Deconvolution of RNA-Seq Analysis of Hyperbaric Oxygen-Treated Mice Lungs Reveals Mesenchymal Cell Subtype Changes
Abstract
1. Introduction
2. Results
2.1. Repetitive HBO Exposure Has Little Impact on Mice Lung Morphology and Body Weight
2.2. Repetitive HBO Exposure Shows Mild Effects on Gene Expression in Mice Lungs.
2.3. Repetitive HBO Exposure Has Little Effect on the Relative Composition of Different Cell Types of Mice Lungs
2.4. Deconvolution Based Upon Fibrotic Lung Cell Profiles Reveals Mesenchymal Cell Subtype Changes
2.5. GO Enrichment Analysis Identifies Different Roles of the Eight Mesenchymal Cell Types
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. HBO Exposure
4.3. Morphological Examination
4.4. RNA Isolation, Library Construction and Sequencing
4.5. RNA-Seq Data Analysis
4.6. Real-Time qPCR Analysis
4.7. Single-Cell Data Analysis
4.8. CIBERSORTx Analysis
4.9. Pathway Enrichment Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HBO | Hyperbaric oxygen |
| DEG | Differentially expressed gene |
| PCA | Principal components analysis |
| GSEA | gene set enrichment analysis |
| FDR | false discovery rate |
| t-SNE | t-distributed stochastic neighborhood embedding |
| RNA-seq | RNA-sequencing |
| scRNA-seq | Single cell RNA-sequencing |
| padj | adjusted p-value |
| MCA | Mouse Cell Atlas |
| AECII | alveolar epithelial cell II |
| ATA | Atmosphere Absolute |
| atm | atmosphere |
| H/E | hematoxylin-eosin |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Poff, A.M.; Kernagis, D.; D’Agostino, D.P. Hyperbaric environment: Oxygen and cellular damage versus protection. Compr. Physiol. 2016, 7, 213–234. [Google Scholar] [CrossRef]
- Wedgwood, S.; Warford, C.; Agvateesiri, S.C.; Thai, P.; Berkelhamer, S.K.; Perez, M.; Underwood, M.A.; Steinhorn, R.H. Postnatal growth restriction augments oxygen-induced pulmonary hypertension in a neonatal rat model of bronchopulmonary dysplasia. Pediatr. Res. 2016, 80, 894–902. [Google Scholar] [CrossRef]
- Hummler, J.K.; Dapaah-Siakwan, F.; Vaidya, R.; Zambrano, R.; Luo, S.; Chen, S.; Kerr, N.; de Rivero Vaccari, J.P.; Keane, R.W.; Dietrich, W.D.; et al. Inhibition of Rac1 signaling downregulates inflammasome activation and attenuates lung injury in neonatal rats exposed to hyperoxia. Neonatology 2017, 111, 280–288. [Google Scholar] [CrossRef]
- Chen, X.; Orriols, M.; Walther, F.J.; Laghmani, E.H.; Hoogeboom, A.M.; Hogen-Esch, A.C.B.; Hiemstra, P.S.; Folkerts, G.; Goumans, M.-J.T.H.; Ten Dijke, P.; et al. Bone Morphogenetic Protein 9 protects against neonatal hyperoxia-induced impairment of alveolarization and pulmonary inflammation. Front. Physiol. 2017, 8, 486. [Google Scholar] [CrossRef]
