Mdm2 and MdmX RING Domains Play Distinct Roles in the Regulation of p53 Responses: A Comparative Study of Mdm2 and MdmX RING Domains in U2OS Cells
Abstract
1. Introduction
2. Results
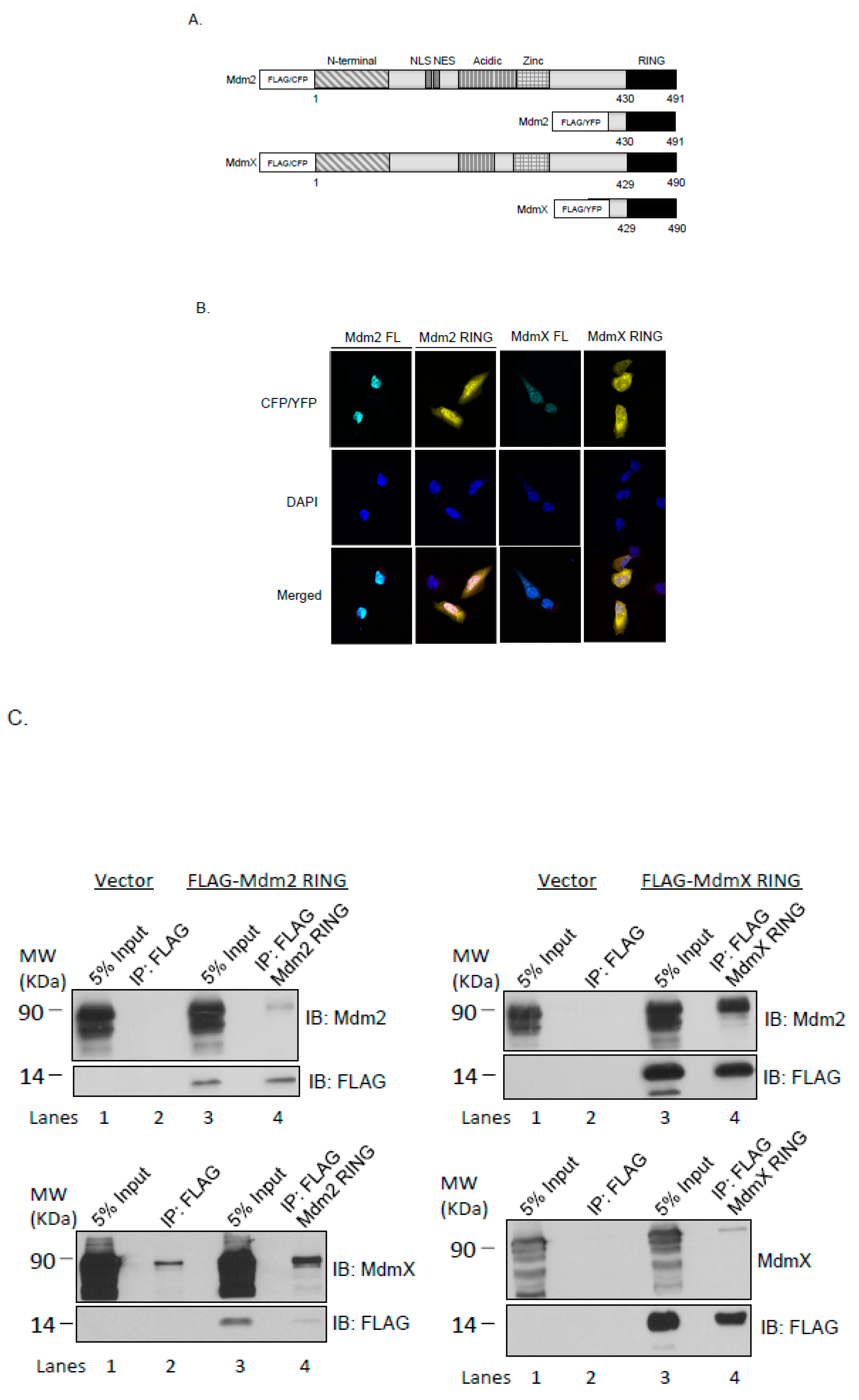
2.1. Cellular Localization of the Ectopically Expressed Mdm2 and MdmX RING Domains in U2OS Cells
2.2. Mdm2 RING and MdmX RING Domains Interact with the Full-Length Mdm2 and MdmX
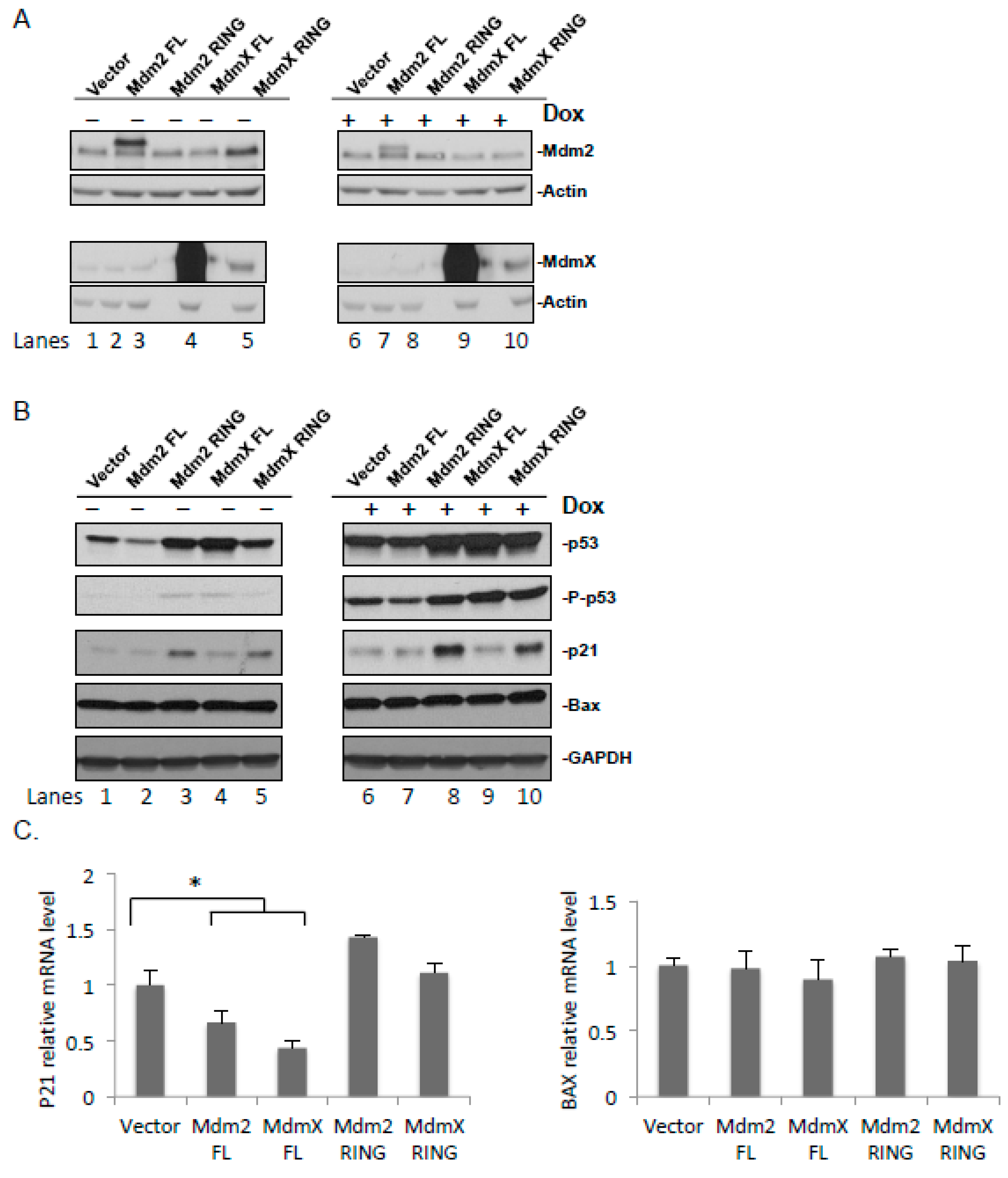
2.3. Ectopically Expressed Mdm2 and MdmX RING Domains Affect the Stability and Function of Endogenous Mdm2 and MdmX
2.4. Effect of the Ectopic Mdm2 and MdmX RING Domains on the Cellular Level of p53 under Normal and DNA Damage Conditions
2.5. Effect of the Ectopic Mdm2 and MdmX RING Domains on p53 Target Genes
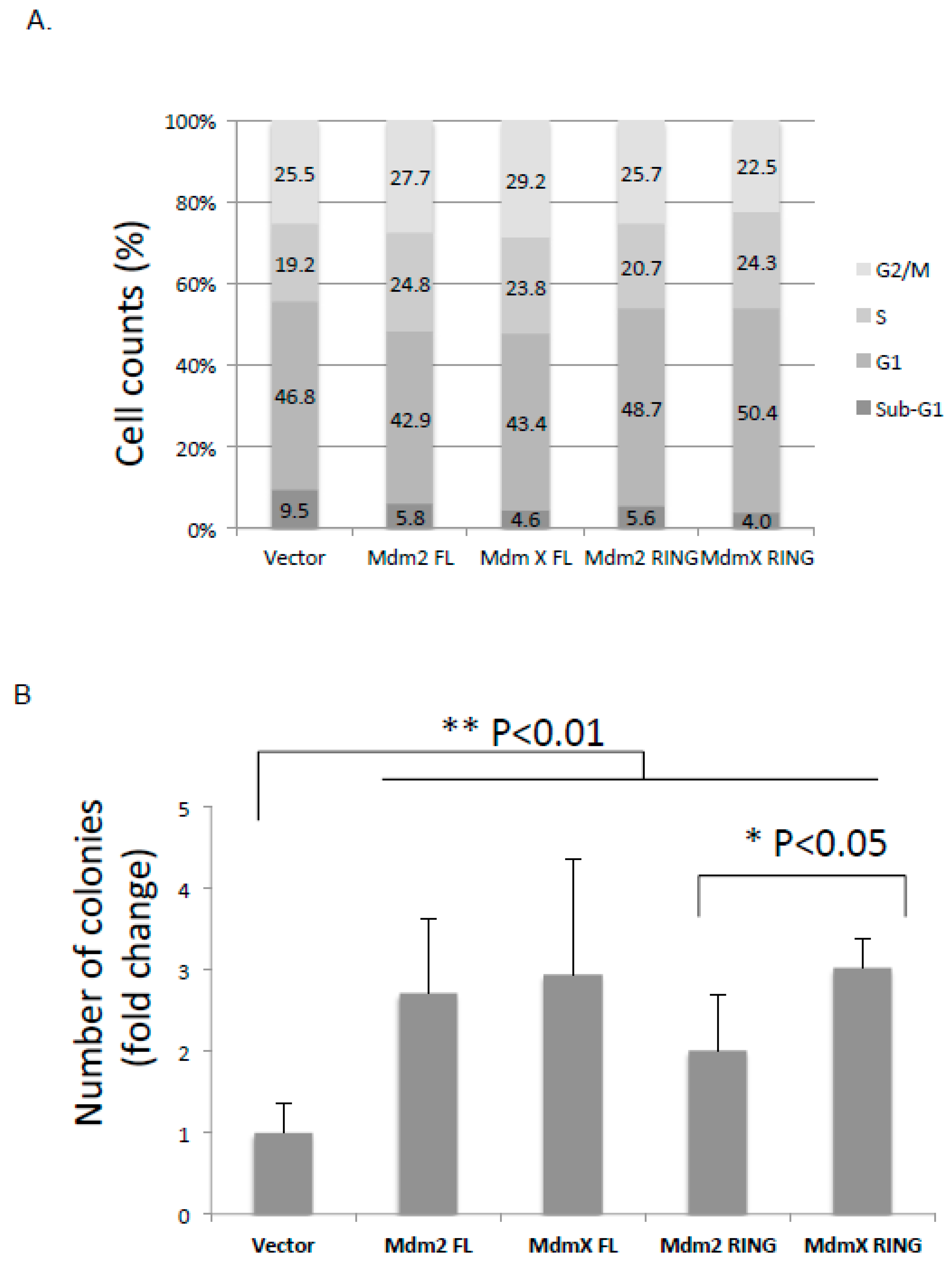
2.6. Effect of the Ectopic Mdm2 and MdmX RING Domains on U2OS Cell Cycle and Cell Growth
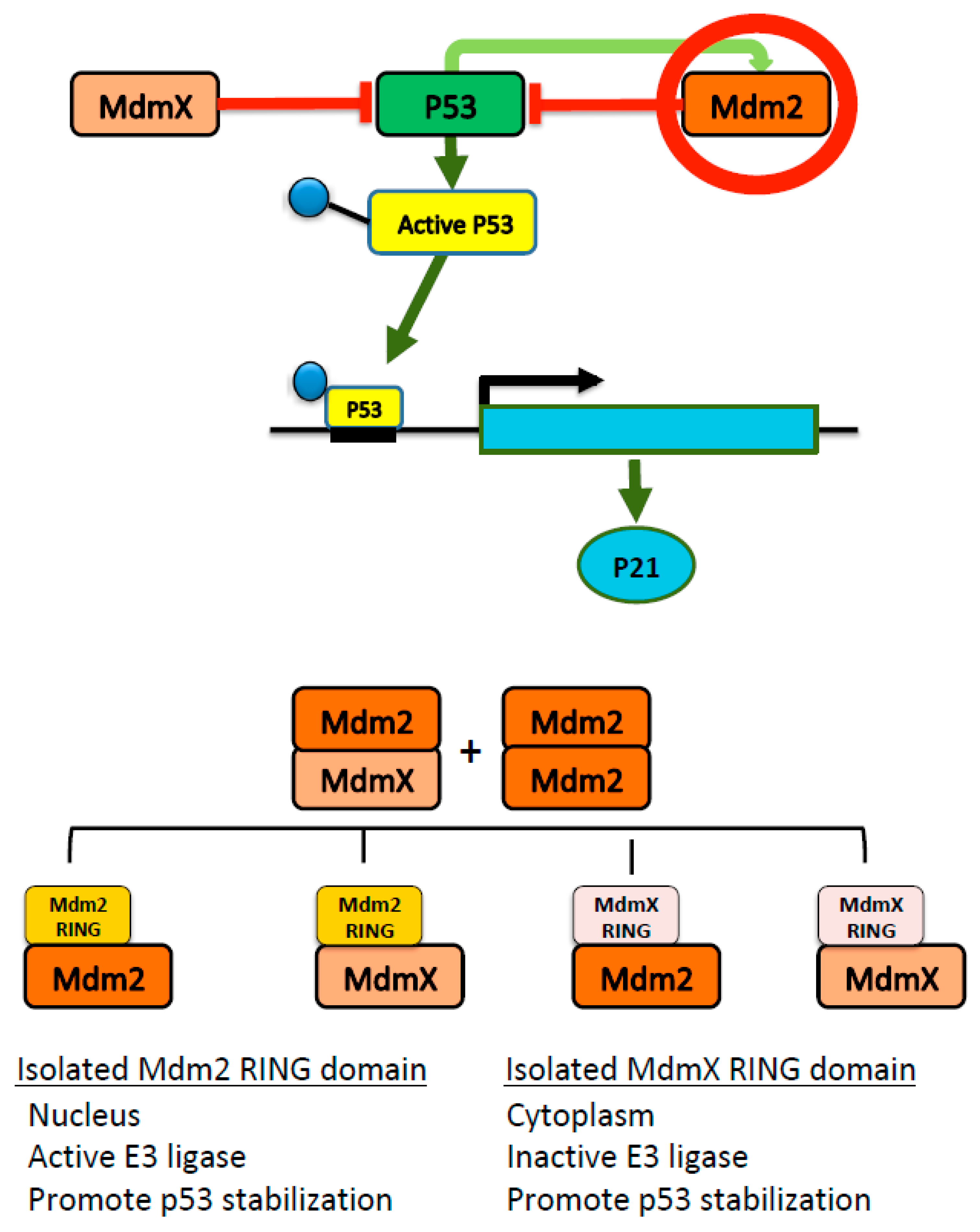
3. Discussion
4. Materials and Methods
4.1. Plasmids
4.2. Cell Culture and Transfection
4.3. Antibodies
4.4. Immunoblotting
4.5. Fluorescent Microscopy
4.6. Co-Immunoprecipitation
4.7. RNA Expression Analysis
4.8. Cell Cycle Analysis
4.9. Clonogenic Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lane, D.P.; Fischer, P.M. Turning the key on p53. Nature 2004, 427, 789–790. [Google Scholar] [CrossRef]
- Toledo, F.; Wahl, G.M. Regulating the p53 pathway: In vitro hypotheses, in vivo veritas. Nat. Rev. Cancer 2006, 6, 909–923. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef]
