Adeno-Associated Viral Vectors as Versatile Tools for Parkinson’s Research, Both for Disease Modeling Purposes and for Therapeutic Uses
Abstract
1. AAV Landscapes
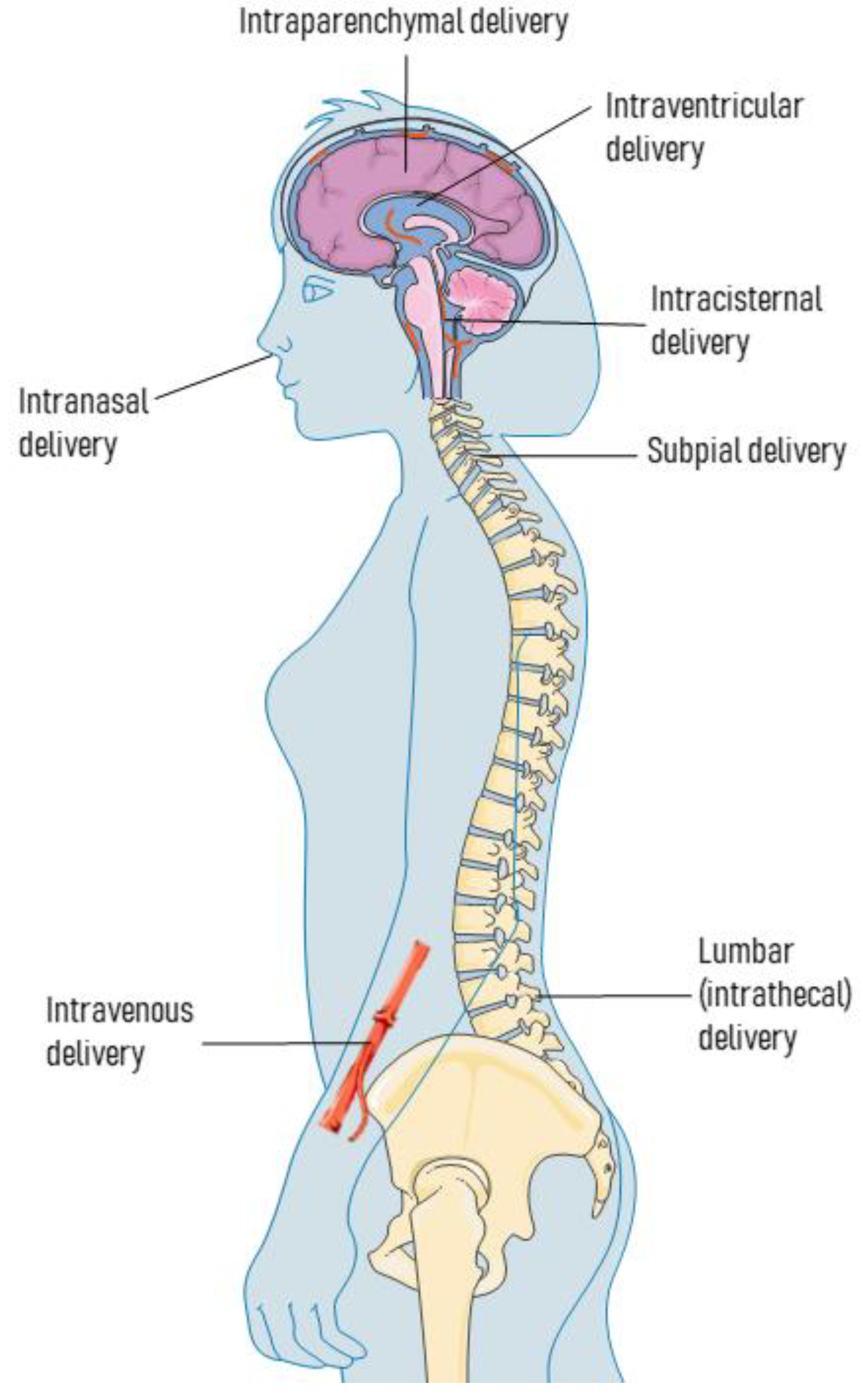
2. Routes for AAV Delivery
2.1. Intraparenchymal Deliveries
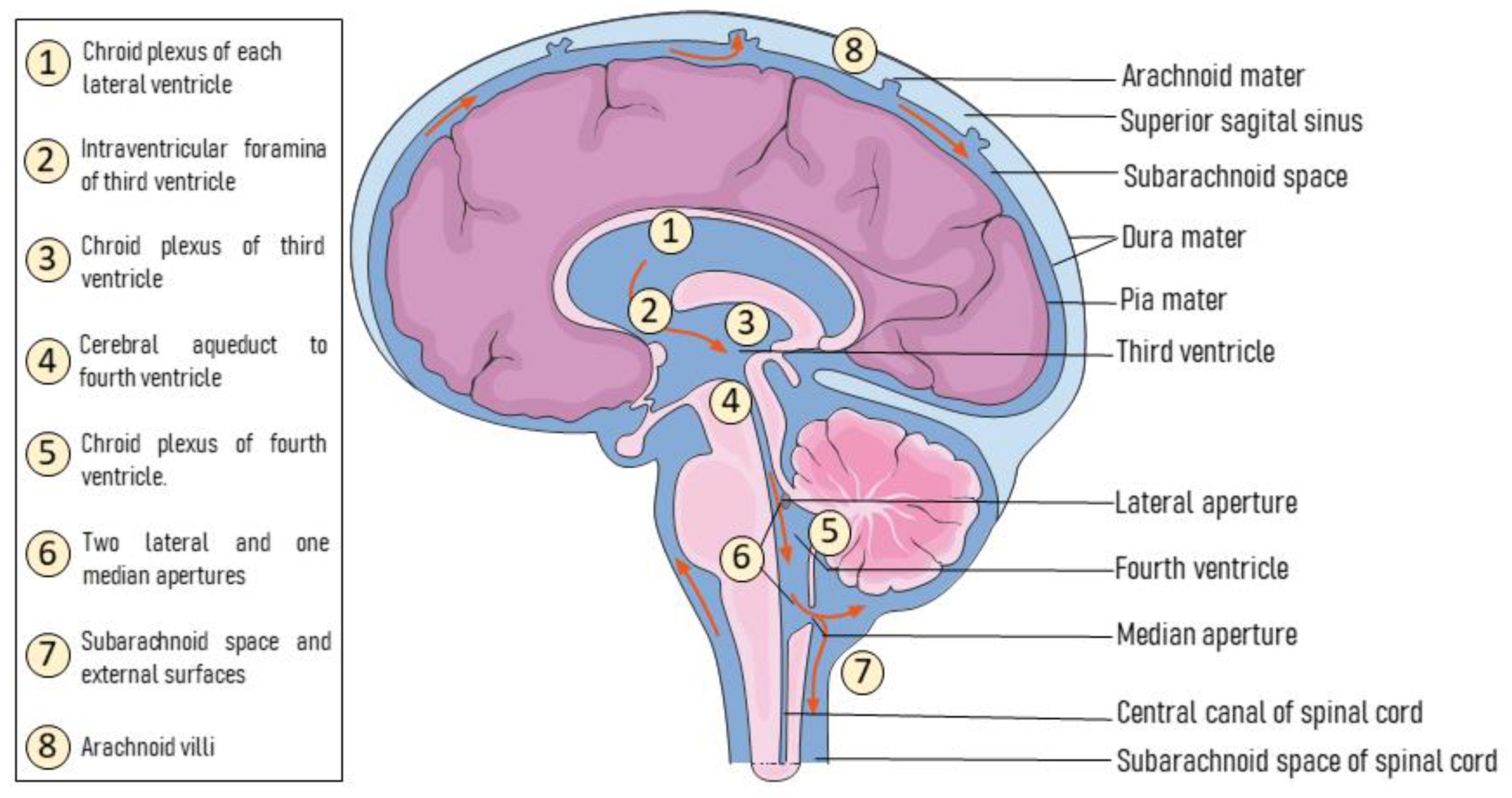
2.2. Intra-CSF Deliveries
2.3. Intravenous Deliveries
2.4. Other Delivery Routes
3. AAVs for Animal Modeling of Parkinson’s Disease
3.1. Models Based on AAV-Mediated α-syn Overexpression
3.2. Models Based on AAV-Mediated Neuromelanin Overexpression
3.3. Other AAV-Based Models
4. Use of AAV-Mediated Gene Transfer for Therapeutic Purposes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murlidharan, G.; Samulski, R.J.; Asokan, A. Biology of Adeno-Associated Viral Vectors in the Central Nervous System. Front. Mol. Neurosci. 2014, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Atchison, R.W.; Casto, B.C.; Hammon, W.M. Adenovirus-Associated Defective Virus Particles. Science 1965, 149, 754–756. [Google Scholar] [CrossRef]
- Tseng, Y.-S.; Agbandje-McKenna, M. Mapping the AAV Capsid Host Antibody Response toward the Development of Second Generation Gene Delivery Vectors. Front. Immunol. 2014, 5, 9. [Google Scholar] [CrossRef]
- Salganik, M.; Hirsch, M.L.; Samulski, R.J. Adeno-Associated Virus as a Mammalian DNA Vector. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, J.; Nobre, R.J.; Pereira de Almeida, L. Gene Therapy for the CNS Using AAVs: The Impact of Systemic Delivery by AAV9. J. Control. Release 2016, 241, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Haberman, R.P.; McCown, T.J.; Samulski, R.J. Inducible Long-Term Gene Expression in Brain with Adeno-Associated Virus Gene Transfer. Gene Ther. 1998, 5, 1604–1611. [Google Scholar] [CrossRef]
- McCarty, D.M.; Young, S.M.; Samulski, R.J. Integration of Adeno-Associated Virus (AAV) and Recombinant AAV Vectors. Annu. Rev. Genet. 2004, 38, 819–845. [Google Scholar] [CrossRef] [PubMed]
- Flotte, T.R.; Carter, B.J. Adeno-Associated Virus Vectors for Gene Therapy. Gene Ther. 1995, 2, 357–362. [Google Scholar]
- Carter, P.J.; Samulski, R.J. Adeno-Associated Viral Vectors as Gene Delivery Vehicles. Int. J. Mol. Med. 2000, 6, 17–27. [Google Scholar] [CrossRef]
- Gaj, T.; Sirk, S.J.; Shui, S.-L.; Liu, J. Genome-Editing Technologies: Principles and Applications. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Hadaczek, P.; Eberling, J.L.; Pivirotto, P.; Bringas, J.; Forsayeth, J.; Bankiewicz, K.S. Eight Years of Clinical Improvement in MPTP-Lesioned Primates after Gene Therapy with AAV2-HAADC. Mol. Ther. 2010, 18, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Shera, D.; McPhee, S.W.J.; Francis, J.S.; Kolodny, E.H.; Bilaniuk, L.T.; Wang, D.-J.; Assadi, M.; Goldfarb, O.; Goldman, H.W.; et al. Long-Term Follow-up after Gene Therapy for Canavan Disease. Sci. Transl. Med. 2012, 4, 165ra163. [Google Scholar] [CrossRef]
- Aly, A.E.-E.; Waszczak, B.L. Intranasal Gene Delivery for Treating Parkinson’s Disease: Overcoming the Blood–Brain Barrier. Expert Opin. Drug Deliv. 2015, 12, 1923–1941. [Google Scholar] [CrossRef]
- Rehman, S.; Nabi, B.; Zafar, A.; Baboota, S.; Ali, J. Intranasal Delivery of Mucoadhesive Nanocarriers: A Viable Option for Parkinson’s Disease Treatment? Expert Opin. Drug Deliv. 2019, 16, 1355–1366. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- de Lima, M.C.P.; da Cruz, M.T.G.; Cardoso, A.L.C.; Simoes, S.; de Almeida, L.P. Liposomal and Viral Vectors for Gene Therapy of the Central Nervous System. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 453–465. [Google Scholar] [CrossRef]
- Gao, G.; Zhong, L.; Danos, O. Exploiting Natural Diversity of AAV for the Design of Vectors with Novel Properties. Methods Mol. Biol. 2011, 807, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Haery, L.; Deverman, B.E.; Matho, K.S.; Cetin, A.; Woodard, K.; Cepko, C.; Guerin, K.I.; Rego, M.A.; Ersing, I.; Bachle, S.M.; et al. Adeno-Associated Virus Technologies and Methods for Targeted Neuronal Manipulation. Front. Neuroanat. 2019, 13, 93. [Google Scholar] [CrossRef]
- Powell, S.K.; Samulski, R.J.; McCown, T.J. AAV Capsid-Promoter Interactions Determine CNS Cell-Selective Gene Expression In Vivo. Mol. Ther. 2020, 28, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, M.O.; McCown, T.J.; Powell, S.K.; El-Nahal, H.G.; Daw, T.; Basso, M.A.; Sommer, M.A.; Samulski, R.J. Adeno-Associated Virus Capsid-Promoter Interactions in the Brain Translate from Rat to the Nonhuman Primate. Hum. Gene Ther. 2020, 31, 1155–1168. [Google Scholar] [CrossRef]
- Pignataro, D.; Sucunza, D.; Vanrell, L.; Lopez-Franco, E.; Dopeso-Reyes, I.G.; Vales, A.; Hommel, M.; Rico, A.J.; Lanciego, J.L.; Gonzalez-Aseguinolaza, G. Adeno-Associated Viral Vectors Serotype 8 for Cell-Specific Delivery of Therapeutic Genes in the Central Nervous System. Front. Neuroanat. 2017, 11, 2. [Google Scholar] [CrossRef]
- Pignataro, D.; Sucunza, D.; Rico, A.J.; Dopeso-Reyes, I.G.; Roda, E.; Rodríguez-Perez, A.I.; Labandeira-Garcia, J.L.; Broccoli, V.; Kato, S.; Kobayashi, K.; et al. Gene Therapy Approaches in the Non-Human Primate Model of Parkinson’s Disease. J. Neural. Transm. 2018, 125, 575–589. [Google Scholar] [CrossRef]
- Brady, M.L.; Raghavan, R.; Singh, D.; Anand, P.J.; Fleisher, A.S.; Mata, J.; Broaddus, W.C.; Olbricht, W.L. In Vivo Performance of a Microfabricated Catheter for Intraparenchymal Delivery. J. Neurosci. Methods 2014, 229, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Burger, C.; Gorbatyuk, O.S.; Velardo, M.J.; Peden, C.S.; Williams, P.; Zolotukhin, S.; Reier, P.J.; Mandel, R.J.; Muzyczka, N. Recombinant AAV Viral Vectors Pseudotyped with Viral Capsids from Serotypes 1, 2, and 5 Display Differential Efficiency and Cell Tropism after Delivery to Different Regions of the Central Nervous System. Mol. Ther. 2004, 10, 302–317. [Google Scholar] [CrossRef]
- Joniec-Maciejak, I.; Ciesielska, A.; Wawer, A.; Sznejder-Pachołek, A.; Schwenkgrub, J.; Cudna, A.; Hadaczek, P.; Bankiewicz, K.S.; Członkowska, A.; Członkowski, A. The Influence of AAV2-Mediated Gene Transfer of Human IL-10 on Neurodegeneration and Immune Response in a Murine Model of Parkinson’s Disease. Pharmacol. Rep. 2014, 66, 660–669. [Google Scholar] [CrossRef]
- Theodore, S.; Cao, S.; McLean, P.J.; Standaert, D.G. Targeted Overexpression of Human Alpha-Synuclein Triggers Microglial Activation and an Adaptive Immune Response in a Mouse Model of Parkinson Disease. J. Neuropathol. Exp. Neurol. 2008, 67, 1149–1158. [Google Scholar] [CrossRef]
