P.D. Bicarbonate transport and extracellular carbonic anhydrase in marine diatoms. Physiol. Plant. 2008, 133, 106–116. [Google Scholar] [CrossRef]
- Smith-Harding, T.J.; Beardall, J.; Mitchell, J.G. The role of external carbonic anhydrase in photosynthesis during growth of the marine diatom Chaetoceros muelleri. J. Phycol. 2017, 53, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.-P.; Gao, K. Interactions of anthropogenic stress factors on marine phytoplankton. Front. Environ. Sci. 2015, 3. [Google Scholar] [CrossRef]
- Gao, K.; Campbell, D.A. Photophysiological responses of marine diatoms to elevated CO2 and decreased pH: A review. Funct. Plant Biol. 2014, 41, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, S.; Amoroso, G.; Riebesell, U.; Sültemeyer, D. CO2 and HCO3- uptake in marine diatoms acclimated to different CO2 concentrations. Limnol. Oceanogr. 2001, 46, 1378–1391. [Google Scholar] [CrossRef]
- Shi, D.; Hong, H.; Su, X.; Liao, L.; Chang, S.; Lin, W. The physiological response of marine diatoms to ocean acidification: Differential roles of seawater pCO2 and pH. J. Phycol. 2019, 55, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Jin, P.; Zou, D.; Liu, Y.; Xia, J. Responses of carbonic anhydrases and Rubisco to abrupt CO2 changes of seawater in two marine diatoms. Environ. Sci. Pollut. Res. 2019, 26, 16388–16395. [Google Scholar] [CrossRef] [PubMed]
- Riebesell, U.; Zondervan, I.; Rost, B.; Tortell, P.D.; Zeebe, R.E.; Morel, F.M. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 2000, 407, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Carbon fixation and carbon availability in marine phytoplankton. Photosynth. Res. 1994, 39, 259–273. [Google Scholar] [CrossRef]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef]
- Young, J.N.; Heureux, A.M.C.; Sharwood, R.E.; Rickaby, R.E.M.; Morel, F.M.M.; Whitney, S.M. Large variation in the Rubisco kinetics of diatoms reveals diversity among their carbon-concentrating mechanisms. J. Exp. Bot. 2016, 67, 3445–3456. [Google Scholar] [CrossRef] [PubMed]
- Badger, M.R.; Andrews, T.J.; Whitney, S.M.; Ludwig, M.; Yellowlees, D.C.; Leggat, W.; Price, G.D. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can. J. Bot. 1998, 76, 1052–1071. [Google Scholar] [CrossRef]
- Heureux, A.M.C.; Young, J.N.; Whitney, S.M.; Eason-Hubbard, M.R.; Lee, R.B.Y.; Sharwood, R.E.; Rickaby, R.E.M. The role of Rubisco kinetics and pyrenoid morphology in shaping the CCM of haptophyte microalgae. J. Exp. Bot. 2017, 68, 3959–3969. [Google Scholar] [CrossRef] [PubMed]
- Husic, D.W.; Husic, H.D.; Tolbert, N.E.; Black, C.C. The oxidative photosynthetic carbon cycle or C2 cycle. Crit. Rev. Plant Sci. 1987, 5, 45–100. [Google Scholar] [CrossRef]
- Samukawa, M.; Shen, C.; Hopkinson, B.M.; Matsuda, Y. Localization of putative carbonic anhydrases in the marine diatom, Thalassiosira pseudonana. Photosynth. Res. 2014, 121, 235–249. [Google Scholar] [CrossRef]
- Tachibana, M.; Allen, A.E.; Kikutani, S.; Endo, Y.; Bowler, C.; Matsuda, Y. Localization of putative carbonic anhydrases in two marine diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana. Photosynth. Res. 2011, 109, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, B.M.; Meile, C.; Shen, C. Quantification of Extracellular Carbonic Anhydrase Activity in Two Marine Diatoms and Investigation of Its Role. Plant Physiol. 2013, 162, 1142–1152. [Google Scholar] [CrossRef]
