Mut9p-LIKE KINASE Family Members: New Roles of the Plant-Specific Casein Kinase I in Plant Growth and Development
Abstract
1. Introduction
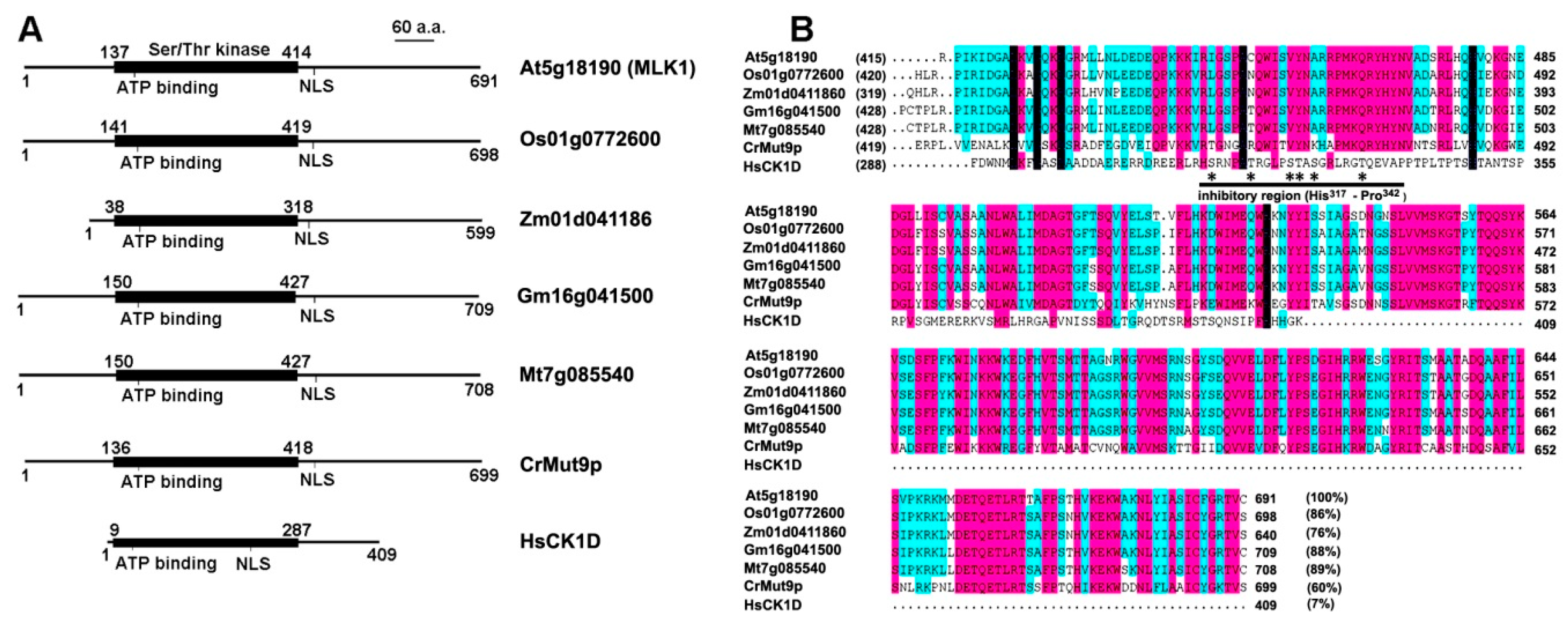
2. Plant-Specific CK1 in Arabidopsis and Major Crops
3. Regulation of CK1s Expression and Activity
4. Target Proteins Phosphorylated by MLK Homologs
4.1. Histones Targeted by MLK Famliy Members
4.2. Signaling Components Targeted by Plant CK1
5. Biological Functions of Plant CK1
5.1. Light Signaling
5.2. Circadian Clock
5.3. Phytohormone
5.4. Plant Stress Response
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AEL | Arabidopsis early-flowering 1-like |
| CCA1 | CIRCADIAN CLOCK-ASSOCIATED 1 |
| CDC7 | Cell division control protein 7 |
| CK1 | Casein kinase I |
| CRY2 | CRYPTOCHROME 2 |
| DREB2A | Dehydration-responsive element-binding protein 2A |
| ELF3/4 | EARLY FLOWERING 3/4 |
| Ghd7 | Grain number, plant height, and heading date 7 |
| H3T3ph | Phosphorylation of histone H3 at threonine 3 |
| Hd16 | Heading date 16 |
| LTG1 | Low temperature growth 1 |
| MLK | MUT9-like kinase |
| PIF | PHYTOCHROME INTERACTING FACTOR |
| PPK | Photoregulatory protein kinase |
| PRR | PSEUDO-RESPONSE REGULATOR |
| PYR1/PYL | PYRABACTIN RESISTANCE1/PYR1-LIKE |
| SLR1 | SLENDER RICE 1 |
| TCP15 | TEOSINTE BRANCHED1-CYCLOIDEA-PCF transcription factor 15 |
| TOC1 | TIMING OF CAB EXPRESSION 1 |
References
- Kumar, R.; Tao, M. Multiple forms of casein kinase from rabbit erythrocytes. Biochim. Biophys. Acta 1975, 410, 87–98. [Google Scholar] [CrossRef]
- Chen, H.H.; Qu, L.; Xu, Z.H.; Zhu, J.K.; Xue, H.W. El1-like casein kinases suppress aba signaling and responses by phosphorylating and destabilizing the aba receptors pyr/pyls in arabidopsis. Mol. Plant 2018, 11, 706–719. [Google Scholar] [CrossRef]
- Dai, C.; Xue, H.W. Rice early flowering1, a cki, phosphorylates della protein slr1 to negatively regulate gibberellin signalling. Embo J. 2010, 29, 1916–1927. [Google Scholar] [CrossRef]
- Hori, K.; Ogiso-Tanaka, E.; Matsubara, K.; Yamanouchi, U.; Ebana, K.; Yano, M. Hd16, a gene for casein kinase i, is involved in the control of rice flowering time by modulating the day-length response. Plant. J. 2013, 76, 36–46. [Google Scholar] [CrossRef]
- Mulekar, J.J.; Huq, E. Expanding roles of protein kinase ck2 in regulating plant growth and development. J. Exp. Bot. 2014, 65, 2883–2893. [Google Scholar] [CrossRef]
- Lee, J.Y. Versatile casein kinase 1: Multiple locations and functions. Plant. Signal. Behav. 2009, 4, 652–654. [Google Scholar] [CrossRef]
- van Ooijen, G.; Hindle, M.; Martin, S.F.; Barrios-Llerena, M.; Sanchez, F.; Bouget, F.Y.; O’Neill, J.S.; Le Bihan, T.; Millar, A.J. Functional analysis of casein kinase 1 in a minimal circadian system. PLoS ONE 2013, 8, e70021. [Google Scholar] [CrossRef]
- Schittek, B.; Sinnberg, T. Biological functions of casein kinase 1 isoforms and putative roles in tumorigenesis. Mol. Cancer 2014, 13, 231. [Google Scholar] [CrossRef]
- Knippschild, U.; Gocht, A.; Wolff, S.; Huber, N.; Lohler, J.; Stoter, M. The casein kinase 1 family: Participation in multiple cellular processes in eukaryotes. Cell. Signal. 2005, 17, 675–689. [Google Scholar] [CrossRef]