- Cao, S.; Theodore, S.; Standaert, D.G. Fcγ Receptors Are Required for NF-ΚB Signaling, Microglial Activation and Dopaminergic Neurodegeneration in an AAV-Synuclein Mouse Model of Parkinson’s Disease. Mol. Neurodegener. 2010, 5, 42. [Google Scholar] [CrossRef]
- Thome, A.D.; Standaert, D.G.; Harms, A.S. Fractalkine Signaling Regulates the Inflammatory Response in an α-Synuclein Model of Parkinson Disease. PLoS ONE 2015, 10, e0140566. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Tao, Q.; Chen, S.; Le, W. Adeno-Associated Virus Type 2 Vector-Mediated Glial Cell Line-Derived Neurotrophic Factor Gene Transfer Induces Neuroprotection and Neuroregeneration in a Ubiquitin-Proteasome System Impairment Animal Model of Parkinson’s Disease. Neurodegener. Dis. 2013, 11, 113–128. [Google Scholar] [CrossRef]
- Steidinger, T.U.; Slone, S.R.; Ding, H.; Standaert, D.G.; Yacoubian, T.A. Angiogenin in Parkinson Disease Models: Role of Akt Phosphorylation and Evaluation of AAV-Mediated Angiogenin Expression in MPTP Treated Mice. PLoS ONE 2013, 8, e56092. [Google Scholar] [CrossRef]
- Hadaczek, P.; Wu, G.; Sharma, N.; Ciesielska, A.; Bankiewicz, K.; Davidow, A.L.; Lu, Z.-H.; Forsayeth, J.; Ledeen, R.W. GDNF Signaling Implemented by GM1 Ganglioside; Failure in Parkinson’s Disease and GM1-Deficient Murine Model. Exp. Neurol. 2015, 263, 177–189. [Google Scholar] [CrossRef]
- Klein, R.L.; Hamby, M.E.; Sonntag, C.F.; Millard, W.J.; King, M.A.; Meyer, E.M. Measurements of Vector-Derived Neurotrophic Factor and Green Fluorescent Protein Levels in the Brain. Methods 2002, 28, 286–292. [Google Scholar] [CrossRef]
- Bäck, S.; Peränen, J.; Galli, E.; Pulkkila, P.; Lonka-Nevalaita, L.; Tamminen, T.; Voutilainen, M.H.; Raasmaja, A.; Saarma, M.; Männistö, P.T.; et al. Gene Therapy with AAV2-CDNF Provides Functional Benefits in a Rat Model of Parkinson’s Disease. Brain Behav. 2013, 3, 75–88. [Google Scholar] [CrossRef]
- Ciesielska, A.; Mittermeyer, G.; Hadaczek, P.; Kells, A.P.; Forsayeth, J.; Bankiewicz, K.S. Anterograde Axonal Transport of AAV2-GDNF in Rat Basal Ganglia. Mol. Ther. 2011, 19, 922–927. [Google Scholar] [CrossRef]
- Ciesielska, A.; Sharma, N.; Beyer, J.; Forsayeth, J.; Bankiewicz, K. Carbidopa-Based Modulation of the Functional Effect of the AAV2-HAADC Gene Therapy in 6-OHDA Lesioned Rats. PLoS ONE 2015, 10, e0122708. [Google Scholar] [CrossRef]
- Moon, H.C.; Won, S.Y.; Kim, E.G.; Kim, H.K.; Cho, C.B.; Park, Y.S. Effect of Optogenetic Modulation on Entopeduncular Input Affects Thalamic Discharge and Behavior in an AAV2-α-Synuclein-Induced Hemiparkinson Rat Model. Neurosci. Lett. 2018, 662, 129–135. [Google Scholar] [CrossRef]
- Gasmi, M.; Herzog, C.D.; Brandon, E.P.; Cunningham, J.J.; Ramirez, G.A.; Ketchum, E.T.; Bartus, R.T. Striatal Delivery of Neurturin by CERE-120, an AAV2 Vector for the Treatment of Dopaminergic Neuron Degeneration in Parkinson’s Disease. Mol. Ther. 2007, 15, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, M.; Brandon, E.P.; Herzog, C.D.; Wilson, A.; Bishop, K.M.; Hofer, E.K.; Cunningham, J.J.; Printz, M.A.; Kordower, J.H.; Bartus, R.T. AAV2-Mediated Delivery of Human Neurturin to the Rat Nigrostriatal System: Long-Term Efficacy and Tolerability of CERE-120 for Parkinson’s Disease. NeuroBiol. Dis. 2007, 27, 67–76. [Google Scholar] [CrossRef]
- Johnston, L.C.; Su, X.; Maguire-Zeiss, K.; Horovitz, K.; Ankoudinova, I.; Guschin, D.; Hadaczek, P.; Federoff, H.J.; Bankiewicz, K.; Forsayeth, J. Human Interleukin-10 Gene Transfer Is Protective in a Rat Model of Parkinson’s Disease. Mol. Ther. 2008, 16, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Nordström, U.; Kügler, S.; Brundin, P. Pre-Existing Immunity to Adeno-Associated Virus (AAV)2 Limits Transgene Expression Following Intracerebral AAV2-Based Gene Delivery in a 6-Hydroxydopamine Model of Parkinson’s Disease. J. Gene Med. 2014, 16, 300–308. [Google Scholar] [CrossRef]
- Zheng, D.; Jiang, X.; Zhao, J.; Duan, D.; Zhao, H.; Xu, Q. Subthalamic HGAD65 Gene Therapy and Striatum TH Gene Transfer in a Parkinson’s Disease Rat Model. Neural. Plast. 2013, 2013, 263287. [Google Scholar] [CrossRef][Green Version]
- Fitzsimons, H.L.; Riban, V.; Bland, R.J.; Wendelken, J.L.; Sapan, C.V.; During, M.J. Biodistribution and Safety Assessment of AAV2-GAD Following Intrasubthalamic Injection in the Rat. J. Gene Med. 2010, 12, 385–398. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, B.R.; Rocha, E.M.; Castro, S.L.; Greenamyre, J.T. Protection from α-Synuclein Induced Dopaminergic Neurodegeneration by Overexpression of the Mitochondrial Import Receptor TOM20. NPJ Parkinsons Dis. 2020, 6, 38. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, T.; Gong, X.; Hu, G.; Ding, W.; Wang, X. AAV2-Mediated Striatum Delivery of Human CDNF Prevents the Deterioration of Midbrain Dopamine Neurons in a 6-Hydroxydopamine Induced Parkinsonian Rat Model. Exp. Neurol. 2013, 248, 148–156. [Google Scholar] [CrossRef]
- Herzog, C.D.; Bishop, K.M.; Brown, L.; Wilson, A.; Kordower, J.H.; Bartus, R.T. Gene Transfer Provides a Practical Means for Safe, Long-Term, Targeted Delivery of Biologically Active Neurotrophic Factor Proteins for Neurodegenerative Diseases. Drug Deliv. Transl. Res. 2011, 1, 361–382. [Google Scholar] [CrossRef]
- Herzog, C.D.; Brown, L.; Kruegel, B.R.; Wilson, A.; Tansey, M.G.; Gage, F.H.; Johnson, E.M.; Bartus, R.T. Enhanced Neurotrophic Distribution, Cell Signaling and Neuroprotection Following Substantia Nigral versus Striatal Delivery of AAV2-NRTN (CERE-120). NeuroBiol. Dis. 2013, 58, 38–48. [Google Scholar] [CrossRef]
- Bartus, R.T.; Brown, L.; Wilson, A.; Kruegel, B.; Siffert, J.; Johnson, E.M.; Kordower, J.H.; Herzog, C.D. Properly Scaled and Targeted AAV2-NRTN (Neurturin) to the Substantia Nigra Is Safe, Effective and Causes No Weight Loss: Support for Nigral Targeting in Parkinson’s Disease. NeuroBiol. Dis. 2011, 44, 38–52. [Google Scholar] [CrossRef]
- Wang, C.Y.; Guo, H.Y.; Lim, T.M.; Ng, Y.K.; Neo, H.P.; Hwang, P.Y.K.; Yee, W.-C.; Wang, S. Improved Neuronal Transgene Expression from an AAV-2 Vector with a Hybrid CMV Enhancer/PDGF-Beta Promoter. J. Gene Med. 2005, 7, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Herzog, C.D.; Chu, Y.; Wilson, A.; Brown, L.; Siffert, J.; Johnson, E.M.; Olanow, C.W.; Mufson, E.J.; Kordower, J.H. Bioactivity of AAV2-Neurturin Gene Therapy (CERE-120): Differences between Parkinson’s Disease and Nonhuman Primate Brains. Mov. Disord. 2011, 26, 27–36. [Google Scholar] [CrossRef]
