Abstract
Wound healing is an important process in the human body to protect against external threats. A dysregulation at any stage of the wound healing process may result in the development of various intractable ulcers or excessive scar formation. Numerous factors such as growth factors, cytokines, and chemokines are involved in this process and play vital roles in tissue repair. Moreover, recent studies have demonstrated that lipid mediators derived from membrane fatty acids are also involved in the process of wound healing. Among these lipid mediators, we focus on eicosanoids such as prostaglandins, thromboxane, leukotrienes, and specialized pro-resolving mediators, which are produced during wound healing processes and play versatile roles in the process. This review article highlights the roles of eicosanoids on skin wound healing, especially focusing on the biosynthetic pathways and biological functions, i.e., inflammation, proliferation, migration, angiogenesis, remodeling, and scarring.
1. Introduction
Skin is the largest organ in the entire body, which acts as a physical, chemical, biological, radiological, and thermal barrier system and prevents dehydration from the body [1,2]. Wound healing is an important but complicated process in the body that helps protect against external threats [3]. This process involves three consecutive and overlapping steps, including hemostasis/inflammatory, proliferative, and remodeling phases [4]. An impairment of this process leads to the formation of various intractable ulcers such as diabetic ulcers and pressure ulcers [5]. Conversely, excessive wound healing results in the formation of hypertrophic scars or keloids [6,7,8]. Complete integral wound healing requires the cooperative interactions of numerous factors and multiple cell types. Studies have documented that growth factors, cytokines, and chemokines play vital roles in wound healing processes [9,10,11]. Moreover, recent studies have demonstrated that various lipid mediators such as lysophosphatidic acid, sphingosine-1-phosphate, and eicosanoids are also key players in this process [12,13,14,15,16].
Eicosanoids are one of the most important lipid mediators that are rapidly produced upon cell activation by enzymatic conversion of polyunsaturated fatty acids (PUFAs) with 20 carbon atoms, which are derived from membrane phospholipids by the hydrolytic activity of phospholipase A2 (PLA2), particularly cytosolic PLA2α [17,18]. Among eicosanoids, metabolites derived from omega-6 PUFA arachidonic acid (AA) have been well described in the context of the homeostasis and immune system [19,20]. AA can be metabolized into prostaglandins (PGs) and thromboxane A2 (TxA2) by the cyclooxygenase (COX) pathway [21,22]; leukotrienes (LTs), hydroxyeicosatetraenoic acids (HETEs), and lipoxins (LXs) by the lipoxygenase (LOX) pathway [23,24]; or epoxyeicosatrienoic acids (EETs) by the cytochrome P450 (CYP) pathway [25,26]. These metabolites are produced in a time-dependent manner after injury and can act as either a positive or negative regulator of wound healing. Furthermore, recent studies have reported on biological activities that promote wound healing by specialized pro-resolving mediators (SPMs), such as resolvin series derived from the omega-3 PUFA eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) [27,28].
This review is intended to summarize the biosynthetic pathways and functions of eicosanoids and SPMs in skin wound healing. We also discuss the therapeutic potential of targeting eicosanoid and SPM signaling as a treatment approach for wound healing. A comprehensive understanding of complex eicosanoid interactions would help provide novel insights into the development of novel and precise therapeutic approaches for patients with impaired wound healing.
2. Biosynthetic Pathway of Eicosanoids and SPMs
PLA2s hydrolyze the sn-2 position of phospholipids, which are the major components of cell membranes, and liberate free fatty acids and lysophospholipids [29]. In response to various stimuli, including tissue damage, PUFAs, including AA, EPA, and DHA, are released from membrane phospholipids and can be metabolized into various bioactive lipid mediators. Several enzyme families such as COXs, LOXs, and CYPs are implied in the conversion of PUFAs (Figure 1).
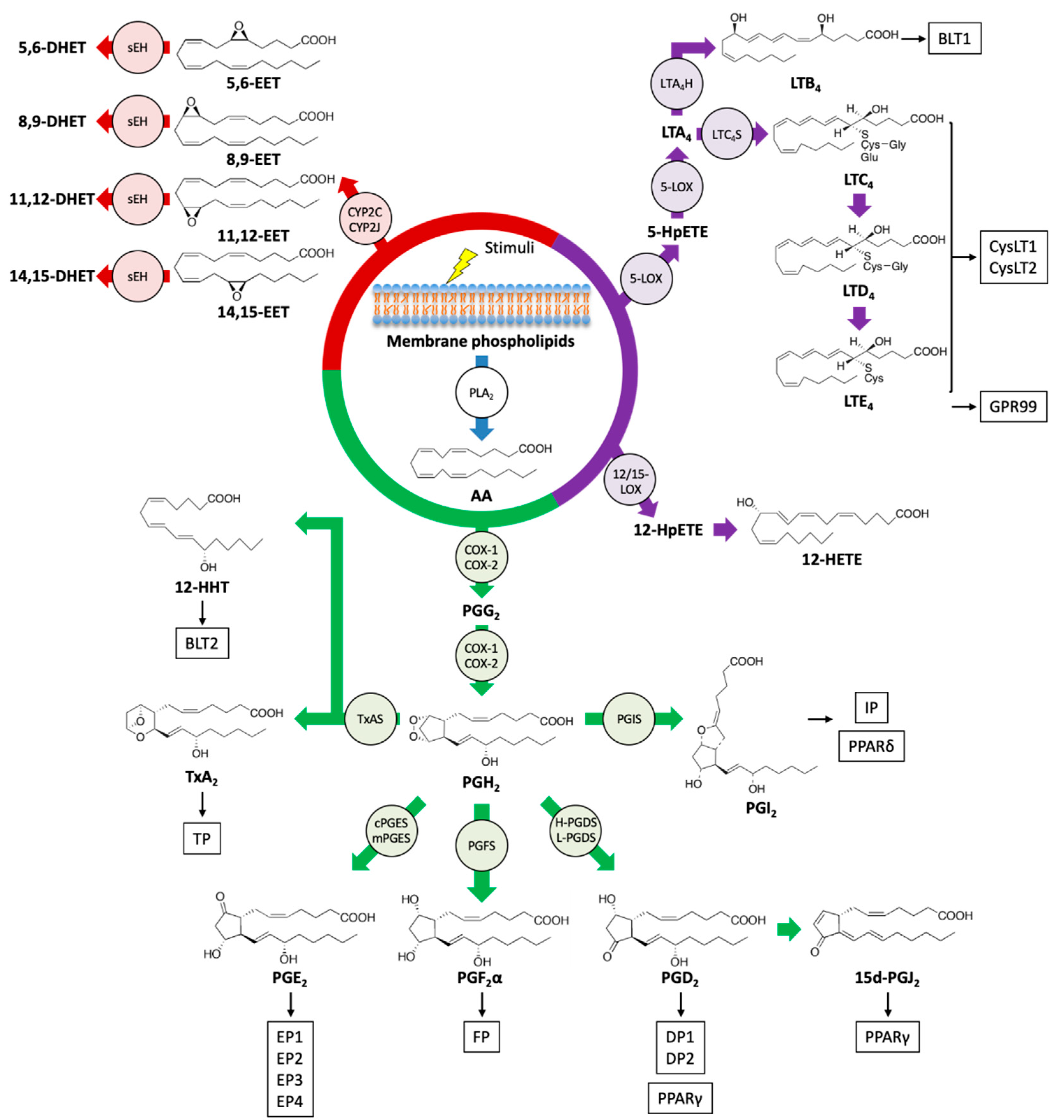
Figure 1.
Biosynthetic pathways and receptors of eicosanoids. Upon stimulation, an omega-6 PUFA arachidonic acid (AA) is liberated from the phospholipids of the cell membrane by the action of phospholipase A2 (PLA2), and AA is metabolized into various bioactive lipid mediators. Prostanoids, leukotrienes (LTs), hydroxyeicosatetraenoic acid (HETEs), and epoxyeicosatrienoic acid (EETs) are formed from AA via cyclooxygenase (COX) (green), a lipoxygenase (LOX) (purple), and cytochrome P450 (CYP) (red) pathways, respectively. Specific eicosanoid receptors and peroxisome proliferator-activated receptors (PPARs) that are potentially activated by eicosanoids are shown in boxes. sEH: soluble epoxide hydrolase; TxAS: thromboxane A synthase; PGIS: prostacyclin synthase; PGFS: prostaglandin F synthase; cPGES: cytosolic prostaglandin E synthase; mPGES: membrane-associated prostaglandin E synthase; H-PGDS: hematopoietic-type prostaglandin D synthase; L-PGDS: lipocalin-type prostaglandin D synthase; LTA4H: LTA4 hydrolase; LTC4S: LTC4 synthase; HpETE: hydroperoxyeicosatetraenoic acid.
Prostanoids are formed by the majority of cells in our bodies through the action of COX enzymes and participate in a variety of physiological and pathological processes [30]. The family of bioactive prostanoids includes PGs (PGD2, PGE2, PGF2α), prostacyclin (PGI2), and TxA2 [31]. COX-1 is constitutively expressed in almost all cell types, whereas COX-2 is inducible by several stimuli, including pro-inflammatory and mitogenic signals, thus implying its role in inflammation and cell proliferation [32]. Through the action of COX enzymes, AA is oxygenated into a hydroperoxy endoperoxide PGG2 and subsequently reduced to a hydroxy endoperoxide PGH2. The synthetic activity of COXs can be inhibited by nonsteroidal anti-inflammatory drugs (NSAIDs), especially celecoxib, a COX-2-selective inhibitor with reduced side effects [33]. In the skin, COX-2 is induced by various stimuli such as injury, mechanical scratching, ultraviolet B irradiation, and cancer [34,35,36,37]. AA is converted into PGH2 by COXs and then into prostanoids by the activity of a range of terminal synthases [38].
