Abstract
The great majority of breast and prostate tumors are hormone-dependent cancers; hence, estrogens and androgens can, respectively, drive their developments, making it possible to use pharmacological therapies in their hormone-dependent phases by targeting the levels of steroid or modulating their physiological activity through their respective nuclear receptors when the tumors relapse. Unfortunately, at some stage, both breast and prostate cancers become resistant to pharmacological treatments that aim to block their receptors, estrogen (ER) or androgen (AR) receptors, respectively. So far, antiestrogens and antiandrogens used in clinics have been designed based on their structural analogies with natural hormones, 17-β estradiol and dihydrotestosterone. Plants are a potential source of drug discovery and the development of new pharmacological compounds. The aim of this review article is to highlight the recent advances in the pharmacological modulation of androgen or estrogen levels, and their activity through their cognate nuclear receptors in prostate or breast cancer and the effects of some plants extracts.
1. Introduction
The second leading cause of mortality worldwide [1], cancer is a complex situation. This is based in part on the extreme heterogeneity of the genetic causes, the levels of the secreted growth factors and circulating hormones, and the interactions between the tumor cells and the surrounding microenvironment [2]. Extensive research over the past 25 years in breast (BCa) and prostate (PCa) cancers have deciphered the molecular mechanisms driven by steroid receptors and elucidated the interplay between genomic and non-genomic activities of these steroid receptors. Altogether, these mechanisms pilot specific gene expression programs that contribute to the tissue homeostasis and the disequilibrium in such a subtle balance that could induce endocrine therapy resistance and cancer progression [3]. The way normal cells transform into cancer cells and how they maintain their malignant state and phenotypes have been associated with genetic and epigenetic deregulations [4]. Interestingly, these alterations are constantly evolving as tumor cells face changing selective pressures such as drug treatments. This is why the discovery and development of new therapeutics is a real challenge for bypassing tumor escapes.
Historically, plants, animals, fungi, and microorganisms have been extensively used as a source of biologically active compounds. Hence, despite the development of chemistry and the introduction of synthetic chemotherapeutics, a substantial part of new pharmaceuticals still remains of a natural origin [5]. Indeed, natural compounds exhibit great diversity in the chemical structures and, thus can act on diverse mechanisms of action through different molecular targets, such as modulating the levels of circulating hormones, blocking or activating nuclear or membrane receptors, or targeting nucleic acids as described for antimicrobial compounds [5]. Globally, evolution has provided numerous suitable candidates for anti-cancer drug discovery due to their pleiotropic activity on target molecules [6]. Among the various types of tumors, hormone-dependent cancers are an exquisite example of what could be done in order to regulate the activity of hormones.
Enzymatically derived from cholesterol (Figure 1), sex steroids are mainly synthetized in the gonads, even though adrenal glands could also produce non-active precursors that may eventually be transformed into active androgens or estrogens [7]. Basically, testosterone is transformed into 5α-dihydrotestosterone (DHT) by the 5α-reductase before acting through AR on prostate growth, while aromatase (present in breast and adipose tissues) acts on testosterone to produce 17β-estradiol (17βE2) acting through ERα. It was also noted that ∆4-androstenedione could be considered as an important adrenal precursor as it could be transformed into testosterone or estrone by 17β hydroxysteroid dehydrogenase (17βHSD).
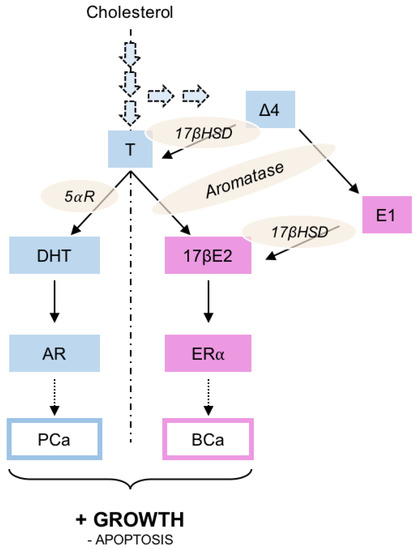
Figure 1.
Schematic representation of the steroid activity on prostate (PCa) or breast (BCa) cancer. Three various levels of control could be pointed out: i) steroid synthesis by inhibiting the enzymatic pathways leading to testosterone or 17β-estradiol production; ii) modulation of the androgen or estrogen receptor activity using specific antagonists; iii) modulation of the steroid receptor activity together with the induction of its degradation/down-regulation. Δ4, Δ4-androstenedion; 5⍺R, 5⍺-reductase; 17βE2, estradiol; 17βHSD, 17β-hydroxy-steroid dehydrogenase; AR, androgen receptor; BCa, breast cancer; E1, estrone; ER⍺, estrogen receptor ⍺; PCa, prostate cancer; T, testosterone.
Schematically, several pathways could be pharmacologically targeted to suppress, or at least reduce, the effects of the steroid hormones in BCa and PCa: (i) the modulation of the enzymes synthetizing testosterone, 17βE2 and DHT namely 17βHSD, aromatase, and 5α-reductase; (ii) specifically preventing the binding of 17βE2 or DHT to their cognate nuclear receptor, respectively, estrogen (ER; NR3A1/2) and androgen (AR; NR3CA) receptors, by the use of specific estrogen (SERMs) or specific androgen (SARMs) receptor modulators; (iii) the specific degradation of ER or AR by antagonists blocking the effect of the natural hormones as well as decreasing the quantity of the receptors, like specific ER degraders or down-regulators (SERDs) or specific AR degraders (SARDs) (Figure 2).
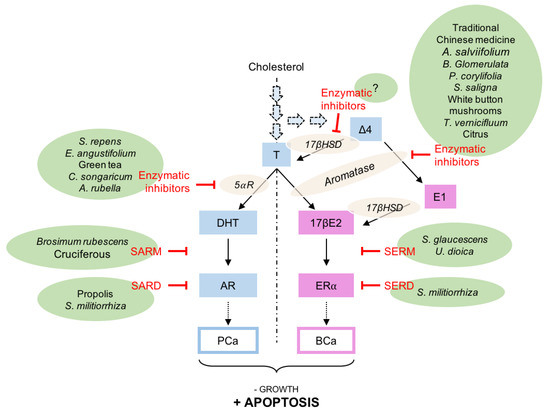
Figure 2.
Examples and sites of activity of natural extracts that could modulate androgen and estrogen receptor activities in prostate (PCa) or breast (BCa) cancer. For more information regarding the isolated molecules that show a significant activity, refer to the main text. Δ4, Δ4-androstenedione; 5⍺R, 5⍺-reductase; 17βE2, estradiol; 17βHSD, 17β-hydroxysteroid dehydrogenase; AR, androgen receptor; BCa, breast cancer; E1, estrone; ER⍺, estrogen receptor ⍺; PCa, prostate cancer; SARM, specific androgen receptor modulator; SARD, specific androgen receptor degrader/down-regulator; SERM, specific estrogen receptor modulator; SERD, specific estrogen receptor degrader/down-regulator; T, testosterone.
This review aims to focus on the pathways that could be targeted to modify the activity of steroids. Some of the natural compounds described as promising therapeutics to treat BCa or PCa will be indicated, as well as some of their pharmacological activity as modulators of AR and ERα levels and modifiers of their hormonal response.