- Donehower, L.A.; Harvey, M.; Slagle, B.L.; McArthur, M.J.; Montgomery, C.A., Jr.; Butel, J.S.; Bradley, A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 1992, 356, 215–221. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, W.; Rajagopal, G.; Levine, A.J. The tumor suppressor p53: Cancer and aging. Cell Cycle 2008, 7, 842–847. [Google Scholar] [CrossRef]
- Fukasawa, K.; Wiener, F.; Vande Woude, G.F.; Mai, S. Genomic instability and apoptosis are frequent in p53 deficient young mice. Oncogene 1997, 15, 1295–1302. [Google Scholar] [CrossRef]
- Murphy, K.L.; Rosen, J.M. Mutant p53 and genomic instability in a transgenic mouse model of breast cancer. Oncogene 2000, 19, 1045–1051. [Google Scholar] [CrossRef]
- Shadfan, M.; Lopez-Pajares, V.; Yuan, Z.M. MDM2 and MDMX: Alone and together in regulation of p53. Transl. Cancer Res. 2012, 1, 88–89. [Google Scholar]
- Freedman, D.A.; Levine, A.J. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell. Biol. 1998, 18, 7288–7293. [Google Scholar] [CrossRef]
- Yu, Z.K.; Geyer, R.K.; Maki, C.G. MDM2-dependent ubiquitination of nuclear and cytoplasmic P53. Oncogene 2000, 19, 5892–5897. [Google Scholar] [CrossRef]
- Tollini, L.A.; Zhang, Y. p53 Regulation Goes Live-Mdm2 and MdmX Co-Star: Lessons Learned from Mouse Modeling. Genes Cancer 2012, 3, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Finch, R.A.; Donoviel, D.B.; Potter, D.; Shi, M.; Fan, A.; Freed, D.D.; Wang, C.Y.; Zambrowicz, B.P.; Ramirez-Solis, R.; Sands, A.T.; et al. mdmx is a negative regulator of p53 activity in vivo. Cancer Res. 2002, 62, 3221–3225. [Google Scholar] [PubMed]
- Jones, S.N.; Roe, A.E.; Donehower, L.A.; Bradley, A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 1995, 378, 206–208. [Google Scholar] [CrossRef]
