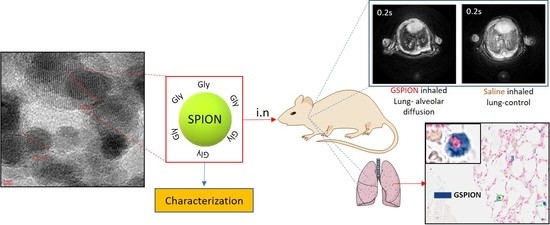
A Novel Approach for Non-Invasive Lung Imaging and Targeting Lung Immune Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of GSPIONs for Evaluating Their Pulmonary Delivery Capability
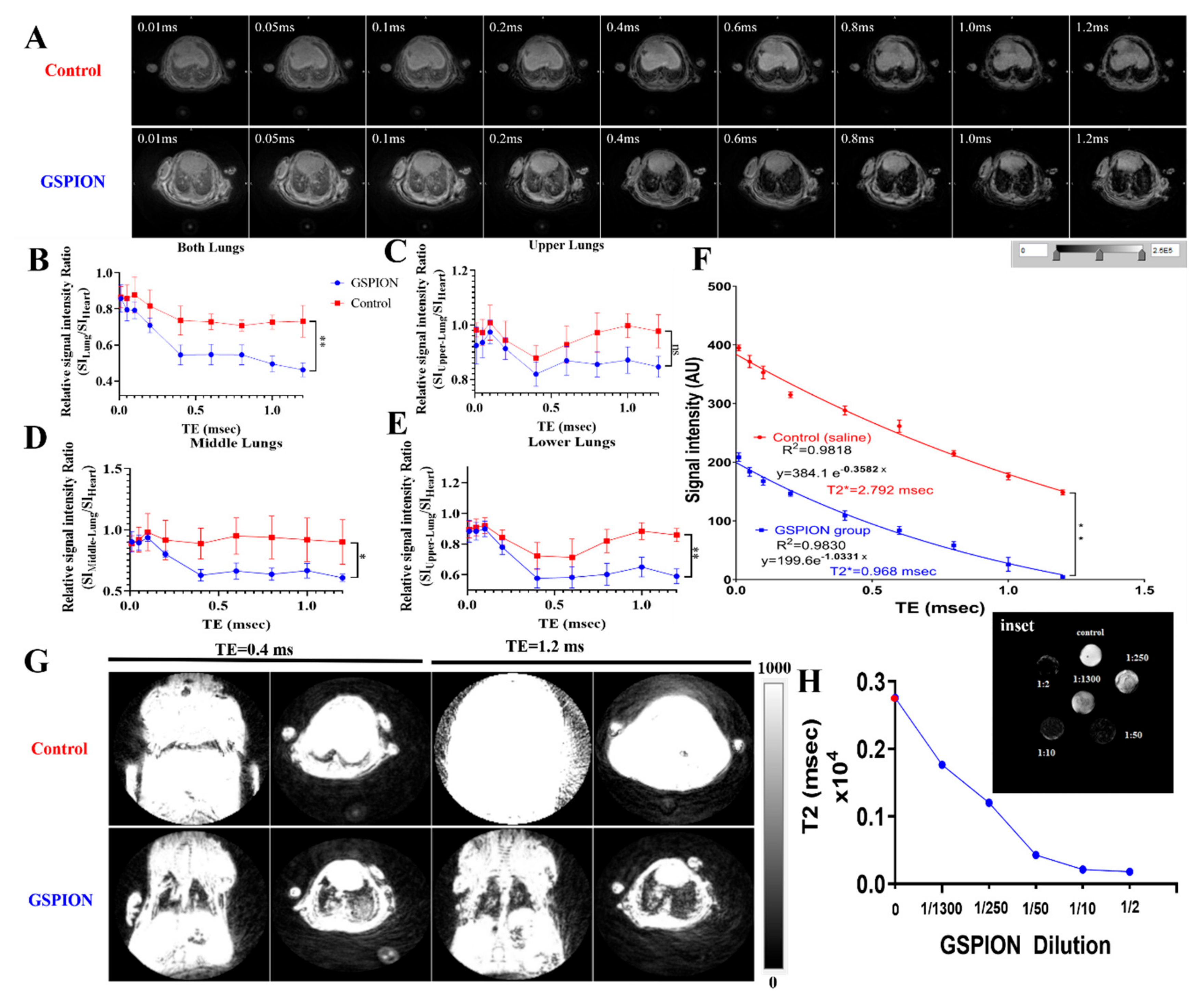
2.2. GSPIONs Are Distributed Throughout the Lung and Show T2* Relaxivity in Lung
2.3. Selective Localization of GSPIONs in the Lung Within Alveolar Macrophages and Neutrophils
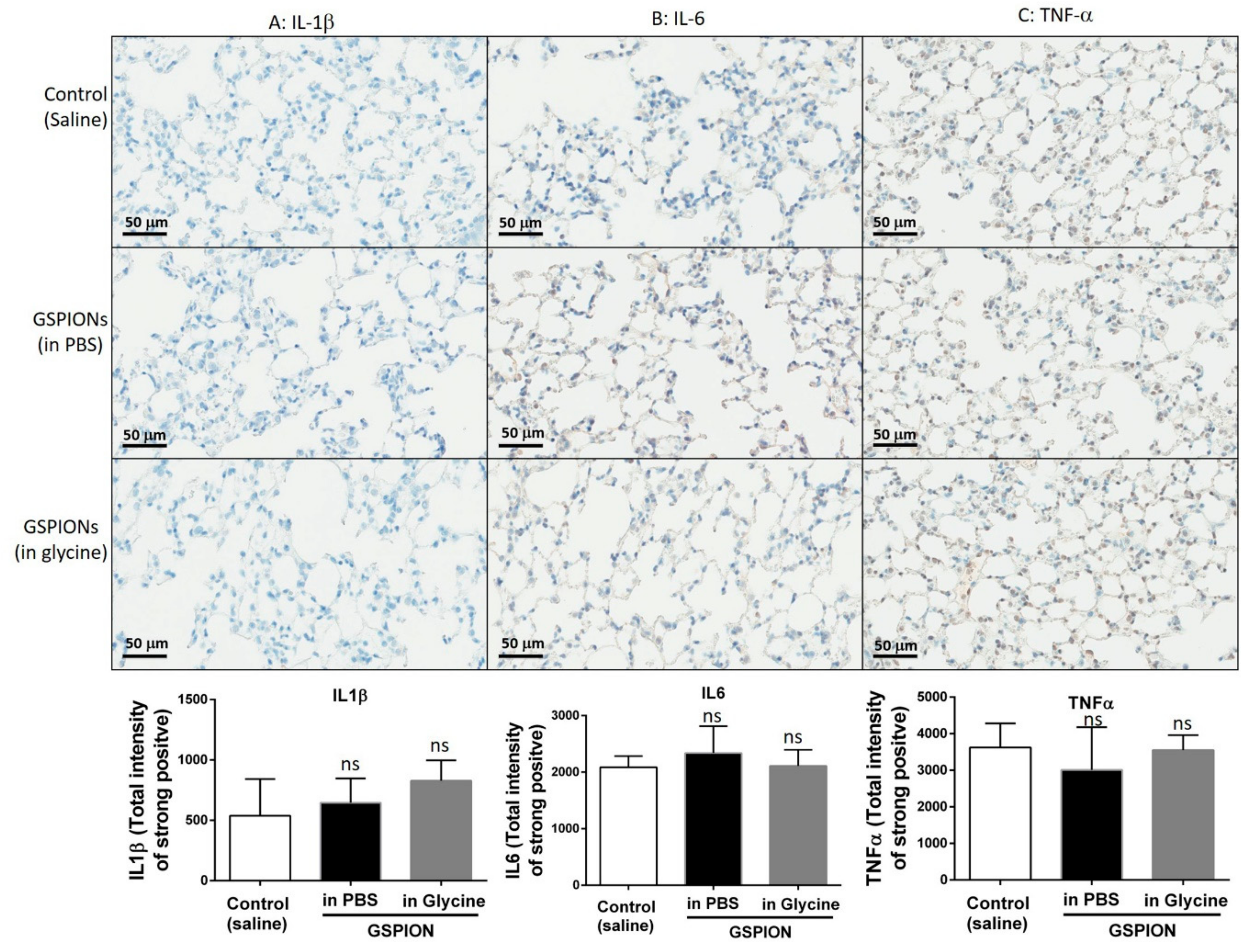
2.4. GSPIONs Do Not Induce Pro-Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of GSPIONs
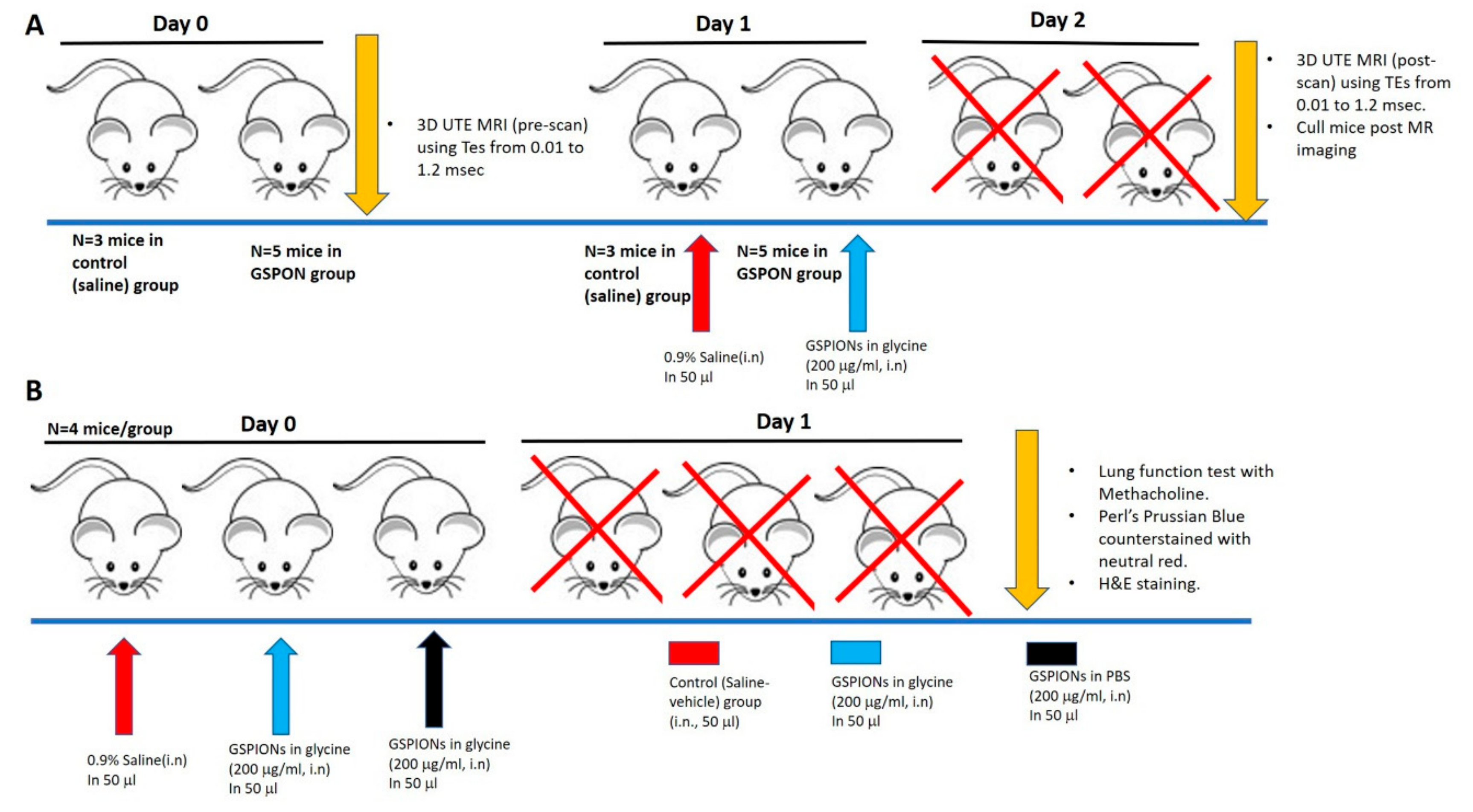
4.2. Study Design and Animal Ethics
4.3. Lung Function Measurement Upon GSPION Sensitization
4.4. Histology and Morphometry
4.4.1. Nanoparticle Uptake by Lung Immune Cells
4.4.2. H&E Staining for Tissue Morphometry
4.5. Non-Invasive Lung 3D Ultra-Short Echo Time Magnetic Resonance Imaging (MRI)
Magnetic Resonance Image Processing and Quantitative Analysis
4.6. Immunohistochemical Analysis of Pro-Inflammatory Cytokines
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GSPIONs | Glycine coated super-paramagnetic iron oxide nanoparticles |
| MRI | Magnetic resonance imaging |
| SI | Signal intensity |
| UTE | Ultra-short echo time |
| AHR | Acute-hyperresponsiveness |
References
- Choi, Y.W.; Munden, R.F.; Erasmus, J.J.; Park, K.J.; Chung, W.K.; Jeon, S.C.; Park, C.K. Effects of Radiation Therapy on the Lung: Radiologic Appearances and Differential Diagnosis. RadioGraphics 2004, 24, 985–997. [Google Scholar] [CrossRef]
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.T.; Hanania, N.A.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. CHEST 2019, 156, 150–162. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef]