- Tortell, P.D.; Payne, C.; Gueguen, C.; Strzepek, R.F.; Boyd, P.W.; Rost, B. Inorganic carbon uptake by Southern Ocean phytoplankton. Limnol. Oceanogr. 2008, 53, 1266–1278. [Google Scholar] [CrossRef]
- Trimborn, S.; Wolf-Gladrow, D.; Richter, K.-U.; Rost, B. The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. J. Exp. Mar. Biol. Ecol. 2009, 376, 26–36. [Google Scholar] [CrossRef]
- Trimborn, S.; Lundholm, N.; Thoms, S.; Richter, K.U.; Krock, B.; Hansen, P.J.; Rost, B. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: The effect of pH-induced changes in seawater carbonate chemistry. Physiol. Plant. 2008, 133, 92–105. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Reinfelder, J.R.; Roberts, S.B.; Chamberlain, C.P.; Lee, J.G.; Yee, D. Zinc and carbon co-limitation of marine phytoplankton. Nature 1994, 369, 740–742. [Google Scholar] [CrossRef]
- Facchini, M.C.; Rinaldi, M.; Decesari, S.; Carbone, C.; Finessi, E.; Mircea, M.; Fuzzi, S.; Ceburnis, D.; Flanagan, R.; Nilsson, E.D.; et al. Primary submicron marine aerosol dominated by insoluble organic colloids and aggregates. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Emerson, S. Enhanced transport of carbon dioxide during gas exchange. In Air-Water Gas Transfer; Jähne, B., Monahan, E., Eds.; AEON Verlag & Studio Hanau: Heidelberg, Germany, 1995; pp. 23–36. [Google Scholar]
- Lee, J.G.; Roberts, S.B.; Morel, F.M.M. Cadmium: A nutrient for the marine diatom Thalassiosira weissflogii. Limnol. Oceanogr. 1995, 40, 1056–1063. [Google Scholar] [CrossRef]
- Lee, J.G.; Morel, F.M.M. Replacement of zinc by cadmium in marine phytoplankton. Mar. Ecol. Prog. Ser. 1995, 127, 305–309. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Price, N.M. The Biogeochemical Cycles of Trace Metals in the Oceans. Science 2003, 300, 944–947. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Lam, P.J.; Saito, M.A. Trace Metal Substitution in Marine Phytoplankton. Annu. Rev. Earth Planet Sci. 2020, 48, 491–517. [Google Scholar] [CrossRef]
- Li, W.; Sunda, W.G.; Lin, W.; Hong, H.; Shi, D. The effect of cell size on cellular Zn and Cd and Zn-Cd-CO2 colimitation of growth rate in marine diatoms. Limnol. Oceanogr. 2020, 65, 2896–2911. [Google Scholar] [CrossRef]
- Subhas, A.V.; Adkins, J.F.; Dong, S.; Rollins, N.E.; Berelson, W.M. The carbonic anhydrase activity of sinking and suspended particles in the North Pacific Ocean. Limnol. Oceanogr. 2019, 65, 637–651. [Google Scholar] [CrossRef]
- Liao, W.-H.; Yang, S.-C.; Ho, T.-Y. Trace metal composition of size-fractionated plankton in the Western Philippine Sea: The impact of anthropogenic aerosol deposition. Limnol. Oceanogr. 2017, 62, 2243–2259. [Google Scholar] [CrossRef]
- Gruber, N.; Clement, D.; Carter, B.R.; Feely, R.A.; van Heuven, S.; Hoppema, M.; Ishii, M.; Key, R.M.; Kozyr, A.; Lauvset, S.K.; et al. The oceanic sink for anthropogenic CO2; from 1994 to 2007. Science 2019, 363, 1193. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A; IPCC: Cambridge, UK, 2014. [Google Scholar]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidification: The other CO2 problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Gladrow, D.A.; Riebesell, U.L.F.; Burkhardt, S.; Bijma, J. Direct effects of CO2 concentration on growth and isotopic composition of marine plankton. Tellus B 1999, 51, 461–476. [Google Scholar] [CrossRef]
