Abstract
Acupuncture is clinically used to treat various diseases and exerts positive local and systemic effects in several nervous system diseases. Advanced molecular and clinical studies have continually attempted to decipher the mechanisms underlying these effects of acupuncture. While a growing understanding of the pathophysiology underlying several nervous system diseases shows it to be related to inflammation and impair cell regeneration after ischemic events, the relationship between the therapeutic mechanism of acupuncture and the p38 MAPK signal pathway has yet to be elucidated. This review discusses the latest advancements in the identification of the effect of acupuncture on the p38 signaling pathway in several nervous system diseases. We electronically searched databases including PubMed, Embase, and the Cochrane Library from their inception to April 2020, using the following keywords alone or in various combinations: “acupuncture”, “p38 MAPK pathway”, “signaling”, “stress response”, “inflammation”, “immune”, “pain”, “analgesic”, “cerebral ischemic injury”, “epilepsy”, “Alzheimer’s disease”, “Parkinson’s disease”, “dementia”, “degenerative”, and “homeostasis”. Manual acupuncture and electroacupuncture confer positive therapeutic effects by regulating proinflammatory cytokines, ion channels, scaffold proteins, and transcription factors including TRPV1/4, Nav, BDNF, and NADMR1; consequently, p38 regulates various phenomena including cell communication, remodeling, regeneration, and gene expression. In this review article, we found the most common acupoints for the relief of nervous system disorders including GV20, GV14, ST36, ST37, and LI4. Acupuncture exhibits dual regulatory functions of activating or inhibiting different p38 MAPK pathways, contributing to an overall improvement of clinical symptoms and function in several nervous system diseases.
1. Introduction
Acupuncture is a form of therapy that has been practiced for more than 3000 years in Asia [1,2]. Medical doctors practice acupuncture under the guidance of meridian theory, which was first recorded in detail in The Yellow Emperor’s Classic of Internal Medicine [2]. To perform acupuncture, doctors use thin and sterile metal needles to penetrate specific stimulation points termed acupoints, and they manipulate the needle to achieve “de qi” status [2]. Both manual and electroacupuncture (EA) are used in medical practice. Acupuncture is generally a safe, easy to perform, [3,4,5] and economical procedure that provides another choice for those who are concerned about the adverse effects of routine managements such as drug prescription [6,7]. Occasional minor problems, such as needles left in patients by mistake, headaches, and drowsiness have been reported after acupuncture treatment; usually, these symptoms are self-resolved after a short rest [1,3,6,7]. Due to the limited number of controlled clinical trials that have been published, the efficacy of acupuncture as treatment for has been questioned [8].
Acupuncture is widely used to treat various diseases and exerts positive effects, including analgesia, at both local and systemic levels [3,8,9,10,11,12]; it improves consciousness and cognition [13,14,15,16,17,18,19,20], and it induces therapeutic effects in several nervous system diseases [8,13,14,15,16,17,18,19,20]. Advanced molecular and clinical studies have continually attempted to decipher the mechanisms underlying these effects of acupuncture [2,7,16,17,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72]. The signal transduction pathways through which acupuncture treats nervous system diseases involves multiple signal pathways, including p38 mitogen-activated protein kinases (p38 MAPKs) [7,33,34,35,36,37,38,39], Raf/MAPK/extracellular signal-regulated kinases (ERK) 1/2 [27,28,29,30,31,32,39], Toll-like receptor 4 (TLR4)/ERK [40,41,42,43,59], phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (Akt) [26,31,38,49], adenyl cyclase (AC)/cyclic-adenosine mono-phosphate (cAMP)/protein kinase A (PKA) [25,31,44,47,50,51,52], apoptosis signal-regulating kinase 1 (ASK1)-c-Jun amino-terminal kinases (JNK)/p38 [7,33,34,35,36,37,38,39,44,47,48,63], and downstream cAMP response element-binding protein (CREB), JNK [7,24,54,55,56,57,58,59,60,61,63], mammalian target of rapamycin (mTOR) [26,44,45,46], nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [41,42,43,44,47,62], and B-cell lymphoma 2 (Bcl-2)/ Bcl-2 associated X (Bax) balance [57,63,64,65,66,67,68,69]. While a growing understanding of the pathophysiology underlying several nervous system diseases shows that it is related to inflammation and the impairment of cell regeneration, the relationship between the therapeutic mechanism of acupuncture and the p38 MAPK signal pathway has yet to be elucidated. A reverse of the detrimental effect of cerebral ischemic or hemorrhagic injury involves the modulation of the ERK/JNK/p38 signal pathway, which leads to anti-apoptosis of the affected brain area. Improvements of Alzheimer’s disease, vascular dementia, and Parkinson’s disease involves depression or inactivation of the p38 MAPK pathway [32,69,70,71,72,73,74,75,76,77,78,79]. The inactivation of interleukin (IL) 1 β (IL-1β)/p38 in the frontal lobe and hippocampus has a positive effect on improving cognition and memory. Moreover, acupuncture exerts analgesic effects through the interference of both the ascending and descending pain signaling pathway. These findings continue to shed light on the pivot role of the p38 signaling pathway in several nervous system diseases. The common signal transduction pathways through which acupuncture treats nervous system diseases are summarized in Figure 1.
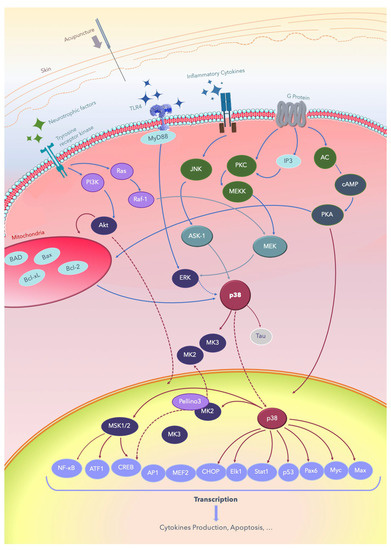
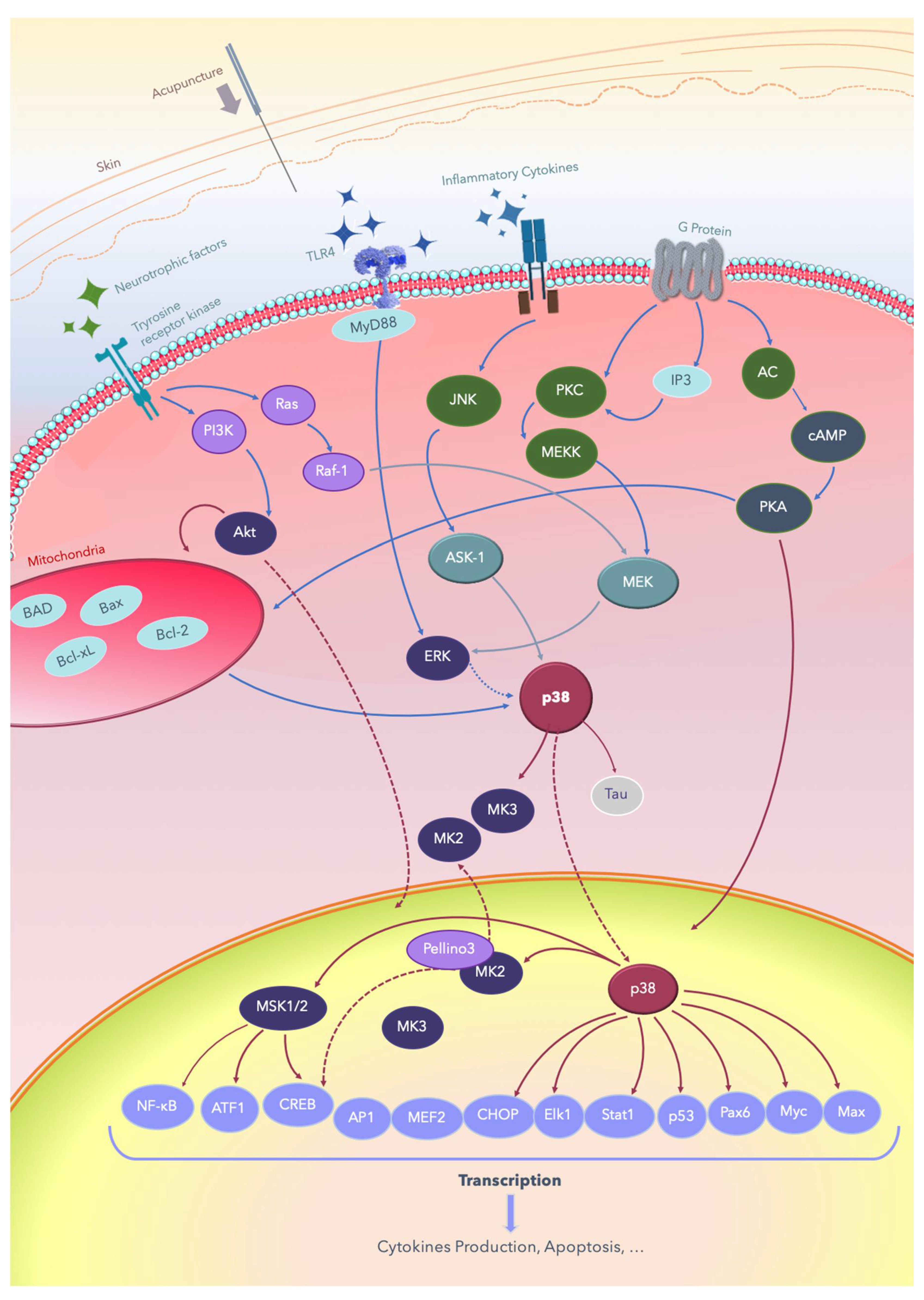
Figure 1.
Summary of the signal transduction pathways through which acupuncture treats nervous system diseases. Acupuncture is applied on acupoints and results in de qi, evoking the excitation of cell membrane receptors, such as the Tyrosine receptor kinase and TLR/ligand, and subsequently producing signal transduction. AC: adenyl cyclase; Akt: protein kinase B; AMPK: AMP-activated protein kinase; ASK-1: apoptosis signal-regulating kinase 1; Bad: Bcl-2-associated death promoter; Bax: Bcl-2 associated X; Bcl-2: B-cell lymphoma 2; Bcl2-xl: B-cell lymphomaextralarge; cAMP: cyclic adenosine monophosphate; CREB: cAMP response element-binding protein; ERK: extracellular signal-regulated kinase; IP3: inositol triphosphate; JNK: c-Jun N-terminal kinases; Elk-1: erythroblast transformation specific (ETS) like-1 protein; Max: a transcription factor coded by the myc-associated factor X; MEF: myocyte-enhancing factor; MEK: MEK kinase; MEKK: MK kinase kinase; MSK: mitogen- and stress-activated protein kinase; Myc: a group of transcription factors coded by regulator genes and a proto-oncogene called Myc; MyD88: myeloid differentiation primary response 88; TLRs: Toll-like receptors; NF-κB: nuclear factor kappa B; Pax6: a paired-box protein encoded by the master gene Pax-6; PI3K: phosphatidylinositol-4,5-bisphosphate 3-kinase; PKA: protein kinase A; PKC: protein kinase C; ATF: activating transcription factor; AP-1: activator protein; CHOP: C/EBP homologous; Stat1: signal transducer and activator of transcription 1.
2. The p38 MAPKs
MAPKs are a large group of evolutionarily conserved proteins in the plant and animal kingdoms. MAPKs have been implicated in diverse cellular processes including cell survival, proliferation, differentiation, and migration.
Three major subfamilies of MAPK proteins have been defined: ERK, JNK, and the p38 MAPKs (Figure 2). The middle amino acid residue of the conserved Thr-Xxx-Tyr dual-phosphorylation domain (dP-consensus) designates a MAPK protein to its cognate subfamily, and the p38 MAPK subfamily bears the Thr-Gly-Tyr (TGY) dual-phosphorylation domain [77,78].
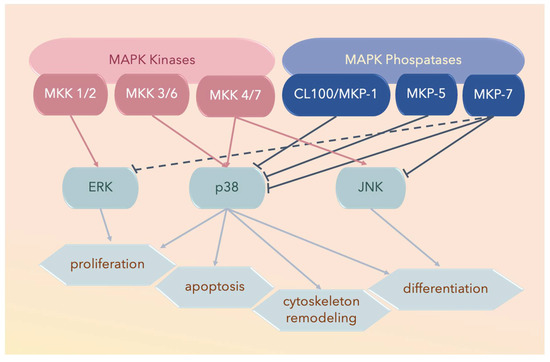
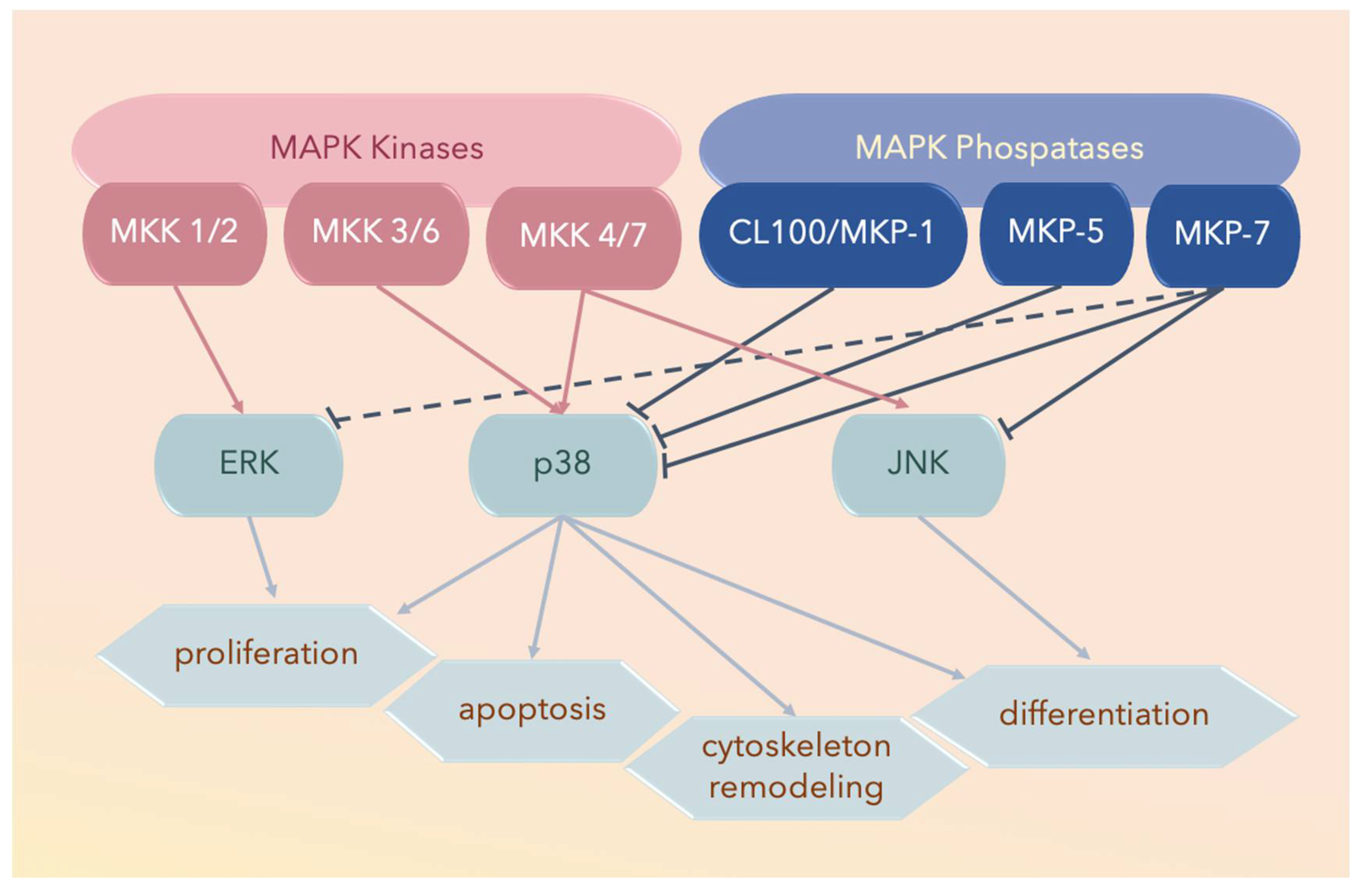
Figure 2.
Three major subfamilies of mitogen-activated protein kinases (MAPKs) include extracellular signal-regulated kinases (ERKs), the c-Jun amino-terminal kinases (JNKs), and the p38 MAPKs. The solid lines ending with arrowheads denote activated proteins, solid lines with blunt ends denote deactivated proteins, and the dotted lines with blunt ends denote partially deactivated proteins.
The most extensively studied MAPK pathways are activated by dual-specificity serine-threonine/tyrosine kinases (STK) termed MAPK kinases (MKKs, MAPKKs, or MAP2Ks), which are activated by MAPKK kinases (MAPKKKs or MAP3Ks). For the p38 MAPK pathway, MKK3 and MKK6 serve as MKKs, and the identified MAPKKKs include MAPKKK2, MAPKKK3, ASK-1, tumor progression locus-2 (Tpl2), and transforming growth factor-β-activated kinase 1 (TAK-1) of the non-canonical transforming growth factor-β (TGFβ) pathway. In general, ERK proteins are primarily activated by growth factors; JNKs are activated by stress-, differentiation-, and growth-related factors; and p38 is activated by stress-related factors [78,79,80,81,82,83,84].
The p38 MAPK family comprises p38α, p38β, p38γ, and p38δ (summarized in Table 1). Considering its central role in developmental programming, cellular adaptation to environmental stress, immune responses, inflammation, tissue regeneration, and tumorigenesis, this protein subfamily has gained increasing attention since its initial discovery. Recent studies using new genetic and pharmacological tools have provided essential information regarding the functions of p38 MAPKs in the pathogenesis of prevalent conditions associated with inflammation, diabetes, neurodegeneration, and cancer [78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99].

Table 1.
Members of the p38 mitogen-activated protein kinase (MAPK) subfamily.
The subfamily p38α is reportedly a homolog of Saccharomyces cerevisiae Hog1, which is an important regulator of osmotic response [77,78,84,100,101,102,103,104,105]. Other p38 MAPK family members, sharing approximately 60% sequence similarity with p38α, were subsequently cloned and named p38β, p38γ, and p38δ [77,78,84,100,101,102,103,104,105].
X-ray crystallographic studies have yielded structural insights into the mechanisms underlying the interaction between an MAPKK and an MAPK [103,107]. Both p38α and p38β are well-conserved at both the gene and protein levels and are important in eliciting innate immunity [77,78,84,101,102,103,104,105]. Some p38α and p38β physiological substrates are summarized in Figure 3; these include transcription factors, transcription factor kinases, cytoskeletal proteins, translational machinery components, and other proteins including metabolic enzymes, glycogen synthase, or cytosolic phospholipase A2 (cPLA2). The p38γ and p38δ MAPK isoforms can phosphorylate typical p38 MAPK substrates including transcription factors activating transcription factor 2 (ATF2), erythroblast transformation specific (ETS) like-1 protein (Elk-1), or stress-activated protein kinase (SAP1). However, they cannot phosphorylate MK2 or MK3, which are suitable substrates for the other two p38 MAPK isoforms [109,110,111].
