Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa
Abstract
1. Introduction
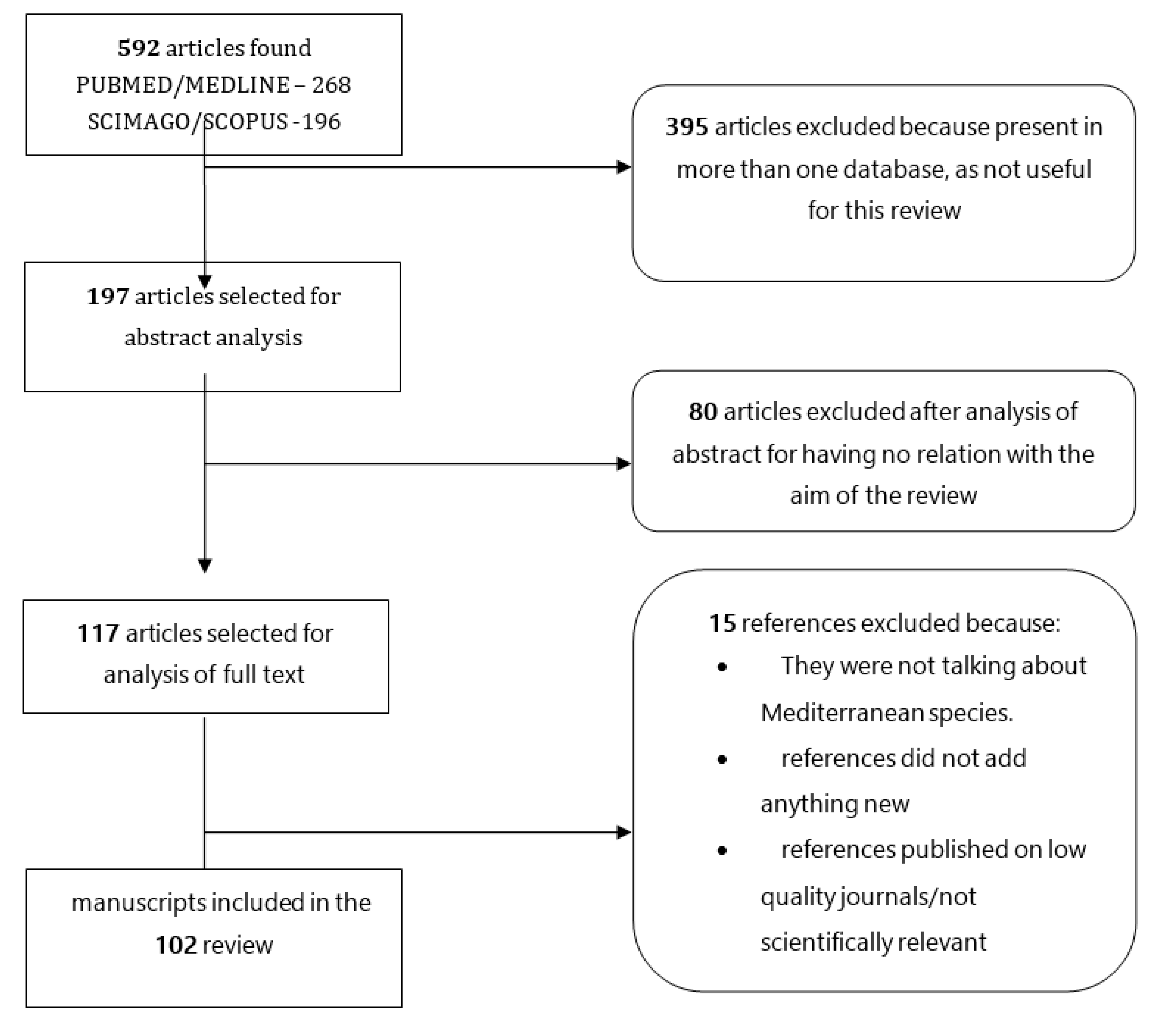
2. Materials and Methods
3. Results
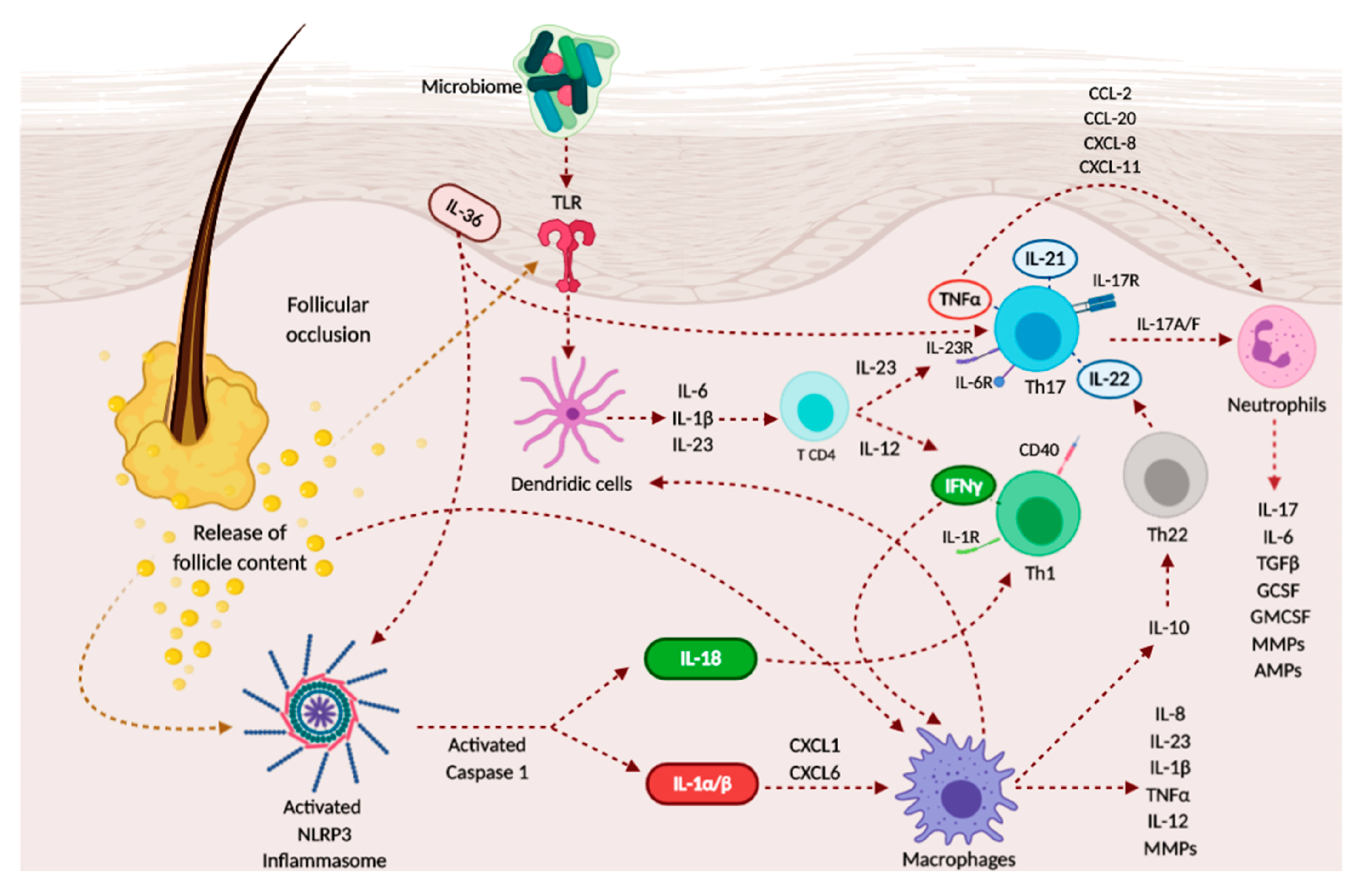
3.1. Immunopathogenesis of Hidradenitis Suppurativa
3.2. Cytokines’ Role
3.2.1. Interleukin (IL)-1 Pathway
3.2.2. TNF-α and IFN-γ
3.2.3. IL-17/IL-23 Axis
3.2.4. IL-6
3.2.5. IL-10
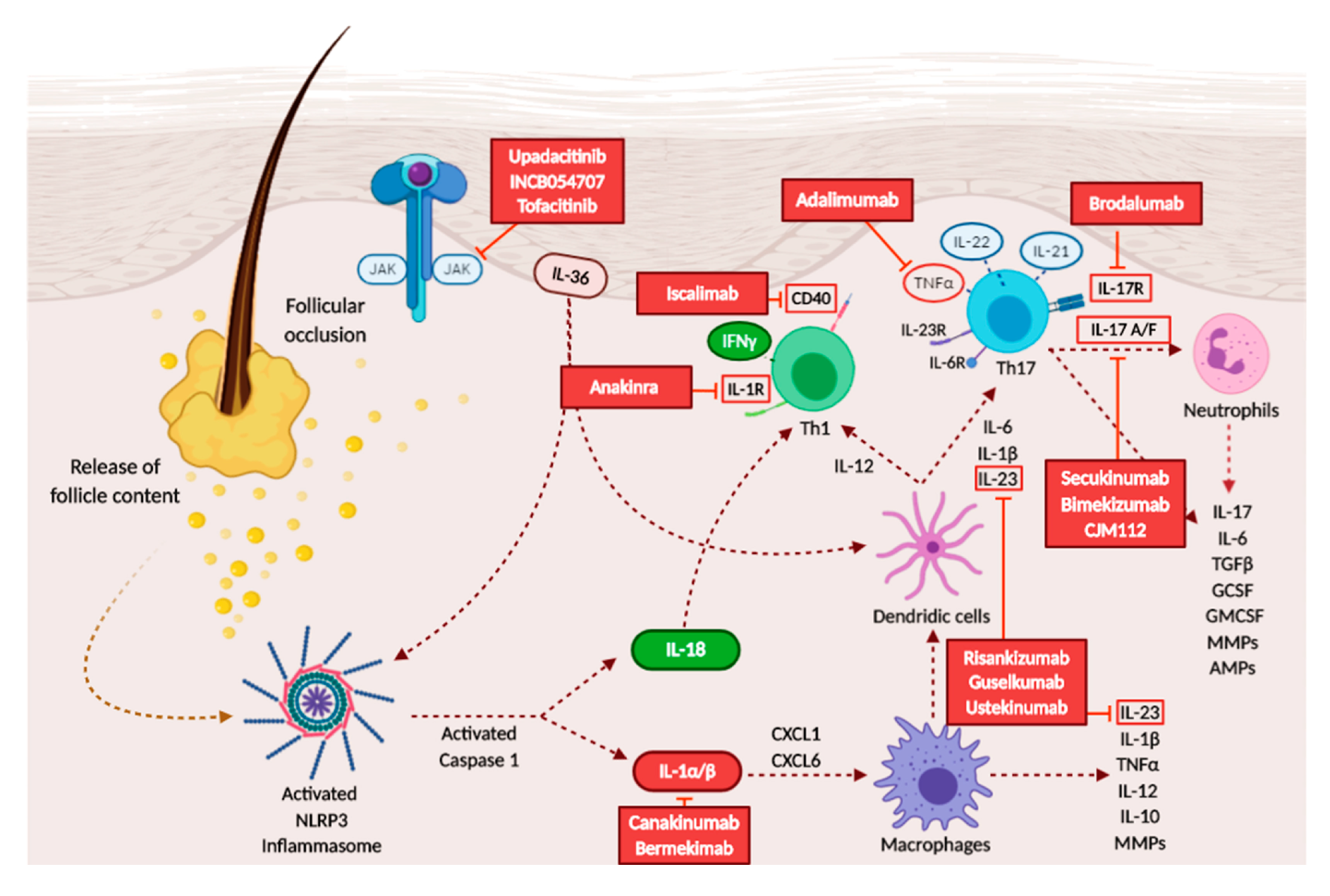
3.3. Overview on Therapies and Treatment Possibilities
3.3.1. TNF-α Inhibitors
3.3.2. IL-1 Inhibitors
3.3.3. Anti-IL-17 Drugs
3.3.4. Anti-IL-23 Drugs
3.3.5. Anti-IL-12/23 Drugs
3.3.6. Janus Kinase (JAK) Inhibitors
3.3.7. Others
3.4. The Role of Microbiome and Biofilms
4. Discussion
Funding
Conflicts of Interest
References
- Goldburg, S.R.; Strober, B.E.; Payette, M.J. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J. Am. Acad. Dermatol. 2020, 82, 1045–1058. [Google Scholar] [CrossRef]
- Del Duca, E.; Pavel, A.B.; Dubin, C.; Song, T.; Wallace, E.B.; Peng, X.; Estrada, Y.D.; Xu, H.; Maari, C.; Jack, C.; et al. Major Differences in Expression of Inflammatory Pathways in Skin from Different Body Sites of Healthy Individuals. J. Investig. Dermatol. 2019, 139, 2228–2232.e10. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Giovanardi, G.; Caro, D.R.C.; Iannone, M.; Garcovich, S.; Dini, V.; De Simone, C.; Franceschini, C.; Oranges, T.; Mingrone, G.; et al. Alexithymia affects patients with hidradenitis suppurativa. Eur. J. Dermatol. 2018, 28, 482–487. [Google Scholar] [CrossRef]
- Cusack, C.; Buckley, C. Etanercept: Effective in the management of hidradenitis suppurativa. Br. J. Dermatol. 2006, 154, 726–729. [Google Scholar] [CrossRef]
- Kraft, J.N.; Searles, G.E. Hidradenitis suppurativa in 64 female patients: Retrospective study comparing oral antibiotics and antiandrogen therapy. J. Cutan. Med. Surg. 2007, 11, 125–131. [Google Scholar] [CrossRef]