- Weiss, A.R.; Liguore, W.A.; Domire, J.S.; Button, D.; McBride, J.L. Intra-Striatal AAV2.Retro Administration Leads to Extensive Retrograde Transport in the Rhesus Macaque Brain: Implications for Disease Modeling and Therapeutic Development. Sci. Rep. 2020, 10, 6970. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.M.; Kells, A.P.; Rosenbluth, K.H.; Salegio, E.A.; Fiandaca, M.S.; Larson, P.S.; Starr, P.A.; Martin, A.J.; Lonser, R.R.; Federoff, H.J.; et al. Interventional MRI-Guided Putaminal Delivery of AAV2-GDNF for a Planned Clinical Trial in Parkinson’s Disease. Mol. Ther. 2011, 19, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Kells, A.P.; Huang, E.J.; Lee, H.S.; Hadaczek, P.; Beyer, J.; Bringas, J.; Pivirotto, P.; Penticuff, J.; Eberling, J.; et al. Safety Evaluation of AAV2-GDNF Gene Transfer into the Dopaminergic Nigrostriatal Pathway in Aged and Parkinsonian Rhesus Monkeys. Hum. Gene Ther. 2009, 20, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Valles, F.; Fiandaca, M.S.; Eberling, J.L.; Starr, P.A.; Larson, P.S.; Christine, C.W.; Forsayeth, J.; Richardson, R.M.; Su, X.; Aminoff, M.J.; et al. Qualitative Imaging of Adeno-Associated Virus Serotype 2-Human Aromatic L-Amino Acid Decarboxylase Gene Therapy in a Phase I Study for the Treatment of Parkinson Disease. Neurosurgery 2010, 67, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Kim, D.; Kim, S.; Kim, S.; Karuppagounder, S.S.; Kwon, S.-H.; Lee, S.; Kam, T.-I.; Lee, S.; Ham, S.; et al. α-Synuclein Accumulation and GBA Deficiency Due to L444P GBA Mutation Contributes to MPTP-Induced Parkinsonism. Mol. Neurodegener. 2018, 13, 1. [Google Scholar] [CrossRef]
- Pajarillo, E.; Johnson, J.; Rizor, A.; Nyarko-Danquah, I.; Adinew, G.; Bornhorst, J.; Stiboller, M.; Schwerdtle, T.; Son, D.-S.; Aschner, M.; et al. Astrocyte-Specific Deletion of the Transcription Factor Yin Yang 1 in Murine Substantia Nigra Mitigates Manganese-Induced Dopaminergic Neurotoxicity. J. Biol. Chem. 2020, 295, 15662–15676. [Google Scholar] [CrossRef]
- Bordia, T.; Perez, X.A.; Heiss, J.; Zhang, D.; Quik, M. Optogenetic Activation of Striatal Cholinergic Interneurons Regulates L-Dopa-Induced Dyskinesias. NeuroBiol. Dis. 2016, 91, 47–58. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bhatt, M.; Butler, D.; De Genst, E.; Dobson, C.M.; Messer, A.; Kordower, J.H. Proteasome-Targeted Nanobodies Alleviate Pathology and Functional Decline in an α-Synuclein-Based Parkinson’s Disease Model. NPJ Parkinsons Dis. 2018, 4, 25. [Google Scholar] [CrossRef]
- Gombash, S.E.; Manfredsson, F.P.; Kemp, C.J.; Kuhn, N.C.; Fleming, S.M.; Egan, A.E.; Grant, L.M.; Ciucci, M.R.; MacKeigan, J.P.; Sortwell, C.E. Morphological and Behavioral Impact of AAV2/5-Mediated Overexpression of Human Wildtype Alpha-Synuclein in the Rat Nigrostriatal System. PLoS ONE 2013, 8, e81426. [Google Scholar] [CrossRef]
- Wagner, L.M.; Nathwani, S.M.; Ten Eyck, P.P.; Aldridge, G.M. Local Cortical Overexpression of Human Wild-Type Alpha-Synuclein Leads to Increased Dendritic Spine Density in Mouse. Neurosci. Lett. 2020, 733, 135051. [Google Scholar] [CrossRef]
- Xilouri, M.; Brekk, O.R.; Landeck, N.; Pitychoutis, P.M.; Papasilekas, T.; Papadopoulou-Daifoti, Z.; Kirik, D.; Stefanis, L. Boosting Chaperone-Mediated Autophagy in Vivo Mitigates α-Synuclein-Induced Neurodegeneration. Brain 2013, 136, 2130–2146. [Google Scholar] [CrossRef]
- Löw, K.; Aebischer, P.; Schneider, B.L. Direct and Retrograde Transduction of Nigral Neurons with AAV6, 8, and 9 and Intraneuronal Persistence of Viral Particles. Hum. Gene Ther. 2013, 24, 613–629. [Google Scholar] [CrossRef]
- Kordower, J.H.; Dodiya, H.B.; Kordower, A.M.; Terpstra, B.; Paumier, K.; Madhavan, L.; Sortwell, C.; Steece-Collier, K.; Collier, T.J. Transfer of Host-Derived α Synuclein to Grafted Dopaminergic Neurons in Rat. NeuroBiol. Dis. 2011, 43, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Decressac, M.; Mattsson, B.; Lundblad, M.; Weikop, P.; Björklund, A. Progressive Neurodegenerative and Behavioural Changes Induced by AAV-Mediated Overexpression of α-Synuclein in Midbrain Dopamine Neurons. NeuroBiol. Dis. 2012, 45, 939–953. [Google Scholar] [CrossRef]
- Decressac, M.; Mattsson, B.; Weikop, P.; Lundblad, M.; Jakobsson, J.; Björklund, A. TFEB-Mediated Autophagy Rescues Midbrain Dopamine Neurons from α-Synuclein Toxicity. Proc. Natl. Acad. Sci. USA 2013, 110, E1817–E1826. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-Salvá, M.; Macchi, F.; Coessens, V.; Deleersnijder, A.; Gérard, M.; Van der Perren, A.; Van den Haute, C.; Baekelandt, V. Alpha-Synuclein-Induced Neurodegeneration Is Exacerbated in PINK1 Knockout Mice. NeuroBiol. Aging 2014, 35, 2625–2636. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Xu, X.; Zhu, R.; Bi, J.; Liu, W.; Feng, X.; Wu, H.; Zhang, H.; Wu, J.; et al. Recombinant AAV8-Mediated Intrastriatal Gene Delivery of CDNF Protects Rats against Methamphetamine Neurotoxicity. Int. J. Med. Sci. 2017, 14, 340–347. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Zhu, R.; Bi, J.; Feng, X.; Liu, W.; Wu, J.; Zhang, H.; Wu, H.; Kong, W.; et al. Therapeutic Efficacy of AAV8-Mediated Intrastriatal Delivery of Human Cerebral Dopamine Neurotrophic Factor in 6-OHDA-Induced Parkinsonian Rat Models with Different Disease Progression. PLoS ONE 2017, 12, e0179476. [Google Scholar] [CrossRef]
- McFarland, N.R.; Dimant, H.; Kibuuka, L.; Ebrahimi-Fakhari, D.; Desjardins, C.A.; Danzer, K.M.; Danzer, M.; Fan, Z.; Schwarzschild, M.A.; Hirst, W.; et al. Chronic Treatment with Novel Small Molecule Hsp90 Inhibitors Rescues Striatal Dopamine Levels but Not α-Synuclein-Induced Neuronal Cell Loss. PLoS ONE 2014, 9, e86048. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-J.; Chen, A.-D.; Chen, H.-C.; Wang, D.-X.; Cai, Y.-T.; Yin, J.; Jing, Y.-H.; Gao, L.-P. Noncanonical Roles of Hα-Syn (A53T) in the Pathogenesis of Parkinson’s Disease: Synaptic Pathology and Neuronal Aging. Neural. Plast. 2020, 2020, 6283754. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, L.; Li, M.; Li, J.; Tran, K.; Ren, L.; He, R.; Xie, J.; Moser, R.P.; Fraser, C.; et al. Adeno-Associated Virus Neutralizing Antibodies in Large Animals and Their Impact on Brain Intraparenchymal Gene Transfer. Mol. Ther. Methods Clin. Dev. 2018, 11, 65–72. [Google Scholar] [CrossRef]
- Hudry, E.; Andres-Mateos, E.; Lerner, E.P.; Volak, A.; Cohen, O.; Hyman, B.T.; Maguire, C.A.; Vandenberghe, L.H. Efficient Gene Transfer to the Central Nervous System by Single-Stranded Anc80L65. Mol. Ther. Methods Clin. Dev. 2018, 10, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Bourdenx, M.; Dovero, S.; Engeln, M.; Bido, S.; Bastide, M.F.; Dutheil, N.; Vollenweider, I.; Baud, L.; Piron, C.; Grouthier, V.; et al. Lack of Additive Role of Ageing in Nigrostriatal Neurodegeneration Triggered by α-Synuclein Overexpression. Acta Neuropathol. Commun. 2015, 3, 46. [Google Scholar] [CrossRef]
