Abstract
Partial or complete obstruction of the urinary tract is a common and challenging urological condition caused by a variety of conditions, including ureteral calculi, ureteral pelvic junction obstruction, ureteral stricture, and malignant ureteral obstruction. The condition, which may develop in patients of any age, induces tubular and interstitial injury followed by inflammatory cell infiltration and interstitial fibrosis, eventually impairing renal function. The serum creatinine level is commonly used to evaluate global renal function but is not sensitive to early changes in the glomerular filtration rate and unilateral renal damage. Biomarkers of acute kidney injury are useful for the early detection and monitoring of kidney injury induced by upper urinary tract obstruction. These markers include levels of neutrophil gelatinase-associated lipocalin (NGAL), monocyte chemotactic protein-1, kidney injury molecule 1, N-acetyl-b-D-glucosaminidase, and vanin-1 in the urine and serum NGAL and cystatin C concentrations. This review summarizes the pathophysiology of kidney injury caused by upper urinary tract obstruction, the roles played by emerging biomarkers of obstructive nephropathy, the mechanisms involved, and the clinical utility and limitations of the biomarkers.
1. Introduction
Upper urinary tract obstruction (UUTO) is a common and challenging urological condition caused by a variety of diseases, such as ureteropelvic junction obstruction (UPJO), ureteral calculi, ureteral strictures, and malignant ureteral obstruction. The condition may occur in patients of any age. Surgical intervention is necessary for moderate to severe cases, depending on the cause of the obstruction.
Hydronephrosis, or swelling in one or both kidneys due to incomplete emptying, is often observed in UUTO patients. However, the extent of hydronephrosis does not necessarily reflect the severity of UUTO. Obstruction may be minimal despite moderate to severe hydronephrosis, or it may be severe without obvious hydronephrosis. Renal scans together with determination of the glomerular filtration rate constitute the standard method of evaluating the presence and severity of UUTO. These examinations can be time-consuming and distressing especially to the child, and are not sensitive or specific enough to identify those kidneys that require treatment in all cases. Additionally, renal scans are expensive and not always available. Therefore, there is a great need for the development of new methods to stratify and monitor patients, and the biomarker research field is a promising approach for this purpose. Urinary as well as serum proteins provide information of the physiological condition in the kidney and have the potential to be used as prognostic tools for early disease detection and the choice of the optimal treatment and monitoring [1]. The present review summarizes the pathophysiology of kidney injury caused by UUTO, the roles played by emerging biomarkers of obstructive nephropathy, the mechanisms involved, and the clinical utility and limitations of the biomarkers.
2. Upper Urinary Tract Obstruction
2.1. UPJO
Congenital obstructive nephropathy reflects maldevelopment of the urinary tract in utero. Most commonly, lesions lie in the ureteropelvic junction (UPJ), causing chronic renal failure. Rapid diagnosis and treatment are essential to preserving function and slowing renal damage. The prevalence is one in 1500 live births [2]. Although UPJO is less common in adults, the condition is not rare [3]. In addition to having a congenital cause, acquired stenosis of the UPJ may follow an upper urinary tract infection, the development of stones, trauma, or ischemia. Vessels that compress or distort the UPJ when crossing the urinary tract may obstruct ureteral outflow in adults.
2.2. Ureteral Calculi
Urinary tract stones are very common. The prevalence is 1–19.1% in Asia, 5–9% in Europe, and 7–13% in North America [4,5]. Although most small stones pass spontaneously, some do not, causing UUTO with or without infection. Surgical intervention (shock wave or ureteroscopic lithotripsy) is required to prevent the impairment of renal function [6]. Even after stones are removed, some patients develop ureteral strictures that may continue to impair renal function.
2.3. Ureteral Strictures
A ureteral stricture is a narrowing of the ureter that causes an obstruction. Strictures cause significant morbidity and mortality from renal failure. Benign strictures are typically caused by ischemia or inflammation. Causes include radiation, trauma associated with calculus impaction, pelvic surgery, and ureteroscopy [7]. Moderate to severe strictures require surgical intervention, such as balloon dilation, endoureterotomy, or stricture resection.
2.4. Malignant Ureteral Obstruction
A malignant ureteral obstruction develops secondary to a malignant tumor. A primary tumor may infiltrate the ureteral wall and compress the ureter, swollen lymph nodes may wrap around the ureter, edema and retroperitoneal fibrosis that develop after radiotherapy may distort the ureter or cause luminal stenosis, or ureter elasticity may be weakened [8]. The condition may be unilateral or bilateral. Clinical removal of the obstruction and rapid improvement in renal function are the aims of treatment. Although ureteral stenting or nephrostomy is performed in severe cases, these procedures reduce the quality of life. Markers of severity are required.
3. The Pathophysiology of Kidney Injury Caused by UUTO (Figure 1)
Urinary tract obstruction affects renal function in many ways. The increase in intratubular hydrostatic pressure [9] triggers renopathogenic effects via three proposed mechanisms: tubular ischemia caused by hypoperfusion, pressure-induced mechanical stretching or compression of tubular cells, and altered urinary shear stress. The latter two mechanisms are likely the primary causes of obstructive renal injury [10], being associated with the dysregulation of many cytokines, growth factors, enzymes, and cytoskeletal proteins. Changes in early renal hemodynamics are followed by structural and functional changes in the entire nephron. The earliest stage of UUTO is associated with an increase in renal blood flow 1–2 h in duration [10]. The intrarenal renin–angiotensin–aldosterone system is then activated, which causes pre- and post-glomerular vasoconstriction and resultant drops in renal blood flow, medullary oxygen tension, and the glomerular filtration rate [11,12]. The increased intra-renal angiotensin II activates nuclear factor kappa B, triggering cytokine release and reactive oxygen species (ROS) production [2,10,13,14]. Adhesion molecules such as selectins attract infiltrating macrophages, monocyte chemotactic protein-1 (MCP-1) is upregulated, and tumor necrosis factor-α (TNF-α) is released. Monocytes and macrophages are attracted to the tubular interstitium of the UUTO kidney [2,14,15]. Activated macrophages infiltrate the interstitium, sustaining the inflammatory response by releasing cytokines such as transforming growth factor-β1 (TGF-β1) and TNF-α and ROS [16]. ROS mediate the profibrotic action of TGF-β1, and renal fibrosis proceeds via the epithelial–mesenchymal transition (EMT) of renal tubular epithelial cells. The outcome is interstitial fibrosis caused by increased deposition of the extracellular matrix, cellular infiltration, tubular apoptosis, and the EMT [17]. The mechanical stretching of tubular cells, ischemia, and oxidative stress that follow ureteral obstruction cause tubular cell death [18,19]. Mild injury triggers apoptosis, while tubulointerstitial atrophy after obstruction causes cell deletion. The apoptotic bodies are phagocytosed by neighboring tubular cells or directly shed into the tubular lumen, reestablishing homeostasis. When the injury is severe, necrosis is likely to be the predominant cause of cell loss [19,20]. Increased apoptosis and/or necrosis activates cell infiltration, interstitial cell proliferation, and interstitial fibrosis (Figure 1).
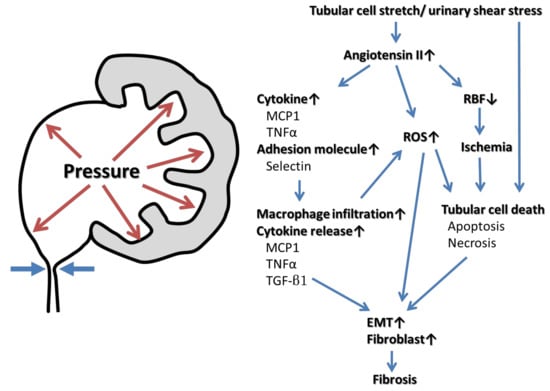
Figure 1.
Mechanisms of UUTO causing kidney injury and fibrosis.
Hydrostatic pressure stretches tubular cells and creates urinary shear stress, inducing intrarenal angiotensin II activation followed by the release of cytokines and adhesion molecules, in turn triggering macrophage infiltration, the production of reactive oxygen species (ROS), and decreased renal blood flow (RBF). The drop in RBF triggers renal ischemia, and the increase of hydrostatic pressure and ROS causes tubular cell death. Monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α) and transforming growth factor-β1 (TGF-β1) released from activated macrophages, ROS, and/or tubular cell death induce the epithelial–mesenchymal transition (EMT) and fibroblast proliferation. Eventually the renal parenchyma is transformed into fibrotic tissue.
