Aryl-4,5-dihydro-1H-pyrazole-1-carboxamide Derivatives Bearing a Sulfonamide Moiety Show Single-digit Nanomolar-to-Subnanomolar Inhibition Constants against the Tumor-associated Human Carbonic Anhydrases IX and XII
Abstract
1. Introduction
2. Results and Discussion
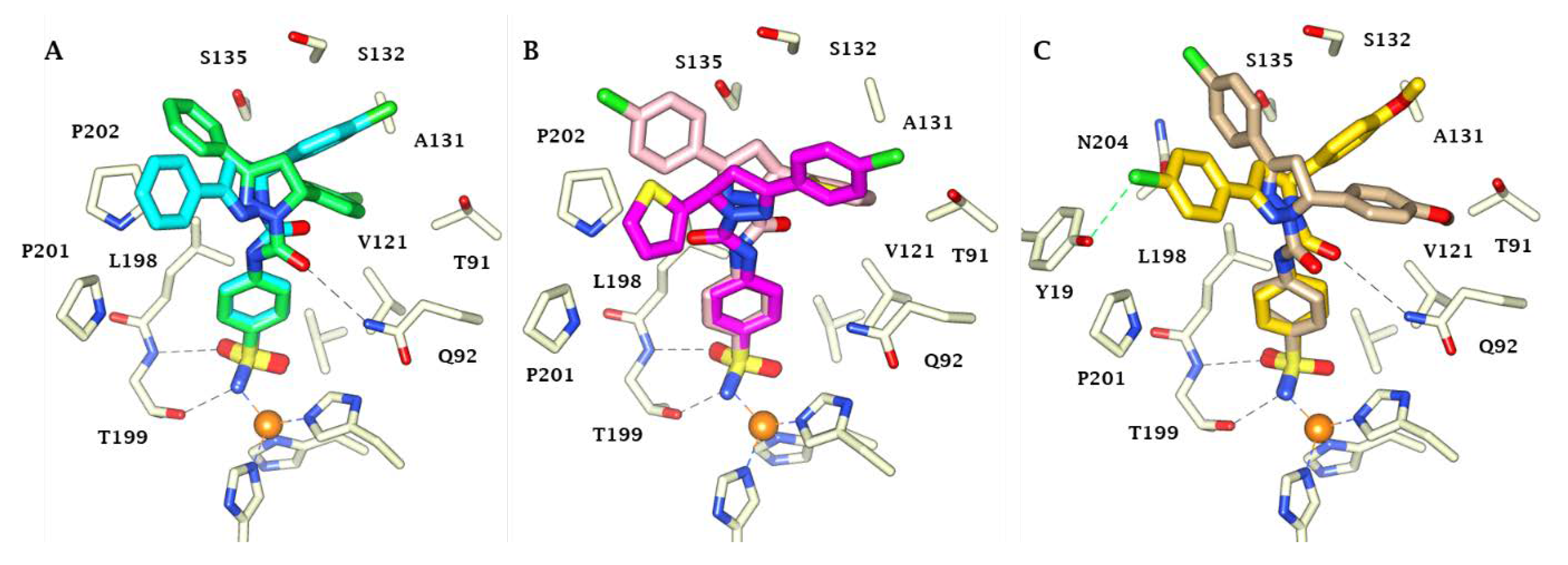
2.1. Chemistry
2.2. Carbonic Anhydrases Inhibition
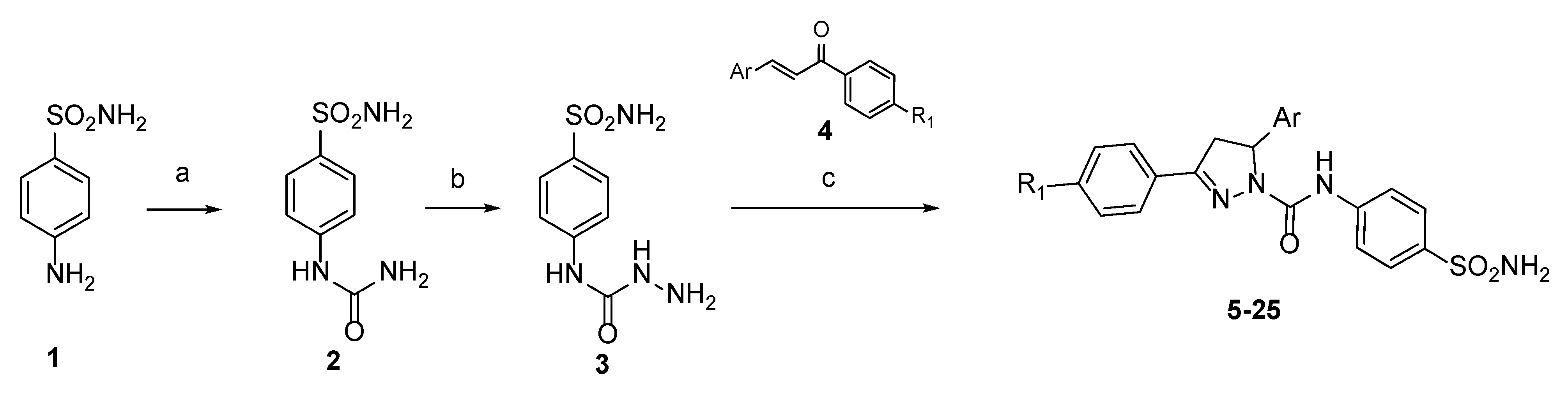
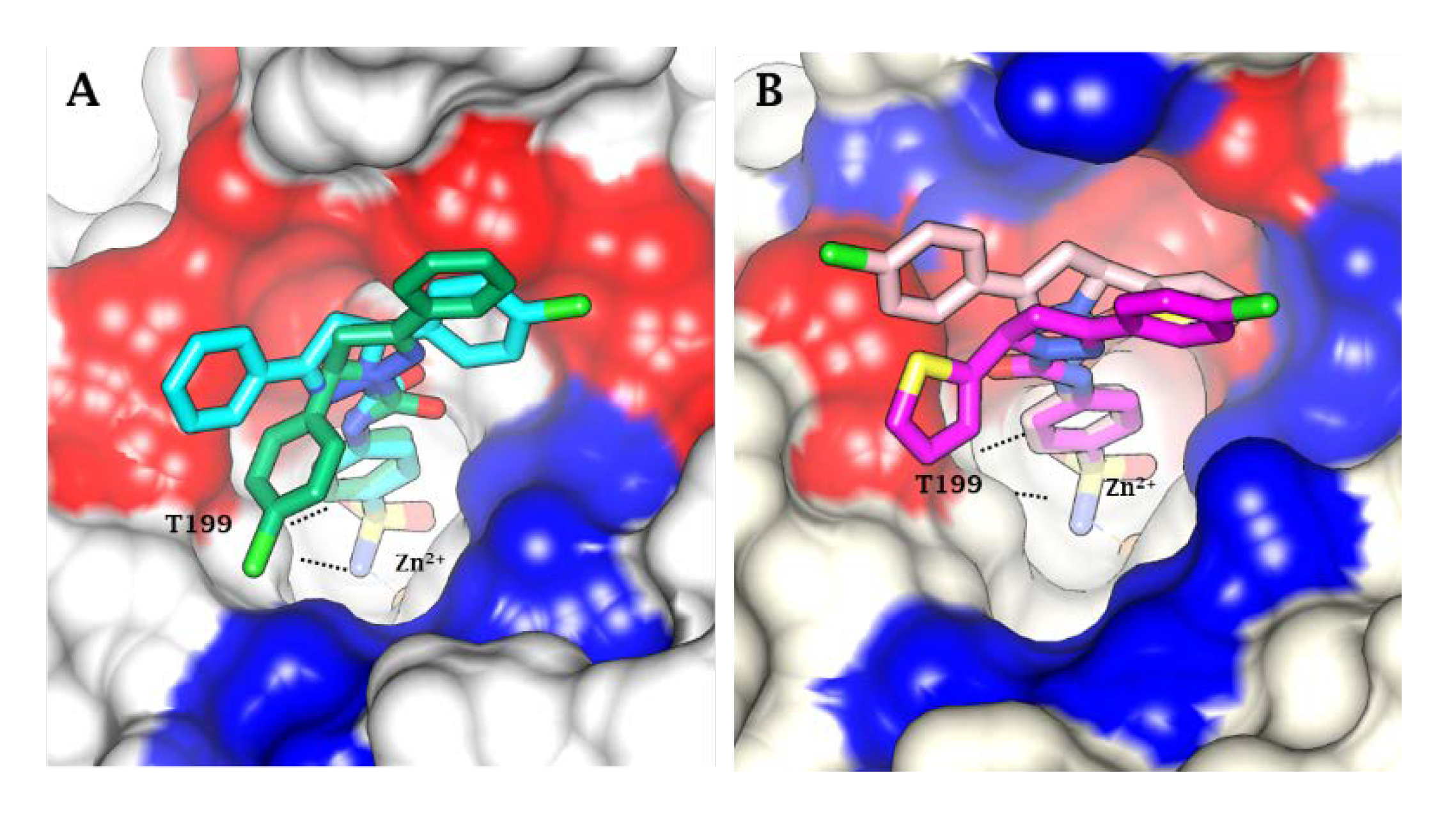
2.3. Molecular Docking
3. Conclusions
4. Experimental Section
4.1. Chemistry
4.1.1. Synthesis of 4-ureidobenzenesulfonamide 2
4.1.2. Synthesis of 4-aminosulfonylphenyl Semicarbazide 3
4.1.3. Synthesis of (E)-1-(4-substituted phenyl)-3-substituted Aromatic prop-2-en-1-one 4 [20,21]
4.1.4. General Procedure for Synthesis of 3-phenyl-5-aryl-N-(4-sulfamoylphenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 5–25 [22]
3,5-Diphenyl-4,5-dihydro-pyrazole-1-carboxylic acid (4-sulfamoyl-phenyl)-amide 5
5-(4-Chlorophenyl)-3-phenyl-4,5-dihydro-pyrazole-1-carboxylic acid (4-sulfamoyl-phenyl)-amide 6
5-(4-Flurophenyl)-3-phenyl-4,5-dihydro-pyrazole-1-carboxylic acid (4-sulfamoyl-phenyl)-amide 7
5-(2-Chloro-6-fluorophenyl)-3-phenyl-4, 5-dihydro-pyrazole-1-carboxylic acid (4-sulfamoyl-phenyl)-amide 8
3-Phenyl-5-thiophene-2-yl-4, 5-dihydro-pyrazole-1-carboxylic acid (4-sulfamoyl-phenyl)-amide 9
5-Anthracene-9-yl-3-phenyl-4,5-dihydro-pyrazole-1-carboxylic acid (4-sulfamoyl-phenyl)-amide 10