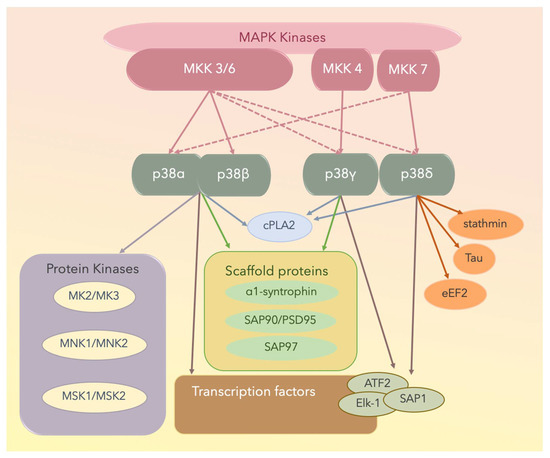
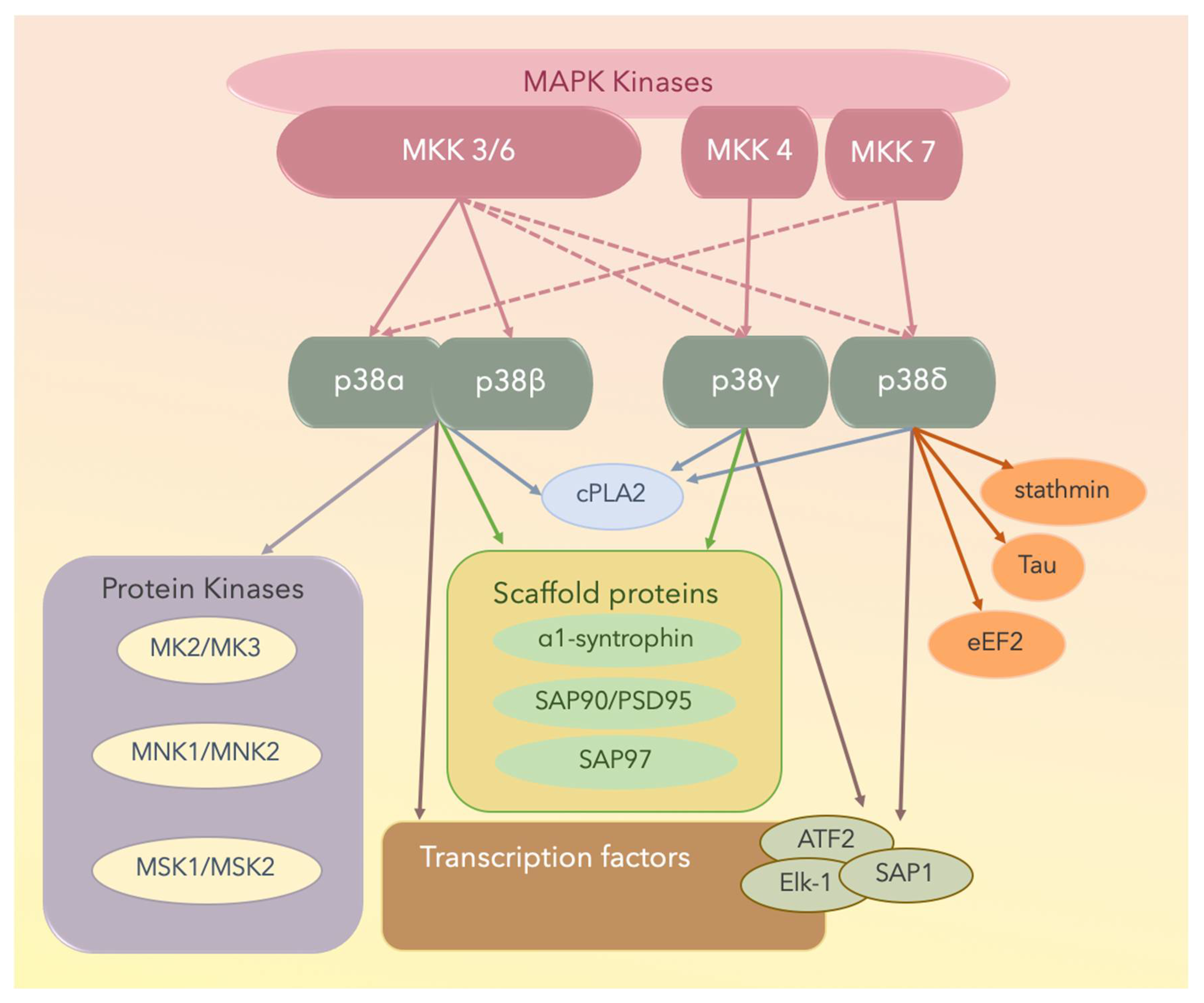
Figure 3.
The four p38 MAPK isoforms are p38α, p38β, p38γ, and p38δ. Solid lines denote activated proteins, and dotted lines denote partially activated proteins. MNK: mitogen-activated protein kinase-interacting protein; MSK: mitogen- and stress-activated protein kinase; cPLA2: cytosolic phospholipase A2; ATF2: the activating transcription factor 2; Elk-1: the [erythroblast transformation specific (ETS)] like-1 protein; SAP1: stress-activated protein 1; PSD: postsynaptic density proteins; eEF2: eukaryotic elongation factor 2.
Of the MAPKs, p38γ has a unique short C-terminal sequence, KETXL, which is ideal for binding PDZ domains in proteins, thus accounting for the regulatory role of p38γ in the localization of cellular elements and interactions with cytoskeletal components. Under stress conditions, p38γ interacts with and activates various scaffold proteins including α1-syntrophin, SAP90/PSD95, and SAP97, which are generally targeted to the plasma membrane cytoskeleton at specialized sites, including the neuromuscular junction and gap junctions through protein–protein interactions [111].
With respect to p38δ, it potentially contributes to cytoskeletal regulation because it phosphorylates the cytoplasmic protein stathmin [110,111], which is a crucial regulator of microtubule dynamics and the cell cycle, by promoting the depolymerization of microtubules and/or preventing the polymerization of tubulin heterodimers [109,111]. Furthermore, eukaryotic elongation factor 2 (eEF2) kinase and microtubule-associated protein tau are substrates of p38δ [109,111]. The four p38 isoforms and the substrates are shown in Figure 3.
These four p38 MAPKs are encoded by different genes and display different histotypic expression patterns, with p38α being ubiquitously expressed at significant levels in most cell types, whereas the others potentially display more histotypic expression patterns. For example, p38β is expressed in the brain, p38γ is expressed in skeletal muscle (neuromuscular junction, gap junction), and p38δ is expressed in endocrine glands [108,109,110,111,112,113,114,115]. The genetic ablation of specific p38 MAPK family members has revealed functional redundancy in this protein subfamily. For example, the osmotic shock-induced phosphorylation of the stress-activated protein 97 (alternatively termed synapse-associated protein 97, SAP97; also known as discs large homolog 1 scaffold protein, hDLG1) is usually mediated by p38γ; however, in the absence of this kinase, other p38 MAPKs can perform this function.
3. MAPK Substrates, Signaling Pathways, and Functions
Nine members of the dual-specificity phosphatases specific for MAPKs, termed MKPs, have been reported. Each member has specific substrates, tissue distribution, and subcellular localization. For example, MAPK phosphatase 7 (MKP-7) binds to and inactivates p38α and p38β through dephosphorylation; however, it does not interact with p38γ and p38δ; MKP-5 and CL100/MKP-1 also bind to p38α and p38β, but not to p38γ or p38δ [115] (Figure 2 and Figure 3).
Several studies have revealed a direct interaction of the N-terminal region of the MAPKK with a docking groove present on the surface of the MAPK distant from the catalytic active site. The second determinant of MAPKK specificity is the structure of the MAPK activation loop harboring the Thr-Xxx-Tyr dual-phosphorylation motif. The specificity of these interactions partly mediates the potential of an individual MAPKK to selectively activate a particular MAPK.
A recent study emphasized that dynamic changes are necessary for enzyme activity [116]. For instance, two MAPKs, ERK2 and p38, are differentially activated owing to differences in dynamism. A comparison of the dynamics of PKA and p38 revealed similarities in their dynamic properties.
3.1. Dual Phosphorylation by MKKs
In yeast, only a single MAPKK appears to activate each MAPK, whereas mammalian MAPK signaling modules include more than one MAPKK. The MAPKKs responsible for activating the p38 MAPK pathways appear to be specific to cell type and stimulus [105,107,114,116]. Brancho and Tanaka et al. investigated the mechanism underlying p38 activation in vivo by examining the effect of disruption of the murine Mkk3, Mkk4, and Mkk6 genes on the p38 MAPK signaling pathway [100]; they found that Mkk3 and Mkk6 are essential for tumor necrosis factor (TNF)-mediated p38 activation. By contrast, ultraviolet (UV) radiation-induced p38 activation was mediated by Mkk3, Mkk4, and Mkk6. Furthermore, they reported that the role of Mkk4 in p38 MAPK activation in fibroblasts was largely redundant with those of Mkk3 and Mkk6. Mkk4 is a potentially important p38 MAPK activator in cells with low levels of Mkk3 and Mkk6. These data indicated that p38 MAPK was regulated by the coordinated and selective action of the three different protein kinases MKK3, MKK4, and MKK6 in response to cytokines and exposure to environmental stress. The inactivation of p38 MAPK was reportedly associated with defects in cell cycle arrest and increased tumorigenesis [117,118,119,120,121,122,123].
Several MAPKKKs have been implicated in the regulation of p38 MAPK signaling, including the mixed-lineage kinases (MLKs), apoptosis signal-regulating kinase 1 (ASK-1), TAK-1, and some members of the MAPK/ERK kinase kinase (MEKK) family. Low-molecular-weight guanosine-5′-triphosphate (GTP)-binding proteins of the Rho subfamily, including Ras-related C3 botulinum toxin substrate 1 (Rac1), the erythrocyte membrane glycoprotein Cd242, the nonlipid modified Ras-related protein (Rit), the transcription termination factor Rho, and heterotrimeric G-protein-coupled receptors (GPCRs) contribute to p38 activation upstream of MAPKKKs [124,125,126,127,128,129,130,131,132,133,134,135]. More recently, a new signaling pathway, different from TAK1, that involves the inhibitor of nuclear factor kappa-B kinase (IκBK), NFκB/p105, and Tpl2 stimulating MKK3/6, and downstream p38 was established in macrophages [135,136,137,138,139]. The p38 MAPK pathways are shown in Figure 4.
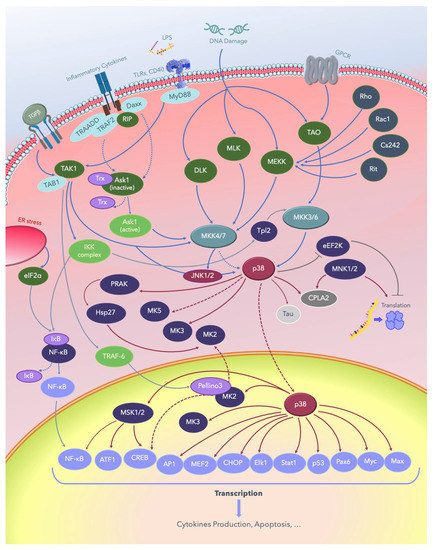
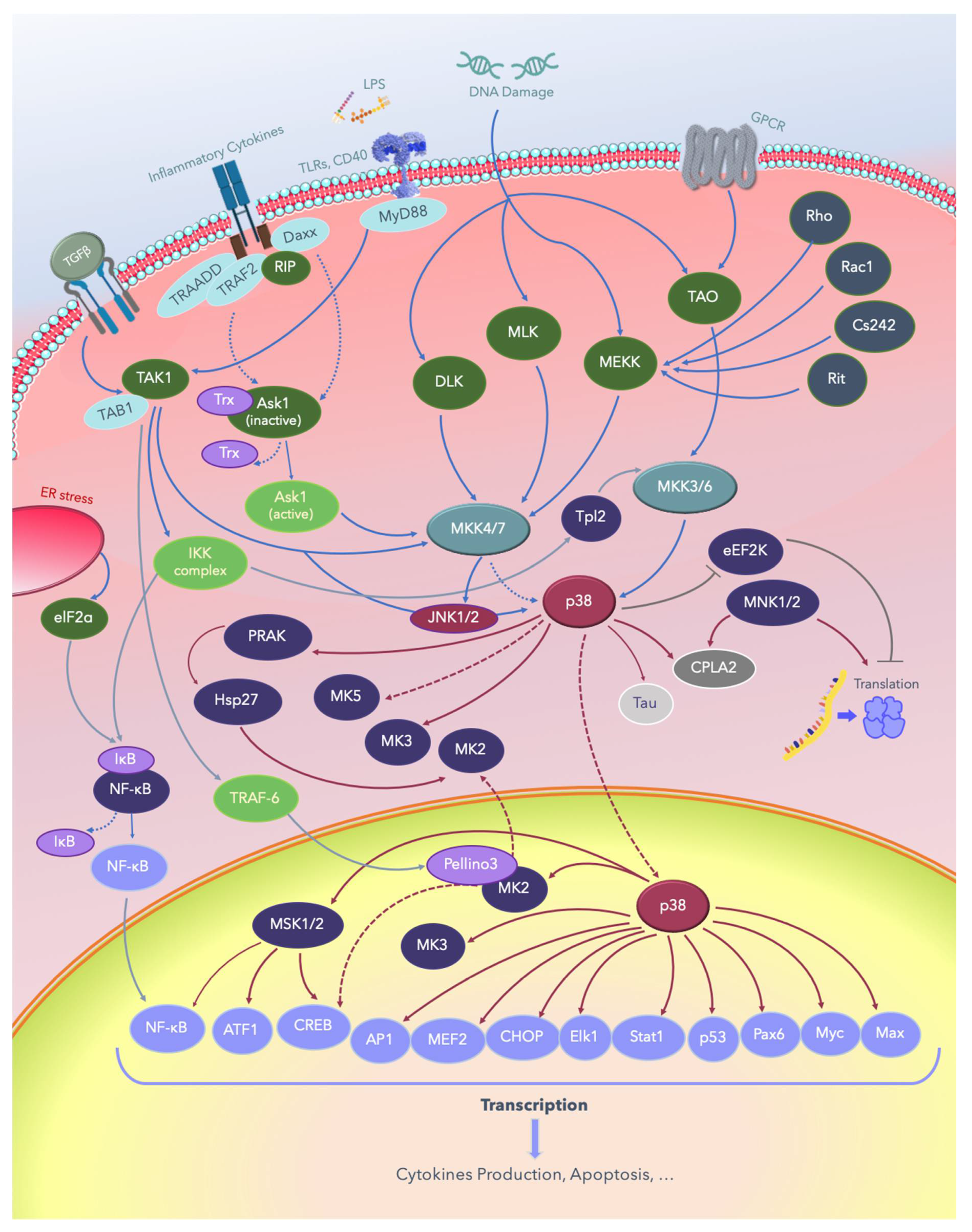
Figure 4.
The p38 MAPK signaling pathways. Solid lines indicate signaling pathways and the proteins involved in them; dotted lines indicate the regulatory mechanisms reported in several studies, and lines with blunt ends indicate pathways inhibiting or deactivating downstream substrates. LPS: lipopolysaccharide; TGFβ: growth factor beta; TLRs: Toll-like receptors; CD40: cluster of differentiation 40 receptors; GPCRs: G-protein-coupled receptors; elF2a: eukaryotic translation initiation factor 2A; ER: endoplasmic reticulum; NF-κB: nuclear factor kappa B; TAB1: TGF-beta-activated kinase1; TRADD: tumor necrosis factor receptor type 1-associated DEATH domain protein; TRAF: tumor necrosis factor receptor (TNFR)-associated factor; Daxx: death domain-associated protein; RIP: receptor-interacting protein kinases; MyD88: myeloid differentiation primary response 88; PRAK: p38-regulated and -activated kinase; Hsp: heat shock proteins; DLK: dual leucine zipper kinase; MLK: mixed-lineage protein kinase; MEKK: MEKK kinase; MEK: MEK kinase; MKK: MK kinase; MNK: mitogen-activated protein kinase-interacting protein; TAO: thousand and one amino acids; eEF2K: eukaryotic elongation factor 2 kinase; cPLA2, cytosolic phospholipase A2; ATF: activating transcription factor; MSK: mitogen- and stress-activated protein kinase; CREB: cAMP response element–binding protein; AP-1: activator protein 1; MEF: myocyte enhancing factor; CHOP: C/EBP homologous protein, a member of the CCAAT/enhancer-binding proteins; Elk-1: erythroblast transformation specific (ETS) like-1 protein; Stat1: signal transducer and activator of transcription 1; Pax6: a paired-box protein encoded by the master gene Pax-6; Myc: a group of transcription factors coded by a regulator genes and proto-oncogene called Myc; Max: a transcription factor coded by the myc-associated factor X.
3.2. Autophosphorylation
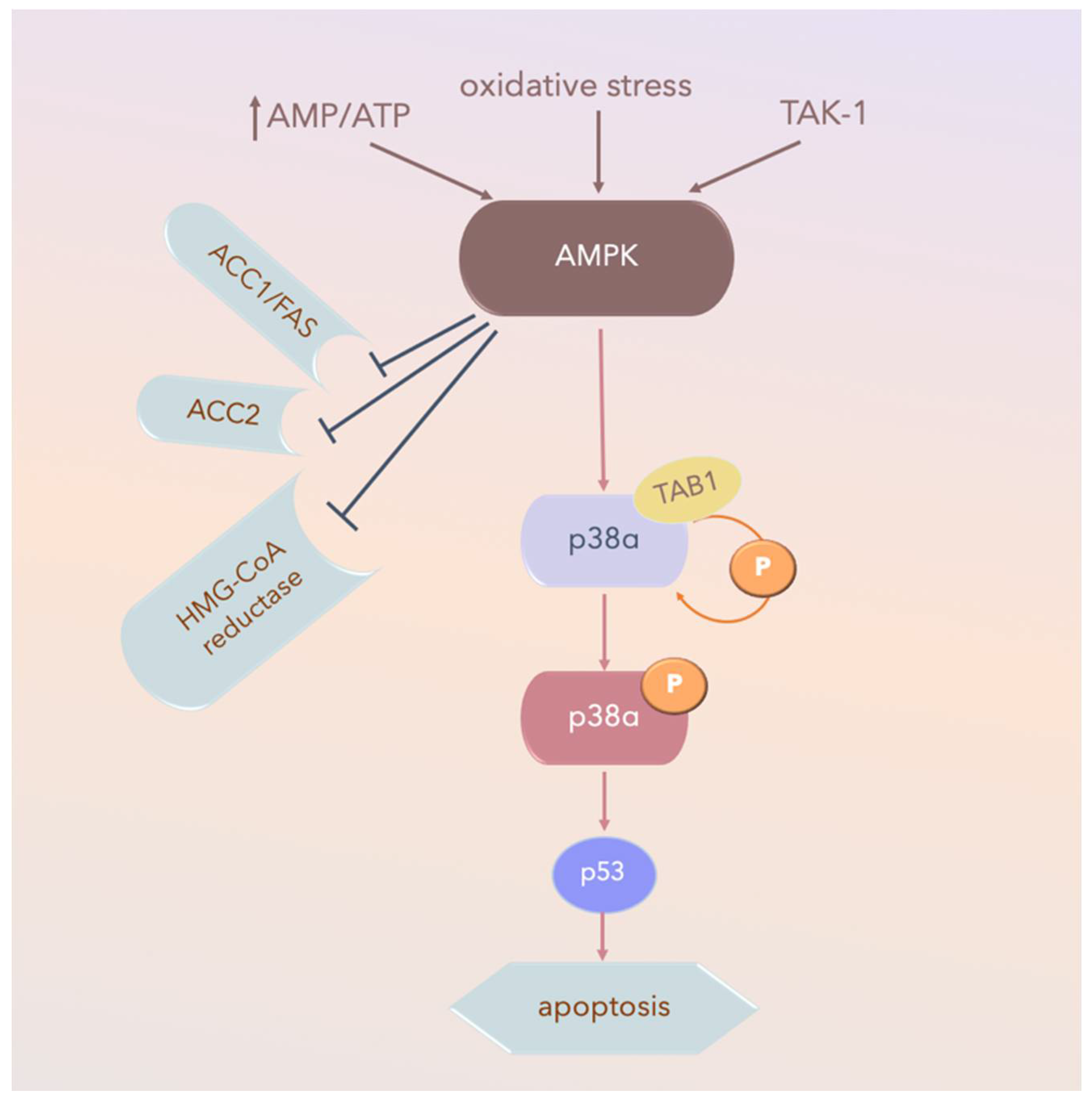
MKK-independent activation is achieved through autophosphorylation and activation of p38α after interaction with TGFβ-activated kinase 1 (TAB1), which appears to activate p38α via the 5’ AMP-activated protein kinase (AMPK) in ischemic heart tissue (Figure 5). TAB1 sequesters p38α in the cytosol, thus potentially preventing some MKK-activated p38α functions. However, this mechanism does not contribute to p38 MAPK activation in fibroblasts or epithelial cells under the same conditions [134].