- Jemec, G.B.; Wendelboe, P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J. Am. Acad. Dermatol. 1998, 39, 971–974. [Google Scholar] [CrossRef]
- Strober, B.E.; Kim, C.; Siu, K. Efalizumab for the treatment of refractory hidradenitis suppurativa. J. Am. Acad. Dermatol. 2007, 57, 1090–1091. [Google Scholar] [CrossRef]
- Finley, E.M.; Ratz, J.L. Treatment of hidradenitis suppurativa with carbon dioxide laser excision and second-intention healing. J. Am. Acad. Dermatol. 1996, 34, 465–469. [Google Scholar] [CrossRef]
- Nistico, S.P.; Del Duca, E.; Farnetani, F.; Guida, S.; Pellacani, G.; Rajabi-Estarabadi, A.; Nouri, K. Removal of unwanted hair: Efficacy, tolerability, and safety of long-pulsed 755-nm alexandrite laser equipped with a sapphire handpiece. Lasers Med. Sci. 2018, 33, 1479–1483. [Google Scholar] [CrossRef]
- van der Zee, H.H.; Prens, E.P.; Boer, J. Deroofing: A tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J. Am. Acad. Dermatol. 2010, 63, 475–480. [Google Scholar] [CrossRef]
- Sabat, R.; Jemec, G.B.E.; Matusiak, L.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Primers 2020, 6, 18. [Google Scholar] [CrossRef]
- Kurokawa, I.; Nishijima, S.; Kusumoto, K.; Senzaki, H.; Shikata, N.; Tsubura, A. Immunohistochemical study of cytokeratins in hidradenitis suppurativa (acne inversa). J. Int. Med. Res. 2002, 30, 131–136. [Google Scholar] [CrossRef]
- A Study of Bermekimab in Patients with Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT03512275 (accessed on 30 April 2018).
- Biomarkers in Hidradenitis Suppurativa Participants Receiving Brodalumab. Available online: https://ClinicalTrials.gov/show/NCT03960268 (accessed on 23 May 2019).