- Migliorini, D.; Lazzerini Denchi, E.; Danovi, D.; Jochemsen, A.; Capillo, M.; Gobbi, A.; Helin, K.; Pelicci, P.G.; Marine, J.C. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol. Cell. Biol. 2002, 22, 5527–5538. [Google Scholar] [CrossRef]
- Montes de Oca Luna, R.; Wagner, D.S.; Lozano, G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995, 378, 203–206. [Google Scholar] [CrossRef]
- Chavez-Reyes, A.; Parant, J.M.; Amelse, L.L.; de Oca Luna, R.M.; Korsmeyer, S.J.; Lozano, G. Switching mechanisms of cell death in mdm2- and mdm4-null mice by deletion of p53 downstream targets. Cancer Res. 2003, 63, 8664–8669. [Google Scholar]
- Parant, J.; Chavez-Reyes, A.; Little, N.A.; Yan, W.; Reinke, V.; Jochemsen, A.G.; Lozano, G. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat. Genet. 2001, 29, 92–95. [Google Scholar] [CrossRef]
- Huang, L.; Yan, Z.; Liao, X.; Li, Y.; Yang, J.; Wang, Z.G.; Zuo, Y.; Kawai, H.; Shadfan, M.; Ganapathy, S.; et al. The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 12001–12006. [Google Scholar] [CrossRef]
- Pant, V.; Xiong, S.; Iwakuma, T.; Quintas-Cardama, A.; Lozano, G. Heterodimerization of Mdm2 and Mdm4 is critical for regulating p53 activity during embryogenesis but dispensable for p53 and Mdm2 stability. Proc. Natl. Acad. Sci. USA 2011, 108, 11995–12000. [Google Scholar] [CrossRef]
- Migliorini, D.; Danovi, D.; Colombo, E.; Carbone, R.; Pelicci, P.G.; Marine, J.C. Hdmx recruitment into the nucleus by Hdm2 is essential for its ability to regulate p53 stability and transactivation. J. Biol. Chem. 2002, 277, 7318–7323. [Google Scholar] [CrossRef]