2. PCa and BCa, Two Hormone-Dependent Cancers
Prostate cancer (PCa) has a long natural history from the diagnosis to the death caused by cancer progression [8]. While androgens have been described to be necessary for the development and maintenance of the prostate gland; they are also responsible for the development of the tumor [9]. Schematically, a prostate tumor is composed of multiple epithelial cell types, inter-mingled with various fibroblasts, neuroendocrine cells, endothelial cells, macrophages and lymphocytes, all of them interacting to influence treatment responses in a patient-specific manner [10]. Androgens and their receptor (AR), thus play a key role in the development of prostate tissue by guiding cytodifferentiation and homeostasis of normal or tumor luminal epithelial cells. Various risk factors may lead to prostate carcinogenesis, including infectious agents [11], contamination by heavy metals [12], alcohol, and tobacco consumption [13]. Based on its heterogeneity, the management of the patients diagnosed with a PCa depends on the stage of the tumor, the age of diagnosis, and the expected quality of life (for an extensive review see [14]). Hence in local PCa, which represents about 80% of diagnosed PCa disease [15], the European Association of Urology guidelines propose a radical prostatectomy to patients with low to high-risk PCa since they have a life expectancy >10 years. Radiation therapy is a suitable option for low-risk PCa and should be used in combination with androgen deprivation therapy (ADT) for intermediate/high-risk localized and locally advanced PCa. In advanced and metastatic PCa (about 5% of the tumors), the median survival, even heterogeneous, is around 42 months. The first line standard approach is ADT [16]. Besides blocking androgen effects that will be developed above, treatment with gonadotropin releasing hormone (GnRH) analogs results in a significant decrease of luteinizing (LH)/follicle-stimulating (FSH) hormone secretions and then testosterone production. Together with ADT, chemotherapy may be performed as well [14]. Despite ADT, most patients experience tumor growth recovery within a median of 18 to 24 months and progress to a lethal stage called castration-resistant PCa (CRPC). The emergence of this aggressive form of PCa is diagnosed when blood PSA increases despite low serum testosterone. CRPC is followed by a further progression of the disease with the appearance of new symptoms and bone or soft tissue lesions [17].
As for prostate tropism and androgens, the growth of BCa is mainly related to in situ levels of estrogens and the stimulation of local growth factors. Genetic factors are highly important, defining host susceptibility through polymorphisms, for example, related to the enzymes that affect hormone levels, estrogen/progesterone receptors, and protein synthesis [18]. DNA methylation can mimic the effects of germline mutations in cancer predisposition genes such as breast cancer type 2 susceptibility (BRCA2) [19]. A variety of interrelated genetic, environmental, and physiological factors appears to be associated with increased risk of breast cancer, but no single factor or combination of variables presently known is sufficient to explain the etiology of the disease [20]. While androgens have been described as mutagens, estrogens could become endogenous carcinogens via the formation of catechol estrogen quinones, which react with DNA to form specific depurination estrogen-DNA adducts [21], possibly inducing cell transformation and initiation of BCa [21]. Current knowledge about the most aggressive forms of BCa points out the role of specific cells with stem properties located within the tumor and called BCa stem cells [22]. Interestingly, sex steroid receptors involved in BCa etiology and progression could promote BCa stem cell proliferation, dedifferentiation, and migration [22], even though the molecular mechanisms allowing this have not been fully deciphered so far.
Local therapy surgery remains the first step in the treatment of BCa; besides, the development of new conservative procedures has improved the patients’ quality of life as well as their life expectancy [23]. Thereafter, neoadjuvant chemotherapy or adjuvant therapy depends on the tumor characteristics such as cell growth rate, tumor grade, or lymph nodes dissemination. Based on the fact that estrogens have an important role in luminal cell growth, endocrine therapy has been historically developed at the beginning of the 70′s for blocking estrogen receptors with the use of SERMS or SERDs (for an extensive review see [24]). As for PCa, the appearance of a metastatic stage of the disease drastically decreases the life expectancy of the patients. In that situation and together with increasing survival, the aim of the therapy is to maintain the quality of life and to reduce the symptoms. Accordingly, “international guidelines” recommend endocrine therapy as the first therapeutic choice in patients with human epidermal growth factor receptor 2 (HER2)-negative luminal metastatic BCa unless a visceral crisis or another life-threatening situation requires chemotherapy [25]. These treatments, targeting the estrogen signaling pathways include SERMs, SERDs, and aromatase inhibitors [24].
3. The Activity of Androgens and Estrogens
Altogether, 5α-reductase, aromatase, and 17βHSD are theoretically the main enzymes to be targeted to decrease the levels of DHT and 17βE2 and to reduce the progression of hormone-dependent PCa and BCa.
Once synthesized, DHT and 17βE2 act through their cognate nuclear receptors AR (nuclear receptor subfamily 3, group C, member 4, NR3C4) and ERα (NR3A1), respectively. ERβ (NR3A2) is a second estrogen receptor; however, conversely to ERα whose expression increases at the early stages of BCa and acts as a tumor promoter, ERβ is reduced during carcinogenesis and cancer progression and seems to act as a tumor suppressor [26]. Altogether, these nuclear receptors display ligand-modulated transcription [27]. The binding of DHT or 17βE2 within the ligand-binding pocket of AR or ERα, respectively, induces a conformational modification of the receptor, it’s shuttling from the cytoplasm to the nucleus, and its binding to specific DNA sequences located in its target genes. This binding and the recruitment of co-activators regulate the expression of specific genes involved in breast and prostate epithelium homeostasis [28]. Hence, Nelson et al. identified a program of androgen-responsive genes in the neoplastic prostate epithelium [29] and classified them into several physiological pathways: (i) metabolism, such as those regulating the fatty acid (sterol response element-binding protein, fatty acid synthase, stearoyl-CoA desaturase) and the cholesterol (3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA)-synthase, HMG-CoA-reductase, 3-β-hydroxysterol-Δ-24 reductase) homeostasis; (ii) transport or trafficking, such as the transcript encoding the FK-506 binding-protein FKBP5 (FKBP51); (iii) cell proliferation or differentiation. AR is not the only nuclear receptor that could be involved in the progression of PCa. Hence, Gail Prins’ group has shown that early exposure to estrogens and estrogen-like compounds could also increase PCa incidence through ERα [30,31].
A parallel can again be drawn between PCa/androgens and BCa/estrogens. Approximately 70% of all BCa express ERα, progesterone receptor (PgR; NR3C3), or both, and such tumors are considered hormone receptor-positive [32]. Several genetic programs have linked estrogens to BCa (for a review, see [33]). For example, 17βE2 facilitates the G1/S phase transition by the over-accumulation of cyclin D1 and its activation or could also modulate the tumor surrounding immune cells. Not only 17βE2 can drive ERα activity; indeed, Siersbaek et al. [34] pointed out that membrane-receptors and other steroid receptors could also modulate ERα function in BCa.
Targeting androgen synthesis and the AR pathway has been and remains central to PCa pharmacology therapy [35,36]. 5α-androstane-3β, 17β-diol (3β-Adiol), synthesized from testosterone in the prostate, inhibit PCa cell proliferation, migration, and invasion, acting as an anti-proliferative/anti-metastatic agent. Hence 3β-Adiol is unable to bind AR; it exerts its protective activity by interacting with ERβ [37]. Interestingly, the combination of the phytoestrogens genistein, quercetin, and biochanin A inhibits the growth of androgen-responsive prostate cancer cells (LNCaP) as well as DU-145 and PC-3, two androgen-insensitive prostate cancer cells [38]. Subsequent mechanistic studies in PC-3 cells indicated that the action of phytoestrogens was mediated both through ER-dependent and ER-independent pathways [38]. Bicalutamide that acts as a pure antagonist in parental LNCaP cells showed agonistic effects on AR transactivation activity in LNCaP-abl cells and was not able to block the effects of androgen in these cells. The non-steroidal AR blocker hydroxyflutamide exerted stimulatory effects on AR activity in both LNCaP and LNCaP-abl cells; however, the induction of reporter gene activity by hydroxyflutamide was 2.4- to 4-fold higher in the LNCaP-abl subline [39].
Lead compound 16-(4,6-Dimethyl-1,2-dihydro-1,3,5-triazin-2-yl)-17-chloro-Δ1,3,5(10), 16-estratetraen-3-ol displayed selectivity in ERα-positive breast cancer cells. At 10 μM concentration, this heterosteroid inhibited 50% of the E2-mediated ERα activity and led to partial ERα down-regulation. Docking studies suggested that the binding mode of this molecule was within the ER pocket [40].