5-(2,4-Dichlorophenyl)-3-phenyl-4, 5-dihydro-pyrazole-1-carboxylic acid (4-sulfamoyl-phenyl)-amide 11
3-(4-Chlorophenyl)-5-phenyl-N-(4-sulfamoylphenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 12
3-(4-Chlorophenyl)-N-(4-sulfamoylphenyl)-5-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 13
3,5-Bis(4-chlorophenyl)-N-(4-sulfamoylphenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 14
3-(4-Chlorophenyl)-5-(4-fluorophenyl)-N-(4-sulfamoylphenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 15
5-(2-Chlorophenyl)-3-(4-chlorophenyl)-N-(4-sulfamoylphenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 16
3-(4-Chlorophenyl)-5-(2,4-dichlorophenyl)-N-(4-sulfamoylphenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 17
3-(4-Chlorophenyl)-N-(4-sulfamoylphenyl)-5-(thiophen-2-yl)-4,5-dihydro-1H-pyrazole-1-carboxamide 18
5-(2-Chloro-6-fluorophenyl)-3-(4-chlorophenyl)-N-(4-sulfamoylphenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 19
3-(4-Chlorophenyl)-5-(2-hydroxyphenyl)-N-(4-sulfamoylphenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 20
3-(4-Chlorophenyl)-5-(4-methoxyphenyl)-N-(4-sulfamoylphenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 21
3-(4-Chlorophenyl)-5-(3,4-dimethoxyphenyl)-N-(4-sulfamoylphenyl)-4,5-dihydro-1H-pyrazole-1-carboxamide 22
3-(4-Bromophenyl)-5-phenyl-45-dihydro-pyrazole-1-carboxylic acid (4-sulfamoyl-phenyl)-amide 23
