Abstract
Vascular smooth muscle cells (VSMCs) are major components of blood vessels. They regulate physiological functions, such as vascular tone and blood flow. Under pathological conditions, VSMCs undergo a remodeling process known as phenotypic switching. During this process, VSMCs lose their contractility and acquire a synthetic phenotype, where they over-proliferate and migrate from the tunica media to the tunica interna, contributing to the occlusion of blood vessels. Since their discovery as effector proteins of cyclic adenosine 3′,5′-monophosphate (cAMP), exchange proteins activated by cAMP (EPACs) have been shown to play vital roles in a plethora of pathways in different cell systems. While extensive research to identify the role of EPAC in the vasculature has been conducted, much remains to be explored to resolve the reported discordance in EPAC’s effects. In this paper, we review the role of EPAC in VSMCs, namely its regulation of the vascular tone and phenotypic switching, with the likely involvement of reactive oxygen species (ROS) in the interplay between EPAC and its targets/effectors.
1. Introduction
Cyclic adenosine 3′,5′-monophosphate (cAMP) is one of the most studied second messengers that play a critical role in intracellular signaling transduction. It controls a wide variety of cellular responses including cell proliferation, migration, differentiation and apoptosis. Particularly in the cardiovasculature, cAMP’s role has been overwhelmingly documented [1,2,3,4,5,6,7,8,9,10,11]. cAMP is generated from ATP by the action of adenylyl cyclase (AC) isoforms, either membrane-bound or soluble [12,13]. The intracellular level of cAMP depends on its production by ACs and its degradation by cAMP phosphodiesterases (PDEs), which catalyze the hydrolysis of cAMP into 5′-adenosine monophosphate (5′-AMP) [14]. In addition to ACs and PDEs, the intracellular level of cAMP is regulated by A-kinase anchoring proteins (AKAPs), scaffolding proteins that sequester cAMP and its relevant signaling components into defined subcellular compartments [15]. This compartmentalization helps in sustaining localized pools of cAMP to effectively modulate the cellular actions of this second messenger [16,17].
A decade after the discovery of cAMP, protein kinase A (PKA) was identified as the downstream effector mediating cAMP signaling [18]. Later in 1998, two independent research groups discovered a novel cAMP effector family, currently known as exchange proteins activated by cAMP (EPACs) [19,20]. EPAC proteins, EPAC1 and EPAC2, act as guanine nucleotide exchange factors (GEFs) for the small Ras-like GTPases, Rap1 and Rap2. Although EPAC and PKA may act independently, they are often associated with the same biological process wherein they could mediate synergistic or opposite effects. The discovery of EPAC is relatively new; however, it has been shown to significantly modulate a plethora of pathways in different cell systems. It can control key cellular processes, such as cell proliferation, migration and apoptosis [1,3,16,21]. Many studies have demonstrated an important role of EPAC in the cardiovascular system. In addition to its role in physiology, EPAC is a key contributor to several cardiovascular pathologies [22,23].
Vascular smooth muscle cells (VSMCs) are major components of blood vessels and are located in the tunica media, the middle layer of a vessel wall. These cells are integral to the function of a blood vessel, both under physiologic and pathophysiologic conditions [3,24]. In healthy vessels, VSMCs contribute to vasotone and the regulation of blood flow. The onset of certain pathological conditions, such as atherosclerosis or hypertension, could trigger VSMCs to undergo a remodeling process known as phenotypic switching, where they lose their contractile phenotype and acquire a synthetic one [24]. VSMCs of a synthetic phenotype excessively proliferate and migrate towards the tunica intima of vessel wall. In the tunica intima, VSMCs contribute to the formation of atheroma in atherosclerosis and neointimal hyperplasia during restenosis [24,25,26]. There are some discrepancies regarding the role of EPAC in cardiovascular pathologies, where some studies report EPAC as a mediator of VSMCs phenotypic switching, while others describe a protective role.
In the present review, we aim to focus on the role of EPAC in VSMCs, mainly its involvement in the vascular tone and phenotypic switching. We provide a detailed and critical discussion of the targeted pathways and the underlying mechanisms involved in VSMC remodeling in in vitro and in vivo models of hypertension and neointimal hyperplasia.
2. EPAC
2.1. Genes and Expression
EPAC proteins, also known as cAMP-GEF proteins, comprise two isoforms, EPAC1 and EPAC2, which are encoded by two independent genes. EPAC1 is encoded by RAPGEF3 gene in humans and is ubiquitously expressed, particularly in the heart, blood vessels and kidney [19]. On the other hand, EPAC2, encoded by RAPGEF4 gene in humans, is prominently expressed in the brain and adrenal glands [19,20]. Alternative splicing of RAPGEF4 gene gives rise to three EPAC2 variants, designated as EPAC2A, EPAC2B, and EPAC2C [27]. These variants differ in their structure and tissue-specific expression. While EPAC2A is broadly expressed in the brain, pituitary and pancreas [20,27], EPAC2B is detected in the adrenal gland [28] and EPAC2C is liver specific [29].
2.2. Structure and Activation
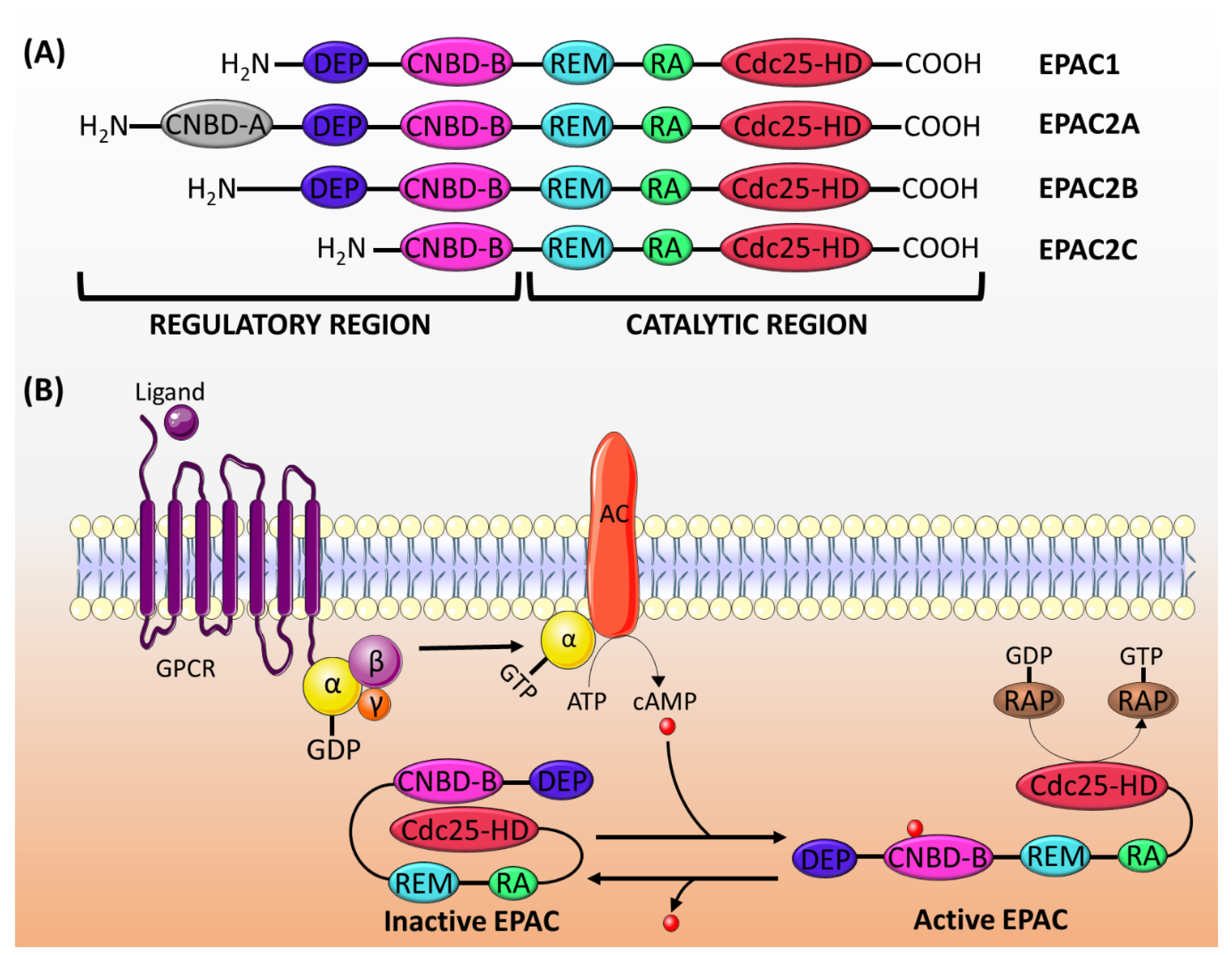
EPAC proteins are characterized by sequence homology to GEFs (for Ras and Rap) and to cAMP-binding sites. The structure of EPAC consists of a C-terminal catalytic region and an N-terminal regulatory region (Figure 1A). The catalytic region is similar in all isoforms and comprises a Ras-exchange motif (REM) domain, a Ras-association (RA) domain, and a cell division cycle 25 homology domain (Cdc25-HD) [16]. The REM domain, which is found in all Ras- and Rap-specific GEFs, is involved in the stabilization of the active conformation of EPAC [19,30]. On the other hand, the RA domain helps target EPAC to the membrane [31]. The Cdc25-HD is responsible for the GEF activity of EPAC [32].