- Niezink, A.G.H.; de Jong, R.A.; Muijs, C.T.; Langendijk, J.A.; Widder, J. Pulmonary Function Changes After Radiotherapy for Lung or Esophageal Cancer: A Systematic Review Focusing on Dose-Volume Parameters. Oncology 2017, 22, 1257–1264. [Google Scholar] [CrossRef]
- Kim, H.; Pyo, H.; Noh, J.M.; Lee, W.; Park, B.; Park, H.Y.; Yoo, H. Preliminary result of definitive radiotherapy in patients with non-small cell lung cancer who have underlying idiopathic pulmonary fibrosis: comparison between X-ray and proton therapy. Radiat. Oncol. 2019, 14, 19. [Google Scholar] [CrossRef]
- Abratt, R.P.; Morgan, G.W.; Silvestri, G.; Willcox, P. Pulmonary complications of radiation therapy. Clin. Chest Med. 2004, 25, 167–177. Clin. Chest Med. 2004, 25, 167–177. [Google Scholar] [CrossRef]
- Inoue, K.-i.; Koike, E.; Yanagisawa, R.; Hirano, S.; Nishikawa, M.; Takano, H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol. Appl. Pharmacol. 2009, 237, 306–316. [Google Scholar] [CrossRef]
- Inoue, K.-I.; Yanagisawa, R.; Koike, E.; Nishikawa, M.; Takano, H. Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: Possible role of oxidative stress. Free. Radic. Biol. Med. 2010, 48, 924–934. [Google Scholar] [CrossRef]
- Alessandrini, F.; Schulz, H.; Takenaka, S.; Lentner, B.; Karg, E.; Behrendt, H.; Jakob, T. Effects of ultrafine carbon particle inhalation on allergic inflammation of the lung. J. Allergy Clin. Immunol. 2006, 117, 824–830. [Google Scholar] [CrossRef]
- De Haar, C.; Hassing, I.; Böl, M.; Bleumink, R.; Pieters, R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin. Exp. Allergy 2006, 36, 1469–1479. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Cai, K.; Hou, Y.; Hu, Y.; Zhao, L.; Luo, Z.; Shi, Y.; Lai, M.; Yang, W.; Liu, P. Correlation of the Cytotoxicity of TiO2 Nanoparticles with Different Particle Sizes on a Sub-200-nm Scale. Small 2011, 7, 3026–3031. [Google Scholar] [CrossRef]