- A Placebo-Controlled Study of the Safety of INCB054707 in Participants with Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT03607487 (accessed on 31 July 2018).
- A Study of the Safety of INCB054707 in Participants with Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT03569371 (accessed on 26 June 2018).
- A Study to Test the Efficacy, Safety and Pharmacokinetics of Bimekizumab in Subjects with Moderate to Severe Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT03248531 (accessed on 14 August 2017).
- Anakinra as a Treatment for Hydradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT01516749 (accessed on 25 January 2012).
- Efficacy, Safety, and Pharmacokinetics Study of CJM112 in Hidradenitis Suppurativa Patients. Available online: https://ClinicalTrials.gov/show/NCT02421172 (accessed on 20 April 2015).
- Study to Assess the Safety and Efficacy of Infliximab to Treat Hidradenitis Suppurtativa. Available online: https://ClinicalTrials.gov/show/NCT00795574 (accessed on 21 November 2008).
- Etanercept in Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT00329823 (accessed on 25 May 2006).
- A Study to Evaluate the Efficacy, Safety, and Tolerability of Guselkumab for the Treatment of Participants with Moderate to Severe Hidradenitis Suppurativa (HS). Available online: https://ClinicalTrials.gov/show/NCT03628924 (accessed on 14 August 2018).
- Studying Complement Inhibition in Patients with Moderate to Severe Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT03001622 (accessed on 10 December 2016).
- Short-Term Safety, Efficacy and Mode of Action of Apremilast in Moderate Suppurative Hidradenitis. Available online: https://ClinicalTrials.gov/show/NCT03049267 (accessed on 10 February 2017).
- Exploratory Trial Evaluating Cosentyx (Secukinumab) for Patients with Moderate-To-Severe Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT03099980 (accessed on 11 July 2016).
- Etanercept for Treatment of Hidradenitis. Available online: https://ClinicalTrials.gov/show/NCT00107991 (accessed on 12 April 2005).
- Open-Label Study of Adalimumab in Japanese Subjects with Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT02904902 (accessed on 19 September 2016).
- MABP1 in Hidradenitis Suppurativa Refractory to Adalimumab. Available online: https://ClinicalTrials.gov/show/NCT02643654 (accessed on 10 December 2015).
- Single Center Study of Apremilast for the Treatment of Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT02695212 (accessed on 1 March 2016).
- A Proof of Concept Study to Evaluate the Effectiveness of Ustekinumab in Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT01704534 (accessed on 11 October 2012).
- Efficacy and Safety Study of IFX-1 in Patients with Moderate to Severe Hidradenitis Suppurativa (HS). Available online: https://ClinicalTrials.gov/show/NCT03487276 (accessed on 26 February 2018).
- Anakinra in Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT01558375 (accessed on 20 March 2012).
- Open-Label Study of the Safety and Efficacy of Adalimumab in the Treatment of Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT01635764 (accessed on 5 September 2017).
- Safety and Efficacy of Adalimumab (Humira) for Hidradenitis Suppurativa (HS) Peri-Surgically. Available online: https://ClinicalTrials.gov/show/NCT02808975 (accessed on 22 June 2016).
- Study of Adalimumab in Subjects with Moderate to Severe Chronic Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT00918255 (accessed on 11 June 2009).
- Efficacy and Safety Study of Adalimumab in Treatment of Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT01468207 (accessed on 9 November 2011).
- Efficacy and Safety Study of Adalimumab in the Treatment of Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT01468233 (accessed on 9 November 2011).
- To Assess the Efficacy and Safety of Adalimumab in Subjects with Moderate to Severe Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT00827996 (accessed on 12 February 2007).
- Comparison of PK and Tolerability of MSB11022 Administered by AI or PFS. Available online: https://ClinicalTrials.gov/show/NCT04018599 (accessed on 12 July 2019).
- A Global Study Comparing Risankizumab to Placebo in Adult Participants with Moderate to Severe Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT03926169 (accessed on 24 April 2019).
- A Study of Oral Upadacitinib Tablet Compared to Placebo in Adult Participants with Moderate to Severe Hidradenitis Suppurativa to Assess Change in Disease Symptoms. Available online: https://ClinicalTrials.gov/show/NCT04430855 (accessed on 12 June 2020).
- A Study to Test the Efficacy and Safety of Bimekizumab in Study Participants with Moderate to Severe Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT04242498 (accessed on 27 January 2020).
- Extension Study to Assess Effects of Non-Interrupted Versus Interrupted and Long Term Treatment of Two Dose Regimes of Secukinumab in Subjects with Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT04179175 (accessed on 27 November 2019).
- Study of Efficacy and Safety of Two Secukinumab Dose Regimens in Subjects with Moderate to Severe Hidradenitis Suppurativa (HS). Available online: https://ClinicalTrials.gov/show/NCT03713632 (accessed on 22 October 2018).
- A Study to Evaluate the Safety and Efficacy of PF-06650833, PF-06700841, and PF 06826647 in Adults with Hidradenitis Suppurativa. Available online: https://ClinicalTrials.gov/show/NCT04092452 (accessed on 17 September 2019).
- Tofacitinib for Immune Skin Conditions in Down Syndrome. Available online: https://ClinicalTrials.gov/show/NCT04246372 (accessed on 29 January 2020).
- Wolk, K.; Join-Lambert, O.; Sabat, R. Aetiology and pathogenesis of hidradenitis suppurativa. Br. J. Dermatol. 2020. [Google Scholar] [CrossRef]
- Frew, J.W.; Hawkes, J.E.; Krueger, J.G. Topical, systemic and biologic therapies in hidradenitis suppurativa: Pathogenic insights by examining therapeutic mechanisms. Ther. Adv. Chronic. Dis. 2019, 10. [Google Scholar] [CrossRef]
- Vossen, A.; van der Zee, H.H.; Prens, E.P. Hidradenitis Suppurativa: A Systematic Review Integrating Inflammatory Pathways Into a Cohesive Pathogenic Model. Front. Immunol. 2018, 9, 2965. [Google Scholar] [CrossRef]
- van der Zee, H.H.; de Ruiter, L.; Boer, J.; van den Broecke, D.G.; den Hollander, J.C.; Laman, J.D.; Prens, E.P. Alterations in leucocyte subsets and histomorphology in normal-appearing perilesional skin and early and chronic hidradenitis suppurativa lesions. Br. J. Dermatol. 2012, 166, 98–106. [Google Scholar] [CrossRef]
- Tsaousi, A.; Witte, E.; Witte, K.; Rowert-Huber, H.J.; Volk, H.D.; Sterry, W.; Wolk, K.; Schneider-Burrus, S.; Sabat, R. MMP8 Is Increased in Lesions and Blood of Acne Inversa Patients: A Potential Link to Skin Destruction and Metabolic Alterations. Mediat. Inflamm. 2016, 2016, 4097574. [Google Scholar] [CrossRef]