- Perez, M.; Wisniewska, K.; Lee, K.J.; Cardona, H.J.; Taylor, J.M.; Farrow, K.N. Dose-dependent effects of glucocorticoids on pulmonary vascular development in a murine model of hyperoxic lung injury. Pediatr. Res. 2016, 79, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Morikawa, S.; Kitahara, S.; Yoshii, A.; Uchiyama, A.; Kusuda, S.; Ezaki, T. Morphological characterization of pulmonary microvascular disease in bronchopulmonary dysplasia caused by hyperoxia in newborn mice. Med. Mol. Morphol. 2018, 51, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Plafki, C.; Peters, P.; Almeling, M.; Welslau, W.; Busch, R. Complications and side effects of hyperbaric oxygen therapy. Aviat. Space Environ. Med. 2000, 71, 119–124. [Google Scholar] [PubMed]
- Dejmek, J.; Kohoutova, M.; Kripnerova, M.; Cedikova, M.; Tuma, Z.; Babuska, V.; Bolek, L.; Kuncova, J. Repeated exposure to hyperbaric hyperoxia affects mitochondrial functions of the lung fibroblasts. Physiol. Res. 2018, 67, S633–S643. [Google Scholar] [CrossRef]
- Ay, H.; Topal, T.; Uysal, B.; Ozler, M.; Oter, S.; Korkmaz, A.; Dündar, K. Time-dependent course of hyperbaric oxygen-induced oxidative effects in rat lung and erythrocytes. Clin. Exp. Pharmacol. Physiol. 2007, 34, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Oter, S.; Topal, T.; Sadir, S.; Ozler, M.; Uysal, B.; Ay, H.; Yaren, H.; Korkmaz, A.; Akin, A. Oxidative stress levels in rats following exposure to oxygen at 3 atm for 0-120 min. Aviat. Space Environ. Med. 2007, 78, 1108–1113. [Google Scholar] [CrossRef]
- Simsek, K.; Ay, H.; Topal, T.; Ozler, M.; Uysal, B.; Ucar, E.; Acikel, C.H.; Yesilyurt, O.; Korkmaz, A.; Oter, S.; et al. Long-term exposure to repetitive hyperbaric oxygen results in cumulative oxidative stress in rat lung tissue. Inhal. Toxicol. 2011, 23, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Kang, B.H.; Wan, F.J.; Chen, C.S.; Huang, K.L. Hyperbaric oxygen attenuates lipopolysaccharide-induced acute lung injury. Intensive Care Med. 2002, 28, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.-C.; Wu, C.-P.; Chu, S.-J.; Kang, B.-H.; Huang, K.-L. Effect of hyperbaric oxygen on endotoxin-induced lung injury in rats. Shock 2004, 21, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Tsai, P.S.; Wang, T.Y.; Huang, C.L.; Huang, C.J. Hyperbaric oxygen attenuation of lipopolysaccharide-induced acute lung injury involves heme oxygenase-1. Acta Anaesthesiol. Scand. 