- Wang, X.; Hoekstra, M.F.; DeMaggio, A.J.; Dhillon, N.; Vancura, A.; Kuret, J.; Johnston, G.C.; Singer, R.A. Prenylated isoforms of yeast casein kinase i, including the novel yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase. Mol. Cell. Biol. 1996, 16, 5375–5385. [Google Scholar] [CrossRef]
- Gross, S.D.; Anderson, R.A. Casein kinase i: Spatial organization and positioning of a multifunctional protein kinase family. Cell. Signal. 1998, 10, 699–711. [Google Scholar] [CrossRef]
- Zhai, L.; Graves, P.R.; Robinson, L.C.; Italiano, M.; Culbertson, M.R.; Rowles, J.; Cobb, M.H.; DePaoli-Roach, A.A.; Roach, P.J. Casein kinase i gamma subfamily. Molecular cloning, expression, and characterization of three mammalian isoforms and complementation of defects in the saccharomyces cerevisiae yck genes. J. Biol. Chem. 1271, 270, 12717–12724. [Google Scholar] [CrossRef]
- Green, C.L.; Bennett, G.S. Identification of four alternatively spliced isoforms of chicken casein kinase i alpha that are all expressed in diverse cell types. Gene 1998, 216, 189–195. [Google Scholar] [CrossRef]
- Casas-Mollano, J.A.; Jeong, B.R.; Xu, J.; Moriyama, H.; Cerutti, H. The mut9p kinase phosphorylates histone h3 threonine 3 and is necessary for heritable epigenetic silencing in chlamydomonas. Proc. Natl. Acad. Sci. USA 2008, 105, 6486–6491. [Google Scholar] [CrossRef]
- Ni, W.; Xu, S.L.; Gonzalez-Grandio, E.; Chalkley, R.J.; Huhmer, A.F.R.; Burlingame, A.L.; Wang, Z.Y.; Quail, P.H. Ppks mediate direct signal transfer from phytochrome photoreceptors to transcription factor pif3. Nat. Commun. 2017, 8, 15236. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Q.; Deng, W.; Wang, X.; Piao, M.; Cai, D.; Li, Y.; Barshop, W.D.; Yu, X.; Zhou, T.; et al. Molecular basis for blue light-dependent phosphorylation of arabidopsis cryptochrome 2. Nat. Commun. 2017, 8, 15234. [Google Scholar] [CrossRef]
- Kim, M.J.; Go, Y.S.; Lee, S.B.; Kim, Y.S.; Shin, J.S.; Min, M.K.; Hwang, I.; Suh, M.C. Seed-expressed casein kinase i acts as a positive regulator of the sefad2 promoter via phosphorylation of the sebhlh transcription factor. Plant. Mol. Biol. 2010, 73, 425–437. [Google Scholar] [CrossRef]
- Min, L.; Zhu, L.; Tu, L.; Deng, F.; Yuan, D.; Zhang, X. Cotton ghcki disrupts normal male reproduction by delaying tapetum programmed cell death via inactivating starch synthase. Plant. J. 2013, 75, 823–835. [Google Scholar] [CrossRef]
- Lehti-Shiu, M.D.; Shiu, S.H. Diversity, classification and function of the plant protein kinase superfamily. Philos. Trans. R. Soc. London. Ser. Biol. Sci. 2012, 367, 2619–2639. [Google Scholar] [CrossRef]
- Uehara, T.N.; Mizutani, Y.; Kuwata, K.; Hirota, T.; Sato, A.; Mizoi, J.; Takao, S.; Matsuo, H.; Suzuki, T.; Ito, S.; et al. Casein kinase 1 family regulates prr5 and toc1 in the arabidopsis circadian clock. Proc. Natl. Acad. Sci. USA 2019, 116, 11528–11536. [Google Scholar] [CrossRef]