4. Pharmacological Treatments of BCa and PCa
Altogether and based on the previous data, the main pharmacological treatments aim to reduce the levels of active steroids by (i) inhibiting in vivo synthesis from the cholesterol, (ii) blocking AR and ERα by specific antagonists and (iii) specific degraders (Figure 2). Note that the inhibition of de novo cholesterol synthesis by statins will not be covered in this review (Table 1).
4.1. Modulation of the Enzymes Involved in 17βE2 and DHT Synthesis
Inhibition of aromatase, the enzyme responsible for converting androgens to estrogens, is therapeutically useful for the endocrine treatment of hormone-dependent BCa [41] or PCa [42]. Melatonin, at physiological concentrations, decreases aromatase activity and expression in the human breast cancer cells MCF-7 [43]. A cell-free in vitro assay confirmed that melatonin, as well as 2-methyl indole hydrazones, binds the catalytic site of aromatase [44]. The synthetic aromatase inhibitor and steroid-derivative exemestane [45] is prescribed to postmenopausal women with advanced BCa whose disease has progressed despite tamoxifen therapy [46]. Anastrozole [47], is also a potent aromatase inhibitor. Even though it does not use the same mechanistic as exemestane, anastrozole displays the same effect as adjuvant treatment for hormone receptor-positive early BCa [48]. New aromatase inhibitors based on the testosterone skeleton could decrease aromatase stability, disrupt the cell cycle progression of MCF7 cells, and induce their apoptosis through the mitochondrial pathway [49]. The same efficacy and effects were found with 7α-substituted steroid molecules [50]. In addition, the steroids 3β-hydroxyandrost-4-en-17-one, androst-4-en-17-one, 4α,5α-epoxyandrostan-17-one, and 5α-androst-2-en-17-one, obtained from modifications in the A-ring of androstenedione, inhibit aromatase, and decrease cell viability [51]. Altogether, 5α-androst-3-en-17-one and 3α, 4α-epoxy-5α-androstan-17-one are the most potent irreversible aromatase inhibitors [52].
Even though currently recommended in clinical guidelines for benign prostatic hyperplasia or for the treatment of androgenic alopecia, the potential use of 5αR inhibitors has been questioned these last years in PCa. Indeed, as indicated in Figure 2, the possible intratumoral reduction of testosterone into the potent DHT, which critically contributes to the progression of PCa and its castration-resistant stage, has driven this hypothesis. Finasteride [53] and its analog dutasteride [54] are the two 4-azasteroids that decrease the prostatic DHT concentration by 85 to 90%. Finasteride has been described to lower the risk of low-grade PCa but seems to increase the risk of high grade, and has no effect on overall survival. Based on a limited number of patients, dutasteride could be more efficient in treating CRPC [55]. However, altogether, no impact of 5αR inhibitor on survival has been found in people with PCa [56].
17β-hydroxysteroid dehydrogenases (17βHSDs) catalyze the interconversions between active 17β-hydroxysteroids and less-active 17-ketosteroids and modulate the availability of biologically active estrogens and androgens in breast and prostate [57]. Among them, 17βHSD type 1 is essential for the production of 17βE2. These enzymes are thus theoretically exquisite targets to reduce the production of 17βE2. However, if several steroidal and non-steroidal compounds are able to reduce HSD17B1 activity in vitro, the list of in vivo validated inhibitors is much shorter [58], and finally, there is no 17βHSD type 1 inhibitor currently used for the treatment of BCa.
17α-hydroxylase/C17-20-lyase (CYP17A1) converts pregnane steroids into androgens like testerosterone. Abiraterone acetate [59] is used in metastatic castration-resistant PCa [60] to block the biosynthesis of androgens by inhibiting CYP17A1 activity.
4.2. Antagonists of ERα and AR Transcriptional Activities
The use of selective estrogen receptor modulators (SERMs) dates to the late 1960s and early 1970s when positive clinical outcomes were reported with the use of antiestrogenic agents such as tamoxifen [61], a trans-isomere of 1(p-β-dimethylaminoethoxy-phenyl)-1-2-diphenylnut-1-ene, which has complex pharmacology. Apart from being metabolized into numerous biologically active compounds, it is an estrogen agonist-antagonist depending on its competitive binding to ERα. Raloxifene, a benzothiophene SERM, initially failed to be used in women treated with a BCa; however, it is now used to decrease the incidence of invasive BCa in postmenopausal women who have a higher risk to develop the disease [62]. SERMs tamoxifen and raloxifene were also approved for the chemoprevention in women with a high risk of breast cancer [63].
Based on the analogy with SERMs, SARMs have been developed to block the transcriptional activity of AR. Non-steroidal antiandrogens were first introduced in 1989 in clinical practice as a treatment for advanced and metastatic PCa [64]. The first-generation of antiandrogens bicalutamide [65], flutamide [66], and nilutamide [67] efficiently block AR, and thereby, inhibit tumor growth with similar efficacy, even though bicalutamide is better tolerated and more stable antiandrogen currently used in clinical practice [68]. The second generation of SARMs is represented by enzalutamide [69]. In contrast to the first generation of AR blockers, enzalutamide also inhibits the shuttling of AR from the cytoplasm to the nucleus, and thus impairs AR binding to DNA [70]. Since enzalutamide, apalutamide and darolutamide [71] have been approved by FDA and EMA for the treatment of metastatic PCa and non CRPC, respectively.
4.3. Antagonists of the Steroid Receptors and Inducers of Their Degradation
While ERα and AR blockers have been currently used for decades, it came to the evidence that these nuclear receptors were able to go back to a transcriptionally active state despite the presence of their specific antagonists, hence making the tumor resistant to chemical castration. The idea was, thus, to develop a molecule able to inhibit the binding of the bona fide ligand to the ligand-binding pocket of the receptor and, at the same time, to induce its proteasomal degradation. Hence, the possibility for the remaining receptor to be activated even in the presence of the antagonist would have been decreased.
Fulvestrant, a 7α-alkylsulphinyl analog of 17βE2, is distinctly different in chemical structure from the nonsteroidal structures of tamoxifen, raloxifene, and other SERMs. The binding affinity of fulvestrant to ERα is 89% that of the natural ligand [72] and significantly greater than the affinity of tamoxifen. SERD of first-generation, fulvestrant, also impairs ER-dimerization and its translocation to the nucleus. More importantly, the unstable fulvestrant-ERα complex is unstable and rapidly degraded, inducing in parallel a decrease in the amount of ERα encoding mRNA [73], which explains its robustly effective antitumor activity in preclinical models of BCa [74]. Fulvestrant was first approved in 2002 as monotherapy for the treatment of postmenopausal patients with positive ER metastatic BCa whose cancer had progressed following anti-estrogen therapy [75]. Furthermore, neoangiogenesis is impaired by intraductal fulvestrant treatment [76]. When added to anastrozole, fulvestrant co-treatment improves the overall survival of patients with metastatic hormone receptor-positive BCa compared with anastrozole alone [77]. Novel SERDs have also been designed based on the 6-OH-benzothiophene scaffold common to arzoxifene, another SERM, and raloxifene [78]; however, these molecules did not reach clinical use so far.
Conversely to the clinically available SERDs, no equivalence of specific androgen receptor degraders or down-regulators (SARDs) have been used so far in patients with a PCa. However, darulotamide derivatives have been screened in cell culture. It appears that two isolated compounds have the ability to act as specific AR antagonists more efficiently than enzalutamide, as well as down-regulators in inducing the degradation of the receptor and decreasing AR mRNA [79]. These quinoline or purine derived-darulotamide could, thus, be considered as lead compounds for the synthesis of new SARDs to be used for the treatment of CRPC.