2005, 49, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-J.; Li, M.-H.; Hsu, C.-W.; Tsai, S.-H.; Lin, S.-H.; Huang, K.-L. Influence of hyperbaric oxygen on tumor necrosis factor-alpha and nitric oxide production in endotoxin-induced acute lung injury in rats. Pulm. Pharmacol. Ther. 2007, 20, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J.; Mason, S.W.; Taira, M.T.; Hasson, G.E.; Naito, M.S.; Yamaguchi, K.T. Effect of radical scavengers and hyperbaric oxygen on smoke-induced pulmonary edema. Undersea Hyperb. Med. 1994, 21, 21–30. [Google Scholar]
- Feng, Y.; Zhang, Z.; Li, Q.; Li, W.; Xu, J.; Cao, H. Hyperbaric oxygen preconditioning protects lung against hyperoxic acute lung injury in rats via heme oxygenase-1 induction. Biochem. Biophys. Res. Commun. 2015, 456, 549–554. [Google Scholar] [CrossRef]
- Yu, Q.-H.; Zhang, P.-X.; Liu, Y.; Liu, W.; Yin, N. Hyperbaric oxygen preconditioning protects the lung against acute pancreatitis induced injury via attenuating inflammation and oxidative stress in a nitric oxide dependent manner. Biochem. Biophys. Res. Commun. 2016, 478, 93–100. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Harokopos, V.; Paparountas, T.; Oikonomou, N.; Chatziioannou, A.; Vilaras, G.; Tsiambas, E.; Karameris, A.; Bouros, D.; Aidinis, V. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1alpha in disease pathogenesis. Am. J. Respir. Crit. Care Med. 2007, 176, 1108–1119. [Google Scholar] [CrossRef]
- Higgins, D.F.; Kimura, K.; Bernhardt, W.M.; Shrimanker, N.; Akai, Y.; Hohenstein, B.; Saito, Y.; Johnson, R.S.; Kretzler, M.; Cohen, C.D.; et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Investig. 2007, 117, 3810–3820. [Google Scholar] [CrossRef]
- Goodwin, J.; Choi, H.; Hsieh, M.H.; Neugent, M.L.; Ahn, J.M.; Hayenga, H.N.; Singh, P.K.; Shackelford, D.B.; Lee, I.K.; Shulaev, V.; et al. Targeting Hypoxia-Inducible Factor-1alpha/Pyruvate Dehydrogenase Kinase 1 axis by dichloroacetate suppresses bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 216–231. [Google Scholar] [CrossRef]
- Shimoda, L.A.; Semenza, G.L. HIF and the lung: Role of hypoxia-inducible factors in pulmonary development and disease. Am. J. Respir. Crit. Care Med. 2011, 183, 152–156. [Google Scholar] [CrossRef]
- Aquino-Galvez, A.; Gonzalez-Avila, G.; Jimenez-Sanchez, L.L.; Maldonado-Martinez, H.A.; Cisneros, J.; Toscano-Marquez, F.; Castillejos-Lopez, M.; Torres-Espindola, L.M.; Velazquez-Cruz, R.; Rodriguez, V.H.O.; et al. Dysregulated expression of hypoxia-inducible factors augments myofibroblasts differentiation in idiopathic pulmonary fibrosis. Respir. Res. 2019, 20, 130. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15. [Google Scholar] [CrossRef]