- Vedantam, P.; Huang, G.; Tzeng, T.R.J. Size-dependent cellular toxicity and uptake of commercial colloidal gold nanoparticles in DU-145 cells. Cancer Nanotechnol. 2013, 4, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ariano, P.; Zamburlin, P.; Mortera, R.; Ghiazza, M.; Lovisolo, D.; Gilardino, A.; Onida, B.; Tomatis, M.; Fubini, B. Interaction of Spherical Silica Nanoparticles with Neuronal Cells: Size-Dependent Toxicity and Perturbation of Calcium Homeostasis. Small 2011, 7, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Van Rijt, S.H.; Bein, T.; Meiners, S.; Soborg, C.; Ruhwald, M.; Andersen, P.H.; Ravn, P. Medical nanoparticles for next generation drug delivery to the lungs. Eur. Respir. J. 2014, 44, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Braet, F.; Wisse, E.; Bomans, P.; Frederik, P.; Geerts, W.J.C.; Koster, A.; Soon, L.; Ringer, S. Contribution of high-resolution correlative imaging techniques in the study of the liver sieve in three-dimensions. Microsc. Res. Tech. 2007, 70, 230–242. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle–Cell Interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Ponzoni, M.; Pastorino, F.; Di Paolo, D.; Perri, P.; Brignole, C. Targeting Macrophages as a Potential Therapeutic Intervention: Impact on Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2018, 19, 1953. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M. Macrophage Recognition of Crystals and Nanoparticles. Front. Immunol. 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Boer, J.C.; Plebanski, M.; Selomulya, C.; Royce, S. Insights into endotoxin-mediated lung inflammation and future treatment strategies. Expert Rev. Respir. Med. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed]