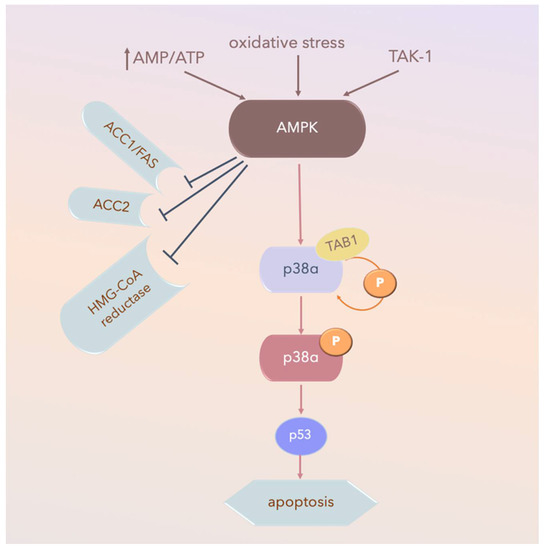
Figure 5.
AMPK-activated-p38α pathways in ischemic heart tissue. Arrow: activated; TAK-1: transforming growth factor-β-activated kinase 1; AMPK: 5’ AMP-activated protein kinase; P: phospho-; AMP: adenosine mono-phosphate; ATP: adenosine tri-phosphate; AMP/ATP: AMP/ATP ratio; ACC1: Acetyl-CoA carboxylase 1; Fas signal pathway, Fas and Fas Ligand (FasL) regulate cell death; HMG-CoA reductase: a rate-controlling enzyme of the mevalonate pathway responsible for cholesterol and other isoprenoid biosynthesis.
Another MKK-independent mechanism underlying p38α activation has been observed in T-cell stimulation, wherein p38α is activated by T-cell antigen receptor (TCR)-mediated stimulation through p38α phosphorylation on a noncanonical activating residue, Tyr323. This activated p38α alters its structural conformation, phosphorylating third-party substrates and its TGY motif [115].
4. TRPV1 and the p38 Pathway
Transient receptor potential vanilloid receptors (TRPVs) are mechanosensitive channels highly associated with nervous system functions including pain, memory, and mechanical sensations. Furthermore, capsaicin receptor TRPV1 is a key regulator of pain and inflammation and is upregulated in microglia in the brain, especially in the anterior cingulate cortex [138,139,140]. The stimulation of microglial TRPV1 induces cortical microglial activation and indirectly enhances glutamatergic neuronal transmission by promoting the shedding of extracellular microglial microvesicles (release of vesicles in the extracellular space [141]). Moreover, in the cortex of mice with neuropathic pain, TRPV1 affects the intrinsic electrical properties of neurons and synaptic strength [142,143]. This signal transduction can be inhibited by the p38 MAPK inhibitor SB203580. Thus, p38 MAPK is a downstream TRPV1 activator whose phosphorylation plays an essential role in microglial microvesicle shedding by activating P2X purinoceptor 7 (P2X7) ATP receptor [138,139,140,141,142,143]. Whereas TRPV1 mediates communication between microglia and neurons, inhibition of the phosphorylation of its downstream p38 MAPK inhibits sphingosine metabolism [140,141,142,143].
Furthermore, several studies have examined biomarkers of nerve damage, including astrocytic marker glial fibrillary acidic protein (GFAP), microglial marker ionized calcium-binding adapter molecule 1 (Iba-1), S100 calcium-binding protein B (S100B), and the receptor for advanced glycation end-products (RAGE), revealing marked upregulation of these molecules in the dorsal root ganglion (DRG) and spinal cord dorsal horn (SCDH) of Complete Freund’s adjuvant (CFA)-treated mice. This inflammatory effect was reversed through electroacupuncture, which achieved an equivalent result to that of TRV1 gene deletion [143].
5. Brain-Derived Neurotrophic Factor and p38 Pathways
Microglia cells are resident macrophages in the central nervous system (CNS) with a small soma with thin and branched processes. In the case of neural injury, their processes rapidly migrate toward the site of injury. In peripheral nerve injury, spinal cord microglia are activated. Spinal P2X4 receptors (P2X4Rs), phosphorylated p38 MAPK (p-p38-MAPK), and brain-derived neurotrophic factor (BDNF) are upregulated in spared nerve injury (SNI) rats [144]. BDNF signals to neurons in spinal lamina I and increases intracellular chloride concentration, thereby counteracting gamma-aminobutyric acid (GABA)- and glycine-mediated inhibition in these cells. The disinhibition unmasks innocuous inputs to lamina I neurons and facilitates their responses to noxious inputs [145]. In other studies, BDNF/TrkB was reported to promote inflammation in spinal cord injury through the p38 signaling pathway in the murine model [40,146]. Moreover, an upregulation of BDNF expression was observed in rats when applying electroacupuncture (EA) to acupoints GV20 and GV14, which later revealed that EA counteract caspase-3-dependent neuronal apoptosis by activating the Raf-1/MEK1/2/ERK1/2/p90RSK/Bad signaling pathway [147].
These results suggest that increasing the expression of BDNF synthesis, the downregulation of P2X4Rs, and the inhibition of p38 phosphorylation might account for the therapeutic effects induced by acupuncture in nerves injury.
6. Acupuncture and the Effects of Electric Fields on Nerve Regeneration
Previous in vitro and a few in vivo studies have reported positive nerve growth-promoting effects of electric fields where the cathode was placed toward the distal end of the injured nerve stumps [148,149,150,151,152,153,154]. Chen et al. reported that regenerated nerves in the electrical stimulation group may have a more mature ultrastructure compared with those in the control group [155]. Furthermore, they reported low regeneration success in patients receiving electrical stimulation relative to controls [155].
Together with several previous clinical studies [155,156,157,158], these studies show that patients with nerve anastomosis should not receive any electrical stimulation for rehabilitation until their reconnected nerve stumps have grown into a more mature stage of regeneration. One of the mechanisms underlying electroacupuncture (EA) is the relief of acute pain through the release of opiates to activate μ-, δ-, and κ-opioid receptors; furthermore, it can regulate persistent pain by activating μ- and δ-opioid receptors [25]. These findings explain the application of EA in treating neuropathic pain when pharmacotherapeutic approaches are ineffective.
Furthermore, the analgesic effect of low-frequency (2 Hz) EA is exerted on the noradrenergic descending pathway, involving the modulation of spinal GABAergic nerves (GABAA). Administering EA at 2 Hz at acupoint ST36-37 reportedly relieved neuropathic pain through long-term depression (LTD) of the C-fiber [159]. This phenomenon could be blocked by N-methyl-D-aspartate (NMDA) and an opioid receptor antagonist. By contrast, high-frequency (100 Hz) EA induces long-term potentiation (LTP) of endogenous GABAergic (GABAB) and the serotonergic inhibitory systems through modulation of μ-opioid and 5-hydroxytryptamine 1 (5-HT1) receptors [25,160]. Other studies have reported that delivering EA at acupoints GV14 and GV20 exerts neuroprotective effects by activating CREB, BDNF [161], and α7 nicotinic acetylcholine receptor (α7 nAChR) [162] while simultaneously reducing S100B-mediated neurotoxicity [163]. Moreover, EA at acupoint ST36 can evoke excitatory signals in either the peripheral nervous system or CNS in vivo [163].
7. Inflammatory and Neuropathic Pain
7.1. Inflammatory Pain
Protein kinase C (PKC) is rapidly activated by heat or bradykinin and translocates, assisted by scaffolding proteins, from intracellular compartments to the plasma membrane. In rat DRG, the TRPV1 signaling pathways involve the phosphatidylinositol 3-kinases (PI3K), PKC, and calmodulin-dependent protein kinase II (CaMKII) [143,163]. The p38α MAPK was first recognized for its role in inflammation where it regulates the biosynthesis of proinflammatory cytokines IL-1 and TNFα [77,78,98,99,100,101,102,103,104,105,115,164,165,166,167,168,169]. After noxious stimulation, activated p38 (phospho-p38) is increased in the spinal cord and DRG neurons and continues to propagate pain signaling by phosphorylating transcription factors and proinflammatory cytokines including TNF-α [168,169]. Inflammation is induced through the activation of these cytokines, followed by their interactions with their cognate receptors and via the small GTPases (e.g., Rac1 and CDC42), leading to p38 activation [169].
7.2. Neuropathic Pain
In contrast to physiological pain, pathological pain does not depend on the presence of tissue-damaging stimuli. Neuropathic pain can be agonizing, potentially persisting over long periods, and it is often resistant to known painkillers. Increasing evidence indicates that spinal microglia react and undergo a series of changes that directly influence the establishment of painful peripheral neuropathy [168,169,170]. After nerve damage, purinergic P2X4 receptors (nonselective cation channels activated by extracellular ATP) are upregulated in spinal microglia in a manner dependent on transcription factors interferon regulatory factor 8 and 5 (IRF8 and IRF5), both of which are expressed in microglia after peripheral nerve injury [171,172]. Furthermore, in spinal microglia, the response to extracellular stimuli results in signal transduction through intracellular signaling cascades involving p38 and ERK. Inhibition of the function or expression of these microglial molecules suppresses the aberrant excitability of dorsal horn neurons and neuropathic pain [172].
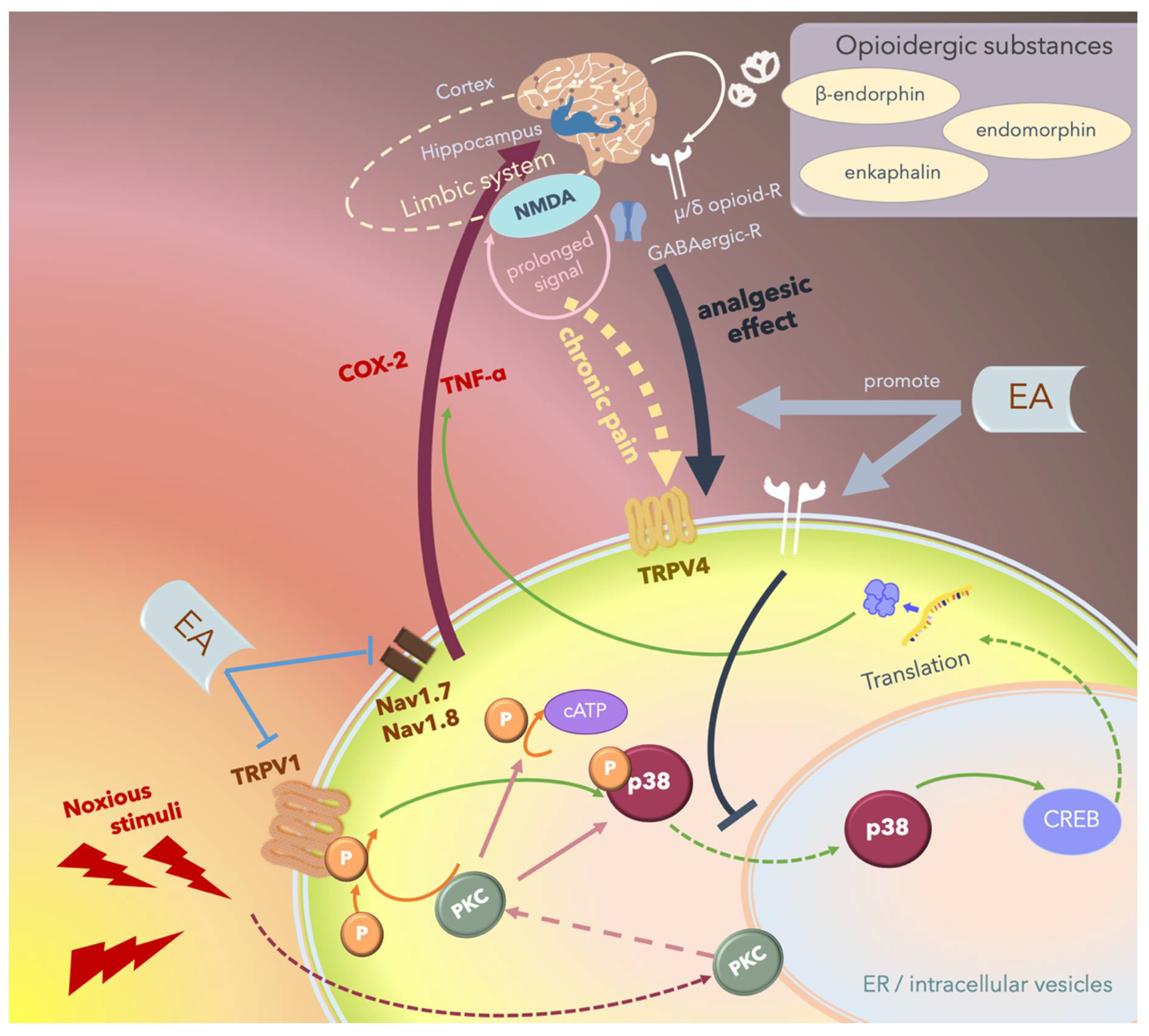
Since MAPKs significantly contribute to the development of hyperalgesia, the inhibition of any of the three pathways, namely the ERK, p38, and JNK pathways, can rescue inflammatory or neuropathic pain. Several studies have reported that 2- and 15-Hz EA can downregulate cerebral TRPV4 expression and attenuate chronic constriction injury (CCI)-induced neuropathic pain in an animal model [173,174,175,176,177,178,179]. Furthermore, Huang et al. reported that EA modulates both excitatory and inhibitory neurotransmitters to relieve neuropathic pain in the higher brain regions [175]. The hippocampus plays an integral role in the transition from acute to chronic pain in the limbic system by activating NMDA receptors and subsequently prolonging acute nociceptive stimuli that continue activating the descending pathways of pain (Figure 6).
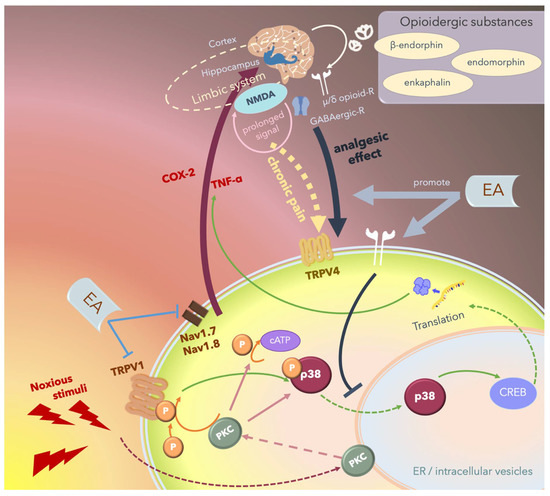
Figure 6.
Schematic of the potential mechanism underlying the electroacupuncture (EA)-mediated analgesic effect. Noxious stimuli including heat or proinflammatory cytokines are transduced by the transient receptor potential vanilloid 1 (TRPV1) into the cells, thus phosphorylating and activating p38, which translocates from the cytosol to the nucleus and promotes transcription by affecting cAMP response element-binding protein (CREB), thus upregulating specific proteins including inflammatory cytokines or inducing apoptosis. These noxious stimuli can also directly activate protein kinase C (PKC). Nociception regulation by TRPV1 and Nav1.7/1.8 receptor stimulation in the peripheral nerves propagates the signal through the ascending pathway and upregulates proinflammatory cytokines including cyclooxygenase-2 (COX-2) and tumor necrosis factor-α (TNFα) in the central nervous system, and the signal is then perceived as “pain”. Chronic pain re-emerges when nociceptive signals cause prolonged stimulation through hippocampal N-methyl-D-aspartate receptor (NMDA) receptors and some other areas of the limbic system, and the signals are transmitted through the descending pathway, stimulating TRPV4 and triggering pain. Endogenic analgesic mechanisms involve the release of opioidergic substances that bind to γ-aminobutyric acidergic (GABAergic) receptors and μ- or δ-opioid receptors that act locally in the central nervous system and inhibit the descending pain pathway. EA exerts therapeutic effects by inhibiting the ascending pain pathway and intracellular p38-mediated inflammatory pathway by stimulating peripheral opioid receptors. Furthermore, EA promotes endogenic analgesic mechanisms, thus exerting immediate local analgesic effects and rescuing CNS-induced chronic pain.
7.3. Post-Operation Pain
Presurgical sham-, low-, and high-frequency EA treatments were reported to significantly reduce the postoperative patient-controlled analgesia morphine requirement; moreover, both low- and high-frequency EA decreased opioid-related side effects including nausea and dizziness throughout the first postoperative day [176,177]. Among these studies, ST36 and LI4 were the most frequent acupoints manipulated to achieve analgesic effects [summarized in Table 2].

Table 2.
The effect of acupuncture on inflammatory and neuropathic pain.
Furthermore, overexpression of the voltage-gated sodium channel (Nav), TRPV1, and acid-sensing ion channel 3 (ASIC3) in DRG neurons in response to proinflammatory cytokines is a key component of inflammatory pain [179,180,181,182,183,184,185,186,187,188,189,190,191,192]. Low-frequency (2-Hz) EA at acupoint ST36 can reduce inflammatory pain by attenuating ASIC3 overexpression in peripheral DRG neurons. ASIC3 downregulation potentially prolongs the clinical benefits of EA. Goldman et al. reported that acupuncture stimulates adenosine A1 receptor and induces adenosine release, thus relieving inflammation and neuropathic pain, which was not observed in mice lacking adenosine A1 receptors [193]. These results suggest that the opioid and adenosine pathways potentially contribute to analgesia in both manual acupuncture and EA.
7.4. Migraine
Migraine is a complex disorder; each episode begins with prodromes and aura (transient focal neurological symptoms). The origin of recurrent headache accompanied by visual or sensory symptoms is speculated to involve the hypothalamus, brain stem, and cortex. Current theories suggest that migraine is a neurovascular disorder involving cortical spreading depression, neurogenic inflammation, and vasodilation [194,195]. Owing to the disease itself or its genetic underpinnings, the brains of individuals who experience migraine are altered structurally and functionally; these molecular, anatomical, and functional abnormalities provide a neuronal substrate for extreme sensitivity to fluctuations in homeostasis, decreased adaptability, and recurrent headaches [194].