- Tan, S.T.; Dai, C.; Liu, H.T.; Xue, H.W. Arabidopsis casein kinase1 proteins ck1.3 and ck1.4 phosphorylate cryptochrome2 to regulate blue light signaling. Plant. Cell 2013, 25, 2618–2632. [Google Scholar] [CrossRef]
- Wang, Z.; Casas-Mollano, J.A.; Xu, J.; Riethoven, J.J.; Zhang, C.; Cerutti, H. Osmotic stress induces phosphorylation of histone h3 at threonine 3 in pericentromeric regions of arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2015, 112, 8487–8492. [Google Scholar] [CrossRef]
- Cheong, J.K.; Virshup, D.M. Casein kinase 1: Complexity in the family. Int. J. Biochem. Cell Biol. 2011, 43, 465–469. [Google Scholar] [CrossRef]
- Tuazon, P.T.; Traugh, J.A. Casein kinase i and ii--multipotential serine protein kinases: Structure, function, and regulation. Adv. Second Messenger Phosphoprot. Res. 1991, 23, 123–164. [Google Scholar]
- Cerutti, H.; Casas-Mollano, J.A. Histone h3 phosphorylation: Universal code or lineage specific dialects? Epigenetics 2009, 4, 71–75. [Google Scholar] [CrossRef]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An "electronic fluorescent pictograph" browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef]
- Hanks, S.K.; Hunter, T. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. Faseb J. 1995, 9, 576–596. [Google Scholar] [CrossRef]
- Knippschild, U.; Kruger, M.; Richter, J.; Xu, P.; Garcia-Reyes, B.; Peifer, C.; Halekotte, J.; Bakulev, V.; Bischof, J. The ck1 family: Contribution to cellular stress response and its role in carcinogenesis. Front. Oncol. 2014, 4, 96. [Google Scholar] [CrossRef]
- Chijiwa, T.; Hagiwara, M.; Hidaka, H. A newly synthesized selective casein kinase i inhibitor, n-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase i from bovine testis. J. Biol. Chem. 1989, 264, 4924–4927. [Google Scholar]
- Klimczak, L.J.; Cashmore, A.R. Purification and characterization of casein kinase i from broccoli. Biochem. J. 1993, 293, 283–288. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Z.H.; Luo, D.; Xue, H.W. Roles of oscki1, a rice casein kinase i, in root development and plant hormone sensitivity. Plant. J. 2003, 36, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Badura, L.; Swanson, T.; Adamowicz, W.; Adams, J.; Cianfrogna, J.; Fisher, K.; Holland, J.; Kleiman, R.; Nelson, F.; Reynolds, L.; et al. An inhibitor of casein kinase i epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J. Pharmacol. Exp. Ther. 2007, 322, 730–738. [Google Scholar] [CrossRef]
- Saito, A.N.; Matsuo, H.; Kuwata, K.; Ono, A.; Kinoshita, T.; Yamaguchi, J.; Nakamichi, N. Structure-function study of a novel inhibitor of the casein kinase 1 family in arabidopsis thaliana. Plant. Direct 2019, 3, e00172. [Google Scholar] [CrossRef] [PubMed]
- Graves, P.R.; Roach, P.J. Role of cooh-terminal phosphorylation in the regulation of casein kinase i delta. J. Biol. Chem. 1995, 270, 21689–21694. [Google Scholar] [CrossRef] [PubMed]
- Dahan, J.; Wendehenne, D.; Ranjeva, R.; Pugin, A.; Bourque, S. Nuclear protein kinases: Still enigmatic components in plant cell signalling. New Phytol. 2010, 185, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ding, Y. Mlk1 and mlk2 integrate gibberellins and circadian clock signaling to modulate plant growth. Plant. Signal. Behav. 2018, 13, e1439654. [Google Scholar] [CrossRef]