3-(4-Bromophenyl)-5-(4-chloro phenyl)-4,5-dihydro-pyrazole-1-carboxylic acid (4-sulfamoyl-phenyl)-amide 24
3-(4-Bromo phenyl)-5-(4-fluro phenyl)-4, 5-dihydro-pyrazole-1-carboxylic acid (4-sulfamoyl-phenyl)-amide 25
4.2. CA Inhibition
4.3. Molecular Docking
Author Contributions
Funding
Conflicts of Interest
References
- Smith, K.S.; Jakubzick, C.; Whittam, T.S.; Ferry, J.G. Carbonic anhydrase is an ancient enzyme widespread in prokaryotes. Proc. Natl. Acad. Sci. USA 1999, 96, 15184–15189. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; De Simone, G. Multiple Binding Modes of Inhibitors to Carbonic Anhydrases: How to Design Specific Drugs Targeting 15 Different Isoforms? Chem Rev. 2012, 112, 4421. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T. Carbonic Anhydrases: An Overview. In Carbonic Anhydrases; Nocentini, A., Supuran, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–16. [Google Scholar]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumors as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: A patent review (2008-2018). Expert Opin. Ther. Pat. 2018, 28, 729–740. [Google Scholar] [CrossRef]
- Alterio, V.; Hilvo, M.; Di Fiore, A.; Supuran, C.T.; Pan, P.; Parkkila, S.; Scaloni, A.; Pastorek, J.; Pastorekova, S.; Pedone, C.; et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc. Natl. Acad. Sci. USA 2009, 106, 16233–16238. [Google Scholar] [CrossRef]