A randomized controlled trial investigated the efficacy and tolerability of acupuncture in comparison with topiramate treatment in chronic migraine prophylaxis. The effectiveness of acupuncture was similar to or greater than that of prophylactic pharmacotherapy, with fewer side effects in migraine [194]. The selected acupoints were bilateral BL2, GB20, EX-HN5, and EX-HN3, which are all associated with the trigeminal and cervical dermatomes. Although different combinations of peripheral effects, spinal/supraspinal mechanisms, and cortical and psychological mechanisms potentially contribute to the clinical effects of acupuncture, several other studies have reported that acupuncture potentially exerts curative effects not only through local analgesia but also through anti-inflammation, neuropeptide regulation, cytoskeleton remodeling, cell repair, and improvement in overall homeostasis.
7.5. Transcutaneous Electrical Nerve Stimulation Versus EA
The difference between transcutaneous electrical nerve stimulation (TENS) and EA is that TENS involves the application of the cathode at the affected area rather than at acupoints. Furthermore, TENS is used to reduce mechanical hyperalgesia in knee joint inflammatory pain [196,197], although its effectiveness is only sustained for a short time (several days) [198,199]. The analgesic effect of low-frequency TENS and EA can be attenuated using μ-opioid receptor blockers, thus indicating the involvement of peripheral opiate release in the underlying mechanism [196]; furthermore, the blockade of β-endorphin and corticotropin-releasing factor also reduces EA-induced analgesia.
7.6. Hypothesis Regarding the Long-Term Effect of Acupuncture
Although proinflammatory cytokines are regulated at different levels, the role of the p38α MAPK pathway in posttranscriptional regulation has received increasing attention. Numerous p38 MAPK–regulated mRNAs often contain an adenylate-uridylate-rich element (termed AU-rich element or ARE) in the 3′ untranslated region (3′UTR) [200,201]. The regulation of mRNA stability is particularly important for the expression of proteins involved in inflammatory response. These elements target mRNAs for rapid decay. The decay of ARE-containing reporter mRNAs is blocked upon p38 MAPK activation. Numerous ARE-containing endogenous mRNAs of inflammatory proteins are destabilized upon p38 inhibition. Some studies have reported a common mechanism underlying gene regulation via the p38α MAPK pathway for posttranscriptional regulation by the ARE.
These results are consistent with those of several previous animal and clinical studies reporting that EA improves analgesia during the acute phase posttraumatic healing and pain after major surgery [176,177]. Numerous prospective randomized controlled trials on carpal tunnel syndrome (CTS) have reported that acupuncture at acupoints PC7 and PC6 is as effective and safe as steroid treatment of mild-to-moderate CTS, both in the acute phase and throughout the 1-year follow-up period [187,188]. Based on the analgesic mechanisms of acupuncture and EA, which attenuate the p–p38 signaling pathway, the long-term effect of acupuncture or EA may result from neural modulation through posttranscriptional regulation. The potential mechanism underlying EA-mediated analgesic effects is depicted in Figure 6. The underlying mechanisms and the primary results of these studies are summarized in Table 2.
8. Cerebral Ischemia
During a cerebral ischemia (CI) event, hypoxia is induced in the brain, followed by the upregulation of various cytokines and potentially neurotoxic molecules. Nitric oxide reportedly influences cerebral oxygen vasoreactivity during severe hypoxia [202]. Both manual acupuncture and EA can increase cerebral blood flow [13,14]. Furthermore, 2- and 15-Hz EA at acupoint ST36 increased cerebral blood fluid (CBF) in rats with and without CI [14]. Per the traditional Chinese medicine theory, the Governor Vessel (or the Governor Meridian) directly communicates with the brain. In a previous study, EA at acupoints GV20 and GV14 before artificial mild cerebral ischemia–reperfusion (I/R) injury in an animal model exerted a neuroprotective effect against reperfusion injury [165]. Another neuroprotective mechanism of EA involves the downregulation of astrocytic S100B, which in turn deactivates p38 MAPK and its downstream substrate NF-κB. These effects subsequently reduce oxidative/nitrative stress and inhibit the TNF-α/tumor necrosis factor receptor type 1-associated DEATH domain protein (TRADD)/ Fas-associated protein with death domain (FADD)/cleaved caspase-8/cleaved caspase-3 apoptotic pathway in the ischemic cortical penumbra after reperfusion [89,201,202,203,204,205,206,207]. Both 5- and 25-Hz EA at acupoints GV20 and GV16 effectively downregulated reactive astrocytosis to exert neuroprotective effects against cerebral infarction-induced neuronal apoptosis, probably by activating the p38 MAPK/CREB signaling pathway [203].
Heat shock proteins (Hsp) significantly contribute to cellular regenerative processes in injured tissues because Hsp is upregulated during stem cell differentiation; however, the depletion or inhibition of Hsp70/Hsc70 impairs this process [206]. Hsp27 and Hsp70 reportedly contribute to intracellular protein repair after acute CI [206,207,208,209,210,211]. The dual phosphorylation of p38 MAPK was increased through early ischemia in an in vitro study wherein Hsp27 served as a terminal substrate of the p38 MAPK cascade. A later study indicated that among the p38 MAPKs, p38γ is the principal isoform responsible for the phosphorylation of HSF1 at Ser326 (S326) in cells (phosphorylation at S326 is a hallmark for HSF1 activation) [212], thus contributing to mitotic progression. A protease–mass spectrometry approach unexpectedly revealed that p38 MAPK also catalyzes the phosphorylation of HSF1 at S303/307, which is a repressive posttranslational modification [212,213]. Hsp70 bound MK2 to regulate MK2–p38MAPK interaction in the stem cells, and the essential regions required for Hsp70–MK2 interaction have been identified [206,209,210,211,212,213]. Taken together, Hsp can regulate cell differentiation by interacting with MK2 to stabilize p38 MAPK.
Another study administered 2 Hz EA the GV20 and ST36 acupoints in rat models of cerebral I/R injury and observed lowered peak levels of adrenocorticotrophic hormone and HSP70, suggesting that EA may inhibit excessive stress, reduce inflammation, and promote neural repair, thus facilitating healing in ischemic stroke [211]. The mechanisms and main results of the identified articles are summarized in Table 3.

Table 3.
The p38 signaling pathway in cerebral ischemic stroke.
9. Epilepsy and Seizure
Epilepsy is characterized by an abnormal electric discharge of the brain, especially in the hippocampus. Epilepsy is usually associated with neuronal loss, astrocyte proliferation, mossy fiber sprouting, and synaptic reorganization in the hippocampus [40,214,215,216,217,218,219]. Pharmacological treatment approaches, including those employing Western medicine and Chinese herbal medicine, have been attempted to treat or prevent the harmful effect of seizures. For example, Uncaria rhynchophylla is a herb used in traditional Chinese medicine for epilepsy management; its therapeutic effect was reportedly associated with attenuated mossy fiber sprouting, astrocyte proliferation, S100B protein overexpression, and increased hippocampal neuronal survival [219].
Acupuncture has been applied for clinically managing epilepsy. Previous studies have reported that auricular acupuncture positively influences drug-resistant epilepsy patients, potentially through vagus nerve stimulation, attenuating the hyperactive hippocampus, regulating inflammatory cytokine pathways, protecting the hippocampus from apoptosis, and ameliorating the sprouting of mossy fibers in the hippocampus [40,218,220,221]. Several antiepileptic mechanisms have been proposed, including the downregulation of JNK or ERK1/2 and pro-inflammatory factors (IL-1β, IL-6, TNF-α) in the cerebral cortex and hippocampus [216]. Another study suggested that auricular EA and EA at ST36-ST37 achieved therapeutic effects by reducing hippocampal hyperactivity and the transient receptor potential cation channel subfamily A member 1 (TRPA1), PKCε, PKCα, and pERK1/2 signaling pathways [40]. Moreover, auricular EA with EA at ST36–ST37 exerted long-term (6-week observation in the study) beneficial effects by reducing the anti-inflammatory response in numerous cyclooxygenase-2 (COX-2) immunoreactive cells and hippocampal COX-2 levels [218].
Acupuncture exerts antiepileptic effects by inducing anatomical changes and changes in neurotransmitter, inflammatory cytokine, and transcription factor levels. Regarding studies to date, the related mechanisms primarily involve the suppression of TRPA1/pERK and TLR4/ERK pathways and activation of the PI3K/Akt pathway. Although some studies have reported the inactivation of the TLR4 pathway, accompanied by a reduction in pCaMKIIα, pERK, pp38, pJNK, and pNFκB levels [40], the role of p38 and its association with the aforementioned signaling pathways remain unclear. These mechanisms and the primary results of the aforementioned studies are summarized in Table 4.

Table 4.
The effect of acupuncture on epileptic seizures.
10. Motion Sickness
External noxious stimulation alters intracellular signal transduction pathways in primary afferents, potentially contributing to pain hypersensitivity. This pathway proceeds via TRPVs, especially TRPV1 and TRPV4, leading to the rapid phosphorylation of p38 MAPK in the afferent neurons and the induction of hyperalgesia. Recent studies have revealed that thermal stimulation may not be the only trigger for TRPV activation [222,223]. Kaolin consumption was quantified as a behavioral response of an emetic reflex (pica behavior) in a murine model. A previous study reported that TRPV1 levels were significantly increased upon stimulation of motion sickness, and EA at PC6 acupoints attenuated hypothalamic TRPV1 levels and exerted an antiemetic effect [222]. The acupoint PC6 is well-known for its therapeutic effect, especially in relieving nausea and vomiting. This study revealed that the mechanism underlying the reduction of motion sickness potentially involves the TRPV1 pathway [222].
11. Degenerative Nerve Diseases
Microglia, the endogenous macrophages of the CNS, monitor their territory through a constant movement of their elaborated thin processes and responses to local stressors and immune disruptions. Clinical studies and preclinical animal models have implicated the dysregulation and overproduction of proinflammatory cytokines from activated microglia in the CNS as contributors to the pathophysiology of chronic neurodegenerative disorders including Alzheimer’s disease [16], Parkinson’s disease, and multiple sclerosis and acute neurodegenerative conditions including traumatic brain injury and stroke. The p38α MAPK pathway helps increase the microglial production of proinflammatory cytokines induced by diverse stressors [16,17,223]. These results indicate the feasibility of targeting p38α MAPK to modulate the production of pro-inflammatory cytokines by the CNS.
Few studies have focused on the effects of androgens on neuroinflammation. Yang et al. investigated the neuroprotective role of androgens (including testosterone and its metabolite dihydrotestosterone, DHT) in lipopolysaccharide (LPS)-induced neuroinflammation, neuronal damage, and behavioral dysfunction [18]. DHT potentially inhibits the LPS-induced release of proinflammatory factors in primary microglia by suppressing the TLR4-mediated NF-κB and p38 signaling pathways, thus protecting neurons from inflammatory damage induced by activated microglia.
Sodium ferulate is used in traditional Chinese medicine (such as the root of Ligusticum chuanxiong and Angelica sinensis) and is speculated to help treat cardiovascular and cerebrovascular diseases by preventing thrombosis. Furthermore, sodium ferulate prevents Aβ-induced MKK3/MKK6-p38-Hsp27 signaling and reduces apoptosis in the rat hippocampus [17].
11.1. Alzheimer’s Disease
The pathological hallmarks of Alzheimer’s disease (AD) are the accumulation of extracellular plaques and intracellular neurofibrillary tangles that are composed of filaments of β-amyloid polymers and the neuronal microtubule-associated protein Tau, respectively. Elevated β-amyloid levels in an AD brain are speculated to induce microglial activation and the consequent release of proinflammatory cytokines induced by the p38 MAPK pathway, potentially contributing to AD pathogenesis together with other disorders including neuronal injury, trauma, ischemia, and the accumulation of oxidants associated with brain aging [16,18,224].
Since more than half of the phosphorylation sites in paired helical filament–Tau are serine and threonine residues followed by proline, members of the MAPK family, especially p38α, potentially play an important role in phosphorylating Tau [16]. Considering that aberrantly activated JNK and p38 MAPKs are reportedly associated with cells containing filamentous Tau in some neurodegenerative diseases, these kinases may contribute to Tau hyperphosphorylation. Moreover, the p38 MAPK activator, MKK6, is active in neurodegenerative diseases, indicating a potential contribution to Tau hyperphosphorylation in these diseases. Recent studies have reported that Tau is a suitable in vitro substrate for p38 isoforms p38δ and p38γ, and its phosphorylation by these two enzymes reduces its ability to promote microtubule assembly [18,225,226,227,228]. Moreover, p38γ overexpression in neuroblastoma induces Tau phosphorylation, which is associated with a reduction in Tau that is associated with the cytoskeleton and an increase in soluble Tau. Furthermore, p38δ is the major Tau kinase in neuroblastoma in response to osmotic shock [227]. This evidence indicates that p38 MAPKs potentially regulate Tau hyperphosphorylation in neurodegenerative diseases and are potentially suitable therapeutic targets for those diseases.
Other studies have focused on the receptor for advanced glycation end products (RAGE), a multiligand member of the immunoglobulin superfamily of cell surface molecules, which serves as a receptor for Aβ on neurons, microglia, astrocytes, and endothelial cells of blood vessels. RAGE is upregulated in brain regions affected by AD. A murine transgenic model revealed that RAGE is a potential therapeutic target for AD [229].
Several studies have reported that EA and manual acupuncture have a therapeutic effect on AD. For example, EA at acupoint GV20 suppresses Aβ generation [228]. Stimulation at acupoints GV14 and BL23 downregulates beta-secretase 1, which is an enzyme that is responsible for Aβ generation in AD and increases hippocampal ATP levels in AD mice [230]. Low-frequency (2-Hz) EA at acupoint GV20 and bilateral acupoints KI3 and ST36 for 15 min once daily for 12 sessions was reported to significantly downregulate p-p38 MAPK and IL-1β mRNA expression in the hippocampus and the frontal lobe [65]. Other studies have reported that EA at GV20 reversed behavioral deficit and LTP impairment, possibly through the N-methyl-D-aspartate receptor 1 (NMDA-R1) and TRPV1 pathway, thus reversing NR1- (one of the NMDA receptor subunits) and TRPV1-mediated neurotoxicity in vascular dementia [208,209]. This implies that 2-Hz EA can rescue p38 MAPK signal transduction, resulting in anti-apoptotic and anti-inflammatory effects that reduce Aβ deposits in the brain and improve learning and memory in AD patients [20].
11.2. Parkinson’s Disease
Parkinson’s disease is a neurodegenerative disorder that causes severe motor impairment owing to loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Several studies have focused on PD pathophysiology and reported potential therapeutic targets for PD.
One of the direct neuroprotective effects is exerted by the protein deglycase DJ-1, which inhibits ASK1 through the nuclear sequestration of death domain–associated protein (Daxx) [230,231,232]. DJ-1 is speculated to scavenge reactive oxidative species (ROS) and subsequently attenuate oxidative stress, thereby reducing cellular ROS burden [233,234]. Furthermore, DJ-1 interacts with p38-regulated/activated kinase (PRAK/MK5) under cellular oxidative stress to facilitate nuclear localization of DJ-1 [235]. Thus, DJ-1 co-localizes with PRAK in the nuclei of NIH3T3 cells. Since DJ-1 lacks both nuclear localization and nuclear export signals, PRAK may be a crucial factor assisting DJ-1 in regulating the cellular localization of Daxx and in ASK1 signaling and concomitant cell death [236].
A recent study reported that EA at acupoint KI3 reduced the excitotoxicity of dopaminergic neurons by regulating the NMDA receptor function and thus may potentially serve as a novel therapeutic approach for PD [237]. The levels of pNR1 and pNR2B, phosphorylated PKA, PKC, α-Ca2+/CaMKII, pERK, and CREB were also reduced following EA [237].
12. Fibromyalgia
Fibromyalgia is a common chronic pain syndrome characterized by chronic widespread mechanical pain. TRPV1 and TRPV4 are believed to play a crucial role in the pathophysiology of fibromyalgia. EA at 2 Hz at bilateral ST36 acupoints was reported to reduce long-lasting mechanical hyperalgesia by downregulating TRPV4, p-p38, p-JNK, and pCREB in the peripheral nervous system and CNS [238,239].
Taken together, acupuncture exerts anti-inflammation, anti-apoptosis, and neuroprotection effects via modulating proinflammatory cytokines, increasing the levels of neurotrophic factors and modulating signaling pathways, such as p38 MAPKs, Raf/MAPK/ERK1/2, TLR4/ERK, PI3K/AKT, AC/cAMP/PKA, ASK1–JNK/p38, and downstream CREB, JNK, m-TOR, NF-κB, and Bcl-2/Bax balance. EA relieves acute pain through inhibiting the TRPV1 signaling and PI3K/PKC/CaMKII pathway. EA also promote the release of opiates to activate μ-, δ-, and κ-opioid receptors, therefore producing immediate and persistent analgesic effects. Furthermore, EA can downregulate TRPV1-mediated inflammation and the release of proinflammatory cytokines, GFAP, Iba-1, and S100B by inhibiting the phosphorylation of p38 MAPK, therefore inhibiting microglial activation that triggers nerve damage and pain. Combining acupuncture to the treatment of a cerebral ischemic/hemorrhagic event can have a positive effect; an upregulation of BDNF synthesis, downregulation of P2X4Rs, and inhibition of p38 phosphorylation leads to the activation of the Raf-1/MEK1/2/ERK1/2/p90RSK/Bad signaling pathway, which contributes to the downregulation of caspase-3-dependent neuronal apoptosis. Acupuncture also inhibits the TNF-α/TRADD/FADD/cleaved caspase-8/cleaved caspase-3 apoptotic pathway in the ischemic cortical penumbra after reperfusion. In the case of treating epilepsy and seizure, several antiepileptic mechanisms have been proposed, including the downregulation of JNK or ERK1/2 and proinflammatory factors (IL-1β, IL-6, TNF-α) in the cerebral cortex and hippocampus. Auricular EA can reduce hippocampal hyperactivity through TRPA1 and PKCε/PKCα/pERK1/2 signaling pathways. Furthermore, acupuncture can regulate p38γ and p38δ and reduce Tau hyperphosphorylation in neurodegenerative diseases. Acupuncture also exhibits a therapeutic effect that was reported to involve the regulation of DJ-1, ASK1, Daxx, phosphorylated PKA, PKC, α-Ca2+/CaMKII, pERK, and CREB. Acupuncture exerts curative effects in migraine through local analgesia, anti-inflammation, neuropeptide regulation, cytoskeleton remodeling, cell repair, and improvement in overall homeostasis. Furthermore, the downregulation of TRPV4, p-p38, p-JNK, and pCREB in the peripheral nervous system and CNS may account for the therapeutic effect of acupuncture in fibromyalgia.