5. Natural Compounds Modulating the Steroid Activity
Several steps could be targeted (Figure 2) to decrease or to abolish androgens and estrogens activity on their respective nuclear receptors in PCa or in BCa. It is impossible to list all the molecules used in folk medicine and suspected to modify the activity of the steroids. However, some significant examples have been chosen from the published literature (Table 1).

Table 1.
Examples of molecules for the synthesis and activity of androgens and estrogens.
5.1. Natural Compounds Inhibiting the Steroid Synthesis
Fifteen natural products were screened in traditional Chinese medicine [80], and seven of them showed potent inhibition of aromatase: naringin, apigenin, berberine, palmatine, bavachin, jatrorrhizine and bavachinin. These compounds have been classified as flavone (apigenin), flavonone (naringin, bavachin, bavachinin), or isoquinoline alkaloid (berberine, palmatine, jatrorrhizine). On these bases, indole derivatives have been synthesized as aromatase inhibitors [81]. Alangium salviifolium, commonly known as sage-leaved alangium, is a flowering plant in the Cornaceae family. Most of the isolated cardinane sesquiterpenes are potent aromatase inhibitors [82]. The modulation of the flavonoid skeleton increases the anti-aromatase effect [83]. The hexane extract of the leaves of Brassaiopsis glomerulata, a large shrub found in Indonesia, presents significant aromatase inhibition [84]. Psoralea corylifolia, a plant used in Indian and Chinese traditional medicines, contains a significant amount of bakuchiol, a meroterpene used for its antiandrogenic activity, which shows moderate anti-aromatase activity [85]. Sarcococca saligna, the sweet box or Christmas box, native from Northern Pakistan, contains phytochemical constituents such as alkaloid-C, dictyophlebine, sarcovagine-D and saracodine, which inhibit aromatase [86]. Shu-Gan-Liang-Xue decoction (SGLXD) is a traditional Chinese herbal formula with a potential dual aromatase-sulfatase inhibitor by simultaneously down-regulating the expressions of aromatase and sulfatase in BCa cell cultures [87]. SGLXD also has anti-tumor effects on the BCa cells ZR-75-1 by inhibiting aromatase and steroid sulfatase [88]. Butein, a natural chalcone found in Toxicodendron vernicifluum, shows anti-aromatase activity and could potentially represent a natural alternative for the chemoprevention or therapy of BCa [89]. Not only green plants but also white button mushroom could contain interesting phytochemicals as their aqueous extract inhibits aromatase activity and proliferation of MCF-7 cells [90]. Obacunone, a natural limonoid present in citrus fruits, affects aromatase activity, increases apoptosis, and induces G1 cell cycle arrest [91].
Even though the use of 5αR inhibitors in PCa is controversial, it is noteworthy that numerous extracts present the capacity to inhibit the bioconversion of testosterone into DHT. Historically, lipophilic extracts of Serenoa repens, commonly known as saw palmetto, were the first to be associated with a strong inhibition of the DHT synthesis [92]. Epilobium angustifolium, a native of the temperate northern hemisphere, could present some 5α-reductase inhibition as its ethanolic extract directly inhibits the proliferation of the PZ-HPV-7 cells that are sensitive to DHT [93]. Hiipakka et al. [94] identified the green tea (−)-epigallocatechin gallate as well as other natural flavonoids such as biochanin A, daidzein, genistein and kaempferol as potent inhibitors of the 5α-reductase type 2. Adina rubella, a shrub found in Korea, presents caffeic acid and grandifloroside from leaves that have potent inhibitory activity against 5α-reductase [95]. Cynomorium songaricum, a parasitic perennial flowering plant, contains polyphenolic constituents that significantly inhibit rat prostate enlargement, improved the pathological feature and reduced the thickness of the smooth muscle layer [96]. The mechanism encompasses, in part, the inhibition of the 5αR activity, together with the decrease of AR and ERα mRNA accumulation and the increase of ERβ mRNA [96]. Altogether, and despite the until now low potential of using 5αR inhibitors in the treatment of PCa, the discovery of specific inhibitors from natural extracts is still of interest [97].
5.2. Natural Compounds Acting as AR or ERα Antagonists
So far, only a few natural compounds have been described as having an anti-AR activity. Methanolic extracts of the bark of Brosimum rubescens, also known as palo de sangre in Peru, has shown potent inhibitory activity towards DHT binding to AR [98]. Tanshinone IIA, one of the most abundant constituents of the root of the Chinese sage Salvia militiorrhiza, reduces the accumulation of ERα and AR mRNA in prostate cell lines. In fact, Tanshinone IIA can inhibit the growth of stromal and epithelial cells in vitro and in vivo by a mechanism that may involve arresting the cell cycle and downregulating ERα and AR accumulation [100]. The fact that AR is downregulated is in favor of a SARD role for Tanshinone IIA. We studied the effects of the ethanolic extract of propolis on the phenotype of LNCaP cells [101]. This extract reduces cell survival, induces apoptosis, and blocks the cell cycle at the G0/G1 phase. Interestingly, this ethanolic extract decreases the accumulation of AR and the secreted amount of the androgen target prostate-specific antigen (PSA) in LNCaP cells. This anti-androgen activity was also shown on the androgen target genes Fkbp5 and Sgk1. Finally, the capacity of propolis to block AR functioning was demonstrated in transient transfections using the human AR. Altogether, the ethanolic extract of propolis displays SARD activity that needs to be further investigated in preclinical models [101]. The 3,3′-diindolylmethane is a major digestive product of indole-3-carbinol, a potential anticancer component of cruciferous vegetables. It suppresses cell proliferation of LNCaP cells and inhibits the stimulatory effect of DHT on DNA synthesis. It also blocks AR translocation into the nucleus by strong competitive inhibition of the DHT binding [99]. Bisbibenzyl compounds riccardin C and D as well as marchantin, all extracted from various liverwort species, decrease AR expression at mRNA and protein levels, leading to the suppression of AR transcriptional activity. However, these effects seem to be linked to proteasome inhibition and autophagy activation in LNCaP cells rather than to the AR-binding effect [104].
In BCa, a cycloartane triterpenoid isolated from Schisandra glaucescens, a magnolia vine native to Asia and North America, shows ER antagonistic effects [102]. Otherwise, phytoestrogens, mycoestrogens, and xenoestrogens bind ER in intact cells, but display marked differences in their ability to induce end products of estrogen action and to regulate cell proliferation [105]. All of the different classes of these estrogen-like molecules stimulate cell proliferation at concentrations that half-saturated ER; the fact that EC50s are lower than those of 17βE2 explain their slight antagonist effects in the presence of the natural ligand, as exemplified by genistein found in soy foods [105]. Soy phytoestrogens are non-steroid molecules whose structural similarity lends them the ability to mimic with a lower efficacy the effects of 17βE2 [106]. Extracts from Urtica dioica, often known as common nettle, has some anti-estrogen activity [103]; however, its active compound has not been identified so far. Altogether, natural estrogen-like molecules are numerous. To help in the screening of which ones could have a pro- or an anti-ER activity, Powers and Setzer [107] have developed a molecular docking approach to identify the estrogen activity of phytochemicals, which allowed the study of almost 600 of them. They identified that the prenylation of flavonoids often results in anti-estrogenic activity.
6. Conclusions
Altogether, molecules extracted from a natural environment are the source of the identification of compounds that could serve as lead compounds for building more active drugs. Interestingly, such natural compounds can target the various levels that control androgen or estrogen concentration, AR and ERα transcriptional activity and degradation. The main negative point is, however, that the great majority of these compounds have been tested in vitro or in cell culture, but few have been studied in preclinical models. On the other hand, it is difficult from an ethical point of view to study thousands of these molecules in preclinical models. One solution would be to perform a pre-screening using in silico models and to realize in vivo tests in an ex vivo 3D model [108] or in non-mammalian models of tumors, such as the drosophila for PCa [109].