- Su, Y.; Wang, S.; Zhang, F.; Zheng, H.; Liu, Y.; Huang, T.; Ding, Y. Phosphorylation of histone h2a at serine 95: A plant-specific mark involved in flowering time regulation and h2a.Z deposition. Plant. Cell 2017, 29, 2197–2213. [Google Scholar] [CrossRef]
- Heazlewood, J.L.; Durek, P.; Hummel, J.; Selbig, J.; Weckwerth, W.; Walther, D.; Schulze, W.X. Phosphat: A database of phosphorylation sites in arabidopsis thaliana and a plant-specific phosphorylation site predictor. Nucleic Acids Res. 2008, 36, D1015–D1021. [Google Scholar] [CrossRef]
- Gao, J.; Agrawal, G.K.; Thelen, J.J.; Xu, D. P3db: A plant protein phosphorylation database. Nucleic Acids Res. 2009, 37, D960–D962. [Google Scholar] [CrossRef]
- Arsova, B.; Schulze, W.X. Current status of the plant phosphorylation site database phosphat and its use as a resource for molecular plant physiology. Front. Plant Sci. 2012, 3, 132. [Google Scholar] [CrossRef]
- Gallien, S.; Duriez, E.; Crone, C.; Kellmann, M.; Moehring, T.; Domon, B. Targeted proteomic quantification on quadrupole-orbitrap mass spectrometer. Mol. Cell. Proteom. 2012, 11, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Luger, K. Structure and dynamic behavior of nucleosomes. Curr. Opin. Genet. Dev. 2003, 13, 127–135. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Q.; Su, H.; Birchler, J.A.; Han, F. Histone phosphorylation: Its role during cell cycle and centromere identity in plants. Cytogenet. Genome Res. 2014, 143, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, A.; Seiser, C. Sensing core histone phosphorylation—A matter of perfect timing. Biochim. Et Biophys. Acta 2014, 1839, 711–718. [Google Scholar] [CrossRef]
- Rossetto, D.; Avvakumov, N.; Cote, J. Histone phosphorylation: A chromatin modification involved in diverse nuclear events. Epigenetics 2012, 7, 1098–1108. [Google Scholar] [CrossRef]
- Bigeard, J.; Rayapuram, N.; Pflieger, D.; Hirt, H. Phosphorylation-dependent regulation of plant chromatin and chromatin-associated proteins. Proteomics 2014, 14, 2127–2140. [Google Scholar] [CrossRef]
- Lopez, R.; Sarg, B.; Lindner, H.; Bartolome, S.; Ponte, I.; Suau, P.; Roque, A. Linker histone partial phosphorylation: Effects on secondary structure and chromatin condensation. Nucleic Acids Res. 2015, 43, 4463–4476. [Google Scholar] [CrossRef]
- Perez-Cadahia, B.; Drobic, B.; Khan, P.; Shivashankar, C.C.; Davie, J.R. Current understanding and importance of histone phosphorylation in regulating chromatin biology. Curr. Opin. Drug Discov. Dev. 2010, 13, 613–622. [Google Scholar]
- Moraes, I.; Casas-Mollano, J.A. Histone h3 phosphorylation in plants and other organisms. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications; Alvarez-Venegas, R.l., Penã, C.D.l., Casas-Mollano, J.A., Eds.; Springer: Cham/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2014; pp. 47–70. [Google Scholar]