The products generated by the LOX pathway are important regulators of innate immunity and inflammation, which can promote both pro-inflammatory and anti-inflammatory responses [39,40]. Mammals have several types of LOX isoforms [41]. AA can be oxygenated by 5-LOX to yield 5-hydroperoxyeicosatetraenoic acid (5-HpETE) and then converted into an unstable intermediate, leukotriene A4 (LTA4). LTs consist of two groups, LTB4 and peptide-conjugated cysteinyl LTs (CysLTs; LTC4, LTD4, and LTE4). LTB4 and LTC4 are produced from LTA4 by the action of LTA4 hydrolase (LTA4H) and LTC4 synthase (LTC4S), respectively, and LTC4 can be further metabolized into LTD4 and LTE4 [42]. Alternatively, HpETEs generated by LOXs can be reduced by peroxidases to the monohydroxy fatty acids HETEs. The frequent sites of oxidation of AA are 5-, 8-, 12-, and 15-carbon positions, and these reactions are catalyzed by 5-, 8-, 12-, and 15-LOX enzymes, respectively. The oxidation at another position can be catalyzed by COX and CYP enzymes [24]. Moreover, the sequential reaction of 5-LOX and 12- or 15-LOX generates LXs (LXA4 and LXB4) from AA through interactions with leukocytes and other cells such as platelets and epithelial cells. LXs are categorized as a member of SPMs and act to resolve inflammatory responses [43,44].
EETs are epoxide derivatives of AA formed through the action of CYP epoxygenases, which are abundant in endothelial cells. There are four isomers of EET, 5,6-, 8,9-, 11,12-, and 14,15-EET, that function as autocrine and paracrine mediators and are implicated in vascular relaxation, anti-inflammatory effects, and angiogenesis. Soluble epoxide hydrolase (sEH), which metabolizes EETs into inactive dihydroxyeicosatrienoic acids (DHET), attenuates several functional effects of EETs [45].
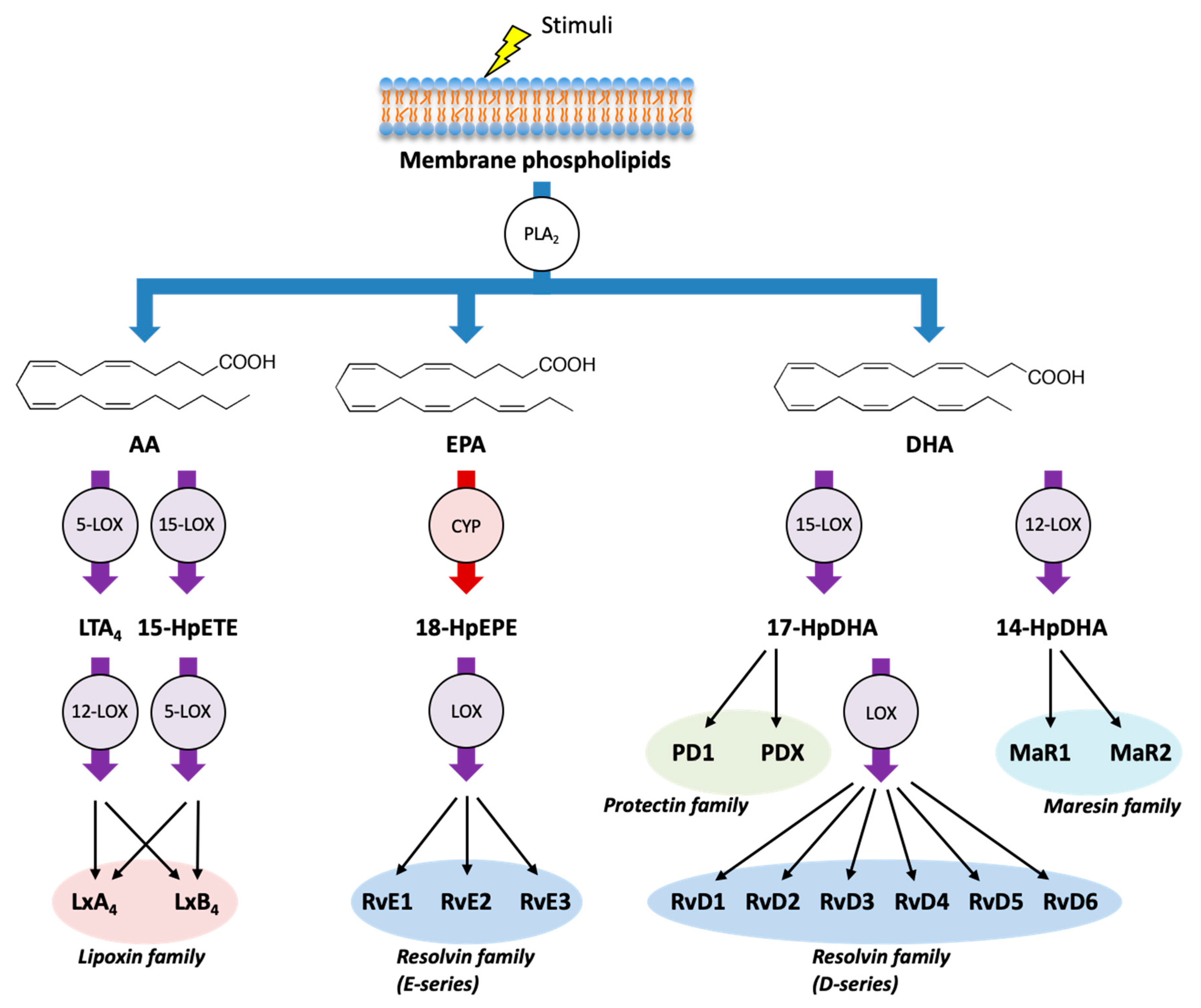
In addition to AA, LOXs and CYPs can metabolize the omega-3 PUFA EPA and DHA and can produce several classes of SPMs (Figure 2). E-series of resolvins (RvE1, RvE2, and RvE3) are formed from EPA through the combination of LOX and CYP enzymes, whereas D-series of resolvins (RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6) are formed from DHA through the combination of LOX enzymes. Moreover, DHA can be metabolized into another class of SPMs such as protectins (PD1 and PDX) and maresins (MaR1 and MaR2) [28,46].
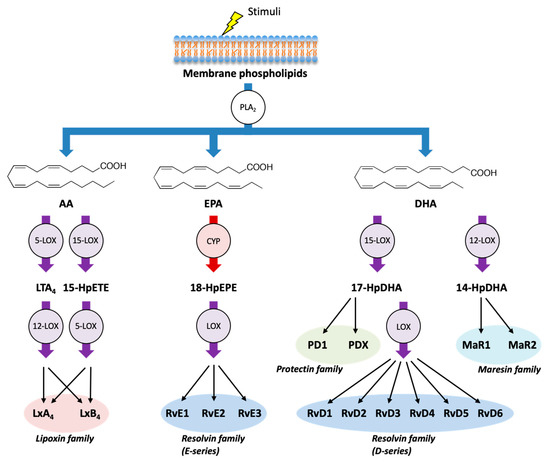
Figure 2.
Biosynthetic pathways of specialized pro-resolving mediators (SPMs).In addition to AA, LOX pathway (purple) and CYP pathway (red) can metabolize the omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) into various SPMs, which act as endogenous immunoresolvents upon stimulation. Lipoxins (LxA4 and LxB4) are formed from AA, E-series of resolvins (RvE1, RvE2, and RvE3) are formed from EPA, and D-series of resolvins (RvD1, RvD2, RvD3, RvD4, RvD5, and RvD6), protectins (PD1 and PDX), and maresins (MaR1 and MaR2) are formed from DHA. HpEPE: hydroperoxyeicosapentaenoic acid; HpDHA: hydroperoxydocosahexaenoic acid.