4. Imaging Studies and Their Limitations
Technetium 99m (99mTc) mertiatide, 99mTc diethylene triamine penta-acetic acid, or 99mTc dimercaptosuccinic acid renal scans (with or without diuresis) are commonly used to evaluate the presence and severity of UUTO in patients with hydronephrosis. Patients are divided into those with no, partial, or complete obstruction and with or without renal function [21,22,23]. Urgent surgical relief of complete obstruction is essential, otherwise the kidney will rapidly become nonfunctional. A partial obstruction is a resistance to outflow that, if left untreated, will lead to a loss of kidney function. If renal function is lost, surgery is not considered unless the kidney may be infected. Although renal scans are the standard method of evaluating the presence and severity of UUTO, they are expensive and expose patients to radiation, and repeat scans should be avoided. Furthermore, they do not reveal kidney damage per se, and the equipment is not widely available.
5. Biomarkers of UUTO
Urinary and serum biomarkers facilitate the evaluation of renal damage in UUTO patients. An ideal biomarker is assessed noninvasively in a simple manner, is highly sensitive and specific in terms of early detection, and exhibits a wide dynamic range and cutoff values, allowing for risk stratification. Diagnostic utility improves when pelvic urine samples (compared to bladder urine) are used [23,24]. However, the collection of renal pelvic urine is invasive, requiring the placement of an indwelling ureteral catheter via cystoscopy under X-ray guidance, and thus it is difficult to repeatedly collect renal pelvic urine. Biomarkers of kidney injury evaluate glomerular function and renal tubular damage. Serum creatinine (SCr) and cystatin C are representative glomerular function biomarkers. Levels of neutrophil gelatinase-associated lipocalin (NGAL), MCP-1, kidney injury molecule 1 (KIM-1), N-acetyl-b-D-glucosaminidase (NAG), and liver type fatty acid-binding protein (L-FABP) are used to evaluate proximal tubule damage.
5.1. Biomarkers of Glomerular Function
5.1.1. SCr
SCr concentrations are widely used to assess kidney function. However, accumulating evidence indicates that measurements of SCr levels do not always detect kidney disease early, and individual variability in SCr generation rates limits the utility of these tests in terms of identifying and assessing the severity of kidney injury [25]. Furthermore, UUTO often affects the unilateral upper urinary tract. The contralateral kidney can compensate for the loss of renal function.
5.1.2. Cystatin C
Cystatin C is an endogenous cysteine protease inhibitor of molecular weight 13.3 kDa secreted by most nucleated cells [26]. It is an ideal filtration marker, being produced at a stable rate, freely filtered without tubular secretion, and completely catabolized in the proximal tubule [27]. Cystatin C is distributed only in the extracellular space and thus reflects changes in the glomerular filtration rate more precisely than creatinine, which is distributed in all body water [28]. In one study, the serum cystatin C level strongly predicted all-cause acute kidney injury (AKI). The area under the curve (AUC; the receiver operating characteristic curve [ROC]) was 0.89 [29]. Use of the urine cystatin C level for early detection of AKI after cardiac surgery allows for the diagnosis of tubular damage and dysfunction [30]. Serum cystatin C is a useful biomarker for AKI in patients in the intensive care unit (ICU) [31,32] with contrast-induced AKI [29,33]. In one study, preoperative serum cystatin C levels were significantly higher in children with UPJO compared to controls and decreased after surgery (Table 1) [26], and the AUC-ROC value of serum cystatin C indicating UPJO was 0.72 (Table 1) [26,34]. In another study, serum cystatin C levels increased in adults with ureteral calculi as hydronephrosis increased and differed significantly between patients with no and mild hydronephrosis, while SCr levels did not [35]. Multivariate logistic regression showed that only the serum cystatin C level was an independent risk factor for hydronephrosis. By contrast, the urine cystatin C level is less useful as a UUTO biomarker. In two independent studies of children with UUTO, urine cystatin C levels did not differ between patients and controls (Table 1) [24,36].

Table 1.
Urinary and serum biomarkers for pediatric and adults UUTO.
5.2. Biomarkers of Renal Tubular Damage
5.2.1. NGAL
Human NGAL, a ubiquitous 25 kDa protein, was initially isolated from human neutrophils [37]. NGAL is expressed in small amounts in cells other than neutrophils, including lung, spleen, and kidney cells, and is thought to inhibit bacterial growth, scavenge iron, and induce epithelial growth [38]. NGAL can be secreted by epithelial cells, and it is markedly elevated in patients exhibiting an inflammatory immune response and defects in lipid metabolism, intracellular iron transport, renal tubular repair, or differentiation of kidney progenitor cells into tubular epithelial cells [39]. In the kidney, NGAL is secreted into the urine from the ascending limb of the loop of Henle to the collecting ducts, being synthesized in the distal nephron [40]. NGAL is small, freely filtered, and easily assayed in urine. The urine NGAL level is an early and sensitive biomarker of kidney injury [41]. The serum or urine NGAL level is a clinically useful biomarker of various types of AKI, including AKI after kidney transplantation [42], contrast medium-induced AKI [43], and AKI in critical care settings [44]. In children with UUTO, urine NGAL levels are significantly higher in bladder urine and/or renal pelvic urine compared to controls, correlate inversely with worsening obstruction, and decrease after surgery (Table 1) [26,36,45,46,47,48,49]. The AUC-ROC value for UUTO in children is 0.61–0.90 for bladder urine NGAL [26,36,45,46,47,48]. In adults with UUTO, the urine NGAL level increases in those with obstructive nephropathy (AUC-ROCs of 0.70 for bladder urine and 0.76 for renal pelvic urine) and decreases after relief of the obstruction [23,50,51]. Serum NGAL levels are significantly higher than in controls [45,50]. However, the use of NGAL as a biomarker of kidney injury induced by UUTO has several limitations. Age affects the predictive performance: NGAL better predicts AKI in children than in adults [52]. Serum and urine NGAL levels may be influenced by conditions other than UUTO, including chronic hypertension, systemic infection, inflammation, anemia, hypoxia, or malignancy [53,54,55]. The many sources of NGAL can render it difficult to identify the underlying pathology [40].
5.2.2. MCP-1
MCP-1, a 13 kDa protein, is a potent attractant of monocytes and a member of the CC subfamily [56]. It is produced by many types of cells, including epithelial, endothelial, and smooth muscle cells; fibroblasts, astrocytes, and monocytes; and microglial cells. MCP-1 recruits monocytes, memory T-cells, and dendritic cells to sites of tissue injury and infection; monocytes and macrophages are the major sources of MCP-1 [57]. MCP-1 mRNA is undetectable in the normal kidney, but MCP-1 gene expression is markedly increased at the tubulointerstitial level in UPJO biopsy samples and correlates with the extent of monocyte infiltration [58,59]. In one experimental study, mice deficient in MCP-1 exhibited significantly decreased survival and increased renal damage after ischemia/reperfusion-induced renal tubular injury in the absence of macrophage accumulation [60]. Kidneys and primary tubular epithelial cells from such mice exhibited increased apoptosis after ischemia, which indicates that MCP-1 protects the kidney from the acute inflammatory response that develops after kidney injury. MCP-1 is one of the most promising biomarkers of kidney injury [61]. Elevated MCP-1 levels are associated with immune system-mediated kidney injury [62,63], diabetic nephropathy [64], and autosomal-dominant polycystic kidney disease [65]. In a mouse model of UUTO, serum and urine MCP-1 levels increased significantly compared to those of control mice [66]. mRNA expression and urinary excretion of MCP-1 correlate with the extent of the obstruction, subsequent renal damage, and hydronephrosis. Urine levels of MCP-1 decrease after release of the obstruction [67]. Urine MCP-1 levels are significantly increased in UPJO groups compared to controls and fall significantly after surgery [24,36,46,59,68,69,70]. The AUC-ROC values in terms of the presence of UUTO are 0.70–0.93 for bladder urine MCP-1 and 0.89 for renal pelvic urine MCP-1 (Table 1) [24,36,46,68,69]. An inverse correlation is evident between the level of MCP-1 in renal pelvic urine and mertiatide clearance by the affected kidney [59,69]. Urine MCP-1 levels usefully distinguish between UPJO (which requires pyeloplasty) and the absence of an obstructive dilation of the renal pelvis. They can be used to monitor the resolution of kidney damage after surgery for UUTO [36]. Serum MCP-1 levels increase in patients with AKI after cardiac surgery and in those with chronic kidney damage [60,71,72], but no study has yet evaluated the serum MCP-1 level as a biomarker of UUTO in a clinical setting.