- Mizoi, J.; Kanazawa, N.; Kidokoro, S.; Takahashi, F.; Qin, F.; Morimoto, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Heat-induced inhibition of phosphorylation of the stress-protective transcription factor dreb2a promotes thermotolerance of arabidopsis thaliana. J. Biol. Chem. 2019, 294, 902–917. [Google Scholar] [CrossRef]
- Park, Y.I.; Do, K.H.; Kim, I.S.; Park, H.H. Structural and functional studies of casein kinase i-like protein from rice. Plant. Cell Physiol. 2012, 53, 304–311. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, Y.; Zhao, Y.; Huang, S.; Yuan, M.; Zhao, Y.; Guo, Y. Casein kinase1-like protein2 regulates actin filament stability and stomatal closure via phosphorylation of actin depolymerizing factor. Plant. Cell 2016, 28, 1422–1439. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Hu, Q.; Li, Y.; Xu, J.; Ma, Y.; Zhu, L.; Yang, X.; Zhang, X. Leafy cotyledon1-casein kinase i-tcp15-phytochrome interacting factor4 network regulates somatic embryogenesis by regulating auxin homeostasis. Plant. Physiol. 2015, 169, 2805–2821. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Xu, S.L.; Chalkley, R.J.; Pham, T.N.; Guan, S.; Maltby, D.A.; Burlingame, A.L.; Wang, Z.Y.; Quail, P.H. Multisite light-induced phosphorylation of the transcription factor pif3 is necessary for both its rapid degradation and concomitant negative feedback modulation of photoreceptor phyb levels in arabidopsis. Plant. Cell 2013, 25, 2679–2698. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.T.; Koo, B.H.; Kim, D.; Yoo, S.C.; Paek, N.C. Casein kinases i and 2alpha phosphorylate oryza sativa pseudo-response regulator 37 (osprr37) in photoperiodic flowering in rice. Mol. Cells 2015, 38, 81–88. [Google Scholar] [PubMed]
- Park, H.H. Casein kinase i-like protein linked to lipase in plant. Plant. Signal. Behav. 2012, 7, 719–721. [Google Scholar] [CrossRef]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and phytochrome interacting factors. Plant. Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Wang, X.; Zuo, Z.; Oka, Y.; Lin, C. New insights into the mechanisms of phytochrome-cryptochrome coaction. New Phytol. 2018, 217, 547–551. [Google Scholar] [CrossRef]
- Salome, P.A. Know your histone (zip) code: Flowering time and phosphorylation of histone h2a on serine 95. Plant. Cell 2017, 29, 2084–2085. [Google Scholar] [CrossRef]
- Huang, H.; Alvarez, S.; Bindbeutel, R.; Shen, Z.; Naldrett, M.J.; Evans, B.S.; Briggs, S.P.; Hicks, L.M.; Kay, S.A.; Nusinow, D.A. Identification of evening complex associated proteins in arabidopsis by affinity purification and mass spectrometry. Mol. Cell. Proteom. 2016, 15, 201–217. [Google Scholar] [CrossRef]
- Kim, J.I.; Park, J.E.; Zarate, X.; Song, P.S. Phytochrome phosphorylation in plant light signaling. Photochem. Photobiol. Sci. 2005, 4, 681–687. [Google Scholar] [CrossRef]