- Mohr, K. Aggregation Behavior of Polystyrene-Nanoparticles in Human Blood Serum and its Impact on the in vivo Distribution in Mice. J. Nanomed. Nanotechnol. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2008, 61, 158–171. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Kao, T.H.; Tsai, T.Y.; Chiu, C.P.; Kumar, R.; Chen, B.H. The synthesis and characterization of poly(γ-glutamic acid)-coated magnetite nanoparticles and their effects on antibacterial activity and cytotoxicity. Nanotechnology 2011, 22, 075101. [Google Scholar] [CrossRef]
- Chakraborty, A.; Boer, J.C.; Plebanski, M.; Selomulya, C. Amino Acid Functionalized Inorganic Nanoparticles as Cutting-Edge Therapeutic and Diagnostic Agents. Bioconjugate Chem. 2017, 29, 657–671. [Google Scholar] [CrossRef]
- Chakraborty, A.; Royce, S.G.; Selomulya, C.; Plebanski, M. Glycine microparticles loaded with functionalized nanoparticles for pulmonary delivery. Int. J. Pharm. 2019, 570, 118654. [Google Scholar] [CrossRef]
- Lee, H.J.; Jang, K.-S.; Jang, S.; Kim, J.W.; Yang, H.-M.; Jeong, Y.Y.; Kim, J.-D. Poly(amino acid)s micelle-mediated assembly of magnetite nanoparticles for ultra-sensitive long-term MR imaging of tumors. Chem. Commun. 2010, 46, 3559–3561. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Park, C.W.; Woo, M.A.; Kim, M.I.; Jo, Y.M.; Park, H.G.; Kim, J.D. HER2/neu Antibody Conjugated Poly(amino acid)-Coated Iron Oxide Nanoparticles for Breast Cancer MR Imaging. Biomacromolecules 2010, 11, 2866–2872. [Google Scholar] [CrossRef]
- Mazuel, F.; Espinosa, A.; Luciani, N.; Reffay, M.; Le Borgne, R.; Motte, L.; Desboeufs, K.; Michel, A.; Pellegrino, T.; Lalatonne, Y.; et al. Massive Intracellular Biodegradation of Iron Oxide Nanoparticles Evidenced Magnetically at Single-Endosome and Tissue Levels. ACS Nano 2016, 10, 7627–7638. [Google Scholar] [CrossRef]
- Bloem, J.L.; Reijnierse, M.; Huizinga, T.W.J.; Mil, A.V.D.H.-V. MR signal intensity: staying on the bright side in MR image interpretation. RMD Open 2018, 4, e000728. [Google Scholar] [CrossRef]