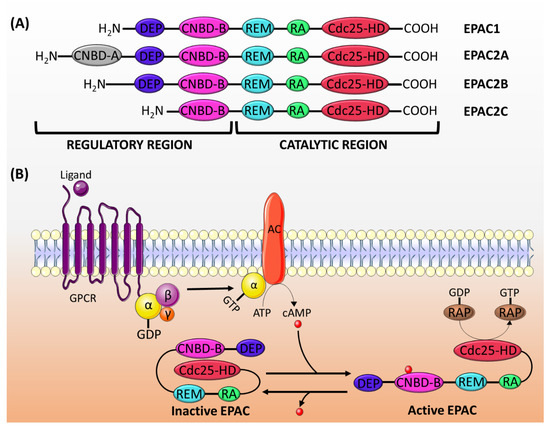
Figure 1.
(A) The structure of EPAC proteins. EPAC is made up of a catalytic region and a regulatory region, each of which is divided into different domains. The catalytic region in all EPAC isoforms comprises three domains: REM, RA and Cdc25-HD. The regulatory region consists of two domains: DEP and CNBD-B. EPAC2A has an addition CNBD-A domain, whereas EPAC2C lacks the DEF domain. (B) The mechanism of EPAC proteins activation: the activation of adenylyl cyclase (AC) by the Gα subunit of Gs protein induces the production of cAMP, which binds to the CNBD-B within the regulatory region of EPAC. This binding induces a conformational change releasing the auto-inhibitory effect, and it permits the binding of Rap1/2 to the catalytic domain (Cdc25-HD) and its subsequent activation by the GEF activity of EPAC. GPCR: G-protein coupled receptor.
The regulatory region differs among isoforms, though it generally consists of two domains: a disheveled/EGL-10/pleckstrin (DEP) domain and a cAMP-nucleotide binding-B domain (CNBD-B) [16]. The DEP domain, which is not present in EPAC2C, is necessary for the translocation of EPAC from the cytosol to the membrane [33]. The CNBD-B, as its name implies, is the binding site for cAMP. EPAC2A has an additional CNBD-A, which has a lower affinity to cAMP, and probably plays a role in the translocation of EPAC2A to the plasma membrane [34].
In its inactive state, the regulatory region of EPAC auto-inhibits its catalytic activity [19]. The cAMP binding domain acts as an inhibitory domain by binding to the Cdc25-HD and hindering the accessibility of Rap to the catalytic region. cAMP binding to CNBD induces a conformational change, which releases the auto-inhibition and promotes the binding of Rap to the Cdc25-HD motif [33] (Figure 1B).
3. The Role of EPAC in VSMCs
3.1. Vascular Tone
The regulation of the contraction and relaxation of VSMCs is critical for controlling the vasotone and blood pressure [24]. Therefore, changes in the dynamics of contraction or relaxation states may lead to pathological conditions such as hypertension. The intracellular second messenger, cAMP, modulates the vascular tone either by preventing the increase in Ca2+ concentration inside the cell or by reducing the Ca2+ sensitivity of the contractile proteins leading to the relaxation of the vessel wall [35,36].
cAMP signaling mediates vasorelaxation by the phosphorylation and inhibition of RhoA [37,38], activation of the myosin light chain phosphatase (MLCP) [39,40] as well as reduction of the inhibitory phosphorylation of myosin phosphatase-targeting subunit (MYPT1) [41,42]. The cAMP modulation of the vascular tone was attributed solely to PKA until the discovery of the novel cAMP effector, EPAC. In general, the activation of RhoA, a key regulator of the cytoskeleton, and its effector Rho kinase (ROCK), which phosphorylates and inhibits MYPT1, plays a role in mediating VSMCs contraction [43,44]. While calcium influx through CaV1.2 channels and subsequent MLCK activation is an important requirement for smooth muscle constriction [45,46], a significant body of literature highlighted the importance of RhoA-mediated calcium sensitization and actin cytoskeleton reorganization in maintaining arteriolar constriction [47,48] and providing a graded tonic response to intra-vascular pressure changes and stimulation by humoral mediators affording the auto-regulation of blood flow [49,50]. In fact, some studies have gone so far to show that the obligate dependence of vascular contractility on extracellular calcium influx is not necessarily tied to its downstream effect on MLCK and increased myosin light chain phosphorylation [51].
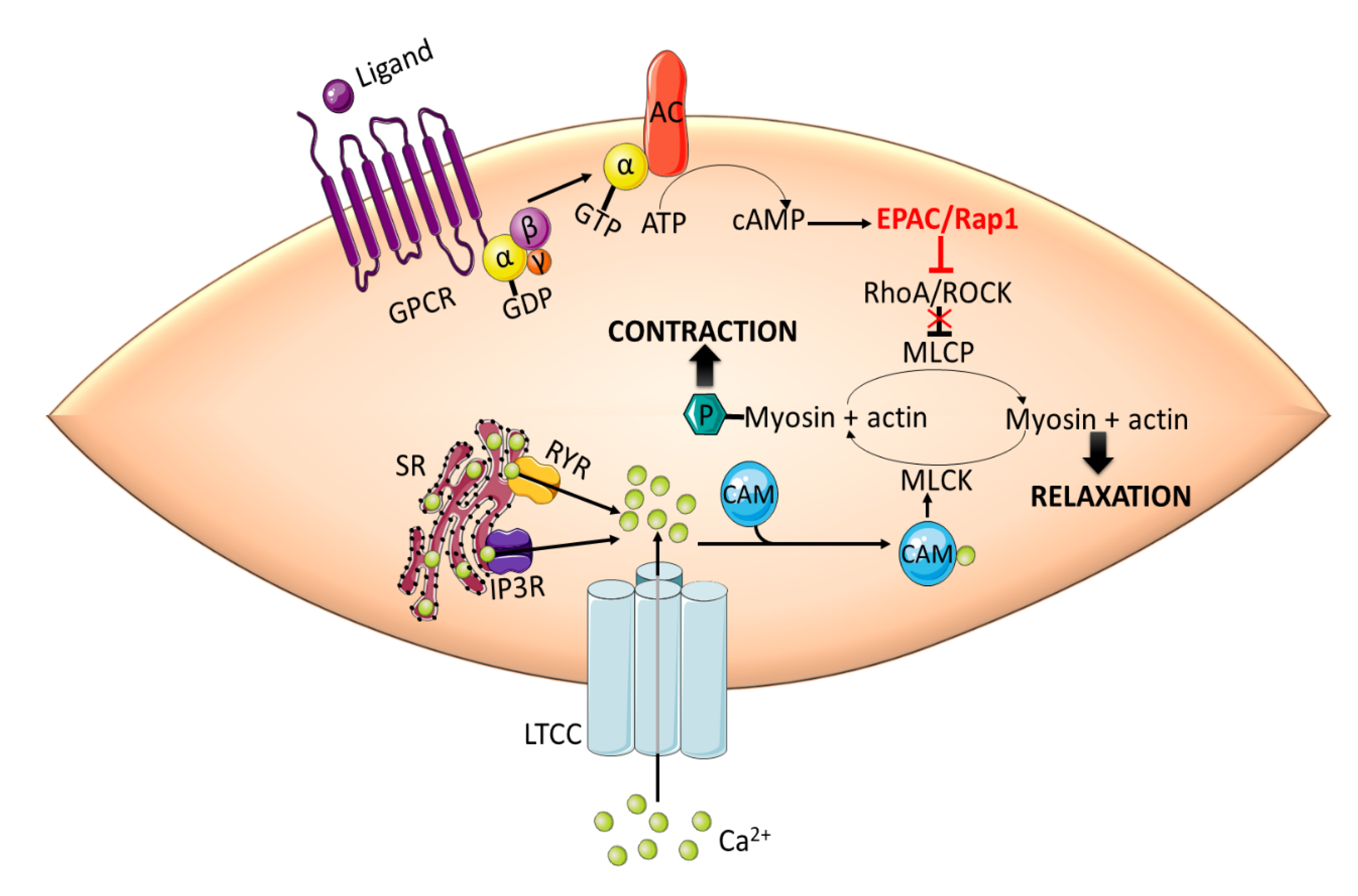
Inhibition of phosphatase activity increases the phosphorylation of myosin regulatory light chain (RLC20) and force generation, leading to contraction [52,53]. Intracellular cAMP signaling, mediated by PKA and EPAC, induces vasorelaxation in large vessels by inhibiting RhoA/ROCK signaling. cAMP-mediated activation of EPAC results in Rap1-dependent Ca2+ desensitization and relaxation in rat aortic VSMCs [54]. EPAC/Rap1-mediated vasorelaxation is accomplished by the inhibition of RhoA, thus disinhibiting the MLCP activity and decreasing the phosphorylation of RLC20 (Figure 2).