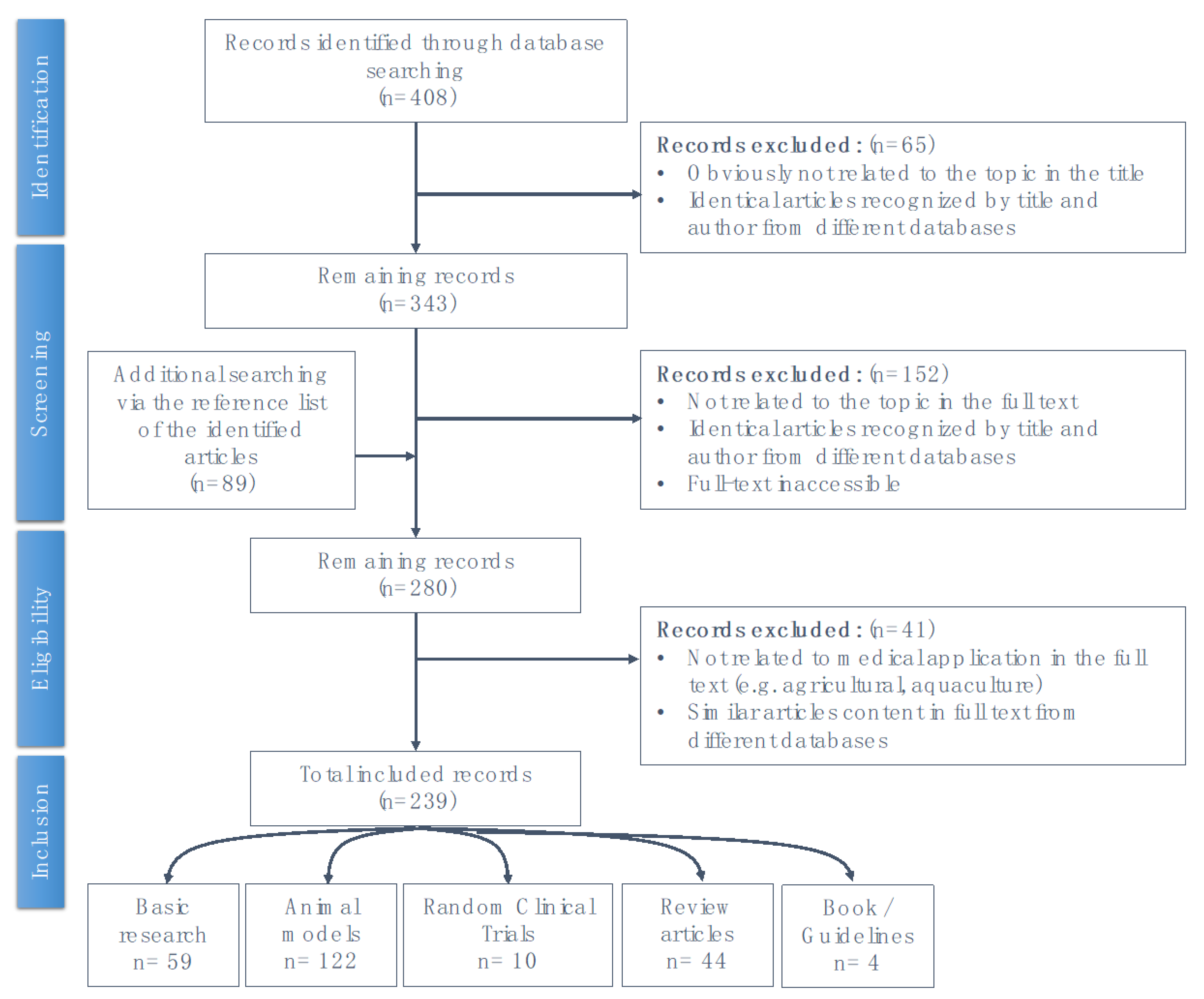
This literature review based on the results of previous studies where we electronically searched databases including PubMed, Embase, and the Cochrane Library from their inception to April 2020, using the following keywords alone or in various combinations: “acupuncture”, “p38 MAPK pathway”, “signaling”, “stress response”, “inflammation”, “immune”, “pain”, “analgesic”, “cerebral ischemic injury”, “epilepsy”, “Alzheimer’s disease”, “Parkinson’s disease”, “dementia”, “degenerative”, and “homeostasis”. The schematic explanation of the searching process is presented as Appendix A.
13. Conclusions
Among the aforementioned cell pathways, the p38 MAPK signaling pathway plays a role in the therapeutic effect of acupuncture in several nervous system diseases. EA inhibits the ascending pain pathway and intracellular p38-mediated inflammatory pathway by stimulating peripheral opioid receptors. Furthermore, EA promotes endogenic analgesic mechanisms, thus exerting immediate local analgesic effects and rescuing CNS-induced chronic pain. Acupuncture also counteracts p38 MAPK signal transduction, reduces Aβ deposits in the brain, and improves learning and memory in AD patients.
Acupuncture exhibits a dual regulatory function through the activation or inhibition of different p38 MAPK pathways and contributes to an overall improvement of clinical symptoms and physiological functions in several nervous system diseases. The p38 MAPK pathway potentially induces both protective and inhibitory effects in highly similar systems. Further studies are required to identify and characterize the various substrates of these kinases involved in cell differentiation/cell repair, neurotoxicity, and neurodegeneration, thus potentially furthering the current understanding of the mechanism underlying acupuncture in treating these diseases.
Author Contributions
Data curation, T.-H.W.; writing—original draft preparation, T.-H.W.; review and editing, C.-L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Chinese Medicine Research Center, China Medical University from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan (CMRC-CENTER-0).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| A1R | adenosine A1 receptor |
| α7 nAChR | α7 nicotinic acetylcholine receptor |
| AC | adenyl cyclase |
| ACC1 | acetyl-CoA carboxylase (ACC) 1 is a biotin-dependent enzyme that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA |
| AD | Alzheimer’s disease |
| Akt (PKB) | protein kinase B (PKB) is also known as Akt |
| Alk5 | TGFβ type I receptor kinase |
| AMP | adenosine mono-phosphate |
| AMPK | 5’ AMP-activated protein kinase |
| AP-1 | activator protein 1 |
| ARE | AU-rich elements |
| ASIC3 | acid-sensing ion channel 3 |
| ASK-1 | apoptosis signal-regulating kinase 1 |
| ATF | activating transcription factor |
| ATP | adenosine tri-phosphate |
| BACE1 | beta-secretase 1, an enzyme responsible for Aβ generation in Alzheimer’s disease |
| Bad | BCL2 associated agonist of cell death; a protein involved in initiating apoptosis. |
| Bax | Bcl-2 associated X |
| Bac-2 | B-cell lymphoma 2 |
| BDNF | brain-derived neurotrophic factor |
| CaMKII | calmodulin-dependent protein kinase II |
| CCI | chronic constriction injury |
| Cd242 | The 40- to 42-kiloDalton red cell membrane glycoprotein bearing the ICAM-4 antigen named by the LW blood system. |
| CFA-treated | Complete Freund’s adjuvant injections produced significant mechanical and thermal hyperalgesia in mice. |
| c-Fos | A proto-oncogene that is expressed within some neurons following depolarization. |
| CHOP | C/EBP homologous protein; belongs to the family of CCAAT/enhancer-binding proteins (C/EBPs) and is involved in the regulation of genes that encode proteins involved in proliferation |
| c-Jun | A proto-oncogene that with c-Fos forms the AP-1 early response transcription factor that regulates gene expression in response to extracellular stimuli. |
| CL100 | The human CL100 gene is induced in skin fibroblasts in response to oxidative/heat stress and growth factors. The CL100 gene encodes a dual specificity (Tyr/Thr) protein phosphatase that specifically inactivates MAPKs. |
| CLCA3A2 | chloride channel accessory 3A2 |
| CLIC3 | chloride intracellular channel 3 |
| Cot (Tp2) | cancer Osaka thyroid oncogene (=Tpl-2) |
| COX-2 | cyclooxygenase-2 |
| cPLA2 | cytosolic phospholipase A2 |
| CREB | cAMP response element-binding protein |
| CRF | corticotropin-releasing factor |
| CWP | components of calcium wave propagation |
| DLK (MAP3K12) | dual leucine zipper kinase, also known as MAP3K12 |
| DJ-1 | a protein deglycase |
| DLG1 (SAP97) | discs large homolog 1 scaffold protein |
| Daxx | the death-associated protein |
| DHT | dihydrotestosterone |
| DRG | dorsal root ganglion |
| EA | electroacupuncture |
| elF2a | eukaryotic translation initiation factor 2A; functions by a separate mechanism in eukaryotic translation |
| eEF2 | eukaryotic elongation factor 2 |
| eEF2K | eukaryotic elongation factor 2 kinase |
| elF2α | a subunit of the heterotrimeric eIF2 complex |
| Elk-1 | erythroblast transformation specific (ETS) like-1 protein |
| ERK | extracellular signal–regulated kinases |
| ETS | erythroblast transformation specific protein, or E26 transformation-specific, or E-twenty-six transcription factors family |
| FADD | Fas-associated protein with death domain; also called MORT1 |
| Fas pathway | Fas and Fas Ligand (FasL) are involved in the regulation of cell death. |
| GABA | gamma-aminobutyric acid |
| GFAP | glial fibrillary acidic protein; a marker of astrocytes |
| GPCRs | G-protein-coupled receptors |
| HMG-CoA Reductase | 3-hydroxy-3-methyl-glutaryl-CoA reductase or HMGR is the rate-controlling enzyme of the mevalonate pathway, responsible for cholesterol and other isoprenoid biosynthesis. |
| Hsc70 | heat shock cognate 70 |
| Hsp | heat shock proteins |
| 5-HT | 5-hydroxytryptamine |
| Iba-1 | ionized calcium–binding adapter molecule 1 |
| IκB | inhibitor of nuclear factor kappa-B |
| IκB | inhibitor of nuclear factor kappa-B kinase |
| IKKs | I-kappa-B kinases |
| IL-1β | Interleukin 1 beta is a member of the interleukin 1 family of cytokines produced by activated macrophages. |
| IRF | interferon regulatory factor |
| JNK | c-Jun amino-terminal kinases |
| LTD | long-term depression; a term in neurophysiology describing an activity-dependent reduction in the efficacy of neuronal synapses lasting hours or longer following a long patterned stimulus. |
| LTP | long-term potentiation; a persistent strengthening of synapses based on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between two neurons. |
| MA | manual acupuncture |
| MAPK | mitogen-activated protein kinase |
| MAPKK (MAP2K, MKK) | MAPK kinase |
| MAPKKK (MAP3K) | MAPKK kinase |
| Max | a transcription factor coded by the myc-associated factor X |
| MEF | myocyte enhancing factor |
| MEK (MKK) | MAPK/ERK kinase |
| MEKK | MEK kinase |
| mGluR3 | metabotropic glutamate receptor 3 |
| MKP | MAPK-phosphatase |
| MAPKAPK-2 | mitogen-activated protein kinase-activated protein kinase 2 |
| MLK | mixed-lineage protein kinase |
| MNK | mitogen activated protein kinase–interacting protein |
| Myc | A group of transcription factors coded by a regulator genes and proto-oncogenes called Myc |
| MyD88 | myeloid differentiation primary response 88 |
| MSK | mitogen and stress activated protein kinase |
| mTOR | mammalian target of rapamycin |
| Nav | voltage-gated sodium channel |
| NFκB | nuclear factor-kappa-B |
| P105 | The 105 kD protein is a Rel protein-specific transcription inhibitor encoded by the NFKB1 gene |
| PAG | periaqueductal gray area |
| NIH3Ts cells | A cell line derived from mouse embryonic fibroblasts. |
| NMDA | N-methyl-D-aspartate, an amino acid derivative |
| PKB | Protein kinase B; also known as Akt |
| NO | nitric oxide |
| NR1 | NMDA receptor subunits includes NR1 and NR2Bi |
| Pax6 | Paired-box protein Pax-6, also known as aniridia type II protein (AN2) or oculorhombin; a “master control” gene for the development of eyes and other sensory organs |
| P2RX7 | P2X purinoceptor 7 is a protein belonging to the family of purinoceptors for ATP. |
| P2X4Rs | P2X4 receptors are a subtype of ionotropic ATP receptors |
| P2Y1, -2 | human purinergic G protein-coupled receptors |
| PD | Parkinson’s disease |
| Pellino | A group of proteins extremely well conserved during evolution; Pellinos interact with key mediators in TLR/IL-1R-induced signaling pathways. |
| PHF-tau | paired helical filament–tau protein |
| PI3K | phosphoinositide 3-kinases; also called phosphatidylinositol 3-kinases |
| PKC | protein kinase C |
| PSD | postsynaptic density protein |
| PRAK | p38-regulated and -activated kinase |
| Rac2 | Ras-related C3 botulinum toxin substrate 2 |
| RAF | rapidly accelerated fibrosarcoma-related oncogene |
| Raf1 | v-raf-1 murine leukemia viral oncogene homolog 1 |
| RAGE | The receptor for advanced glycation end products is a member of the immunoglobulin superfamily of cell surface molecules. |
| RCT study | randomized controlled trial study |
| Rel | A proto-oncogene protein encoded by the REL gene. It is a member of the NF-κB family of transcription factors and contains a Rel homology domain (RHD) at its N-terminus and two C-terminal transactivation domains. |
| Rho | Rho (ρ) factor is a protein that acts in bacterial cells to mediate termination of transcription at distinct sites, which mediates the dissociation of the RNA from the very stable ternary transcription complex. |
| Rit | Ras-like protein in tissues |
| RIP | receptor-interacting protein kinases; a class of serine/threonine protein kinases |
| ROS | reactive oxidative species |
| S100B | S100 calcium-binding protein B |
| SAP | stress-activated protein or synapse-associated protein; SAP90 [(synapse-associated protein 90 is also known as PSD-95 (postsynaptic density-95)] |
| SAPK | stress-activated protein kinase |
| Smac/DIABLO | second mitochondrial-derived activator of caspase/direct inhibitor of apoptosis protein-binding protein with low isoelectric point |
| SNpc | substantia nigra pars compacta |
| Stat1 | signal transducer and activator of transcription 1 |
| STK | serine-threonine/tyrosine kinases |
| TAES | transcutaneous acupoint electrical stimulation |
| TAK-1 | transforming growth factor-β-activated kinase 1 |
| TAO | thousand and one amino acids |
| TAB1 | TGF-beta-activated kinase |
| TGFβ | transforming growth factor-β |
| TLR4 | toll-like receptor 4 |
| TNF | tumor necrosis factor |
| Tpl2 | tumor progression locus 2, also known as COT or MAP3K8 |
| TRADD | tumor necrosis factor receptor type 1-associated DEATH domain protein |
| TRAF6 | tumor necrosis factor receptor (TNFR)-associated factor 6 |
| TrkB | tyrosine kinase receptor B |
| TRPA1 | transient receptor potential cation channel subfamily A member 1 |
| TRPV | transient receptor potential vanilloid receptors |
| 3′UTR | 3′untranslated region |
Appendix A
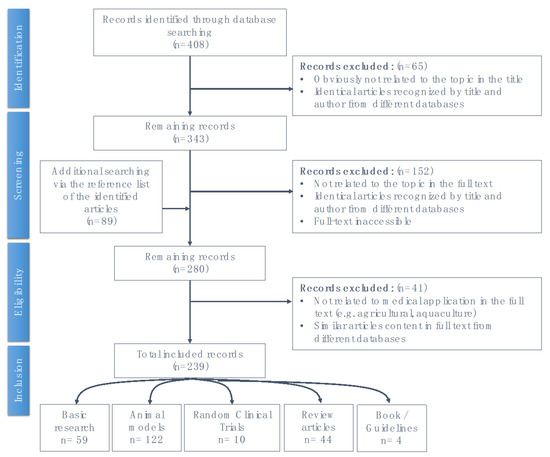
Figure A1.
Schematic of the literature review search process.
Figure A1.
Schematic of the literature review search process.
References
- Zhou, W.; Benharash, P. Effects and mechanisms of acupuncture based on the principle of meridians. J. Acupunct. Meridian Stud. 2014, 7, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, N.L.; Wu, A.Q. The Yellow Emperor’s Canon of Internal Medicine, 1st ed.; China Science & Technology. Press: Beijing, China, 1997; 1999, 2nd edition. [Google Scholar]
- Bannerman, R.H. Acupuncture: The WHO View; World Health Organization: Geneva, Switzerland, 1979; Volume 12, p. 27e8. [Google Scholar]
- Park, J.; Sohn, Y.; White, A.R.; Lee, H. The safety of acupuncture during pregnancy: A systematic review. Acupunct. Med. 2014, 32, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hao, Z.; Zhang, L.L.; Guo, Q. Efficacy and safety of acupuncture in children: An overview of systematic reviews. Pediatr. Res. 2015, 78, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.Y.; Miller, D.W.; Bolash, B.; Bauer, M.; McDonal, J.; Faggert, S.; He, H.; Li, Y.M.; Matecki, A.; Camardella, L.; et al. Acupuncture’s role in solving the opioid epidemic: Evidence, cost-effectiveness, and care availability for acupuncture as a primary, nonpharmacologic method for pain relief and management–white paper. J. Integr. Med. 2017, 15, 411–425. [Google Scholar] [CrossRef]
- Acupuncture: In Depth. National Center for Complementary and Alternative Medicine. Available online: https://nccih.nih.gov/health/acupuncture/introduction (accessed on 25 January 2020).