- Thom, S.R. Oxidative stress is fundamental to hyperbaric oxygen therapy. J. Appl. Physiol. 2009, 106, 988–995. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Kalin, T.V.; Xu, Y.; Kalinichenko, V.V. Building and regenerating the lung cell by cell. Physiol. Rev. 2019, 99, 513–554. [Google Scholar] [CrossRef]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Han, X.; Wang, R.; Zhou, Y.; Fei, L.; Sun, H.; Lai, S.; Saadatpour, A.; Zhou, Z.; Chen, H.; Ye, F.; et al. Mapping the mouse cell atlas by microwell-seq. Cell 2018, 172, 1091–1107. [Google Scholar] [CrossRef]
- Lederer, D.J.; Martinez, F.J. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef]
- Barratt, S.L.; Creamer, A.; Hayton, C.; Chaudhuri, N. Idiopathic pulmonary fibrosis (IPF): An overview. J. Clin. Med. 2018, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Wang, Y.; Deng, N.; Huang, G.; Taghavifar, F.; Geng, Y.; Liu, N.; Kulur, V.; Yao, C.; Chen, P.; et al. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep. 2018, 22, 3625–3640. [Google Scholar] [CrossRef] [PubMed]
- Tahedl, D.; Wirkes, A.; Tschanz, S.A.; Ochs, M.; Mühlfeld, C. How common is the lipid body-containing interstitial cell in the mammalian lung? Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L386–L394. [Google Scholar] [CrossRef] [PubMed]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L.M. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Ruggieri, S.; Annese, T.; Crivellato, E. Surface markers: An identity card of endothelial cells. Microcirculation 2019, e12587. [Google Scholar] [CrossRef] [PubMed]
- Bochaton-Piallat, M.-L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000Research 2016, 5, 752. [Google Scholar] [CrossRef]
- Segundo-Val, I.S.; Sanz-Lozano, C.S. Introduction to the gene expression analysis. Methods Mol. Biol. 2016, 1434, 29–43. [Google Scholar] [CrossRef]
- Lee, J.-H.; Tammela, T.; Hofree, M.; Choi, J.; Marjanovic, N.D.; Han, S.; Canner, D.; Wu, K.; Paschini, M.; Bhang, D.H.; et al. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell 2017, 170, 1149–1163. [Google Scholar] [CrossRef]
- Zepp, J.A.; Zacharias, W.J.; Frank, D.B.; Cavanaugh, C.A.; Zhou, S.; Morley, M.P.; Morrisey, E.E. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 2017, 170, 1134–1148. [Google Scholar] [CrossRef]
