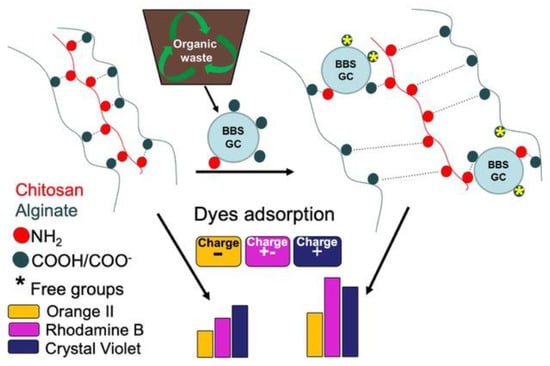
The Innovation Comes from the Sea: Chitosan and Alginate Hybrid Gels and Films as Sustainable Materials for Wastewater Remediation
Abstract
1. Introduction
2. Results and Discussion
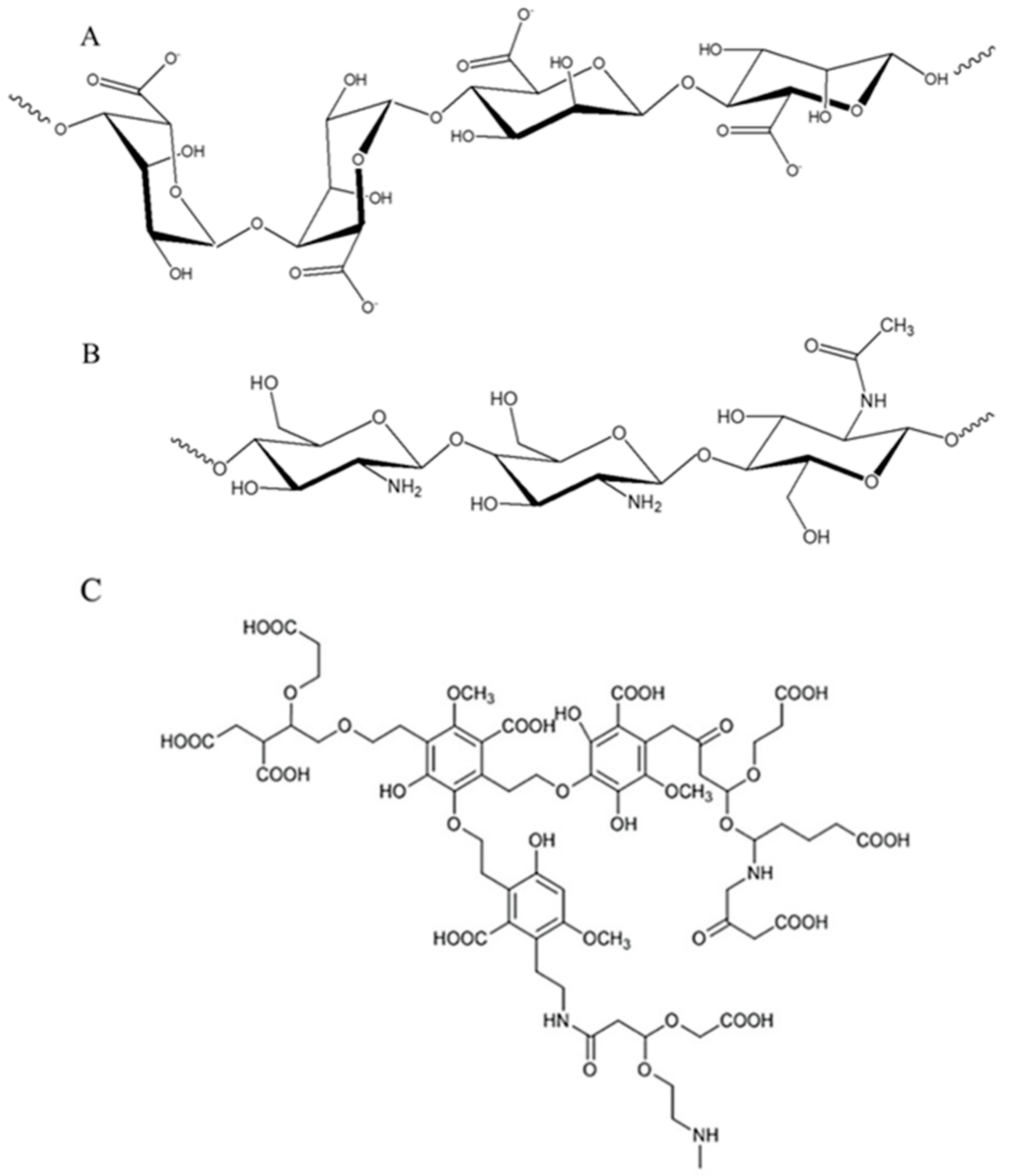
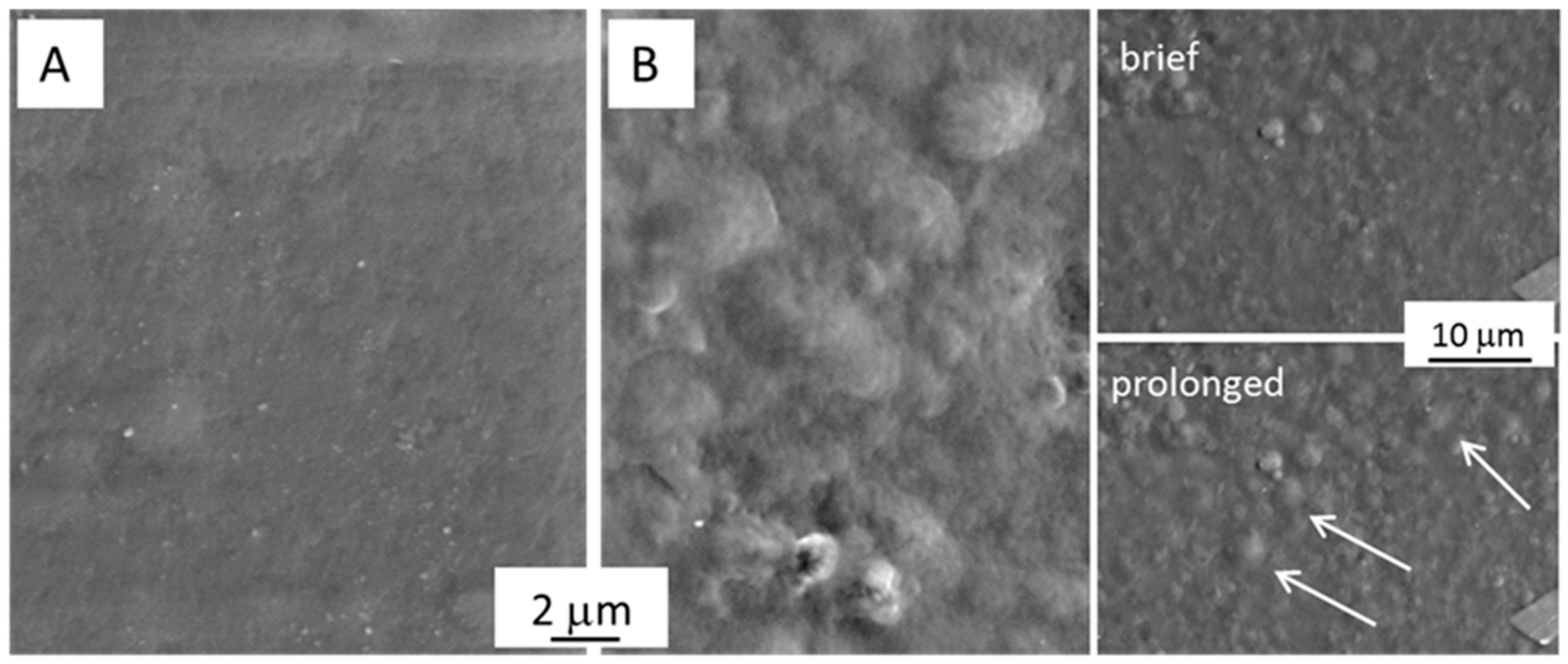
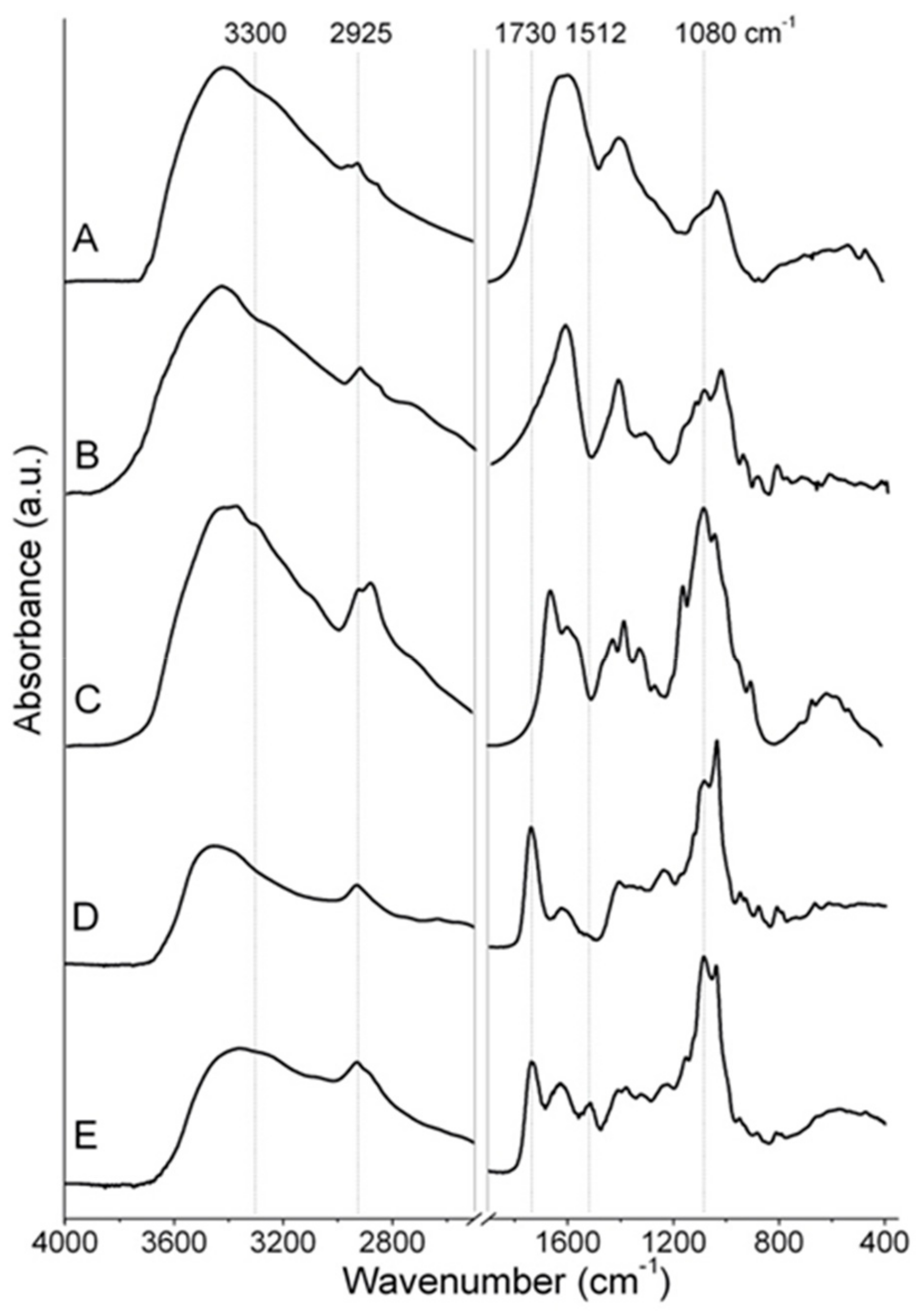
