Acid Soil Improvement Enhances Disease Tolerance in Citrus Infected by Candidatus Liberibacter asiaticus
Abstract
1. Introduction
2. Results
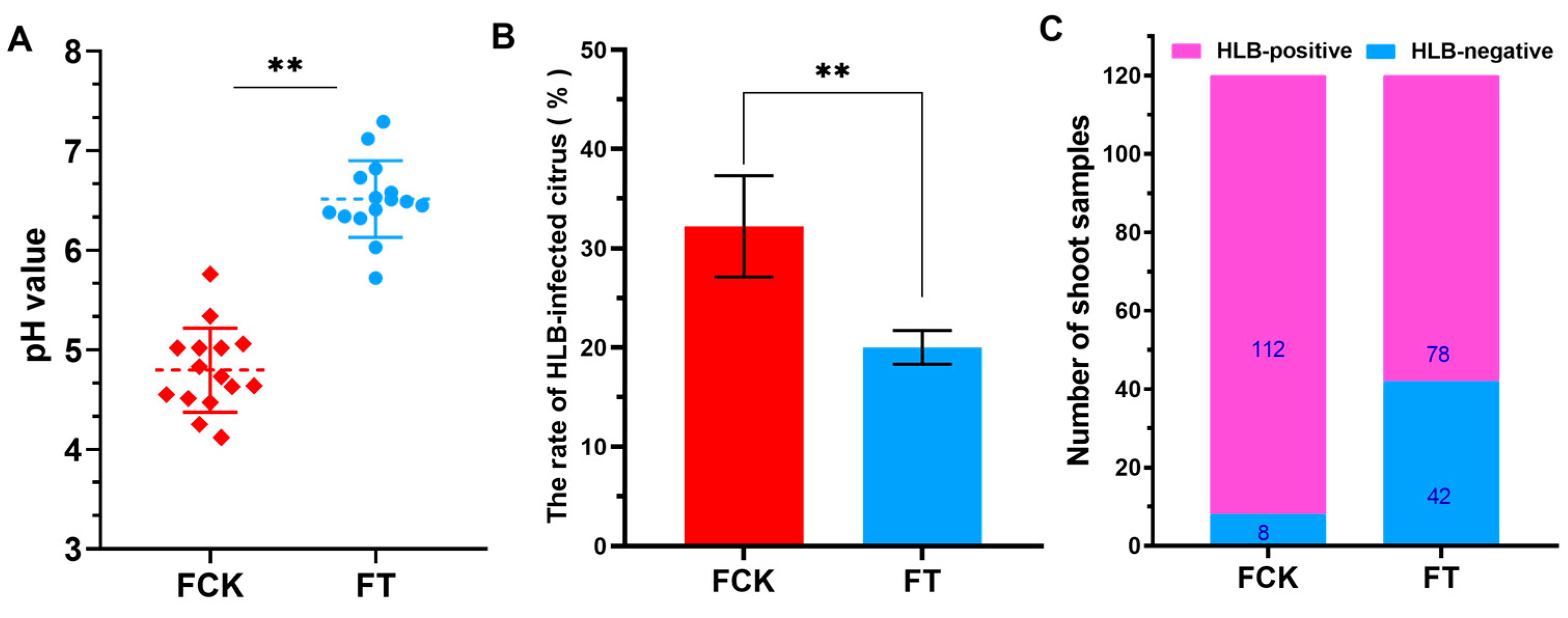
2.1. Effect of Soil Ameliorators on pH Value of Acidic Soil in Citrus Orchard