- Linke, K.; Mace, P.D.; Smith, C.A.; Vaux, D.L.; Silke, J.; Day, C.L. Structure of the MDM2/MDMX RING domain heterodimer reveals dimerization is required for their ubiquitylation in trans. Cell Death Differ. 2008, 15, 841–848. [Google Scholar] [CrossRef]
- Mancini, F.; Gentiletti, F.; D’Angelo, M.; Giglio, S.; Nanni, S.; D’Angelo, C.; Farsetti, A.; Citro, G.; Sacchi, A.; Pontecorvi, A.; et al. MDM4 (MDMX) overexpression enhances stabilization of stress-induced p53 and promotes apoptosis. J. Biol. Chem. 2004, 279, 8169–8180. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zong, C.S.; Xia, W.; Wei, Y.; Ali-Seyed, M.; Li, Z.; Broglio, K.; Berry, D.A.; Hung, M.C. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol. Cell. Biol. 2006, 26, 7269–7282. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zong, C.S.; Xia, W.; Yamaguchi, H.; Ding, Q.; Xie, X.; Lang, J.Y.; Lai, C.C.; Chang, C.J.; Huang, W.C.; et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat. Cell Biol. 2008, 10, 138–148. [Google Scholar] [CrossRef]
- Yap, D.B.; Hsieh, J.K.; Chan, F.S.; Lu, X. mdm2: A bridge over the two tumour suppressors, p53 and Rb. Oncogene 1999, 18, 7681–7689. [Google Scholar] [CrossRef]
- Mancini, F.; Teveroni, E.; Di Conza, G.; Monteleone, V.; Arisi, I.; Pellegrino, M.; Buttarelli, M.; Pieroni, L.; D’Onofrio, M.; Urbani, A.; et al. MDM4 actively restrains cytoplasmic mTORC1 by sensing nutrient availability. Mol. Cancer 2017, 16, 55. [Google Scholar] [CrossRef]
- Fan, C.; Wang, X. Mdm2 Splice isoforms regulate the p53/Mdm2/Mdm4 regulatory circuit via RING domain-mediated ubiquitination of p53 and Mdm4. Cell Cycle 2017, 16, 660–664. [Google Scholar] [CrossRef]