Author Contributions
B.B., J.S. and J.-M.A.L. designed the review; B.B., A.A.Z., C.d.J. and J.-M.A.L. analyzed the data. B.B., S.B., C.d.J. and J.-M.A.L. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this study was supported by Conférence Episcopale Italienne (CEI), Union Economique Monétaire Ouest Africaine (UEMOA) and Ambassade de France au Burkina Faso (Grant stage SSHN n°948600C) for B.B., A.A.Z., J.S.; Région Auvergne, Fond Européen de Développement Régional (FEDER), Plan National de Recherche sur les Perturbateurs Endocriniens (13-MRES-PNRPE-1-CVS043), and Plan-Cancer 2016 for J.-M.A.L. and S.B., and Ligue contre le Cancer Rhone Alpes Auvergne et Saone et Loire for C.d.J., The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Jean-Marc A. Lobaccaro is an editor at IJMS.
Abbreviations
| Δ4 | Δ4-androstenedion |
| 5⍺R | 5⍺-reductase |
| 17βE2 | estradiol |
| 17βHSD | 17β-hydroxy-steroid dehydrogenase |
| AR | androgen receptor |
| BCa | breast cancer |
| E1 | estrone |
| ER⍺ | estrogen receptor ⍺ |
| PCa | prostate cancer |
| SARM | specific androgen receptor modulator |
| SARD | specific androgen receptor degrader/down-regulator |
| SERM | specific estrogen receptor modulator |
| SERD | specific estrogen receptor degrader/down-regulator |
| T | testosterone |
References
- Lordan, R.; Tsoupras, A.; Zabetakis, I. The Potential Role of Dietary Platelet-Activating Factor Inhibitors in Cancer Prevention and Treatment. Adv. Nutr. Bethesda Md 2019, 10, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Gandellini, P.; Andriani, F.; Merlino, G.; D’Aiuto, F.; Roz, L.; Callari, M. Complexity in the tumour microenvironment: Cancer associated fibroblast gene expression patterns identify both common and unique features of tumour-stroma crosstalk across cancer types. Semin. Cancer Biol. 2015, 35, 96–106. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Chimento, A.; Montalto, F.I.; Casaburi, I.; Sirianni, R.; Pezzi, V. Steroid Receptor Signallings as Targets for Resveratrol Actions in Breast and Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1087. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Elemento, O. Cancer systems biology: Embracing complexity to develop better anticancer therapeutic strategies. Oncogene 2015, 34, 3215. [Google Scholar] [CrossRef] [PubMed]
- Godzieba, M.; Ciesielski, S. Natural DNA Intercalators as Promising Therapeutics for Cancer and Infectious Diseases. Curr. Cancer Drug Targets 2019. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Rasul, A.; Sarfraz, A.; Sarfraz, I.; Hussain, G.; Anwar, H.; Riaz, A.; Liu, S.; Wei, W.; Li, J.; et al. Salvianolic acid A & B: Potential cytotoxic polyphenols in battle against cancer via targeting multiple signaling pathways. Int. J. Biol. Sci. 2019, 15, 2256–2264. [Google Scholar] [CrossRef]
- El-Hajjaji, F.-Z.; Oumeddour, A.; Pommier, A.J.C.; Ouvrier, A.; Viennois, E.; Dufour, J.; Caira, F.; Drevet, J.R.; Volle, D.H.; Baron, S.; et al. Liver X receptors, lipids and their reproductive secrets in the male. Biochim. Biophys. Acta 2011, 1812, 974–981. [Google Scholar] [CrossRef]
- Hayashi, T.; Fujita, K.; Matsushita, M.; Nonomura, N. Main Inflammatory cells and potentials of anti-inflammatory agents in prostate cancer. Cancers 2019, 11, 1153. [Google Scholar] [CrossRef]
- Katsogiannou, M.; Ziouziou, H.; Karaki, S.; Andrieu, C.; de Villeneuve, M.H.; Rocchi, P. The hallmarks of castration-resistant prostate cancers. Cancer Treat. Rev. 2015, 41, 588–597. [Google Scholar] [CrossRef]
- Maitland, N.J.; Frame, F.M.; Rane, J.K.; Erb, H.H.; Packer, J.R.; Archer, L.K.; Pellacani, D. Resolution of cellular heterogeneity in human prostate cancers: Implications for diagnosis and treatment. Adv. Exp. Med. Biol. 2019, 1164, 207–224. [Google Scholar] [CrossRef]
- Villani, S.; Gagliano, N.; Procacci, P.; Sartori, P.; Comar, M.; Provenzano, M.; Favi, E.; Ferraresso, M.; Ferrante, P.; Delbue, S. Characterization of an in vitro model to study the possible role of polyomavirus BK in prostate cancer. J. Cell. Physiol. 2019, 234, 11912–11922. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, V.; Miozzi, E.; Loreto, C.; Matera, S.; Fenga, C.; Avola, R.; Ledda, C. Cadmium exposure and prostate cancer: Insights, mechanisms and perspectives. Front. Biosci. Landmark Ed. 2018, 23, 1687–1700. [Google Scholar] [CrossRef] [PubMed]
- Viner, B.; Barberio, A.M.; Haig, T.R.; Friedenreich, C.M.; Brenner, D.R. The individual and combined effects of alcohol consumption and cigarette smoking on site-specific cancer risk in a prospective cohort of 26,607 adults: Results from Alberta’s Tomorrow Project. Cancer Causes Control 2019. [Google Scholar] [CrossRef] [PubMed]
- Bousset, L.; Rambur, A.; Fouache, A.; Bunay, J.; Morel, L.; Lobaccaro, J.-M.A.; Baron, S.; Trousson, A.; de Joussineau, C. New Insights in Prostate Cancer Development and Tumor Therapy: Modulation of Nuclear Receptors and the Specific Role of Liver X Receptors. Int. J. Mol. Sci. 2018, 19, 2545. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA. Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Kuller, L.H. The etiology of breast cancer--from epidemiology to prevention. Public Health Rev. 1995, 23, 157–213. [Google Scholar]
- Dugué, P.-A.; Dowty, J.G.; Joo, J.E.; Wong, E.M.; Makalic, E.; Schmidt, D.F.; English, D.R.; Hopper, J.L.; Pedersen, J.; Severi, G.; et al. Heritable methylation marks associated with breast and prostate cancer risk. Prostate 2018, 78, 962–969. [Google Scholar] [CrossRef]
- Petrakis, N.L. Genetic factors in the etiology of breast cancer. Cancer 1977, 39, 2709–2715. [Google Scholar] [CrossRef]
- Gaikwad, N.W.; Yang, L.; Muti, P.; Meza, J.L.; Pruthi, S.; Ingle, J.N.; Rogan, E.G.; Cavalieri, E.L. The molecular etiology of breast cancer: Evidence from biomarkers of risk. Int. J. Cancer 2008, 122, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Medici, N.; Bilancio, A.; Migliaccio, A.; Castoria, G. Breast cancer stem cells: The role of sex steroid receptors. World J. Stem Cells 2019, 11, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Kiderlen, M.; Ponti, A.; Tomatis, M.; Boelens, P.G.; Bastiaannet, E.; Wilson, R.; van de Velde, C.J.H.; Audisio, R.A. eusomaDB Working Group Variations in compliance to quality indicators by age for 41,871 breast cancer patients across Europe: A European Society of Breast Cancer Specialists database analysis. Eur. J. Cancer Oxf. Engl. 1990 2015, 51, 1221–1230. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet Lond. Engl. 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Cardoso, F.; Costa, A.; Norton, L.; Senkus, E.; Aapro, M.; André, F.; Barrios, C.H.; Bergh, J.; Biganzoli, L.; Blackwell, K.L.; et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast Edinb. Scotl. 2014, 23, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.-Å. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors, RXR, and the big bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef]