- Hsieh, C.L. Acupuncture as treatment for nervous system diseases. BioMed 2012, 2, 51–57. [Google Scholar] [CrossRef]
- He, X.R.; Wang, Q.; Li, P.P. Acupuncture and moxibustion for cancer-related fatigue: A systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 3067–3074. [Google Scholar] [CrossRef]
- Cox, J.; Varatharajan, S.; Côté, P.; Optima Collaboration. Effectiveness of acupuncture therapies to manage musculoskeletal disorders of the extremities: A systematic review. J. Orthop. Sports Phys. Ther. 2016, 46, 409–429. [Google Scholar] [CrossRef]
- Forde, J.C.; Jaffe, E.; Stone, B.V.; Te, A.E.; Espinosa, G.; Chughtai, B. The role of acupuncture in managing overactive bladder: A review of the literature. Int. Urogynecol. J. 2016, 27, 1645–1651. [Google Scholar] [CrossRef]
- Yao, Q.; Li, S.; Liu, X.; Qin, Z.; Liu, Z. The effectiveness and safety of acupuncture for patients with chronic urticaria: A systematic review. Biomed Res. Int. 2016, 2016, 5191729. [Google Scholar] [CrossRef]
- Bäcker, M.; Hammes, M.G.; Valet, M.; Deppe, M.; Valet, M.; Conrad, B.; Tölle, T.R.; Doboset, G. Different modes of manual acupuncture stimulation differentially modulate cerebral blood flow velocity, arterial blood pressure and heart rate in human subjects. [published correction appears in Neurosci. Lett. 2003, 6, 337, 117]. Neurosci. Lett. 2002, 333, 203–206. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Chang, Q.Y.; Lin, I.H.; Lin, J.G.; Liu, C.H.; Tang, N.Y.; Lane, H.Y. The study of electroacupuncture on cerebral blood flow in rats with and without cerebral ischemia. Am. J. Chin. Med. 2006, 34, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.; Lin, Y.; Tang, N.; Cheng, C.Y.; Hsieh, C.L. Electric stimulation of the ears ameliorated learning and memory impairment in rats with cerebral ischemia-reperfusion injury. Sci. Rep. 2016, 6, 20381. [Google Scholar] [CrossRef] [PubMed]
- Bachstetter, A.D.; Xing, B. Microglial p38alpha MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Abeta). J. Neuroinflammation 2011, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Fan, Y. Effects of sodium ferulate on amyloid-beta-induced MKK3/MKK6-p38 MAPK-Hsp27 signal pathway and apoptosis in rat hippocampus. Acta Pharm. Sin. 2006, 27, 1309–1316. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, R. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 2020, 104814. [Google Scholar] [CrossRef]
- Yan, S.D.; Bierhaus, A. RAGE and Alzheimer’s disease: A progression factor for amyloid-beta-induced cellular perturbation? J. Alzheimers Dis 2009, 16, 833–843. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, A.; Shao, S.; Zhou, Y.; Xiong, B.; Li, Z. Electroacupuncture ameliorates cognitive impairment by inhibiting the JNK signaling pathway in a mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2020, 12, 23. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Wang, G.; Li, M.; Zhang, Z.; Gu, G. The effects of electroacupuncture on TH1/TH2 cytokine mRNA expression and mitogen-activated protein kinase signaling pathways in the splenic T cells of traumatized rats. Anesth Analg. 2009, 109, 1666–1673. [Google Scholar] [CrossRef]
- Cheng, K.J. Neurobiological mechanisms of acupuncture for some common illnesses: A clinician’s perspective. J. Acupunct. Meridian Stud. 2014, 7, 105–114. [Google Scholar] [CrossRef]
- Xue, X.; You, Y.; Tao, J.; Ye, X.; Huang, J.; Yang, S.; Lin, Z.; Hong, Z.; Peng, J.; Chen, L. Electro-acupuncture at points of Zusanli and Quchi exerts anti-apoptotic effect through the modulation of PI3K/Akt signaling pathway. Neurosci. Lett. 2014, 558, 14–19. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Liang, F.; Liu, J.; Li, J.; Lu, J.; Fu, Y.; Chen, Q.; Has, Q.; Wu, S. Effects of electroacupuncture on electrocardiogram, myocardial pathological morphology and PI3K/Akt pathway in rats with chronic myocardial ischemia. Zhongguo Zhen Jiu 2016, 36, 389–395. [Google Scholar]
- Hwang, I.K.; Chung, J.Y.; Yoo, D.Y.; Yi, S.S.; Youn, H.Y.; Seong, J.K.; Soon, Y.S. Effects of electroacupuncture at Zusanli and Baihui on brain-derived neurotrophic factor and cyclic AMP response element-binding protein in the hippocampal dentate gyrus. J. Vet. Med. Sci. 2010, 72, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qin, F.; Liu, A.; Sun, Q.; Wang, Q.; Xie, S.; Lu, S.; Zhang, D.; Lu, Z. Electro-acupuncture attenuates the mice premature ovarian failure via mediating PI3K/AKT/mTOR pathway. Life Sci. 2019, 217, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, Q.; Hu, B.; Peng, Z.; Zhao, Y.; Ma, L.; Xiong, L.; Lu, Y.; Zhu, X.; Chen, S. Involvement of ERK 1/2 activation in electroacupuncture pretreatment via cannabinoid CB1 receptor in rats. Brain Res. 2010, 1360, 1–7. [Google Scholar] [CrossRef]
- Xie, G.; Yang, S.; Chen, A.; Lan, L.; Lin, Z.; Gao, Y.; Huang, J.; Lin, J.; Peng, J.; Tao, J.; et al. Electroacupuncture at Quchi and Zusanli treats cerebral ischemia-reperfusion injury through activation of ERK signaling. Exp. Ther. Med. 2013, 5, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, J.; Chun, L.; Zhou, G.; Xu, X.; Zhang, X.; Lan, X. Effect of electroacupuncture on cell apoptosis and erk signal pathway in the hippocampus of adult rats with cerebral ischemia-reperfusion. Evid. Based Complementary Altern. Med. 2015, 414965. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, C.; Zhou, G.; Yang, L.; Jiang, G.; Chen, J.; Li, Q.; Zhan, Z.; Xu, X.; Zhang, X. Effects of electroacupuncture on the cortical extracellular signal regulated kinase pathway in rats with cerebral ischaemia/reperfusion. Acupunct. Med. 2017, 35, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, Q.; Lin, X.; Boriongan, C.; He, Z.; Tan, J.; Cao, C.; Zhou, S. Brain-derived neurotrophic factor signaling pathway: Modulation by acupuncture in telomerase knockout mice. Altern. Health Med. 2015, 21, 36–46. [Google Scholar]
- Wang, S.J.; Ma, J.; Gong, Y.X.; Wang, Y.C.; Zeng, X.L.; Liang, Y.; Sun, G.J. Effect of electroacupuncture intervention on ERK 1/2 signaling and TNF-alpha and IL-1beta protein levels in the substantia nigra in rats with Parkinson’s disease. Zhen Ci Yan Jiu 2014, 39, 456–460. [Google Scholar]
- Lan, X.; Zhang, X.; Zhou, G.P.; Wu, C.X.; Li, C.; Xu, X.H. Electroacupuncture reduces apoptotic index and inhibits p38 mitogen-activated protein kinase signaling pathway in the hippocampus of rats with cerebral ischemia/reperfusion injury. Neural Regen. Res. 2017, 12, 409–416. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, S.D.; Wang, M.M.; Dong, F.; Feng, Y.S.; Zhang, F. Electroacupuncture alleviated neuronal apoptosis following ischemic stroke in rats via midkine and ERK/JNK/p38 signaling pathway. J. Mol. Neurosci. 2018, 66, 26–36. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Yang, S.; Huang, J.; Xue, X.; Zheng, Y.; Shang, G.; Tao, J.; Chen, L. Electroacupunctre improves motor impairment via inhibition of microglia-mediated neuroinflammation in the sensorimotor cortex after ischemic stroke. Life Sci. 2016, 151, 313–322. [Google Scholar] [CrossRef]
- Xu, J.; She, Y.; Su, N.; Zhang, R.; Lao, L.; Xu, S. Effects of electroacupuncture on chronic unpredictable mild stress rats depression-like behavior and expression of p-ERK/ERK and p-P38/P38. Evid. Based Complement Altern. Med. 2015, 2015, 650729. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, Z.; Lu, J.; Zhao, B.; Fei, Y.; Li, J.; Jiang, H.; Sun, L.; Wang, Y.; Sun, Y.; et al. The Role of MAPK and dopaminergic synapse signaling pathways in antidepressant effect of electroacupuncture pretreatment in chronic restraint stress rats. Evid. Based Complementary Altern. Med. 2017, 2017, 2357653. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.M.; Dong, X.; Tu, Y.; Liu, P. A microarray study of chronic unpredictable mild stress rat blood serum with electro-acupuncture intervention. Neurosci. Lett. 2016, 627, 160–167. [Google Scholar] [CrossRef]
- Zhao, G.; Li, D.; Ding, X.; Li, L. Nerve growth factor pretreatment inhibits lidocaine-induced myelin damage via increasing BDNF expression and inhibiting p38 mitogen activation in the rat spinal cord. Mol. Med. Rep. 2017, 16, 4678–4684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liao, E.T.; Lin, Y.W.; Huang, C.P.; Tang, N.Y.; Hsieh, C.L. Electric stimulation of ear reduces the effect of Toll-like receptor 4 signaling pathway on kainic acid-induced epileptic seizures in rats. Biomed. Res. Int. 2018, 2018, 5407256. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yang, S.; Liu, J.; Huang, J.; Peng, J.; Lin, J.; Tao, J.; Chen, L. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol. Med. Rep. 2013, 7, 1516–1522. [Google Scholar] [CrossRef]
- Lan, L.; Tao, J.; Chen, A.; Xie, G.; Huang, J.; Lin, J.; Peng, J.; Chen, L. Electroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathway. Int. J. Mol. Med. 2013, 31, 75–80. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, Y.; Wang, X.; Ye, X. Effect of electroacupuncture on the expression of mTOR and eIF4E in hippocampus of rats with vascular dementia. Neurol. Sci. 2013, 34, 1093–1097. [Google Scholar] [CrossRef]
- Xu, T.; Li, W.; Liang, Y.; Yang, Z.; Liu, J.; Wang, Y.; Su, N. Neuroprotective effects of electro acupuncture on hypoxic-ischemic encephalopathy in newborn rats Ass. Pak. J. Pharm. Sci. 2014, 27 (Suppl. 6), 1991–2000. [Google Scholar] [PubMed]
- Oh, J.; Kim, Y.; Kim, S.; Lee, B.; Jang, J.; Kwon, S.; Park, H. Acupuncture modulates stress response by the mTOR signaling pathway in a rat post-traumatic stress disorder model. Sci. Rep. 2018, 8, 11864. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhuo, P.; Li, L.; Jin, H.; Lin, B.; Zhang, Y.; Liang, S.; Wu, J.; Huang, J.; Wang, Z.; et al. Activation of brain glucose metabolism ameliorating cognitive impairment in APP/PS1 transgenic mice by electroacupuncture. Free. Radic. Biol. Med. 2017, 112, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Kim, H.N.; Jang, J.Y.; Park, C.; Lee, J.H.; Shin, H.K.; Choi, Y.H.; Choi, B.T. Effects of electroacupuncture on apoptotic pathways in a rat model of focal cerebral ischemia. Int. J. Mol. Med. 2013, 32, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Lawan, A.; Bennett, A.M. Mitogen-activated protein kinase regulation in hepatic metabolism. Trends Endocrinol. Metab. 2017, 28, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Kim, S.T.; Doo, A.R.; Park, J.Y.; Moon, W.; Chae, Y.; Yin, C.S.; Lee, H.; Park, H.J. Phosphatidylinositol 3-kinase/Akt signaling pathway mediates acupuncture-induced dopaminergic neuron protection and motor function improvement in a mouse model of Parkinson’s disease. Int. J. Neurosci. 2011, 121, 562–569. [Google Scholar] [CrossRef]
- Kim, S.N.; Doo, A.R.; Park, J.Y.; Bae, H.; Chae, Y.; Shim, I.; Lee, H.; Moon, W.; Lee, H.; Park, H.J. Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson’s disease. PLoS ONE 2011, 6, e27566. [Google Scholar] [CrossRef]
- Li, Q.Q.; Shi, G.X.; Yang, J.W.; Li, Z.H.; Zhang, Z.H.; He, T.; Wang, J.; Liu, L.Y.; Liu, C.Z. Hippocampal cAMP/PKA/CREB is required for neuroprotective effect of acupuncture. Physiol. Behav. 2015, 139, 482–490. [Google Scholar] [CrossRef]
- Lu, F.; Zhu, H.M.; Xie, J.J.; Zhou, H.H.; Chen, Y.L.; Hu, J.Y. Effects of electroacupuncture on behavior, plasma COR and expressions of PKA and PKC in hippocampus of the depression model rat. Zhongguo Zhen Jiu 2008, 28, 214–218. [Google Scholar]
- Liu, J.J.; Wu, Z.F.; Sun, J.; Jiang, L.; Jiang, S.; Fu, W.B. Role of AC-cAMP-PKA cascade in antidepressant action of electroacupuncture treatment in rats. Evid. Based Complementary Altern. Med. 2012, 2012, 932414. [Google Scholar] [CrossRef]
- Lin, R.; Lin, Y.; Tao, J.; Chen, B.; You, K.; Chen, J.; Li, X.; Chen, L.D. Electroacupuncture ameliorates learning and memory in rats with cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting p-CREB expression in the hippocampus. Mol. Med. Rep. 2015, 12, 6807–6814. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, Y.; Kim, H.; Shin, Y.I.; Shin, H.K.; Choi, B.T. Electroacupuncture ameliorates memory impairments by enhancing oligodendrocyte regeneration in a mouse model of prolonged cerebral hypoperfusion. Sci. Rep. 2016, 6, 28646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, R.; Tao, J.; Wu, Y.; Chen, B.; You, K.; Chen, J.; Li, X.; Chen, L.D. Electroacupuncture improves cognitive ability following cerebral ischemia reperfusion injury via CaM-CaMKIV-CREB signaling in the rat hippocampus. Exp. Ther. Med. 2016, 12, 777–782. [Google Scholar] [CrossRef]
- Yun, Y.C.; Jang, D.; Yoon, S.B.; Kim, D.; Choi, D.H.; Kwon, O.S.; Lee, Y.M.; Youn, D. Laser acupuncture exerts neuroprotective effects via regulation of Creb, Bdnf, Bcl-2, and Bax gene expressions in the hippocampus. Evid. Based Complementary Altern. Med. 2017, 2017, 7181637. [Google Scholar] [CrossRef]
- Pak, M.E.; Jung, D.H.; Lee, H.J.; Shin, M.J.; Kim, S.Y.; Shin, Y.B.; Yun, Y.J.; Shin, H.K.; Choi, B.T. Combined therapy involving electroacupuncture and treadmill exercise attenuates demyelination in the corpus callosum by stimulating oligodendrogenesis in a rat model of neonatal hypoxia-ischemia. Exp. Neurol. 2018, 300, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liang, J.; Wang, J.R.; Hu, L.; Tu, Y.; Guo, J.Y. Acupuncture activates ERK-CREB pathway in rats exposed to chronic unpredictable mild stress. Evid. Based Complementary Altern. Med. 2013, 2013, 469765. [Google Scholar] [CrossRef]
- Dimaras, H.; Gallie, B.L. Retinoblastoma Protein, Biological and Clinical Functions. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 4046–4050. [Google Scholar] [CrossRef]
- Duval, D.; Trouillas, M.; Thibault, C.; Dembelé, D.; Diemunsch, F.; Reinhardt, B.; Mertz, A.L.; Dierich, A.; Boeuf, H. Apoptosis and differentiation commitment: Novel insights revealed by gene profiling studies in mouse embryonic stem cells. Cell Death Differ. 2005, 13, 564–575. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Meng, H.; Li, J.; Yang, X.; Zhao, B.; Sun, Y.; Bro, T. Antidepressant mechanism research of acupuncture: Insights from a genome-wide transcriptome analysis of frontal cortex in rats with chronic restraint stress. Evid. Based Complementary Altern. Med. 2017, 2017, 1676808. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Yang, S.; Huang, J.; Feng, X.; Peng, J.; Lin, Z.; Liu, W.; Tao, W.; Chen, L. Electroacupuncture inhibits apoptosis of peri-ischemic regions via modulating p38, extracellular signal-regulated kinase (ERK1/2), and c-Jun N terminal kinases (JNK) in cerebral ischemia-reperfusion-injured rats. Med. Sci. Monit. 2018, 24, 4395–4404. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, L.; Tang, H.; Gao, X.; Wang, Y.; Guo, K.; Kong, J.; Yang, C. Electroacupuncture improves neurobehavioral function and brain injury in rat model of intracerebral hemorrhage. Brain Res. Bull. 2017, 131, 123–132. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, X.; Li, P.; Itoua, E.S.; Moukassa, D.; Ndinga Andely, F. Effects of acupuncture on mRNA levels of apoptotic factors in perihematomal brain tissue during the acute phase of cerebral hemorrhage. Med. Sci. Monit. 2017, 23, 1522–1532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.Y.; Xu, L.; Liu, C.L.; Huang, L.S.; Zhu, X.Y. Electroacupuncture Intervention inhibits the decline of learning-memory ability and overexpression of cleaved Caspase-3 and Bax in hippocampus induced by isoflurane in APPswe/PS 1. Zhen Ci Yan Jiu 2016, 41, 24–30. [Google Scholar]
- Wang, T.; Liu, C.Z.; Yu, J.C.; Jiang, W.; Han, J.X. Acupuncture protected cerebral multi-infarction rats from memory impairment by regulating the expression of apoptosis related genes Bcl-2 and Bax in hippocampus. Physiol. Behav. 2009, 96, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.J.; Huang, L.N.; Wang, R.H.; An, J.M.; Zhang, M. Effects of scalp-acupuncture on astrocyte apoptosis in hippocampal CA 1 region in rats with vascular dementia. Zhen Ci Yan Jiu 2015, 40, 6–12. [Google Scholar] [PubMed]
- Lai, H.C.; Chang, Q.Y.; Hsieh, C.L. Signal transduction pathways of acupuncture for treating some nervous system diseases. Evid. Based Complement. Altern. Med. 2019, 2019, 2909632. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Q.; Zhu, S.X.; Zhang, Y.; Wang, F.; Zhu, Q.Y. Effects of transcutaneous electrostimulation of auricular points on behavior and hippocampal IL-1 β and TNF-α expression in temporal lobe epilepsy rats. Acupunct. Res. 2016, 41, 283–290. [Google Scholar]
- Ding, X.; Yu, J.; Yu, T.; Fu, Y.; Han, J. Acupuncture regulates the aging-related changes in gene profile expression of the hippocampus in senescence-accelerated mouse (SAMP10). Neurosci. Lett. 2006, 399, 11–16. [Google Scholar] [CrossRef]
- Zhang, M.; Xv, G.; Wang, W.; Meng, D.; Ji, Y. Electroacupuncture improves cognitive deficits and activates PPAR-γ in a rat model of Alzheimer’s disease. Acupunct. Med. 2017, 35, 44–51. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, X.; Yu, J.; Han, J.; Yu, T.; Shi, J.; Zhao, L.; Nie, K. Acupuncture for patients with mild to moderate Alzheimer’s disease: A randomized controlled trial. BMC Complement Altern. Med. 2017, 17, 556. [Google Scholar] [CrossRef]
- Zhou, S.; Dong, L.; He, Y.; Xiao, H. Acupuncture plus herbal medicine for Alzheimer’s disease: A systematic review and meta-analysis. Am. J. Chin. Med. 2017, 45, 1327–1344. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, X.; Du, S.; Yan, C.; Yang, N.; Lin, L.; Shi, G.; Liu, C. Anti-oxidative and anti-apoptotic effects of acupuncture: Role of thioredoxin-1 in the hippocampus of vascular dementia rats. Neuroscience 2018, 379, 281–291. [Google Scholar] [CrossRef]
- Wang, S.; Fang, J.; Ma, J.; Wang, Y.; Zeng, X.; Zhou, D.; Sun, G. Influence of electroacupuncture on p38-mitogen activated protein kinase in substantia nigra cells of rats with Parkinson disease model. Zhongguo Zhen Jiu 2013, 33, 329–333. [Google Scholar] [PubMed]