- Jacob, A.G.; O’Brien, D.; Singh, R.K.; Comiskey, D.F., Jr.; Littleton, R.M.; Mohammad, F.; Gladman, J.T.; Widmann, M.C.; Jeyaraj, S.C.; Bolinger, C.; et al. Stress-induced isoforms of MDM2 and MDM4 correlate with high-grade disease and an altered splicing network in pediatric rhabdomyosarcoma. Neoplasia 2013, 15, 1049–1063. [Google Scholar] [CrossRef]
- Schuster, K.; Fan, L.; Harris, L.C. MDM2 splice variants predominantly localize to the nucleoplasm mediated by a COOH-terminal nuclear localization signal. Mol. Cancer Res. 2007, 5, 403–412. [Google Scholar] [CrossRef]
- Volk, E.L.; Fan, L.; Schuster, K.; Rehg, J.E.; Harris, L.C. The MDM2-a splice variant of MDM2 alters transformation in vitro and the tumor spectrum in both Arf- and p53-null models of tumorigenesis. Mol. Cancer Res. 2009, 7, 863–869. [Google Scholar] [CrossRef]
- Giglio, S.; Mancini, F.; Gentiletti, F.; Sparaco, G.; Felicioni, L.; Barassi, F.; Martella, C.; Prodosmo, A.; Iacovelli, S.; Buttitta, F.; et al. Identification of an aberrantly spliced form of HDMX in human tumors: A new mechanism for HDM2 stabilization. Cancer Res. 2005, 65, 9687–9694. [Google Scholar] [CrossRef]
- Evans, S.C.; Viswanathan, M.; Grier, J.D.; Narayana, M.; El-Naggar, A.K.; Lozano, G. An alternatively spliced HDM2 product increases p53 activity by inhibiting HDM2. Oncogene 2001, 20, 4041–4049. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef]
- He, G.; Siddik, Z.H.; Huang, Z.; Wang, R.; Koomen, J.; Kobayashi, R.; Khokhar, A.R.; Kuang, J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 2005, 24, 2929–2943. [Google Scholar] [CrossRef]