- Reischl, S.; Kramer, A. Kinases and phosphatases in the mammalian circadian clock. FEBS Lett. 2011, 585, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, F.; Wang, S.; Su, Y.; Jiang, P.; Cheng, R.; Ji, X.; Hou, S.; Ding, Y. Mlk1 and mlk2 coordinate rga and cca1 activity to regulate hypocotyl elongation in arabidopsis thaliana. Plant. Cell 2017, 30, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Sato, A.; Fujimoto, K.J.; Matsuo, H.; Yanai, T.; Kinoshita, T.; Nakamichi, N. 3, 4-dibromo-7-azaindole modulates arabidopsis circadian clock by inhibiting casein kinase 1 activity. Plant. Cell Physiol. 2019, 60, 2360–2368. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wu, F.Q.; Wu, W.; Wang, H.J.; Zheng, X.M.; Zhang, Y.; Chen, X.; Zhou, K.; Jin, M.; Cheng, Z.; et al. Rice ltg1 is involved in adaptive growth and fitness under low ambient temperature. Plant. J. 2014, 78, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Hsu, C.L.; Hu, C.W.; Ko, S.Y.; Hsieh, H.L.; Huang, H.C.; Juan, H.F. Integrating phosphoproteomics and bioinformatics to study brassinosteroid-regulated phosphorylation dynamics in arabidopsis. BMC Genom. 2015, 16, 533. [Google Scholar] [CrossRef]
- Probst, A.V.; Mittelsten Scheid, O. Stress-induced structural changes in plant chromatin. Curr. Opin. Plant. Biol. 2015, 27, 8–16. [Google Scholar] [CrossRef]
- Kim, J.M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant. Sci. 2015, 6, 114. [Google Scholar] [CrossRef]
- Sokol, A.; Kwiatkowska, A.; Jerzmanowski, A.; Prymakowska-Bosak, M. Up-regulation of stress-inducible genes in tobacco and arabidopsis cells in response to abiotic stresses and aba treatment correlates with dynamic changes in histone h3 and h4 modifications. Planta 2007, 227, 245–254. [Google Scholar] [CrossRef]
- Izabel, M.; Casas-Mollano, J.A. Histone h3 phosphorylation in plants and other organisms. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications. Transcriptional Regulation and Chromatin Remodelling in Plants; Alvarez-Venegas, R.l., Penã, C.D.l., Casas-Mollano, J.A., Eds.; Springer: London, UK, 2014; pp. 47–65. [Google Scholar]
- Houben, A.; Demidov, D.; Caperta, A.D.; Karimi, R.; Agueci, F.; Vlasenko, L. Phosphorylation of histone h3 in plants—A dynamic affair. Biochim. Biophys. Acta 2007, 1769, 308–315. [Google Scholar] [CrossRef]
- Wirthmueller, L.; Asai, S.; Rallapalli, G.; Sklenar, J.; Fabro, G.; Kim, D.S.; Lintermann, R.; Jaspers, P.; Wrzaczek, M.; Kangasjarvi, J.; et al. Arabidopsis downy mildew effector harxl106 suppresses plant immunity by binding to radical-induced cell death1. New Phytol. 2018, 220, 232–248. [Google Scholar] [CrossRef]
- Li, Y.; Min, L.; Zhang, L.; Hu, Q.; Wu, Y.; Li, J.; Xie, S.; Ma, Y.; Zhang, X.; Zhu, L. Promoters of arabidopsis casein kinase i-like 2 and 7 confer specific high-temperature response in anther. Plant. Mol. Biol. 2018, 98, 33–49. [Google Scholar] [CrossRef] [PubMed]
| Species | Genome Size | No. of Ser/Thr Kinase | No. of MLK Homolog | |
| Eudicotylendons | ||||
| Glycine max | 1.1 Gb | 29 | 10 | |
| Nicotiaa attenuata | 2.5 Gb | 16 | 7 | |
| Medicago truncatula | 465 Mb | 20 | 6 | |
| Populus trichocarpa | 500 Mb | 17 | 7 | |
| Arabidopsis thaliana | 135 Mb | 17 | 4 | |
| Monocotylendons | ||||
| Triticum aestivum | ~17 Gb | 21 | 15 | |
| Hordeum vulgare | 5.3 Gb | 8 | 5 | |
| Oryza sativa | 500 Mb | 15 | 6 | |
| Sorghum bicolor | 700 Mb | 7 | 7 | |
| Zea mays | 2.4 Gb | 8 | 5 | |
| Lycopodiophyta | ||||