2.2. Effect of Acid Soil Improvement on Management of HLB Disease in Southern China
2.2.1. HLB Infections Rate
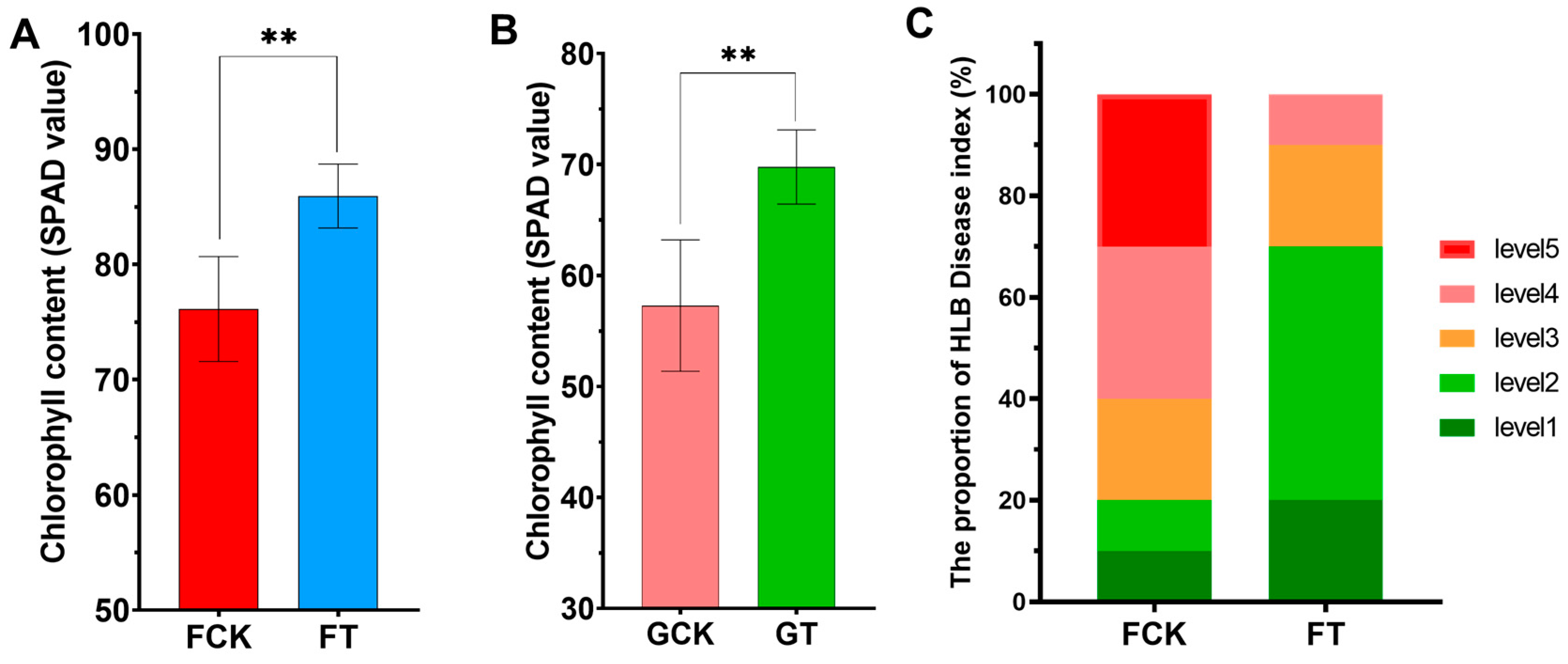
2.2.2. Chlorophyll Content and Disease Index
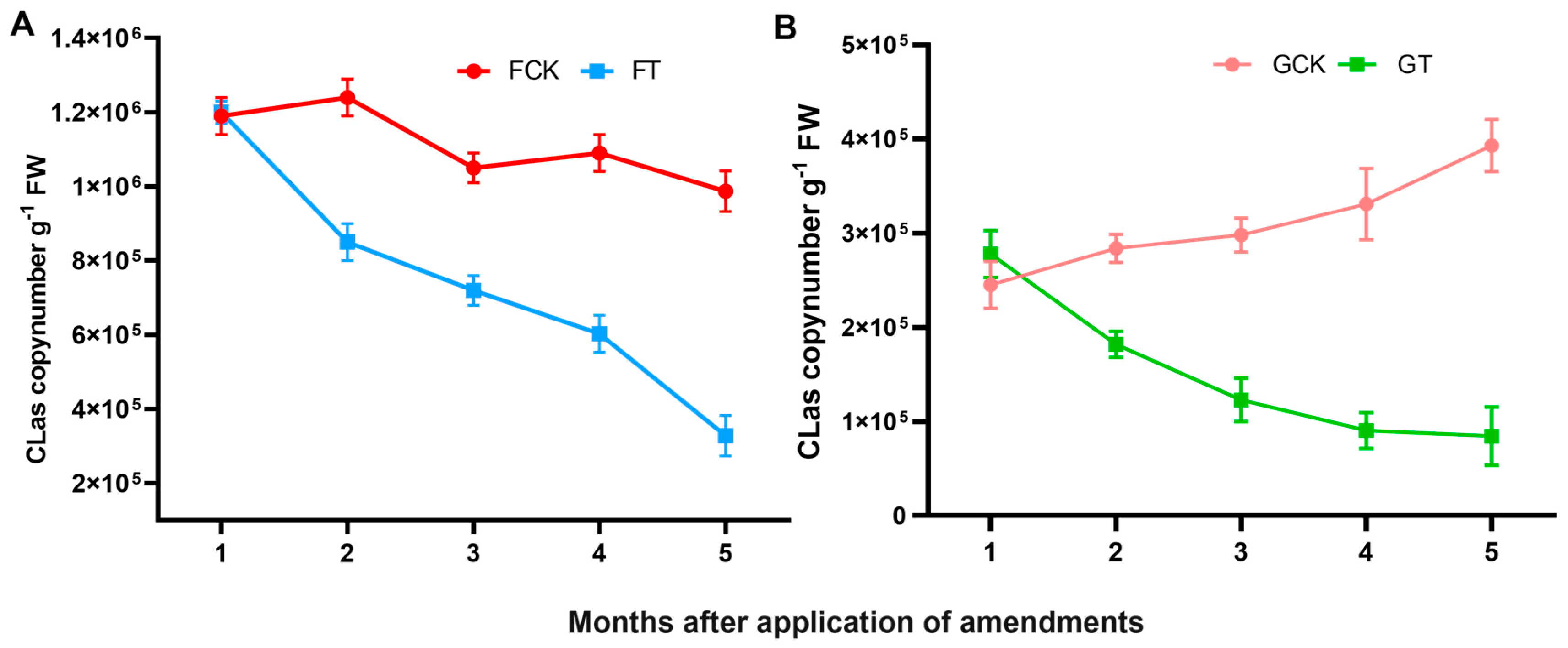