- Kato, S.; Nishimura, K.-I.; Ochi, M.; Shimmura, H.; Mori, J.-I. Bone and calcium metabolism associated with malignancy. The function of sex hormone receptors in sex hormone-dependent cancers. Clin. Calcium 2018, 28, 1457–1463. [Google Scholar]
- Nelson, P.S.; Clegg, N.; Arnold, H.; Ferguson, C.; Bonham, M.; White, J.; Hood, L.; Lin, B. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc. Natl. Acad. Sci. USA 2002, 99, 11890–11895. [Google Scholar] [CrossRef]
- Majumdar, S.; Rinaldi, J.C.; Malhotra, N.R.; Xie, L.; Hu, D.-P.; Gauntner, T.D.; Grewal, H.S.; Hu, W.-Y.; Kim, S.H.; Katzenellenbogen, J.A.; et al. Differential actions of estrogen receptor α and β via Nongenomic signaling in human prostate stem and progenitor cells. Endocrinology 2019, 160, 2692–2708. [Google Scholar] [CrossRef]
- Prins, G.S.; Hu, W.-Y.; Shi, G.-B.; Hu, D.-P.; Majumdar, S.; Li, G.; Huang, K.; Nelles, J.L.; Ho, S.-M.; Walker, C.L.; et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 2014, 155, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.G.; Dickler, M.N. Endocrine resistance in hormone-responsive breast cancer: Mechanisms and therapeutic strategies. Endocr. Relat. Cancer 2016, 23, R337–R352. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.M.; Dickler, M.N. Estrogen receptor-positive breast cancer: Exploiting signaling pathways implicated in endocrine resistance. Oncologist 2018, 23, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Siersbæk, R.; Kumar, S.; Carroll, J.S. Signaling pathways and steroid receptors modulating estrogen receptor α function in breast cancer. Genes Dev. 2018, 32, 1141–1154. [Google Scholar] [CrossRef]
- Proverbs-Singh, T.; Feldman, J.L.; Morris, M.J.; Autio, K.A.; Traina, T.A. Targeting the androgen receptor in prostate and breast cancer: Several new agents in development. Endocr. Relat. Cancer 2015, 22, R87–R106. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, E.; Bergh, A.; Wikström, P. Clinical relevance of androgen receptor alterations in prostate cancer. Endocr. Connect. 2017, 6, R146–R161. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, M.; Crippa, V.; Messi, E.; Tetel, M.J.; Poletti, A. Modulators of estrogen receptor inhibit proliferation and migration of prostate cancer cells. Pharmacol. Res. 2014, 79, 13–20. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, V.; Jain, A.; Jain, R.K.; Maikhuri, J.P.; Gupta, G. Synergistic chemoprotective mechanisms of dietary phytoestrogens in a select combination against prostate cancer. J. Nutr. Biochem. 2011, 22, 723–731. [Google Scholar] [CrossRef]
- Culig, Z.; Hoffmann, J.; Erdel, M.; Eder, I.E.; Hobisch, A.; Hittmair, A.; Bartsch, G.; Utermann, G.; Schneider, M.R.; Parczyk, K.; et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br. J. Cancer 1999, 81, 242–251. [Google Scholar] [CrossRef]
- Scherbakov, A.M.; Komkov, A.V.; Komendantova, A.S.; Yastrebova, M.A.; Andreeva, O.E.; Shirinian, V.Z.; Hajra, A.; Zavarzin, I.V.; Volkova, Y.A. Steroidal pyrimidines and dihydrotriazines as novel classes of anticancer agents against hormone-dependent breast cancer cells. Front. Pharmacol. 2017, 8, 979. [Google Scholar] [CrossRef]
- Brueggemeier, R.W.; O’Reilly, J.M.; Lovely, C.J.; Ward, P.J.; Quinn, A.L.; Baker, D.; Darby, M.V.; Gu, X.J.; Gilbert, N.E. Biochemistry and pharmacology of 7alpha-substituted androstenediones as aromatase inhibitors. J. Steroid Biochem. Mol. Biol. 1997, 61, 247–254. [Google Scholar] [CrossRef]
- Brodie, A.; Njar, V.; Macedo, L.F.; Vasaitis, T.S.; Sabnis, G. The Coffey Lecture: Steroidogenic enzyme inhibitors and hormone dependent cancer. Urol. Oncol. 2009, 27, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; Martínez-Campa, C.; Mediavilla, M.D.; Sánchez-Barceló, E.J. Melatonin modulates aromatase activity in MCF-7 human breast cancer cells. J. Pineal Res. 2005, 38, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ozcan-Sezer, S.; Ince, E.; Akdemir, A.; Ceylan, Ö.Ö.; Suzen, S.; Gurer-Orhan, H. Aromatase inhibition by 2-methyl indole hydrazone derivatives evaluated via molecular docking and in vitro activity studies. Xenobiotica Fate Foreign Compd. Biol. Syst. 2019, 49, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Giudici, D.; Ornati, G.; Briatico, G.; Buzzetti, F.; Lombardi, P.; di Salle, E. 6-Methylenandrosta-1,4-diene-3,17-dione (FCE 24304): A new irreversible aromatase inhibitor. J. Steroid Biochem. 1988, 30, 391–394. [Google Scholar] [CrossRef]
- Pagani, O.; Regan, M.M.; Walley, B.A.; Fleming, G.F.; Colleoni, M.; Láng, I.; Gomez, H.L.; Tondini, C.; Burstein, H.J.; Perez, E.A.; et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 2014, 371, 107–118. [Google Scholar] [CrossRef]
- Plourde, P.V.; Dyroff, M.; Dukes, M. Arimidex: A potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res. Treat. 1994, 30, 103–111. [Google Scholar] [CrossRef]
- De Placido, S.; Gallo, C.; De Laurentiis, M.; Bisagni, G.; Arpino, G.; Sarobba, M.G.; Riccardi, F.; Russo, A.; Del Mastro, L.; Cogoni, A.A.; et al. Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 474–485. [Google Scholar] [CrossRef]
- Augusto, T.V.; Amaral, C.; Varela, C.L.; Bernardo, F.; da Silva, E.T.; Roleira, F.F.M.; Costa, S.; Teixeira, N.; Correia-da-Silva, G. Effects of new C6-substituted steroidal aromatase inhibitors in hormone-sensitive breast cancer cells: Cell death mechanisms and modulation of estrogen and androgen receptors. J. Steroid Biochem. Mol. Biol. 2019, 195, 105486. [Google Scholar] [CrossRef]
- Amaral, C.; Varela, C.L.; Maurício, J.; Sobral, A.F.; Costa, S.C.; Roleira, F.M.F.; Tavares-da-Silva, E.J.; Correia-da-Silva, G.; Teixeira, N. Anti-tumor efficacy of new 7α-substituted androstanes as aromatase inhibitors in hormone-sensitive and resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2017, 171, 218–228. [Google Scholar] [CrossRef]
- Amaral, C.; Varela, C.; Azevedo, M.; da Silva, E.T.; Roleira, F.M.F.; Chen, S.; Correia-da-Silva, G.; Teixeira, N. Effects of steroidal aromatase inhibitors on sensitive and resistant breast cancer cells: Aromatase inhibition and autophagy. J. Steroid Biochem. Mol. Biol. 2013, 135, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cepa, M.; Correia-da-Silva, G.; Tavares da Silva, E.J.; Roleira, F.M.F.; Hong, Y.; Chen, S.; Teixeira, N.A. Molecular mechanisms of aromatase inhibition by new A, D-ring modified steroids. Biol. Chem. 2008, 389, 1183–1191. [Google Scholar] [CrossRef]
