Sewage Sludge ZnCl2-Activated Carbon Intercalated MgFe–LDH Nanocomposites: Insight of the Sorption Mechanism of Improved Removal of Phenol from Water
Abstract
1. Introduction
2. Results
2.1. Characterization of AC–MgFe Composites
2.2. Phenol adsorption Performance (qe) of AC–MgFe Composites at Varied Initial pH
3. Discussion
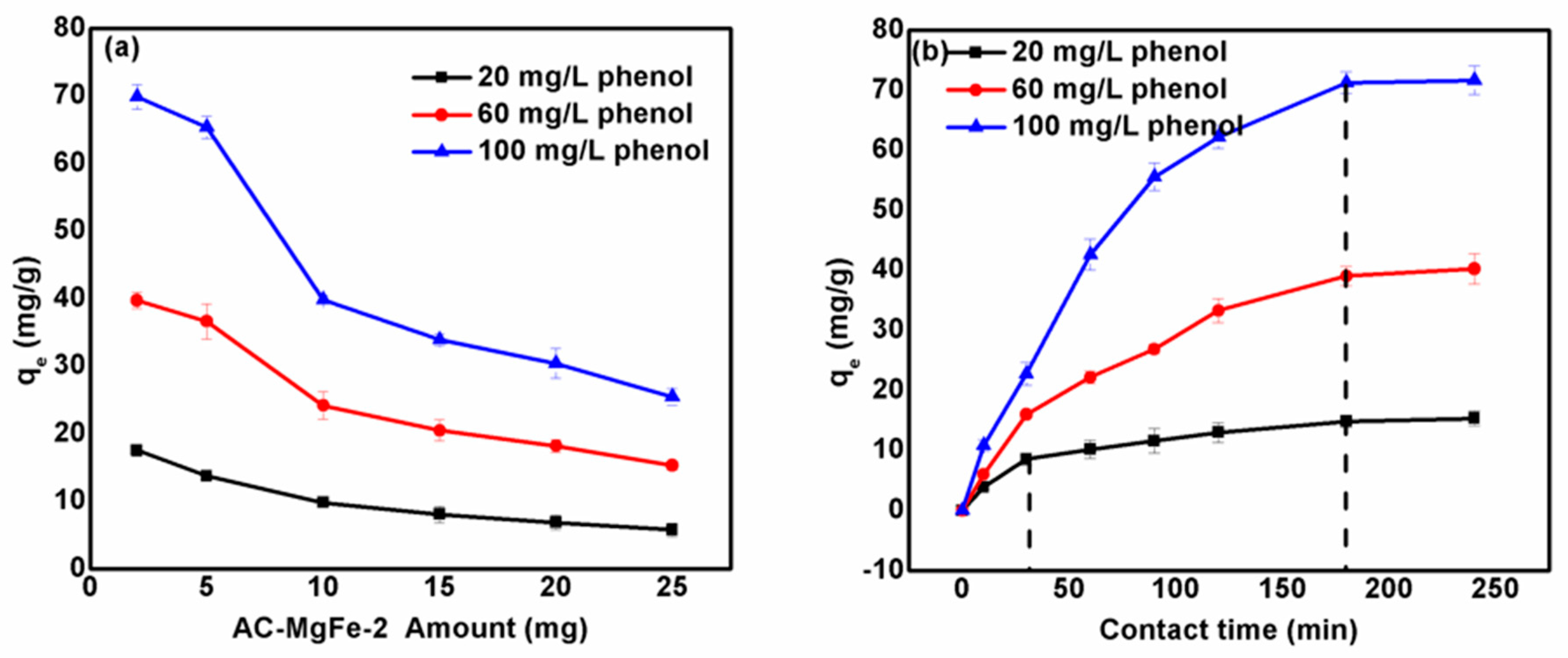
3.1. Influence of AC–MgFe-2 Dosage and Adsorption Contact Time on Phenol Uptake, qe
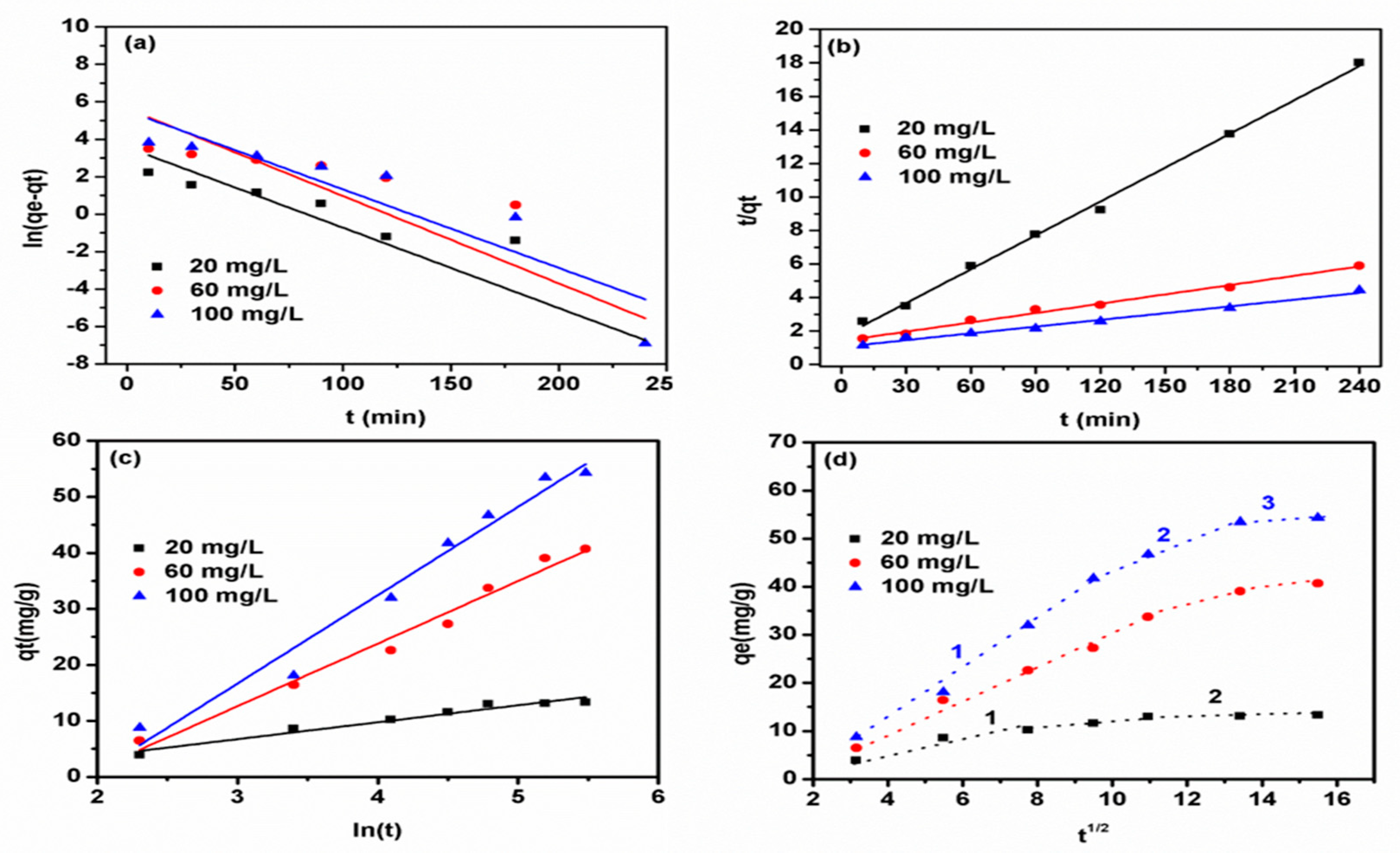
3.2. Phenol Adsorption Kinetics
3.3. Phenol Adsorption Isotherms
3.4. Phenol Adsorption Thermodynamic
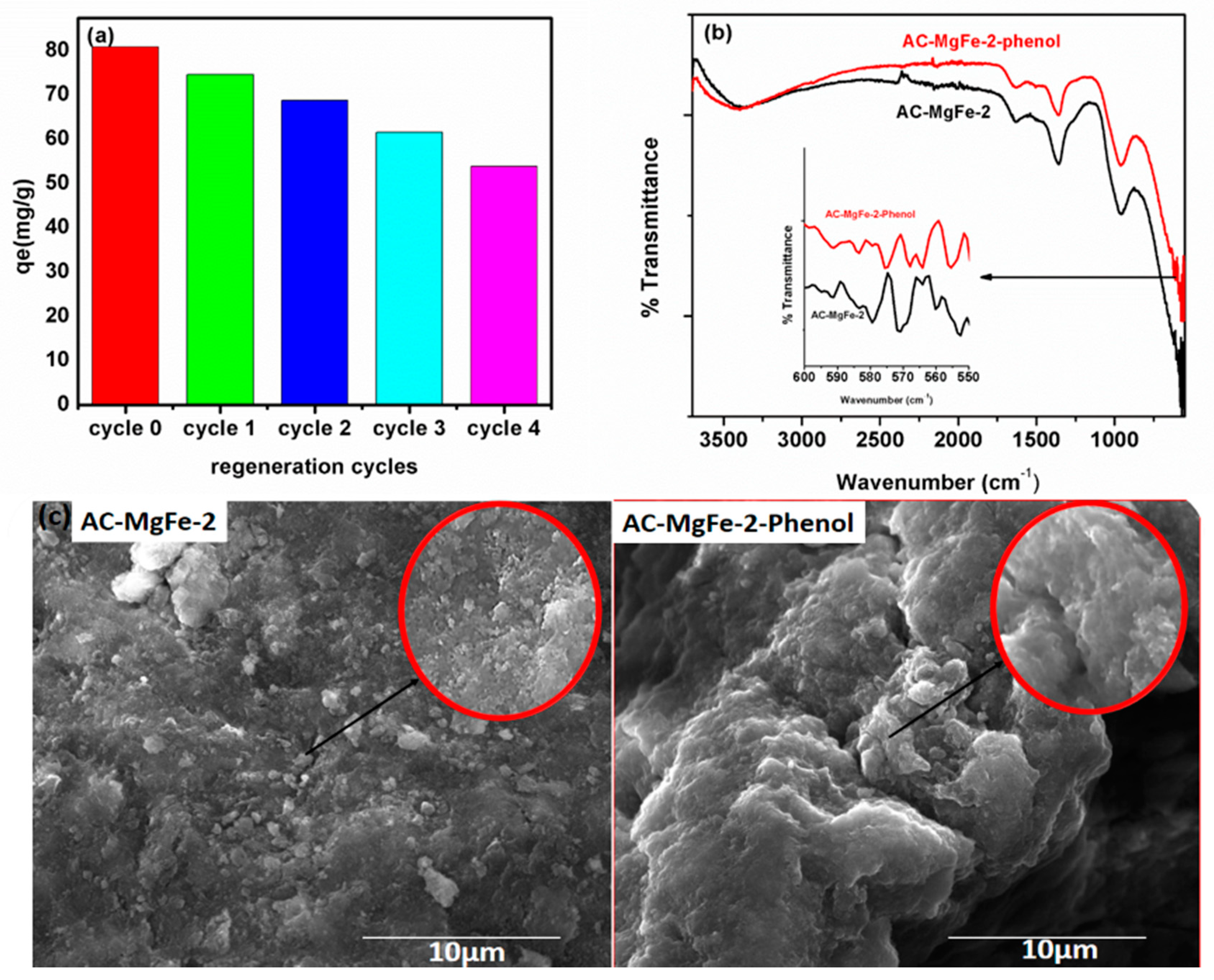
3.5. Reusability of AC–MgFe Composite
3.6. Adsorption Mechanism of Phenol Onto AC–MgFe Composite
3.7. Comparison with Other Adsorbents
4. Materials and Methods
4.1. Materials
4.2. Syntheses of ZnCl2-Activated Carbon Decorated MgFe LDH (AC–MgFe) Composites
4.3. AC–MgFe Composites Characterization
4.4. Phenol Uptake from Water Experiment
4.5. Evaluation of Phenol Sorption Mechanism onto Activated Carbon–MgFe Composite
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schweitzer, L.; Noblet, J. Water contamination and pollution. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–290. [Google Scholar]
- Mu’azu, N.D.; Jarrah, N.; Essa, M.H. Binary adsorption of phenol and O-cresol from aqueous solution on date palm pits based activated carbon: A fixed-bed column study. Desalin. Water Treat. 2017, 58, 192–201. [Google Scholar] [CrossRef]
- Jarraha, N.; Mu’azua, N.D.; Essac, M.H.; Zubaira, M. Response surface modeling and optimization of sludge activated carbon production conditions for phenolic compounds removal from water. Desalin. Water Treat. 2017, 100, 320–332. [Google Scholar] [CrossRef]