5.2.3. KIM-1
KIM-1 is a type I membrane protein of 104 kDa composed of a 14 kDa membrane-bound fragment and a 90 kDa soluble portion [73]. It was isolated from T-cells, exhibits various functions, and was termed T-cell immunoglobulin-and-mucin-domain-containing molecule-1 (TIM-1) [73]. Normal kidney tissue rarely expresses KIM-1, but kidneys acutely injured by ischemia, hypoxia, toxicity, or renal tubular interstitial/polycystic kidney disease do [74]. The ectodomain of KIM-1 (90 kD) is cleaved by matrix metalloproteinases and is found in urine after injury to the kidney proximal tubules [75]. Acute KIM-1 overexpression in proximal, renal tubular epithelial cells after ischemia, hypoxia, or toxicity promotes the transformation of these cells into semi-professional phagocytic cells. KIM-1 is a phosphatidylserine receptor of the liposome surface and identifies both apoptotic bodies and phosphatidylserine, triggering further phagocytosis [74,76]. The upregulation of KIM-1 by injured tubular epithelial cells facilitates the clearance of apoptotic cells, protecting against AKI. Apart from mediating phagocytosis, KIM-1 assists in repairing injury to cells [77]. It is a valuable biomarker of AKI. Urine and/or serum KIM-1 levels increase after ischemic kidney injury [75] and in patients with diabetic nephropathy [78], IgA nephropathy [79], and kidney injury after renal transplantation [80]. In an animal model, serum and urine KIM-1 levels were useful for the early diagnosis of obstructive nephropathy-induced AKI [81,82]. In a mouse model, serum KIM-1 levels increased after UUTO, peaking on day 3, and remained detectable for 14 days [82]. In a rat model, the urine KIM-1 level began to increase on day 1 after UUTO and remained high until day 7 [81]. In children with UUTO, urine KIM1 levels correlated inversely with worsening obstruction and decreased after surgery [34,36,45,48,49]. The AUC-ROC value for the prediction of childhood UUTO is 0.65–0.89 for the bladder urine KIM-1 level (Table 1) [34,36,45,49]. In adults with UUTO, the urine KIM-1 level is a useful marker of obstructive nephropathy (AUCs of 0.57–0.73 for bladder urine and 0.88 for renal pelvic urine) [23,50,51]. Xie found that the urine KIM-1 level after surgery to treat UUTO predicted renal function deterioration [83].
5.2.4. NAG
NAG, a 130–140 kDa protein, is a lysosomal enzyme distributed in various human tissue [84]. NAG is not filtered through the glomeruli. In the kidney, it is found predominantly in lysosomes of proximal tubular cells. The small amount of NAG normally present in the urine is exocytosed by these cells. Although the function of NAG in the kidney remains unknown, it is a marker of tubular cell function or damage [85]. Increased NAG excretion in urine is caused exclusively by proximal tubular cell injury. Accumulating evidence indicates that urine NAG levels correlate with exposure to nephrotoxic drugs, delayed allograft nephropathy, diabetic nephropathy, and AKI [85]. Urine NAG levels are elevated in patients with upper urinary tract infection, nephrolithiasis, and reflux nephropathy [86,87]. In children with UUTO, urine NAG levels were significantly higher in those with hydronephrosis (with or without a vesicoureteral reflux) than healthy controls or cystitis patients [88,89]. The NAG level in renal pelvic urine is 7-fold higher and that in bladder urine 1.7-fold higher than in normal controls [89]. Mohammad found that the AUC-ROC value for bladder NAG was 0.67 in children with UUTO [68]. Skalova reported that although the urine NAG level was significantly higher in patients with hydronephrosis compared to healthy controls, there were no differences between children with unilateral and bilateral hydronephrosis and no correlation between the urine NAG level and the grade of hydronephrosis [90]. In summary, urine NAG levels usefully detect childhood UUTO but do not reflect its severity. In one study of adults with UUTO, levels of NAG in bladder and renal pelvic urine were 2.5- and five-fold higher than those of normal controls (AUC-ROC values of 0.74 for bladder urine and 0.91 for renal pelvic urine) and decreased after treatment [23].
5.2.5. L-FABP
L-FABP, which is expressed by both the normal and diseased human kidney, has been found in both the convoluted and straight portions of human proximal tubules [91]. Mammalian intracellular FABP is a 14 kDa protein encoded by a member of a large multigene family within a superfamily of lipid-binding proteins [92]. Nine tissue-specific FABPs have been identified: L (liver), I (intestinal), H (muscle and heart), A (adipocyte), E (epidermal), IL (ileal), B (brain), M (myelin), and T (testis). All FABPs primarily regulate fatty acid metabolism and intracellular transport [93]. L-FABP is expressed not only in the liver but also in the intestine, pancreas, stomach, lung, and kidney [94]. Serum and/or urine L-FABP levels are useful biomarkers of kidney injury after renal transplantation [95], in critical care patients with AKI [96], and in those with contrast-induced AKI [97] and diabetic nephropathy [98]. However, the utility of L-FABP for predicting UUTO remains controversial. Xie found that urine L-FABP levels after UUTO surgery predicted the deterioration of renal function [83]. Furthermore, in one study of patients with vesicoureteral refluxes, the urine L-FABP level was significantly higher than in controls [99]. However, Noyan found that urine L-FABP levels did not differ significantly between children with hydronephrosis and controls [48].
5.3. Novel Biomarkers of UUTO
5.3.1. Vanin-1
Vanin-1, a 53 kDa protein, is expressed in the brush borders of the proximal tubule of the kidney [100]. By catabolizing pantetheine to cysteamine and pantothenic acid (a precursor of coenzymes), it has roles in metabolism and energy production. The function of kidney vanin-1 remains to be established. However, the fact that vanin-1 is located specifically in the brush borders suggests that the enzyme plays a pivotal role in pantothenic acid salvage and recycling. The proximal tubular cells bear microvilli with large apical surface areas within which many transporters and channels are found [101]. Vanin-1 in cellular membranes is anchored to glycosylphosphatidylinositol. The anchor may be cleaved and soluble vanin-1 then secreted or released into the extracellular matrix in response to various stimuli [102].
Urine vanin-1 levels are increased in patients with drug-induced AKI [103] and UUTO [23], and in rat models with high salt-induced kidney damage [104], diabetic nephropathy [105], and UUTO [102]. UUTO inhibits urine flow and increases intratubular pressure, causing renal tubular damage. Vanin-1 is then secreted into the urine by renal tubular cells. The level of vanin-1 in renal pelvic urine correlates highly with the severity of urinary tract obstruction [23]. The level of vanin-1 in renal pelvic urine is highly predictive (AUC-ROC value 0.98) of adult UUTO, more predictive than NGAL, KIM-1, or NAG levels. Vanin-1 levels decrease following UUTO relief in patients with moderate to severe UUTO [23].
5.3.2. α-Glutathione S-Transferase (GST)
GST, a 28 kDa protein, is a cytosolic enzyme. The isoforms α and π (α-GST, π-GST) are typical of the human kidney [106]. α-GST is expressed in proximal tubular epithelial cells, and π-GST is expressed in distal tubular epithelial cells [45]. Both isoforms of GST are released from injured cells into the urine and were recently suggested to be promising biomarkers of kidney injury [107] in the context of cyclosporine-induced nephrotoxicity, cadmium exposure, administration of nephrotoxic antibiotics, acute transplant rejection [106], and critical illness in the ICU [106,108]. Recently, the utility of the α-GST and π-GST level in terms of predicting UUTO was explored. Children with UPJO exhibited significantly higher urinary α-GST excretion than controls. Urinary AUC-ROC values for UUTO detection were 0.90 for α-GST and 0.3 for π-GST. The predictive performance of α-GST was superior to that of urinary NGAL or KIM-1 [45].
5.3.3. Tissue inhibitor of metalloproteinases-2 (TIMP-2)/ insulin-like growth factor-binding protein 7 (IGFBP7)
TIMP-2 and IGFBP7 are new AKI biomarkers. In 2014, Food and Drug Administration approved TIMP-2/ IGFBP7 to be used in ICU patients to predict the risk of developing moderate to severe AKI [109]. TIMP-2 has a molecular weight of approximately 24 kDa and IGFBP7 has a molecular mass of 29 kDa [110]. Both of them are expressed and secreted by renal tubular cells, and involved in G1 cell cycle arrest during the early phases of cellular stress or injury caused by various insults (e.g., sepsis, ischemia, oxidative stress, and toxins) [111]. TIMP-2/ IGFBP7 shows the best accuracy among AKI biomarkers in patients with various types of AKI condition including AKI after kidney transplantation and AKI in critical care settings, sepsis and platinum-based chemotherapy, and chronic kidney damage induced by diabetes mellitus and congestive heart failure [112,113]. However, no study has yet evaluated the TIMP-2/ IGFBP7 as a biomarker of UUTO in an animal study or a clinical setting, and therefore it is urgently necessary.