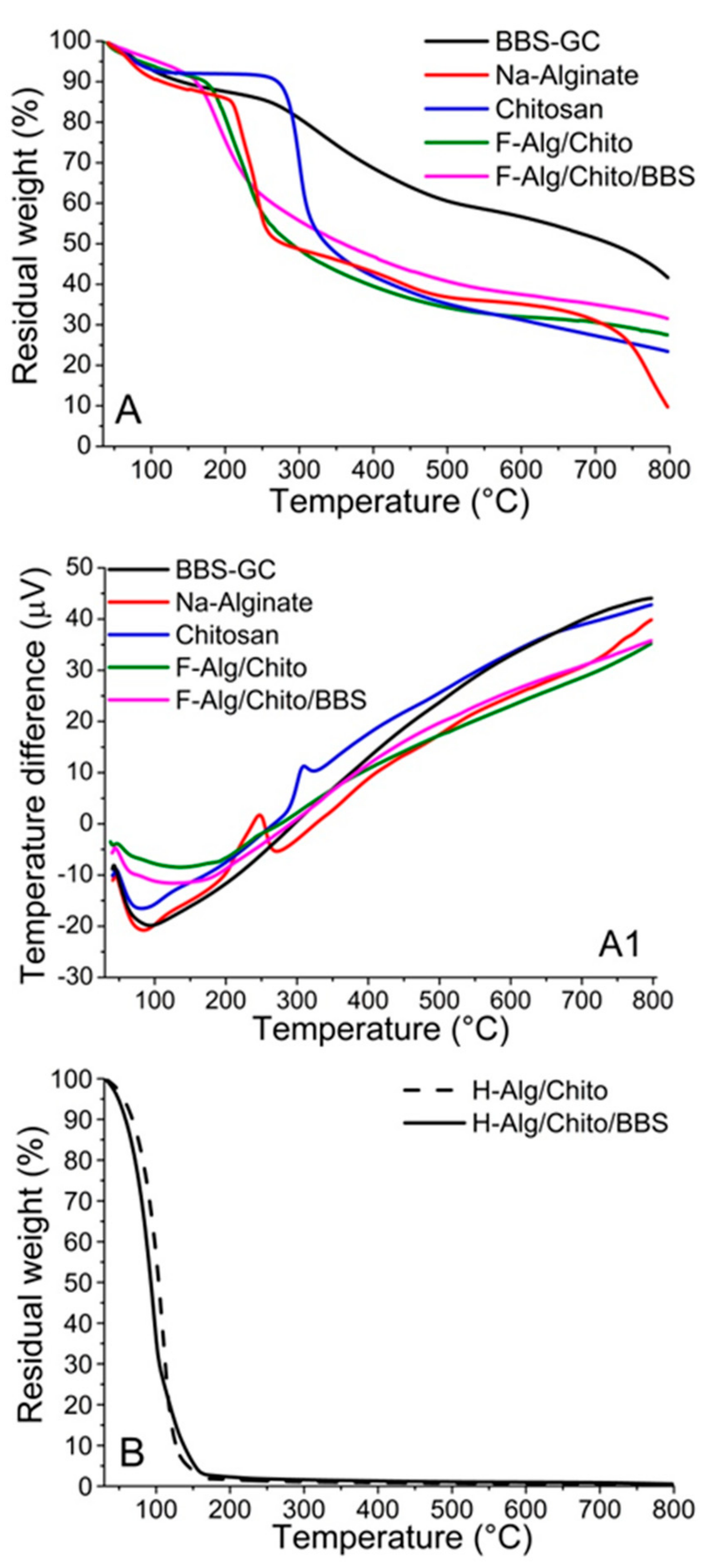
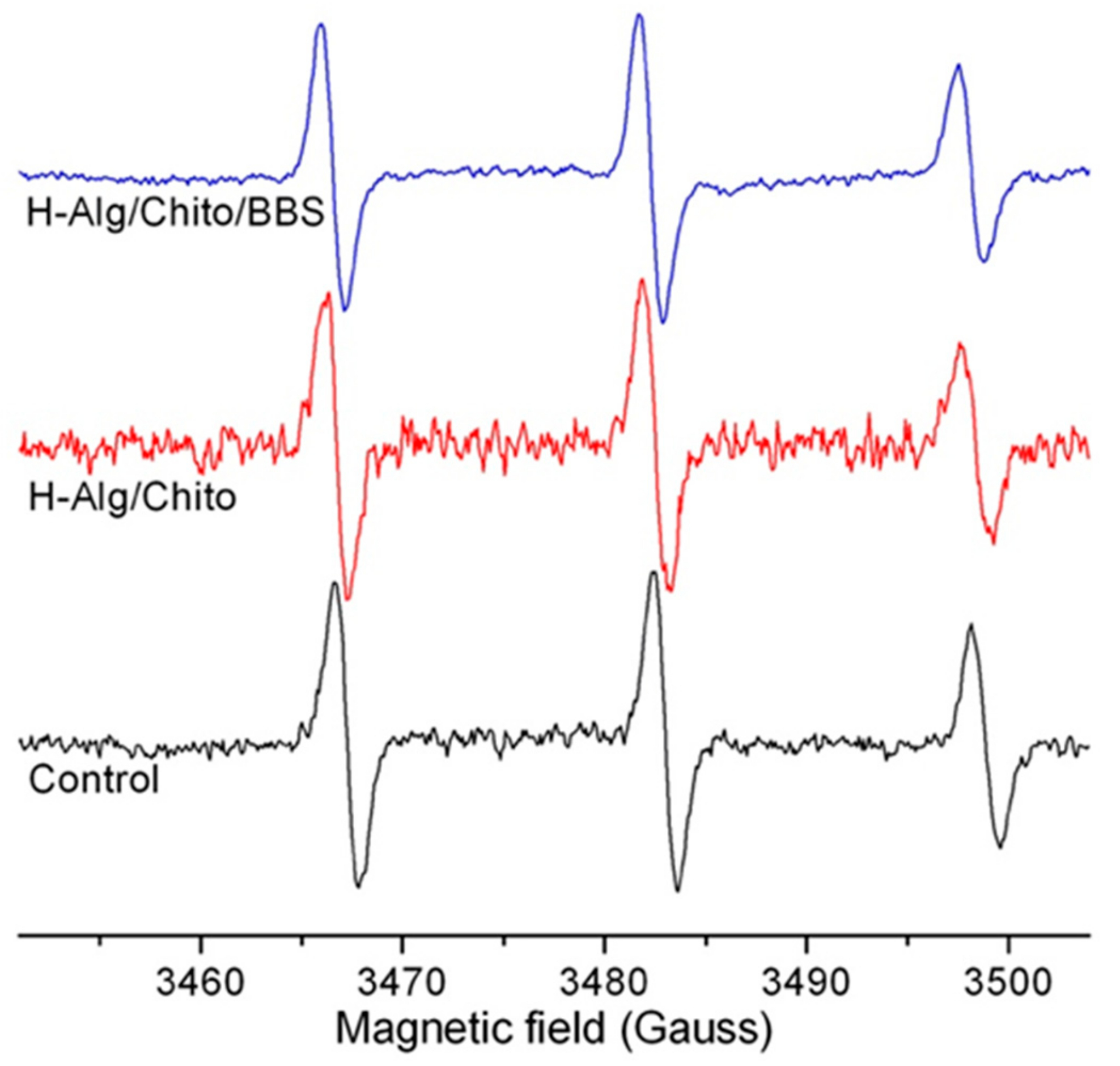
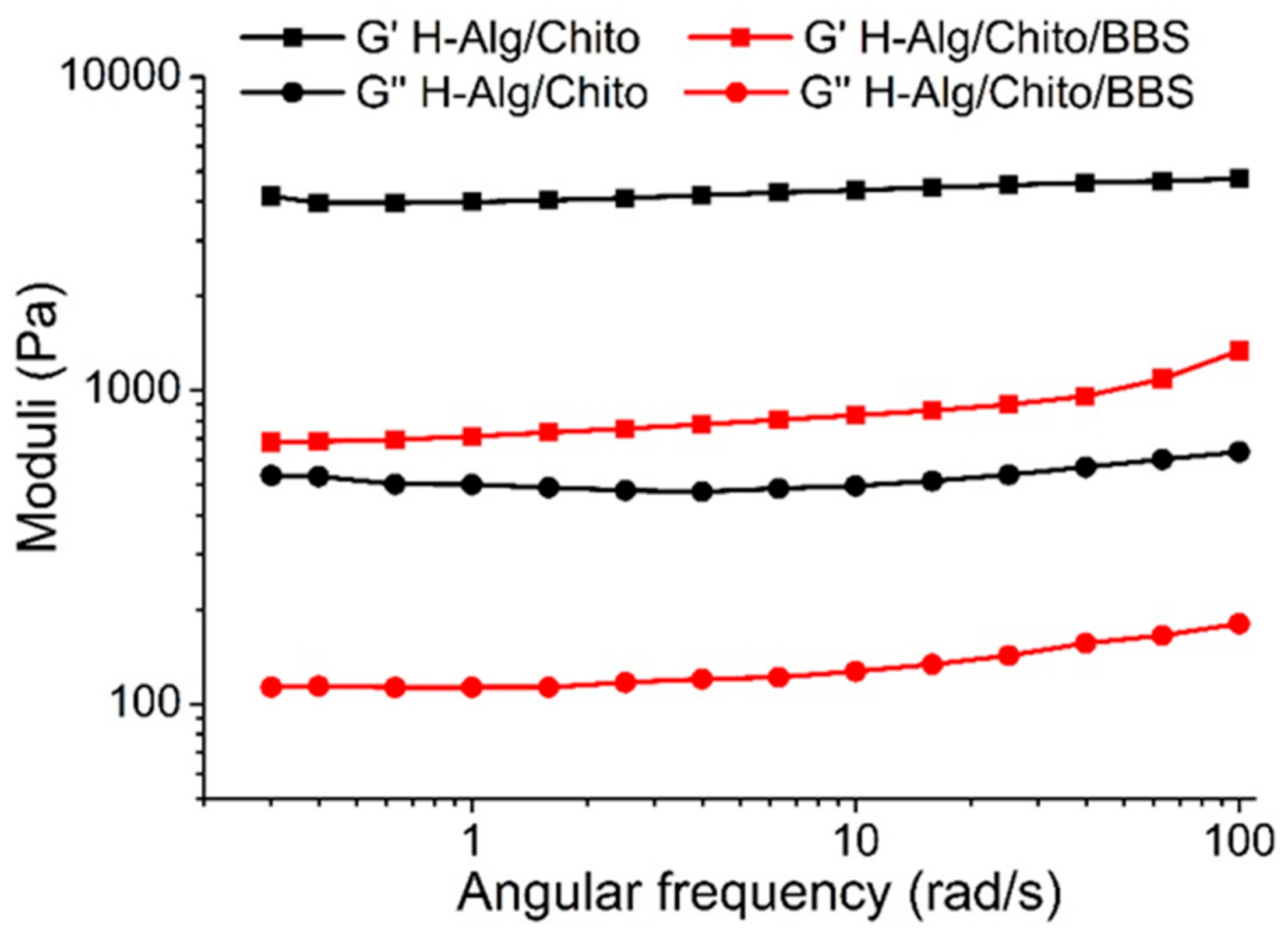
2.1. Physicochemical Characterizations
2.2. Adsorption Experiments
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BBS-GC | Biowaste-derived substances isolated from green compost |
| CV | Crystal Violet |
| DTA | Differential Thermal Analysis (DTA) |
| EPR | Electron Paramagnetic Resonance |
| F-Alg/Chito | Film of Alginate and Chitosan |
| F-Alg/Chito/BBS | Film of Alginate, Chitosan and BBS-GC |
| FTIR | Fourier transform infrared spectroscopy |
| H-Alg/Chito | Hydrogels of Alginate and Chitosan |
| H-Alg/Chito/BBS | Hydrogels of Alginate, Chitosan and BBS-GC |
| OII | Orange II |
| RH | Rhodamine B |
| RT | Room Temperature |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analyses |
References
- U.N. Department of Public Information Sustainable Development Goals for People and Planet: Sustainable Development Knowledge Platform. Available online: https://sustainabledevelopment.un.org/?menu=1300 (accessed on 20 November 2019).