- Tabula Muris Consortium; Overall coordination; Logistical coordination; Organ collection and processing; Library preparation and sequencing; Computational data analysis; Cell type annotation; Writing group; Supplemental text writing group; Principal investigators. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 2018, 562, 367–372. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Anurag, M.; Zhu, M.; Huang, C.; Vasaikar, S.; Wang, J.; Hoog, J.; Burugu, S.; Gao, D.; Suman, V.; Zhang, X.H.; et al. Immune Checkpoint Profiles in Luminal B Breast Cancer (Alliance). J. Natl. Cancer Inst. 2019, djz213. [Google Scholar] [CrossRef]
- Peng, D.; Wang, L.; Li, H.; Cai, C.; Tan, Y.; Xu, B.; Le, H. An immune infiltration signature to predict the overall survival of patients with colon cancer. IUBMB Life 2019, 71, 1760–1770. [Google Scholar] [CrossRef]
- Takeshita, T.; Yan, L.; Asaoka, M.; Rashid, O.; Takabe, K. Late recurrence of breast cancer is associated with pro-cancerous immune microenvironment in the primary tumor. Sci. Rep. 2019, 9, 16942. [Google Scholar] [CrossRef]
- Cao, Y.; Tang, W.; Tang, W. Immune cell infiltration characteristics and related core genes in lupus nephritis: Results from bioinformatic analysis. BMC Immunol. 2019, 20, 37. [Google Scholar] [CrossRef]
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [CrossRef]
- Raa, A.; Stansberg, C.; Steen, V.M.; Bjerkvig, R.; Reed, R.K.; Stuhr, L.E.B. Hyperoxia retards growth and induces apoptosis and loss of glands and blood vessels in DMBA-induced rat mammary tumors. BMC Cancer 2007, 7, 23. [Google Scholar] [CrossRef]
- Stuhr, L.E.B.; Raa, A.; Oyan, A.M.; Kalland, K.H.; Sakariassen, P.O.; Petersen, K.; Bjerkvig, R.; Reed, R.K. Hyperoxia retards growth and induces apoptosis, changes in vascular density and gene expression in transplanted gliomas in nude rats. J. Neurooncol. 2007, 85, 191–202. [Google Scholar] [CrossRef]
- Stenmark Kurt, R.; Fagan Karen, A.; Frid Maria, G. Hypoxia-induced pulmonary vascular remodeling. Circ. Res. 2006, 99, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Kim, K.M.; Yang, J.H.; Kim, J.Y.; Park, S.J.; Kim, S.J.; Kim, J.K.; Cho, I.J.; Ki, S.H. Induction of REDD1 via AP-1 prevents oxidative stress-mediated injury in hepatocytes. Free Radic. Biol. Med. 2018, 124, 221–231. [Google Scholar] [CrossRef] [PubMed]
- He, Y.W.; Li, H.; Zhang, J.; Hsu, C.L.; Lin, E.; Zhang, N.; Guo, J.; Forbush, K.A.; Bevan, M.J. The extracellular matrix protein mindin is a pattern-recognition molecule for microbial pathogens. Nat. Immunol. 2004, 5, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Li, H.; He, Y.W. The extracellular matrix protein mindin serves as an integrin ligand and is critical for inflammatory cell recruitment. Blood 2005, 106, 3854–3859. [Google Scholar] [CrossRef]