- Mozeika, E.; Pilmane, M.; Nurnberg, B.M.; Jemec, G.B. Tumour necrosis factor-alpha and matrix metalloproteinase-2 are expressed strongly in hidradenitis suppurativa. Acta Derm. Venereol. 2013, 93, 301–304. [Google Scholar] [CrossRef]
- Hoffman, L.K.; Ghias, M.H.; Lowes, M.A. Pathophysiology of hidradenitis suppurativa. Semin. Cutan. Med. Surg. 2017, 36, 47–54. [Google Scholar] [CrossRef]
- Ring, H.C.; Bay, L.; Nilsson, M.; Kallenbach, K.; Miller, I.M.; Saunte, D.M.; Bjarnsholt, T.; Tolker-Nielsen, T.; Jemec, G.B. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br. J. Dermatol. 2017, 176, 993–1000. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Antonopoulou, A.; Petropoulou, C.; Mouktaroudi, M.; Spyridaki, E.; Baziaka, F.; Pelekanou, A.; Giamarellou, H.; Stavrianeas, N.G. Altered innate and adaptive immune responses in patients with hidradenitis suppurativa. Br. J. Dermatol. 2007, 156, 51–56. [Google Scholar] [CrossRef]
- van der Zee, H.H.; de Ruiter, L.; van den Broecke, D.G.; Dik, W.A.; Laman, J.D.; Prens, E.P. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: A rationale for targeting TNF-alpha and IL-1beta. Br. J. Dermatol. 2011, 164, 1292–1298. [Google Scholar] [CrossRef]
- von Laffert, M.; Helmbold, P.; Wohlrab, J.; Fiedler, E.; Stadie, V.; Marsch, W.C. Hidradenitis suppurativa (acne inversa): Early inflammatory events at terminal follicles and at interfollicular epidermis. Exp. Dermatol. 2010, 19, 533–537. [Google Scholar] [CrossRef]
- Seyed Jafari, S.M.; Hunger, R.E.; Schlapbach, C. Hidradenitis Suppurativa: Current Understanding of Pathogenic Mechanisms and Suggestion for Treatment Algorithm. Front. Med. 2020, 7, 68. [Google Scholar] [CrossRef]
- Kelly, G.; Sweeney, C.M.; Tobin, A.M.; Kirby, B. Hidradenitis suppurativa: The role of immune dysregulation. Int. J. Dermatol. 2014, 53, 1186–1196. [Google Scholar] [CrossRef]
- Kelly, G.; Hughes, R.; McGarry, T.; van den Born, M.; Adamzik, K.; Fitzgerald, R.; Lawlor, C.; Tobin, A.M.; Sweeney, C.M.; Kirby, B. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br. J. Dermatol. 2015, 173, 1431–1439. [Google Scholar] [CrossRef]
- van der Zee, H.H.; Laman, J.D.; Boer, J.; Prens, E.P. Hidradenitis suppurativa: Viewpoint on clinical phenotyping, pathogenesis and novel treatments. Exp. Dermatol. 2012, 21, 735–739. [Google Scholar] [CrossRef]
- Franza, L.; Carusi, V.; Altamura, S.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Conti, P.; Pandolfi, F. Interrelationship between inflammatory cytokines (IL-1, IL-6, IL-33, IL-37) and acquired immunity. J. Biol. Regul. Homeost. Agents 2019, 33, 1321–1326. [Google Scholar]
- Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Di Emidio, P.; Conti, P. CAR-T cell therapy causes inflammation by IL-1 which activates inflammatory cytokine mast cells: Anti-inflammatory role of IL-37. J. Biol. Regul. Homeost. Agents 2019, 33, 1981–1985. [Google Scholar]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Di Emidio, P.; Ronconi, G.; Toniato, E. IL-1 induces throboxane-A2 (TxA2) in COVID-19 causing inflammation and micro-thrombi: Inhibitory effect of the IL-1 receptor antagonist (IL-1Ra). J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Conti, P.; Gallenga, C.E.; Tete, G.; Caraffa, A.; Ronconi, G.; Younes, A.; Toniato, E.; Ross, R.; Kritas, S.K. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents 2020, 34, 333–338. [Google Scholar] [PubMed]
- Witte-Handel, E.; Wolk, K.; Tsaousi, A.; Irmer, M.L.; Mossner, R.; Shomroni, O.; Lingner, T.; Witte, K.; Kunkel, D.; Salinas, G.; et al. The IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J. Investig. Dermatol. 2019, 139, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Hessam, S.; Sand, M.; Gambichler, T.; Bechara, F.G. Correlation of inflammatory serum markers with disease severity in patients with hidradenitis suppurativa (HS). J. Am. Acad. Dermatol. 2015, 73, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Ardon, C.B.; Wang, C.; Prens, E.P.; van Straalen, K.R. Non-invasive assessment of cytokine and antimicrobial peptide levels in Hidradenitis Suppurativa using transdermal analysis patches. Br. J. Dermatol. 2020. [Google Scholar] [CrossRef]
- Queen, D.; Ediriweera, C.; Liu, L. Function and Regulation of IL-36 Signaling in Inflammatory Diseases and Cancer Development. Front. Cell Dev. Biol. 2019, 7, 317. [Google Scholar] [CrossRef]
- Hessam, S.; Sand, M.; Gambichler, T.; Skrygan, M.; Ruddel, I.; Bechara, F.G. Interleukin-36 in hidradenitis suppurativa: Evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br. J. Dermatol. 2018, 178, 761–767. [Google Scholar] [CrossRef]
- Di Caprio, R.; Balato, A.; Caiazzo, G.; Lembo, S.; Raimondo, A.; Fabbrocini, G.; Monfrecola, G. IL-36 cytokines are increased in acne and hidradenitis suppurativa. Arch. Dermatol. Res. 2017, 309, 673–678. [Google Scholar] [CrossRef]
- Teng, X.; Hu, Z.; Wei, X.; Wang, Z.; Guan, T.; Liu, N.; Liu, X.; Ye, N.; Deng, G.; Luo, C.; et al. IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J. Immunol. 2014, 192, 1815–1823. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Stoeckman, A.K.; Wu, G.; Boeckermann, A.N.; Azam, T.; Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Hao, R.; Kalabokis, V.; et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc. Natl. Acad. Sci. USA 2012, 109, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Frew, J.W.; Hawkes, J.E.; Krueger, J.G. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Research 2018, 7, 1930. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Alhusayen, R.; Amini-Nik, S. The critical role of macrophages in the pathogenesis of hidradenitis suppurativa. Inflamm. Res. 2017, 66, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.T.; Flood, K.S.; Porter, M.L.; Kimball, A.B. TNF-alpha inhibitors in the treatment of hidradenitis suppurativa. Ther. Adv. Chronic. Dis. 2019, 10. [Google Scholar] [CrossRef]
- Kyriakou, A.; Trigoni, A.; Galanis, N.; Sotiriadis, D.; Patsatsi, A. Efficacy of adalimumab in moderate to severe hidradenitis suppurativa: Real life data. Dermatol. Rep. 2018, 10, 7859. [Google Scholar] [CrossRef]
- Moran, B.; Sweeney, C.M.; Hughes, R.; Malara, A.; Kirthi, S.; Tobin, A.M.; Kirby, B.; Fletcher, J.M. Hidradenitis Suppurativa Is Characterized by Dysregulation of the Th17:Treg Cell Axis, Which Is Corrected by Anti-TNF Therapy. J. Investig. Dermatol. 2017, 137, 2389–2395. [Google Scholar] [CrossRef]
- Vossen, A.; van der Zee, H.H.; Tsoi, L.C.; Xing, X.; Devalaraja, M.; Gudjonsson, J.E.; Prens, E.P. Novel cytokine and chemokine markers of hidradenitis suppurativa reflect chronic inflammation and itch. Allergy 2019, 74, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Banerjee, A.; McNish, S.; Shanmugam, V.K. Interferon-gamma (IFN-gamma) is Elevated in Wound Exudate from Hidradenitis Suppurativa. Immunol. Investig. 2017, 46, 149–158. [Google Scholar] [CrossRef]