- Sun, X.; Yu, X.; Zhang, L.; Zhao, W.; Wang, M.; Zhang, Y.; Li, X.; Gao, R.; Breger, L.S.; Dovero, S.; et al. Comparison of the Expression and Toxicity of AAV2/9 Carrying the Human A53T α-Synuclein Gene in Presence or Absence of WPRE. Heliyon 2021, 7, e06302. [Google Scholar] [CrossRef]
- Pabon, M.M.; Jernberg, J.N.; Morganti, J.; Contreras, J.; Hudson, C.E.; Klein, R.L.; Bickford, P.C. A Spirulina-Enhanced Diet Provides Neuroprotection in an α-Synuclein Model of Parkinson’s Disease. PLoS ONE 2012, 7, e45256. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hao, F.; He, J.; Lu, T.; Klein, R.L.; Zhao, L.-R.; Duan, W.-M. Sequential Adeno-Associated Viral Vector Serotype 9-Green Fluorescent Protein Gene Transfer Causes Massive Inflammation and Intense Immune Response in Rat Striatum. Hum. Gene Ther. 2016, 27, 528–543. [Google Scholar] [CrossRef]
- Hao, F.; Yang, C.; Chen, S.-S.; Wang, Y.-Y.; Zhou, W.; Hao, Q.; Lu, T.; Hoffer, B.; Zhao, L.-R.; Duan, W.-M.; et al. Long-Term Protective Effects of AAV9-Mesencephalic Astrocyte-Derived Neurotrophic Factor Gene Transfer in Parkinsonian Rats. Exp. Neurol. 2017, 291, 120–133. [Google Scholar] [CrossRef]
- Subbarayan, M.S.; Hudson, C.; Moss, L.D.; Nash, K.R.; Bickford, P.C. T Cell Infiltration and Upregulation of MHCII in Microglia Leads to Accelerated Neuronal Loss in an α-Synuclein Rat Model of Parkinson’s Disease. J. Neuroinflammation 2020, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, W.-H.; Chen, S.-S.; Ma, B.-F.; Li, B.; Lu, T.; Qu, T.-Y.; Klein, R.L.; Zhao, L.-R.; Duan, W.-M. Pre-Immunization with an Intramuscular Injection of AAV9-Human Erythropoietin Vectors Reduces the Vector-Mediated Transduction Following Re-Administration in Rat Brain. PLoS ONE 2013, 8, e63876. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.-Q.; Ma, B.-F.; Zhao, L.-R.; Tatom, J.B.; Li, B.; Jiang, L.-X.; Klein, R.L.; Duan, W.-M. AAV9-Mediated Erythropoietin Gene Delivery into the Brain Protects Nigral Dopaminergic Neurons in a Rat Model of Parkinson’s Disease. Gene Ther. 2010, 17, 83–94. [Google Scholar] [CrossRef]
- Cresto, N.; Gaillard, M.-C.; Gardier, C.; Gubinelli, F.; Diguet, E.; Bellet, D.; Legroux, L.; Mitja, J.; Auregan, G.; Guillermier, M.; et al. The C-Terminal Domain of LRRK2 with the G2019S Mutation Is Sufficient to Produce Neurodegeneration of Dopaminergic Neurons in Vivo. NeuroBiol. Dis. 2020, 134, 104614. [Google Scholar] [CrossRef]
- Albaugh, D.L.; Smith, Y.; Galvan, A. Comparative Analyses of Transgene Expression Patterns after Intra-striatal Injections of RAAV2-retro in Rats and Rhesus Monkeys: A Light and Electron Microscopic Study. Eur. J. Neurosci. 2020, 52, 4824–4839. [Google Scholar] [CrossRef]
- Tordo, J.; O’Leary, C.; Antunes, A.S.L.M.; Palomar, N.; Aldrin-Kirk, P.; Basche, M.; Bennett, A.; D’Souza, Z.; Gleitz, H.; Godwin, A.; et al. A Novel Adeno-Associated Virus Capsid with Enhanced Neurotropism Corrects a Lysosomal Transmembrane Enzyme Deficiency. Brain 2018, 141, 2014–2031. [Google Scholar] [CrossRef]
- Naidoo, J.; Stanek, L.M.; Ohno, K.; Trewman, S.; Samaranch, L.; Hadaczek, P.; O’Riordan, C.; Sullivan, J.; San Sebastian, W.; Bringas, J.R.; et al. Extensive Transduction and Enhanced Spread of a Modified AAV2 Capsid in the Non-Human Primate CNS. Mol. Ther. 2018, 26, 2418–2430. [Google Scholar] [CrossRef]
- Davidsson, M.; Wang, G.; Aldrin-Kirk, P.; Cardoso, T.; Nolbrant, S.; Hartnor, M.; Mudannayake, J.; Parmar, M.; Björklund, T. A Systematic Capsid Evolution Approach Performed in Vivo for the Design of AAV Vectors with Tailored Properties and Tropism. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef]
- Hammond, S.L.; Leek, A.N.; Richman, E.H.; Tjalkens, R.B. Cellular Selectivity of AAV Serotypes for Gene Delivery in Neurons and Astrocytes by Neonatal Intracerebroventricular Injection. PLoS ONE 2017, 12, e0188830. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.J.; Nagabhushan Kalburgi, S.; McCown, T.J.; Jude Samulski, R. Global CNS Gene Delivery and Evasion of Anti-AAV-Neutralizing Antibodies by Intrathecal AAV Administration in Non-Human Primates. Gene Ther. 2013, 20, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Vulchanova, L.; Schuster, D.J.; Belur, L.R.; Riedl, M.S.; Podetz-Pedersen, K.M.; Kitto, K.F.; Wilcox, G.L.; McIvor, R.S.; Fairbanks, C.A. Differential Adeno-Associated Virus Mediated Gene Transfer to Sensory Neurons Following Intrathecal Delivery by Direct Lumbar Puncture. Mol. Pain 2010, 6, 31. [Google Scholar] [CrossRef]
- Towne, C.; Pertin, M.; Beggah, A.T.; Aebischer, P.; Decosterd, I. Recombinant Adeno-Associated Virus Serotype 6 (RAAV2/6)-Mediated Gene Transfer to Nociceptive Neurons through Different Routes of Delivery. Mol. Pain 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Samaranch, L.; Salegio, E.A.; San Sebastian, W.; Kells, A.P.; Bringas, J.R.; Forsayeth, J.; Bankiewicz, K.S. Strong Cortical and Spinal Cord Transduction after AAV7 and AAV9 Delivery into the Cerebrospinal Fluid of Nonhuman Primates. Hum. Gene Ther. 2013, 24, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Amado, D.A.; Rieders, J.M.; Diatta, F.; Hernandez-Con, P.; Singer, A.; Mak, J.T.; Zhang, J.; Lancaster, E.; Davidson, B.L.; Chen-Plotkin, A.S. AAV-Mediated Progranulin Delivery to a Mouse Model of Progranulin Deficiency Causes T Cell-Mediated Toxicity. Mol. Ther. 2019, 27, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Bey, K.; Ciron, C.; Dubreil, L.; Deniaud, J.; Ledevin, M.; Cristini, J.; Blouin, V.; Aubourg, P.; Colle, M.-A. Efficient CNS Targeting in Adult Mice by Intrathecal Infusion of Single-Stranded AAV9-GFP for Gene Therapy of Neurological Disorders. Gene Ther. 2017, 24, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Marmion, D.J.; McBride, J.L.; Manfredsson, F.P.; Butler, D.; Messer, A.; Kordower, J.H. Enhanced CNS Transduction from AAV.PHP.EB Infusion into the Cisterna Magna of Older Adult Rats Compared to AAV9. Gene Ther. 2021. [Google Scholar] [CrossRef]
- Ohno, K.; Samaranch, L.; Hadaczek, P.; Bringas, J.R.; Allen, P.C.; Sudhakar, V.; Stockinger, D.E.; Snieckus, C.; Campagna, M.V.; San Sebastian, W.; et al. Kinetics and MR-Based Monitoring of AAV9 Vector Delivery into Cerebrospinal Fluid of Nonhuman Primates. Mol. Ther. Methods Clin. Dev. 2019, 13, 47–54. [Google Scholar] [CrossRef]
- Samaranch, L.; Salegio, E.A.; San Sebastian, W.; Kells, A.P.; Foust, K.D.; Bringas, J.R.; Lamarre, C.; Forsayeth, J.; Kaspar, B.K.; Bankiewicz, K.S. Adeno-Associated Virus Serotype 9 Transduction in the Central Nervous System of Nonhuman Primates. Hum. Gene Ther. 2012, 23, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, C.; Bell, P.; Vite, C.H.; Louboutin, J.-P.; Grant, R.; Bote, E.; Yu, H.; Pukenas, B.; Hurst, R.; Wilson, J.M. Widespread Gene Transfer in the Central Nervous System of Cynomolgus Macaques Following Delivery of AAV9 into the Cisterna Magna. Mol. Ther. Methods Clin. Dev. 2014, 1, 14051. [Google Scholar] [CrossRef] [PubMed]