- Descargues, P.; Deraison, C.; Bonnart, C.; Kreft, M.; Kishibe, M.; Ishida-Yamamoto, A.; Elias, P.; Barrandon, Y.; Zambruno, G.; Sonnenberg, A.; et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat. Genet. 2005, 37, 56–65. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, Q.R.; Pan, Z.Z.; Song, Y.; Su, P.; Niu, Y.P.; Sun, Y.M.; Liu, D. Hyperbaric Oxygen Improves Functional Recovery of the Injured Spinal Cord by Inhibiting Inflammation and Glial Scar Formation. Am. J. Phys. Med. Rehab. 2019, 98, 914–920. [Google Scholar] [CrossRef]
- Bosco, G.; Vezzani, G.; Sposta, S.M.; Rizzato, A.; Enten, G.; Abou-samra, A.; Malacrida, S.; Quartesan, S.; Vezzoli, A.; Camporesi, E. Hyperbaric oxygen therapy ameliorates osteonecrosis in patients by modulating inflammation and oxidative stress. J. Enzyme Inhib. Med. Chem. 2018, 33, 1501–1505. [Google Scholar] [CrossRef]
- Oyaizu, T.; Enomoto, M.; Yamamoto, N.; Tsuji, K.; Horie, M.; Muneta, T.; Sekiya, I.; Okawa, A.; Yagishita, K. Hyperbaric oxygen reduces inflammation, oxygenates injured muscle, and regenerates skeletal muscle via macrophage and satellite cell activation. Sci. Rep. 2018, 8, 1288. [Google Scholar] [CrossRef]
- Lin, K.C.; Niu, K.C.; Tsai, K.J.; Kuo, J.R.; Wang, L.C.; Chio, C.C.; Chang, C.P. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J. Trauma Acute Care Surg. 2012, 72, 650–659. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Chang, Q.; Cox, R.A.; Gong, X.M.; Gould, L.J. Hyperbaric oxygen attenuates apoptosis and decreases inflammation in an ischemic wound model. J. Investig. Dermatol. 2008, 128, 2102–2112. [Google Scholar] [CrossRef]
- Wilson, H.D.; Wilson, J.R.; Fuchs, P.N. Hyperbaric oxygen treatment decreases inflammation and mechanical hypersensitivity in an animal model of inflammatory pain. Brain Res. 2006, 1098, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.P.; Qu, B.; Harding, K.G. The effects of hyperbaric oxygen on cultured fibroblasts. J. Wound Care 1994, 3, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.P.; Harding, K.G. Stimulation of glycosaminoglycan synthesis in cultured fibroblasts by hyperbaric oxygen. Br. J. Dermatol. 1994, 131, 630–633. [Google Scholar] [CrossRef]
- Gajendrareddy, P.K.; Junges, R.; Cygan, G.; Zhao, Y.; Marucha, P.T.; Engeland, C.G. Increased oxygen exposure alters collagen expression and tissue architecture during ligature-induced periodontitis. J. Periodontal Res. 2017, 52, 644–649. [Google Scholar] [CrossRef]