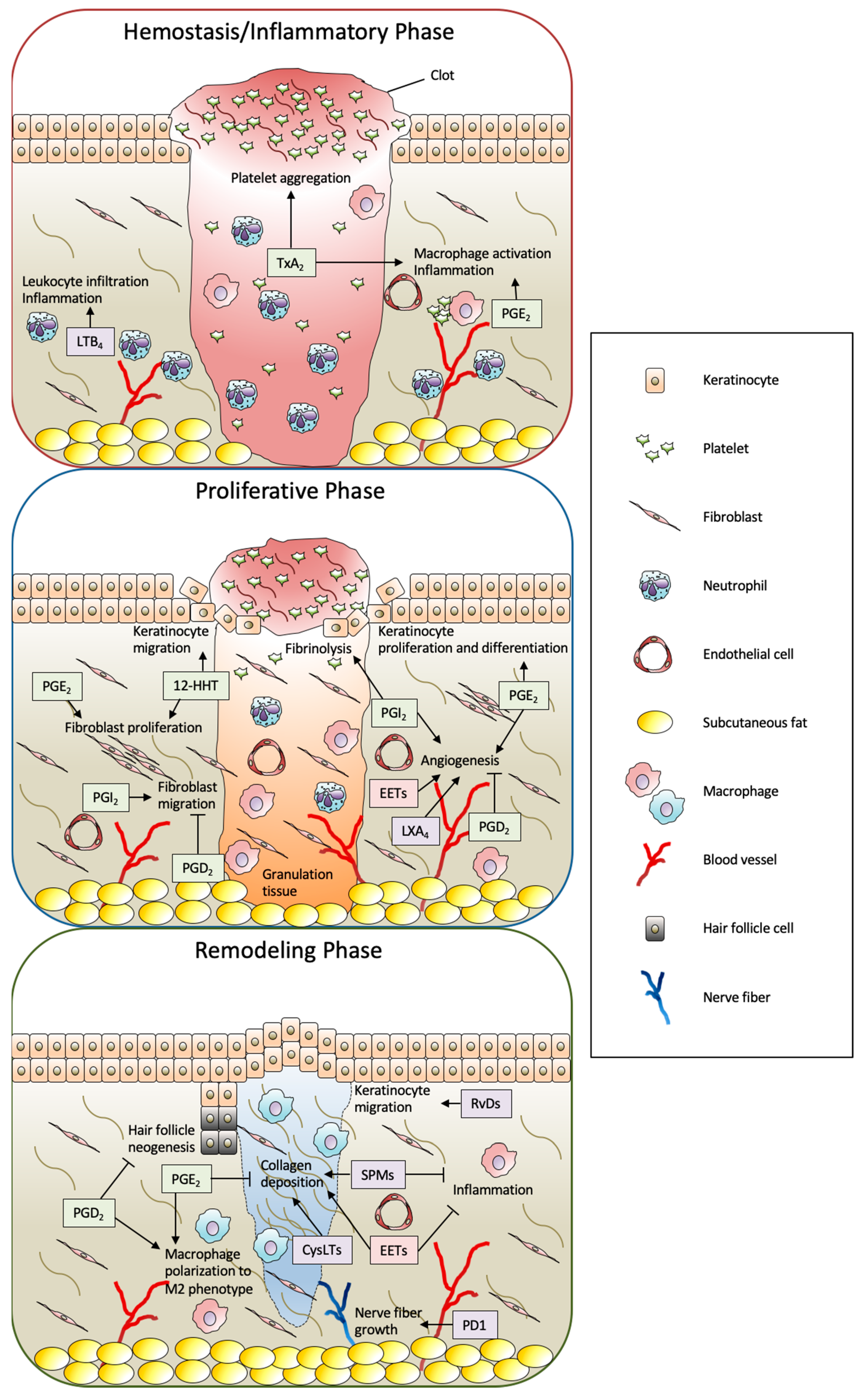
Altogether, in the context of wound healing, a wide variety of eicosanoids and SPMs are produced by the wound stimuli and play multiple roles in the wound healing process (Figure 3). The detailed functions of eicosanoids and SPMs are described in the following sections.
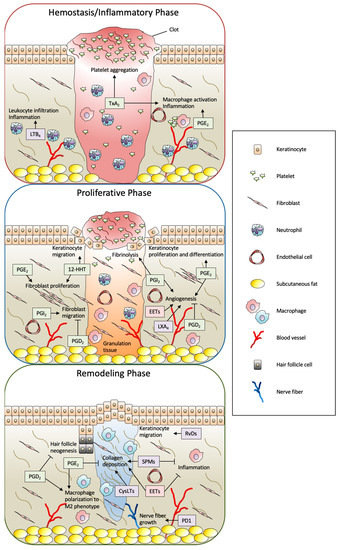
Figure 3.
Summary of biological functions of eicosanoids and SPMs in full-thickness wound healing. The process of skin wound healing consists of three consecutive and overlapping steps, including hemostasis/inflammatory, proliferative, and remodeling phases. In this process, a wide variety of eicosanoids and SPMs are produced from skin-resident cells and infiltrated cells by the wound stimuli and play multiple roles. The metabolites formed by the COX, LOX, and CYP pathways are shown as green, purple, and red boxes, respectively. PG: prostaglandin; Tx: thromboxane; LT; leukotriene; 12-HHT: 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid; EET: epoxyeicosatrienoic acid; LX: lipoxin; Rv: resolvin; PD1: protectin D1; SPM: specialized pro-resolving mediator; CysLT: cysteinyl leukotriene.
3. Functions of Eicosanoids and SPMs in Skin Wound Healing
3.1. COX Metabolites
3.1.1. TxA2
The cellular source of each eicosanoid is dependent on organs and cell types [47]. In the context of wound healing, TxA2 is abundantly produced by activated platelets through the action of TxA2 synthase (TxAS) immediately after the injury and contributes to platelet activation and irreversible platelet aggregation for hemostasis [48,49]. In fact, the lack of G protein-coupled thromboxane-prostanoid (TP) receptor in mice resulted in prolonged bleeding time and TP agonist caused no detectable aggregation of these mice platelets [50]. Administration of TP antagonist was found to significantly inhibit platelet aggregation in humans [51]. Platelet aggregation is an essential system to stop bleeding to protect our body against external threats and is involved in efficient wound healing [52,53].
In addition, TxA2 produced by activated platelets was found to induce the synthesis of the pro-inflammatory cytokine interleukin (IL)-6 and PGE2 and suppress the expression of the anti-inflammatory macrophage marker CD206 in macrophages through the activation of the TP receptor in a cutaneous inflammation mouse model [54]. Moreover, studies have demonstrated that TxA2 can be synthesized in microvascular endothelial cells under COX-2-induced condition and promotes endothelial migration and angiogenesis [55,56].
Altogether, the TxA2/TP axis might be involved in wound healing as a platelet aggregant in the hemostasis/inflammatory phase as well as a mediator of inflammation and tissue regeneration in subsequent phases.
3.1.2. 12(S)-Hydroxyheptadeca-5Z,8E,10E-Trienoic Acid (12-HHT)
TxAS catalyzes the conversion of PGH2 into 12-HHT and malondialdehyde in an equimolar ratio into TxA2. Although 12-HHT had long been considered as merely a by-product of TxA2 synthesis, recent studies have demonstrated that it is a natural endogenous ligand for the low-affinity LTB4 receptor BLT2 [57]. BLT2 is abundantly expressed in epidermal keratinocytes, and the 12-HHT/BLT2 axis promotes keratinocyte migration through the production of tumor necrosis factor α (TNFα) and matrix metalloproteinases (MMPs) and thereby accelerates skin wound closure in mice [58].
In addition, it has been reported that BLT2 agonist directly promotes keratinocyte migration and indirectly enhances fibroblast proliferation by increasing the keratinocyte production of transforming growth factor-β1 (TGF-β1) and basic fibroblast growth factor (bFGF) and thus accelerated wound closure in a rat model of streptozotocin-induced diabetes [59]. Importantly, the delay in wound healing caused by the administration of NSAIDs represented by aspirin was abrogated in BLT2-deficient mice.
These results suggest a novel mechanism underlying the aspirin-dependent delay in wound healing and provide a promising novel therapeutic potential of BLT2 agonist for the treatment of intractable ulcers [58,60,61].
3.1.3. PGE2
As mentioned earlier, COX-2 is an inducible enzyme and rapidly induced by the stimulation of injury, leading to the enhanced production of PGs, including PGE2. It has been reported that cytosolic PGE2 synthase (cPGES) can be coupled with COX-1 and participates in the maintenance of tissue homeostasis and immediate PGE2 synthesis [62]. In contrast, membrane-associated PGE2 synthases (mPGESs) are functionally coupled with COX-2 and essential components for delayed PGE2 biosynthesis, which may be associated with inflammation [63]. PGE2 is the most abundant PG in various tissues and plays versatile roles in physiological and pathological actions by activating four E-prostanoid (EP) receptors (EP1-4), which are categorized as G protein-coupled receptors (GPCRs) [64].
In the early phase of wound healing, immigrating macrophages and stromal cells produce PGE2, which induces angiogenesis and proliferation of human fibroblasts through the induction of bFGF in fibroblasts, thereby accelerating wound repair [65,66]. In fact, in diabetic ob/ob mice, the concentration of PGE2 was reduced in the cutaneous wound [67]. In association with that study, prostaglandin transporter (PGT), which mediates PG catabolism and degradation in the cytosol, is upregulated by hyperglycemia, resulting in diminished PGE2 signaling [68].
On the other hand, PGE2 and its metabolite, 13,14-dihydro-15-keto-PGE2, which are rich in wound fluid, induce the expression of oncostatin M (OSM) in wound-site macrophages through the activation of EP4. OSM is structurally and functionally related to the IL-6 family and acts as an anti-inflammatory cytokine by attenuating the expression of TNFα and IL-1β, thereby improving wound healing [69]. Moreover, studies have reported that PGE2 affects the proliferation and differentiation of keratinocytes through EP2 and EP4, which increase intracellular cAMP concentration [70,71,72,73,74].
In the late phase of wound healing, the transition of the pro-inflammatory M1 phenotype macrophage to M2 regenerating phenotype is required to achieve normal wound healing, and impaired switching of M1 to M2 phenotype could lead to the formation of a nonhealing wound [75]. PGE2 promotes M2 macrophage polarization through the cyclic AMP-responsive element binding (CREB)-mediated induction of Krupple-like factor 4 (KLF4) [76]. In fact, the topical application of PGE2 containing chitosan hydrogel was found to accelerate wound closure by ameliorating inflammation by promoting the M2 phenotypic transformation of macrophages [77].
Moreover, the PGE2/EP2 axis regulates the balance of MMPs and the tissue inhibitor of MMP (TIMP), resulting in the inhibition of the TGF-β1-induced collagen synthesis in dermal fibroblasts, thereby reducing hypertrophic scar formation [78].