5.4. Comparison of Biomarkers
Several studies have compared the utility of NAGL, KIM-1, and/or L-FABP levels as biomarkers of childhood UUTO. In studies, urine and/or serum NAGL levels outperformed urine KIM-1 or L-FABP levels [48,49]. In one study, striking increases in serum and urine NGAL levels were evident in patients with obstructive nephropathy, whereas urine KIM-1 levels did not differ significantly between patients and controls, which suggests that KIM-1 is not sensitive in this setting [50]. In another study, urine NGAL levels were significantly higher in patients with both hydronephrosis and obstruction than in those with hydronephrosis but no obstruction or normal controls. Urine KIM-1 and L-FABP levels did not differ significantly among the groups [48]. Patients with renal colic who also exhibited hydronephrosis had significantly higher urine NAG and NGAL, but not KIM-1, levels than did patients without hydronephrosis [114]. In a mouse model of ischemia/reperfusion kidney injury, serum and urine KIM-1 levels increased during the acute phase and declined gradually in the chronic phase, while serum and urine NGAL levels increased continuously during the transition from AKI to chronic kidney disease, which suggests that NGAL is a valuable biomarker in this setting [41]. This may explain why NGAL is a better biomarker of UUTO than KIM-1. However, one predictive model of worsening kidney function after surgery found that urine KIM-1 and L-FABP levels more reliably predicted kidney deterioration after surgical removal of ureteral stones than did urine NGAL levels [83].
MCP-1 is one of the best biomarkers of childhood UUTO. In one study, urine MCP-1 levels were significantly higher in a pyeloplasty group than a non-obstruction group, while urine NGAL and KIM-1 levels did not differ significantly between the groups [36]. In another study, urine MCP-1 levels were significantly higher in patients with hydronephrosis who required surgery than in those who did not; urine NAG levels did not differ significantly between the groups [68]. In one study, the AUC-ROC values of bladder urine MCP-1 and NGAL in children with UPJO were 0.89 and 0.90, respectively, higher than those of bladder urine interleukin-6 (0.78) or TGF-β1 (0.67) [46].
5.5. Panel Assessment of Biomarkers
No single biomarker is specific for UUTO, and given the multifactorial nature of obstruction, not all obstructions can be identified using a single biomarker [24]. In children with UPJO, combined NGAL/MCP-1 assessment improved diagnostic performance compared to assessment of either biomarker alone [46]. In another study on such children, the AUC-ROC values were 0.63 for SCr, 0.72 for serum cystatin C, 0.80 for urinary NGAL, 0.70 for urinary KIM-1, and 0.70 for urinary cystatin C. The AUC-ROCs of combinations of these biomarkers were higher than those of the single biomarkers, being highest (0.88) for urinary NGAL + urinary cystatin C + serum cystatin C [34]. In critically ill patients with AKI, a combination of urine NGAL and L-FABP levels, sepsis status, blood lactate level, and stratification using the Acute Physiology and Chronic Health Evaluation score improved AKI predictive performance (AUC-ROC 0.94) compared to NGAL alone (AUC-ROC 0.86) or L-FABP alone (AUC-ROC 0.84) [43]. In a model predicting worsening kidney function after surgery in UUTO patients, the AUC-ROC of the preoperative combination of urinary biomarkers L-FABP, KIM-1, and NGAL was 0.97, higher than the highest AUC of a single biomarker (0.91 for L-FABP) [83].
6. Current Limitations and Future Directions
Most obstructions of the upper urinary tract are unilateral. Reduced glomerular filtration in the affected kidney and the obstruction per se decreases the amount of any biomarker that reaches the bladder, which explains why the biomarker AUC-ROCs of bladder urine are generally lower than those of renal pelvic urine [23,24]. Combinations of serum and bladder biomarker levels may thus be optimal. However, serum values of biomarkers have been less studied than urine values in UUTO patients. Only serum cystatin C and NGAL levels are clinically useful. Serum markers that are highly predictive of UUTO should be sought. Combinations of serum and urinary biomarkers facilitate the diagnosis of UUTO, risk stratification, clinical decision making, and monitoring.
Age affects the predictive performance of biomarkers [52]. Acute and/or chronic kidney injury is a condition frequently found in elderly population with comorbidities, which may alter the value of biomarkers. This would be the reason why NGAL better predicts AKI in children than in adults [52].
Both urinary and serum UUTO biomarkers lack specificity. Increases may be associated with conditions other than UUTO or even non-kidney conditions. Increases in MCP-1 are associated with liver cirrhosis [115] and sleep apnea syndrome [116], increases in NGAL are associated with cardiovascular ischemia, heart failure, atherosclerosis, and pneumonia [117,118], and increases in L-FABP are associated with various liver diseases [119,120]. However, panel assessment may compensate for the lack of specificity.
7. Conclusions
Renal scans are standard in evaluations of the presence and severity of UUTO, but they are expensive, are not always available, and expose patients to radiation. Many urinary and serum biomarkers have been studied in children and adults with UUTO. MCP-1 and NGAL, the most extensively studied, are the most likely to be optimal. Recently, novel biomarkers (vanin-1 and α-GST) have outperformed traditional biomarkers in terms of evaluating UUTO, but further work must explore whether this is the case in all UUTO settings. No single biomarker is adequately sensitive or specific. Panel assessment affords mutual biomarker compensation and improves predictive performance. The obstruction per se and reduced glomerular filtration in the affected kidney decrease the amount of any biomarker reaching the bladder, limiting the performance of bladder urine biomarkers. However, combinations of serum and bladder urinary biomarkers improve performance. Panel assessment of urinary and serum biomarkers facilitates the diagnosis of UUTO, risk stratification, clinical decision making, and monitoring.
Author Contributions
Development of the idea: S.W. Review and Editing: S.W. and K.H. Supervision: T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AKI | Acute kidney injury |
| AUC | Area under curve |
| GST | Glutathione S-transferases |
| IGFBP7 | Insulin-like growth factor-binding protein 7 |
| KIM-1 | Kidney injury molecule 1 |
| L-FABP | Liver type fatty acid-binding protein |
| MCP-1 | Monocyte chemotactic protein-1 |
| NAG | N-acetyl-b-D-glucosaminidase |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| ROC | Receiver operating characteristic |
| ROS | Reactive oxygen species |
| SCr | Serum creatinine |
| TNF-α | Tumor necrosis factor-α |
| TIMP-2 | Tissue inhibitor of metalloproteinases-2 |
| TGF-β1 | Transforming growth factor-β1 |
| UPJ | Ureteropelvic junction |
| UPJO | Ureteropelvic junction obstruction |
| UUTO | Upper urinary tract obstruction |
References
- Mesrobian, H.-G.O.; Mitchell, M.E.; See, W.A.; Halligan, B.D.; Carlson, B.E.; Greene, A.S.; Wakim, B.T. Candidate Urinary Biomarker Discovery in Ureteropelvic Junction Obstruction: A Proteomic Approach. J. Urol. 2010, 184, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, R.L.; Thornhill, B.A.; Forbes, M.S.; Kiley, S.C. Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr. Nephrol. 2009, 25, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Grasso, M.; Caruso, R.P.; Phillips, C.K. UPJ Obstruction in the Adult Population: Are Crossing Vessels Significant? Rev. Urol. 2001, 3, 42–51. [Google Scholar] [PubMed]
- Liu, Y.; Chen, Y.; Liao, B.; Luo, D.; Wang, K.-J.; Li, H.; Zeng, G. Epidemiology of urolithiasis in Asia. Asian J. Urol. 2018, 5, 205–214. [Google Scholar] [CrossRef]
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef]
- Türk, C.; Neisius, A.; Petrik, A.; Seitz, C.; Skolarikos, A.; Thomas, K. EAU Guideline for Urolithiasis 2020. Available online: https://uroweb.org/guideline/urolithiasis/ (accessed on 15 June 2020).