- Andre-Levigne, D.; Modarressi, A.; Pignel, R.; Bochaton-Piallat, M.L.; Pittet-Cuenod, B. Hyperbaric oxygen therapy promotes wound repair in ischemic and hyperglycemic conditions, increasing tissue perfusion and collagen deposition. Wound Repair Regen. 2016, 24, 954–965. [Google Scholar] [CrossRef]
- Finotello, F.; Rieder, D.; Hackl, H.; Trajanoski, Z. Next-generation computational tools for interrogating cancer immunity. Nat. Rev. Genet. 2019, 20, 724–746. [Google Scholar] [CrossRef] [PubMed]
- Luca, B.A.; Alizadeh, A.A.; Diehn, M.; Newman, A.M.; Gentles, A.J.; Steen, C.B. Atlas of clinically-distinct cell states and cellular ecosystems across human solid tumors. J. ImmunoTher. Cancer 2019, 7 (Suppl. 1). [Google Scholar] [CrossRef]
- Shi, A.; Kasumova, G.G.; Michaud, W.A.; Cintolo-Gonzales, J.; Martínez, M.D.; Ohmura, J.; Mehta, A.; Chien, I.; Frederick, D.T.; Cohen, S.; et al. Plasma-derived exosomal analysis and deconvolution enables prediction and tracking of melanoma checkpoint blockade response. bioRxiv 2019. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Kaller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Kim, D.; Landmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Proc, G.P.D. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]

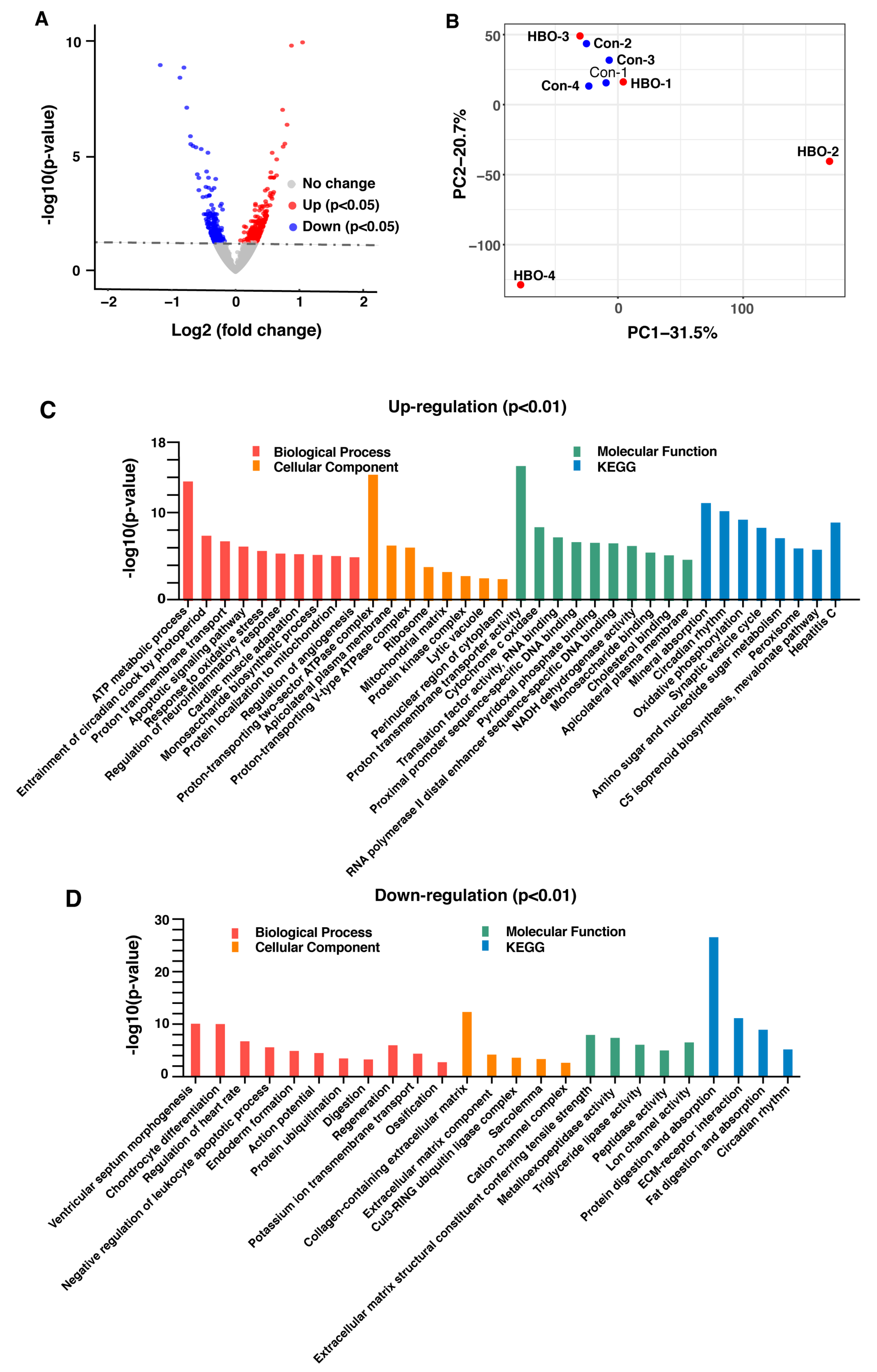
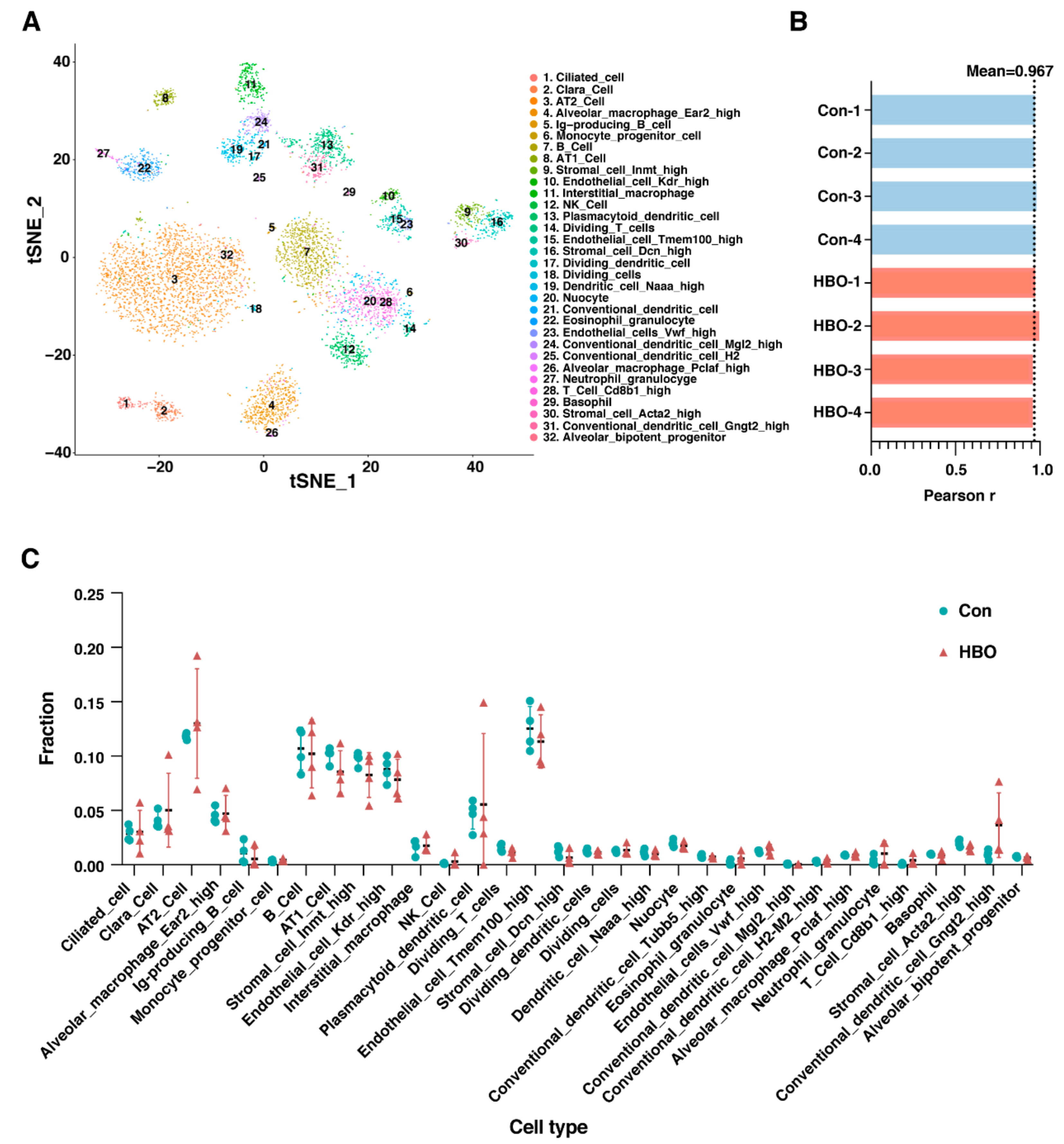
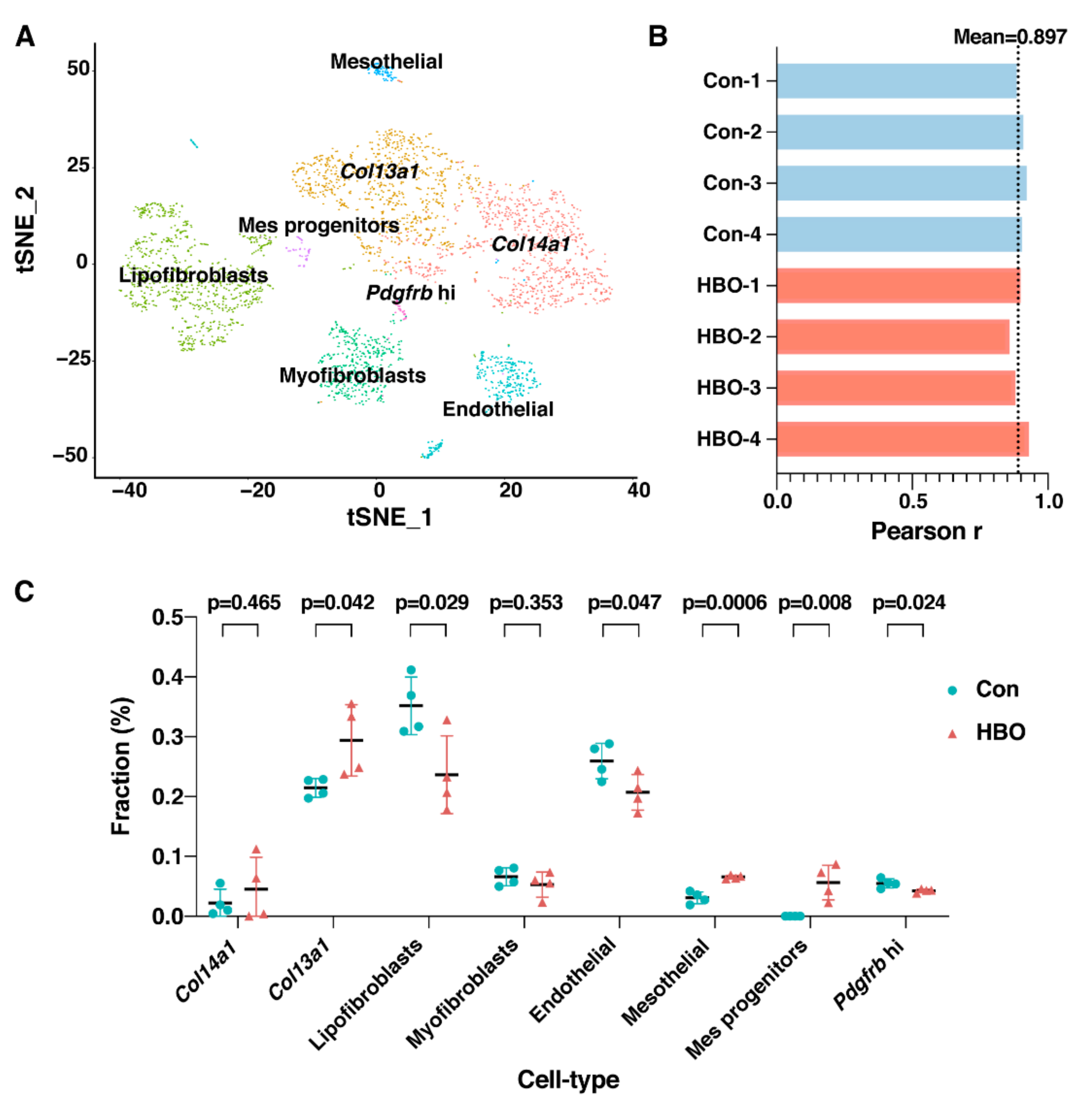
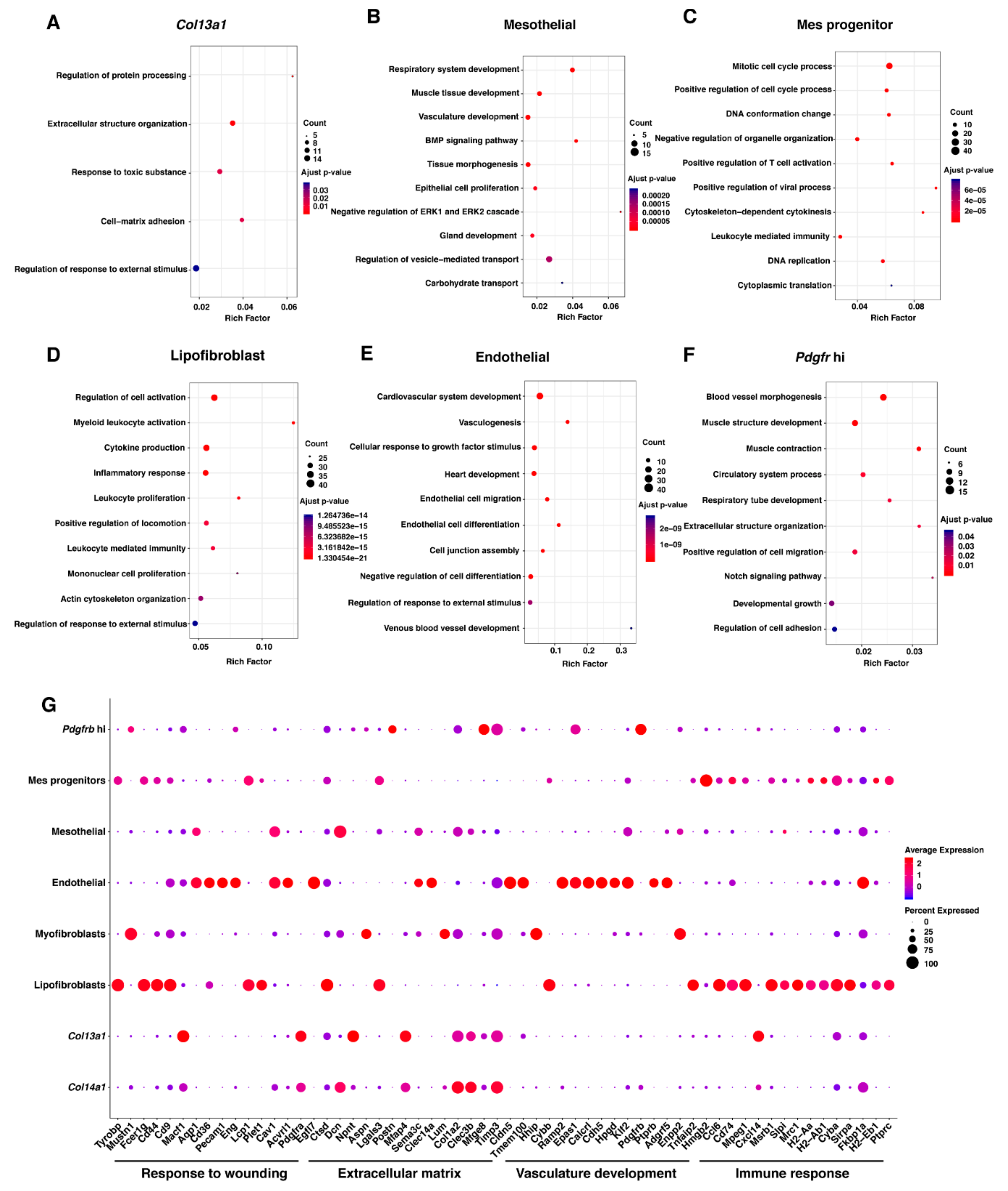
| GO Process | NES | FDR q-Value |
|---|---|---|
| Cytosolic ribosome | 2.73 | <0.0001 |
| Establishment of protein localization to endoplasmic reticulum | 2.58 | <0.0001 |
| Ribosomal subunit | 2.52 | <0.0001 |
| Protein localization to endoplasmic reticulum | 2.51 | <0.0001 |
| Translational initiation | 2.47 | <0.0001 |
| Ribosome | 2.38 | <0.0001 |
| Nuclear transcribed mRNA catabolic process nonsense mediated decay | 2.36 | <0.0001 |
| Cytosolic large ribosomal subunit | 2.33 | <0.0001 |
| Cytosolic small ribosomal subunit | 2.29 | <0.0001 |
| Structural constituent of ribosome | 2.28 | <0.0001 |
| Mitochondrial electron transport NADH to ubiquinone | 2.27 | <0.0001 |
| NADH dehydrogenase activity | 2.26 | <0.0001 |
| NADH Dehydrogenase complex | 2.26 | <0.0001 |
| Oxidoreductase activity acting on NADPH quinone or similar compound as acceptor | 2.24 | 1.28E-04 |
| Cytosolic part | 2.25 | 1.37E-04 |
| Small ribosomal subunit | 2.25 | 1.46E-04 |
| Oxidative phosphorylation | 2.21 | 2.43E-04 |
| Respiratory chain | 2.20 | 2.86E-04 |
| Multi organism metabolic process | 2.19 | 4.10E-04 |
| Large ribosomal subunit | 2.19 | 4.32E-04 |