- Xing, R.; Zhang, F.; Xie, J.; Aronova, M.; Zhang, G.; Guo, N.; Huang, X.; Sun, X.; Liu, G.; Bryant, L.H.; et al. Polyaspartic acid coated manganese oxide nanoparticles for efficient liver MRI. Nanoscale 2011, 3, 4943–4945. [Google Scholar] [CrossRef]
- Roos, J.E.; McAdams, H.P.; Kaushik, S.S.; Driehuys, B. Hyperpolarized Gas MR Imaging. Magn. Reson. Imaging Clin. North Am. 2015, 23, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Wyszogrodzka, G.; Dorozynski, P.; Gil, B.; Roth, W.J.; Strzempek, M.; Marszałek, B.; Węglarz, W.; Menaszek, E.; Strzempek, W.; Kulinowski, P. Iron-Based Metal-Organic Frameworks as a Theranostic Carrier for Local Tuberculosis Therapy. Pharm. Res. 2018, 35, 144. [Google Scholar] [CrossRef] [PubMed]
- Wyszogrodzka-Gaweł, G.; Dorozynski, P.; Giovagnoli, S.; Strzempek, W.; Pesta, E.; Węglarz, W.; Gil, B.; Menaszek, E.; Kulinowski, P. An Inhalable Theranostic System for Local Tuberculosis Treatment Containing an Isoniazid Loaded Metal Organic Framework Fe-MIL-101-NH2-From Raw MOF to Drug Delivery System. Pharmaceutics 2019, 11, 687. [Google Scholar] [CrossRef]
- Altes, T.A.; Eichinger, M.; Puderbach, M. Magnetic Resonance Imaging of the Lung in Cystic Fibrosis. Proc. Am. Thorac. Soc. 2007, 4, 321–327. [Google Scholar] [CrossRef]
- Takahashi, M.; Togao, O.; Obara, M.; Van Cauteren, M.; Ohno, Y.; Doi, S.; Kuro-O, M.; Malloy, C.; Hsia, C.C.; Dimitrov, I. Ultra-short echo time (UTE) MR imaging of the lung: Comparison between normal and emphysematous lungs in mutant mice. J. Magn. Reson. Imaging 2010, 32, 326–333. [Google Scholar] [CrossRef]
- Johnson, K.M.; Fain, S.B.; Schiebler, M.; Nagle, S. Optimized 3D ultrashort echo time pulmonary MRI. Magn. Reson. Med. 2012, 70, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Barick, K.C.; Hassan, P. Glycine passivated Fe3O4 nanoparticles for thermal therapy. J. Colloid Interface Sci. 2012, 369, 96–102. [Google Scholar] [CrossRef]