| Selaginella moellendorffii | 110 Mb | 5 | 4 | |
| Embryophyta | ||||
| Marchantia polymorpha | 280 Mb | 2 | 1 | |
| Physcomitrella patens | 511 Mb | 7 | 2 | |
| Chlorophyta | ||||
| Chlamydomonas reinhardtii | 120 Mb | 2 | 2 | |
| Ostreococcus lucimarinus | 13.2 Mb | 1 | 1 | |
| Amborellales | ||||
| Amborella trichopoda | 870 Mb | 3 | 3 | |
| CKIs | Substrates | Phosphorylation Sites | Biological Role | Species | References |
|---|---|---|---|---|---|
| Mut9p | H3 | T3 | Repress transcription of euchromatic loci | Chlamydomonus | [14] |
| MLK1/2 | H3 | T3 | Probably for heterochromatic organization maintenance | Arabidopsis | [22] |
| MLK4 | H2A | S95 | Promote flowering by interacting with CCA1 | Arabidopsis | [37] |
| PPK1 | CRY2 | S506, S523, S525, S526 S598, S599, S605 | Destabilize or activate blue-light dependent photoreceptor CRY2 | Arabidopsis | [16] |
| PPK1 | PIF3 | S58, S102, S151-3, S250 S253 S266, S269 | Facilitate red light-dependent degradation of photoreceptor PIF3 | Arabidopsis | [15,54] |
| PPK1 | PIF3 | S323, S40/43/45/46, S162, S283-290, S482/T483, T500/T501 | Facilitate light-independent degradation of photoreceptor PIF3 | Arabidopsis | [15,54] |
| AEL1-4 | PYL1 | S59, T71, S91, S109, S112, T133S136, T138, S182, S203 | Promote ubiquitination and degradation of ABA receptors PYR/PYLs | Arabidopsis | [2] |
| EL1 | SLR1 | S196, S510 | Destabilize SLR1 protein in GA signaling | Rice | [3] |
| Hd16 | Ghd7; PRR37 | ? | Inhibit photoperiodic flowering | Rice | [4,55] |
| CK1.3/1.4 | CRY2 | S587, T603 | Promote blue light-induced degradation of CRY2 | Arabidopsis | [21] |
| CKL4 | PPR5, TOC1 | ? | Inhibit the expression of PRR5 and TOC1 | Arabidopsis | [20] |
| CKL2 | ADF4 | ? | Inhibit actin filament disassembly | Arabidopsis | [52] |
| OsCKL | lipase | ? | Regulate lipase activity | Rice | [51,56] |
| GhCKL | TCP15 | ? | Regulate GhPIF4 and disrupts auxin homeostasis | Cotton | [53] |
| CKL | SebHLH | ? | Enhance SebHLH-mediated transactivation of SeFAD2 gene | Sesame | [17] |
| CKL | ? | ? | Function in time keeping | Ostreococcus | [7] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Wang, Z. Mut9p-LIKE KINASE Family Members: New Roles of the Plant-Specific Casein Kinase I in Plant Growth and Development. Int. J. Mol. Sci. 2020, 21, 1562. https://doi.org/10.3390/ijms21051562
Kang J, Wang Z. Mut9p-LIKE KINASE Family Members: New Roles of the Plant-Specific Casein Kinase I in Plant Growth and Development. International Journal of Molecular Sciences. 2020; 21(5):1562. https://doi.org/10.3390/ijms21051562
Chicago/Turabian StyleKang, Junmei, and Zhen Wang. 2020. "Mut9p-LIKE KINASE Family Members: New Roles of the Plant-Specific Casein Kinase I in Plant Growth and Development" International Journal of Molecular Sciences 21, no. 5: 1562. https://doi.org/10.3390/ijms21051562
APA StyleKang, J., & Wang, Z. (2020). Mut9p-LIKE KINASE Family Members: New Roles of the Plant-Specific Casein Kinase I in Plant Growth and Development. International Journal of Molecular Sciences, 21(5), 1562. https://doi.org/10.3390/ijms21051562