- Fiksel, J.; Lal, R. Transforming waste into resources for the Indian economy. Environ. Dev. 2018, 26, 123–128. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Huang, Y. Role of alginate in antibacterial finishing of textiles. Int. J. Biol. Macromol. 2017, 94, 466–473. [Google Scholar] [CrossRef]
- Ouwerx, C.; Velings, N.; Mestdagh, M.M.; Axelos, M.A.V. Physico-chemical properties and rheology of alginate gel beads formed with various divalent cations. Polym. Gels Netw. 1998, 6, 393–408. [Google Scholar] [CrossRef]
- Mihranyan, A. Cellulose from cladophorales green algae: From environmental problem to high-tech composite materials. J. Appl. Polym. Sci. 2011, 119, 2449–2460. [Google Scholar] [CrossRef]
- Guerretta, F.; Magnacca, G.; Franzoso, F.; Ivanchenko, P.; Nisticò, R. Sodium alginate conversion into char via pyrolysis at the onset temperature. Mater. Lett. 2019, 234, 339–342. [Google Scholar] [CrossRef]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Chitosan and alginate types of bio-membrane in fuel cell application: An overview. J. Power Sources 2015, 289, 71–80. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Nisticò, R. Aquatic-derived biomaterials for a sustainable future: A European opportunity. Resources 2017, 6, 65. [Google Scholar] [CrossRef]
- Sila, A.; Mlaik, N.; Sayari, N.; Balti, R.; Bougatef, A. Chitin and chitosan extracted from shrimp waste using fish proteases aided process: Efficiency of chitosan in the treatment of unhairing effluents. J. Polym. Environ. 2014, 22, 78–87. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; de Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Nisticò, R.; Faga, M.G.; Gautier, G.; Magnacca, G.; D’Angelo, D.; Ciancio, E.; Piacenza, G.; Lamberti, R.; Martorana, S. Physico-chemical characterization of functionalized polypropylenic fibers for prosthetic applications. Appl. Surf. Sci. 2012, 258, 7889–7896. [Google Scholar] [CrossRef]
- Nisticò, R.; Franzoso, F.; Cesano, F.; Scarano, D.; Magnacca, G.; Parolo, M.E.; Carlos, L. Chitosan-derived iron oxide systems for magnetically guided and efficient water purification processes from polycyclic aromatic hydrocarbons. ACS Sustain. Chem. Eng. 2017, 5, 793–801. [Google Scholar] [CrossRef]
- Magnacca, G.; Guerretta, F.; Vizintin, A.; Benzi, P.; Valsania, M.C.; Nisticò, R. Preparation, characterization and environmental/electrochemical energy storage testing of low-cost biochar from natural chitin obtained via pyrolysis at mild conditions. Appl. Surf. Sci. 2018, 427, 883–893. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A review on latest innovations in natural gums based hydrogels: Preparations & applications. Int. J. Biol. Macromol. 2019, 136, 870–890. [Google Scholar] [PubMed]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Chubar, N. New inorganic (an)ion exchangers based on Mg-Al hydrous oxides: (alkoxide-free) sol-gel synthesis and characterisation. J. Colloid Interface Sci. 2011, 357, 198–209. [Google Scholar] [CrossRef]
- Vijaya, Y.; Popuri, S.R.; Boddu, V.M.; Krishnaiah, A. Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr. Polym. 2008, 72, 261–271. [Google Scholar] [CrossRef]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef]