- Egorova, O.; Mis, M.; Sheng, Y. A site-directed mutagenesis study of the MdmX RING domain. Biochem. Biophys. Res. Commun. 2014, 447, 696–701. [Google Scholar] [CrossRef]
- De Graaf, P.; Little, N.A.; Ramos, Y.F.; Meulmeester, E.; Letteboer, S.J.; Jochemsen, A.G. Hdmx protein stability is regulated by the ubiquitin ligase activity of Mdm2. J. Biol. Chem. 2003, 278, 38315–38324. [Google Scholar] [CrossRef]
- Nomura, K.; Klejnot, M.; Kowalczyk, D.; Hock, A.K.; Sibbet, G.J.; Vousden, K.H.; Huang, D.T. Structural analysis of MDM2 RING separates degradation from regulation of p53 transcription activity. Nat. Struct. Mol. Biol. 2017, 24, 578–587. [Google Scholar] [CrossRef]
- Tournillon, A.S.; Lopez, I.; Malbert-Colas, L.; Naski, N.; Olivares-Illana, V.; Fahraeus, R. The alternative translated MDMX(p60) isoform regulates MDM2 activity. Cell Cycle 2015, 14, 449–458. [Google Scholar] [CrossRef]
- Tao, W.; Levine, A.J. P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc. Natl. Acad. Sci. USA 1999, 96, 6937–6941. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.; Chen, J. DNA damage induces MDMX nuclear translocation by p53-dependent and -independent mechanisms. Mol. Cell. Biol. 2002, 22, 7562–7571. [Google Scholar] [CrossRef]
- Gu, J.; Kawai, H.; Nie, L.; Kitao, H.; Wiederschain, D.; Jochemsen, A.G.; Parant, J.; Lozano, G.; Yuan, Z.M. Mutual dependence of MDM2 and MDMX in their functional inactivation of p53. J. Biol. Chem. 2002, 277, 19251–19254. [Google Scholar] [CrossRef]