- Michałowicz, J.; Duda, W. Phenols-Sources and Toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Lyu, J.; Park, J.; Pandey, L.K.; Choi, S.; Lee, H.; De Saeger, J.; Depuydt, S.; Han, T. Testing the toxicity of metals, phenol, effluents, and receiving waters by root elongation in Lactuca sativa L. Ecotoxicol. Environ. Saf. 2018, 149, 225–232. [Google Scholar] [CrossRef]
- Mu’azu, N.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of Phenolic Compounds from Water Using Sewage Sludge-Based Activated Carbon Adsorption: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1094. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Kazeem, T.S.; Zubair, M.; Al-Harthi, M. Bentonite-layered double hydroxide composite for enhanced aqueous adsorption of Eriochrome Black T. Appl. Clay Sci. 2018, 161, 23–34. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Jiao, Y.; Xia, Y.; Xia, L.; Wang, Z.; Zhang, W.; Wang, K. Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto graphene. Mater. Res. Bull. 2012, 47, 1898–1904. [Google Scholar] [CrossRef]
- Yu, J.-G.; Zhao, X.-H.; Yang, H.; Chen, X.-H.; Yang, Q.; Yu, L.-Y.; Jiang, J.-H.; Chen, X.-Q. Aqueous adsorption and removal of organic contaminants by carbon nanotubes. Sci. Total Environ. 2014, 482, 241–251. [Google Scholar] [CrossRef]
- Sarkar, B.; Rusmin, R.; Ugochukwu, U.C.; Mukhopadhyay, R.; Manjaiah, K.M. Modified clay minerals for environmental applications. In Modified Clay and Zeolite Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–127. [Google Scholar]