- Bey, K.; Deniaud, J.; Dubreil, L.; Joussemet, B.; Cristini, J.; Ciron, C.; Hordeaux, J.; Le Boulc’h, M.; Marche, K.; Maquigneau, M.; et al. Intra-CSF AAV9 and AAVrh10 Administration in Nonhuman Primates: Promising Routes and Vectors for Which Neurological Diseases? Mol. Ther. Methods Clin. Dev. 2020, 17, 771–784. [Google Scholar] [CrossRef]
- Hordeaux, J.; Dubreil, L.; Deniaud, J.; Iacobelli, F.; Moreau, S.; Ledevin, M.; Le Guiner, C.; Blouin, V.; Le Duff, J.; Mendes-Madeira, A.; et al. Efficient Central Nervous System AAVrh10-Mediated Intrathecal Gene Transfer in Adult and Neonate Rats. Gene Ther. 2015, 22, 316–324. [Google Scholar] [CrossRef]
- Liguore, W.A.; Domire, J.S.; Button, D.; Wang, Y.; Dufour, B.D.; Srinivasan, S.; McBride, J.L. AAV-PHP.B Administration Results in a Differential Pattern of CNS Biodistribution in Non-Human Primates Compared with Mice. Mol. Ther. 2019, 27, 2018–2037. [Google Scholar] [CrossRef]
- Galvan, A.; Petkau, T.L.; Hill, A.M.; Korecki, A.J.; Lu, G.; Choi, D.; Rahman, K.; Simpson, E.; Leavitt, B.R.; Smith, Y. Intracerebroventricular Administration of AAV9-PHP.B SYN1-EmGFP Induces Widespread Transgene Expression in the Mouse and Monkey CNS. Hum. Gene Ther. 2021. [Google Scholar] [CrossRef]
- Iwata, N.; Sekiguchi, M.; Hattori, Y.; Takahashi, A.; Asai, M.; Ji, B.; Higuchi, M.; Staufenbiel, M.; Muramatsu, S.; Saido, T.C. Global Brain Delivery of Neprilysin Gene by Intravascular Administration of AAV Vector in Mice. Sci. Rep. 2013, 3, 1472. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.Y.; de Vin, F.; Duqué, S.I.; Fripont, S.; Castaldo, S.A.; Bouhuijzen-Wenger, J.; Holt, M.G. Widespread Transduction of Astrocytes and Neurons in the Mouse Central Nervous System after Systemic Delivery of a Self-Complementary AAV-PHP.B Vector. Gene Ther. 2018, 25, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.R.; Harris, A.F.; Cabral, D.J.; Keeler, A.M.; Sapp, E.; Ferreira, J.S.; Gray-Edwards, H.L.; Johnson, J.A.; Johnson, A.K.; Su, Q.; et al. Widespread Central Nervous System Gene Transfer and Silencing After Systemic Delivery of Novel AAV-AS Vector. Mol. Ther. 2016, 24, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.-H.; Ho, J.W.-T.; Lo, P.K.; Tin, C. Targeted Transgene Activation in the Brain Tissue by Systemic Delivery of Engineered AAV1 Expressing CRISPRa. Mol. Ther. Nucleic Acids 2019, 16, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Challis, R.C.; Ravindra Kumar, S.; Chan, K.Y.; Challis, C.; Beadle, K.; Jang, M.J.; Kim, H.M.; Rajendran, P.S.; Tompkins, J.D.; Shivkumar, K.; et al. Systemic AAV Vectors for Widespread and Targeted Gene Delivery in Rodents. Nat. Protoc. 2019, 14, 379–414. [Google Scholar] [CrossRef]
- Morabito, G.; Giannelli, S.G.; Ordazzo, G.; Bido, S.; Castoldi, V.; Indrigo, M.; Cabassi, T.; Cattaneo, S.; Luoni, M.; Cancellieri, C.; et al. AAV-PHP.B-Mediated Global-Scale Expression in the Mouse Nervous System Enables GBA1 Gene Therapy for Wide Protection from Synucleinopathy. Mol. Ther. 2017, 25, 2727–2742. [Google Scholar] [CrossRef]
- Miyanohara, A.; Kamizato, K.; Juhas, S.; Juhasova, J.; Navarro, M.; Marsala, S.; Lukacova, N.; Hruska-Plochan, M.; Curtis, E.; Gabel, B.; et al. Potent Spinal Parenchymal AAV9-Mediated Gene Delivery by Subpial Injection in Adult Rats and Pigs. Mol. Ther. Methods Clin. Dev. 2016, 3, 16046. [Google Scholar] [CrossRef]
- Bravo-Hernandez, M.; Tadokoro, T.; Navarro, M.R.; Platoshyn, O.; Kobayashi, Y.; Marsala, S.; Miyanohara, A.; Juhas, S.; Juhasova, J.; Skalnikova, H.; et al. Spinal Subpial Delivery of AAV9 Enables Widespread Gene Silencing and Blocks Motoneuron Degeneration in ALS. Nat. Med. 2020, 26, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Uytingco, C.R.; Green, W.W.; McIntyre, J.C.; Ukhanov, K.; Zimmerman, A.D.; Shively, D.T.; Zhang, L.; Nishimura, D.Y.; Sheffield, V.C.; et al. Gene Therapeutic Reversal of Peripheral Olfactory Impairment in Bardet-Biedl Syndrome. Mol. Ther. 2017, 25, 904–916. [Google Scholar] [CrossRef]
- Hocquemiller, M.; Giersch, L.; Audrain, M.; Parker, S.; Cartier, N. Adeno-Associated Virus-Based Gene Therapy for CNS Diseases. Hum. Gene Ther. 2016, 27, 478–496. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Hudry, E.; Maguire, C.A.; Sena-Esteves, M.; Breakefield, X.O.; Grandi, P. Viral Vectors for Therapy of Neurologic Diseases. Neuropharmacology 2017, 120, 63–80. [Google Scholar] [CrossRef]
- Lonser, R.R.; Akhter, A.S.; Zabek, M.; Elder, J.B.; Bankiewicz, K.S. Direct Convective Delivery of Adeno-Associated Virus Gene Therapy for Treatment of Neurological Disorders. J. Neurosurg 2020, 1–13. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef]
- Samaranch, L.; Blits, B.; San Sebastian, W.; Hadaczek, P.; Bringas, J.; Sudhakar, V.; Macayan, M.; Pivirotto, P.J.; Petry, H.; Bankiewicz, K.S. MR-Guided Parenchymal Delivery of Adeno-Associated Viral Vector Serotype 5 in Non-Human Primate Brain. Gene Ther. 2017, 24, 253–261. [Google Scholar] [CrossRef]
- Christine, C.W.; Bankiewicz, K.S.; Van Laar, A.D.; Richardson, R.M.; Ravina, B.; Kells, A.P.; Boot, B.; Martin, A.J.; Nutt, J.; Thompson, M.E.; et al. Magnetic Resonance Imaging-Guided Phase 1 Trial of Putaminal AADC Gene Therapy for Parkinson’s Disease. Ann. Neurol. 2019, 85, 704–714. [Google Scholar] [CrossRef]
- Muramatsu, S.; Fujimoto, K.; Kato, S.; Mizukami, H.; Asari, S.; Ikeguchi, K.; Kawakami, T.; Urabe, M.; Kume, A.; Sato, T.; et al. A Phase I Study of Aromatic L-Amino Acid Decarboxylase Gene Therapy for Parkinson’s Disease. Mol. Ther. 2010, 18, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- McFarthing, K.; Prakash, N.; Simuni, T. Clinical trial highlights: 1. Gene therapy for parkinson’s, 2. Phase 3 study in focus-intec pharma’s accordion pill, 3. Clinical trials resources. J. Parkinsons Dis. 2019, 9, 251–264. [Google Scholar] [CrossRef]
- Nutt, J.G.; Curtze, C.; Hiller, A.; Anderson, S.; Larson, P.S.; Van Laar, A.D.; Richardson, R.M.; Thompson, M.E.; Sedkov, A.; Leinonen, M.; et al. Aromatic L-Amino Acid Decarboxylase Gene Therapy Enhances Levodopa Response in Parkinson’s Disease. Mov. Disord. 2020, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, M.S.; Saarma, M. The GDNF Family: Signalling, Biological Functions and Therapeutic Value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef]
- Bäckman, C.M.; Shan, L.; Zhang, Y.J.; Hoffer, B.J.; Leonard, S.; Troncoso, J.C.; Vonsatel, P.; Tomac, A.C. Gene Expression Patterns for GDNF and Its Receptors in the Human Putamen Affected by Parkinson’s Disease: A Real-Time PCR Study. Mol. Cell Endocrinol. 2006, 252, 160–166. [Google Scholar] [CrossRef]