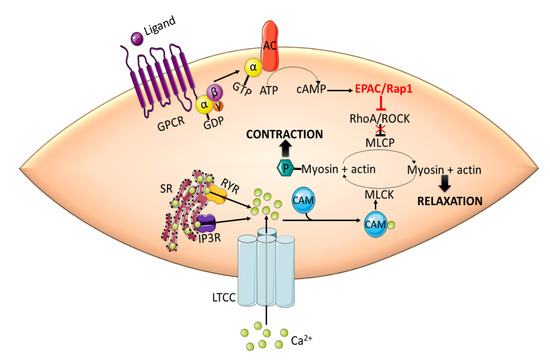
Figure 2.
EPAC promotes vasorelaxation in vascular smooth muscle cells (VSMCs) by inhibiting RhoA/ROCK signaling. Activation of RhoA/ROCK phosphorylates MLCP and inhibits its phosphatase activity. This in turn increases the phosphorylation of MLC by MLCK, which is activated by Ca2+-CaM complex, and induces contraction. cAMP-mediated activation of EPAC/Rap1 releases the inhibitory effect of RhoA/ROCK on MLCP leading to the dephosphorylation of MLC and subsequent relaxation. CaM: calmodulin; GPCR: G-protein coupled receptor; IP3R: inositol 1,4,5-triphosphate receptor; LTCC: L-type calcium channel; MLC: myosin light chain; MLCK: myosin light chain kinase; RYR: ryanodine receptor; SR: sarcoplasmic reticulum.
Another mechanism to promote vasorelaxation is by regulating intracellular levels of Ca2+. During vasorelaxation, the activation of EPAC increases the level of cytosolic Ca2+ by promoting its release through the ryanodine receptors of the sarcoplasmic reticulum and induces the activation of large conductance Ca2+-sensitive K+ (BKCa) channels [55]. Active BKCa channels evoke membrane hyperpolarization and limit the entry of Ca2+ by decreasing the activity of voltage-gated Ca2+ channels [56,57]. Surprisingly, EPAC-mediated increase in intracellular Ca2+ has been shown to inhibit ATP-sensitive potassium (KATP) channels activity through a Ca2+-sensitive protein phosphatase 2B (calcineurin)-dependent mechanism promoting arterial constriction [58]. Moreover, ryanodine receptor-mediated intra-cellular Ca2+ release has been implicated in the development of Ca2+ waves in VSMCs that are necessary for the maintenance of myogenic contractility in resistance arterioles [59]. Whether this effect is dependent on EPAC remains to be elucidated.
Physiologically, cAMP activates KATP channels by the virtue of its ability to stimulate PKA-dependent phosphorylation at the pore-forming and regulatory subunits of KATP, leading to vasorelaxation [60]. Interestingly, the inhibitory effect of EPAC on KATP channels has been only detected in the absence of PKA [58]. Therefore, one could speculate that EPAC promotes its inhibitory effect in pathological conditions where PKA signaling is disrupted. In rat aortic smooth muscle cells, EPAC promoted vasorelaxation by contributing to the cAMP-induced depletion of Ca2+ from intracellular stores and inhibition of store-operated calcium entry (SOCE). This effect of EPAC is only evident in combination with PKA activation [61]. By depleting intracellular Ca2+ stores, EPAC and PKA reduce Ca2+ availability for vasoconstriction.
In addition to its involvement in cAMP-induced endothelium-independent vasorelaxation, EPAC also contributes to vasorelaxation in the presence of an intact endothelium. Both EPAC and PKA could enhance the activity of endothelial nitric oxide synthase (eNOS), leading to an increase in nitric oxide (NO) production [55,62,63]. Contextually, knockout of Rap1, an effector of EPAC, inhibits NO-dependent vasorelaxation, resulting in heightened vasoconstriction and eventual hypertension [64]. This clearly implicates the EPAC-Rap1 signaling in endothelial dysfunction and its sequelae.
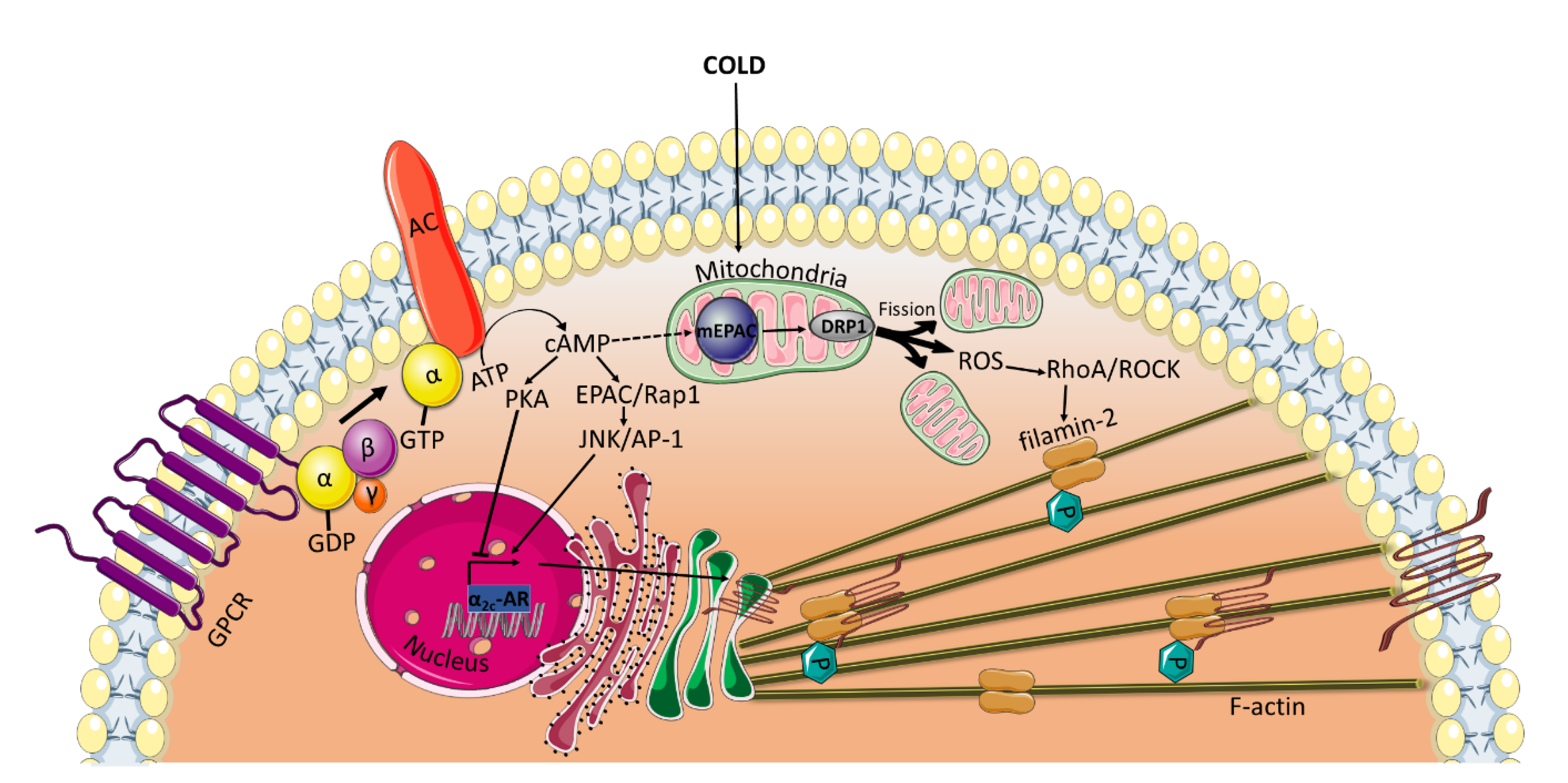
All of the previously mentioned studies, which reveal an EPAC-mediated vasorelaxation, have been conducted using VSMCs from large vessels. Interestingly, under certain conditions, such as cold temperatures, EPAC has been found to mediate cold-induced constriction of human dermal arterioles [6,7]. In microvascular smooth muscle cells (microVSMCs) extracted from these vessels, EPAC/Rap1 increases α2C-adrenoreceptor (α2C-AR) expression by activating c-Jun N-terminal kinase (JNK)/activating protein-1 (AP-1) signaling [6,7], whereas PKA suppresses cAMP-induced activation of this receptor [4] (Figure 3). α2C-ARs have once been considered silent receptors, largely due to intracellular entrapment within the endoplasmic reticulum and Golgi compartment, where they are not available for their agonist [65]. However, in response to cold stimuli, α2C-ARs are translocated from their intracellular localization to the cell surface, where they induce vasoconstriction [4,66]. EPAC/Rap1 not only increases α2C-ARs expression but also contributes to the translocation of the receptor to the cell surface by activation of RhoA/ROCK signaling, reorganization of the actin cytoskeleton and increase in F-actin [6,67]. Rap1/RhoA/ROCK has been found to phosphorylate filamin-2, an actin cross-linker, at Ser2113 leading to the translocation of α2C-ARs [5].
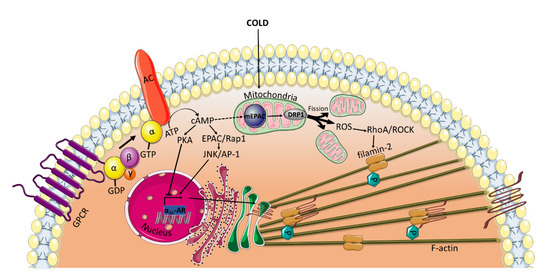
Figure 3.