- Whittington, D.A.; Waheed, A.; Ulmasov, B.; Shah, G.N.; Grubb, J.H.; Sly, W.S.; Christianson, D.W. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc. Natl. Acad. Sci. USA 2001, 98, 9545–9550. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Carta, F.; Monti, S.M.; De Simone, G. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med. Res. Rev. 2018, 38, 1799–1836. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Parkkila, S.; Zavada, J. Tumor-associated carbonic anhydrases and their clinical significance. Adv. Clin. Chem. 2006, 42, 167–216. [Google Scholar] [PubMed]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist. J. Enzyme Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin. Drug Discov. 2019, 14, 1175–1197. [Google Scholar] [CrossRef] [PubMed]
- Lomelino, C.L.; Andring, J.T.; McKenna, R. Crystallography and its impact on carbonic anhydrase research. Int. J. Med. Chem. 2018, 2018, 9419521. [Google Scholar] [CrossRef] [PubMed]
- Pacchiano, F.; Carta, F.; McDonald, P.C.; Lou, Y.; Vullo, D.; Scozzafava, A.; Dedhar, S.; Supuran, C.T. Ureido-Substituted Benzenesulfonamides Potently Inhibit Carbonic Anhydrase IX and Show Antimetastatic Activity in a Model of Breast Cancer Metastasis. J. Med. Chem. 2011, 54, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Welichem Biotech Incorporated. Safety Study of SLC-0111 in Subjects with Advanced Solid Tumours; NCT02215850; Welichem Biotech Incorporated: Vancouver, BC, Canada.