- Tran, H.; Arsovska, O.; Paterson, R.F.; Chew, B.H. Evaluation of risk factors and treatment options in patients with ureteral stricture disease at a single institution. Can. Urol. Assoc. J. 2015, 9, 921–924. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.-Y.; Zhang, Z.-H.; Xu, P.-C.; Chen, D.-G.; Fan, X.-H.; Ma, J.-C.; Xu, Y.-P. Malignant ureteral obstruction: Experience and comparative analysis of metallic versus ordinary polymer ureteral stents. World J. Surg. Oncol. 2019, 17, 74. [Google Scholar] [CrossRef]
- Chevalier, R.L. Chronic Partial Ureteral Obstruction in the Neonatal Guinea Pig. II. Pressure Gradients Affecting Glomerular Filtration Rate. Pediatr. Res. 1984, 18, 1271–1277. [Google Scholar] [CrossRef]
- Klein, J.; Gonzalez, J.; Miravete, M.; Caubet, C.; Chaaya, R.; Decramer, S.; Bandin, F.; Bascands, J.L.; Buffin-Meyer, B.; Schanstra, J.P. Congenital ureteropelvic junction obstruction: Human disease and animal models. Int. J. Exp. Pathol. 2010, 92, 168–192. [Google Scholar] [CrossRef]
- Cachat, F.; Lange-Sperandio, B.; Chang, A.Y.; Kiley, S.C.; Thornhill, B.A.; Forbes, M.S.; Chevalier, R.L. Ureteral obstruction in neonatal mice elicits segment-specific tubular cell responses leading to nephron loss11See Editorial by Woolf, p. 761. Kidney Int. 2003, 63, 564–575. [Google Scholar] [CrossRef]
- Klimova, E.M.; Aparicio-Trejo, O.E.; Tapia, E.; Pedraza-Chaverri, J. Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments. Biomolecules 2019, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, R.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.; Woodward, M.; Coward, R.J. The molecular biology of pelvi-ureteric junction obstruction. Pediatr. Nephrol. 2017, 33, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.; Jao, H.-Y.; Hsu, J.-D.; Lee, Y.-R.; Wu, M.-J.; Kao, Y.-L.; Lee, H.-J. Apple polyphenols reduce inflammation response of the kidneys in unilateral ureteral obstruction rats. J. Funct. Foods 2014, 11, 1–11. [Google Scholar] [CrossRef]
- Madsen, M.G. Urinary biomarkers in hydronephrosis. Dan. Med. J. 2013, 60, B4582. [Google Scholar] [PubMed]
- Xia, Z.-E.; Xi, J.-L.; Shi, L. 3,3′-Diindolylmethane ameliorates renal fibrosis through the inhibition of renal fibroblast activation in vivo and in vitro. Ren. Fail. 2018, 40, 447–454. [Google Scholar] [CrossRef]
- Nilsson, L.; Madsen, K.; Krag, S.; Frøkiær, J.; Jensen, B.L.; Nørregaard, R. Disruption of cyclooxygenase type 2 exacerbates apoptosis and renal damage during obstructive nephropathy. Am. J. Physiol. Physiol. 2015, 309, F1035–F1048. [Google Scholar] [CrossRef]
- Mei, W.; Peng, Z.; Tang, D.; Yang, H.; Tao, L.; Lu, M.; Liu, C.; Deng, Z.; Xiao, Y.; Liu, J.; et al. Peroxiredoxin 1 inhibits the oxidative stress induced apoptosis in renal tubulointerstitial fibrosis. Nephrology 2015, 20, 832–842. [Google Scholar] [CrossRef]
- Xu, Y.; Ruan, S.; Wu, X.; Chen, H.; Zheng, K.; Fu, B. Autophagy and apoptosis in tubular cells following unilateral ureteral obstruction are associated with mitochondrial oxidative stress. Int. J. Mol. Med. 2013, 31, 628–636. [Google Scholar] [CrossRef]
- Taylor, A.T. Radionuclides in nephrourology, Part 2: Pitfalls and diagnostic applications. J. Nucl. Med. 2014, 55, 786–798. [Google Scholar] [CrossRef]
- Akbal, C.; Şahan, A.; Garayev, A.; Şekerci, Ç.A.; Sulukaya, M.; Alpay, H.; Tarcan, T.; Şimşek, F. Assessment of Differential Renal Function in Children with Hydronephrosis: Comparison of DMSA and MAG-3. J. Urol. Surg. 2015, 2, 129–134. [Google Scholar] [CrossRef]
- Washino, S.; Hosohata, K.; Oshima, M.; Okochi, T.; Konishi, T.; Nakamura, Y.; Saito, K.; Miyagawa, T. A Novel Biomarker for Acute Kidney Injury, Vanin-1, for Obstructive Nephropathy: A Prospective Cohort Pilot Study. Int. J. Mol. Sci. 2019, 20, 899. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.G.; Nørregaard, R.; Palmfeldt, J.; Olsen, L.H.; Frøkiær, J.; Jørgensen, T.M. Epidermal growth factor and monocyte chemotactic peptide-1: Potential biomarkers of urinary tract obstruction in children with hydronephrosis. J. Pediatr. Urol. 2013, 9, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Giacoman, S.; Madero, M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J. Nephrol. 2015, 4, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Pavlaki, A.; Printza, N.; Farmaki, E.; Stabouli, S.; Taparkou, A.; Sterpi, M.; Dotis, J.; Papachristou, F. The role of urinary NGAL and serum cystatin C in assessing the severity of ureteropelvic junction obstruction in infants. Pediatr. Nephrol. 2019, 35, 163–170. [Google Scholar] [CrossRef]
- Inker, L.A.; Okparavero, A. Cystatin C as a marker of glomerular filtration rate. Curr. Opin. Nephrol. Hypertens. 2011, 20, 631–639. [Google Scholar] [CrossRef]
- Slort, P.R.; Ozden, N.; Pape, L.; Offner, G.; Tromp, W.F.; Wilhelm, A.J.; Bokenkamp, A. Comparing cystatin C and creatinine in the diagnosis of pediatric acute renal allograft dysfunction. Pediatr. Nephrol. 2011, 27, 843–849. [Google Scholar] [CrossRef]
- Yong, Z.; Pei, X.; Zhu, B.; Yuan, H.; Zhao, W. Predictive value of serum cystatin C for acute kidney injury in adults: A meta-analysis of prospective cohort trials. Sci. Rep. 2017, 7, 41012. [Google Scholar] [CrossRef]
- Koyner, J.L.; Vaidya, V.S.; Bennett, M.R.; Ma, Q.; Worcester, E.; Akhter, S.A.; Raman, J.; Jeevanandam, V.; O’Connor, M.F.; Devarajan, P.; et al. Urinary Biomarkers in the Clinical Prognosis and Early Detection of Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2010, 5, 2154–2165. [Google Scholar] [CrossRef]
- Chen, S.; Shi, J.-S.; Yibulayin, X.; Wu, T.-S.; Yang, X.-W.; Zhang, J.; Baiheti, P. Cystatin C is a moderate predictor of acute kidney injury in the early stage of traumatic hemorrhagic shock. Exp. Ther. Med. 2015, 10, 237–240. [Google Scholar] [CrossRef]
- Haase-Fielitz, A.; Bellomo, R.; Devarajan, P.; Story, D.F.; Matalanis, G.; Dragun, D.; Haase, M. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery—A prospective cohort study. Crit. Care Med. 2009, 37, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.N.; Juneja, M.; Patel, H.; Shah, K.H.; Konat, A.; Thakkar, B.M.; Madan, T.; Prajapati, J. Diagnostic accuracy of serum cystatin C for early recognition of contrast induced nephropathy in Western Indians undergoing cardiac catheterization. Indian Hear. J. 2016, 69, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Kostic, D.; Beozzo, G.; Couto, S.B.D.; Kato, A.; Lima, L.; Palmeira, P.; Krebs, V.; Bunduki, V.; Francisco, R.P.; Zugaib, M.; et al. The role of renal biomarkers to predict the need of surgery in congenital urinary tract obstruction in infants. J. Pediatr. Urol. 2019, 15, 242.e1–242.e9. [Google Scholar] [CrossRef]