3.1.4. PGD2
The synthesis of PGD2 from PGH2 is manipulated by two PGD synthase (PGDS) subtypes, hematopoietic-type (H-PGDS) and lipocalin-type (L-PGDS), which are evolutionarily different from each other [79]. Although the pro-inflammatory and anti-inflammatory functions of PGD2 are well known, its direct function in wound healing remains incompletely understood [80]. In the skin, PGD2 mitigated the pruritic behavior in an atopic dermatitis mouse model and accelerated the recovery of cutaneous barrier function after mechanical scratching through the activation of the D-prostanoid (DP) receptor DP1 [81,82,83].
Studies have well established that PGD2 and its J-ring-type metabolites, especially 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), act as a ligand for peroxisome proliferator-activated receptor γ (PPARγ) [84,85]. The activation of PPARγ in monocytes induced the differentiation into anti-inflammatory M2 macrophages, whereas the genetic deletion of PPARγ in macrophages resulted in impaired wound healing, suggesting that PGD2 and its metabolites participate in the resolution of inflammation and promotion of wound closure [86,87]. In fact, it has been demonstrated that the topical application of 15d-PGJ2 to the cutaneous wound of diabetic db/db mice enhanced the activity of PPARγ in wound-site macrophages and accelerated wound closure with reduced inflammation [88].
In contrast, the PGD2/DP1 axis diminished the migration of fibroblasts [89]. Although fibroblast migration is essential for tissue repair, excessive accumulation of fibroblasts can cause aberrant tissue reconstitution such as fibrosis or keloids, thus implying the modulating function of PGD2 in tissue regeneration.
Moreover, the PGD2/DP1 axis is known to exert suppressive functions in VEGF- or IL-1β-induced vascular leakage and angiogenesis [90]. Consistent with the suppressive effects, L-PGDS-derived PGD2 was found to inhibit hair follicle neogenesis through DP2 receptor (also known as a chemoattractant receptor homologous molecule expressed on type 2 T-helper cells; CRTH2 or GPR44) signaling during wound repair [91]. In fact, both L-PGDS gene expression and PGD2 level are significantly elevated in the bald scalp compared to those in the haired scalp in humans, and the topical application of PGD2 inhibited hair growth in both mice and humans through DP2 signaling [92].
3.1.5. PGF2α
Since several decades ago, the PGF2α/F-prostanoid receptor (FP) signal has been known to induce labor and control intraocular pressure [93,94]. Although several studies have demonstrated that PGF2α is produced in the sites of injury, atopic dermatitis, and psoriasis, the function and significance of FP in the skin are less understood [95,96,97]. Recent research has reported the hypertrichotic effect of FP agonist, thereby implying the involvement of PGF2α in tissue remodeling; however, the molecular mechanisms underlying the hair growth remain unknown [98].
3.1.6. PGI2
PGI2 (also known as prostacyclin) is primarily produced in endothelial cells by the action of prostacyclin synthase (PGIS) from PGH2 and is rapidly converted by nonenzymatic processes into an inactive metabolite, 6-keto PGF1α [99]. It is well known that PGI2 acts as a vasodilator, maintaining the appropriate blood flow to peripheral tissues [100]. Although hemostasis is an indispensable mechanism required for regular wound healing as described earlier, it is important to remove the clot for the subsequent process. In fact, stimulation of I-prostanoid receptor (IP) promotes fibrinolysis by enhancing the production of urokinase-type plasminogen activator (uPA) in fibroblasts and accelerating fibroblast migration [101].
On the other hand, interestingly, the PGI2 analogs iloprost and carbaprostacyclin, which activate both IP and PPARδ, are known to have angiogenic function through the induction of vascular endothelial growth factor (VEGF), whereas the IP-specific agonist cicaprost does not possess such function [102,103].
3.2. LOX Metabolites
3.2.1. Leukotrienes (LTB4 and CysLTs; LTC4, LTD4, and LTE4)
LTB4 binds to its receptor BLT1, and the most important function of the LTB4/BLT1 axis is its potent chemotactic effect for several immune cells [40,104]. In the initial inflammatory phase of wound healing, inflammatory cells, especially neutrophils, are recruited to numerous chemotactic factors generated from injured tissues to clean up the injured sites and initiate subsequent inflammatory reactions. However, although the inflammatory process is an important component of the wound repair process in response to tissue damage, excessive production of LTB4 has been found to cause uncontrolled neutrophil chemotaxis and insufficient bacterial clearance in the skin of diabetic mice [105]. BLT2 was initially identified as a low-affinity receptor for LTB4, but it is now considered as the receptor for 12-HHT as mentioned earlier [57,106].
CysLTs are produced primarily by activated eosinophils, basophils, mast cells, and macrophages and are involved in bronchoconstriction and allergic response. CysLT receptor (CysLT1 and CysLT2) antagonists are reported to be clinically efficacious in patients with asthma or allergic rhinitis [107]. Moreover, recent studies found GPR99 as a potential novel receptor for LTE4, which is the most stable and abundant CysLT at the site of inflammation, and the intradermal injection of LTE4 induced vascular permeability in wild-type mice ear but not in GPR99-deficient mice ear [108,109].
Several studies have demonstrated that the 5-LOX/LTs axis is involved in impaired cutaneous wound healing. Genetic deficiency or pharmacological inhibition of 5-LOX in mice resulted in decreased reactive oxygen species (ROS) formation in fibroblasts by upregulating the expression of heme oxygenase-1 (HO-1), thereby accelerating the process of wound repair [110]. Furthermore, BLT1 antagonists or CysLT receptor antagonists, as well as 5-LOX deficiency, was found to improve wound closure by modulating the inflammatory response [111]. In addition, increased systemic levels of LTB4 and pro-inflammatory cytokines were observed in streptozotocin-induced type 1 diabetic mice with delayed wound healing compared to those in healthy mice. Genetic 5-LOX deletion in diabetic mice resulted in improved wound healing with increased frequency of alternative M2 macrophage population [112].
On the other hand, it has been reported that CysLTs directly promoted collagen production, and IL-6 and granulocyte macrophage colony-stimulating factor (GM-CSF) are released from fibroblasts through the activation of CysLT2 but not CysLT1, thereby inducing keratinocyte proliferation in ovalbumin-sensitized skin [113].
Altogether, these studies suggest that the inhibition of 5-LOX activity or the selective antagonism of BLT1 or CysLTs is beneficial for mitigating inflammation and could be a therapeutic alternative for patients with unresolved cutaneous healing such as a diabetic ulcer.
3.2.2. HETEs
The production of HETEs from AA is manipulated by the activity of various LOXs. The major HETEs identified in the human skin are 12- and 15-LOX products [114]. Although the presence of wide variations of HETE isoforms in the skin is known, the physiological roles of HETEs in wound repair largely remain unknown. It has been reported that 12-HETE treatment enhanced keratinocyte proliferation and chemotaxis to 12-HETE, implicating its participation in wound healing [115,116]. BLT2 is known to be activated by high 12-HETE concentration [117]; however, its role in the skin function of 12-HETE is unknown.
3.3. CYP Metabolites (EETs)
CYP epoxygenases (CYP2C8 and CYP2J2 in humans) are primarily expressed in endothelial cells and can catalyze the epoxidation of AA to produce EETs. It has been reported that EETs are bioactive lipid mediators that possess vasodilative, anti-inflammatory, and angiogenic effects [45]. In addition, recent studies have demonstrated their direct function in the promotion of skin wound healing.
The forced expression of human CYP2C8 or CYP2J2 in mice endothelial cells (Tie2-promoter driven) and the deficiency of sEH resulted in accelerated cutaneous wound closure [118,119]. In the same manner, the systemic administration of 11,12-EET, 14,15-EET, and sEH inhibitor displayed similar effects, including increased vascularization, compared to those in control mice as evaluated by histological analyses [118]. Topical application of 11,12-EET or 14,15-EET in mice ears was found to ameliorate ischemic wound healing with a significant elevation of the expression of VEGF, TGF-β1, and stromal cell-derived factor 1α (SDF-1α) [120]. The expression of CYP2C65 and CYP2J6 was significantly reduced in the granulation tissues of diabetic ob/ob mice, and the injection of 11,12-EET resulted in mitigated inflammatory reactions and increased collagen deposition, leading to accelerated wound healing [121]. Moreover, the administration of sEH inhibitor or a combination of sEH inhibitor and EETs was found to improve the engraftment of transplanted skin graft with increased vascularization [122].
These studies suggest the therapeutic potential of the treatment of EETs and/or sEH inhibitor with the functions of neovascularization and anti-inflammatory effect in various pathologies of wound healing.
3.4. SPMs (Lipoxins, Resolvins, Protectins, and Maresins)
SPMs are also produced through the metabolism of PUFAs and implicated in the orchestration of resolution of inflammation. Recent studies suggest that numerous chronic inflammatory pathologies are caused by the incomplete resolution of inflammation, and stimulating the resolution pathways with SPMs could be beneficial in multiple disease states, including skin injury [123,124].
Topical application of LXA4 or microparticle-encapsulated LXA4 to the dorsal wound of a rat model promoted wound closure with attenuated inflammatory responses and enhanced angiogenesis and collagen accumulation through its receptor ALX [125]. Furthermore, LXA4 directly modulated fibroblast proliferation and migration, thus implying the promotion of wound healing without scarring [126].