- Tortell, P.D.; DiTullio, G.R.; Sigman, D.M.; Morel, F.M. CO2 effects on taxonomic composition and nutrient utilization in an Equatorial Pacific phytoplankton assemblage. Mar. Ecol. Prog. Ser. 2002, 236, 37–43. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Laufkötter, C.; Vogt, M.; Gruber, N. Long-term trends in ocean plankton production and particle export between 1960–2006. Biogeosciences 2013, 10, 7373–7393. [Google Scholar] [CrossRef]
- Rost, B.; Kranz, S.; Richter, K.-U.; Tortell, P.D. Isotope disequilibrium and mass spectrometric studies of inorganic carbon acquisition by phytoplankton. Limnol. Oceanogr. Methods 2007, 5, 328–337. [Google Scholar] [CrossRef]
- Roberts, K.; Granum, E.; Leegood, R.C.; Raven, J.A. C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiol. 2007, 145, 230–235. [Google Scholar] [CrossRef]
- Del Prete, S.; Vullo, D.; De Luca, V.; Supuran, C.T.; Capasso, C. Biochemical characterization of the δ-carbonic anhydrase from the marine diatom Thalassiosira weissflogii, TweCA. J. Enzyme Inhib. Med. Chem. 2014, 29, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Elzenga, J.T.M.; Prins, H.B.A.; Stefels, J. The role of extracellular carbonic anhydrase activity in inorganic carbon utilization of Phaeocystis globosa (Prymnesiophyceae): A comparison with other marine algae using the isotopic disequilibrium technique. Limnol. Oceanogr. 2000, 45, 372–380. [Google Scholar] [CrossRef]
- Shi, Q.; Xiahou, W.; Wu, H. Photosynthetic responses of the marine diatom Thalassiosira pseudonana to CO2-induced seawater acidification. Hydrobiologia 2017, 788, 361–369. [Google Scholar] [CrossRef]
- Tortell, P.D.; Morel, F.M. Sources of inorganic carbon for phytoplankton in the eastern Subtropical and Equatorial Pacific Ocean. Limnol. Oceanogr. 2002, 47, 1012–1022. [Google Scholar] [CrossRef]
- Young, J.N.; Kranz, S.A.; Goldman, J.A.; Tortell, P.D.; Morel, F.M. Antarctic phytoplankton down-regulate their carbon-concentrating mechanisms under high CO2 with no change in growth rates. Mar. Ecol. Prog. Ser. 2015, 532, 13–28. [Google Scholar] [CrossRef]
- Kranz, S.A.; Young, J.N.; Hopkinson, B.M.; Goldman, J.A.; Tortell, P.D.; Morel, F.M. Low temperature reduces the energetic requirement for the CO2 concentrating mechanism in diatoms. New Phytol. 2015, 205, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Deppeler, S.; Petrou, K.; Schulz, K.G.; Westwood, K.; Pearce, I.; McKinlay, J.; Davidson, A. Ocean acidification of a coastal Antarctic marine microbial community reveals a critical threshold for CO2 tolerance in phytoplankton productivity. Biogeosciences 2018, 15, 209–231. [Google Scholar] [CrossRef]
- Rahlff, J.; Khodami, S.; Voskuhl, L.; Humphreys, M.; Stolle, C.; Arbizu, P.M.; Wurl, O.; Ribas-Ribas, M. Short-term responses to ocean acidification: Effects on relative abundance of eukaryotic plankton from the tropical Timor Sea. Mar. Ecol. Prog. Ser. 2020, 658, 59–74. [Google Scholar] [CrossRef]