- Sudduth, S.L.; Koronkowski, M.J. Finasteride: The first 5 alpha-reductase inhibitor. Pharmacotherapy 1993, 13, 309–325. [Google Scholar] [PubMed]
- Andriole, G.L.; Bostwick, D.G.; Brawley, O.W.; Gomella, L.G.; Marberger, M.; Montorsi, F.; Pettaway, C.A.; Tammela, T.L.; Teloken, C.; Tindall, D.J.; et al. Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 2010, 362, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Matayoshi, Y.; Sato, Y.; Nagase, Y. Effect of dutasteride on castration-resistant prostate cancer. Mol. Clin. Oncol. 2018, 8, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Hirshburg, J.M.; Kelsey, P.A.; Therrien, C.A.; Gavino, A.C.; Reichenberg, J.S. Adverse effects and safety of 5-alpha reductase inhibitors (Finasteride, Dutasteride): A systematic review. J. Clin. Aesthetic Dermatol. 2016, 9, 56–62. [Google Scholar]
- Vihko, P.; Härkönen, P.; Oduwole, O.; Törn, S.; Kurkela, R.; Porvari, K.; Pulkka, A.; Isomaa, V. 17 beta-hydroxysteroid dehydrogenases and cancers. J. Steroid Biochem. Mol. Biol. 2002, 83, 119–122. [Google Scholar] [CrossRef]
- Hilborn, E.; Stål, O.; Jansson, A. Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: With a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget 2017, 8, 30552–30562. [Google Scholar] [CrossRef]
- Barrie, S.E.; Potter, G.A.; Goddard, P.M.; Haynes, B.P.; Dowsett, M.; Jarman, M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase). J. Steroid Biochem. Mol. Biol. 1994, 50, 267–273. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef]
- Cole, M.P.; Jones, C.T.; Todd, I.D. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br. J. Cancer 1971, 25, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-I.; Kim, T.; Kim, J.-E.; Lee, J.; Heo, J.; Lee, N.-R.; Kim, N.-J.; Inn, K.-S. NJK14013, a novel synthetic estrogen receptor-α agonist, exhibits estrogen receptor-independent, tumor cell-specific cytotoxicity. Int. J. Oncol. 2015, 47, 280–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ball, S.; Arevalo, M.; Juarez, E.; Payne, J.D.; Jones, C. Breast cancer chemoprevention: An update on current practice and opportunities for primary care physicians. Prev. Med. 2019, 129, 105834. [Google Scholar] [CrossRef] [PubMed]
- Helsen, C.; Van den Broeck, T.; Voet, A.; Prekovic, S.; Van Poppel, H.; Joniau, S.; Claessens, F. Androgen receptor antagonists for prostate cancer therapy. Endocr. Relat. Cancer 2014, 21, T105–T118. [Google Scholar] [CrossRef] [PubMed]
- Furr, B.J.; Valcaccia, B.; Curry, B.; Woodburn, J.R.; Chesterson, G.; Tucker, H. ICI 176,334: A novel non-steroidal, peripherally selective antiandrogen. J. Endocrinol. 1987, 113, R7–R9. [Google Scholar] [CrossRef] [PubMed]
- Peets, E.A.; Henson, M.F.; Neri, R. On the mechanism of the anti-androgenic action of flutamide (alpha-alpha-alpha-trifluoro-2-methyl-4′-nitro-m-propionotoluidide) in the rat. Endocrinology 1974, 94, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Séguin, C.; Cusan, L.; Bélanger, A.; Kelly, P.A.; Labrie, F.; Raynaud, J.P. Additive inhibitory effects of treatment with an LHRH agonist and an antiandrogen on androgen-dependent tissues in the rat. Mol. Cell. Endocrinol. 1981, 21, 37–41. [Google Scholar] [CrossRef]
- Schellhammer, P.; Sharifi, R.; Block, N.; Soloway, M.; Venner, P.; Patterson, A.L.; Sarosdy, M.; Vogelzang, N.; Jones, J.; Kolvenbag, G. Maximal androgen blockade for patients with metastatic prostate cancer: Outcome of a controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy. Casodex Combination Study Group. Urology 1996, 47, 54–60; discussion 80–84. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Fizazi, K.; Massard, C.; Bono, P.; Jones, R.; Kataja, V.; James, N.; Garcia, J.A.; Protheroe, A.; Tammela, T.L.; Elliott, T.; et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): An open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014, 15, 975–985. [Google Scholar] [CrossRef]
- Wakeling, A.E.; Dukes, M.; Bowler, J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991, 51, 3867–3873. [Google Scholar] [PubMed]
- Osborne, C.K.; Wakeling, A.; Nicholson, R.I. Fulvestrant: An oestrogen receptor antagonist with a novel mechanism of action. Br. J. Cancer 2004, 90 (Suppl. 1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Wardell, S.E.; Yllanes, A.P.; Chao, C.A.; Bae, Y.; Andreano, K.J.; Desautels, T.K.; Heetderks, K.A.; Blitzer, J.T.; Norris, J.D.; McDonnell, D.P. Pharmacokinetic and pharmacodynamic analysis of fulvestrant in preclinical models of breast cancer to assess the importance of its estrogen receptor-α degrader activity in antitumor efficacy. Breast Cancer Res. Treat. 2019, 179, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Mahtani, R.L. A role for fulvestrant monotherapy in the first-line treatment of ER+ metastatic breast cancer? Breast J. 2019, 26, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, C.; Pai, P.; Korangath, P.; Sun, S.; Merino, V.F.; Yuan, J.; Li, S.; Nie, G.; Stearns, V.; et al. Intraductal fulvestrant for therapy of ERα-positive ductal carcinoma in situ of the breast: A preclinical study. Carcinogenesis 2019, 40, 903–913. [Google Scholar] [CrossRef]
- Burki, T.K. Fulvestrant plus anastrozole for metastatic breast cancer. Lancet Oncol. 2019, 20, e247. [Google Scholar] [CrossRef]
- Xiong, R.; Zhao, J.; Gutgesell, L.M.; Wang, Y.; Lee, S.; Karumudi, B.; Zhao, H.; Lu, Y.; Tonetti, D.A.; Thatcher, G.R.J. Novel selective estrogen receptor Downregulators (SERDs) developed against treatment-resistant breast cancer. J. Med. Chem. 2017, 60, 1325–1342. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, P.; Hu, M.; Yang, L.; Yan, G.; Xu, R.; Deng, Y.; Li, X.; Chen, Y. Discovery and biological evaluation of darolutamide derivatives as inhibitors and down-regulators of wild-type AR and the mutants. Eur. J. Med. Chem. 2019, 182, 111608. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Z. Screening of aromatase inhibitors in traditional Chinese medicines by electrophoretically mediated microanalysis in a partially filled capillary. J. Sep. Sci. 2013, 36, 2691–2697. [Google Scholar] [CrossRef]
- Pingaew, R.; Mandi, P.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, molecular docking, and QSAR study of sulfonamide-based indoles as aromatase inhibitors. Eur. J. Med. Chem. 2018, 143, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Pailee, P.; Prachyawarakorn, V.; Ruchirawat, S.; Mahidol, C. Bioactive cardinane sesquiterpenes from the stems of Alangium salviifolium. Chem. Asian J. 2015, 10, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, S.; Fagnere, C.; Pouget, C.; Buxeraud, J.; Chulia, A.-J. New 7,8-benzoflavanones as potent aromatase inhibitors: Synthesis and biological evaluation. Bioorg. Med. Chem. 2008, 16, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Su, B.; Riswan, S.; Fong, H.H.S.; Brueggemeier, R.W.; Pezzuto, J.M.; Kinghorn, A.D. Isolation and Characterization of Aromatase Inhibitors from Brassaiopsis glomerulata (Araliaceae). Phytochem. Lett. 2009, 2, 29–33. [Google Scholar] [CrossRef]