It has been reported that RvE1 binds to BLT1 as a partial agonist and attenuates LTB4/BLT1 signals to modulate leukocyte infiltration as well as stimulating the RvE1 receptor ChemR23 to regulate migration and cytokine production of macrophages and dendritic cells [127,128]. RvE1, RvD1, and RvD2 accelerated dorsal wound closure with a more mature collagen organization upon topical application [129]. Moreover, treatment with RvD1, RvD2, or RvD4 promoted re-epithelialization during skin injury and thus accelerated the process of wound healing. The enhancement of re-epithelialization by RvD1 or RvD2 treatment was abolished by the genetic deficiency of RvD1 receptor (ALX/FPR2) or RvD2 receptor (DRV2/GPR18), which are expressed on keratinocytes, respectively. It has been reported that the PI3K-AKT-mTOR-S6 pathway is involved in the downstream of RvD2 signal [27]. In addition, RvD2 was found to inhibit TGF-β-induced fibroblast proliferation and migration in an in vitro scratch model, thus suggesting its modulating function in the formation of fibrosis [126].
Although PD1 production in diabetic mice wound was decreased, PD1 treatment enhanced the process of wound healing by promoting nerve fiber growth, re-epithelialization, collagen deposition, and reduced inflammation [130].
Injection of MaR1 was found to exhibit a protective function in mice skin against UVB irradiation-induced edema, neutrophil recruitment, collagen degradation, and pro-inflammatory cytokine induction [131]. Another study reported that topical application of MaR1 onto tooth extraction sockets accelerated wound closure by promoting re-epithelialization and inducing an increased ratio of CD206-positive M2-like macrophages [132].
Altogether, recent studies have demonstrated the beneficial effects of the treatment of SPMs in the process of skin wound healing, particularly in conditions that impair the resolution of inflammation, such as diabetes. Development of treatment strategies for wound healing based on SPM pathways may offer distinct advantages over traditional anti-inflammatory therapies that disturb tissue repair.
4. Summary and Perspectives
Wound healing is a complex process involving multiple factors such as growth factors, cytokines, chemokines, and lipid mediators at each stage of healing. Despite the increase in the number of patients with impaired wound healing, there are limited mechanism-based therapeutic alternatives for the treatment of intractable wounds. For instance, recombinant human bFGF (Fiblast Spray), which is indicated for decubitus and skin ulcers, including burns and leg ulcers, is available [133]. Therefore, it is necessary to develop treatment options from different perspectives.
In this review, we have summarized the biosynthetic process and functions of lipid mediators derived from PUFAs such as AA, EPA, and DHA, and recent studies have described the involvement of eicosanoids and SPMs with versatile functions in skin wound healing (Table 1). For example, among AA metabolites, several COX or CYP products demonstrated a positive effect on skin wound healing through various activities, including modulation of clotting, macrophage polarization, keratinocyte migration, and angiogenesis, and the metabolites produced by the 5-LOX pathway such as LTs delayed the process of skin wound healing partially via the mechanisms of excessive inflammation, including neutrophil recruitment and reduced macrophage polarization to M2 phenotype. Furthermore, SPMs accelerated wound healing, as indicated by the promotion of resolution, without causing defects in the initial inflammation. As it is relatively easy to administer topical treatments to skin wounds, targeting the eicosanoid and SPM signals could be promising as an alternative strategy for patients with acute injury or surgery-induced injury as well as chronic wounds with aberrant inflammation or impaired vascularization.

Table 1.
Overview of eicosanoids and SPMs involved in skin wound healing.
However, in general, eicosanoids are rapidly degraded by non-enzymic pathway or enzymatic metabolism in vivo [134,135]. Thus, the development of a formulation strategy that could prolong the release of eicosanoids might have a better effect on tissue repair and regeneration [136]. Indeed, the topical application of PGE2 containing chitosan hydrogel or microparticle-encapsulated LXA4 demonstrated improved efficacy compared to PGE2 or LXA4 alone [77,125].
On the other hand, wound healing might be promoted by the inhibition of eicosanoids production such as LTs that have a negative effect on the processes [110,111,112]. An aspect that must be noted is that because eicosanoids and SPMs have a common precursor, modifying one pathway will affect the other. Therefore, a much more comprehensive study such as a time-course study of lipidomics during skin wound healing is required to better understand the complex interaction of lipid mediators.
Furthermore, the specific receptors of several lipid mediators, including EETs, and the majority of SPMs are still ambiguous. GPR40, also known as free fatty acid receptor-1 (FFAR-1), is a low-affinity receptor of EETs and mediates vascular actions with micromolar concentration of EETs [137]. Regarding SPM receptors, it has been reported that LXA4, RvD1, and RvD3 can stimulate both ALX/FPR2 and DRV1/GPR32 receptors [138]. In addition, recent studies have demonstrated that GPR37 and LGR6 are the receptors for PD1 and MaR1, respectively [139,140]. However, the receptors for the other subtypes of SPMs have not been identified, and it is also not clear whether EETs or SPM/receptor signals are functional under physiological or pathological conditions. To gain a better understanding of the physiological functions of these mediators and receptors for the development of clinical applications, it is indispensable to clarify the molecular mechanisms underlying the phenomenon of wound healing.
Author Contributions
K.Y. designed and wrote the manuscript. T.O. and T.Y. advised, conceived the concept, and improved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by MEXT/JSPS KAKENHI, Grant Numbers: 19K07357 (to T.O.), 15H05904, 18H02627, and 19KK0199 (to T.Y.), and in part by Sato Pharmaceutical Co., Ltd.
Acknowledgments
We would like to thank Yoshiki Ito and Masatsugu Oh-hora for their useful comments.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PUFA | polyunsaturated fatty acid |
| PLA2 | phospholipase A2 |
| PG | Prostaglandin |
| TxA2 | thromboxane A2 |
| AA | arachidonic acid |
| COX | Cyclooxygenase |
| LT | Leukotriene |
| HETE | hydroxyeicosatetraenoic acid |
| LX | Lipoxin |
| LOX | lipoxygenase |
| EET | epoxyeicosatrienoic acid |
| CYP | cytochrome P450 |
| SPM | specialized pro-resolving mediator |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| NSAID | nonsteroidal anti-inflammatory drug |
| HpETE | hydroperoxyeicosatetraenoic acid |
| CysLT | cysteinyl leukotriene |
| LTA4H | LTA4 hydrolase |
| LTC4S | LTC4 synthase |
| MaR1 | maresin 1 |
| TxAS | thromboxane A synthase |
| IL-6, 1β | interleukin-6, 1β |
| 12-HHT | 12(S)-hydroxyheptadeca-5Z,8E,10E-trienoic acid |
| TNFα | tumor necrosis factor α |
| MMP | matrix metalloproteinase |
| TGF-β1 | transforming growth factor-β1 |
| bFGF | basic fibroblast growth factor |
| cPGES | cytosolic prostaglandin E synthase |
| mPGES | membrane-associated prostaglandin E synthase |
| GPCR | G-protein coupled receptor |
| PGT | prostaglandin transporter |
| OSM | oncostatin M |
| CREB | cyclic AMP-responsive element binding |
| KLF4 | Krupple-like factor 4 |
| TIMP | tissue inhibitor of matrix metalloproteinase |
| H-PGDS | hematopoietic-type prostaglandin D synthase |
| L-PGDS | lipocalin-type prostaglandin D synthase |
| PPARγ, δ | peroxisome proliferator-activated receptor γ, δ |
| PGIS | prostacyclin synthase |
| uPA | urokinase-type plasminogen activator |
| VEGF | vascular endothelial growth factor |
| ROS | reactive oxygen species |
| HO-1 | heme oxygenase-1 |
| GM-CSF | granulocyte macrophage colony-stimulating factor |
| sEH | soluble epoxide hydrolase |
| DHET | dihydroxyeicosatrienoic acids |
| SDF-1α | stromal cell-derived factor 1α |
References
- Jean, K. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–401. [Google Scholar]
- Ludriksone, L.; Garcia Bartels, N.; Kanti, V.; Blume-Peytavi, U.; Kottner, J. Skin barrier function in infancy: A systematic review. Arch. Dermatol. Res 2014, 306, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Canedo-Dorantes, L.; Canedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Grinnell, F.; Gilchrest, B.; Maddox, Y.T.; Moshell, A. Workshop on the Pathogenesis of Chronic Wounds. J. Investig. Dermatol. 1994, 102, 125–127. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Rosique, R.G.; Rosique, M.J.; Farina Junior, J.A. Curbing Inflammation in Skin Wound Healing: A Review. Int. J. Inflamm. 2015, 2015, 316235. [Google Scholar] [CrossRef]