- Björklund, A.; Kirik, D.; Rosenblad, C.; Georgievska, B.; Lundberg, C.; Mandel, R.J. Towards a Neuroprotective Gene Therapy for Parkinson’s Disease: Use of Adenovirus, AAV and Lentivirus Vectors for Gene Transfer of GDNF to the Nigrostriatal System in the Rat Parkinson Model. Brain Res. 2000, 886, 82–98. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Thomas, K.; Sarkar, A.; Siddiqui, M.S.; et al. AAV2-GAD Gene Therapy for Advanced Parkinson’s Disease: A Double-Blind, Sham-Surgery Controlled, Randomised Trial. Lancet Neurol. 2011, 10, 309–319. [Google Scholar] [CrossRef]
- Kaplitt, M.G.; Feigin, A.; Tang, C.; Fitzsimons, H.L.; Mattis, P.; Lawlor, P.A.; Bland, R.J.; Young, D.; Strybing, K.; Eidelberg, D.; et al. Safety and Tolerability of Gene Therapy with an Adeno-Associated Virus (AAV) Borne GAD Gene for Parkinson’s Disease: An Open Label, Phase I Trial. Lancet 2007, 369, 2097–2105. [Google Scholar] [CrossRef]
- Feigin, A.; Kaplitt, M.G.; Tang, C.; Lin, T.; Mattis, P.; Dhawan, V.; During, M.J.; Eidelberg, D. Modulation of Metabolic Brain Networks after Subthalamic Gene Therapy for Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2007, 104, 19559–19564. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, M.; Tang, C.C.; LeWitt, P.A.; Rezai, A.R.; Leehey, M.A.; Ojemann, S.G.; Flaherty, A.W.; Eskandar, E.N.; Kostyk, S.K.; Sarkar, A.; et al. Long-Term Follow-up of a Randomized AAV2-GAD Gene Therapy Trial for Parkinson’s Disease. JCI Insight 2017, 2, e90133. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Kaplitt, M.G.; Fitzsimons, H.L.; Zuzga, D.S.; Liu, Y.; Oshinsky, M.L.; During, M.J. Subthalamic GAD Gene Therapy in a Parkinson’s Disease Rat Model. Science 2002, 298, 425–429. [Google Scholar] [CrossRef]
- Marks, W.J.; Ostrem, J.L.; Verhagen, L.; Starr, P.A.; Larson, P.S.; Bakay, R.A.; Taylor, R.; Cahn-Weiner, D.A.; Stoessl, A.J.; Olanow, C.W.; et al. Safety and Tolerability of Intraputaminal Delivery of CERE-120 (Adeno-Associated Virus Serotype 2-Neurturin) to Patients with Idiopathic Parkinson’s Disease: An Open-Label, Phase I Trial. Lancet Neurol. 2008, 7, 400–408. [Google Scholar] [CrossRef]
- Bartus, R.T.; Baumann, T.L.; Siffert, J.; Herzog, C.D.; Alterman, R.; Boulis, N.; Turner, D.A.; Stacy, M.; Lang, A.E.; Lozano, A.M.; et al. Safety/Feasibility of Targeting the Substantia Nigra with AAV2-Neurturin in Parkinson Patients. Neurology 2013, 80, 1698–1701. [Google Scholar] [CrossRef]
- Green, F.; Samaranch, L.; Zhang, H.S.; Manning-Bog, A.; Meyer, K.; Forsayeth, J.; Bankiewicz, K.S. Axonal Transport of AAV9 in Nonhuman Primate Brain. Gene Ther. 2016, 23, 520–526. [Google Scholar] [CrossRef]
- Tervo, D.G.R.; Hwang, B.-Y.; Viswanathan, S.; Gaj, T.; Lavzin, M.; Ritola, K.D.; Lindo, S.; Michael, S.; Kuleshova, E.; Ojala, D.; et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 2016, 92, 372–382. [Google Scholar] [CrossRef]
- Cohen-Pfeffer, J.L.; Gururangan, S.; Lester, T.; Lim, D.A.; Shaywitz, A.J.; Westphal, M.; Slavc, I. Intracerebroventricular Delivery as a Safe, Long-Term Route of Drug Administration. Pediatr. Neurol. 2017, 67, 23–35. [Google Scholar] [CrossRef]
- Hinderer, C.; Bell, P.; Katz, N.; Vite, C.H.; Louboutin, J.-P.; Bote, E.; Yu, H.; Zhu, Y.; Casal, M.L.; Bagel, J.; et al. Evaluation of Intrathecal Routes of Administration for Adeno-Associated Viral Vectors in Large Animals. Hum. Gene Ther. 2018, 29, 15–24. [Google Scholar] [CrossRef]
- Yang, B.; Li, S.; Wang, H.; Guo, Y.; Gessler, D.J.; Cao, C.; Su, Q.; Kramer, J.; Zhong, L.; Ahmed, S.S.; et al. Global CNS Transduction of Adult Mice by Intravenously Delivered RAAVrh.8 and RAAVrh.10 and Nonhuman Primates by RAAVrh.10. Mol. Ther. 2014, 22, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Manfredsson, F.P.; Rising, A.C.; Mandel, R.J. AAV9: A Potential Blood-Brain Barrier Buster. Mol. Ther. 2009, 17, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Forsayeth, J.R.; Bankiewicz, K.S. AAV9: Over the Fence and into the Woods. Mol. Ther. 2011, 19, 1006–1007. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Pravdo, P.L.; Simpson, B.P.; Kumar, S.R.; Chan, K.Y.; Banerjee, A.; Wu, W.-L.; Yang, B.; Huber, N.; Pasca, S.P.; et al. Cre-Dependent Selection Yields AAV Variants for Widespread Gene Transfer to the Adult Brain. Nat. Biotechnol. 2016, 34, 204–209. [Google Scholar] [CrossRef]
- Hordeaux, J.; Wang, Q.; Katz, N.; Buza, E.L.; Bell, P.; Wilson, J.M. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J. Mice. Mol. Ther. 2018, 26, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Hudry, E.; Vandenberghe, L.H. Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron 2019, 101, 839–862. [Google Scholar] [CrossRef]
- Jackson, K.L.; Dayton, R.D.; Klein, R.L. AAV9 Supports Wide-Scale Transduction of the CNS and TDP-43 Disease Modeling in Adult Rats. Mol. Ther. Methods Clin. Dev. 2015, 2, 15036. [Google Scholar] [CrossRef]
- Dayton, R.D.; Grames, M.S.; Klein, R.L. More Expansive Gene Transfer to the Rat CNS: AAV PHP.EB Vector Dose-Response and Comparison to AAV PHP.B. Gene Ther. 2018, 25, 392–400. [Google Scholar] [CrossRef]
- Huang, Q.; Chan, K.Y.; Tobey, I.G.; Chan, Y.A.; Poterba, T.; Boutros, C.L.; Balazs, A.B.; Daneman, R.; Bloom, J.M.; Seed, C.; et al. Delivering Genes across the Blood-Brain Barrier: LY6A, a Novel Cellular Receptor for AAV-PHP.B Capsids. PLoS ONE 2019, 14, e0225206. [Google Scholar] [CrossRef]
- Batista, A.R.; King, O.D.; Reardon, C.P.; Davis, C.; Shankaracharya; Philip, V.; Gray-Edwards, H.; Aronin, N.; Lutz, C.; Landers, J.; et al. Ly6a Differential Expression in Blood-Brain Barrier Is Responsible for Strain Specific Central Nervous System Transduction Profile of AAV-PHP.B. Hum. Gene Ther. 2020, 31, 90–102. [Google Scholar] [CrossRef]
- Spindler, K.R.; Welton, A.R.; Lim, E.S.; Duvvuru, S.; Althaus, I.W.; Imperiale, J.E.; Daoud, A.I.; Chesler, E.J. The Major Locus for Mouse Adenovirus Susceptibility Maps to Genes of the Hematopoietic Cell Surface-Expressed LY6 Family. J. Immunol. 2010, 184, 3055–3062. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Konno, A.; Mochizuki, R.; Shinohara, Y.; Nitta, K.; Okada, Y.; Hirai, H. Intravenous Administration of the Adeno-Associated Virus-PHP.B Capsid Fails to Upregulate Transduction Efficiency in the Marmoset Brain. Neurosci. Lett. 2018, 665, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Uytingco, C.R.; Martens, J.R. Intranasal Delivery of Adenoviral and AAV Vectors for Transduction of the Mammalian Peripheral Olfactory System. Methods Mol. Biol. 2019, 1950, 283–297. [Google Scholar] [CrossRef]