- Krens, S.F.; Spaink, H.P.; Snaar-Jagalska, B. Functions of the MAPK family in vertebrate-development. FEBS Lett. 2006, 580, 4984–4990. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Ajibade, A.A.; Wang, Q. TAK1 negatively regulates NF-kappaB and p38 MAP kinase activation in Gr-1+CD11b+ neutrophils. Immunity 2012, 36, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Brand, S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: New immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut 2009, 58, 1152–1167. [Google Scholar] [CrossRef]
- Hannigan, M.; Zhan, L. The role of p38 MAP kinase in TGF-beta1-induced signal transduction in human neutrophils. Biochem. Biophys. Res. Commun. 1998, 346, 55–58. [Google Scholar] [CrossRef]
- Heineke, J.; Molkentin, J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006, 77, 589–600. [Google Scholar] [CrossRef]
- Shiojima, I.; Walsh, K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006, 20, 3347–3365. [Google Scholar] [CrossRef]
- Shioi, T.; Kang, P.M. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000, 19, 2537–2548. [Google Scholar] [CrossRef]
- Tanike, M.; Yamaguchi, O. Apoptosis signal-regulating kinase 1/p38 signaling pathway negatively regulates physiological hypertrophy. Circulation 2008, 117, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Becattini, B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metab. 2017, 6, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Davis, R.J. Cell Signaling and Stress Responses. Cold Spring Harb. Perspect Biol. 2016, 8. [Google Scholar] [CrossRef]
- Ning, C.; Wang, X. Chicory inulin ameliorates type 2 diabetes mellitus and suppresses JNK and MAPK pathways in vivo and in vitro. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Koh, A.; Molinaro, A. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef] [PubMed]
- Eagleton, M.J. Inflammation in abdominal aortic aneurysms: Cellular infiltrate and cytokine profiles. Vascular 2012, 20, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Hussien, H.; Soekhoe, R.G. Collagen degradation in the abdominal aneurysm: A conspiracy of matrixmetalloproteinase and cysteine collagenases. Am. J. Pathol. 2007, 170, 809–817. [Google Scholar] [CrossRef]
- Saratzis, A.; Abbas, A.A. Abdominal aortic aneurysm: A review of the genetic basis. Angiology 2011, 62, 18–32. [Google Scholar] [CrossRef]
- Saratzis, A.; Kitas, G.D. Can statins suppress the development of abdominal aortic aneurysms? A review of the current evidence. Angiology 2010, 61, 137–144. [Google Scholar] [CrossRef]
- Papalambros, E.; Sigala, F. Immunohistochemical expression of metalloproteinases MMP-2 and MMP-9 in abdominal aortic aneurysms: Correlation with symptoms and aortic diameter. Int. J. Mol. Med. 2003, 12, 965–968. [Google Scholar] [CrossRef]
- Petersen, E.; Gineitis, A. Activity of matrix metalloproteinase-2 and -9 in abdominal aortic aneurysms. Relation to size and rupture. Eur. J. Vasc. Endovasc. Surg. 2000, 20, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Q.; Li, W. MCP-1 Stimulates MMP-9 expression via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth muscle cells. Cell Physiol. Biochem. 2014, 34, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, C. Metabolism: A Novel Shared Link between Diabetes Mellitus and Alzheimer’s Disease. J. Diabetes Res. 2020, 2020, 4981814. [Google Scholar] [CrossRef]
- Kyriskis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Am. J. Physiol. 2001, 81, 808–869. [Google Scholar] [CrossRef]
- Lang, E.; Bissinger, R.; Qadri, S.M.; Lang, F. Suicidal death of erythrocytes in cancer and its chemotherapy: A potential target in the treatment of tumor-associated anemia. Int. J. Cancer 2017, 141, 1522–1528. [Google Scholar] [CrossRef]
- Brancho, D.; Tanaka, N.; Jaeschke, A.; Ventura, J.-J.; Kelkar, N.; Tanaka, Y.; Kyuuma, M.; Takeshita, T.; Flavell, R.A.; Davis, R.J. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003, 17, 1969–1978. [Google Scholar] [CrossRef]
- Krishna, M.; Narang, H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol. Life Sci. 2008, 65, 3525–3544. [Google Scholar] [CrossRef] [PubMed]
- Bardwell, L. Mechanisms of MAPK signalling specificity. Biochem. Soc. Trans. 2006, 34, 837–841. [Google Scholar] [CrossRef]
- Akella, R.; Moon, T.M.; Goldsmith, E.J. Unique MAP kinase binding sites. Biochim. Biophys. Acta 2008, 1784, 48–55. [Google Scholar] [CrossRef][Green Version]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochim. J. 2010, 429, 403–417. [Google Scholar] [CrossRef]
- Bonney, E.A. Mapping out p38MAPK. Am. J. Reporod. Immunol. 2017, 77. [Google Scholar] [CrossRef]
- Wang, S.; Ding, L.; Ji, H.; Xu, Z.; Liu, Q.; Zheng, Y. The role of p38 MAPK in the development of diabetic cardiomyopathy. Int. J. Mol. Sci. 2016, 17, 1037. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.I.; Xu, B.E.; Cobb, M.H.; Goldsmith, E.J. Crystal structures of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Mol. Cell 2002, 9, 1241–1249. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Fanning, L.J.; Barry, O.P. p38δ MAPK: Emerging Roles of a Neglected Isoform. Int. J. Cell Biol. 2014, 2014, 272689. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Arthur, J.S.C.; Kuma, Y.; Peggie, M.; Carr, J.; Murray-Tait, V.; Centeno, F.; Goedert, M.; Morrice, N.A.; Cuenda, A. p38γ regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005, 24, 1134–1145. [Google Scholar] [CrossRef]
- Parker, C.G.; Hunt, J.; Diener, K.; McGinley, M.; Soriano, B.; Keesler, G.A.; Bray, J.; Yao, Z.; Wang, X.S.; Kohno, T.; et al. Identification of stathmin as a novel substrate for p38 Delta. Biochem. Biophys. Res. Commun. 1998, 249, 791–796. [Google Scholar] [CrossRef]
- Rubin, C.I.; Atweh, G.F. The role of stathmin in the regulation of the cell cycle. J. Cell Biochem. 2004, 93, 242–250. [Google Scholar] [CrossRef]
- Knebel, A.; Morrice, N.; Cohen, P. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38δ. EMBO J. 2001, 20, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Hasegawa, M.; Jakes, R.; Lawler, S.; Cuenda, A.; Cohen, P. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Lett. 1997, 409, 57–62. [Google Scholar] [CrossRef]
- Masuda, K.; Shima, H.; Watanabe, M.; Kikuchi, K. MKP-7, a novel mitogen-activated protein kinase phosphatase, functions as a shuttle protein. J. Biol. Chem. 2001, 276, 39002–39011. [Google Scholar] [CrossRef]
- Kumar, G.S.; Clarkson, M.W.; Kunze, M.B.A.; Granata, D.; Wand, A.J.; Lindorff-Larsen, K.; Page, R.; Peti, W. Dynamic activation and regulation of the mitogen-activated protein kinase p38. Proc. Natl. Acad. Sci. USA 2018, 115, 4655–4660. [Google Scholar] [CrossRef]
- Cuenda, A.; Rousseau, S. p38 MAP-Kinases pathway regulation, function and role in human disease. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; McGowan, C.H.; He, L.; Downey, J.S.; Fearns, C.; Wang, Y.; Huang, S.; Han, J. Involvement of the MKK6-p38gamma cascade in gamma-radiation-induced cell cycle arrest. Mol. Cell Biol. 2000, 20, 4543–4552. [Google Scholar] [CrossRef]
- Zhu, K.Q.; Zhang, S.J. Involvement of ATM/ATR-p38 MAPK cascade in MNNG induced G1-S arrest. World J. Gastroenterol. 2003, 9, 2073–2077. [Google Scholar] [CrossRef]
- Tang, J.; Qi, X.; Mercola, D.; Han, J.; Chen, G. Essential role of p38gamma in K-Ras transformation independent of phosphorylation. J. Biol. Chem. 2005, 280, 23910–23917. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.L.; Wu, Z.; Zhang, P.; Wood, L.D.; Bhakta, K.S.; Han, J.; Feramisco, J.R.; Karin, M.; Wang, J.Y. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000, 14, 574–584. [Google Scholar] [PubMed]
- Campbell, R.M.; Anderson, B.D.; Brooks, N.A.; Brooks, H.B.; Chan, E.M.; De Dios, A.; Gilmour, R.; Graff, J.R.; Jambrina, E.; Mader, M.; et al. Characterization of LY2228820 dimesylate, a potent and selective inhibitor of p38 MAPK with antitumor activity. Mol. Cancer 2014, 13, 364–374. [Google Scholar] [CrossRef]
- Maimon, A.; Mogilevsky, M.; Shilo, A.; Golan-Gerstl, R.; Obiedat, A.; Ben-Hur, V.; Lebenthal-Loinger, I.; Stein, I.; Reich, R.; Beenstock, J.; et al. Mnk2 alternative splicing modulates the p38-MAPK pathway and impacts Ras-induced transformation. Cell Rep. 2014, 7, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Azijli, K.; Yuvaraj, S.; van Roosmalen, I.; Flach, K.; Giovannetti, E.; Peters, G.J.; de Jong, S.; Kruyt, F.A. MAPK p38 and JNK have opposing activities on TRAIL-induced apoptosis activation in NSCLC H460 cells that involves RIP1 and caspase-8 and is mediated by Mcl-1. Apoptosis 2013, 18, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Rozengurt, E. PKD, PKD2, and p38 MAPK mediate Hsp27 Serine-82 phosphorylation induced by neurotensin in pancreatic cancer PANC-1 cells. J. Cell Biochem. 2008, 103, 648–662. [Google Scholar] [CrossRef]
- Cánovas, B.; Igea, A.; Sartori, A.A.; Gomis, R.R.; Paull, T.T.; Isoda, M.; Pérez-Montoyo, H.; Serra, V.; González-Suárez, E.; Stracker, T.H.; et al. Targeting p38alpha increases DNA damage, chromosome instability, and the anti-tumoral response to taxanes in breast cancer cells. Cancer Cell 2018, 33, 1094–1110 e1098. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Ala-aho, R.; Jokilehto, T.; Peltonen, J.; Kallajoki, M.; Grenman, R.; Jaakkola, P.; Westermarck, J.; Kähäri, V.-M. p38α and p38δ mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene 2007, 26, 5267–5279. [Google Scholar] [CrossRef] [PubMed]
- Ringshausen, I.; Dechow, T.; Schneller, F.; Weick, K.; Oelsner, M.; Peschel, C.; Decker, T. Constitutive activation of the MAPkinase p38 is critical for MMP-9 production and survival of B-CLL cells on bone marrow stromal cells. Leukemia 2004, 18, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Jiang, B.H.; Leonard, S.S.; Corum, L.; Zhang, Z.; Roberts, J.R.; Antonini, J.; Zheng, J.Z.; Flynn, D.C.; Castranova, V.; et al. p38 signaling-mediated hypoxia-inducible factor 1alpha and vascular endothelial growth factor induction by Cr(VI) in DU145 human prostate carcinoma cells. J. Biol. Chem. 2002, 277, 45041–45048. [Google Scholar] [CrossRef]
- Kim, E.S.; Sohn, Y.W.; Moon, A. TGF-beta-induced transcriptional activation of MMP-2 is mediated by activating transcription factor (ATF)2 in human breast epithelial cells. Cancer Lett. 2007, 252, 147–156. [Google Scholar] [CrossRef]
- Hong, I.K.; Kim, Y.M.; Jeoung, D.I.; Kim, K.C.; Lee, H. Tetraspanin CD9 induces MMP-2 expression by activating p38 MAPK, JNK and c-Jun pathways in human melanoma cells. Exp. Mol. Med. 2005, 37, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Villa-Diaz, L.G.; Miyano, T. Activation of p38 MAPK during porcine oocyte maturation. Biol. Reprod. 2004, 71, 691–696. [Google Scholar] [CrossRef]
- Lu, H.T.; Yang, D.D.; Wysk, M.; Gatti, E.; Mellman, I.; Davis, R.J.; Flavell, R.A. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999, 18, 1845–1857. [Google Scholar] [CrossRef]
- Tanaka, N.; Kamanaka, M.; Enslen, H.; Dong, C.; Wysk, M.; Davis, R.J.; Flavelle, R.A. Differential involvement of p38 mitogen-activated protein kinase kinases MKK3 and MKK6 in T-cell apoptosis. EMBO Rep. 2002, 3, 785–791. [Google Scholar] [CrossRef]
- Menon, M.B.; Gaestel, M. TPL2 meets p38MAPK: Emergence of a novel positive feedback loop in inflammation. Biochem. J. 2016, 473, 2995–2999. [Google Scholar] [CrossRef]
- Xu, D.; Matsumoto, M.L.; MacKenzie, B.S.; Zarrin, A.A. TPL2 kinase action and control of inflammation. Pharm. Res. 2018, 129, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, L.; Li, B.; Yang, J.; Huen, M.S.Y.; Pan, X.; Tsao, S.W.; Cheung, A.L.M. F-box only protein 31 (FBXO31) negatively regulates p38 mitogen-activated protein kinase (MAPK) signaling by mediating lysine 48-linked ubiquitination and degradation of mitogen-activated protein kinase kinase 6 (MKK6). J. Biol. Chem. 2014, 289, 21508–21518. [Google Scholar] [CrossRef] [PubMed]
- Tanno, M.; Bassi, R. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: Evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ. Res. 2003, 93, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Amantini, C.; Mosca, M.; Nabissi, M.; Lucciarini, R.; Caprodossi, S.; Arcella, A.; Giangaspero, F.; Santoni, G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem. 2007, 102, 977–990. [Google Scholar] [CrossRef]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiù, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E.; et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 2017, 8, 15292. [Google Scholar] [CrossRef]
- Surowy, C.S.; Kym, P.R.; Reilly, R.M. Biochemical Pharmacology of TRPV1: Molecular intergrator of pain signals. In Vanilloid Receptor TRPV1 in Drug Discovery: Targeting Pain and other Pathological Disorders, 2nd ed.; Gomtsyan, A., Faltynek, C.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, (published simultaneously in Canada); 2010; pp. 119–120. [Google Scholar]
- Bianco, F.; Perrotta, C.; Novellino, L.; Francolini, M.; Riganti, L.; Menna, E.; Saglietti, L.; Schuchman, E.H.; Furlan, R.; Clementi, E.; et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009, 28, 1043–1054. [Google Scholar] [CrossRef]
- Suter, M.R.; Berta, T.; Gao, Y.J.; Decosterd, I.; Ji, R.R. Large A-Fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: Different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol. Pain 2009, 5, 53. [Google Scholar] [CrossRef]
- Liao, H.Y.; Hsieh, C.L.; Huang, C.P. Electroacupuncture Attenuates CFA-induced Inflammatory Pain by suppressing Nav1.8 through S100B, TRPV1, Opioid, and Adenosine Pathways in Mice. Sci. Rep. 2017, 13, 42531. [Google Scholar] [CrossRef]
- Zhou, T.T.; Wu, J.R.; Chen, Z.Y.; Liu, Z.X.; Miao, B. Effects of dexmedetomidine on P2X4Rs, p38-MAPK and BDNF in spinal microglia in rats with spared nerve injury. Brain Res. 2014, 1568, 21–30. [Google Scholar] [CrossRef]
- Keller, A.F.; Beggs, S.; Salter, M.W.; De Koninck, Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol. Pain. 2007, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Deng, G.; Huang, H. The activation of BDNF reduced inflammation in a spinal cord injury model by TrkB/p38 MAPK signaling. Exp. Med. 2019, 17, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Lin, J.G.; Su, S.Y.; Tang, N.Y.; Kao, S.T.; Hsieh, C.L. Electroacupuncture-like stimulation at Baihui and Dazhui acupoints exerts neuroprotective effects through activation of the brain-derived neurotrophic factor-mediated MEK1/2/ERK1/2/p90RSK/bad signaling pathway in mild transient focal cerebral ischemia in rats. BMC Complement Altern. Med. 2014, 14, 92. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, L.F.; Poo, M.M. Neurites grow faster towards the cathode than the anode in a steady field. J. Exp. Zool. 1979, 209, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Poo, M.M. Orientation of neurite growth by extracellular electric fields. J. Neurosci. 1982, 2, 483–496. [Google Scholar] [CrossRef]
- Kerns, J.M.; Fakhouri, A.J.; Weinrib, H.P.; Freeman, J.A. Electrical stimulation of nerve regeneration in the rat: The early effects evaluated by a vibrating probe and electron microscopy. Neuroscience 1991, 40, 93–107. [Google Scholar] [CrossRef]
- Kerns, J.M.; Lucchinetti, C. Electrical field effects on crushed nerve regeneration. Exp. Neurol. 1991, 117, 71–80. [Google Scholar] [CrossRef]
- Todorov, A.T.; Yogev, D.; Qi, P.; Fendler, J.H.; Rodziewicz, G.S. Electric-field-induced reconnection of severed axons. Brain. Res. 1992, 582, 329–334. [Google Scholar] [CrossRef]
- Pomeranz, B.; Campbell, J.J. Weak electric current accelerates motoneuron regeneration in the sciatic nerve of ten-month-old rats. Brain. Res. 1993, 603, 271–278. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hu, C.L.; Hsieh, C.L.; Lin, J.G.; Tsai, C.C.; Chen, T.H.; Yao, C.H. Effects of percutaneous electrical stimulation on peripheral nerve regeneration using silicone rubber chambers. J. Biomed. Mater. Res. 2001, 57, 541–549. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hsieh, C.L.; Tsai, C.C.; Chen, T.H.; Cheng, W.C.; Hu, C.L.; Yao, C.H. Increased success of peripheral nerve regeneration using silicone rubber chambers filled with an extracellular gel containing collagen, laminin and fibronectin. Biomaterials 2000, 21, 1541–1547. [Google Scholar] [CrossRef]
- Greer, R.; Daniel, J.; Uemura, E.; Kudej, R.; Chen, Y.S.; Chung, C.H. Use of a multiple lumen cuff for nerve regeneration. Mat. Res. Soc. Symp. Proc. 1994, 331, 3–11. [Google Scholar] [CrossRef]
- Chen, Y.S.; Miller, C.; Greer, M.H.; Quinones, M.; Greer, R.T. Development of a multiple-lumen nerve cuff utilizing growth stimulant patterns for controlled regeneration. Mat. Res. Soc. Symp. Proc. 1999, 550, 303–312. [Google Scholar] [CrossRef]
- Zhang, R.; Lao, L.; Ren, K.; Berman, B.M. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 2014, 120, 482–503. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.T.; Silva, M.L.; Prado, W.A. Analgesia induced by 2- or 100-Hz electroacupuncture in the rat tail-flick test depends on the activation of different descending pain inhibitory mechanisms. J. Pain 2011, 12, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, F.; Li, X.; Yang, Q.; Li, X.; Xu, N.; Huang, Y.; Zhang, Q.; Gou, X.; Chen, S.; et al. Electroacupuncture pretreatment attenuates cerebral ischemic injury through α7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats. J. Neuroinflammation 2012, 9, 24. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lin, J.G.; Tang, N.Y.; Kao, S.T.; Hsieh, C.L. Electroacupuncture-like stimulation at the Baihui (GV20) and Dazhui (GV14) acupoints protects rats against subacute-phase cerebral ischemia-reperfusion injuries by reducing S100B-mediated neurotoxicity. PLoS ONE 2014, 9, e91426. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, M.Y.; Hsieh, C.L.; Wu, S.Y.; Hsu, H.C.; Lin, Y.W. TRPV1 is a responding channel for acupuncture manipulation in mice peripheral and central nerve system. Cell. Physiol. Biochem. 2018, 49, 1813–1824. [Google Scholar] [CrossRef]