- Nitzsche, R.; Groengroeft, A.; Kraume, M. Separation of lignin from beech wood hydrolysate using polymeric resins and zeolites–Determination and application of adsorption isotherms. Sep. Purif. Technol. 2019, 209, 491–502. [Google Scholar] [CrossRef]
- Yang, X.; Tang, W.; Liu, X.; Du, H.; Wu, Y.; Zhang, J. Synthesis of mesoporous silica from coal slag and CO2 for phenol removal. J. Clean. Prod. 2019, 208, 1255–1264. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon-a critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; Rahman, A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard. Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, A.; Duran, C.; Senturk, H.B.; Soylak, M.; Ozdes, D.; Serencam, H.; Imamoglu, M. Adsorption of Phenol from Aqueous Solution on a Low-Cost Activated Carbon Produced from Tea Industry Waste: Equilibrium, Kinetic, and Thermodynamic Study. J. Chem. Eng. Data 2012, 57, 2733–2743. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Zhang, F.; Zhou, J.; Wu, J.; Alsaedi, A.; Hayat, T.; Li, J. Insight into the mechanism of adsorption of phenol and resorcinol on activated carbons with different oxidation degrees. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 22–30. [Google Scholar] [CrossRef]
- Liadi, M.A.; Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O.; Al-Harthi, M.A.; Essa, M.H. Comparative performance study of ZnCl2 and NaOH sludge based activated carbon for simultaneous aqueous uptake of phenolic compounds. Int. J. Environ. Anal. Chem. 2020, 1–25. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, X.; Chen, Q.; Ruan, C.; Leng, Y. Enhanced removal performance by the core-shell zeolites/MgFe-layered double hydroxides (LDHs) for municipal wastewater treatment. Environ. Sci. Pollut. Res. 2016, 23, 6749–6757. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Cai, W.; Zhao, R.; Yuan, J. Hierarchically porous NiAl-LDH nanoparticles as highly efficient adsorbent for p-nitrophenol from water. Appl. Surf. Sci. 2015, 349, 897–903. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Mantilla, A.; Bañuelos, F.; Fernández, J.L.; Gómez, R. Improved Photocatalytic Degradation of Phenolic Compounds With ZnAl Mixed Oxides Obtained from LDH Materials. Top. Catal. 2011, 54, 257–263. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J.; Ntho, T. Synthesis and characterization of an efficient and stable Al/Fe pillared clay catalyst for the catalytic wet air oxidation of phenol. RSC Adv. 2018, 8, 30115–30124. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Li, G.; Zhang, B.; Zhang, B.; Qiu, S.; Li, Y.; Wu, T. Calcined products of Mg-Al layered double hydroxides/single-walled carbon nanotubes nanocomposites for expeditious removal of phenol and 4-chlorophenol from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 143–153. [Google Scholar] [CrossRef]
- Kazeem, T.S.; Zubair, M.; Daud, M.; Mu’azu, N.D.; Al-Harthi, M.A. Graphene/ternary layered double hydroxide composites: Efficient removal of anionic dye from aqueous phase. Korean J. Chem. Eng. 2019, 36, 1057–1068. [Google Scholar] [CrossRef]
- Jarrah, N.; Mu’azu, N.D.; Zubair, M.; Al-Harthi, M. Enhanced adsorptive performance of Cr (VI) onto layered double hydroxide-bentonite composite: Isotherm, kinetic and thermodynamic studies. Sep. Sci. Technol. 2019, 1–13. [Google Scholar] [CrossRef]