- Franzoso, F.; Nisticò, R.; Cesano, F.; Corazzari, I.; Turci, F.; Scarano, D.; Bianco Prevot, A.; Magnacca, G.; Carlos, L.; Mártire, D.O. Biowaste-derived substances as a tool for obtaining magnet-sensitive materials for environmental applications in wastewater treatments. Chem. Eng. J. 2017, 310, 307–316. [Google Scholar] [CrossRef]
- Deganello, F.; Tummino, M.L.; Calabrese, C.; Testa, M.L.; Avetta, P.; Fabbri, D.; Prevot, A.B.; Montoneri, E.; Magnacca, G. A new, sustainable LaFeO3 material prepared from biowaste-sourced soluble substances. New J. Chem. 2015, 39, 877–885. [Google Scholar] [CrossRef]
- Nisticò, R.; Barrasso, M.; Carrillo Le Roux, G.A.; Seckler, M.M.; Sousa, W.; Malandrino, M.; Magnacca, G. Biopolymers from composted biowaste as stabilizers for the synthesis of spherical and homogeneously sized silver nanoparticles for textile applications on natural fibers. ChemPhysChem 2015, 16, 3902–3909. [Google Scholar] [CrossRef] [PubMed]
- Tummino, M.L.; Testa, M.L.; Malandrino, M.; Gamberini, R.; Prevot, A.B.; Magnacca, G.; Laurenti, E. Green waste-derived substances immobilized on SBA-15 silica: Surface properties, adsorbing and photosensitizing activities towards organic and inorganic substrates. Nanomaterials 2019, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Sadraei, R.; Paganini, M.C.; Calza, P.; Magnacca, G. An easy synthesis for preparing bio-based hybrid adsorbent useful for fast adsorption of polar pollutants. Nanomaterials 2019, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Favaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textiles dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Mani, S.; Chowdhary, P.; Bharagava, R.N. Textile wastewater dyes: Toxicity profile and treatment approaches. In Emerging and Eco-Friendly Approaches for Waste Management; Bharagawa, R., Chowdhary, P., Eds.; Springer: Singapore, 2019; pp. 219–244. [Google Scholar]
- Azari, A.; Noorisepehr, M.; Dehghanifard, E.; Karimyan, K.; Hashemi, S.Y.; Kalhori, E.M.; Norouzi, R.; Agarwal, S.; Gupta, V.K. Experimental design, modeling and mechanism of cationic dyes biosorption on to magnetic chitosan-lutaraldehyde composite. Int. J. Biol. Macromol. 2019, 15, 633–645. [Google Scholar] [CrossRef]
- Kousha, M.; Daneshvar, E.; Sohrabi, M.S.; Jokar, M.; Bhatnagar, A. Adsorption of acid orange II dye by raw and chemically modified brown macroalfa Stoechospermum marginatum. Chem. Eng. J. 2012, 192, 67–76. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Samantilleke, A.P.; Parpot, P.; Fernandes, F.; Pastor, M.; Correia, A.; Luis, E.A.; Chivanga Barros, A.A.; Teixeira, V. Visible light induced enhanced photocatalytic degradation of industrial effluents (Rhodamine B) in aqueous media using TiO2 nanoparticles. J. Nanomater. 2016, 2016, 4396175. [Google Scholar] [CrossRef]
- Hayeeye, F.; Sattar, M.; Chinpa, W.; Sirichote, O. Kinetics and thermodynamics of Rhodamine B adsorption by gelatin/activated carbon composite beads. Colloids Surf. A 2017, 513, 259–266. [Google Scholar] [CrossRef]