- Wolk, K.; Warszawska, K.; Hoeflich, C.; Witte, E.; Schneider-Burrus, S.; Witte, K.; Kunz, S.; Buss, A.; Roewert, H.J.; Krause, M.; et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: Pathogenetic mechanisms in acne inversa. J. Immunol. 2011, 186, 1228–1239. [Google Scholar] [CrossRef]
- Frew, J.W.; Navrazhina, K.; Grand, D.; Sullivan-Whalen, M.; Gilleaudeau, P.; Garcet, S.; Ungar, J.; Krueger, J.G. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: An open-label cohort study. J. Am. Acad. Dermatol. 2020, 83, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, C.; Hanni, T.; Yawalkar, N.; Hunger, R.E. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J. Am. Acad. Dermatol. 2011, 65, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Gaffen, S.L. Interleukin 17 Family Cytokines: Signaling Mechanisms, Biological Activities, and Therapeutic Implications. Cold Spring Harb. Perspect. Biol. 2018, 10, a028522. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Boniotto, M.; Guguin, A.; Surenaud, M.; Jean-Louis, F.; Tisserand, P.; Ortonne, N.; Hersant, B.; Bosc, R.; Poli, F.; et al. Intrinsic Defect in Keratinocyte Function Leads to Inflammation in Hidradenitis Suppurativa. J. Investig. Dermatol. 2016, 136, 1768–1780. [Google Scholar] [CrossRef]
- Lima, A.L.; Karl, I.; Giner, T.; Poppe, H.; Schmidt, M.; Presser, D.; Goebeler, M.; Bauer, B. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br. J. Dermatol. 2016, 174, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, L.; Szczech, J.; Bieniek, A.; Nowicka-Suszko, D.; Szepietowski, J.C. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: Implications for treatment with anti-IL-17 agents. J. Am. Acad. Dermatol. 2017, 76, 670–675. [Google Scholar] [CrossRef]
- Thomi, R.; Schlapbach, C.; Yawalkar, N.; Simon, D.; Yerly, D.; Hunger, R.E. Elevated levels of the antimicrobial peptide LL-37 in hidradenitis suppurativa are associated with a Th1/Th17 immune response. Exp. Dermatol. 2018, 27, 172–177. [Google Scholar] [CrossRef]
- Yao, Y.; Thomsen, S.F. The role of interleukin-17 in the pathogenesis of hidradenitis suppurativa. Dermatol. Online J. 2017, 23, 1–7. [Google Scholar]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar] [CrossRef]
- Lyakh, L.; Trinchieri, G.; Provezza, L.; Carra, G.; Gerosa, F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol. Rev. 2008, 226, 112–131. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. Targeting interleukin-6: All the way to treat autoimmune and inflammatory diseases. Int. J. Biol. Sci. 2012, 8, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Bechara, F.G.; Sand, M.; Skrygan, M.; Kreuter, A.; Altmeyer, P.; Gambichler, T. Acne inversa: Evaluating antimicrobial peptides and proteins. Ann. Dermatol. 2012, 24, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Khammari, A.; Brocard, A.; Moyse, D.; Blouin, E.; Guillet, G.; Leonard, F.; Knol, A.C. Hidradenitis suppurativa: The role of deficient cutaneous innate immunity. Arch. Dermatol. 2012, 148, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xiao, X.; He, Y.; Zhang, X.; Li, C.; Mao, Q.; Wu, X.; Wang, B. Increased serum interleukin-6 levels in patients with hidradenitis suppurativa. Postepy Dermatol. Alergol. 2017, 34, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, A.; Samstein, R.M.; Treuting, P.; Liang, Y.; Pils, M.C.; Heinrich, J.M.; Jack, R.S.; Wunderlich, F.T.; Bruning, J.C.; Muller, W.; et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 2011, 34, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Fukaya, T.; Fukui, T.; Uto, T.; Takagi, H.; Nasu, J.; Miyanaga, N.; Arimura, K.; Nakamura, T.; Koseki, H.; Choijookhuu, N.; et al. Pivotal Role of IL-22 Binding Protein in the Epithelial Autoregulation of Interleukin-22 Signaling in the Control of Skin Inflammation. Front. Immunol. 2018, 9, 1418. [Google Scholar] [CrossRef]
- Sabat, R.; Ouyang, W.; Wolk, K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat. Rev. Drug Discov. 2014, 13, 21–38. [Google Scholar] [CrossRef]
- Jones, D.; Banerjee, A.; Berger, P.Z.; Gross, A.; McNish, S.; Amdur, R.; Shanmugam, V.K. Inherent differences in keratinocyte function in hidradenitis suppurativa: Evidence for the role of IL-22 in disease pathogenesis. Immunol. Investig. 2018, 47, 57–70. [Google Scholar] [CrossRef]
- Jimenez-Gallo, D.; de la Varga-Martinez, R.; Ossorio-Garcia, L.; Albarran-Planelles, C.; Rodriguez, C.; Linares-Barrios, M. The Clinical Significance of Increased Serum Proinflammatory Cytokines, C-Reactive Protein, and Erythrocyte Sedimentation Rate in Patients with Hidradenitis Suppurativa. Mediat. Inflamm. 2017, 2017, 2450401. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Wenzel, J.; Tsaousi, A.; Witte-Handel, E.; Babel, N.; Zelenak, C.; Volk, H.D.; Sterry, W.; Schneider-Burrus, S.; Sabat, R. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br. J. Dermatol. 2017, 177, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Sobell, J.M.; Zouboulis, C.C.; Gu, Y.; Williams, D.A.; Sundaram, M.; Teixeira, H.D.; Jemec, G.B. HiSCR (Hidradenitis Suppurativa Clinical Response): A novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.K.; Dellavalle, R.P.; Bigby, M.; Callen, J.P. Systematic reviews: Grading recommendations and evidence quality. Arch. Dermatol. 2008, 144, 97–99. [Google Scholar] [CrossRef]
- Gupta, A.K.; Studholme, C. Adalimumab (Humira) for the Treatment of Hidradenitis Suppurativa. Skin Therapy Lett. 2016, 21, 1–4. [Google Scholar]
- Kimball, A.B.; Okun, M.M.; Williams, D.A.; Gottlieb, A.B.; Papp, K.A.; Zouboulis, C.C.; Armstrong, A.W.; Kerdel, F.; Gold, M.H.; Forman, S.B.; et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N. Engl. J. Med. 2016, 375, 422–434. [Google Scholar] [CrossRef]
- Menter, A.; Tyring, S.K.; Gordon, K.; Kimball, A.B.; Leonardi, C.L.; Langley, R.G.; Strober, B.E.; Kaul, M.; Gu, Y.; Okun, M.; et al. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J. Am. Acad. Dermatol. 2008, 58, 106–115. [Google Scholar] [CrossRef]
- Knight, D.M.; Trinh, H.; Le, J.; Siegel, S.; Shealy, D.; McDonough, M.; Scallon, B.; Moore, M.A.; Vilcek, J.; Daddona, P.; et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol. Immunol. 1993, 30, 1443–1453. [Google Scholar] [CrossRef]
- Ghias, M.H.; Johnston, A.D.; Kutner, A.J.; Micheletti, R.G.; Hosgood, H.D.; Cohen, S.R. High-dose, high-frequency infliximab: A novel treatment paradigm for hidradenitis suppurativa. J. Am. Acad. Dermatol. 2020, 82, 1094–1101. [Google Scholar] [CrossRef]
- Grant, A.; Gonzalez, T.; Montgomery, M.O.; Cardenas, V.; Kerdel, F.A. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: A randomized, double-blind, placebo-controlled crossover trial. J. Am. Acad. Dermatol. 2010, 62, 205–217. [Google Scholar] [CrossRef]