- Hardy, C.L.; LeMasurier, J.S.; Belz, G.T.; Scalzo-Inguanti, K.; Yao, J.; Xiang, S.; Kanellakis, P.; Bobik, A.; Strickland, D.H.; Rolland, J.M.; et al. Inert 50-nm Polystyrene Nanoparticles That Modify Pulmonary Dendritic Cell Function and Inhibit Allergic Airway Inflammation. J. Immunol. 2011, 188, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Pušnik, K.; Peterlin, M.; Kralj-Cigic, I.; Marolt, G.; Kogej, K.; Mertelj, A.; Gyergyek, S.; Makovec, D. Adsorption of Amino Acids, Aspartic Acid, and Lysine onto Iron-Oxide Nanoparticles. J. Phys. Chem. C 2016, 120, 14372–14381. [Google Scholar] [CrossRef]
- Liu, L.; Sha, R.; Yang, L.; Zhao, X.; Zhu, Y.; Gao, J.; Zhang, Y.; Zhang, Y. Impact of Morphology on Iron Oxide Nanoparticles-Induced Inflammasome Activation in Macrophages. ACS Appl. Mater. Interfaces 2018, 10, 41197–41206. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Rokstad, A.M.; Ali, S.; Lambris, J.; E Mollnes, T.; Nilsen, A.M.; Stenvik, J. Iron oxide nanoparticles induce cytokine secretion in a complement-dependent manner in a human whole blood model. Int. J. Nanomed. 2017, 12, 3927–3940. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Peters, J.I.; Williams, R.O. Influence of particle size on regional lung deposition – What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-Mediated Pulmonary Drug Delivery: A Review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082–2113. [Google Scholar] [CrossRef]
- Katsnelson, B.A.; Privalova, L.; Sutunkova, M.; Tulakina, L.G.; Pichugova, S.V.; Beykin, J.B.; Khodos, M.J. Interaction of iron oxide Fe3O4 nanoparticles and alveolar macrophages in vivo. Bull. Exp. Biol. Med. 2012, 152, 627–629. [Google Scholar] [CrossRef]
- Gannon, M.C.; Nuttall, J.A.; Nuttall, F.Q. The metabolic response to ingested glycine1–3. Am. J. Clin. Nutr. 2002, 76, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Vásquez, N.; Zamudio, P.; Cerón, E.; Vanda, B.; Zenteno, E.; Carvajal-Sandoval, G. Effect of glycine in streptozotocin-induced diabetic rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 134, 521–527. [Google Scholar] [CrossRef]