- Asadi, S.; Eris, S.; Azizian, S. Alginate-based hydrogel beads as a biocompatible and efficient adsorbent for dye removal from aqueous solutions. ACS Omega 2018, 3, 15140–15148. [Google Scholar] [CrossRef]
- Abbas, M.; Harrache, Z.; Trari, M. Removal of gentian violet in aqueous solution by activated carbon equilibrium, kinetics, and thermodynamic study. Adsorpt. Sci. Technol. 2019, 37, 566–589. [Google Scholar] [CrossRef]
- Fabryanty, R.; Valencia, C.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Kurniawan, A.; Ju, Y.-H.; Ismadji, S. Removal of cristal violet dye by adsorption using bentonite—Alginate composite. J. Environ. Chem. Eng. 2017, 5, 5677–5687. [Google Scholar] [CrossRef]
- Conzatti, G.; Faucon, D.; Castel, M.; Ayadi, F.; Cavalie, S.; Tourrette, A. Alginate/chitosan polyelectrolyte complexes: A comparative study of the influence of the drying step on physicochemical properties. Carbohydr. Polym. 2017, 172, 142–151. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Chitosan-sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Sartori, C.; Finch, D.S.; Ralph, B.; Gilding, K. Determination of the cation content of alginate thin films by FTi.r. spectroscopy. Polymer 1997, 38, 43–51. [Google Scholar] [CrossRef]
- Abreu, F.O.M.S.; Bianchini, C.; Forte, M.M.C.; Kist, T.B.L. Influence of the composition and preparation method on the morphology and swelling behavior of alginate-chitosan hydrogels. Carbohydr. Polym. 2008, 74, 283–289. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- De Britto, D.; Campana-Filho, S.P. A kinetic study on the thermal degradation of N,N,N-trimethylchitosan. Polym. Degrad. Stab. 2004, 84, 353–361. [Google Scholar] [CrossRef]
- Avetta, P.; Nisticò, R.; Faga, M.G.; D’Angelo, D.; Aimo Boot, E.; Lamberti, R.; Martorana, S.; Calza, P.; Fabbri, D.; Magnacca, G. Hernia-repair prosthetic devices functionalised with chitosan and ciprofloxacin coating: Controlled release and antibacterial activity. J. Mater. Chem. B 2014, 2, 5287–5294. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate-chitosan complex formed with different polymers ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclet. Quim. 2004, 29, 57–63. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, H.G. Thermal and carbothermic decomposition of Na2CO3 and Li2CO3. Metall. Mater. Trans. B 2001, 32, 17–24. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Pasparakis, G.; Bouropoulos, N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate-chitosan beads. Int. J. Pharm. 2006, 323, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Štrancar, J.; Šentjurc, M.; Schara, M. Fast and accurate characterization of biological membranes by EPR spectral simulations of nitroxides. J. Magn. Reson. 2000, 142, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a phenomenological definition of the term “gel”. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Jean, L.; Lee, C.F.; Hodder, P.; Hawkins, N.; Vaux, D.J. Dynamics of the formation of a hydrogel by a pathogenic amyloid peptide: Islet amyloid polypeptide. Sci. Rep. 2016, 6, 32124. [Google Scholar] [CrossRef]