- Imbert, C.; Bezard, E.; Guitraud, S.; Boraud, T.; Gross, C.E. Comparison of Eight Clinical Rating Scales Used for the Assessment of MPTP-Induced Parkinsonism in the Macaque Monkey. J. Neurosci. Methods 2000, 96, 71–76. [Google Scholar] [CrossRef]
- Eslamboli, A.; Romero-Ramos, M.; Burger, C.; Bjorklund, T.; Muzyczka, N.; Mandel, R.J.; Baker, H.; Ridley, R.M.; Kirik, D. Long-Term Consequences of Human Alpha-Synuclein Overexpression in the Primate Ventral Midbrain. Brain 2007, 130, 799–815. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional Neuroanatomy of the Basal Ganglia. Cold Spring Harb. Perspect Med. 2012, 2, a009621. [Google Scholar] [CrossRef] [PubMed]
- Marmion, D.J.; Kordower, J.H. α-Synuclein Nonhuman Primate Models of Parkinson’s Disease. J. Neural. Transm. 2018, 125, 385–400. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. Alpha-Synuclein in Filamentous Inclusions of Lewy Bodies from Parkinson’s Disease and Dementia with Lewy Bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [PubMed]
- Visanji, N.P.; Brotchie, J.M.; Kalia, L.V.; Koprich, J.B.; Tandon, A.; Watts, J.C.; Lang, A.E. α-Synuclein-Based Animal Models of Parkinson’s Disease: Challenges and Opportunities in a New Era. Trends Neurosci. 2016, 39, 750–762. [Google Scholar] [CrossRef]
- Kirik, D.; Annett, L.E.; Burger, C.; Muzyczka, N.; Mandel, R.J.; Björklund, A. Nigrostriatal Alpha-Synucleinopathy Induced by Viral Vector-Mediated Overexpression of Human Alpha-Synuclein: A New Primate Model of Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2003, 100, 2884–2889. [Google Scholar] [CrossRef]
- Lo Bianco, C.; Ridet, J.-L.; Schneider, B.L.; Deglon, N.; Aebischer, P. Alpha -Synucleinopathy and Selective Dopaminergic Neuron Loss in a Rat Lentiviral-Based Model of Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10813–10818. [Google Scholar] [CrossRef]
- Kirik, D.; Rosenblad, C.; Burger, C.; Lundberg, C.; Johansen, T.E.; Muzyczka, N.; Mandel, R.J.; Björklund, A. Parkinson-like Neurodegeneration Induced by Targeted Overexpression of Alpha-Synuclein in the Nigrostriatal System. J. Neurosci. 2002, 22, 2780–2791. [Google Scholar] [CrossRef]
- Koprich, J.B.; Johnston, T.H.; Reyes, M.G.; Sun, X.; Brotchie, J.M. Expression of Human A53T Alpha-Synuclein in the Rat Substantia Nigra Using a Novel AAV1/2 Vector Produces a Rapidly Evolving Pathology with Protein Aggregation, Dystrophic Neurite Architecture and Nigrostriatal Degeneration with Potential to Model the Pathology of Parkinson’s Disease. Mol. Neurodegener. 2010, 5, 43. [Google Scholar] [CrossRef]
- Koprich, J.B.; Johnston, T.H.; Huot, P.; Reyes, M.G.; Espinosa, M.; Brotchie, J.M. Progressive Neurodegeneration or Endogenous Compensation in an Animal Model of Parkinson’s Disease Produced by Decreasing Doses of Alpha-Synuclein. PLoS ONE 2011, 6, e17698. [Google Scholar] [CrossRef]
- Koprich, J.B.; Johnston, T.H.; Reyes, G.; Omana, V.; Brotchie, J.M. Towards a Non-Human Primate Model of Alpha-Synucleinopathy for Development of Therapeutics for Parkinson’s Disease: Optimization of AAV1/2 Delivery Parameters to Drive Sustained Expression of Alpha Synuclein and Dopaminergic Degeneration in Macaque. PLoS ONE 2016, 11, e0167235. [Google Scholar] [CrossRef]
- Sucunza, D.; Rico, A.J.; Roda, E.; Collantes, M.; González-Aseguinolaza, G.; Rodríguez-Pérez, A.I.; Peñuelas, I.; Vázquez, A.; Labandeira-García, J.L.; Broccoli, V.; et al. Glucocerebrosidase Gene Therapy Induces Alpha-Synuclein Clearance and Neuroprotection of Midbrain Dopaminergic Neurons in Mice and Macaques. Int. J. Mol. Sci. 2021, 22, 4825. [Google Scholar] [CrossRef]
- Yang, W.; Wang, G.; Wang, C.-E.; Guo, X.; Yin, P.; Gao, J.; Tu, Z.; Wang, Z.; Wu, J.; Hu, X.; et al. Mutant Alpha-Synuclein Causes Age-Dependent Neuropathology in Monkey Brain. J. Neurosci. 2015, 35, 8345–8358. [Google Scholar] [CrossRef]
- Braak, E.; Sandmann-Keil, D.; Rüb, U.; Gai, W.P.; de Vos, R.A.; Steur, E.N.; Arai, K.; Braak, H. Alpha-Synuclein Immunopositive Parkinson’s Disease-Related Inclusion Bodies in Lower Brain Stem Nuclei. Acta Neuropathol. 2001, 101, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Carbajal, I.; Laguna, A.; Romero-Giménez, J.; Cuadros, T.; Bové, J.; Martinez-Vicente, M.; Parent, A.; Gonzalez-Sepulveda, M.; Peñuelas, N.; Torra, A.; et al. Brain Tyrosinase Overexpression Implicates Age-Dependent Neuromelanin Production in Parkinson’s Disease Pathogenesis. Nat. Commun. 2019, 10, 973. [Google Scholar] [CrossRef]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B.; et al. Mutations in LRRK2 Cause Autosomal-Dominant Parkinsonism with Pleomorphic Pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Dusonchet, J.; Kochubey, O.; Stafa, K.; Young, S.M.; Zufferey, R.; Moore, D.J.; Schneider, B.L.; Aebischer, P. A Rat Model of Progressive Nigral Neurodegeneration Induced by the Parkinson’s Disease-Associated G2019S Mutation in LRRK2. J. Neurosci. 2011, 31, 907–912. [Google Scholar] [CrossRef]
- Lee, B.D.; Shin, J.-H.; VanKampen, J.; Petrucelli, L.; West, A.B.; Ko, H.S.; Lee, Y.-I.; Maguire-Zeiss, K.A.; Bowers, W.J.; Federoff, H.J.; et al. Inhibitors of Leucine-Rich Repeat Kinase-2 Protect against Models of Parkinson’s Disease. Nat. Med. 2010, 16, 998–1000. [Google Scholar] [CrossRef]
- Haque, M.E.; Mount, M.P.; Safarpour, F.; Abdel-Messih, E.; Callaghan, S.; Mazerolle, C.; Kitada, T.; Slack, R.S.; Wallace, V.; Shen, J.; et al. Inactivation of Pink1 Gene in Vivo Sensitizes Dopamine-Producing Neurons to 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) and Can Be Rescued by Autosomal Recessive Parkinson Disease Genes, Parkin or DJ-1. J. Biol. Chem. 2012, 287, 23162–23170. [Google Scholar] [CrossRef]
- Wagner, J.A.; Messner, A.H.; Moran, M.L.; Daifuku, R.; Kouyama, K.; Desch, J.K.; Manley, S.; Norbash, A.M.; Conrad, C.K.; Friborg, S.; et al. Safety and Biological Efficacy of an Adeno-Associated Virus Vector-Cystic Fibrosis Transmembrane Regulator (AAV-CFTR) in the Cystic Fibrosis Maxillary Sinus. Laryngoscope 1999, 109, 266–274. [Google Scholar] [CrossRef]
- Mandel, T.E.; Koulmanda, M.; Cozzi, E.; Waterworth, P.; Tolan, M.; Langford, G.; White, D.J. Transplantation of Normal and DAF-Transgenic Fetal Pig Pancreas into Cynomolgus Monkeys. Transplant. Proc. 1997, 29, 940. [Google Scholar] [CrossRef]
- Bartus, R.T.; Weinberg, M.S.; Samulski, R.J. Parkinson’s Disease Gene Therapy: Success by Design Meets Failure by Efficacy. Mol. Ther. 2014, 22, 487–497. [Google Scholar] [CrossRef]