- Moulin, V. Growth factors in skin wound healing. Eur. J. Cell Biol. 1995, 68, 1–7. [Google Scholar]
- Steed, D.L. The Role of Growth Factors in Wound Healing. Surg. Clin. 1997, 77, 575–586. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Esser-von Bieren, J. Eicosanoids in tissue repair. Immunol. Cell Biol. 2019, 97, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.-P.; Yin, J.; Yu, F.-S.X. Lysophosphatidic Acid Promoting Corneal Epithelial Wound Healing by Transactivation of Epidermal Growth Factor Receptor. Investig. Ophthalmol. Vis. Sci. 2007, 48, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Aoki, H.; Mukhopadhyay, P.; Tsuge, T.; Yamamoto, H.; Matsumoto, N.M.; Toyohara, E.; Okubo, Y.; Ogawa, R.; Takabe, K. Sphingosine-1-Phosphate Facilitates Skin Wound Healing by Increasing Angiogenesis and Inflammatory Cell Recruitment with Less Scar Formation. Int. J. Mol. Sci. 2019, 20, 3381. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.M.; Han, H.J. Autotaxin-LPA axis regulates hMSC migration by adherent junction disruption and cytoskeletal rearrangement via LPAR1/3-dependent PKC/GSK3beta/beta-catenin and PKC/Rho GTPase pathways. Stem Cells 2015, 33, 819–832. [Google Scholar] [CrossRef]
- Aoki, M.; Aoki, H.; Ramanathan, R.; Hait, N.C.; Takabe, K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediat. Inflamm. 2016, 2016, 8606878. [Google Scholar] [CrossRef]
- Dennis, E.A. Phospholipase A2 in eicosanoid generation. Am. J. Respir. Crit. Care Med. 2000, 161, S32–S35. [Google Scholar] [CrossRef]
- Shimizu, T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef]
- Esser-von Bieren, J. Immune-regulation and -functions of eicosanoid lipid mediators. Biol. Chem. 2017, 398, 1177–1191. [Google Scholar] [CrossRef]
- López, D.E.; Ballaz, S.J. The Role of Brain Cyclooxygenase-2 (Cox-2) Beyond Neuroinflammation: Neuronal Homeostasis in Memory and Anxiety. Mol. Neurobiol. 2020, 57, 5167–5176. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Vane, J.R. Prostaglandins: Their Disappearance from and Release into the Circulation. Nature 1967, 216, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Kirkby, N.S. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br. J. Pharmacol. 2019, 176, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B. Leukotrienes: Mediators of immediate hypersensitivity reactions and inflammation. Science 1983, 220, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Chacos, N.; Werringloer, J.; Prough, R.A.; Estabrook, R.W. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 5362–5366. [Google Scholar] [CrossRef]
- Jamieson, K.L.; Endo, T.; Darwesh, A.M.; Samokhvalov, V.; Seubert, J.M. Cytochrome P450-derived eicosanoids and heart function. Pharmacol. Ther. 2017, 179, 47–83. [Google Scholar] [CrossRef]
- Hellmann, J.; Sansbury, B.E.; Wong, B.; Li, X.; Singh, M.; Nuutila, K.; Chiang, N.; Eriksson, E.; Serhan, C.N.; Spite, M. Biosynthesis of D-Series Resolvins in Skin Provides Insights into their Role in Tissue Repair. J. Investig. Dermatol. 2018, 138, 2051–2060. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Dennis, E.A. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994, 269, 13057–13060. [Google Scholar]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Hirata, T.; Narumiya, S. Prostanoids as regulators of innate and adaptive immunity. Adv. Immunol. 2012, 116, 143–174. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 AND 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.D.; Talley, J.J.; Bertenshaw, S.R.; Carter, J.S.; Collins, P.W.; Docter, S.; Graneto, M.J.; Lee, L.F.; Malecha, J.W.; Miyashiro, J.M.; et al. Synthesis and Biological Evaluation of the 1,5-Diarylpyrazole Class of Cyclooxygenase-2 Inhibitors: Identification of 4-[5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib). J. Med. Chem. 1997, 40, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Futagami, A.; Ishizaki, M.; Fukuda, Y.; Kawana, S.; Yamanaka, N. Wound healing involves induction of cyclooxygenase-2 expression in rat skin. Lab. Investig. 2002, 82, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Arai, I.; Futaki, N.; Hashimoto, Y.; Honma, Y.; Nakaike, S. Role of COX-1 and COX-2 on skin PGs biosynthesis by mechanical scratching in mice. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 1–8. [Google Scholar] [CrossRef]
- Buckman, S.Y.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef]
- Higashi, Y.; Kanekura, T.; Kanzaki, T. Enhanced expression of cyclooxygenase (COX)-2 in human skin epidermal cancer cells: Evidence for growth suppression by inhibiting COX-2 expression. Int. J. Cancer 2000, 86, 667–671. [Google Scholar] [CrossRef]
- Noverr, M.C.; Erb-Downward, J.R.; Huffnagle, G.B. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin. Microbiol. Rev. 2003, 16, 517–533. [Google Scholar] [CrossRef]
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar] [CrossRef]
- Yokomizo, T.; Nakamura, M.; Shimizu, T. Leukotriene receptors as potential therapeutic targets. J. Clin. Investig. 2018, 128, 2691–2701. [Google Scholar] [CrossRef]
- Kuhn, H.; Banthiya, S.; van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta 2015, 1851, 308–330. [Google Scholar] [CrossRef] [PubMed]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Sheppard, K.A. Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J. Clin. Investig. 1990, 85, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Serhan, C.N.; Dahlen, S.E.; Drazen, J.M.; Hay, D.W.; Rovati, G.E.; Shimizu, T.; Yokomizo, T.; Brink, C. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 2006, 58, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A. Arachidonic acid cytochrome P450 epoxygenase pathway. J. Lipid Res. 2009, 50, S52–S56. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Hamberg, M.; Samuelsson, B. On the formation and effects of thromboxane A2 in human platelets. Acta Physiol. Scand. 1976, 98, 285–294. [Google Scholar] [CrossRef]
- Ogletree, M.L. Overview of physiological and pathophysiological effects of thromboxane A2. Fed. Proc 1987, 46, 133–138. [Google Scholar]
- Thomas, D.W.; Mannon, R.B.; Mannon, P.J.; Latour, A.; Oliver, J.A.; Hoffman, M.; Smithies, O.; Koller, B.H.; Coffman, T.M. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J. Clin. Investig. 1998, 102, 1994–2001. [Google Scholar] [CrossRef]
- Lau, C.S.; Khan, F.; McLaren, M.; Bancroft, A.; Walker, M.; Belch, J.J.F. The effects of thromboxane receptor blockade on platelet aggregation and digital skin blood flow in patients with secondary Raynaud’s syndrome. Rheumatol. Int. 1991, 11, 163–168. [Google Scholar] [CrossRef]
- Leslie, M. Beyond Clotting: The Powers of Platelets. Science 2010, 328, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, Y.H. The roles of platelets in inflammation, immunity, wound healing and malignancy. Int. J. Clin. Exp. Med. 2016, 9, 5347–5358. [Google Scholar]
- Pierre, S.; Linke, B.; Suo, J.; Tarighi, N.; Del Turco, D.; Thomas, D.; Ferreiros, N.; Stegner, D.; Frölich, S.; Sisignano, M.; et al. GPVI and Thromboxane Receptor on Platelets Promote Proinflammatory Macrophage Phenotypes during Cutaneous Inflammation. J. Investig. Dermatol. 2017, 137, 686–695. [Google Scholar] [CrossRef]
- Daniel, T.O.; Liu, H.; Morrow, J.D.; Crews, B.C.; Marnett, L.J. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999, 59, 4574–4577. [Google Scholar] [PubMed]
- Nie, D.; Lamberti, M.; Zacharek, A.; Li, L.; Szekeres, K.; Tang, K.; Chen, Y.; Honn, K.V. Thromboxane A2 Regulation of Endothelial Cell Migration, Angiogenesis, and Tumor Metastasis. Biochem. Biophys. Res. Commun. 2000, 267, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Iizuka, Y.; Okazaki, H.; Yokomizo, T.; Taguchi, R.; Shimizu, T. 12(S)-Hydroxyheptadeca-5Z, 8E, 10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2. J. Exp. Med. 2008, 205, 759–766. [Google Scholar] [CrossRef]
- Liu, M.; Saeki, K.; Matsunobu, T.; Okuno, T.; Koga, T.; Sugimoto, Y.; Yokoyama, C.; Nakamizo, S.; Kabashima, K.; Narumiya, S.; et al. 12-Hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. J. Exp. Med. 2014, 211, 1063–1078. [Google Scholar] [CrossRef]
- Luo, L.; Tanaka, R.; Kanazawa, S.; Lu, F.; Hayashi, A.; Yokomizo, T.; Mizuno, H. A synthetic leukotriene B4 receptor type 2 agonist accelerates the cutaneous wound healing process in diabetic rats by indirect stimulation of fibroblasts and direct stimulation of keratinocytes. J. Diabetes Its Complicat. 2017, 31, 13–20. [Google Scholar] [CrossRef][Green Version]