- Kusaka, T.; Nakayama, M.; Nakamura, K.; Ishimiya, M.; Furusawa, E.; Ogasawara, K. Effect of Silica Particle Size on Macrophage Inflammatory Responses. PLOS ONE 2014, 9, e92634. [Google Scholar] [CrossRef] [PubMed]
- Bogart, L.K.; Pourroy, G.; Murphy, C.J.; Puntes, V.; Pellegrino, T.; Rosenblum, D.; Peer, D.; Levy, R. Nanoparticles for Imaging, Sensing, and Therapeutic Intervention. Acs Nano 2014, 8, 3107–3122. [Google Scholar] [CrossRef]
- Gossuin, Y.; Gillis, P.; Hocq, A.; Vuong, Q.L.; Roch, A. Magnetic resonance relaxation properties of superparamagnetic particles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Klug, G.; Kampf, T.; Bloemer, S.; Bremicker, J.; Ziener, C.H.; Heymer, A.; Gbureck, U.; Rommel, E.; Nöth, U.; Schenk, W.A.; et al. Intracellular and extracellular T1 and T2 relaxivities of magneto-optical nanoparticles at experimental high fields. Magn. Reson. Med. 2010, 64, 1607–1615. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Mattijssen, F.; De Wit, N.J.; Georgiadi, A.; Hooiveld, G.J.; Van Der Meer, R.; He, Y.; Qi, L.; Köster, A.; Tamsma, J.T.; et al. Angptl4 Protects against Severe Proinflammatory Effects of Saturated Fat by Inhibiting Fatty Acid Uptake into Mesenteric Lymph Node Macrophages. Cell Metab. 2010, 12, 580–592. [Google Scholar] [CrossRef]
- Hardy, C.L.; LeMasurier, J.S.; Mohamud, R.; Yao, J.; Xiang, S.; Rolland, J.M.; O’Hehir, R.; Plebanski, M. Differential Uptake of Nanoparticles and Microparticles by Pulmonary APC Subsets Induces Discrete Immunological Imprints. J. Immunol. 2013, 191, 5278–5290. [Google Scholar] [CrossRef]
- Eynden, J.V.D.; Ali, S.S.; Horwood, N.J.; Carmans, S.; Brone, B.; Hellings, N.; Steels, P.; Harvey, R.J.; Rigo, J.-M. Glycine and Glycine Receptor Signalling in Non-Neuronal Cells. Front. Mol. Neurosci. 2009, 2, 9. [Google Scholar] [CrossRef]