- Jin, Y.; Lee, H.; Zeng, S.X.; Dai, M.S.; Lu, H. MDM2 promotes p21waf1/cip1 proteasomal turnover independently of ubiquitylation. EMBO J. 2003, 22, 6365–6377. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Li, M.; Agrawal, S.; Chen, X.; Zhang, R. MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J. Biol. Chem. 2004, 279, 16000–16006. [Google Scholar] [CrossRef]
- Jin, Y.; Zeng, S.X.; Sun, X.X.; Lee, H.; Blattner, C.; Xiao, Z.; Lu, H. MDMX promotes proteasomal turnover of p21 at G1 and early S phases independently of, but in cooperation with, MDM2. Mol. Cell. Biol. 2008, 28, 1218–1229. [Google Scholar] [CrossRef]
- Lee, J.H.; Lu, H. 14-3-3Gamma inhibition of MDMX-mediated p21 turnover independent of p53. J. Biol. Chem. 2011, 286, 5136–5142. [Google Scholar] [CrossRef]
- Priest, C.; Prives, C.; Poyurovsky, M.V. Deconstructing nucleotide binding activity of the Mdm2 RING domain. Nucleic Acids Res. 2010, 38, 7587–7598. [Google Scholar] [CrossRef]
- Alt, J.R.; Bouska, A.; Fernandez, M.R.; Cerny, R.L.; Xiao, H.; Eischen, C.M. Mdm2 binds to Nbs1 at sites of DNA damage and regulates double strand break repair. J. Biol. Chem. 2005, 280, 18771–18781. [Google Scholar] [CrossRef]
- Riscal, R.; Schrepfer, E.; Arena, G.; Cisse, M.Y.; Bellvert, F.; Heuillet, M.; Rambow, F.; Bonneil, E.; Sabourdy, F.; Vincent, C.; et al. Chromatin-Bound MDM2 Regulates Serine Metabolism and Redox Homeostasis Independently of p53. Mol. Cell 2016, 62, 890–902. [Google Scholar] [CrossRef]
- Wienken, M.; Moll, U.M.; Dobbelstein, M. Mdm2 as a chromatin modifier. J. Mol. Cell. Biol. 2017, 9, 74–80. [Google Scholar] [CrossRef]
- Carrillo, A.M.; Bouska, A.; Arrate, M.P.; Eischen, C.M. Mdmx promotes genomic instability independent of p53 and Mdm2. Oncogene 2015, 34, 846–856. [Google Scholar] [CrossRef]