- Mao, W.; Liu, S.; Wang, K.; Wang, M.; Shi, H.; Liu, Q.; Bao, M.; Peng, B.; Geng, J. Cystatin C in Evaluating Renal Function in Ureteral Calculi Hydronephrosis in Adults. Kidney Blood Press. Res. 2019, 45, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Karakus, S.; Oktar, T.; Kucukgergin, C.; Kalelioğlu, I.; Seckin, S.; Atar, A.; Ander, H.; Ziylan, O. Urinary IP-10, MCP-1, NGAL, Cystatin-C, and KIM-1 Levels in Prenatally Diagnosed Unilateral Hydronephrosis: The Search for an Ideal Biomarker. Urology 2016, 87, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhu, B.; Yuan, H.; Zhao, W. Evaluation of serum neutrophil gelatinase-associated lipocalin in older patients with chronic kidney disease. Aging Med. 2020, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zwiers, A.J.M.; De Wildt, S.N.; Van Rosmalen, J.; De Rijke, Y.B.; Buijs, E.A.B.; Tibboel, D.; Cransberg, K. Urinary neutrophil gelatinase-associated lipocalin identifies critically ill young children with acute kidney injury following intensive care admission: A prospective cohort study. Crit. Care 2015, 19, 1–14. [Google Scholar] [CrossRef]
- Liu, X.; Guan, Y.; Xu, S.; Li, Q.; Sun, Y.; Han, R.; Jiang, C. Early Predictors of Acute Kidney Injury: A Narrative Review. Kidney Blood Press. Res. 2016, 41, 680–700. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.M.; Mori, K.; Li, J.Y.; Kalandadze, A.; Cohen, D.J.; Devarajan, P.; Barasch, J. Dual Action of Neutrophil Gelatinase–Associated Lipocalin. J. Am. Soc. Nephrol. 2007, 18, 407–413. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Q.; Wen, J.; Chen, T.; He, L.; Wang, Y.; Yin, J.; Wu, R.; Xue, R.; Li, S.; et al. Ischemic Duration and Frequency Determines AKI-to-CKD Progression Monitored by Dynamic Changes of Tubular Biomarkers in IRI Mice. Front. Physiol. 2019, 10, 153. [Google Scholar] [CrossRef]
- Cappuccilli, M.; Capelli, I.; Comai, G.; Cianciolo, G.; La Manna, G. Neutrophil Gelatinase-Associated Lipocalin as a Biomarker of Allograft Function After Renal Transplantation: Evaluation of the Current Status and Future Insights. Artif. Organs 2017, 42, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Nusca, A.; Miglionico, M.; Proscia, C.; Ragni, L.; Carassiti, M.; Pepe, F.L.; Di Sciascio, G. Early prediction of contrast-induced acute kidney injury by a "bedside" assessment of Neutrophil Gelatinase-Associated Lipocalin during elective percutaneous coronary interventions. PLoS ONE 2018, 13, e0197833. [Google Scholar] [CrossRef] [PubMed]
- Asada, T.; Isshiki, R.; Hayase, N.; Sumida, M.; Inokuchi, R.; Noiri, E.; Nangaku, M.; Yahagi, N.; Doi, K. Impact of clinical context on acute kidney injury biomarker performances: Differences between neutrophil gelatinase-associated lipocalin and L-type fatty acid-binding protein. Sci. Rep. 2016, 6, 33077. [Google Scholar] [CrossRef] [PubMed]
- Bieniaś, B.; Sikora, P. Potential Novel Biomarkers of Obstructive Nephropathy in Children with Hydronephrosis. Dis. Markers 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, L.; Li, Q.; Li, S.; Luo, X.; Zhang, C.; Wu, B.; Brooks, J.D.; Sun, H. Elevated urinary lipocalin-2, interleukin-6 and monocyte chemoattractant protein-1 levels in children with congenital ureteropelvic junction obstruction. J. Pediatr. Urol. 2019, 15, 44.e1–44.e7. [Google Scholar] [CrossRef]
- Gupta, S.; Jackson, A.R.; DaJusta, D.; McLeod, D.; Alpert, S.; Jayanthi, V.R.; McHugh, K.; Schwaderer, A.; Becknell, B.; Ching, C. Urinary antimicrobial peptides: Potential novel biomarkers of obstructive uropathy. J. Pediatr. Urol. 2018, 14, 238.e1–238.e6. [Google Scholar] [CrossRef]
- Noyan, A.; Parmaksiz, G.; Dursun, H.; Ezer, S.S.; Anarat, R.; Cengiz, N.; Parmaksız, G. Urinary NGAL, KIM-1 and L-FABP concentrations in antenatal hydronephrosis. J. Pediatr. Urol. 2015, 11, 249.e1–249.e6. [Google Scholar] [CrossRef]
- Wasilewska, A.; Taranta-Janusz, K.; Debek, W.; Zoch-Zwierz, W.; Kuroczycka-Saniutycz, E. KIM-1 and NGAL: New markers of obstructive nephropathy. Pediatr. Nephrol. 2011, 26, 579–586. [Google Scholar] [CrossRef]
- Urbschat, A.; Gauer, S.; Paulus, P.; Reissig, M.; Weipert, C.; Ramos-Lopez, E.; Hofmann, R.; Hadji, P.; Geiger, H.; Obermüller, N. Serum and urinary NGAL but not KIM-1 raises in human postrenal AKI. Eur. J. Clin. Investig. 2014, 44, 652–659. [Google Scholar] [CrossRef]
- Olvera-Posada, D.; Dayarathna, T.; Dion, M.; Alenezi, H.; Sener, A.; Denstedt, J.D.; Pautler, S.E.; Razvi, H. KIM-1 Is a Potential Urinary Biomarker of Obstruction: Results from a Prospective Cohort Study. J. Endourol. 2017, 31, 111–118. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin: A promising biomarker for human acute kidney injury. Biomark. Med. 2010, 4, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin: New paths for an old shuttle. Cancer Ther. 2007, 5, 463–470. [Google Scholar] [PubMed]
- Choi, J.W.; Fujii, T.; Fujii, N. Elevated Plasma Neutrophil Gelatinase-Associated Lipocalin Level as a Risk Factor for Anemia in Patients with Systemic Inflammation. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Mao, X.; Niu, Y.; Tang, B.; Shen, H. Usefulness and limitations of neutrophil gelatinase-associated lipocalin in the assessment of kidney diseases. J. Lab. Precis. Med. 2018, 3, 1. [Google Scholar] [CrossRef]
- Gu, L.; Tseng, S.C.; Rollins, B.J. Monocyte chemoattractant protein-1. Chem. Immunol. 1999, 72, 7–29. [Google Scholar]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef]
- Grandaliano, G.; Gesualdo, L.; Ranieri, E.; Monno, R.; Montinaro, V.; Marra, F.; Schena, F.P. Monocyte chemotactic peptide-1 expression in acute and chronic human nephritides: A pathogenetic role in interstitial monocytes recruitment. J. Am. Soc. Nephrol. 1996, 7, 906–913. [Google Scholar]
- Grandaliano, G.; Gesualdo, L.; Bartoli, F.; Ranieri, E.; Monno, R.; Leggio, A.; Paradies, G.; Caldarulo, E.; Infante, B.; Schena, F.P. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int. 2000, 58, 182–192. [Google Scholar] [CrossRef]
- Stroo, I.; Claessen, N.; Teske, G.J.D.; Butter, L.M.; Florquin, S.; Leemans, J.C. Deficiency for the Chemokine Monocyte Chemoattractant Protein-1 Aggravates Tubular Damage after Renal Ischemia/Reperfusion Injury. PLoS ONE 2015, 10, e0123203. [Google Scholar] [CrossRef]
- Moledina, D.G.; Isguven, S.; McArthur, E.; Thiessen-Philbrook, H.; Garg, A.X.; Shlipak, M.; Whitlock, R.; Kavsak, P.A.; Coca, S.G.; Parikh, C.R.; et al. Plasma Monocyte Chemotactic Protein-1 Is Associated With Acute Kidney Injury and Death After Cardiac Operations. Ann. Thorac. Surg. 2017, 104, 613–620. [Google Scholar] [CrossRef]
- Tam, F.W.K.; Ong, A.C. Renal monocyte chemoattractant protein-1: An emerging universal biomarker and therapeutic target for kidney diseases? Nephrol. Dial. Transplant. 2019, 35, 198–203. [Google Scholar] [CrossRef]
- Kronbichler, A.; Kerschbaum, J.; Gründlinger, G.; Leierer, J.; Mayer, G.; Rudnicki, M.A. Evaluation and validation of biomarkers in granulomatosis with polyangiitis and microscopic polyangiitis. Nephrol. Dial. Transplant. 2015, 31, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Nowak, N.; Skupien, J.; Smiles, A.M.; Yamanouchi, M.; Niewczas, M.; Galecki, A.T.; Duffin, K.L.; Breyer, M.D.; Pullen, N.; Bonventre, J.V.; et al. Markers of early progressive renal decline in type 2 diabetes suggest different implications for etiological studies and prognostic tests development. Kidney Int. 2018, 93, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J.; Chapman, A.B.; Blais, J.; Czerwiec, F.S.; Devuyst, O.; Gansevoort, R.T.; Higashihara, E.; Krasa, H.; Zhou, W.; Ouyang, J.; et al. Tolvaptan suppresses monocyte chemotactic protein-1 excretion in autosomal-dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2017, 32, 969–975. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munshi, R.; Johnson, A.; Siew, E.D.; Ikizler, T.A.; Ware, L.B.; Wurfel, M.M.; Himmelfarb, J.; Zager, R.A. MCP-1 Gene Activation Marks Acute Kidney Injury. J. Am. Soc. Nephrol. 2010, 22, 165–175. [Google Scholar] [CrossRef]