EPAC mediates cold-induced constriction in microVSMCs. Extreme cold temperatures induce the release of reactive oxygen species (ROS) form the mitochondria. Alternatively, ROS may be generated by DRP1-induced mitochondrial fission driven by mEPAC. ROS then activates RhoA/ROCK pathway, which phosphorylates filamin-2 required for the translocation of α2C-ARs from the transGolgi to the cell surface where they induce vasoconstriction. EPAC also plays a role in increasing the α2C-ARs transcription by activating JNK/AP-1 and in the translocation of the receptor by activating RhoA/ROCK. DRP1: Dynamin-related protein; mEPAC: mitochondrial EPAC.
The opposite positive regulatory role of EPAC/Rap1 on RhoA/ROCK in microVSMCs may be driven indirectly via mitochondrial interaction. It has been shown that the upstream regulator of EPAC, cAMP, can be transported into the mitochondria and accumulate in the matrix [68]. The transported cAMP may activate the mitochondrially localized EPAC, known to contain an NH2-terminal mitochondrial-targeting sequence essential for EPAC co-localization with the mitochondria [69]. Several lines of evidence have shown that mitochondrial EPAC is a key role player in many physiological and pathophysiological conditions. For example, EPAC contributes to modulating the phosphorylation of dynamin-related protein (DRP1) [70], which plays a critical role in mitochondrial fission/fusion dynamics [71]. Mitochondrial fission has been shown to be implicated in the generation of reactive oxygen species (ROS) [72,73], which are known to activate Rho/ROCK in VSMCs [73,74] (Figure 3). This hypothesis can be corroborated by the findings of Hui et al., who demonstrated that in a mouse carotid artery ligation model, the genetic ablation of mitochondrial EPAC mitigates DRP1-mediated mitochondrial fission and ROS production [71].
Redox signaling in mediating the differential effects of EPAC on vascular tone merits further investigation. Nonetheless, the role of EPAC in vascular tone appears to be vascular bed-specific, where it contributes to vasorelaxation in large vessels and to vasoconstriction in microvessels. This could be in part due to the absence in large vessels of α2C-ARs, which are known to mediate vasoconstriction [75,76].
3.2. Phenotypic Switching: VSMCs Proliferation and Migration
VSMC proliferation and migration are critical events involved in normal vessel growth and wound healing process as well as pathological conditions, such as atherosclerosis and neointimal hyperplasia [26]. In response to vascular injury, activated VSMCs lose their contractility and acquire a synthetic phenotype. This phenotype is characterized by increased proliferation, migration, extracellular matrix synthesis and expression of synthetic markers [24]. Therefore, both proliferation and migration must be tightly regulated to prevent the development of pathological conditions, including neointimal hyperplasia, atherosclerosis and hypertension.
There are some controversies regarding the role of cAMP and its effectors, PKA and EPAC, in cell proliferation and migration. Depending on the cell type, some studies reported an inhibitory role [77,78,79], whereas others revealed the opposite [80,81,82]. In the vasculature, cAMP is known to negatively regulate VSMC proliferation [83]. The anti-proliferative effect of cAMP was first only attributed to its effector PKA [84,85,86]. However, in rat aortic VSMCs, activation of PKA alone was insufficient to mediate cAMP-dependent cell-cycle arrest. EPAC and PKA have been shown to synergistically reduce cell proliferation by inhibiting the expression of cyclin D1 and S-phase kinase-associated protein 2 (Skp2), regulators of G1-S cell cycle phase, and attenuating mitogen-activated protein kinases (MAPKs), extracellular signal-regulated protein kinase 1/2 (ERK1/2) and JNK signaling [86]. In addition, EPAC and PKA induce a stellate morphology and cytoskeletal disruption in these cells [87]. Although Rap1 promotes cell-cycle progression in VSMCs [88], EPAC has been found to mediate its inhibitory effects in a Rap1-independent manner [88]. In another study, EPAC and PKA were also shown to synergistically reduce the expression of early growth response 1 (Egr1) [89], which has a role in cell proliferation [90,91]. By inhibiting Rac-1, EPAC and PKA promote actin-cytoskeleton remodeling and nuclear export of ERK1/2 leading to dephosphorylation of the serum response factor (SRF) co-factor Elk. This, in turn, decreases the activity of Egr1 leading to inhibition of VSMC proliferation [89].
In addition to acting in synergy with PKA, EPAC can inhibit cell proliferation in a PKA-independent manner. By downregulating the early gene nuclear receptor NR4A1, EPAC has been shown to mediate the anti-proliferative effect of adenosine A2B receptors in primary human coronary artery smooth muscle cells [92]. EPAC and PKA can also mediate opposing effects. While PKA promotes cell proliferation and hyaluronic acid production in ductus arteriosus smooth muscle cells, leading to neointimal formation [93], the treatment of these cells with the EPAC agonist, 8-pCPT-2′-O-Me-cAMP, inhibits cell proliferation and induces no change in the production of hyaluronic acid [94].
Besides its anti-proliferative effect, cAMP also plays a protective role in VSMCs by inhibiting cell migration [95,96,97]. The increase in cAMP production by beraprost, a prostacyclin analog, inhibits growth factor-induced migration of human VSMCs from saphenous veins [98]. Interestingly, therapeutically relevant concentrations of beraprost activate EPAC but not PKA. It has been reported that the EPAC/Rap1 pathway impedes RhoA signaling, preventing actin-cytoskeletal changes and subsequent migration. Notably, although high concentrations of beraprost can activate both EPAC and PKA, cell proliferation has not been detected [98]. Likewise, in rat primary thoracic aorta VSMCs, the activation of EPAC using low concentrations of an EPAC analog inhibits cell migration. EPAC mediates its inhibition via the pharmacological blockade of multiple kinase signals, such as MAPK, phosphoinositide 3-kinase (PI3K) and Rac, and by increasing paxillin and modulating focal adhesion proteins, both of which are involved in cell motility [99,100].
Surprisingly, other studies have reported a role of EPAC in increasing VSMC proliferation and migration and promoting neointimal formation. Contradictory to the treatment with a PKA analog, treatment of rat aortic VSMCs with an EPAC activator significantly enhanced cell migration. In addition, overexpression of EPAC facilitated the development of neointimal formation in an ex vivo model [101]. Platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF) are both released by VSMCs and other cells present at the vascular injury site and contribute to cell migration and intimal thickening [102,103]. Kato et al. revealed that EPAC deficiency inhibits migration and attenuates neointimal formation by decreasing PDGF-induced intracellular Ca2+ elevation and suppressing cofilin-mediated lamellipodia formation [104]. The same group later reported that EPAC also contributes to bFGF-induced migration of VSMCs by activating the downstream signaling of PI3K/ protein kinase B (Akt) [105,106]. In a mouse carotid artery ligation model, the knockout of EPAC or its pharmacological inhibition significantly decreases neointimal formation by inhibiting VSMCs migration and proliferation [70]. Looking into the molecular mechanism of EPAC-mediated neointimal formation, EPAC increases the activity of PI3K/Akt signaling and induces mitochondrial fission and ROS production, both of which play a role in VSMCs activation during vascular remodeling [70,107,108].