- Durrani, S.K.; Naz, S.; Mehmood, M.; Nadeem, M.; Siddique, M. Structural, impedance and Mössbauer studies of magnesium ferrite synthesized via sol-gel auto-combustion process. J. Saudi Chem. Soc. 2017, 21, 899–910. [Google Scholar] [CrossRef]
- Zubair, M.; Shehzad, F.; Al-Harthi, M.A. Impact of modified graphene and microwave irradiation on thermal stability and degradation mechanism of poly (styrene-co-methyl meth acrylate). Thermochim. Acta 2016, 633, 48–55. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the size and internal structure of colloidal particles by means of röntgen. Nachr. Ges. Wiss. Göttingen. 1918, 2, 96–100. [Google Scholar]
- Kang, D.; Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. Performance and mechanism of Mg/Fe layered double hydroxides for fluoride and arsenate removal from aqueous solution. Chem. Eng. J. 2013, 228, 731–740. [Google Scholar] [CrossRef]
- Morrell, D.G. Catalysis of Organic Reactions; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Mehta, D.; Mazumdar, S.; Singh, S.K. Magnetic adsorbents for the treatment of water/wastewater—A review. J. Water Process Eng. 2015, 7, 244–265. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E. Effect of micropore size distribution on phenol adsorption on steam activated carbons. Adsorption 2016, 22, 599–607. [Google Scholar] [CrossRef]
- Nasiruddin Khan, M.; Sarwar, A. Determination of points of zero charge of natural and treated adsorbents. Surf. Rev. Lett. 2007, 14, 461–469. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Lee, Y.J. Hydrothermal synthesis of hierarchically structured birnessite-type MnO2/biochar composites for the adsorptive removal of Cu (II) from aqueous media. Bioresour. Technol. 