- Stephan, M.; Conrad, S.; Eggert, T.; Heuer, R.; Fernandez, S.; Huland, H. Urinary concentration and tissue messenger RNA expression of monocyte chemoattractant protein-1 as an indicator of the degree of hydronephrotic atrophy in partial ureteral obstruction. J. Urol. 2002, 167, 1497–1502. [Google Scholar] [CrossRef]
- Mohammadjafari, H.; Rafiei, A.; Mousavi, S.A.; Alaee, A.; Yeganeh, Y. Role of Urinary Levels of Endothelin-1, Monocyte Chemotactic Peptide-1, andN-Acetyl Glucosaminidase in Predicting the Severity of Obstruction in Hydronephrotic Neonates. Korean J. Urol. 2014, 55, 670–676. [Google Scholar] [CrossRef]
- Taranta-Janusz, K.; Wasilewska, A.; Debek, W.; Waszkiewicz-Stojda, M. Urinary cytokine profiles in unilateral congenital hydronephrosis. Pediatr. Nephrol. 2012, 27, 2107–2113. [Google Scholar] [CrossRef]
- Bartoli, F.; Penza, R.; Aceto, G.; Niglio, F.; D’Addato, O.; Pastore, V.; Campanella, V.; Magaldi, S.; Lasalandra, C.; Di Bitonto, G.; et al. Urinary epidermal growth factor, monocyte chemotactic protein-1, and beta2-microglobulin in children with ureteropelvic junction obstruction. J. Pediatr. Surg. 2011, 46, 530–536. [Google Scholar] [CrossRef]
- Musiał, K.; Bargenda, A.; Drożdż, D.; Zwolińska, D. New Markers of Inflammation and Tubular Damage in Children with Chronic Kidney Disease. Dis. Markers 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- Gregg, L.P.; Tio, M.C.; Li, X.; Adams-Huet, B.; De Lemos, J.A.; Hedayati, S.S. Association of Monocyte Chemoattractant Protein-1 with Death and Atherosclerotic Events in Chronic Kidney Disease. Am. J. Nephrol. 2018, 47, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney Injury Molecule-1 (KIM-1), a Putative Epithelial Cell Adhesion Molecule Containing a Novel Immunoglobulin Domain, Is Up-regulated in Renal Cells after Injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, J.; Prayogo, G.W.; Cao, W.; Wu, Y.; Jia, Z.; Zhang, A. Understanding kidney injury molecule 1: A novel immune factor in kidney pathophysiology. Am. J. Transl. Res. 2019, 11, 1219–1229. [Google Scholar]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Asseldonk, E.J.; Humphreys, B.D.; Gunaratnam, L.; Duffield, J.S.; Bonventre, J.V. Kidney injury molecule–1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Investig. 2008, 118, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Ismail, O.Z.; Zhang, X.; Bonventre, J.V.; Gunaratnam, L. G protein α12 (Gα12) is a negative regulator of kidney injury molecule-1-mediated efferocytosis. Am. J. Physiol. Physiol. 2016, 310, F607–F620. [Google Scholar] [CrossRef]
- Satirapoj, B.; Pooluea, P.; Nata, N.; Supasyndh, O. Urinary biomarkers of tubular injury to predict renal progression and end stage renal disease in type 2 diabetes mellitus with advanced nephropathy: A prospective cohort study. J. Diabetes Complicat. 2019, 33, 675–681. [Google Scholar] [CrossRef]
- Peters, H.P.; Waanders, F.; Meijer, E.; Brand, J.V.D.; Steenbergen, E.J.; Van Goor, H.; Wetzels, J.F. High urinary excretion of kidney injury molecule-1 is an independent predictor of end-stage renal disease in patients with IgA nephropathy. Nephrol. Dial. Transplant. 2011, 26, 3581–3588. [Google Scholar] [CrossRef]
- Zhang, P.; Rothblum, L.; Han, W.; Blasick, T.; Potdar, S.; Bonventre, J.V. Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int. 2008, 73, 608–614. [Google Scholar] [CrossRef]
- Jin, Y.; Shao, X.; Sun, B.; Miao, C.; Li, Z.; Shi, Y. Urinary kidney injury molecule-1 as an early diagnostic biomarker of obstructive acute kidney injury and development of a rapid detection method. Mol. Med. Rep. 2017, 15, 1229–1235. [Google Scholar] [CrossRef]
- Tian, L.; Shao, X.; Xie, Y.; Wang, Q.; Che, X.; Zhang, M.; Xu, W.; Xu, Y.; Mou, S.; Ni, Z. Kidney Injury Molecule-1 is Elevated in Nephropathy and Mediates Macrophage Activation via the Mapk Signalling Pathway. Cell. Physiol. Biochem. 2017, 41, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xue, W.; Shao, X.; Che, X.; Xu, W.; Ni, Z.; Mou, S. Analysis of a Urinary Biomarker Panel for Obstructive Nephropathy and Clinical Outcomes. PLoS ONE 2014, 9, e112865. [Google Scholar] [CrossRef] [PubMed]
- Skálová, S. The diagnostic role of urinary N-acetyl-beta-D-glucosaminidase (NAG) activity in the detection of renal tubular impairment. Acta Medica 2005, 48, 75–80. [Google Scholar] [PubMed]
- Bosomworth, M.P.; Aparicio, S.R.; Hay, A.W. Urine N-acetyl- -D-glucosaminidase—A marker of tubular damage? Nephrol. Dial. Transplant. 1999, 14, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Lee, M.; Park, S.; Lee, Y.H.; Jin, S.M.; Kim, J.H.; Lee, B.W. Elevated urinary N-acetyl-beta-D-glucosaminidase is associated with high glycoalbumin-to-hemoglobin A1c ratio in type 1 diabetes patients with early diabetic kidney disease. Sci. Rep. 2018, 8, 6710. [Google Scholar] [CrossRef]
- Demir, A.D.; Goknar, N.; Oktem, F.; Ozkaya, E.; Yazici, M.; Torun, E.; Vehapoglu, A.; Kucukkoc, M. Renal tubular function and urinary N-acetyl-beta-d-glucosaminidase and kidney injury molecule-1 levels in asthmatic children. Int. J. Immunopathol. Pharmacol. 2016, 29, 626–631. [Google Scholar] [CrossRef]
- Ali, R.J.; Al-Obaidi, F.H.; Arif, H.S. The Role of Urinary N-acetyl Beta-D-glucosaminidase in Children with Urological Problems. Oman Med. J. 2014, 29, 285–288. [Google Scholar] [CrossRef]
- Carr, M.C.; Peters, C.A.; Retik, A.B.; Mandell, J. Urinary levels of the renal tubular enzyme N-acetyl-beta-D-glucosaminidase in unilateral obstructive uropathy. J. Urol. 1994, 151, 442–445. [Google Scholar] [CrossRef]
- Skálová, S.; Rejtar, P.; Kutilek, S. Increased urinary N-acetyl-beta-D-glucosaminidase activity in children with hydronephrosis. Int. Braz. Urol. 2007, 33, 80–86. [Google Scholar] [CrossRef]
- Kamijo-Ikemori, A.; Sugaya, T.; Matsui, K.; Yokoyama, T.; Kimura, K. Roles of human liver type fatty acid binding protein in kidney disease clarified using hL-FABP chromosomal transgenic mice. Nephrology 2011, 16, 539–544. [Google Scholar] [CrossRef]