- Orenstein, L.A.V.; Nguyen, T.V.; Damiani, G.; Sayed, C.; Jemec, G.B.E.; Hamzavi, I. Medical and Surgical Management of Hidradenitis Suppurativa: A Review of International Treatment Guidelines and Implementation in General Dermatology Practice. Dermatology 2020, 236, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Moreland, L.W.; Baumgartner, S.W.; Schiff, M.H.; Tindall, E.A.; Fleischmann, R.M.; Weaver, A.L.; Ettlinger, R.E.; Cohen, S.; Koopman, W.J.; Mohler, K.; et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N. Engl. J. Med. 1997, 337, 141–147. [Google Scholar] [CrossRef]
- Pelekanou, A.; Kanni, T.; Savva, A.; Mouktaroudi, M.; Raftogiannis, M.; Kotsaki, A.; Giamarellos-Bourboulis, E.J. Long-term efficacy of etanercept in hidradenitis suppurativa: Results from an open-label phase II prospective trial. Exp. Dermatol. 2010, 19, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.A.; Dommasch, E.; Treat, J.; Sciacca-Kirby, J.; Chachkin, S.; Williams, J.; Shin, D.B.; Leyden, J.J.; Vittorio, C.; Gelfand, J.M. A prospective clinical trial of open-label etanercept for the treatment of hidradenitis suppurativa. J. Am. Acad. Dermatol. 2009, 60, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Tzanetakou, V.; Kanni, T.; Giatrakou, S.; Katoulis, A.; Papadavid, E.; Netea, M.G.; Dinarello, C.A.; van der Meer, J.W.M.; Rigopoulos, D.; Giamarellos-Bourboulis, E.J. Safety and Efficacy of Anakinra in Severe Hidradenitis Suppurativa: A Randomized Clinical Trial. JAMA Dermatol. 2016, 152, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.; Marescassier, H.; Gabay, C.; Pittet, B.; Laffitte, E. Long-term therapy with anakinra in hidradenitis suppurativa in three patients. Int. J. Dermatol. 2019, 58, e208–e209. [Google Scholar] [CrossRef]
- Leslie, K.S.; Tripathi, S.V.; Nguyen, T.V.; Pauli, M.; Rosenblum, M.D. An open-label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J. Am. Acad. Dermatol. 2014, 70, 243–251. [Google Scholar] [CrossRef]
- Russo, V.; Alikhan, A. Failure of Anakinra in a Case of Severe Hidradenitis Suppurativa. J. Drugs Dermatol. 2016, 15, 772–774. [Google Scholar]
- Gottlieb, A.; Natsis, N.E.; Kerdel, F.; Forman, S.; Gonzalez, E.; Jimenez, G.; Hernandez, L.; Kaffenberger, J.; Guido, G.; Lucas, K.; et al. A Phase II Open-Label Study of Bermekimab in Patients with Hidradenitis Suppurativa Shows Resolution of Inflammatory Lesions and Pain. J. Investig. Dermatol. 2020, 140, 1538–1545 e2. [Google Scholar] [CrossRef]
- Calverley, P.M.A.; Sethi, S.; Dawson, M.; Ward, C.K.; Finch, D.K.; Penney, M.; Newbold, P.; van der Merwe, R. A randomised, placebo-controlled trial of anti-interleukin-1 receptor 1 monoclonal antibody MEDI8968 in chronic obstructive pulmonary disease. Respir. Res. 2017, 18, 153. [Google Scholar] [CrossRef]
- Houriet, C.; Seyed Jafari, S.M.; Thomi, R.; Schlapbach, C.; Borradori, L.; Yawalkar, N.; Hunger, R.E. Canakinumab for Severe Hidradenitis Suppurativa: Preliminary Experience in 2 Cases. JAMA Dermatol. 2017, 153, 1195–1197. [Google Scholar] [CrossRef] [PubMed]
- Canakinumab. Drugs and Lactation Database (LactMed); Canakinumab: Bethesda, MD, USA, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK500580/ (accessed on 6 September 2020).
- Lim, S.Y.D.; Oon, H.H. Systematic review of immunomodulatory therapies for hidradenitis suppurativa. Biologics 2019, 13, 53–78. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.Z.; Ro, T.; Jolly, P.; Sayed, C.J. Non-response to Interleukin-1 Antagonist Canakinumab in Two Patients with Refractory Pyoderma Gangrenosum and Hidradenitis Suppurativa. J. Clin. Aesthet. Dermatol. 2017, 10, 36–38. [Google Scholar] [PubMed]
- Schuch, A.; Fischer, T.; Boehner, A.; Biedermann, T.; Volz, T. Successful Treatment of Severe Recalcitrant Hidradenitis Suppurativa with the Interleukin-17A Antibody Secukinumab. Acta Derm. Venereol. 2018, 98, 151–152. [Google Scholar] [CrossRef]
- Thorlacius, L.; Theut Riis, P.; Jemec, G.B.E. Severe hidradenitis suppurativa responding to treatment with secukinumab: A case report. Br. J. Dermatol. 2018, 179, 182–185. [Google Scholar] [CrossRef]
- Foulkes, A.C.; Warren, R.B. Brodalumab in psoriasis: Evidence to date and clinical potential. Drugs Context 2019, 8, 212570. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef]
- Bai, F.; Li, G.G.; Liu, Q.; Niu, X.; Li, R.; Ma, H. Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Immunol. Res. 2019, 2019, 2546161. [Google Scholar]
- Kovacs, M.; Podda, M. Guselkumab in the treatment of severe hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e140–e141. [Google Scholar] [CrossRef]
- Takeda, K.; Kikuchi, K.; Kanazawa, Y.; Yamasaki, K.; Aiba, S. Ustekinumab treatment for hidradenitis suppurativa. J. Dermatol. 2019, 46, 1215–1218. [Google Scholar] [CrossRef]
- Blok, J.L.; Li, K.; Brodmerkel, C.; Horvatovich, P.; Jonkman, M.F.; Horvath, B. Ustekinumab in hidradenitis suppurativa: Clinical results and a search for potential biomarkers in serum. Br. J. Dermatol. 2016, 174, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Pozo-Roman, T.; Sanchez-Velicia, L.; Vega-Gutierrez, J.; Arias-Santiago, S.; Molina-Leyva, A. Ustekinumab in the treatment of patients with hidradenitis suppurativa: Multicenter case series and systematic review. J. Dermatol. Treat. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Ubina, S.; Jimenez-Gallo, D.; Villegas-Romero, I.; Rodriguez-Mateos, M.E.; Linares-Barrios, M. Effectiveness of ustekinumab for moderate-to-severe hidradenitis suppurativa: A case series. J. Dermatol. Treat. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Serra Lopez-Matencio, J.M.; Morell Baladron, A.; Castaneda, S. JAK-STAT inhibitors for the treatment of immunomediated diseases. Med. Clin. 2019, 152, 353–360. [Google Scholar] [CrossRef]
- Savage, K.T.; Santillan, M.R.; Flood, K.S.; Charrow, A.; Porter, M.L.; Kimball, A.B. Tofacitinib shows benefit in conjunction with other therapies in recalcitrant hidradenitis suppurativa patients. JAAD Case Rep. 2020, 6, 99–102. [Google Scholar] [CrossRef]
- Genovese, M.C.; Smolen, J.S.; Weinblatt, M.E.; Burmester, G.R.; Meerwein, S.; Camp, H.S.; Wang, L.; Othman, A.A.; Khan, N.; Pangan, A.L.; et al. Efficacy and Safety of ABT-494, a Selective JAK-1 Inhibitor, in a Phase IIb Study in Patients With Rheumatoid Arthritis and an Inadequate Response to Methotrexate. Arthritis Rheumatol. 2016, 68, 2857–2866. [Google Scholar] [CrossRef]
- Bianchi, L.; Del Duca, E.; Romanelli, M.; Saraceno, R.; Chimenti, S.; Chiricozzi, A. Pharmacodynamic assessment of apremilast for the treatment of moderate-to-severe plaque psoriasis. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1121–1128. [Google Scholar] [CrossRef]
- Dattola, A.; Del Duca, E.; Saraceno, R.; Gramiccia, T.; Bianchi, L. Safety evaluation of apremilast for the treatment of psoriasis. Expert Opin. Drug Saf. 2017, 16, 381–385. [Google Scholar] [CrossRef]