- Pollack, S.V. Systemic drugs and nutritional aspects of wound healing. Clin. Dermatol. 1984, 2, 68–80. [Google Scholar] [CrossRef]
- Kaushal, M.; Gopalan Kutty, N.; Mallikarjuna Rao, C. Wound healing activity of NOE-aspirin: A pre-clinical study. Nitric Oxide 2007, 16, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, T.; Nakatani, Y.; Semmyo, N.; Murakami, M.; Kudo, I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 2000, 275, 32775–32782. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Naraba, H.; Tanioka, T.; Semmyo, N.; Nakatani, Y.; Kojima, F.; Ikeda, T.; Fueki, M.; Ueno, A.; Oh, S.; et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000, 275, 32783–32792. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef]
- Sakai, Y.; Fujita, K.; Sakai, H.; Mizuno, K. Prostaglandin E2 regulates the expression of basic fibroblast growth factor messenger RNA in normal human fibroblasts. Kobe J. Med. Sci. 2001, 47, 35–45. [Google Scholar]
- Fairweather, M.; Heit, Y.I.; Buie, J.; Rosenberg, L.M.; Briggs, A.; Orgill, D.P.; Bertagnolli, M.M. Celecoxib inhibits early cutaneous wound healing. J. Surg. Res. 2015, 194, 717–724. [Google Scholar] [CrossRef]
- Kämpfer, H.; Schmidt, R.; Geisslinger, G.; Pfeilschifter, J.; Frank, S. Wound inflammation in diabetic ob/ob mice: Functional coupling of prostaglandin biosynthesis to cyclooxygenase-1 activity in diabetes-impaired wound healing. Diabetes 2005, 54, 1543–1551. [Google Scholar] [CrossRef]
- Syeda, M.M.; Jing, X.; Mirza, R.H.; Yu, H.; Sellers, R.S.; Chi, Y. Prostaglandin transporter modulates wound healing in diabetes by regulating prostaglandin-induced angiogenesis. Am. J. Pathol. 2012, 181, 334–346. [Google Scholar] [CrossRef]
- Ganesh, K.; Das, A.; Dickerson, R.; Khanna, S.; Parinandi, N.L.; Gordillo, G.M.; Sen, C.K.; Roy, S. Prostaglandin E(2) induces oncostatin M expression in human chronic wound macrophages through Axl receptor tyrosine kinase pathway. J. Immunol. 2012, 189, 2563–2573. [Google Scholar] [CrossRef]
- Lowe, N.J.; Stoughton, R.B. Effects of topical prostaglandin E analogue on normal hairless mouse epidermal DNA synthesis. J. Investig. Dermatol. 1977, 68, 134–137. [Google Scholar] [CrossRef]
- Pentland, A.P.; Needleman, P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J. Clin. Investig. 1986, 77, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.B.; Pillai, S.; Goldyne, M.E. Endogenous prostaglandin E2 modulates calcium-induced differentiation in human skin keratinocytes. Prostaglandins Leukot. Essent. Fat. Acids 1993, 49, 777–781. [Google Scholar] [CrossRef]
- Konger, R.L.; Malaviya, R.; Pentland, A.P. Growth regulation of primary human keratinocytes by prostaglandin E receptor EP2 and EP3 subtypes. Biochim. Biophys. Acta 1998, 1401, 221–234. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Kotwal, G.J.; Chien, S. Macrophage Differentiation in Normal and Accelerated Wound Healing. Results Probl. Cell Differ. 2017, 62, 353–364. [Google Scholar] [CrossRef]
- Luan, B.; Yoon, Y.-S.; Le Lay, J.; Kaestner, K.H.; Hedrick, S.; Montminy, M. CREB pathway links PGE2 signaling with macrophage polarization. Proc. Natl. Acad. Sci. USA 2015, 112, 15642–15647. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Zhang, X.; Zhu, D.; Qi, X.; Cao, X.; Fang, Y.; Che, Y.; Han, Z.C.; He, Z.X.; et al. Prostaglandin E2 hydrogel improves cutaneous wound healing via M2 macrophages polarization. Theranostics 2018, 8, 5348–5361. [Google Scholar] [CrossRef]
- Zhao, J.; Shu, B.; Chen, L.; Tang, J.; Zhang, L.; Xie, J.; Liu, X.; Xu, Y.; Qi, S. Prostaglandin E2 inhibits collagen synthesis in dermal fibroblasts and prevents hypertrophic scar formation in vivo. Exp. Dermatol. 2016, 25, 604–610. [Google Scholar] [CrossRef]
- Helliwell, R.J.; Adams, L.F.; Mitchell, M.D. Prostaglandin synthases: Recent developments and a novel hypothesis. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 101–113. [Google Scholar] [CrossRef]
- Joo, M.; Sadikot, R.T. PGD synthase and PGD2 in immune resposne. Mediat. Inflamm. 2012, 2012, 503128. [Google Scholar] [CrossRef]
- Arai, I.; Takano, N.; Hashimoto, Y.; Futaki, N.; Sugimoto, M.; Takahashi, N.; Inoue, T.; Nakaike, S. Prostanoid DP1 receptor agonist inhibits the pruritic activity in NC/Nga mice with atopic dermatitis. Eur. J. Pharmacol. 2004, 505, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Honma, Y.; Arai, I.; Hashimoto, Y.; Futaki, N.; Sugimoto, M.; Tanaka, M.; Nakaike, S. Prostaglandin D2 and prostaglandin E2 accelerate the recovery of cutaneous barrier disruption induced by mechanical scratching in mice. Eur. J. Pharmacol. 2005, 518, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Honma, Y.; Arai, I.; Sakurai, T.; Futaki, N.; Hashimoto, Y.; Sugimoto, M.; Nakanishi, Y.; Nakaike, S. Effects of indomethacin and dexamethasone on mechanical scratching-induced cutaneous barrier disruption in mice. Exp. Dermatol. 2006, 15, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Lenhard, J.M.; Willson, T.M.; Patel, I.; Morris, D.C.; Lehmann, J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 1995, 83, 813–819. [Google Scholar] [CrossRef]
- Forman, B.M.; Tontonoz, P.; Chen, J.; Brun, R.P.; Spiegelman, B.M.; Evans, R.M. 15-Deoxy-Δ12,14-Prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell 1995, 83, 803–812. [Google Scholar] [CrossRef]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Dièvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef]
- Chen, H.; Shi, R.; Luo, B.; Yang, X.; Qiu, L.; Xiong, J.; Jiang, M.; Liu, Y.; Zhang, Z.; Wu, Y. Macrophage peroxisome proliferator-activated receptor B deficiency delays skin wound healing through impairing apoptotic cell clearance in mice. Cell Death Dis. 2015, 6, e1597. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Novak, M.L.; Urao, N.; Sui, A.; Ennis, W.J.; Koh, T.J. Macrophage PPARgamma and impaired wound healing in type 2 diabetes. J. Pathol. 2015, 236, 433–444. [Google Scholar] [CrossRef]
- Kohyama, T.; Liu, X.D.; Wen, F.Q.; Kim, H.J.; Takizawa, H.; Rennard, S.I. Prostaglandin D2 inhibits fibroblast migration. Eur. Respir. J. 2002, 19, 684–689. [Google Scholar] [CrossRef]
- Murata, T.; Lin, M.I.; Aritake, K.; Matsumoto, S.; Narumiya, S.; Ozaki, H.; Urade, Y.; Hori, M.; Sessa, W.C. Role of prostaglandin D2 receptor DP as a suppressor of tumor hyperpermeability and angiogenesis in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 20009–20014. [Google Scholar] [CrossRef]
- Nelson, A.M.; Loy, D.E.; Lawson, J.A.; Katseff, A.S.; Fitzgerald, G.A.; Garza, L.A. Prostaglandin D2 inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. J. Investig. Dermatol. 2013, 133, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 2012, 4, 126ra134. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.G. Induction of term labor with intravenous PGF2α: A review. Prostaglandins 1973, 4, 765–774. [Google Scholar] [CrossRef]
- Lee, P.Y.; Shao, H.; Xu, L.A.; Qu, C.K. The effect of prostaglandin F2 alpha on intraocular pressure in normotensive human subjects. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1474–1477. [Google Scholar]
- Hernández-Cueto, C.; Vieira, D.N.; Girela, E.; Marques, E.; Calvo, M.D.; Villalobos, M.; Oliveira de Sà, F.; Villanueva, E. Prostaglandin F2a (PGF2a): An inadequate marker of the vitality of wounds? Int. J. Legal Med. 1994, 106, 312–314. [Google Scholar] [CrossRef]
- Muller, K.; Krieg, P.; Marks, F.; Furstenberger, G. Expression of PGF2α receptor mRNA in normal, hyperplastic and neoplastic skin. Carcinogenesis 2000, 21, 1063–1066. [Google Scholar] [CrossRef][Green Version]
- Lee, H.-C.; Tominaga, M.; Yasukawa, K.; Ohba, M.; Takahashi, N.; Honda, K.; Okuno, T.; Takamori, K.; Yokomizo, T. Dietary supplementation of omega-3 fatty acid eicosapentaenoic acid does not ameliorate pruritus in murine models of atopic dermatitis and psoriasis. J. Dermatol. Sci. 2019, 95, 130–133. [Google Scholar] [CrossRef]
- Stjernschantz, J.W. From PGF2α-Isopropyl Ester to Latanoprost: A Review of the Development of Xalatan The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1134–1145. [Google Scholar]
- Vane, J.; Corin, R.E. Prostacyclin: A Vascular Mediator. Eur. J. Vasc. Endovasc. Surgery 2003, 26, 571–578. [Google Scholar] [CrossRef]
- Jackson, W.F.; König, A.; Dambacher, T.; Busse, R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am. J. Physiol. 1993, 264, H238–H243. [Google Scholar] [CrossRef]
- Hatane, T.; Yoshida, E.; Kawano, J.; Sugiki, M.; Onitsuka, T.; Maruyama, M. Prostaglandin I2 analog enhances the expression of urokinase-type plasminogen activator and wound healing in cultured human fibroblast. Biochim. Biophys. Acta 1998, 1403, 189–198. [Google Scholar] [CrossRef]