- Kim, T.K.; Eberwine, J.H. Mammalian cell transfection: The present and the future. Anal. Bioanal. Chem. 2010, 397, 3173–3178. [Google Scholar] [CrossRef]
- Jacob, A.G.; Singh, R.K.; Comiskey, D.F., Jr.; Rouhier, M.F.; Mohammad, F.; Bebee, T.W.; Chandler, D.S. Stress-induced alternative splice forms of MDM2 and MDMX modulate the p53-pathway in distinct ways. PLoS ONE 2014, 9, e104444. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorova, O.; Lau, H.H.; McGraphery, K.; Sheng, Y. Mdm2 and MdmX RING Domains Play Distinct Roles in the Regulation of p53 Responses: A Comparative Study of Mdm2 and MdmX RING Domains in U2OS Cells. Int. J. Mol. Sci. 2020, 21, 1309. https://doi.org/10.3390/ijms21041309
Egorova O, Lau HH, McGraphery K, Sheng Y. Mdm2 and MdmX RING Domains Play Distinct Roles in the Regulation of p53 Responses: A Comparative Study of Mdm2 and MdmX RING Domains in U2OS Cells. International Journal of Molecular Sciences. 2020; 21(4):1309. https://doi.org/10.3390/ijms21041309
Chicago/Turabian StyleEgorova, Olga, Heather HC Lau, Kate McGraphery, and Yi Sheng. 2020. "Mdm2 and MdmX RING Domains Play Distinct Roles in the Regulation of p53 Responses: A Comparative Study of Mdm2 and MdmX RING Domains in U2OS Cells" International Journal of Molecular Sciences 21, no. 4: 1309. https://doi.org/10.3390/ijms21041309
APA StyleEgorova, O., Lau, H. H., McGraphery, K., & Sheng, Y. (2020). Mdm2 and MdmX RING Domains Play Distinct Roles in the Regulation of p53 Responses: A Comparative Study of Mdm2 and MdmX RING Domains in U2OS Cells. International Journal of Molecular Sciences, 21(4), 1309. https://doi.org/10.3390/ijms21041309