- Tortell, P.D.; Martin, C.L.; Corkum, M.E. Inorganic carbon uptake and intracellular assimilation by subarctic Pacific phytoplankton assemblages. Limnol. Oceanogr. 2006, 51, 2102–2110. [Google Scholar] [CrossRef]
- Trimborn, S.; Brenneis, T.; Sweet, E.; Rost, B. Sensitivity of Antarctic phytoplankton species to ocean acidification: Growth, carbon acquisition, and species interaction. Limnol. Oceanogr. 2013, 58, 997–1007. [Google Scholar] [CrossRef]
- Neven, I.A.; Stefels, J.; van Heuven, S.M.A.C.; de Baar, H.J.W.; Elzenga, J.T.M. High plasticity in inorganic carbon uptake by Southern Ocean phytoplankton in response to ambient CO2. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 2636–2646. [Google Scholar] [CrossRef][Green Version]
- Cassar, N.; Laws, E.A.; Bidigare, R.R.; Popp, B.N. Bicarbonate uptake by Southern Ocean phytoplankton. Glob. Biogeochem. 2004, 18, GB2003. [Google Scholar] [CrossRef]
- Silverman, D.; Tu, C. Buffer dependence of carbonic anhydrase catalyzed oxygen-18 exchange at equilibrium. J. Am. Chem. Soc. 1975, 97, 2263–2269. [Google Scholar] [CrossRef]
- Hopkinson, B.M.; Dupont, C.L.; Allen, A.E.; Morel, F.M.M. Efficiency of the CO2 concentrating mechanism of diatoms. Proc. Natl. Acad. Sci. USA 2011, 108, 3830–3837. [Google Scholar] [CrossRef] [PubMed]
- Tortell, P.D.; Payne, C.D.; Li, Y.; Trimborn, S.; Rost, B.; Smith, W.O.; Riesselman, C.; Dunbar, R.B.; Sedwick, P.; DiTullio, G.R. CO2 sensitivity of Southern Ocean phytoplankton. Geophys. Res. Lett. 2008, 35, L04605. [Google Scholar] [CrossRef]
- Reinfelder, J.R. Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annu. Rev. Mar. Sci. 2011, 3, 291–315. [Google Scholar] [CrossRef]
- Sett, S.; Schulz, K.G.; Bach, L.T.; Riebesell, U. Shift towards larger diatoms in a natural phytoplankton assemblage under combined high-CO2 and warming conditions. J. Plankton Res. 2018, 40, 391–406. [Google Scholar] [CrossRef]
- Gao, K.; Xu, J.; Gao, G.; Li, Y.; Hutchins, D.A.; Huang, B.; Wang, L.; Zheng, Y.; Jin, P.; Cai, X.; et al. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat. Clim. Chang. 2012, 2, 519–523. [Google Scholar] [CrossRef]
- Passow, U.; Carlson, C.A. The biological pump in a high CO2 world. Mar. Ecol. Prog. Ser. 2012, 470, 249–271. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Effect of Zn, Mn, and Fe on Cd accumulation in phytoplankton: Implications for oceanic Cd cycling. Limnol. Oceanogr. 2000, 45, 1501–1516. [Google Scholar] [CrossRef]
- Gao, K.; Helbling, E.W.; Häder, D.-P.; Hutchins, D.A. Responses of marine primary producers to interactions between ocean acidification, solar radiation, and warming. Mar. Ecol. Prog. Ser. 2012, 470, 167–189. [Google Scholar] [CrossRef]
- Qu, L.; Campbell, D.A.; Gao, K. Ocean acidification interacts with growth light to suppress CO2 acquisition efficiency and enhance mitochondrial respiration in a coastal diatom. Mar. Pollut. Bull. 2021, 163, 112008. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, K.; Villafañe, V.; Helbling, E. Ocean acidification mediates photosynthetic response to UV radiation and temperature increase in the diatom Phaeodactylum tricornutum. Biogeosciences 2012, 9, 3931–3942. [Google Scholar] [CrossRef]