- Pola, R.; Gaetani, E.; Flex, A.; Aprahamian, T.R.; Bosch-MarcÈ, M.; Losordo, D.; Smith, R.C.; Pola, P. Comparative analysis of the in vivo angiogenic properties of stable prostacyclin analogs: A possible role for peroxisome proliferator-activated receptors. J. Mol. Cell. Cardiol. 2004, 36, 363–370. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Lu, T.; d’Uscio, L.V.; Lam, C.F.; Lee, H.C.; Katusic, Z.S. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ. Res. 2008, 103, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, T.; Izumi, T.; Chang, K.; Takuwa, Y.; Shimizu, T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 1997, 387, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.L.; Wang, S.; Dejani, N.N.; Klopfenstein, N.; Winfree, S.; Filgueiras, L.; McCarthy, B.P.; Territo, P.R.; Serezani, C.H. Excessive localized leukotriene B4 levels dictate poor skin host defense in diabetic mice. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Yokomizo, T.; Kato, K.; Terawaki, K.; Izumi, T.; Shimizu, T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 2000, 192, 421–432. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Boyce, J.A. Cysteinyl leukotrienes and their receptors: Cellular distribution and function in immune and inflammatory responses. J. Immunol. 2004, 173, 1503–1510. [Google Scholar] [CrossRef]
- Maekawa, A.; Kanaoka, Y.; Xing, W.; Austen, K.F. Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 16695–16700. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Maekawa, A.; Austen, K.F. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J. Biol. Chem. 2013, 288, 10967–10972. [Google Scholar] [CrossRef]
- Brogliato, A.R.; Moor, A.N.; Kesl, S.L.; Guilherme, R.F.; Georgii, J.L.; Peters-Golden, M.; Canetti, C.; Gould, L.J.; Benjamim, C.F. Critical role of 5-lipoxygenase and heme oxygenase-1 in wound healing. J. Invest. Dermatol. 2014, 134, 1436–1445. [Google Scholar] [CrossRef]
- Guimaraes, F.R.; Sales-Campos, H.; Nardini, V.; da Costa, T.A.; Fonseca, M.T.C.; Junior, V.R.; Sorgi, C.A.; da Silva, J.S.; Chica, J.E.L.; Faccioli, L.H.; et al. The inhibition of 5-Lipoxygenase (5-LO) products leukotriene B4 (LTB4) and cysteinyl leukotrienes (cysLTs) modulates the inflammatory response and improves cutaneous wound healing. Clin. Immunol. 2018, 190, 74–83. [Google Scholar] [CrossRef]
- Ramalho, T.; Filgueiras, L.; Silva-Jr, I.A.; Pessoa, A.F.M.; Jancar, S. Impaired wound healing in type 1 diabetes is dependent on 5-lipoxygenase products. Sci. Rep. 2018, 8, 14164. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; He, R.; Kanaoka, Y.; ElKhal, A.; Kawamoto, S.; Lewis, C.N.; Austen, K.F.; Geha, R.S. Eosinophil-derived leukotriene C4 signals via type 2 cysteinyl leukotriene receptor to promote skin fibrosis in a mouse model of atopic dermatitis. Proc. Natl. Acad. Sci. USA 2012, 109, 4992–4997. [Google Scholar] [CrossRef]
- Raja, S.K.; Sivamani, K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef]
- Chan, C.C.; Duhamel, L.; Ford-Hutchison, A. Leukotriene B4 and 12-hydroxyeicosatetraenoic acid stimulate epidermal proliferation in vivo in the guinea pig. J. Investig. Dermatol. 1985, 85, 333–334. [Google Scholar] [CrossRef]
- Ruzicka, T. The role of the epidermal 12-hydroxyeicosatetraenoic acid receptor in the skin. Eicosanoids 1992, 5, S63–S65. [Google Scholar]
- Yokomizo, T.; Kato, K.; Hagiya, H.; Izumi, T.; Shimizu, T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J. Biol. Chem. 2001, 276, 12454–12459. [Google Scholar] [CrossRef]
- Panigrahy, D.; Kalish, B.T.; Huang, S.; Bielenberg, D.R.; Le, H.D.; Yang, J.; Edin, M.L.; Lee, C.R.; Benny, O.; Mudge, D.K.; et al. Epoxyeicosanoids promote organ and tissue regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13528–13533. [Google Scholar] [CrossRef]
- Sander, A.L.; Sommer, K.; Neumayer, T.; Fleming, I.; Marzi, I.; Barker, J.H.; Frank, J.; Jakob, H. Soluble epoxide hydrolase disruption as therapeutic target for wound healing. J. Surg. Res. 2013, 182, 362–367. [Google Scholar] [CrossRef]
- Sommer, K.; Jakob, H.; Badjlan, F.; Henrich, D.; Frank, J.; Marzi, I.; Sander, A.L. 11,12 and 14,15 epoxyeicosatrienoic acid rescue deteriorated wound healing in ischemia. PLoS ONE 2019, 14, e0209158. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Chai, J.; Zhang, Y.; Yu, C.; Pan, Z.; Gao, P.; Zong, C.; Guan, Q.; Fu, Y.; et al. Cytochrome P450 (CYP) epoxygenases as potential targets in the management of impaired diabetic wound healing. Lab. Investig. 2017, 97, 782–791. [Google Scholar] [CrossRef]
- Supp, D.M.; Hahn, J.M.; McFarland, K.L.; Combs, K.A.; Lee, K.S.; Inceoglu, B.; Wan, D.; Boyce, S.T.; Hammock, B.D. Soluble Epoxide Hydrolase Inhibition and Epoxyeicosatrienoic Acid Treatment Improve Vascularization of Engineered Skin Substitutes. Plast Reconstr. Surg. Glob. Open 2016, 4, e1151. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Hellmann, J.; Tang, Y.; Spite, M. Proresolving lipid mediators and diabetic wound healing. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 104–108. [Google Scholar] [CrossRef]
- Reis, M.B.; Pereira, P.A.T.; Caetano, G.F.; Leite, M.N.; Galvao, A.F.; Paula-Silva, F.W.G.; Frade, M.A.C.; Faccioli, L.H. Lipoxin A4 encapsulated in PLGA microparticles accelerates wound healing of skin ulcers. PLoS ONE 2017, 12, e0182381. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.S.; Kantarci, A.; Zarrough, A.; Hasturk, H.; Leung, K.P.; Van Dyke, T.E. LXA4 actions direct fibroblast function and wound closure. Biochem. Biophys. Res. Commun. 2015, 464, 1072–1077. [Google Scholar] [CrossRef]
- Arita, M.; Bianchini, F.; Aliberti, J.; Sher, A.; Chiang, N.; Hong, S.; Yang, R.; Petasis, N.A.; Serhan, C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005, 201, 713–722. [Google Scholar] [CrossRef]
- Arita, M.; Ohira, T.; Sun, Y.P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef]
- Menon, R.; Krzyszczyk, P.; Berthiaume, F. Pro-Resolution Potency of Resolvins D1, D2 and E1 on Neutrophil Migration and in Dermal Wound Healing. Nano Life 2017, 07, 1750002. [Google Scholar] [CrossRef]
- Hong, S.; Tian, H.; Lu, Y.; Laborde, J.M.; Muhale, F.A.; Wang, Q.; Alapure, B.V.; Serhan, C.N.; Bazan, N.G. Neuroprotectin/protectin D1: Endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am. J. Physiol Cell Physiol 2014, 307, C1058–C1067. [Google Scholar] [CrossRef]
- Cezar, T.L.C.; Martinez, R.M.; Rocha, C.D.; Melo, C.P.B.; Vale, D.L.; Borghi, S.M.; Fattori, V.; Vignoli, J.A.; Camilios-Neto, D.; Baracat, M.M.; et al. Treatment with maresin 1, a docosahexaenoic acid-derived pro-resolution lipid, protects skin from inflammation and oxidative stress caused by UVB irradiation. Sci. Rep. 2019, 9, 3062. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Yu, S.H.; Fretwurst, T.; Larsson, L.; Sugai, J.V.; Oh, J.; Lehner, K.; Jin, Q.; Giannobile, W.V. Maresin 1 Promotes Wound Healing and Socket Bone Regeneration for Alveolar Ridge Preservation. J. Dent. Res. 2020, 99, 930–937. [Google Scholar] [CrossRef]
- Ito, K.; Ito, S.; Sekine, M.; Abe, M. Reconstruction of the soft tissue of a deep diabetic foot wound with artificial dermis and recombinant basic fibroblast growth factor. Plast. Reconstr. Surg. 2005, 115, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.H.; Ensor, C.M.; Tong, M.; Zhou, H.; Yan, F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002, 68–69, 483–493. [Google Scholar] [CrossRef]
- Clish, C.B.; Levy, B.D.; Chiang, N.; Tai, H.H.; Serhan, C.N. Oxidoreductases in lipoxin A4 metabolic inactivation: A novel role for 15-onoprostaglandin 13-reductase/leukotriene B4 12-hydroxydehydrogenase in inflammation. J. Biol. Chem. 2000, 275, 25372–25380. [Google Scholar] [CrossRef] [PubMed]
- Nicolete, R.; Lima, K.d.M.; Júnior, J.M.R.; Baruffi, M.D.; de Medeiros, A.I.; Bentley, M.V.L.B.; Silva, C.L.; Faccioli, L.H. In vitro and in vivo activities of leukotriene B4-loaded biodegradable microspheres. Prostaglandins Other Lipid Mediat. 2007, 83, 121–129. [Google Scholar] [CrossRef]
- Park, S.K.; Herrnreiter, A.; Pfister, S.L.; Gauthier, K.M.; Falck, B.A.; Falck, J.R.; Campbell, W.B. GPR40 is a low-affinity epoxyeicosatrienoic acid receptor in vascular cells. J. Biol. Chem. 2018, 293, 10675–10691. [Google Scholar] [CrossRef]
- Hansen, T.V.; Vik, A.; Serhan, C.N. The Protectin Family of Specialized Pro-resolving Mediators: Potent Immunoresolvents Enabling Innovative Approaches to Target Obesity and Diabetes. Front. Pharmacol. 2018, 9, 1582. [Google Scholar] [CrossRef]
- Bang, S.; Xie, Y.K.; Zhang, Z.J.; Wang, Z.; Xu, Z.Z.; Ji, R.R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Investig. 2018, 128, 3568–3582. [Google Scholar] [CrossRef]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).