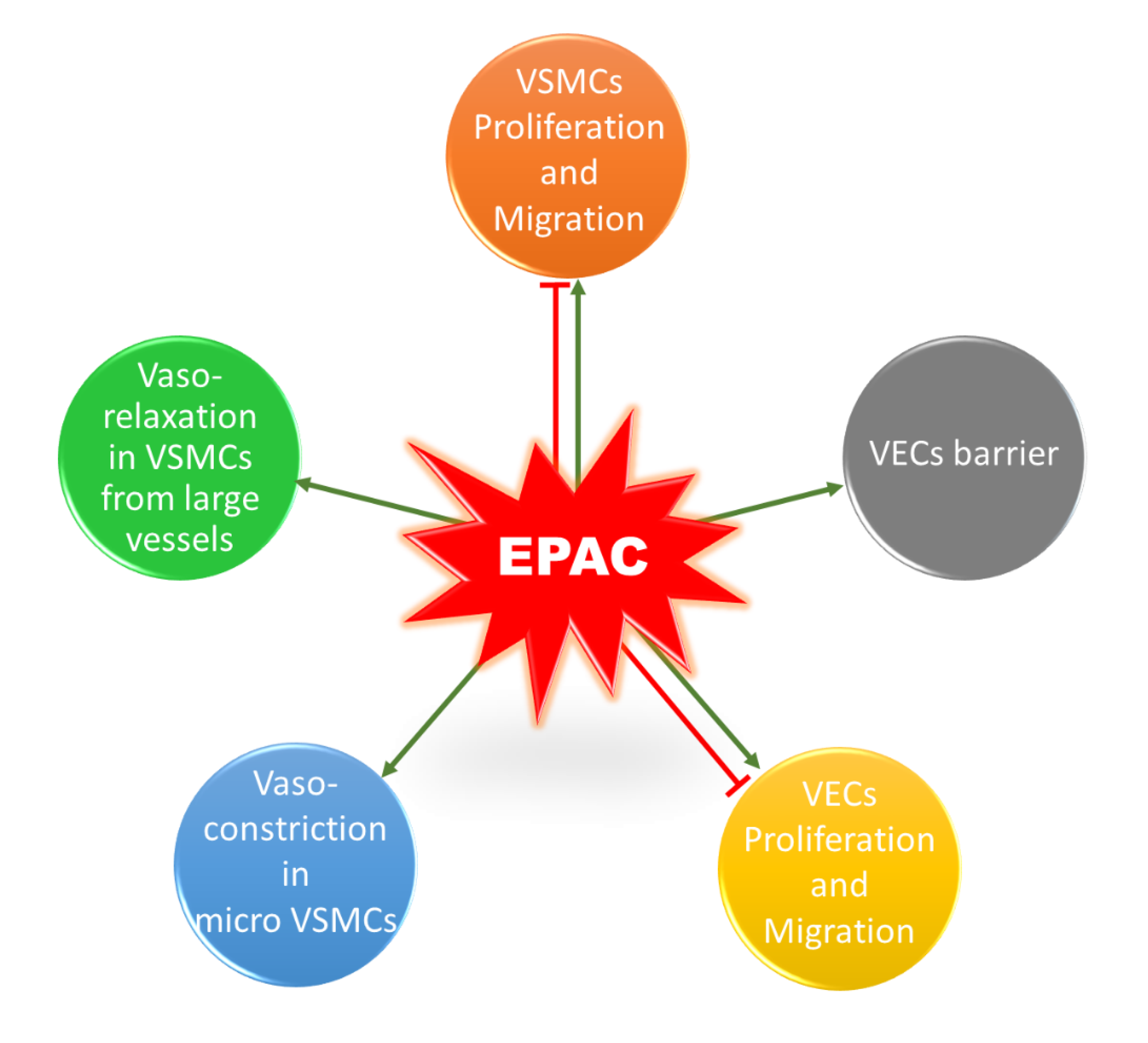
It is worth mentioning that a similar contradictory profile exists for the role of EPAC in vascular endothelial cells (VECs) (Figure 4). EPAC has been shown to differentially mediate adhesion of VECs isolated from microvessels and macrovessels [109]. In addition, EPAC and its effector, Rap1, enhance cell–cell contacts, mediating the vascular endothelial barrier and decreasing cell permeability [110,111]. Regarding the impact of EPAC on VEC proliferation and migration, some studies reported an inhibitory effect, while others showed that EPAC increases VEC proliferation and migration, promoting angiogenesis [112,113,114]. The discrepancy in these studies could arise from differences in model used and vascular beds from which VECs were obtained. Since this does not lie in the scope of our review, details for the role of EPAC in VECs can be found in other reviews [16,22,115].
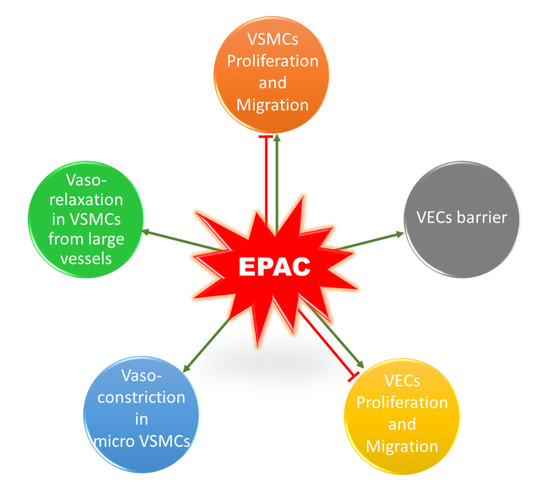
Figure 4.
Role of EPAC in the vasculature. EPAC can promote or inhibit proliferation and migration in VSMCs. In addition, EPAC induces vasorelaxation in VSMCs extracted from large vessels, whereas it mediates cold-induced vasoconstriction in microVSMCs. EPAC has a controversial role in VECs; it can promote or inhibit cell proliferation and migration. EPAC also enhances VECs barrier and decreases permeability.
4. Concluding Remarks
Although the role of EPAC in the vasculature is controversial, there is no doubt that it is a key player in the cardiovasculature. The reasons for this discordance partly lies in the differences between cellular model systems. Indeed, the susceptibility of VSMCs to phenotypic switching can be influenced by their site of origin and vascular bed from where the cells were isolated, in addition to differences in the species, strain, age and gender of the animal model [12].
Nevertheless, since EPAC plays a crucial role in vascular physiology and pathology, it could represent a potential target for drugs designed to treat atherosclerosis and hypertension. It is, therefore, important that future studies identify other downstream effectors that mediate Rap1-independent EPAC signaling to facilitate the development of new therapeutics that may target this family of cAMP effectors. Extensive research is also required to resolve the discrepancies in the role of EPAC in the vasculature and to translate in vitro and in vivo studies into clinical trials.
Author Contributions
N.W., S.A.N., Y.A.-D., R.I., A.B. (Alessandra Bitto), A.F.E.-Y., A.B. (Adnan Badran), F.K., E.B. and A.H.E. contributed to writing the manuscript. N.W., S.A.N. and A.H.E. led the writing of the first draft. A.H.E. conceived, edited, and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the American University of Beirut (Grant: MPP 320133 and Farouk Jabre Award to Ali Eid), and United Arab Emirates University (Grant #: 31S398-UPAR to Yusra Al-Dhaheri).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fardoun, M.M.; Issa, K.; Maaliki, D.; Nasser, S.A.; Baydoun, E.; Eid, A.H. Estrogen increases expression of vascular alpha 2C adrenoceptor through the cAMP/Epac/JNK/AP-1 pathway and potentiates cold-induced vasoconstriction. Vasc. Pharmacol. 2020, 131, 106690. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Samaha, A.A.; Baydoun, S.; Iratni, R.; Eid, A.H. Rhus coriaria L.(Sumac) Evokes Endothelium-Dependent Vasorelaxation of Rat Aorta: Involvement of the cAMP and cGMP Pathways. Front. Pharmacol. [CrossRef] [PubMed]
- Eid, A.H. cAMP induces adhesion of microvascular smooth muscle cells to fibronectin via an Epac-mediated but PKA-independent mechanism. Cell. Physiol. Biochem. 2012, 30, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Chotani, M.A.; Mitra, S.; Eid, A.H.; Han, S.A.; Flavahan, N.A. Distinct cAMP signaling pathways differentially regulate alpha2C-adrenoceptor expression: Role in serum induction in human arteriolar smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H69–H76. [Google Scholar] [CrossRef]
- Motawea, H.K.; Jeyaraj, S.C.; Eid, A.H.; Mitra, S.; Unger, N.T.; Ahmed, A.A.; Flavahan, N.A.; Chotani, M.A. Cyclic AMP-Rap1A signaling mediates cell surface translocation of microvascular smooth muscle alpha2C-adrenoceptors through the actin-binding protein filamin-2. Am. J. Physiol. Cell Physiol. 2013, 305, C829–C845. [Google Scholar] [CrossRef]
- Jeyaraj, S.C.; Unger, N.T.; Eid, A.H.; Mitra, S.; Paul El-Dahdah, N.; Quilliam, L.A.; Flavahan, N.A.; Chotani, M.A. Cyclic AMP-Rap1A signaling activates RhoA to induce alpha(2c)-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2012, 303, C499–C511. [Google Scholar] [CrossRef]
- Eid, A.H.; Chotani, M.A.; Mitra, S.; Miller, T.J.; Flavahan, N.A. Cyclic AMP acts through Rap1 and JNK signaling to increase expression of cutaneous smooth muscle alpha2C-adrenoceptors. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H266–H272. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, Z.; Nasser, S.A.; El-Yazbi, A.; Nasreddine, S.; Eid, A.H. Estrogen and Bisphenol A in Hypertension. Curr. Hypertens. Rep. 2020, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Fardoun, M.; Dehaini, H.; Shaito, A.; Mesmar, J.; El-Yazbi, A.; Badran, A.; Beydoun, E.; Eid, A.H. The hypertensive potential of estrogen: An untold story. Vasc. Pharmacol. 2020, 124, 106600. [Google Scholar] [CrossRef]
- Dehaini, H.; Fardoun, M.; Abou-Saleh, H.; El-Yazbi, A.; Eid, A.A.; Eid, A.H. Estrogen in vascular smooth muscle cells: A friend or a foe? Vasc. Pharmacol. 2018, 111, 15–21. [Google Scholar] [CrossRef]
- Eid, A.H.; Maiti, K.; Mitra, S.; Chotani, M.A.; Flavahan, S.; Bailey, S.R.; Thompson-Torgerson, C.S.; Flavahan, N.A. Estrogen increases smooth muscle expression of alpha2C-adrenoceptors and cold-induced constriction of cutaneous arteries. Am. J. Physiol. 2007, 293, H1955–H1961. [Google Scholar] [CrossRef]
- Tresguerres, M.; Levin, L.R.; Buck, J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 2011, 79, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.M. Regulation and organization of adenylyl cyclases and cAMP. Biochem. J. 2003, 375, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Mika, D.; Leroy, J.; Vandecasteele, G.; Fischmeister, R. PDEs create local domains of cAMP signaling. J. Mol. Cell. Cardiol. 2012, 52, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kritzer, M.D.; Li, J.; Dodge-Kafka, K.; Kapiloff, M.S. AKAPs: The architectural underpinnings of local cAMP signaling. J. Mol. Cell. Cardiol. 2012, 52, 351–358. [Google Scholar] [CrossRef]
- Robichaux, W.G., 3rd; Cheng, X. Intracellular cAMP Sensor EPAC: Physiology, Pathophysiology, and Therapeutics Development. Physiol. Rev. 2018, 98, 919–1053. [Google Scholar] [CrossRef]
- Arora, K.; Sinha, C.; Zhang, W.; Ren, A.; Moon, C.S.; Yarlagadda, S.; Naren, A.P. Compartmentalization of cyclic nucleotide signaling: A question of when, where, and why? Pflug. Arch. 2013, 465, 1397–1407. [Google Scholar] [CrossRef]
- Walsh, D.A.; Perkins, J.P.; Krebs, E.G. An adenosine 3’,5’-monophosphate-dependant protein kinase from rabbit skeletal muscle. J. Biol. Chem. 1968, 243, 3763–3765. [Google Scholar]
- De Rooij, J.; Zwartkruis, F.J.; Verheijen, M.H.; Cool, R.H.; Nijman, S.M.; Wittinghofer, A.; Bos, J.L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998, 396, 474–477. [Google Scholar] [CrossRef]