- Chen, X.; Gao, K. Effect of CO2 concentrations on the activity of photosynthetic CO2 fixation and extracelluar carbonic anhydrase in the marine diatom Skeletonema costatum. Chin. Sci. Bull. 2003, 48, 2616–2620. [Google Scholar] [CrossRef]
- Wu, H.; Gao, K. Ultraviolet radiation stimulated activity of extracellular carbonic anhydrase in the marine diatom Skeletonema costatum. Funct. Plant Biol. 2009, 36, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Jin, P.; Jiang, Y.; Yang, H.; Zhong, J.; Liang, Z.; Guo, Y.; Li, P.; Huang, Q.; Pan, J.; et al. Light alters the responses of two marine diatoms to increased warming. Mar. Environ. Res. 2020, 154, 104871. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Libby, W. Equilibration of atmospheric carbon dioxide with sea water: Possible enzymatic control of the rate. Science 1969, 164, 1395–1397. [Google Scholar] [CrossRef]
- Coleman, J.E. Chemical reactions of sulfonamides with carbonic anhydrase. Annu. Rev. Pharmacol. 1975, 15, 221–242. [Google Scholar] [CrossRef]
- Wurl, O.; Wurl, E.; Miller, L.; Johnson, K.; Vagle, S. Formation and global distribution of sea-surface microlayers. Biogeosciences 2011, 8, 121–135. [Google Scholar] [CrossRef]
- Cunliffe, M.; Engel, A.; Frka, S.; Gašparović, B.; Guitart, C.; Murrell, J.C.; Salter, M.; Stolle, C.; Upstill-Goddard, R.; Wurl, O. Sea surface microlayers: A unified physicochemical and biological perspective of the air–ocean interface. Prog. Oceanogr. 2013, 109, 104–116. [Google Scholar] [CrossRef]
- Wurl, O.; Stolle, C.; Van Thuoc, C.; The Thu, P.; Mari, X. Biofilm-like properties of the sea surface and predicted effects on air–sea CO2 exchange. Prog. Oceanogr. 2016, 144, 15–24. [Google Scholar] [CrossRef]
- Sieburth, J.M.; Willis, P.-J.; Johnson, K.M.; Burney, C.M.; Lavoie, D.M.; Hinga, K.R.; Caron, D.A.; FRENCH, F.W.; Johnson, P.W.; Davis, P.G. Dissolved organic matter and heterotrophic microneuston in the surface microlayers of the North Atlantic. Science 1976, 194, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.T.; Apts, C.W. The sea-surface microlayer: Phytoneuston productivity and effects of atmospheric particulate matter. Mar. Biol. 1984, 82, 293–300. [Google Scholar] [CrossRef]
- Hardy, J.T. The sea surface microlayer: Biology, chemistry and anthropogenic enrichment. Prog. Oceanogr. 1982, 11, 307–328. [Google Scholar] [CrossRef]
- Mustaffa, N.I.H.; Kallajoki, L.; Hillebrand, H.; Wurl, O.; Striebel, M. Sea surface phytoplankton community responses to nutrient and light changes. Mar. Biol. 2020, 167, 1–15. [Google Scholar] [CrossRef]
- Hobson, L.A.; Hanson, C.E.; Holeton, C. An ecological basis for extracellular carbonic anhydrase in marine unicellular algae. J. Phycol. 2001, 37, 717–723. [Google Scholar] [CrossRef]
- Mustaffa, N.I.H.; Striebel, M.; Wurl, O. Enrichment of extracellular carbonic anhydrase in the sea surface microlayer and its effect on air-sea CO2 exchange. Geophys. Res. Lett. 2017, 44, 2017GL075797. [Google Scholar] [CrossRef]
- Wurl, O.; Obbard, J.P. A review of pollutants in the sea-surface microlayer (SML): A unique habitat for marine organisms. Mar. Pollut. Bull. 2004, 48, 1016–1030. [Google Scholar] [CrossRef]
- Ebling, A.M.; Landing, W.M. Trace elements in the sea surface microlayer: Rapid responses to changes in aerosol deposition. Elementa-Sci. Anthrop. 2017, 5, 42. [Google Scholar] [CrossRef]