- Miao, L.; Jiao, C.; Shao, R.; Qi, Y.; Fan, G.; Li, X.; Wang, Y.; Zhu, Y.; Zhang, J.; Gao, X. Bakuchiol suppresses oestrogen/testosterone-induced Benign Prostatic Hyperplasia development through up-regulation of epithelial estrogen receptor β and down-regulation of stromal aromatase. Toxicol. Appl. Pharmacol. 2019, 381, 114637. [Google Scholar] [CrossRef]
- Ali, A.; Jan, N.U.; Ali, S.; Ahmad, B.; Ali, A.; Samrana, S.; Jahan, A.; Ali, H.; Khan, I.A.; Rahim, H.; et al. Steroidal alkaloids efficient aromatase inhibitors with potential for the treatment of post-menopausal breast cancer. Chem. Biol. Drug Des. 2019. [Google Scholar] [CrossRef]
- Fu, X.-S.; Li, P.-P. Shu-Gan-Liang-Xue decoction simultaneously down-regulates expressions of aromatase and steroid Sulfatase in estrogen receptor positive breast cancer Cells. Chin. J. Cancer Res. Chung Kuo Yen Cheng Yen Chiu 2011, 23, 208–213. [Google Scholar] [CrossRef]
- Zhou, N.; Han, S.-Y.; Zhou, F.; Li, P. Anti-tumor effect of Shu-Gan-Liang-Xue decoction in breast cancer is related to the inhibition of aromatase and steroid sulfatase expression. J. Ethnopharmacol. 2014, 154, 687–695. [Google Scholar] [CrossRef]
- Wang, Y.; Chan, F.L.; Chen, S.; Leung, L.K. The plant polyphenol butein inhibits testosterone-induced proliferation in breast cancer cells expressing aromatase. Life Sci. 2005, 77, 39–51. [Google Scholar] [CrossRef]
- Grube, B.J.; Eng, E.T.; Kao, Y.C.; Kwon, A.; Chen, S. White button mushroom phytochemicals inhibit aromatase activity and breast cancer cell proliferation. J. Nutr. 2001, 131, 3288–3293. [Google Scholar] [CrossRef]
- Kim, J.; Jayaprakasha, G.K.; Patil, B.S. Obacunone exhibits anti-proliferative and anti-aromatase activity in vitro by inhibiting the p38 MAPK signaling pathway in MCF-7 human breast adenocarcinoma cells. Biochimie 2014, 105, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Sultan, C.; Terraza, A.; Devillier, C.; Carilla, E.; Briley, M.; Loire, C.; Descomps, B. Inhibition of androgen metabolism and binding by a liposterolic extract of “Serenoa repens B” in human foreskin fibroblasts. J. Steroid Biochem. 1984, 20, 515–519. [Google Scholar] [CrossRef]
- Vitalone, A.; Bordi, F.; Baldazzi, C.; Mazzanti, G.; Saso, L.; Tita, B. Anti-proliferative effect on a prostatic epithelial cell line (PZ-HPV-7) by Epilobium angustifolium L. Farm. Soc. Chim. Ital. 1989 2001, 56, 483–489. [Google Scholar] [CrossRef]
- Hiipakka, R.A.; Zhang, H.-Z.; Dai, W.; Dai, Q.; Liao, S. Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem. Pharmacol. 2002, 63, 1165–1176. [Google Scholar] [CrossRef]
- Yin, J.; Heo, J.H.; Hwang, Y.J.; Le, T.T.; Lee, M.W. Inhibitory nst 5α-reductase associated with benign prostatic hypertrophy. Molecules 2016, 21, 887. [Google Scholar] [CrossRef]
- Tao, R.; Miao, L.; Yu, X.; Orgah, J.O.; Barnabas, O.; Chang, Y.; Liu, E.; Fan, G.; Gao, X. Cynomorium songaricum Rupr demonstrates phytoestrogenic or phytoandrogenic like activities that attenuates benign prostatic hyperplasia via regulating steroid 5-α-reductase. J. Ethnopharmacol. 2019, 235, 65–74. [Google Scholar] [CrossRef]
- Srivilai, J.; Minale, G.; Scholfield, C.N.; Ingkaninan, K. Discovery of natural steroid 5 alpha-reductase inhibitors. Assay Drug Dev. Technol. 2019, 17, 44–57. [Google Scholar] [CrossRef]
- Shirota, O.; Takizawa, K.; Sekita, S.; Satake, M.; Hirayama, Y.; Hakamata, Y.; Hayashi, T.; Yanagawa, T. Antiandrogenic natural Diels−alder-type adducts from Brosimum Rubescens. J. Nat. Prod. 1997, 60, 997–1002. [Google Scholar] [CrossRef]
- Le, H.T.; Schaldach, C.M.; Firestone, G.L.; Bjeldanes, L.F. Plant-derived 3,3′-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J. Biol. Chem. 2003, 278, 21136–21145. [Google Scholar] [CrossRef]
- Wang, C.; Du, X.; Yang, R.; Liu, J.; Xu, D.; Shi, J.; Chen, L.; Shao, R.; Fan, G.; Gao, X.; et al. The prevention and treatment effects of tanshinone IIA on oestrogen/androgen-induced benign prostatic hyperplasia in rats. J. Steroid Biochem. Mol. Biol. 2015, 145, 28–37. [Google Scholar] [CrossRef]
- Zabaiou, N.; Fouache, A.; Trousson, A.; Buñay-Noboa, J.; Marceau, G.; Sapin, V.; Zellagui, A.; Baron, S.; Lahouel, M.; Lobaccaro, J.-M.A. Ethanolic extract of Algerian propolis decreases androgen receptor transcriptional activity in cultured LNCaP cells. J. Steroid Biochem. Mol. Biol. 2019, 189, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jiang, J.; Zhang, S.; Zhu, L.; Zou, J.; Diao, Y.; Xiao, W.; Shan, L.; Sun, H.; Zhang, W.; et al. Discovery of natural estrogen receptor modulators with structure-based virtual screening. Bioorg. Med. Chem. Lett. 2013, 23, 3329–3333. [Google Scholar] [CrossRef] [PubMed]
- Vahlensieck, W.; Fabricius, P.G.; Hell, U. Drug therapy of benign prostatic hyperplasia. Fortschr. Med. 1996, 114, 407–411. [Google Scholar] [PubMed]
- Hu, Z.; Zhang, D.; Wang, D.; Sun, B.; Safoor, A.; Young, C.Y.F.; Lou, H.; Yuan, H. Bisbibenzyls, novel proteasome inhibitors, suppress androgen receptor transcriptional activity and expression accompanied by activation of autophagy in prostate cancer LNCaP cells. Pharm. Biol. 2016, 54, 364–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zava, D.T.; Blen, M.; Duwe, G. Estrogenic activity of natural and synthetic estrogens in human breast cancer cells in culture. Environ. Health Perspect. 1997, 105 (Suppl. 3), 637–645. [Google Scholar] [CrossRef] [PubMed]
- Adjakly, M.; Ngollo, M.; Dagdemir, A.; Judes, G.; Pajon, A.; Karsli-Ceppioglu, S.; Penault-Llorca, F.; Boiteux, J.-P.; Bignon, Y.-J.; Guy, L.; et al. Prostate cancer: The main risk and protective factors-Epigenetic modifications. Ann. Endocrinol. 2015, 76, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.N.; Setzer, W.N. A molecular docking study of phytochemical estrogen mimics from dietary herbal supplements. Silico Pharmacol. 2015, 3, 4. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef]
- Rambur, A.; Lours-Calet, C.; Beaudoin, C.; Buñay, J.; Vialat, M.; Mirouse, V.; Trousson, A.; Renaud, Y.; Lobaccaro, J.-M.; Baron, S.; et al. Sequential Ras/MAPK and PI3K/AKT/mTOR pathways recruitment drives basal extrusion in the prostate-like gland of Drosophila. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).