2018, 260, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Si, W.; Yao, Z.; Feng, R.; Du, B.; Yan, L.; Wei, Q. Adsorption of benzoic acid from aqueous solution by three kinds of modified bentonites. J. Colloid Interface Sci. 2011, 359, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Jarrah, N.; Khalid, A.; Manzar, M.S.; Kazeem, T.S.; Al-Harthi, M.A. Starch-NiFe-layered double hydroxide composites: Efficient removal of methyl orange from aqueous phase. J. Mol. Liq. 2018, 249, 254–264. [Google Scholar] [CrossRef]
- Channa, A.M.; Baytak, S.; Memon, S.Q.; Talpur, M.Y. Equilibrium, kinetic and thermodynamic studies of removal of phenol from aqueous solution using surface engineered chemistry. Heliyon 2019, 5, e01852. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Adio, S.O.; Asif, M.; Dafalla, H. Statistical analysis of phenols adsorption on diethylenetriamine-modified activated carbon. J. Clean. Prod. 2018, 182, 960–968. [Google Scholar] [CrossRef]
- He, X.; Wang, B.; Zhang, Q. Phenols removal from water by precursor preparation for MgAl layered double hydroxide: Isotherm, kinetic and mechanism. Mater. Chem. Phys. 2019, 221, 108–117. [Google Scholar] [CrossRef]
- Lupa, L.; Cocheci, L.; Pode, R.; Hulka, I. Phenol adsorption using Aliquat 336 functionalized Zn-Al layered double hydroxide. Sep. Purif. Technol. 2018, 196, 82–95. [Google Scholar] [CrossRef]
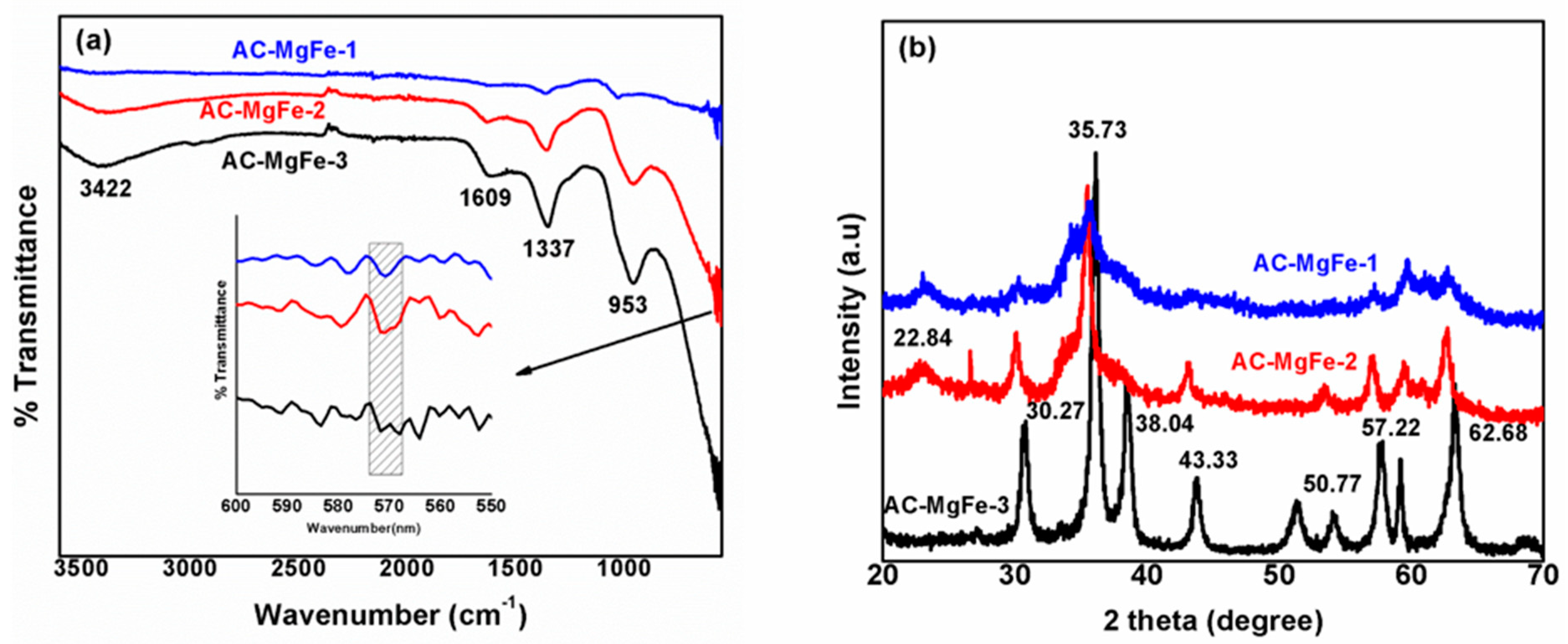
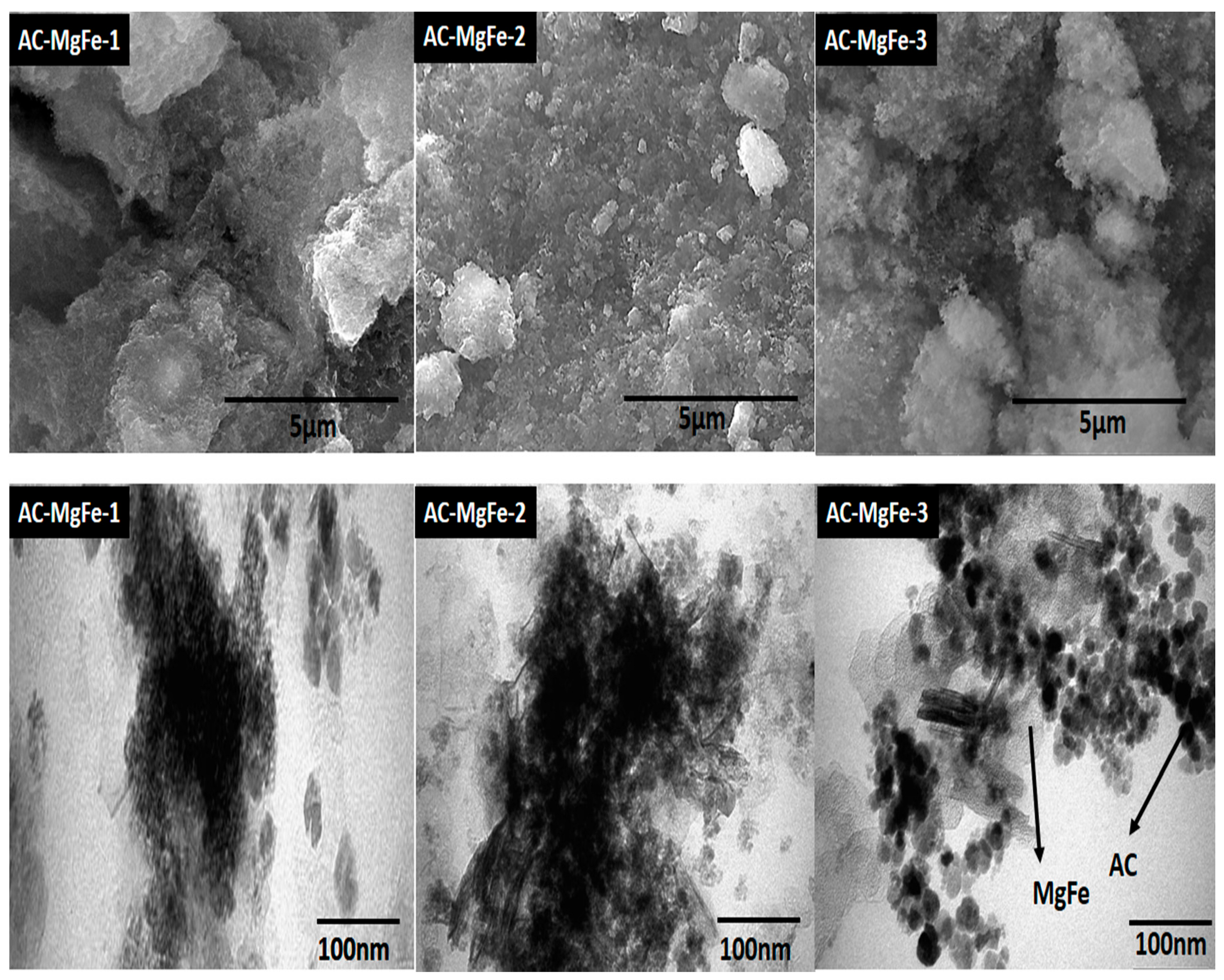



| AC–MgFe-1 | AC–MgFe-2 | AC–MgFe-3 | |
|---|---|---|---|
| BET surface area (m2/g) | 257.22 | 233.76 | 168.92 |
| Pore volume (cm3/g) | 0.23 | 0.27 | 0.21 |