- Mustaffa, N.I.H.; Badewien, T.H.; Ribas-Ribas, M.; Wurl, O. High-resolution observations on enrichment processes in the sea-surface microlayer. Sci. Rep. 2018, 8, 13122. [Google Scholar] [CrossRef]
- Maki, J.S. Neuston Microbiology: Life at the Air–Water Interface. In Encyclopedia of Environmental Microbiology; Bitton, G., Ed.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Watson, A.J.; Schuster, U.; Shutler, J.D.; Holding, T.; Ashton, I.G.C.; Landschützer, P.; Woolf, D.K.; Goddijn-Murphy, L. Revised estimates of ocean-atmosphere CO2 flux are consistent with ocean carbon inventory. Nat. Commun. 2020, 11, 4422. [Google Scholar] [CrossRef]
- Keller, K. Chemical Enhancement of Carbon Dioxide Transfer Across the Air-Sea Interface. Massachusetts Institute of Technology. 1994. Available online: https://dspace.mit.edu/bitstream/handle/1721.1/35997/32162323-MIT.pdf?sequence=2 (accessed on 18 March 2018).
- Feely, R.A.; Wanninkhof, R.; Landschützer, P.; Carter, B.R.; Triñanes, J.A. Global ocean carbon cycle [in “State of the Climate in 2016”]. Bull. Am. Meteorol. Soc. 2017, 98, S89–S92. [Google Scholar] [CrossRef]
- Williams, P.M.; Carlucci, A.F.; Henrichs, S.M.; Van Vleet, E.S.; Horrigan, S.G.; Reid, F.M.H.; Robertson, K.J. Chemical and microbiological studies of sea-surface films in the Southern Gulf of California and off the West Coast of Baja California. Mar. Chem. 1986, 19, 17–98. [Google Scholar] [CrossRef]
- Bischof, K.; Kräbs, G.; Wiencke, C.; Hanelt, D. Solar ultraviolet radiation affects the activity of ribulose-1, 5-bisphosphate carboxylase-oxygenase and the composition of photosynthetic and xanthophyll cycle pigments in the intertidal green alga Ulva lactuca L. Planta 2002, 215, 502–509. [Google Scholar] [CrossRef]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing Ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B Biol. 2014, 137, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.E.; Zepp, R.G.; Lucas, R.M.; Madronich, S.; Austin, A.T.; Ballaré, C.L.; Norval, M.; Sulzberger, B.; Bais, A.F.; McKenzie, R.L. Solar ultraviolet radiation in a changing climate. Nat. Clim. Chang. 2014, 4, 434–441. [Google Scholar] [CrossRef]
- Frew, N.M.; Goldman, J.C.; Dennett, M.R.; Johnson, A.S. Impact of phytoplankton-generated surfactants on air-sea gas exchange. J. Geophys. Res. Oceans 1990, 95, 3337–3352. [Google Scholar] [CrossRef]
- Mustaffa, N.I.H.; Ribas-Ribas, M.; Banko-Kubis, H.M.; Wurl, O. Global reduction of in situ CO2 transfer velocity by natural surfactants in the sea-surface microlayer. Proc. R. Soc. A 2020, 476, 20190763. [Google Scholar] [CrossRef] [PubMed]
- Calleja, M.L.; Duarte, C.M.; Navarro, N.; Agustí, S. Control of air-sea CO2 disequilibria in the subtropical NE Atlantic by planktonic metabolism under the ocean skin. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Wurl, O.; Ekau, W.; Landing, W.M.; Zappa, C.J. Sea surface microlayer in a changing ocean–A perspective. Elementa-Sci. Anthrop. 2017, 5, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Song, S.-H.; Qi, Y.-Z. A comparative study of phytoneuston and the phytoplankton community structure in Daya Bay, South China Sea. J. Sea Res. 2014, 85, 474–482. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).