- Ashwell, J. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat. Rev. Immunol. 2006, 6, 532–540. [Google Scholar] [CrossRef]
- Zhang, C.N.; Rahimnejad, S. Molecular characterization of p38 MAPK from blunt snout bream (Megalobrama amblycephala) and its expression after ammonia stress, and lipoplysaccaride and bacterial challenge. Fish Shellfish Immunol. 2019, 84, 848–856. [Google Scholar] [CrossRef]
- Sreekanth, G.P.; Chuncharunee, A. SB203580 modulates p38 MAPK signaling and Dengue virus-induced liver injury by reducing MAPKAPK2, HSP27, and ATF2 phosporylation. PLoS ONE 2016, 11, e0149486. [Google Scholar] [CrossRef] [PubMed]
- Beardmore, V.A.; Hinton, H.J. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol. Cell Biol. 2005, 25, 10454–10464. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ji, R. Intracellular Signaling in Primary Sensory Neurons and Persistent Pain. Neurochem. Res. 2008, 33, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, K.; Sasakia, M.; Izumia, Y.; Miuraa, M.; Watanabe, M.; Amaya, F. Activation of p38 mitogen-activated protein kinase in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision. Neuroscience 2013, 234, 77–87. [Google Scholar] [CrossRef]
- Tsuda, M. Microglia in the spinal cord and neuropathic pain. J. Diabetes Investig. 2016, 7, 17–26. [Google Scholar] [CrossRef]
- Suzuki, N.; Hasegawa-Moriyama, M. Lidocaine attenuates the development of diabetic-induced tactile allodynia by inhibiting microglial activation. Anesth. Analg. 2011, 113, 941–946. [Google Scholar] [CrossRef]
- Cheng, K.I.; Wang, H.C. Persistent mechanical allodynia positively correlates with an increase in activated microglia and increased P-p38 mitogen-activated protein kinase activation in streptozotocin-induced diabetic rats. Eur. J. Pain 2014, 18, 162–173. [Google Scholar] [CrossRef]
- Hsu, H.C.; Yang, N.Y.; Lin, Y.W.; Ki, T.C.; Liu, H.J.; Hsieh, C.L. Effect of electroacupuncture on rats with chronic constriction injury-Induced neuropathic pain. Sci. World J. 2014, 2014, 129875. [Google Scholar] [CrossRef]
- Jiang, S.W.; Lin, Y.W.; Hsieh, C.L. Electroacupuncture at Hua Tuo Jia Ji acupoints reduced neuropathic pain and increased GABAA receptors in rat spinal cord. Evid. Based Complement Altern. Med. 2018, 2018, 8041820. [Google Scholar] [CrossRef]
- Huang, C.P.; Lin, Y.W.; Lee, D.Y.; Hsieh, C.L. Electroacupuncture relieves CCI-induced neuropathic pain involving excitatory and inhibitory neurotransmitters. Evid. Based Complement Altern. Med. 2019, 2019, 6784735. [Google Scholar] [CrossRef]
- Lin, J.G.; Lo, M.W.; Wen, Y.R.; Hsieh, C.L.; Tsai, S.K.; Sun, W.Z. The effect of high and low frequency electroacupuncture in pain after lower abdominal surgery. Pain 2002, 99, 509–514. [Google Scholar] [CrossRef]
- Wang, B.; Tang, J.; White, P.F.; Naruse, R.; Sloninsky, A.; Kariger, R.; Gold, J.; Wender, R.H. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesth. Analg. 1997, 85, 406–413. [Google Scholar] [PubMed]
- Chen, W.H.; Hsieh, C.L.; Huang, C.P.; Lin, T.J.; Tzen, J.T.C.; Ho, T.Y.; Lin, Y.W. Acid-sensing ion channel 3 mediates peripheral anti-hyperalgesia effects of acupuncture in mice inflammatory pain. J. Biomed. Sci. 2011, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Tzen, J.T.C.; Hsieh, C.L.; Chen, Y.H.; Lin, T.J.; Chen, S.Y.; Lin, Y.W. Attenuation of TRPV1 and TRPV4 expression and function in mouse inflammatory pain models using electroacupuncture. J. Biomed. Sci. 2012, 2012. [Google Scholar] [CrossRef]
- Huang, C.P.; Chen, H.N.; Su, H.L.; Hsieh, C.L.; Chen, W.H.; Lai, Z.R.; Lin, Y.W. Electroacupuncture reduces carrageenan- and CFA-induced inflammatory pain accompanied by changing the expression of Nav1.7 and Nav1.8, rather than Nav1.9, in mice dorsal root ganglia. Evid. Based Complement Altern. Med. 2013, 2013, 312184. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chen, W.H.; Hsueh, C.H.; Lin, Y.W. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: Mechanosensitive TRPV1 as an acupuncture-responding channel. BMC Complement Altern. Med. 2014, 14, 96. [Google Scholar] [CrossRef]
- Lu, K.W.; Hsu, C.K.; Hsieh, C.L.; Yang, J.; Lin, Y.W. Probing the effects and mechanisms of electroacupuncture at ipsilateral or contralateral ST36-ST37 acupoints on CFA-induced inflammatory pain. Sci. Rep. 2016, 24, 22123. [Google Scholar] [CrossRef]
- Liao, H.Y.; Hsieh, C.L.; Huang, C.P.; Lin, Y.W. Electroacupuncture attenuates induction of inflammatory pain by regulating opioid and adenosine pathways in mice. Sci. Rep. 2017, 7, 15679. [Google Scholar] [CrossRef]
- Yang, J.; Hsieh, C.L.; Lin, Y.W. Role of transient receptor potential vanilloid 1 in electroacupuncture analgesia on chronic inflammatory pain in mice. Biomed Res. Int. 2017, 2017, 5068347. [Google Scholar] [CrossRef]
- Yen, C.M.; Wu, T.C.; Hsieh, C.L.; Huang, Y.W.; Lin, Y.W. Distal electroacupuncture at the LI4 acupoint reduces CFA-induced inflammatory pain via the brain TRPV1 signaling pathway. Int. J. Mol. Sci. 2019, 20, 4471. [Google Scholar] [CrossRef]
- Hsu, H.C.; Hsieh, C.L.; Wu, S.Y.; Lin, Y.W. Toll-like receptor 2 plays an essential role in electroacupuncture analgesia in a mouse model of inflammatory pain. Acupunct. Med. 2019, 37, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Hsieh, C.L.; Wang, N.H.; Li, T.C.; Hwang, K.L.; Yu, S.C.; Chang, M.H. Acupuncture in patients with carpal tunnel syndrome: A randomized controlled trial. Clin. J. Pain. 2009, 25, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Wang, N.H.; Li, T.C.; Hsieh, C.L.; Chang, H.H.; Hwang, K.L.; Ko, W.S.; Chang, M.H. A randomized clinical trial of acupuncture versus oral steroids for carpal tunnel syndrome: A long-term follow-up. J. Pain 2011, 12, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Chang, M.H.; Liu, P.E.; Li, T.C.; Hsieh, C.L.; Hwang, K.L.; Chang, H.H. Acupuncture versus topiramate in chronic migraine prophylaxis: A randomized clinical trial. Cephalalgia 2011, 31, 1510–1521. [Google Scholar] [CrossRef]
- Walder, R.Y.; Rasmussen, L.A.; Rainier, J.D.; Light, A.R.; Wemmie, J.A.; Sluka, K.A. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J. Pain 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Black, J.A.; Liu, S.; Tanaka, M.; Cummins, T.R.; Waxman, S.G. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 2004, 108, 237–247. [Google Scholar] [CrossRef]
- Yu, L.; Yang, F.; Luo, H.; Liu, F.Y.; Han, J.S.; Xing, G.G.; Wan, Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol. Pain 2008, 4, 61. [Google Scholar] [CrossRef]
- Goldman, N.; Chen, M.; Fujita, T.; Xu, Q.; Peng, W.; Liu, W.; Jensen, T.; Pei, Y.; Wang, F.; Han, X.; et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat. Neurosci. 2010, 13, 883–888. [Google Scholar] [CrossRef]
- Silberstein, S.D. Migraine pathophysiology and its clinical implications. Cephalalgia 2004, 24 (Suppl. 2), 2–7. [Google Scholar] [CrossRef]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef]
- Sabino, G.S.; Santos, C.M.; Francischi, J.N.; de Resende, M.A. Release of endogenous opioids following transcutaneous electric nerve stimulation in an experimental model of acute inflammatory pain. J. Pain 2008, 9, 157–163. [Google Scholar] [CrossRef] [PubMed]
- King, E.W.; Audette, K.; Athman, G.A.; Nguyen, H.O.; Sluka, K.A.; Fairbanks, C.A. Transcutaneous electrical nerve stimulation activates peripherally located alpha-2A adrenergic receptors. Pain 2005, 115, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, L.; Budelier, K.; Clinesmith, M.; Fiedler, A.; Landstrom, R.; Leeper, B.J.; Moeller, L.; Mutch, S.; O’Dell., K.; Ross, J.; et al. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain 2006, 120, 182–187. [Google Scholar] [CrossRef]
- Sekido, R.; Ishimaru, K.; Sakita, M. Differences of electroacupuncture-induced analgesic effect in normal and inflammatory conditions in rats. Am. J. Chin. Med. 2003, 31, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, M.; Kirkwood, K.L. p38alpha stabilizes interleukin-6 mRNA via multiple AU-rich elements. J. Biol. Chem. 2008, 283, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Tudor, C.; Marchese, F.P. p38 mitogen-activated protein kinase inhibits tristetraprolin-directed decay of the mRNA of the anti-inflammatory cytokine interleukin-10. FEBS Lett. 2009, 583, 1933–1938. [Google Scholar] [CrossRef]
- Berger, C.; von Kummer, R. Does NO regulate the cerebral blood flow response in hypoxia? Acta Neurol. Scand. 2009, 97, 118–125. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lin, J.G.; Tang, N.Y.; Kao, S.T.; Hsieh, C.L. Electroacupuncture at different frequencies (5Hz and 25Hz) ameliorates cerebral ischemia-reperfusion injury in rats: Possible involvement of p38 MAPK-mediated anti-apoptotic signaling pathways. BMC Complement Altern. Med. 2015, 15, 241. [Google Scholar] [CrossRef]
- Armstrong, S.C.; Delacey, M.; Ganote, C.E. Phosphorylation state of hsp27 and p38 MAPK during preconditioning and protein phosphatase inhibitor protection of rabbit cardiomyocytes. J. Mol. Cell Cardiol. 1999, 31, 555–567. [Google Scholar] [CrossRef]
- Marais, E.; Genade, S.; Salie, R.; Huisamen, B.; Maritz, S.; Moolman, J.A.; Lochner, A. The temporal relationship between p38 MAPK and HSP 27 activation in ischaemic and pharmacological preconditioning. Basic Res. Cardiol. 2005, 100, 35–47. [Google Scholar] [CrossRef]
- Fan, W.; Gao, X.K. Hsp70 interacts with mitogen-activated protein kinase (MAPK)-activated protein kinase 2 to regulate p38MAPK stability and myoblast differentiation during skeletal muscle regeneration. Mol. Cell Biol. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.Y.; Lin, Y.W.; Hsieh, C.L. Acupuncture and neuroregeneration in ischemic stroke. Neural Regen. Res. 2018, 13, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sun, H.; Chen, S.H.; Zhang, Y.M.; Piao, Y.L.; Gao, Y. Effects of acupuncture at Baihui (DU20) and Zusanli (ST36) on the expression of heat shock protein 70 and tumor necrosis factor α in the peripheral serum of cerebral ischemia-reperfusion-injured rats. Chin. J. Integr. Med. 2014, 20, 369–374. [Google Scholar] [CrossRef]
- Dayalan Naidu, S.; Sutherland, C.; Zhang, Y.; Risco, A.; de la Vega, L.; Caunt, C.J.; Hastie, C.J.; Lamont, D.J.; Torrente, L.; Chowdhry, S.; et al. Heat Shock Factor 1 is a substrate for p38 mitogen-activated protein kinase. J. Mol. Cell Biol. 2016, 36, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Qiao, A.; Moskophidis, D.; Mivechi, N.F. Modulation of Heat Shock Factor 1 Activity through Silencing of Ser303/Ser307 Phosphorylation Supports a Metabolic Program Leading to Age-Related Obesity and Insulin Resistance. Mol. Cell Biol. 2018, 38, e00095-18. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Wang, X.S. MAPKAPK5, a novel mitogen-activated protein kinase (MAPK)-activated protein kinase, is a substrate of the extracellular-regulated kinase (ERK) and p38 kinase. Biochem. Biophys. Res. Comm. 1998, 243, 492–496. [Google Scholar] [CrossRef]
- Sahadevan, P.; Allen, B.G. MK5: A novel regulator of cardiac fibroblast function? Iubmb Life 2017, 69, 785–794. [Google Scholar] [CrossRef]
- Shiryaev, A.; Moens, U. Mitogen-activated protein kinase p38 and MK2, MK3 and MK5: Ménage à trois or ménage à quatre. Cell Signal. 2010, 22, 1185–1192. [Google Scholar] [CrossRef]
- Liu, C.H.; Lin, Y.W.; Tang, N.Y.; Liu, H.J.; Hsieh, C.L. Neuroprotective Effect of Uncaria rhynchophylla in kainic acid-induced epileptic seizures by modulating hippocampal mossy fiber sprouting, neuron survival, astrocyte proliferation, and S100B expression. Evid. Based Complement Altern. Med. 2012, 2012, 194790. [Google Scholar] [CrossRef]
- Kim, S.T.; Jeon, S.; Park, H.J.; Hong, M.S.; Jeong, W.B.; Kim, J.H.; Kim, Y.; Lee, H.J.; Park, H.J.; Chung, J.H. Acupuncture inhibits kainic acid-induced hippocampal cell death in mice. J. Physiol. Sci. 2008, 58, 31–38. [Google Scholar] [CrossRef]
- Kim, S.T.; Doo, A.R.; Kim, S.N.; Kim, S.Y.; Kim, Y.Y.; Kim, J.H.; Lee, H.; Yin, C.S.; Park, H.J. Acupuncture suppresses kainic acid-induced neuronal death and inflammatory events in mouse hippocampus. J. Physiol. Sci. 2012, 62, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.H.; Kim, D.S.; Jun, Y.L.; Kwon, S.; Park, H.J.; Hahm, D.H.; Lee, H.; Kim, S.T. Proteomic analysis of the effect of acupuncture on the suppression of kainic acid-induced neuronal destruction in mouse hippocampus. Evid. Based Complement Altern. Med. 2013, 2013, 436315. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Lin, Y.W.; Hsu, H.C.; Liu, H.J.; Lin, W.J.; Hsieh, C.L. Electroacupuncture at ST36-ST37 and at ear ameliorates hippocampal mossy fiber sprouting in kainic acid-induced epileptic seizure rats. Biomed Res. Int. 2014, 2014, 756019. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Tang, N.Y.; Liu, C.H.; Hsieh, C.L. Antiepileptic Effect of Uncaria rhynchophylla and Rhynchophylline involved in the initiation of c-Jun N-terminal kinase phosphorylation of MAPK signal pathways in acute seizures of kainic acid-treated rats. Evid. Based Complement Altern. Med. 2013, 2013, 961289. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Hsieh, C.L. Auricular electroacupuncture reduced inflammation-related epilepsy accompanied by altered TRPA1, pPKCα, pPKCε, and pERk1/2 signaling pathways in kainic acid-treated rats. Mediat. Inflamm. 2014, 2014, 493480. [Google Scholar] [CrossRef]
- Liao, E.T.; Tang, N.Y.; Lin, Y.W.; Hsieh, C.L. Long-term electrical stimulation at ear and electro-acupuncture at ST36-ST37 attenuated COX-2 in the CA1 of hippocampus in kainic acid-induced epileptic seizure rats. Sci. Rep. 2017, 7, 472. [Google Scholar] [CrossRef]
- Inprasit, C.; Lin, Y.W.; Huang, C.P.; Wu, S.Y.; Hsieh, C.L. Targeting TRPV1 to relieve motion sickness symptoms in mice by electroacupuncture and gene deletion. Sci. Rep. 2018, 8, 10365. [Google Scholar] [CrossRef] [PubMed]
- Maione, S.; Cristino, L.; Migliozzi, A.; Georgiou, A.; Starowicz, K.; Salt, T.; Marzo, V. TRPV1 channels control synaptic plasticity in the developing superior colliculus. J. Physiol. 2009, 587, 2521–2535. [Google Scholar] [CrossRef]
- Puig, B.; Gomez-Isla, T. Expression of stress-activated kinases c-Jun N-terminal kinase (SAPK/JNK-P) and p38 kinase (p38-P), and tau hyperphosphorylation in neurites surrounding betaA plaques in APP Tg2576 mice. Neuropathol. Appl. Neurobiol. 2004, 30, 491–502. [Google Scholar] [CrossRef]
- Risco, A.; Cuenda, A. New Insights into the p38γ and p38δ MAPK Pathways. J. Signal Transduct. 2012, 2012, 520289. [Google Scholar] [CrossRef]
- Escós, A.; Risco, A. p38γ and p38δ Mitogen Activated Protein Kinases (MAPKs), New Stars in the MAPK Galaxy. Front. Cell Dev. Biol. 2016, 4, 31. [Google Scholar] [CrossRef]
- Maphis, N.; Jiang, S. Selective suppression of the alpha isoform of p38 MAPK rescues late-stage tau pathology. Alzheimers Res. 2016, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Hsieh, C.L. Electroacupuncture at Baihui acupoint (GV20) reverses behavior deficit and long-term potentiation through N-methyl-d-aspartate and transient receptor potential vanilloid subtype 1 receptors in middle cerebral artery occlusion rats. J. Integr. Neurosci. 2010, 9, 269–282. [Google Scholar] [CrossRef]
- Dong, W.; Guo, W.; Zheng, X.; Wang, F.; Chen, Y.; Zhang, W.; Shi, H. Electroacupuncture improves cognitive deficits associated with AMPK activation in SAMP8 mice. Metab. Brain Dis. 2015, 30, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Lee, Y.J. Role of the ASK1-SEK1-JNK1- HIPK1 signal in Daxx trafficking and ASK1 oligomerization. J. Biol. Chem. 2003, 278, 47245–47252. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Nishitoh, H.; Yang, X.; Ichijo, H.; Baltimore, D. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 1988, 281, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Akterin, S.; Cowburn, R.F.; Miranda-Vizuete, A.; Jimenez, A.; Bogdanovic, N.; Winblad, B.; Cedazo-Minguez, A. Involvement of glutaredoxin-1 and thioredoxin-1 in amyloid toxicity and Alzheimer’s disease. Cell Death Differ. 2006, 13, 1454–1465. [Google Scholar] [CrossRef]
- Takahashi-Niki, K.; Niki, T.; Taira, T.; Iguchi-Ariga, S.M.M.; Ariga, H. Reduced anti-oxidative stress activities of DJ-1 mutants found in Parkinson’s disease patients. Biochem. Biophys. Res. Commun. 2004, 320, 389–397. [Google Scholar] [CrossRef]
- Junn, E.; Taniguchi, H.; Jeong, B.S.; Zhao, X.; Ichijo, H.; Mouradian, M.M. Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc. Natl. Acad. Sci. USA 2005, 102, 9691–9696. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Li, X.; Zhong, Y.; Zhong, T.; Liu, Y.; Wang, J.H.; Jiang, Y. PRAK Interacts with DJ-1 and prevents oxidative stress-induced cell death. Oxid. Med. Cell. Longev. 2014, 735618. [Google Scholar] [CrossRef]
- Karunakaran, S.; Diwakar, L. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: Protection by alpha-lipoic acid. FASEB J. 2007, 21, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.W.; Yang, J.; Hsieh, C.L.; Hsu, Y.C.; Lin, Y.W. Electroacupuncture restores spatial learning and downregulates phosphorylated N-methyl-D-aspartate receptors in a mouse model of Parkinson’s disease. Acupunct. Med. 2017, 35, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.G.; Hsieh, C.L.; Lin, Y.W. Analgesic effect of electroacupuncture in a mouse fibromyalgia model: Roles of TRPV1, TRPV4, and pERK. PLoS ONE 2015, 10, e0128037. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.W.; Hsieh, C.L.; Yang, J.; Lin, Y.W. Effects of electroacupuncture in a mouse model of fibromyalgia: Role of N-methyl-D-aspartate receptors and related mechanisms. Acupunct. Med. 2017, 35, 59–68. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).