- Kawasaki, H.; Springett, G.M.; Mochizuki, N.; Toki, S.; Nakaya, M.; Matsuda, M.; Housman, D.E.; Graybiel, A.M. A family of cAMP-binding proteins that directly activate Rap1. Science 1998, 282, 2275–2279. [Google Scholar] [CrossRef]
- Schmidt, M.; Dekker, F.J.; Maarsingh, H. Exchange protein directly activated by cAMP (epac): A multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol. Rev. 2013, 65, 670–709. [Google Scholar] [CrossRef] [PubMed]
- Lezoualc’h, F.; Fazal, L.; Laudette, M.; Conte, C. Cyclic AMP Sensor EPAC Proteins and Their Role in Cardiovascular Function and Disease. Circ. Res. 2016, 118, 881–897. [Google Scholar] [CrossRef] [PubMed]
- Fardoun, M.M.; Nassif, J.; Issa, K.; Baydoun, E.; Eid, A.H. Raynaud’s Phenomenon: A Brief Review of the Underlying Mechanisms. Front. Pharmacol. 2016, 7, 438. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, P.N. Molecular biology of atherosclerosis. Physiol. Rev. 2013, 93, 1317–1542. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jorgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Newby, A.C.; Zaltsman, A.B. Molecular mechanisms in intimal hyperplasia. J. Pathol. 2000, 190, 300–309. [Google Scholar] [CrossRef]
- Hoivik, E.A.; Witsoe, S.L.; Bergheim, I.R.; Xu, Y.; Jakobsson, I.; Tengholm, A.; Doskeland, S.O.; Bakke, M. DNA methylation of alternative promoters directs tissue specific expression of Epac2 isoforms. PLoS ONE 2013, 8, e67925. [Google Scholar] [CrossRef]
- Aumo, L.; Rusten, M.; Mellgren, G.; Bakke, M.; Lewis, A.E. Functional roles of protein kinase A (PKA) and exchange protein directly activated by 3’,5’-cyclic adenosine 5’-monophosphate (cAMP) 2 (EPAC2) in cAMP-mediated actions in adrenocortical cells. Endocrinology 2010, 151, 2151–2161. [Google Scholar] [CrossRef]
- Ueno, H.; Shibasaki, T.; Iwanaga, T.; Takahashi, K.; Yokoyama, Y.; Liu, L.M.; Yokoi, N.; Ozaki, N.; Matsukura, S.; Yano, H.; et al. Characterization of the gene EPAC2: Structure, chromosomal localization, tissue expression, and identification of the liver-specific isoform. Genomics 2001, 78, 91–98. [Google Scholar] [CrossRef]
- Boriack-Sjodin, P.A.; Margarit, S.M.; Bar-Sagi, D.; Kuriyan, J. The structural basis of the activation of Ras by Sos. Nature 1998, 394, 337–343. [Google Scholar] [CrossRef]
- Liu, C.; Takahashi, M.; Li, Y.; Song, S.; Dillon, T.J.; Shinde, U.; Stork, P.J. Ras is required for the cyclic AMP-dependent activation of Rap1 via Epac2. Mol. Cell. Biol. 2008, 28, 7109–7125. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Rensen-de Leeuw, M.; Rehmann, H. Selectivity of CDC25 homology domain-containing guanine nucleotide exchange factors. J. Mol. Biol. 2013, 425, 2782–2794. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, J.; Rehmann, H.; van Triest, M.; Cool, R.H.; Wittinghofer, A.; Bos, J.L. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem 2000, 275, 20829–20836. [Google Scholar] [CrossRef] [PubMed]
- Niimura, M.; Miki, T.; Shibasaki, T.; Fujimoto, W.; Iwanaga, T.; Seino, S. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J. Cell. Physiol. 2009, 219, 652–658. [Google Scholar] [CrossRef]
- Rembold, C.M. Regulation of contraction and relaxation in arterial smooth muscle. Hypertension 1992, 20, 129–137. [Google Scholar] [CrossRef]
- Raeymaekers, L.; Eggermont, J.A.; Wuytack, F.; Casteels, R. Effects of cyclic nucleotide dependent protein kinases on the endoplasmic reticulum Ca2+ pump of bovine pulmonary artery. Cell Calcium 1990, 11, 261–268. [Google Scholar] [CrossRef]
- Oishi, A.; Makita, N.; Sato, J.; Iiri, T. Regulation of RhoA signaling by the cAMP-dependent phosphorylation of RhoGDIalpha. J. Biol. Chem. 2012, 287, 38705–38715. [Google Scholar] [CrossRef]
- Sauzeau, V.; Le Jeune, H.; Cario-Toumaniantz, C.; Smolenski, A.; Lohmann, S.M.; Bertoglio, J.; Chardin, P.; Pacaud, P.; Loirand, G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000, 275, 21722–21729. [Google Scholar] [CrossRef]
- Wu, X.; Haystead, T.A.; Nakamoto, R.K.; Somlyo, A.V.; Somlyo, A.P. Acceleration of myosin light chain dephosphorylation and relaxation of smooth muscle by telokin. Synergism with cyclic nucleotide-activated kinase. J. Biol. Chem. 1998, 273, 11362–11369. [Google Scholar] [CrossRef]
- Wu, X.; Somlyo, A.V.; Somlyo, A.P. Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphate. Biochem. Biophys. Res. Commun. 1996, 220, 658–663. [Google Scholar] [CrossRef]
- Lubomirov, L.T.; Reimann, K.; Metzler, D.; Hasse, V.; Stehle, R.; Ito, M.; Hartshorne, D.J.; Gagov, H.; Pfitzer, G.; Schubert, R. Urocortin-induced decrease in Ca2+ sensitivity of contraction in mouse tail arteries is attributable to cAMP-dependent dephosphorylation of MYPT1 and activation of myosin light chain phosphatase. Circ. Res. 2006, 98, 1159–1167. [Google Scholar] [CrossRef]
- Wooldridge, A.A.; MacDonald, J.A.; Erdodi, F.; Ma, C.; Borman, M.A.; Hartshorne, D.J.; Haystead, T.A. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J. Biol. Chem. 2004, 279, 34496–34504. [Google Scholar] [CrossRef] [PubMed]
- Seko, T.; Ito, M.; Kureishi, Y.; Okamoto, R.; Moriki, N.; Onishi, K.; Isaka, N.; Hartshorne, D.J.; Nakano, T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ. Res. 2003, 92, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Sward, K.; Mita, M.; Wilson, D.P.; Deng, J.T.; Susnjar, M.; Walsh, M.P. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr. Hypertens. Rep. 2003, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Zou, H.; Potocnik, S.J.; Meininger, G.A.; Davis, M.J. Arteriolar smooth muscle mechanotransduction: Ca(2+) signaling pathways underlying myogenic reactivity. J. Appl. Physiol. 2001, 91, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Ratz, P.H.; Hill, M.A. Temporal aspects of Ca(2+) and myosin phosphorylation during myogenic and norepinephrine-induced arteriolar constriction. J. Vasc. Res. 2000, 37, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Domínguez, A.; Colinas, O.; El-Yazbi, A.; Walsh, E.J.; Hill, M.A.; Walsh, M.P.; Cole, W.C. Ca 2+ sensitization due to myosin light chain phosphatase inhibition and cytoskeletal reorganization in the myogenic response of skeletal muscle resistance arteries. J. Physiol. 2013, 591, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Dominguez, A.; El-Yazbi, A.F.; Zhu, H.L.; Colinas, O.; Zhong, X.Z.; Walsh, E.J.; Cole, D.M.; Kargacin, G.J.; Walsh, M.P.; Cole, W.C. Cytoskeletal reorganization evoked by Rho-associated kinase- and protein kinase C-catalyzed phosphorylation of cofilin and heat shock protein 27, respectively, contributes to myogenic constriction of rat cerebral arteries. J. Biol. Chem. 2014, 289, 20939–20952. [Google Scholar] [CrossRef]
- Johnson, R.P.; El-Yazbi, A.F.; Takeya, K.; Walsh, E.J.; Walsh, M.P.; Cole, W.C. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J. Physiol. 2009, 587, 2537–2553. [Google Scholar] [CrossRef]
- El-Yazbi, A.F.; Johnson, R.P.; Walsh, E.J.; Takeya, K.; Walsh, M.P.; Cole, W.C. Pressure-dependent contribution of Rho kinase-mediated calcium sensitization in serotonin-evoked vasoconstriction of rat cerebral arteries. J. Physiol. 2010, 588, 1747–1762. [Google Scholar] [CrossRef]
- El-Yazbi, A.F.; Abd-Elrahman, K.S.; Moreno-Dominguez, A. PKC-mediated cerebral vasoconstriction: Role of myosin light chain phosphorylation versus actin cytoskeleton reorganization. Biochem. Pharmacol. 2015, 95, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C.; Walsh, M.P. Myosin regulatory light chain diphosphorylation slows relaxation of arterial smooth muscle. J. Biol. Chem. 2012, 287, 24064–24076. [Google Scholar] [CrossRef] [PubMed]