- Maloney, N.J.; Zhao, J.; Tegtmeyer, K.; Lee, E.Y.; Cheng, K. Off-label studies on apremilast in dermatology: A review. J. Dermatol. Treat. 2020, 31, 131–140. [Google Scholar] [CrossRef]
- Vossen, A.; van Doorn, M.B.A.; van der Zee, H.H.; Prens, E.P. Apremilast for moderate hidradenitis suppurativa: Results of a randomized controlled trial. J. Am. Acad. Dermatol. 2019, 80, 80–88. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Argyropoulou, M.; Kanni, T.; Spyridopoulos, T.; Otto, I.; Zenker, O.; Guo, R.; Riedemann, N.C. Clinical efficacy of complement C5a inhibition by IFX-1 in hidradenitis suppurativa: An open-label single-arm trial in patients not eligible for adalimumab. Br. J. Dermatol. 2020, 183, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Espie, P.; He, Y.; Koo, P.; Sickert, D.; Dupuy, C.; Chokote, E.; Schuler, R.; Mergentaler, H.; Ristov, J.; Milojevic, J.; et al. First-in-human clinical trial to assess pharmacokinetics, pharmacodynamics, safety, and tolerability of iscalimab, an anti-CD40 monoclonal antibody. Am. J. Transpl. 2020, 20, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J.; Stan, M.N.; Frommer, L.; Gergely, P.; Colin, L.; Amer, A.; Schuhmann, I.; Espie, P.; Rush, J.S.; Basson, C.; et al. A Novel Anti-CD40 Monoclonal Antibody, Iscalimab, for Control of Graves Hyperthyroidism-A Proof-of-Concept Trial. J. Clin. Endocrinol. Metab. 2020, 105, 696–704. [Google Scholar] [CrossRef]
- Mavragani, C.P.; Moutsopoulos, H.M. Sjogren’s syndrome: Old and new therapeutic targets. J. Autoimmun. 2020, 110, 102364. [Google Scholar] [CrossRef] [PubMed]
- Marasca, C.; Tranchini, P.; Marino, V.; Annunziata, M.C.; Napolitano, M.; Fattore, D.; Fabbrocini, G. The pharmacology of antibiotic therapy in hidradenitis suppurativa. Expert Rev. Clin. Pharmacol. 2020, 13, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Hunger, R.E.; Surovy, A.M.; Hassan, A.S.; Braathen, L.R.; Yawalkar, N. Toll-like receptor 2 is highly expressed in lesions of acne inversa and colocalizes with C-type lectin receptor. Br. J. Dermatol. 2008, 158, 691–697. [Google Scholar] [CrossRef]
- Hessam, S.; Sand, M.; Georgas, D.; Anders, A.; Bechara, F.G. Microbial Profile and Antimicrobial Susceptibility of Bacteria Found in Inflammatory Hidradenitis Suppurativa Lesions. Skin Pharmacol. Physiol. 2016, 29, 161–167. [Google Scholar] [CrossRef]
- Bettoli, V.; Manfredini, M.; Massoli, L.; Carillo, C.; Barozzi, A.; Amendolagine, G.; Ruina, G.; Musmeci, D.; Libanore, M.; Curtolo, A.; et al. Rates of antibiotic resistance/sensitivity in bacterial cultures of hidradenitis suppurativa patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 930–936. [Google Scholar] [CrossRef]
- Benzecry, V.; Grancini, A.; Guanziroli, E.; Nazzaro, G.; Barbareschi, M.; Marzano, A.V.; Muratori, S.; Veraldi, S. Hidradenitis suppurativa/acne inversa: A prospective bacteriological study of 46 patients and review of the literature. G. Ital. Dermatol. Venereol. 2018, 155, 459–463. [Google Scholar]
- Sartorius, K.; Killasli, H.; Oprica, C.; Sullivan, A.; Lapins, J. Bacteriology of hidradenitis suppurativa exacerbations and deep tissue cultures obtained during carbon dioxide laser treatment. Br. J. Dermatol. 2012, 166, 879–883. [Google Scholar] [CrossRef]
- Ring, H.C.; Riis Mikkelsen, P.; Miller, I.M.; Jenssen, H.; Fuursted, K.; Saunte, D.M.; Jemec, G.B. The bacteriology of hidradenitis suppurativa: A systematic review. Exp. Dermatol. 2015, 24, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Nikolakis, G.; Liakou, A.I.; Bonovas, S.; Seltmann, H.; Bonitsis, N.; Join-Lambert, O.; Wild, T.; Karagiannidis, I.; Zolke-Fischer, S.; Langner, K.; et al. Bacterial Colonization in Hidradenitis Suppurativa/Acne Inversa: A Cross-sectional Study of 50 Patients and Review of the Literature. Acta Derm. Venereol. 2017, 97, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Ring, H.C.; Thorsen, J.; Jorgensen, A.H.; Bay, L.; Bjarnsholt, T.; Fuursted, K.; Thomsen, S.F.; Jemec, G.B. Predictive Metagenomic Analysis Reveals a Role of Cutaneous Dysbiosis in the Development of Hidradenitis Suppurativa. J. Investig. Dermatol. 2020, 140, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.M.; Cook, L.C.; Zhan, X.; Banerjee, K.; Cong, Z.; Imamura-Kawasawa, Y.; Gettle, S.L.; Longenecker, A.L.; Kirby, J.S.; Nelson, A.M. Loss of Skin Microbial Diversity and Alteration of Bacterial Metabolic Function in Hidradenitis Suppurativa. J. Investig. Dermatol. 2020, 140, 716–720. [Google Scholar] [CrossRef]
- Ring, H.C.; Thorsen, J.; Saunte, D.M.; Lilje, B.; Bay, L.; Riis, P.T.; Larsen, N.; Andersen, L.O.; Nielsen, H.V.; Miller, I.M.; et al. The Follicular Skin Microbiome in Patients With Hidradenitis Suppurativa and Healthy Controls. JAMA Dermatol. 2017, 153, 897–905. [Google Scholar] [CrossRef]
- Naik, H.B.; Jo, J.H.; Paul, M.; Kong, H.H. Skin Microbiota Perturbations Are Distinct and Disease Severity-Dependent in Hidradenitis Suppurativa. J. Investig. Dermatol. 2020, 140, 922–925.e3. [Google Scholar] [CrossRef]
- Guet-Revillet, H.; Coignard-Biehler, H.; Jais, J.P.; Quesne, G.; Frapy, E.; Poiree, S.; Le Guern, A.S.; Le Fleche-Mateos, A.; Hovnanian, A.; Consigny, P.H.; et al. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg. Infect. Dis. 2014, 20, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Ring, H.C.; Sigsgaard, V.; Thorsen, J.; Fuursted, K.; Fabricius, S.; Saunte, D.M.; Jemec, G.B. The microbiome of tunnels in hidradenitis suppurativa patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1775–1780. [Google Scholar] [CrossRef]
- Naik, H.B.; Nassif, A.; Ramesh, M.S.; Schultz, G.; Piguet, V.; Alavi, A.; Lowes, M.A. Are Bacteria Infectious Pathogens in Hidradenitis Suppurativa? Debate at the Symposium for Hidradenitis Suppurativa Advances Meeting, November 2017. J. Investig. Dermatol. 2019, 139, 13–16. [Google Scholar] [CrossRef]
| NCT Clinical Trial | Intervention | Phase | Study Design | Enrollment |
|---|---|---|---|---|
| NCT03512275 [13] | Bermekimab 400 mg | Phase 2 | •Allocation: Non-Randomized | 42 |
| •Intervention Model: Single Group Assignment | ||||
| •Masking: None (Open Label) | ||||
| NCT03960268 [14] | Brodalumab | Phase 1 | •Allocation: N/A | 10 |
| •Intervention Model: Single Group Assignment | ||||
| •Masking: None (Open Label) | ||||
| NCT03607487 [15] | INCB054707 | Phase 2 | •Allocation: Randomized | 36 |
| Placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Triple | ||||
| NCT03569371 [16] | INCB054707 | Phase 2 | •Allocation: N/A | 10 |
| •Intervention Model: Single Group Assignment | ||||
| •Masking: None (Open Label) | ||||
| NCT03248531 [17] | Bimekizumab | Phase 2 | •Allocation: Randomized | 90 |
| Adalimumab | •Intervention Model: Parallel Assignment | |||
| Placebo | •Masking: Quadruple | |||