- Pichake, J.; Kharkar, P.; Ceruso, M.; Supuran, C.T.; Toraskar, M.P. Carbonic Anhydrase Inhibitors: Design, Synthesis and Biological Evaluation of Novel Sulfonyl Semicarbazide Derivatives. ACS Med. Chem. Lett. 2014, 5, 793–796. [Google Scholar] [CrossRef]
- Singasane, N.; Kharkar, P.; Ceruso, M.; Supuran, C.T.; Toraskar, M.P. Inhibition of carbonic anhydrase isoforms I, II, IX and XII with Schiff’s bases incorporating iminoureido moieties. J. Enzyme Inhib. Med. Chem. 2015, 30, 901–907. [Google Scholar] [CrossRef][Green Version]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar]
- Leitans, J.; Kazaks, A.; Balode, A.; Ivanova, J.; Zalubovskis, R.; Supuran, C.T.; Tars, K. Efficient expression and Crystallization system of cancer-associated carbonic anhydrase isoform IX. J. Med. Chem. 2015, 58, 9004–9009. [Google Scholar] [CrossRef]
- Vogel, A.; Furniss, B.; Hannaford, A.; Tatchell, A.R.; Smith, P.W.G. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman: Harlow, UK, 1989. [Google Scholar]
- Singhal, M.; Paul, A.; Singh, H. Design, Synthesis and Biological Evaluation of 2-Methylphenyl Semicarbazone Derivatives. Bangladesh J. Pharmacol. 2011, 6, 1–7. [Google Scholar] [CrossRef][Green Version]
- Khalil, N.; Ahmed, E.; El-Nassan, H. Synthesis, Characterization and Biological Evaluation of Certain 1, 3-Thiazolone Derivatives Bearing Pyrazoline Moiety as Potential Anti-Breast Cancer Agents. Med. Chem. Res. 2013, 22, 1021–1027. [Google Scholar] [CrossRef]
- Krasavin, M.; Shetnev, A.; Baykov, S.; Kalinin, S.; Nocentini, A.; Sharoyko, V.; Poli, G.; Tuccinardi, T.; Korsakov, M.; Tennikova, T.B.; et al. Pyridazinone-substituted benzenesulfonamides display potent inhibition of membrane-bound human carbonic anhydrase IX and promising antiproliferative activity against cancer cell lines. Eur. J. Med. Chem. 2019, 168, 301–314. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; El-Azab, A.S.; Bua, S.; Nocentini, A.; Abu El-Enin, M.A.; Alanazi, M.M.; AlSaif, N.A.; Hefnawy, M.M.; Supuran, C.T. Design, synthesis, and carbonic anhydrase inhibition activity of benzenesulfonamide-linked novel pyrazoline derivatives. Bioorg. Chem. 2019, 87, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Moi, D.; Nocentini, A.; Deplano, A.; Balboni, G.; Supuran, C.T.; Onnis, V. Structure-activity relationship with pyrazoline-based aromatic sulfamates as carbonic anhydrase isoforms I, II, IX and XII inhibitors: Synthesis and biological evaluation. Eur. J. Med. Chem. 2019, 182, 111638. [Google Scholar] [CrossRef] [PubMed]
- Vats, L.; Kumar, R.; Bua, S.; Nocentini, A.; Gratteri, P.; Supuran, C.T.; Sharma, P.K. Continued exploration and tail approach synthesis of benzenesulfonamides containing triazole and dual triazole moieties as carbonic anhydrase I, II, IV and IX inhibitors. Eur. J. Med. Chem. 2019, 183, 111698. [Google Scholar] [CrossRef] [PubMed]
- Rogato, A.; Del Prete, S.; Nocentini, A.; Carginale, V.; Supuran, C.T.; Capasso, C. Phaeodactylum tricornutum as a model organism for testing the membrane penetrability of sulphonamide carbonic anhydrase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Suite Release 2019-1; Schrödinger, LLC: New York, NY, USA, 2019; (a) Prime, v.5.5; Maestro v.11.9; (b) Epik, v.4.7; (c) Impact, v.8.2; (d) Macromodel v.12.3. (e) Glide, v.8.2.