| Micropore volume (DR method) (cm3/g) | 0.18 | 0.18 | 0.17 |
| Pore diameter (based on BJH) (nm) | 3.36 | 3.36 | 3.42 |
| Adsorbent | Co | Pseudo First Order | Pseudo Second Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (exp) | qe | k1 | R2 | qe | k2 × 10−2 | h | R2 | ||
| AC-MgFe-2 | 20 | 14.85 | 35.46 | 0.09 | 0.826 | 19.44 | 0.27 | 0.60 | 0.997 |
| 60 | 40.68 | 280.81 | 0.10 | 0.731 | 54.05 | 0.24 | 0.71 | 0.992 | |
| 100 | 54.3 | 247.2 | 0.09 | 0.836 | 74.62 | 0.17 | 0.94 | 0.987 | |
| Elovich | Intra particle diffusion qt = kd t1/2 + C | ||||||||
| qe (exp) | α | β | R2 | Kp | C | R2 | |||
| AC-MgFe-2 | 20 | 14.85 | 2.25 | 0.06 | 0.967 | 0.71 | 3.79 | 0.799 | |
| 60 | 40.68 | 1.71 | 0.08 | 0.977 | 2.82 | 0.04 | 0.966 | ||
| 100 | 54.3 | 1.39 | 0.33 | 0.952 | 3.95 | 0.71 | 0.933 | ||
| Adsorbent | T (K) | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL | R2 | RMSE | KF | 1/n | R2 | RMSE | ||
| AC-MgFe-2 | 298 | 138.69 | 0.003 | 0.997 | 0.001 | 4.01 | 1.26 | 0.996 | 0.046 |
| 308 | 139.77 | 0.004 | 0.994 | 0.001 | 3.70 | 1.25 | 0.996 | 0.047 | |
| 318 | 141.64 | 0.004 | 0.990 | 0.002 | 3.48 | 1.25 | 0.996 | 0.049 | |
| Adsorbent | pH | Time (Minutes) | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|
| Iron impregnated activated carbon | 7 | 90 | 20 | [35] |
| Diethylenetriamine-modified activated carbon | 3 | 120 | 18.12 | [36] |
| milled MgAl | - | 180 | 82.6 | [37] |
| Aliquat 336 functionalized Zn-Al | 6.5 | 60 | 64.7 | [38] |
| Calcined MgAl/SWCNT | 6 | 3600 | 219.0 | [23] |
| AC-MgFe composite | 6 | 180 | 138.69 | This work |
| T (K) | ∆G (kJ/mol) | ∆H (kJ/mol) | ∆S (kJ/mol K) | |