- Khromov, A.; Choudhury, N.; Stevenson, A.S.; Somlyo, A.V.; Eto, M. Phosphorylation-dependent autoinhibition of myosin light chain phosphatase accounts for Ca2+ sensitization force of smooth muscle contraction. J. Biol. Chem. 2009, 284, 21569–21579. [Google Scholar] [CrossRef]
- Zieba, B.J.; Artamonov, M.V.; Jin, L.; Momotani, K.; Ho, R.; Franke, A.S.; Neppl, R.L.; Stevenson, A.S.; Khromov, A.S.; Chrzanowska-Wodnicka, M.; et al. The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activity. J. Biol. Chem. 2011, 286, 16681–16692. [Google Scholar] [CrossRef] [PubMed]
- Roberts, O.L.; Kamishima, T.; Barrett-Jolley, R.; Quayle, J.M.; Dart, C. Exchange protein activated by cAMP (Epac) induces vascular relaxation by activating Ca2+-sensitive K+ channels in rat mesenteric artery. J. Physiol. 2013, 591, 5107–5123. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Ella, S.R.; Davis, M.J.; Braun, A.P. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. Febs Lett. 2010, 584, 2033–2042. [Google Scholar] [CrossRef]
- Nelson, M.T.; Patlak, J.B.; Worley, J.F.; Standen, N.B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. 1990, 259, C3–C18. [Google Scholar] [CrossRef]
- Purves, G.I.; Kamishima, T.; Davies, L.M.; Quayle, J.M.; Dart, C. Exchange protein activated by cAMP (Epac) mediates cAMP-dependent but protein kinase A-insensitive modulation of vascular ATP-sensitive potassium channels. J. Physiol. 2009, 587, 3639–3650. [Google Scholar] [CrossRef]
- Mufti, R.E.; Brett, S.E.; Tran, C.H.T.; Abd El-Rahman, R.; Anfinogenova, Y.; El-Yazbi, A.; Cole, W.C.; Jones, P.P.; Chen, S.R.W.; Welsh, D.G. Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves. J. Physiol. 2010, 588, 3983–4005. [Google Scholar] [CrossRef]
- Quinn, K.V.; Giblin, J.P.; Tinker, A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ. Res. 2004, 94, 1359–1366. [Google Scholar] [CrossRef]
- Cuinas, A.; Garcia-Morales, V.; Vina, D.; Gil-Longo, J.; Campos-Toimil, M. Activation of PKA and Epac proteins by cyclic AMP depletes intracellular calcium stores and reduces calcium availability for vasoconstriction. Life Sci. 2016, 155, 102–109. [Google Scholar] [CrossRef]
- Garcia-Morales, V.; Luaces-Regueira, M.; Campos-Toimil, M. The cAMP effectors PKA and Epac activate endothelial NO synthase through PI3K/Akt pathway in human endothelial cells. Biochem. Pharmacol. 2017, 145, 94–101. [Google Scholar] [CrossRef]
- Garcia-Morales, V.; Cuinas, A.; Elies, J.; Campos-Toimil, M. PKA and Epac activation mediates cAMP-induced vasorelaxation by increasing endothelial NO production. Vasc. Pharmacol. 2014, 60, 95–101. [Google Scholar] [CrossRef]
- Lakshmikanthan, S.; Zieba, B.J.; Ge, Z.D.; Momotani, K.; Zheng, X.; Lund, H.; Artamonov, M.V.; Maas, J.E.; Szabo, A.; Zhang, D.X.; et al. Rap1b in smooth muscle and endothelium is required for maintenance of vascular tone and normal blood pressure. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1486–1494. [Google Scholar] [CrossRef]
- Daunt, D.A.; Hurt, C.; Hein, L.; Kallio, J.; Feng, F.; Kobilka, B.K. Subtype-specific intracellular trafficking of alpha2-adrenergic receptors. Mol. Pharmacol. 1997, 51, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Chotani, M.A.; Flavahan, S.; Mitra, S.; Daunt, D.; Flavahan, N.A. Silent alpha(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1075–H1083. [Google Scholar] [CrossRef]
- Bailey, S.R.; Eid, A.H.; Mitra, S.; Flavahan, S.; Flavahan, N.A. Rho kinase mediates cold-induced constriction of cutaneous arteries: Role of alpha2C-adrenoceptor translocation. Circ. Res. 2004, 94, 1367–1374. [Google Scholar] [CrossRef]
- Kulinskii, V.I.; Zobova, N.V. Submitochondrial distribution of cAMP during incubation with rat liver mitochondria. Biokhimiia 1985, 50, 1546–1552. [Google Scholar]
- Qiao, J.; Mei, F.C.; Popov, V.L.; Vergara, L.A.; Cheng, X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J. Biol. Chem. 2002, 277, 26581–26586. [Google Scholar] [CrossRef]
- Wang, H.; Robichaux, W.G.; Wang, Z.; Mei, F.C.; Cai, M.; Du, G.; Chen, J.; Cheng, X. Inhibition of Epac1 suppresses mitochondrial fission and reduces neointima formation induced by vascular injury. Sci. Rep. 2016, 6, 36552. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Y.; Li, L. Drp1-Dependent Mitochondrial Fission Plays Critical Roles in Physiological and Pathological Progresses in Mammals. Int. J. Mol. Sci. 2017, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Sheu, S.S.; Robotham, J.L.; Yoon, Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc. Res. 2008, 79, 341–351. [Google Scholar] [CrossRef]
- Jin, L.; Ying, Z.; Webb, R.C. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am. J. Physiol. 2004, 287, H1495–H1500. [Google Scholar] [CrossRef]
- Bailey, S.R.; Mitra, S.; Flavahan, S.; Flavahan, N.A. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am. J. Physiol. 2005, 289, H243–H250. [Google Scholar] [CrossRef] [PubMed]
- Chotani, M.A.; Mitra, S.; Su, B.Y.; Flavahan, S.; Eid, A.H.; Clark, K.R.; Montague, C.R.; Paris, H.; Handy, D.E.; Flavahan, N.A. Regulation of alpha(2)-adrenoceptors in human vascular smooth muscle cells. Am. J. Physiol. 2004, 286, H59–H67. [Google Scholar] [CrossRef]
- Leech, C.J.; Faber, J.E. Different alpha-adrenoceptor subtypes mediate constriction of arterioles and venules. Am. J. Physiol. 1996, 270, H710–H722. [Google Scholar] [CrossRef]
- Dong, H.; Claffey, K.P.; Brocke, S.; Epstein, P.M. Inhibition of breast cancer cell migration by activation of cAMP signaling. Breast Cancer Res. Treat. 2015, 152, 17–28. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, J.; Moon, E.Y. HeLa human cervical cancer cell migration is inhibited by treatment with dibutyryl-cAMP. Anticancer Res. 2014, 34, 3447–3455. [Google Scholar]
- Schmitt, J.M.; Stork, P.J. Cyclic AMP-mediated inhibition of cell growth requires the small G protein Rap1. Mol. Cell. Biol. 2001, 21, 3671–3683. [Google Scholar] [CrossRef]
- Kim, M.O.; Ryu, J.M.; Suh, H.N.; Park, S.H.; Oh, Y.M.; Lee, S.H.; Han, H.J. cAMP Promotes Cell Migration Through Cell Junctional Complex Dynamics and Actin Cytoskeleton Remodeling: Implications in Skin Wound Healing. Stem Cells Dev. 2015, 24, 2513–2524. [Google Scholar] [CrossRef]
- Misra, U.K.; Pizzo, S.V. Epac1-induced cellular proliferation in prostate cancer cells is mediated by B-Raf/ERK and mTOR signaling cascades. J. Cell. Biochem. 2009, 108, 998–1011. [Google Scholar] [CrossRef]
- Hochbaum, D.; Hong, K.; Barila, G.; Ribeiro-Neto, F.; Altschuler, D.L. Epac, in synergy with cAMP-dependent protein kinase (PKA), is required for cAMP-mediated mitogenesis. J. Biol. Chem. 2008, 283, 4464–4468. [Google Scholar] [CrossRef]
- Smith, S.A.; Newby, A.C.; Bond, M. Ending Restenosis: Inhibition of Vascular Smooth Muscle Cell Proliferation by cAM. Cells 2019, 8, 1447. [Google Scholar] [CrossRef]
- Wu, Y.J.; Bond, M.; Sala-Newby, G.B.; Newby, A.C. Altered S-phase kinase-associated protein-2 levels are a major mediator of cyclic nucleotide-induced inhibition of vascular smooth muscle cell proliferation. Circ. Res. 2006, 98, 1141–1150. [Google Scholar] [CrossRef]
- Hayashi, S.; Morishita, R.; Matsushita, H.; Nakagami, H.; Taniyama, Y.; Nakamura, T.; Aoki, M.; Yamamoto, K.; Higaki, J.; Ogihara, T. Cyclic AMP inhibited proliferation of human aortic vascular smooth muscle cells, accompanied by induction of p53 and p21. Hypertension 2000, 35, 237–243. [Google Scholar] [CrossRef]