- Nocentini, A.; Gratteri, P.; Supuran, C.T. Phosphorus versus Sulfur: Discovery of Benzenephosphonamidates as Versatile Sulfonamide-Mimic Chemotypes Acting as Carbonic Anhydrase Inhibitors. Chemistry 2019, 25, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Bonardi, A.; Gratteri, P.; Cerra, B.; Gioiello, A.; Supuran, C.T. Steroids interfere with human carbonic anhydrase activity by using alternative binding mechanisms. J. Enzyme Inhib. Med. Chem. 2018, 33, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Carta, F.; Tanc, M.; Selleri, S.; Supuran, C.T.; Bazzicalupi, C.; Gratteri, P. Deciphering the Mechanism of Human Carbonic Anhydrases Inhibition with Sulfocoumarins: Computational and Experimental Studies. Chemistry 2018, 24, 7840–7844. [Google Scholar] [CrossRef]


| Cmpd | R | Ar | KI (nM) | ||||
|---|---|---|---|---|---|---|---|
| CA I | CA II | CA VII | CA IX | CA XII | |||
| 5 | H | C6H5 | 382 | 67.3 | 33.2 | 1.3 | 7.7 |
| 6 | H | 4-Cl-C6H4 | 36.2 | 8.2 | 7.7 | 1.4 | 1.2 |
| 7 | H | 4-F-C6H4 | 252 | 58.2 | 2.3 | 1.5 | 6.3 |
| 8 | H | 2-Cl-6-F-C6H3 | 38.3 | 5.2 | 2.4 | 1.5 | 1.4 |
| 9 | H | thiophen-2-yl | 185 | 5.2 | 1.9 | 1.4 | 2.5 |
| 10 | H | anthracene-9-yl | 293 | 7.1 | 2.4 | 1.3 | 6.8 |
| 11 | H | 2,4-diCl-C6H3 | 39.5 | 5.4 | 2.5 | 1.4 | 0.84 |
| 12 | Cl | C6H5 | 239 | 233 | 350 | 16.5 | 9.2 |
| 13 | Cl | 4-CH3-C6H4 | 66.7 | 12.3 | 4.5 | 2.9 | 9.2 |
| 14 | Cl | 4-Cl-C6H4 | 237.9 | 12.7 | 3.3 | 11.4 | 1.1 |
| 15 | Cl | 4-F-C6H4 | 616.7 | 71.3 | 3.2 | 2.7 | 1.0 |
| 16 | Cl | 2-Cl-C6H4 | 296.6 | 8.8 | 4.3 | 8 | 1.7 |
| 17 | Cl | 2,4-diCl-C6H3 | 769.6 | 13.5 | 27.6 | 15 | 1.3 |
| 18 | Cl | thiophen-2-yl | 726 | 9.2 | 2.9 | 2.5 | 0.62 |
| 19 | Cl | 2-Cl-6-F-C6H3 | 42.7 | 10.8 | 2.3 | 2.7 | 6.8 |
| 20 | Cl | 2-OH-C6H4 | 74.2 | 7.6 | 2.1 | 76.6 | 0.99 |
| 21 | Cl | 4-OCH3-C6H4 | 367.7 | 9.4 | 2.7 | 2.8 | 0.82 |
| 22 | Cl | 3,4-diOCH3-C6H3 | 165.9 | 9.3 | 2.3 | 2.2 | 1.5 |
| 23 | Br | C6H5 | 44.4 | 6.3 | 2.9 | 1.4 | 6.7 |
| 24 | Br | 4-Cl-C6H4 | 45.5 | 10.7 | 2.7 | 14.3 | 0.89 |
| 25 | Br | 4-F-C6H4 | 38.2 | 5.5 | 2.6 | 1.4 | 2.4 |
| AAZ | - | 250 | 12.1 | 2.5 | 25 | 5.8 | |
| Cmpd | GlideScore (kcal/mol) | Cmpd | GlideScore (kcal/mol) | ||
|---|---|---|---|---|---|
| CA IX | CA XII | CA IX | CA XII | ||
| (S)-5 | −6.82 | −6.02 | (R)-5 | −5.34 | −6.84 |
| (S)-6 | −6.54 | −6.28 | (R)-6 | −5.67 | −7.01 |
| (S)-7 | −6.93 | −5.63 | (R)-7 | −5.39 | −6.49 |
| (S)-8 | −6.28 | −6.45 | (R)-8 | −5.38 | −7.36 |
| (S)-9 | −6.34 | −6.63 | (R)-9 | −5.94 | −7.57 |
| (S)-10 | −7.01 | −6.22 | (R)-10 | −5.68 | −6.82 |
| (S)-11 | −6.35 | −5.96 | (R)-11 | −5.02 | −7.69 |