- Bankiewicz, K.S.; Eberling, J.L.; Kohutnicka, M.; Jagust, W.; Pivirotto, P.; Bringas, J.; Cunningham, J.; Budinger, T.F.; Harvey-White, J. Convection-Enhanced Delivery of AAV Vector in Parkinsonian Monkeys; in Vivo Detection of Gene Expression and Restoration of Dopaminergic Function Using pro-Drug Approach. Exp. Neurol. 2000, 164, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Christine, C.W.; Starr, P.A.; Larson, P.S.; Eberling, J.L.; Jagust, W.J.; Hawkins, R.A.; VanBrocklin, H.F.; Wright, J.F.; Bankiewicz, K.S.; Aminoff, M.J. Safety and Tolerability of Putaminal AADC Gene Therapy for Parkinson Disease. Neurology 2009, 73, 1662–1669. [Google Scholar] [CrossRef]
- Heiss, J.D.; Lungu, C.; Hammoud, D.A.; Herscovitch, P.; Ehrlich, D.J.; Argersinger, D.P.; Sinharay, S.; Scott, G.; Wu, T.; Federoff, H.J.; et al. Trial of Magnetic Resonance-Guided Putaminal Gene Therapy for Advanced Parkinson’s Disease. Mov. Disord. 2019, 34, 1073–1078. [Google Scholar] [CrossRef]
- Warren Olanow, C.; Bartus, R.T.; Baumann, T.L.; Factor, S.; Boulis, N.; Stacy, M.; Turner, D.A.; Marks, W.; Larson, P.; Starr, P.A.; et al. Gene Delivery of Neurturin to Putamen and Substantia Nigra in Parkinson Disease: A Double-Blind, Randomized, Controlled Trial. Ann. Neurol. 2015, 78, 248–257. [Google Scholar] [CrossRef]
- Erlander, M.G.; Tillakaratne, N.J.; Feldblum, S.; Patel, N.; Tobin, A.J. Two Genes Encode Distinct Glutamate Decarboxylases. Neuron 1991, 7, 91–100. [Google Scholar] [CrossRef]
- Sidransky, E.; Nalls, M.A.; Aasly, J.O.; Aharon-Peretz, J.; Annesi, G.; Barbosa, E.R.; Bar-Shira, A.; Berg, D.; Bras, J.; Brice, A.; et al. Multicenter Analysis of Glucocerebrosidase Mutations in Parkinson’s Disease. N. Engl. J. Med. 2009, 361, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.M.; Smith, G.A.; Park, E.; Cao, H.; Brown, E.; Hayes, M.A.; Beagan, J.; McLean, J.R.; Izen, S.C.; Perez-Torres, E.; et al. Glucocerebrosidase Gene Therapy Prevents α-Synucleinopathy of Midbrain Dopamine Neurons. NeuroBiol. Dis. 2015, 82, 495–503. [Google Scholar] [CrossRef] [PubMed]
| Delivery Routes | AAV Serotypes | Animal Species | References |
|---|---|---|---|
| Intraparenchymal | N.A. | Pig | [23] |
| AAV1 | Rat | [24] | |
| AAV2 | Mouse | [25,26,27,28,29,30,31] | |
| Rat | [24,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] | ||
| NHP (M. fascicularis) | [47,49] | ||
| NHP (M. mulatta) | [50,51,52,53] | ||
| AAV5 | Mouse | [54,55,56] | |
| Rat | [24,32,57,58] | ||
| AAV6 | Mouse | [59] | |
| Rat | [60,61,62,63,64] | ||
| AAV7 | Mouse | [65] | |
| Rat | [65] | ||
| AAV8 | Rat | [61,66,67,68] | |
| AAV9 | Mouse | [69,70,71,72,73] | |
| Rat | [61,72,74,75,76,77,78,79,80] | ||
| NHP (M. mulatta) | [72] | ||
| AAV2-retro | NHP (M. mulatta) | [50,81] | |
| AAV-TT | Mouse | [82] | |
| Rat | [82] | ||
| AAV-HBKO | NHP (M. mulatta) | [83] | |
| AAV-MNM | Rat | [84] | |
| Intra-CSF | AAV2.1 | Mouse | [85] |
| AAV2.5 | NHP (M. fascicularis) | [86] | |
| AAV5 | Mouse | [87] | |
| AAV6 | Mouse | [88] | |
| AAV7 | NHP (M. fascicularis) | [89] | |
| AAV8 | Mouse | [87] | |
| AAV9 | Mouse | [85,90,91] | |
| Rat | [92] | ||
| NHP (M. mulatta) | [93,94] | ||
| NHP (M. fascicularis) | [86,89,94,95,96] | ||
| AAVDJ8 | Mouse | [85] | |
| AAVrh10 | Mouse | [91] | |
| Rat | [97] | ||
| NHP (M. fascicularis) | [96] | ||
| AAV-PHP.B | Mouse | [98,99] | |
| NHP (M.mulatta) | [98,99]. | ||
| Intravenous | AAV9 | Mouse | [100,101] |
| NHP (M. fasciularis) | [94] | ||
| NHP (M. mulatta) | [94] | ||
| AAV-AS | Mouse | [102] | |
| AAV1-PHP.B | Mouse | [103] | |
| AAV-PHP.B | Mouse | [98,101,104,105] | |
| NHP (M. mulatta) | [98] | ||
| AAV-PHP.eB | Mouse | [104] | |
| AAV-PHP.S | Mouse | [104] | |
| Subpial | AAV9 | Rat | [106] |
| Pig | [106,107] | ||
| NHP (M. fasciularis) | [107] |
| Clinical Trial Identifier | Duration | Phase | Gene | AAV Serotype | Delivery Routes | Region | Status | References |
|---|---|---|---|---|---|---|---|---|
| NCT01973543 | 2013–2020 | I | AADC | AAV2 | IP | Putamen | Completed | [114] |
| NCT02418598 | 2015–2018 | I/II | AADC | AAV2 | IP | Putamen | Terminated | [115] |
| NCT03065192 | 2017–2021 | I | AADC | AAV2 | IP | Putamen | Active, not recruiting | N.A. (Neurocrine Biosciences). |
| NCT03562494 | 2018–2022 | II | AADC | AAV2 | IP | N.A. | Recruiting | [116] |
| NCT03733496 | 2018–2026 | N.A. | AADC | AAV2 | IP | Putamen | Enrolling, by invitation | [51,114,117] |
| NCT04167540 | 2020–2022 | I | GDNF | AAV2 | IP | Putamen | Recruiting | N.A. (Brain Neurotherapy Bio, Inc.) |
| NCT01621581 | 2013–2022 | I | GDNF | AAV2 | IP | Putamen | Active, not recruiting | [116,118,119,120] |
| NCT00643890 | 2008–2010 | II | GAD | AAV2 | IP | STN | Terminated | [121,122,123,124] |
| NCT00195143 | 2003–2005 | I | GAD | AAV2 | IP | STN | Completed | [122,125] |
| NCT01301573 | 2011–2012 | N.A. | GAD | AAV2 | IP | STN | Terminated | N.A. (Neurologix, Inc.) |
| NCT00252850 | 2005–2007 | I | NRTN | AAV2 | IP | Putamen | Completed | [126] |
| NCT00985517 | 2009–2017 | I/II | NRTN | AAV2 | IP | Putamen | Completed | [127] |
| NCT04127578 | 2020–2027 | I/II | GBA1 | AAV9 | IC | CM | Recruiting | N.A. (Prevail Therapeutics) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajardo-Serrano, A.; Rico, A.J.; Roda, E.; Honrubia, A.; Arrieta, S.; Ariznabarreta, G.; Chocarro, J.; Lorenzo-Ramos, E.; Pejenaute, A.; Vázquez, A.; et al. Adeno-Associated Viral Vectors as Versatile Tools for Parkinson’s Research, Both for Disease Modeling Purposes and for Therapeutic Uses. Int. J. Mol. Sci. 2021, 22, 6389. https://doi.org/10.3390/ijms22126389
Fajardo-Serrano A, Rico AJ, Roda E, Honrubia A, Arrieta S, Ariznabarreta G, Chocarro J, Lorenzo-Ramos E, Pejenaute A, Vázquez A, et al. Adeno-Associated Viral Vectors as Versatile Tools for Parkinson’s Research, Both for Disease Modeling Purposes and for Therapeutic Uses. International Journal of Molecular Sciences. 2021; 22(12):6389. https://doi.org/10.3390/ijms22126389
Chicago/Turabian StyleFajardo-Serrano, Ana, Alberto J. Rico, Elvira Roda, Adriana Honrubia, Sandra Arrieta, Goiaz Ariznabarreta, Julia Chocarro, Elena Lorenzo-Ramos, Alvaro Pejenaute, Alfonso Vázquez, and et al. 2021. "Adeno-Associated Viral Vectors as Versatile Tools for Parkinson’s Research, Both for Disease Modeling Purposes and for Therapeutic Uses" International Journal of Molecular Sciences 22, no. 12: 6389. https://doi.org/10.3390/ijms22126389
APA StyleFajardo-Serrano, A., Rico, A. J., Roda, E., Honrubia, A., Arrieta, S., Ariznabarreta, G., Chocarro, J., Lorenzo-Ramos, E., Pejenaute, A., Vázquez, A., & Lanciego, J. L. (2021). Adeno-Associated Viral Vectors as Versatile Tools for Parkinson’s Research, Both for Disease Modeling Purposes and for Therapeutic Uses. International Journal of Molecular Sciences, 22(12), 6389. https://doi.org/10.3390/ijms22126389