| Electron transport chain | 2.13 | 1.32E-03 |
| NADH dehydrogenase complex assembly | 2.11 | 1.69E-03 |
| Interaction with symbiont | 2.12 | 1.77E-03 |
| Mitochondrial respiratory chain complex I assembly | 2.11 | 1.79E-03 |
| Mitochondrial respiratory chain complex I biogenesis | 2.10 | 2.05E-03 |
| RNA catabolic process | 2.09 | 2.64E-03 |
| Oxidoreductase activity acting on peroxide as acceptor | 2.07 | 3.62E-03 |
| Mitochondrial protein complex | 2.07 | 3.64E-03 |
| Mitochondrial respiratory chain complex assembly | 2.06 | 3.99E-03 |
| Nucleoside triphosphate metabolic process | 2.04 | 5.36E-03 |
| Proteinaceous extracellular matrix | −2.30 | 6.34E-04 |
| Calcium dependent cell adhesion via plasma membrane cell adhesion molecules | −2.45 | 9.50E-04 |
| Extracellular matrix component | −2.38 | 9.51E-04 |
| Delayed rectifier potassium channel activity | −2.25 | 1.19E-03 |
| Homophilic cell adhesion via plasma membrane adhesion molecules | −2.13 | 1.12E-02 |
| Intestinal absorption | −2.12 | 1.20E-02 |
| Basement membrane | −2.14 | 1.25E-02 |
| Cell cell adhesion via plasma membrane adhesion molecules | −2.04 | 3.45E-02 |
| Voltage gated potassium channel activity | −2.04 | 3.83E-02 |
| Extracellular matrix | −2.01 | 4.87E-02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Zhou, Y.; Li, Y.; Hill, C.; Ewing, R.M.; Jones, M.G.; Davies, D.E.; Jiang, Z.; Wang, Y. Deconvolution of RNA-Seq Analysis of Hyperbaric Oxygen-Treated Mice Lungs Reveals Mesenchymal Cell Subtype Changes. Int. J. Mol. Sci. 2020, 21, 1371. https://doi.org/10.3390/ijms21041371
Yuan Y, Zhou Y, Li Y, Hill C, Ewing RM, Jones MG, Davies DE, Jiang Z, Wang Y. Deconvolution of RNA-Seq Analysis of Hyperbaric Oxygen-Treated Mice Lungs Reveals Mesenchymal Cell Subtype Changes. International Journal of Molecular Sciences. 2020; 21(4):1371. https://doi.org/10.3390/ijms21041371
Chicago/Turabian StyleYuan, Yuan, Yilu Zhou, Yali Li, Charlotte Hill, Rob M. Ewing, Mark G. Jones, Donna E. Davies, Zhenglin Jiang, and Yihua Wang. 2020. "Deconvolution of RNA-Seq Analysis of Hyperbaric Oxygen-Treated Mice Lungs Reveals Mesenchymal Cell Subtype Changes" International Journal of Molecular Sciences 21, no. 4: 1371. https://doi.org/10.3390/ijms21041371
APA StyleYuan, Y., Zhou, Y., Li, Y., Hill, C., Ewing, R. M., Jones, M. G., Davies, D. E., Jiang, Z., & Wang, Y. (2020). Deconvolution of RNA-Seq Analysis of Hyperbaric Oxygen-Treated Mice Lungs Reveals Mesenchymal Cell Subtype Changes. International Journal of Molecular Sciences, 21(4), 1371. https://doi.org/10.3390/ijms21041371