| (S)-12 | −5.86 | −6.29 | (R)-12 | −5.49 | −6.57 |
| (S)-13 | −6.57 | −6.07 | (R)-13 | −5.79 | −7.03 |
| (S)-14 | −6.02 | −6.38 | (R)-14 | −5.38 | −7.51 |
| (S)-15 | −6.83 | −5.99 | (R)-15 | −5.46 | −7.36 |
| (S)-16 | −6.12 | −6.23 | (R)-16 | −5.82 | −7.15 |
| (S)- 17 | −5.84 | −6.54 | (R)- 17 | −5.09 | −7.64 |
| (S)- 18 | −6.14 | −6.08 | (R)- 18 | −5.97 | −7.92 |
| (S)- 19 | −6.72 | −6.63 | (R)- 19 | −5.01 | −7.29 |
| (S)- 20 | −5.67 | −6.41 | (R)- 20 | −5.26 | −6.86 |
| (S)- 21 | −6.35 | −6.39 | (R)- 21 | −6.03 | −7.27 |
| (S)- 22 | −6.92 | −6.28 | (R)- 22 | −6.05 | −7.16 |
| (S)- 23 | −6.54 | −6.92 | (R)- 23 | −5.65 | −6.48 |
| (S)- 24 | −6.24 | −6.47 | (R)- 24 | −5.24 | −7.58 |
| (S)- 25 | −6.82 | −6.19 | (R)- 25 | −5.37 | −7.22 |
| AAZ | −6.31 | −7.35 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hargunani, P.; Tadge, N.; Ceruso, M.; Leitans, J.; Kazaks, A.; Tars, K.; Gratteri, P.; Supuran, C.T.; Nocentini, A.; Toraskar, M.P. Aryl-4,5-dihydro-1H-pyrazole-1-carboxamide Derivatives Bearing a Sulfonamide Moiety Show Single-digit Nanomolar-to-Subnanomolar Inhibition Constants against the Tumor-associated Human Carbonic Anhydrases IX and XII. Int. J. Mol. Sci. 2020, 21, 2621. https://doi.org/10.3390/ijms21072621
Hargunani P, Tadge N, Ceruso M, Leitans J, Kazaks A, Tars K, Gratteri P, Supuran CT, Nocentini A, Toraskar MP. Aryl-4,5-dihydro-1H-pyrazole-1-carboxamide Derivatives Bearing a Sulfonamide Moiety Show Single-digit Nanomolar-to-Subnanomolar Inhibition Constants against the Tumor-associated Human Carbonic Anhydrases IX and XII. International Journal of Molecular Sciences. 2020; 21(7):2621. https://doi.org/10.3390/ijms21072621
Chicago/Turabian StyleHargunani, Priya, Nikhil Tadge, Mariangela Ceruso, Janis Leitans, Andris Kazaks, Kaspars Tars, Paola Gratteri, Claudiu T. Supuran, Alessio Nocentini, and Mrunmayee P. Toraskar. 2020. "Aryl-4,5-dihydro-1H-pyrazole-1-carboxamide Derivatives Bearing a Sulfonamide Moiety Show Single-digit Nanomolar-to-Subnanomolar Inhibition Constants against the Tumor-associated Human Carbonic Anhydrases IX and XII" International Journal of Molecular Sciences 21, no. 7: 2621. https://doi.org/10.3390/ijms21072621
APA StyleHargunani, P., Tadge, N., Ceruso, M., Leitans, J., Kazaks, A., Tars, K., Gratteri, P., Supuran, C. T., Nocentini, A., & Toraskar, M. P. (2020). Aryl-4,5-dihydro-1H-pyrazole-1-carboxamide Derivatives Bearing a Sulfonamide Moiety Show Single-digit Nanomolar-to-Subnanomolar Inhibition Constants against the Tumor-associated Human Carbonic Anhydrases IX and XII. International Journal of Molecular Sciences, 21(7), 2621. https://doi.org/10.3390/ijms21072621