- Yamamoto, T.; Noiri, E.; Ono, Y.; Doi, K.; Negishi, K.; Kamijo, A.; Kimura, K.; Fujita, T.; Kinukawa, T.; Taniguchi, H.; et al. Renal L-Type Fatty Acid–Binding Protein in Acute Ischemic Injury. J. Am. Soc. Nephrol. 2007, 18, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M. Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. J. Atheroscler. Thromb. 2019, 26, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Graupera, I.; Coll, M.; Pose, E.; Elia, C.; Piano, S.; Sola, E.; Blaya, D.; Huelin, P.; Solé, C.; Moreira, R.; et al. Adipocyte Fatty-Acid Binding Protein is Overexpressed in Cirrhosis and Correlates with Clinical Outcomes. Sci. Rep. 2017, 7, 1829. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; Kusaka, M.; Kitagawa, F.; Ishii, J.; Fukami, N.; Maruyama, T.; Sasaki, H.; Shiroki, R.; Kurahashi, H.; Hoshinaga, K. Serum liver-type fatty acid-binding protein predicts recovery of graft function after kidney transplantation from donors after cardiac death. Clin. Transplant. 2014, 28, 749–754. [Google Scholar] [CrossRef]
- Parr, S.K.; Clark, A.J.; Bian, A.; Shintani, A.K.; Wickersham, N.E.; Ware, L.B.; Ikizler, T.A.; Siew, E.D. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. 2014, 87, 640–648. [Google Scholar] [CrossRef]
- Connolly, M.; Kinnin, M.; Mc Eneaney, D.; Menown, I.; Kurth, M.J.; Lamont, J.; Morgan, N.; Harbinson, M. Prediction of contrast induced acute kidney injury using novel biomarkers following contrast coronary angiography. QJM Int. J. Med. 2017, 111, 103–110. [Google Scholar] [CrossRef]
- Panduru, N.M.; Forsblom, C.; Saraheimo, M.; Thorn, L.; Bierhaus, A.; Humpert, P.M.; Groop, P.-H. Urinary Liver-Type Fatty Acid–Binding Protein and Progression of Diabetic Nephropathy in Type 1 Diabetes. Diabetes Care 2013, 36, 2077–2083. [Google Scholar] [CrossRef]
- Parmaksız, G.; Noyan, A.; Dursun, H.; Ince, E.; Anarat, R.; Cengiz, N. Role of new biomarkers for predicting renal scarring in vesicoureteral reflux: NGAL, KIM-1, and L-FABP. Pediatr. Nephrol. 2015, 31, 97–103. [Google Scholar] [CrossRef]
- Bartucci, R.; Salvati, A.; Olinga, P.; Boersma, Y.L. Vanin 1: Its Physiological Function and Role in Diseases. Int. J. Mol. Sci. 2019, 20, 3891. [Google Scholar] [CrossRef]
- Wessely, O.; Cerqueira, D.M.; Tran, U.; Kumar, V.; Hassey, J.M.; Romaker, D. The bigger the better: Determining nephron size in kidney. Pediatr. Nephrol. 2013, 29, 525–530. [Google Scholar] [CrossRef]
- Hosohata, K.; Jin, D.; Takai, S.; Iwanaga, K. Vanin-1 in Renal Pelvic Urine Reflects Kidney Injury in a Rat Model of Hydronephrosis. Int. J. Mol. Sci. 2018, 19, 3186. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K.; Washino, S.; Kubo, T.; Natsui, S.; Fujisaki, A.; Kurokawa, S.; Ando, H.; Fujimura, A.; Morita, T. Early prediction of cisplatin-induced nephrotoxicity by urinary vanin-1 in patients with urothelial carcinoma. Toxicology 2016, 360, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K.; Jin, D.; Takai, S.; Iwanaga, K. Involvement of Vanin-1 in Ameliorating Effect of Oxidative Renal Tubular Injury in Dahl-Salt Sensitive Rats. Int. J. Mol. Sci. 2019, 20, 4481. [Google Scholar] [CrossRef] [PubMed]
- Fugmann, T.; Borgia, B.; Révész, C.; Godó, M.; Forsblom, C.; Hamar, P.; Holthofer, H.; Neri, D.; Roesli, C. Proteomic identification of vanin-1 as a marker of kidney damage in a rat model of type 1 diabetic nephropathy. Kidney Int. 2011, 80, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, A.; Appelkvist, E.L.; Dallner, G.; Nilsson, R. Glutathione Transferases in the Urine: Sensitive Methods for Detection of Kidney Damage Induced by Nephrotoxic Agents in Humans. Environ. Health Perspect. 1994, 102, 293. [Google Scholar] [CrossRef]
- Andreucci, M.; Faga, T.; Pisani, A.; Perticone, M.; Michael, A. The ischemic/nephrotoxic acute kidney injury and the use of renal biomarkers in clinical practice. Eur. J. Intern. Med. 2017, 39, 1–8. [Google Scholar] [CrossRef]
- Walshe, C.M.; Odejayi, F.; Ng, S.; Marsh, B. Urinary glutathione S-transferase as an early marker for renal dysfunction in patients admitted to intensive care with sepsis. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2009, 11, 204–209. [Google Scholar]
- Vijayan, A.; Faubel, S.; Askenazi, D.J.; Cerda, J.; Fissell, W.H.; Heung, M.; Humphreys, B.D.; Koyner, J.L.; Liu, K.D.; Mour, G.; et al. Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am. J. Kidney Dis. 2016, 68, 19–28. [Google Scholar] [CrossRef]
- Emlet, D.R.; Pastor-Soler, N.; Marciszyn, A.; Wen, X.; Gomez, H.; Humphries, W.H.; Morrisroe, S.; Volpe, J.K.; Kellum, J.A. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: Differential expression and secretion in human kidney tubule cells. Am. J. Physiol. Physiol. 2016, 312, F284–F296. [Google Scholar] [CrossRef]
- Ortega, L.M.; Heung, M. The use of cell cycle arrest biomarkers in the early detection of acute kidney injury. Is this the new renal troponin? Nefrologia 2018, 38, 361–367. [Google Scholar] [CrossRef]
- Heung, M.; Ortega, L.M.; Chawla, L.S.; Wunderink, R.G.; Self, W.H.; Koyner, J.L.; Shi, J.; Kellum, J.A. Sapphire and Topaz Investigators Common chronic conditions do not affect performance of cell cycle arrest biomarkers for risk stratification of acute kidney injury. Nephrol. Dial. Transplant. 2016, 31, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Ankawi, G.; Zhang, J.; Digvijay, K.; Giavarina, D.; Yin, Y.; Ronco, C. Current understanding and future directions in the application of TIMP-2 and IGFBP7 in AKI clinical practice. Clin. Chem. Lab. Med. 2019, 57, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, M.; Fucucuoglu, D.; Kucuk, S.H.; Erol, M.; Yigit, O.; Bilge, I. Urinary biomarkers in the early detection and follow-up of tubular injury in childhood urolithiasis. Clin. Exp. Nephrol. 2018, 22, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Graupera, I.; Sola, E.; Fabrellas, N.; Moreira, R.; Sole, C.; Huelin, P.; De La Prada, G.; Pose, E.; Ariza, X.; Risso, A.; et al. Urine Monocyte Chemoattractant Protein-1 Is an Independent Predictive Factor of Hospital Readmission and Survival in Cirrhosis. PLoS ONE 2016, 11, e0157371. [Google Scholar] [CrossRef]
- Fanfulla, F.; Rotondi, M.; Morrone, E.; Coperchini, F.; Lodigiani, S.; Trentin, R.; Maccabruni, V.; Chiovato, L. Sleep hypoxia and not obesity is the main determinant of the increasing monocyte chemoattractant protein-1 (MCP-1) in patients with obstructive sleep apnoea. ERJ Open Res. 2017, 3, P70. [Google Scholar] [CrossRef]
- Buonafine, M.; Martinez-Martinez, E.; Jaisser, F. More than a simple biomarker: The role of NGAL in cardiovascular and renal diseases. Clin. Sci. 2018, 132, 909–923. [Google Scholar] [CrossRef]
- Kim, J.W.; Hong, D.Y.; Lee, K.R.; Kim, S.Y.; Baek, K.J.; Park, S.O. Usefulness of plasma neutrophil gelatinase-associated lipocalin concentration for predicting the severity and mortality of patients with community-acquired pneumonia. Clin. Chim. Acta 2016, 462, 140–145. [Google Scholar] [CrossRef]
- Tanoğlu, A.; Beyazit, Y. Liver fatty acid-binding protein may be a useful marker for non-alcoholic fatty liver disease but obesity is a major concern. Wien. Klin. Wochenschr. 2016, 128, 304. [Google Scholar] [CrossRef]
- Eguchi, A.; Hasegawa, H.; Iwasa, M.; Tamai, Y.; Ohata, K.; Oikawa, T.; Sugaya, T.; Takei, Y. Serum Liver-Type Fatty Acid-Binding Protein Is a Possible Prognostic Factor in Human Chronic Liver Diseases From Chronic Hepatitis to Liver Cirrhosis and Hepatocellular Carcinoma. Hepatol. Commun. 2019, 3, 825–837. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).