- Indolfi, C.; Avvedimento, E.V.; Di Lorenzo, E.; Esposito, G.; Rapacciuolo, A.; Giuliano, P.; Grieco, D.; Cavuto, L.; Stingone, A.M.; Ciullo, I.; et al. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat. Med. 1997, 3, 775–779. [Google Scholar] [CrossRef]
- Hewer, R.C.; Sala-Newby, G.B.; Wu, Y.J.; Newby, A.C.; Bond, M. PKA and Epac synergistically inhibit smooth muscle cell proliferation. J. Mol. Cell. Cardiol. 2011, 50, 87–98. [Google Scholar] [CrossRef]
- Li, Q.; Teng, Y.; Wang, J.; Yu, M.; Li, Y.; Zheng, H. Rap1 promotes proliferation and migration of vascular smooth muscle cell via the ERK pathway. Pathol. Res. Pract. 2018, 214, 1045–1050. [Google Scholar] [CrossRef]
- Kimura, T.E.; Duggirala, A.; Hindmarch, C.C.; Hewer, R.C.; Cui, M.Z.; Newby, A.C.; Bond, M. Inhibition of Egr1 expression underlies the anti-mitogenic effects of cAMP in vascular smooth muscle cells. J. Mol. Cell. Cardiol. 2014, 72, 9–19. [Google Scholar] [CrossRef]
- Min, I.M.; Pietramaggiori, G.; Kim, F.S.; Passegue, E.; Stevenson, K.E.; Wagers, A.J. The transcription factor EGR1 controls both the proliferation and localization of hematopoietic stem cells. Cell Stem Cell 2008, 2, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, R.G.; Khachigian, L.M. Locked nucleic acid modified DNA enzymes targeting early growth response-1 inhibit human vascular smooth muscle cell growth. Nucleic Acids Res. 2004, 32, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Mayer, P.; Hinze, A.V.; Harst, A.; von Kugelgen, I. A(2)B receptors mediate the induction of early genes and inhibition of arterial smooth muscle cell proliferation via Epac. Cardiovasc. Res. 2011, 90, 148–156. [Google Scholar] [CrossRef]
- Yokoyama, U.; Minamisawa, S.; Quan, H.; Ghatak, S.; Akaike, T.; Segi-Nishida, E.; Iwasaki, S.; Iwamoto, M.; Misra, S.; Tamura, K.; et al. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J. Clin. Investig. 2006, 116, 3026–3034. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Minamisawa, S.; Quan, H.; Akaike, T.; Suzuki, S.; Jin, M.; Jiao, Q.; Watanabe, M.; Otsu, K.; Iwasaki, S.; et al. Prostaglandin E2-activated Epac promotes neointimal formation of the rat ductus arteriosus by a process distinct from that of cAMP-dependent protein kinase A. J. Biol. Chem. 2008, 283, 28702–28709. [Google Scholar] [CrossRef]
- Kimura, T.E.; Duggirala, A.; Smith, M.C.; White, S.; Sala-Newby, G.B.; Newby, A.C.; Bond, M. The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP. J. Mol. Cell. Cardiol. 2016, 90, 1–10. [Google Scholar] [CrossRef]
- Stone, J.D.; Narine, A.; Tulis, D.A. Inhibition of vascular smooth muscle growth via signaling crosstalk between AMP-activated protein kinase and cAMP-dependent protein kinase. Front. Physiol. 2012, 3, 409. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.; Tsoi, K.; Maurice, D.H. Synergistic inhibition of vascular smooth muscle cell migration by phosphodiesterase 3 and phosphodiesterase 4 inhibitors. Circ. Res. 1998, 82, 852–861. [Google Scholar] [CrossRef]
- McKean, J.S.; Murray, F.; Gibson, G.; Shewan, D.A.; Tucker, S.J.; Nixon, G.F. The cAMP-producing agonist beraprost inhibits human vascular smooth muscle cell migration via exchange protein directly activated by cAMP. Cardiovasc. Res. 2015, 107, 546–555. [Google Scholar] [CrossRef]
- Min, J.; Reznichenko, M.; Poythress, R.H.; Gallant, C.M.; Vetterkind, S.; Li, Y.; Morgan, K.G. Src modulates contractile vascular smooth muscle function via regulation of focal adhesions. J. Cell. Physiol. 2012, 227, 3585–3592. [Google Scholar] [CrossRef]
- Turner, C.E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000, 2, E231–E236. [Google Scholar] [CrossRef]
- Yokoyama, U.; Minamisawa, S.; Quan, H.; Akaike, T.; Jin, M.; Otsu, K.; Ulucan, C.; Wang, X.; Baljinnyam, E.; Takaoka, M.; et al. Epac1 is upregulated during neointima formation and promotes vascular smooth muscle cell migration. Am. J. Physiol. 2008, 295, H1547–H1555. [Google Scholar] [CrossRef]
- Jawien, A.; Bowen-Pope, D.F.; Lindner, V.; Schwartz, S.M.; Clowes, A.W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J. Clin. Investig. 1992, 89, 507–511. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.; Li, G.; Tellides, G.; Simons, M. Fibroblast growth factor (FGF) signaling regulates transforming growth factor beta (TGFbeta)-dependent smooth muscle cell phenotype modulation. Sci. Rep. 2016, 6, 33407. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Yokoyama, U.; Yanai, C.; Ishige, R.; Kurotaki, D.; Umemura, M.; Fujita, T.; Kubota, T.; Okumura, S.; Sata, M.; et al. Epac1 Deficiency Attenuated Vascular Smooth Muscle Cell Migration and Neointimal Formation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2617–2625. [Google Scholar] [CrossRef]
- Kato, Y.; Yokoyama, U.; Fujita, T.; Umemura, M.; Kubota, T.; Ishikawa, Y. Epac1 deficiency inhibits basic fibroblast growth factor-mediated vascular smooth muscle cell migration. J. Physiol. Sci. 2019, 69, 175–184. [Google Scholar] [CrossRef]
- Shigematsu, K.; Koyama, H.; Olson, N.E.; Cho, A.; Reidy, M.A. Phosphatidylinositol 3-kinase signaling is important for smooth muscle cell replication after arterial injury. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2373–2378. [Google Scholar] [CrossRef]
- Wang, L.; Yu, T.; Lee, H.; O’Brien, D.K.; Sesaki, H.; Yoon, Y. Decreasing mitochondrial fission diminishes vascular smooth muscle cell migration and ameliorates intimal hyperplasia. Cardiovasc. Res. 2015, 106, 272–283. [Google Scholar] [CrossRef]
- Clempus, R.E.; Griendling, K.K. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc. Res. 2006, 71, 216–225. [Google Scholar] [CrossRef]
- Netherton, S.J.; Sutton, J.A.; Wilson, L.S.; Carter, R.L.; Maurice, D.H. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ. Res. 2007, 101, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Cullere, X.; Shaw, S.K.; Andersson, L.; Hirahashi, J.; Luscinskas, F.W.; Mayadas, T.N. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 2005, 105, 1950–1955. [Google Scholar] [CrossRef]
- Fukuhara, S.; Sakurai, A.; Sano, H.; Yamagishi, A.; Somekawa, S.; Takakura, N.; Saito, Y.; Kangawa, K.; Mochizuki, N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol. Cell. Biol. 2005, 25, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mei, F.C.; Yang, W.; Wang, H.; Wong, E.; Cai, J.; Toth, E.; Luo, P.; Li, Y.M.; Zhang, W.; et al. Epac1 inhibition ameliorates pathological angiogenesis through coordinated activation of Notch and suppression of VEGF signaling. Sci. Adv. 2020, 6, eaay3566. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Schulze-Hoepfner, F.T.; Hong, J.; Chlenski, A.; Zeitlin, B.D.; Goel, K.; Gomes, S.; Liu, Y.; Abe, M.K.; Nor, J.E.; et al. A novel interplay between Epac/Rap1 and mitogen-activated protein kinase kinase 5/extracellular signal-regulated kinase 5 (MEK5/ERK5) regulates thrombospondin to control angiogenesis. Blood 2009, 114, 4592–4600. [Google Scholar] [CrossRef]
- Namkoong, S.; Kim, C.K.; Cho, Y.L.; Kim, J.H.; Lee, H.; Ha, K.S.; Choe, J.; Kim, P.H.; Won, M.H.; Kwon, Y.G.; et al. Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell. Signal. 2009, 21, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Roberts, O.L.; Dart, C. cAMP signalling in the vasculature: The role of Epac (exchange protein directly activated by cAMP). Biochem. Soc. Trans 2014, 42, 89–97. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).