| NCT01516749 [18] | Anakinra | Phase 2 | •Allocation: N/A •Intervention Model: Single Group Assignment •Masking: None (Open Label) | 6 |
| NCT02421172 [19] | CJM112 | Phase 2 | •Allocation: Randomized | 66 |
| Placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Double (Participant, Investigator) | ||||
| NCT00795574 [20] | Infliximab | Phase 2 | •Allocation: Randomized | 38 |
| Placebo Comparator | •Intervention Model: Crossover Assignment | |||
| •Masking: Quadruple | ||||
| NCT00329823 [21] | Etanercept sc 50 mg per week for 12 weeks | Phase 2 | •Allocation: Non-Randomized | 10 |
| •Intervention Model: Single Group Assignment | ||||
| •Masking: None (Open Label) | ||||
| NCT03628924 [22] | Guselkumab dose 1 | Phase 2 | •Allocation: Randomized | 184 |
| Guselkumab dose 2 | •Intervention Model: Parallel Assignment | |||
| Guselkumab dose 3 | •Masking: Double (Participant, Investigator) | |||
| NCT03001622 [23] | IFX-1 | Phase 2 | •Allocation: N/A | 12 |
| •Intervention Model: Single Group Assignment | ||||
| •Masking: None (Open Label) | ||||
| NCT03049267 [24] | Apremilast | Phase 2 | •Allocation: Randomized | 20 |
| Placebo Oral Tablet | •Intervention Model: Parallel Assignment | |||
| •Masking: Double (Participant, Investigator) | ||||
| NCT03099980 [25] | Secukinumab | Phase 1 | •Allocation: N/A •Intervention Model: Single Group Assignment •Masking: None (Open Label) | 20 |
| NCT00107991 [26] | Etanercept | Phase 2 | •Allocation: N/A | 15 |
| •Intervention Model: Single Group Assignment | ||||
| •Masking: None (Open Label) | ||||
| NCT02904902 [27] | Adalimumab | Phase 3 | •Allocation: N/A | 15 |
| •Intervention Model: Single Group Assignment | ||||
| •Masking: None (Open Label) | ||||
| NCT02643654 [28] | MABp1 | Phase 2 | •Allocation: Randomized | 20 |
| Placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Quadruple | ||||
| NCT02695212 [29] | Apremilast | Phase 2 | •Allocation: N/A •Intervention Model: Single Group Assignment •Masking: None (Open Label) | 20 |
| NCT01704534 [30] | Ustekinumab | Phase 2 | •Allocation: N/A | 20 |
| •Intervention Model: Single Group Assignment | ||||
| •Masking: None (Open Label) | ||||
| NCT03487276 [31] | IFX-1 | Phase 2 | •Allocation: Randomized | 179 |
| Placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Quadruple | ||||
| NCT01558375 [32] | Anakinra | Phase 2 | •Allocation: Randomized | 20 |
| Water for injection | •Intervention Model: Parallel Assignment | |||
| •Masking: Quadruple | ||||
| NCT01635764 [33] | Adalimumab | Phase 3 | •Allocation: N/A •Intervention Model: Single Group Assignment •Masking: None (Open Label) | 508 |
| NCT02808975 [34] | Adalimumab | Phase 4 | •Allocation: Randomized | 206 |
| Placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Quadruple | ||||
| NCT00918255 [35] | Adalimumab | Phase 2 | •Allocation: Randomized | 154 |
| Placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Quadruple | ||||
| NCT01468207 [36] | Adalimumab | Phase 3 | •Allocation: Randomized | 307 |
| placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Double (Participant, Investigator) | ||||
| NCT01468233 [37] | Adalimumab | Phase 3 | •Allocation: Randomized | 326 |
| placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Double (Participant, Investigator) | ||||
| NCT00827996 [38] | Adalimumab | Phase 2 | •Allocation: N/A | 10 |
| •Intervention Model: Single Group Assignment | ||||
| •Masking: None (Open Label) | ||||
| NCT04018599 [39] | 40 mg MSB11022 | Phase 1 | •Allocation: Randomized | 216 |
| •Intervention Model: Parallel Assignment | ||||
| •Masking: None (Open Label) |
| NCT Clinical Trial | Intervention | Phase | Study Design | Enrollment |
|---|---|---|---|---|
| NCT03512275 [13] | CFZ533 | Phase 2 | •Allocation: Randomized | 90 |
| LY006 | •Intervention Model: Parallel Assignment | |||
| Placebo | •Masking: Quadruple | |||
| NCT03926169 [40] | Risankizumab | Phase 2 | •Allocation: Randomized | 220 |
| Placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Quadruple | ||||
| NCT04430855 [41] | Upadacitinib | Phase 2 | •Allocation: Randomized | 60 |
| Placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Quadruple | ||||
| NCT04242498 [42] | Bimekizumab | Phase 3 | •Allocation: Randomized | 460 |
| Placebo | •Intervention Model: Parallel Assignment | |||
| •Masking: Quadruple | ||||
| NCT04179175 [43] | Secukinumab | Phase 3 | •Allocation: Randomized | 745 |
| •Intervention Model: Parallel Assignment | ||||
| •Masking: Triple | ||||
| NCT03713632 [44] | Secukinumab Placebo | Phase 3 | •Allocation: N/A •Intervention Model: Parallel Assignment •Masking: Triple | 471 |
| NCT04092452 [45] | PF-06650833, Placebo | Phase 2 | •Allocation: Randomized | 192 |
| PF-06700841 | •Intervention Model: Parallel Assignment | |||
| PF-06826647 | •Masking: Triple | |||
| NCT04246372 [46] | Tofacitinib | Phase 2 | •Allocation: N/A | 46 |
| •Intervention Model: Single Group Assignment | ||||
| •Masking: None (Open Label) |
| Cytokines | Drugs | Quality of Evidence |
|---|---|---|
| anti-TNF-α | Adalimumab [33,34,35,36,37,38] | A |
| Infliximab [20] | B | |
| Etanercept [26] | B | |
| anti-IL-1 | Anakinra [18] | B |
| MEDI8968 | Ongoing Trial | |
| Canakinumab | C | |
| Bermekimab [13] | B | |
| anti-IL-12/23 | Ustekinumab [30] | Ongoing Trial |
| anti-IL-23 | Guselkumab [22] | Ongoing Trial |
| Risankizumab [40] | Ongoing Trial | |
| anti-IL-17 | Secukinumab [43,44] | Ongoing Trial |
| CJM112 [19] | Ongoing Trial | |
| Bimekizumab [42] | Ongoing Trial | |
| Brodalumab [14,16] | Ongoing Trial | |
| anti-PDE-4 | Apremilast [24] | B |
| anti-C5a | IFX-1 [31] | Ongoing Trial |
| anti-CD20 | Rituximab | C |
| anti-CD40 | Iscalimab [13] | Ongoing Trial |
| anti-JAK | Upadacitinib [41] | Ongoing Trial |
| INCB054707 [15,16] | Ongoing Trial | |
| Tofacitinib [46] | Ongoing Trial |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Duca, E.; Morelli, P.; Bennardo, L.; Di Raimondo, C.; Nisticò, S.P. Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa. Int. J. Mol. Sci. 2020, 21, 8436. https://doi.org/10.3390/ijms21228436
Del Duca E, Morelli P, Bennardo L, Di Raimondo C, Nisticò SP. Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa. International Journal of Molecular Sciences. 2020; 21(22):8436. https://doi.org/10.3390/ijms21228436
Chicago/Turabian StyleDel Duca, Ester, Paola Morelli, Luigi Bennardo, Cosimo Di Raimondo, and Steven Paul Nisticò. 2020. "Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa" International Journal of Molecular Sciences 21, no. 22: 8436. https://doi.org/10.3390/ijms21228436
APA StyleDel Duca, E., Morelli, P., Bennardo, L., Di Raimondo, C., & Nisticò, S. P. (2020). Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa. International Journal of Molecular Sciences, 21(22), 8436. https://doi.org/10.3390/ijms21228436