|---|---|---|---|---|
| AC–MgFe-2 | 298 | −0.39 | ||
| 308 | −0.32 | 3.85 | 0.011 | |
| 318 | −0.16 |
| Sample | AC (g) | Mg:Fe Salts (g) (0.1:0.1)M |
|---|---|---|
| AC–MgFe-1 | 0.5 | 2.54:4.04 |
| AC–MgFe-2 | 0.25 | 2.54:4.04 |
| AC–MgFe-3 | 0.1 | 2.54:4.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu’azu, N.D.; Zubair, M.; Jarrah, N.; Alagha, O.; Al-Harthi, M.A.; Essa, M.H. Sewage Sludge ZnCl2-Activated Carbon Intercalated MgFe–LDH Nanocomposites: Insight of the Sorption Mechanism of Improved Removal of Phenol from Water. Int. J. Mol. Sci. 2020, 21, 1563. https://doi.org/10.3390/ijms21051563
Mu’azu ND, Zubair M, Jarrah N, Alagha O, Al-Harthi MA, Essa MH. Sewage Sludge ZnCl2-Activated Carbon Intercalated MgFe–LDH Nanocomposites: Insight of the Sorption Mechanism of Improved Removal of Phenol from Water. International Journal of Molecular Sciences. 2020; 21(5):1563. https://doi.org/10.3390/ijms21051563
Chicago/Turabian StyleMu’azu, Nuhu Dalhat, Mukarram Zubair, Nabeel Jarrah, Omar Alagha, Mamdouh A. Al-Harthi, and Mohammed H. Essa. 2020. "Sewage Sludge ZnCl2-Activated Carbon Intercalated MgFe–LDH Nanocomposites: Insight of the Sorption Mechanism of Improved Removal of Phenol from Water" International Journal of Molecular Sciences 21, no. 5: 1563. https://doi.org/10.3390/ijms21051563
APA StyleMu’azu, N. D., Zubair, M., Jarrah, N., Alagha, O., Al-Harthi, M. A., & Essa, M. H. (2020). Sewage Sludge ZnCl2-Activated Carbon Intercalated MgFe–LDH Nanocomposites: Insight of the Sorption Mechanism of Improved Removal of Phenol from Water. International Journal of Molecular Sciences, 21(5), 1563. https://doi.org/10.3390/ijms21051563