- McHedlov-Petrosyan, N.O.; Kholin, Y.V. Aggregation of rhodamine B in water. Russ. J. Appl. Chem. 2004, 77, 414–422. [Google Scholar] [CrossRef]
- Palma, D.; Bianco Prevot, A.; Celi, L.; Martin, M.; Fabbri, D.; Magnacca, G.; Chierotti, M.R.; Nisticò, R. Isolation, characterization, and environmental application of bio-based materials as auxiliaries in photocatalytic processes. Catalysts 2018, 8, 197. [Google Scholar] [CrossRef]
- You, R.; Xiao, C.; Zhang, L.; Dong, Y. Versatile particles from water-soluble chitosan and sodium alginate for loading toxic or bioactive substance. Int. J. Biol. Macromol. 2015, 79, 498–503. [Google Scholar] [CrossRef]
- Dual, P.; Kandemir, N.; Wang, J.; Chen, J. Rheological characterization of alginate based hydrogels for tissue engineering. MRS Adv. 2017, 2, 1309–1314. [Google Scholar]
- Bonino, C.A.; Samorezov, J.E.; Jeon, O.; Alsberg, E.; Khan, S.A. Real-time in situ rheology of alginate hydrogel photocrosslinking. Soft Matter 2011, 7, 11510–11517. [Google Scholar] [CrossRef]
- Weng, L.; Chen, X.; Chen, W. Rheological characterization of in situ crosslinkable hydrogels formulated from oxidized dextran and N-carboxyethyl chitosan. Biomacromolecules 2007, 8, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Hashemnejad, S.M.; Kundu, S. Rheological properties and failure of alginate hydrogels with ionic and covalent crosslinks. Soft Matter 2019, 15, 7852–7862. [Google Scholar] [CrossRef]
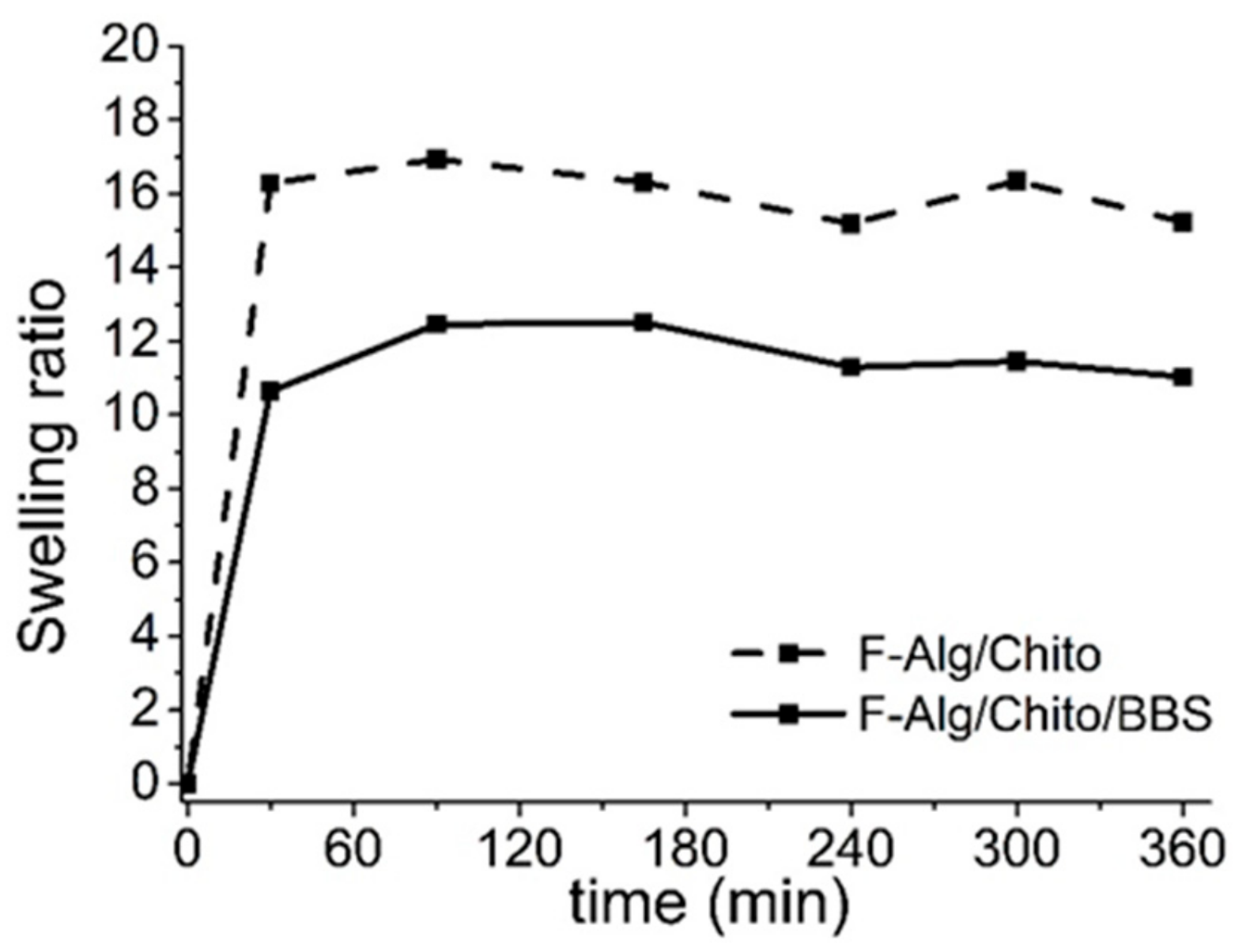
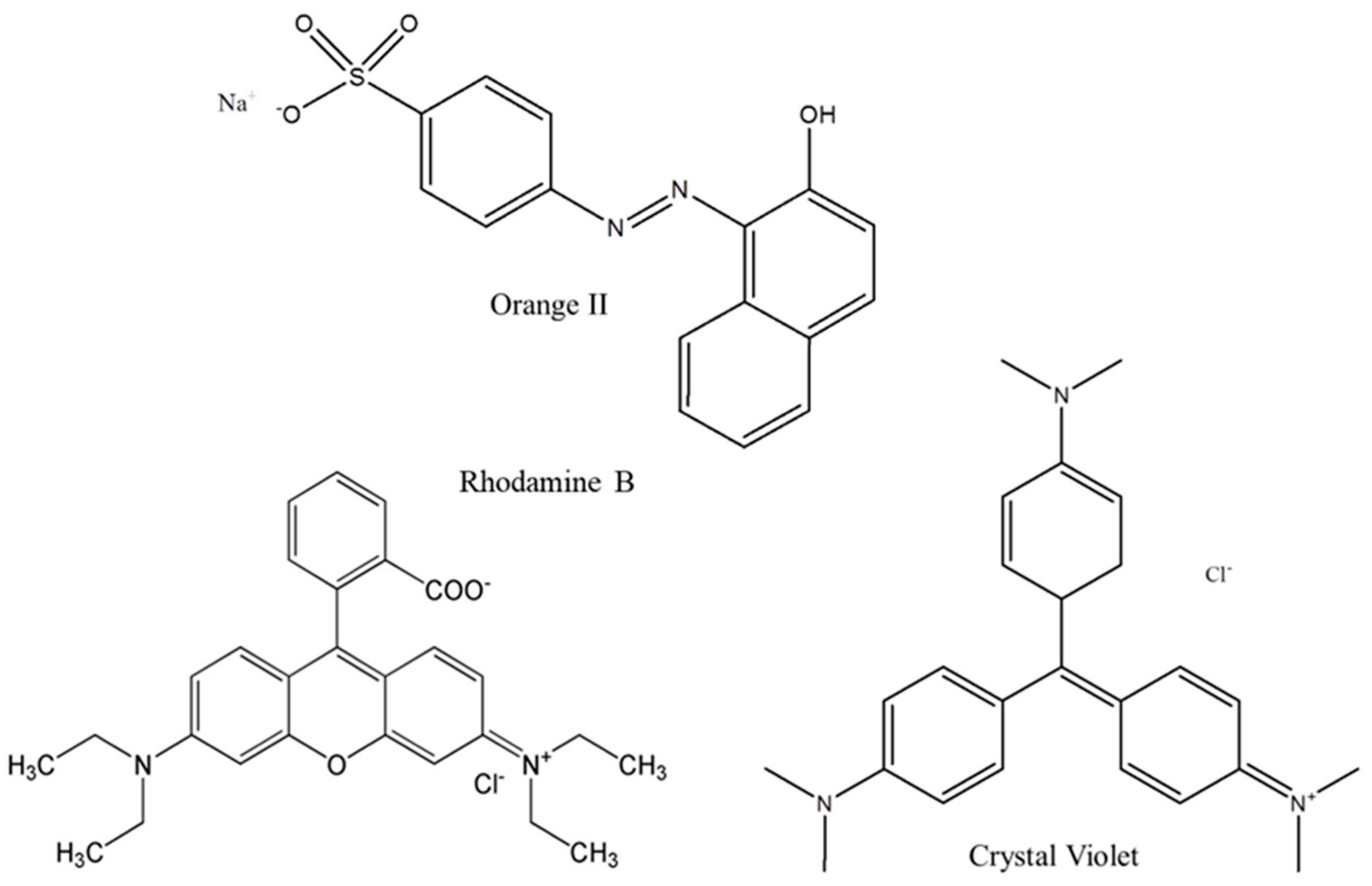
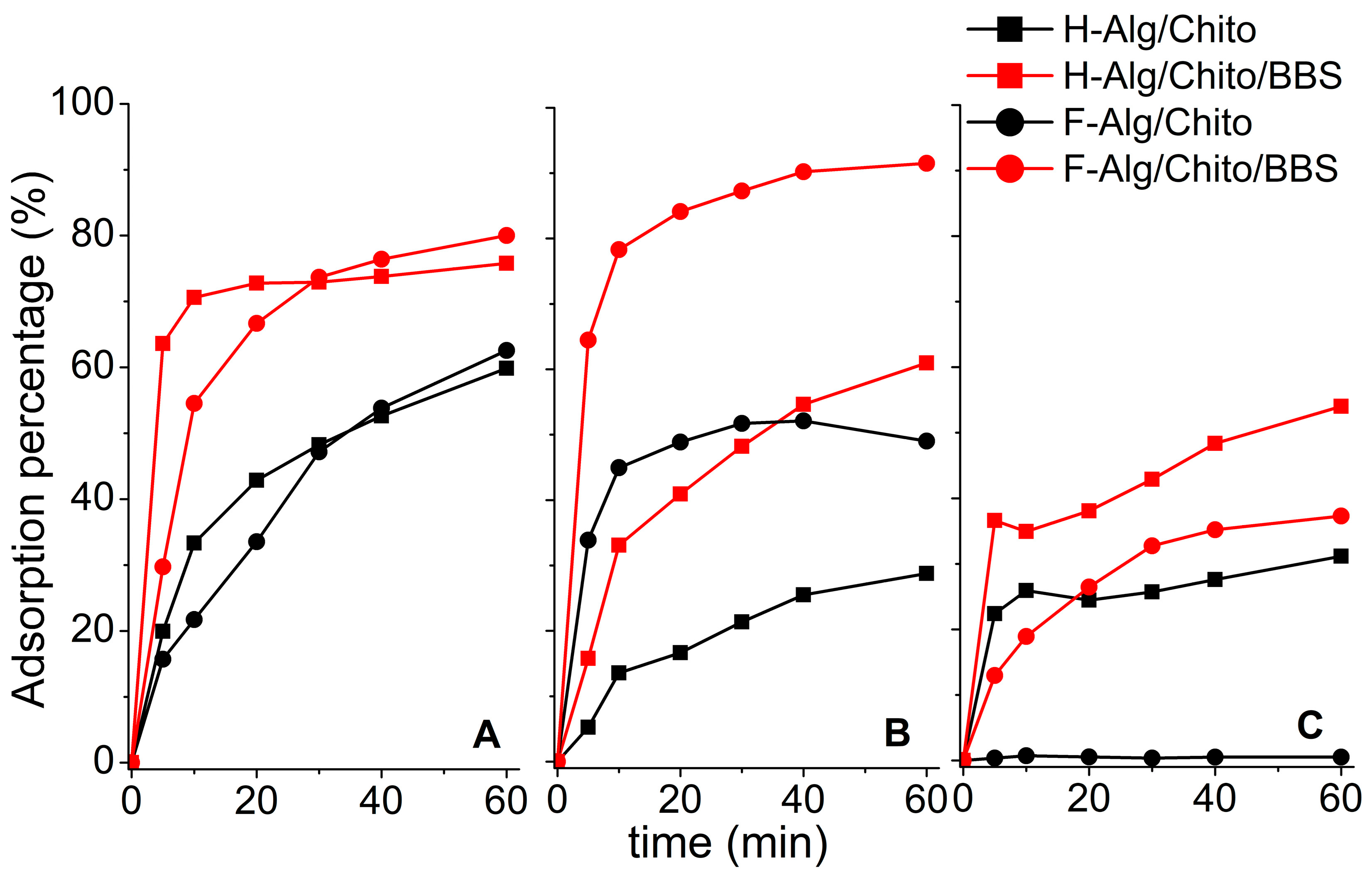
| Sample Compositions (Hydrogels) | Water (%) | Sample Compositions (Dried Films) | Swelling Ratio |
|---|---|---|---|
| Alginate + Chitosan | 98 | Alginate + Chitosan | 16 |
| Alginate + Chitosan + BBS-GC | 97 | Alginate + Chitosan + BBS-GC | 13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tummino, M.L.; Magnacca, G.; Cimino, D.; Laurenti, E.; Nisticò, R. The Innovation Comes from the Sea: Chitosan and Alginate Hybrid Gels and Films as Sustainable Materials for Wastewater Remediation. Int. J. Mol. Sci. 2020, 21, 550. https://doi.org/10.3390/ijms21020550
Tummino ML, Magnacca G, Cimino D, Laurenti E, Nisticò R. The Innovation Comes from the Sea: Chitosan and Alginate Hybrid Gels and Films as Sustainable Materials for Wastewater Remediation. International Journal of Molecular Sciences. 2020; 21(2):550. https://doi.org/10.3390/ijms21020550
Chicago/Turabian StyleTummino, Maria Laura, Giuliana Magnacca, Dafne Cimino, Enzo Laurenti, and Roberto Nisticò. 2020. "The Innovation Comes from the Sea: Chitosan and Alginate Hybrid Gels and Films as Sustainable Materials for Wastewater Remediation" International Journal of Molecular Sciences 21, no. 2: 550. https://doi.org/10.3390/ijms21020550
APA StyleTummino, M. L., Magnacca, G., Cimino, D., Laurenti, E., & Nisticò, R. (2020). The Innovation Comes from the Sea: Chitosan and Alginate Hybrid Gels and Films as Sustainable Materials for Wastewater Remediation. International Journal of Molecular Sciences, 21(2), 550. https://doi.org/10.3390/ijms21020550