- Schmidt, K.F.; Ziu, M.; Schmidt, N.O.; Vaghasia, P.; Cargioli, T.G.; Doshi, S.; Albert, M.S.; Black, P.M.; Carroll, R.S.; Sun, Y. Volume reconstruction techniques improve the correlation between histological and in vivo tumor volume measurements in mouse models of human gliomas. J. NeuroOncol. 2004, 68, 207–215. [Google Scholar] [CrossRef]
- Martin, A.R.; Thompson, B.; Finlay, W.H. MRI Measurement of Regional Lung Deposition in Mice Exposed Nose-Only to Nebulized Superparamagnetic Iron Oxide Nanoparticles. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
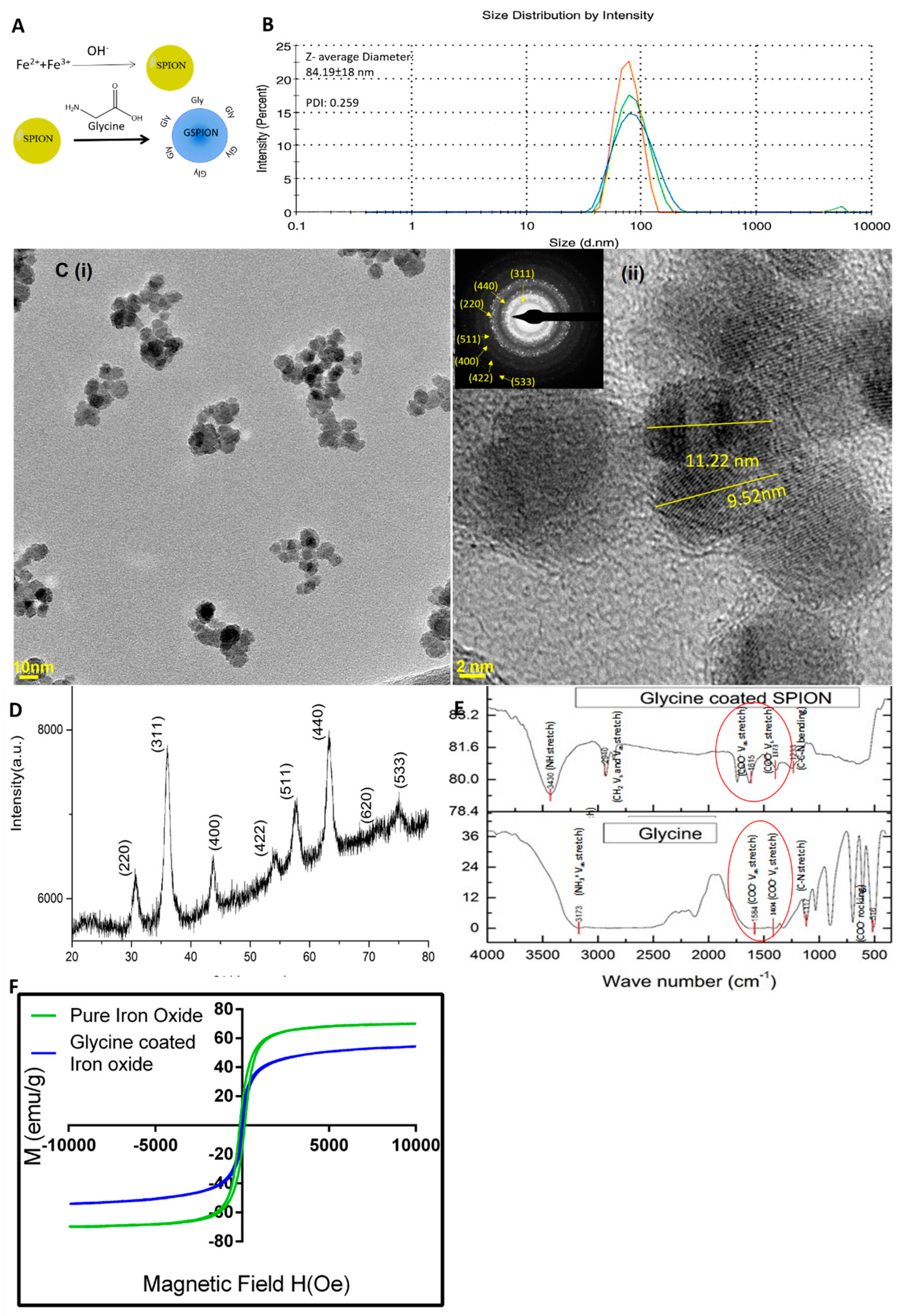

| Name | %N | %C | %H |
|---|---|---|---|
| Glycine coated SPION | 19.10 19.22 | 33.15 33.16 | 6.94 6.94 |
| Pure Glycine | 18.38 18.40 | 32.04 31.76 | 6.81 6.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, A.; Royce, S.G.; Selomulya, C.; Plebanski, M. A Novel Approach for Non-Invasive Lung Imaging and Targeting Lung Immune Cells. Int. J. Mol. Sci. 2020, 21, 1613. https://doi.org/10.3390/ijms21051613
Chakraborty A, Royce SG, Selomulya C, Plebanski M. A Novel Approach for Non-Invasive Lung Imaging and Targeting Lung Immune Cells. International Journal of Molecular Sciences. 2020; 21(5):1613. https://doi.org/10.3390/ijms21051613
Chicago/Turabian StyleChakraborty, Amlan, Simon G. Royce, Cordelia Selomulya, and Magdalena Plebanski. 2020. "A Novel Approach for Non-Invasive Lung Imaging and Targeting Lung Immune Cells" International Journal of Molecular Sciences 21, no. 5: 1613. https://doi.org/10.3390/ijms21051613
APA StyleChakraborty, A., Royce, S. G., Selomulya, C., & Plebanski, M. (2020). A Novel Approach for Non-Invasive Lung Imaging and Targeting Lung Immune Cells. International Journal of Molecular Sciences, 21(5), 1613. https://doi.org/10.3390/ijms21051613