2.2.3. The titre of CLas bacterium
2.2.4. Fruit Yield and Quality
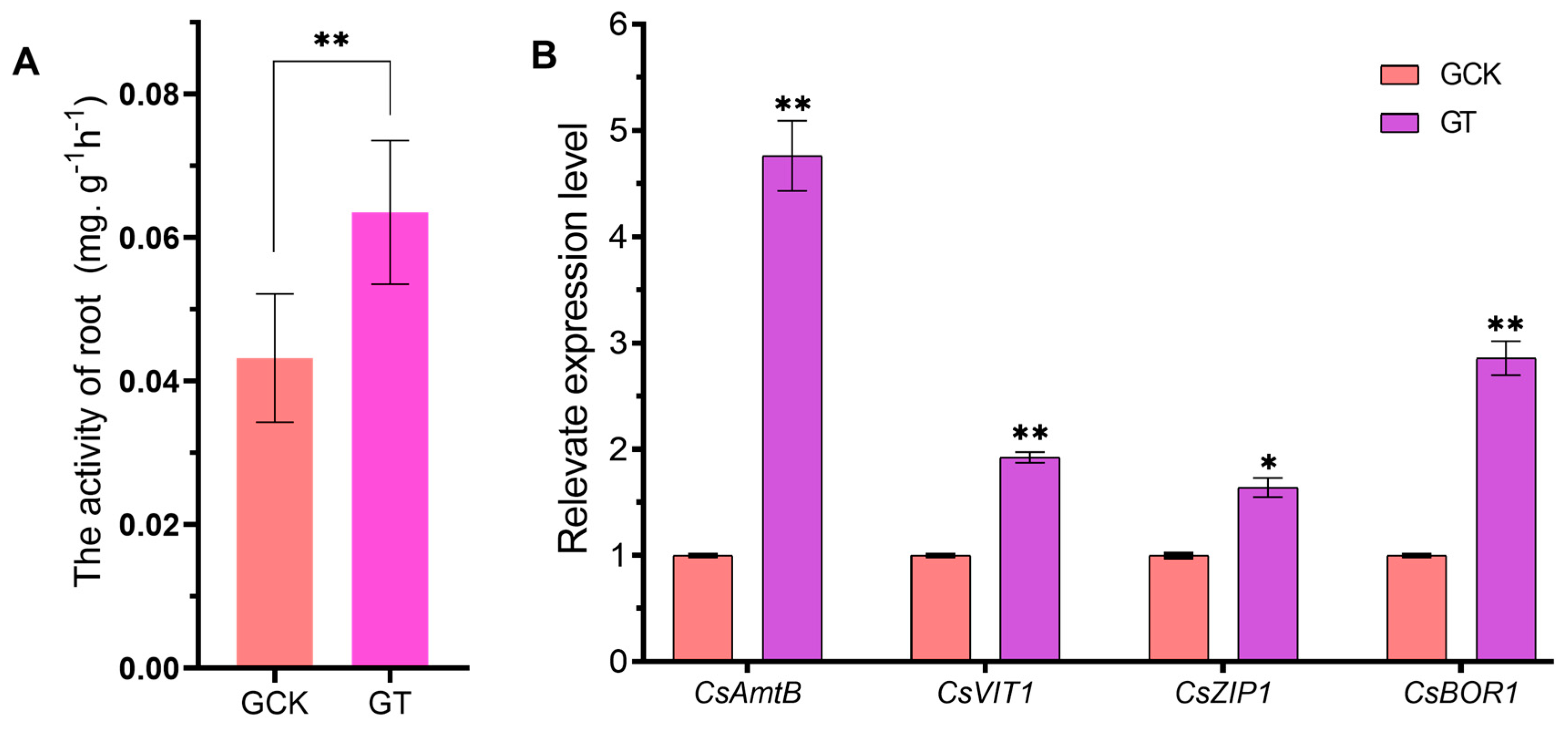
2.3. Effect of Soil pH on the Expression of the Ion Transport-Related Genes and Metabolic Activity in the HLB-Infected Roots
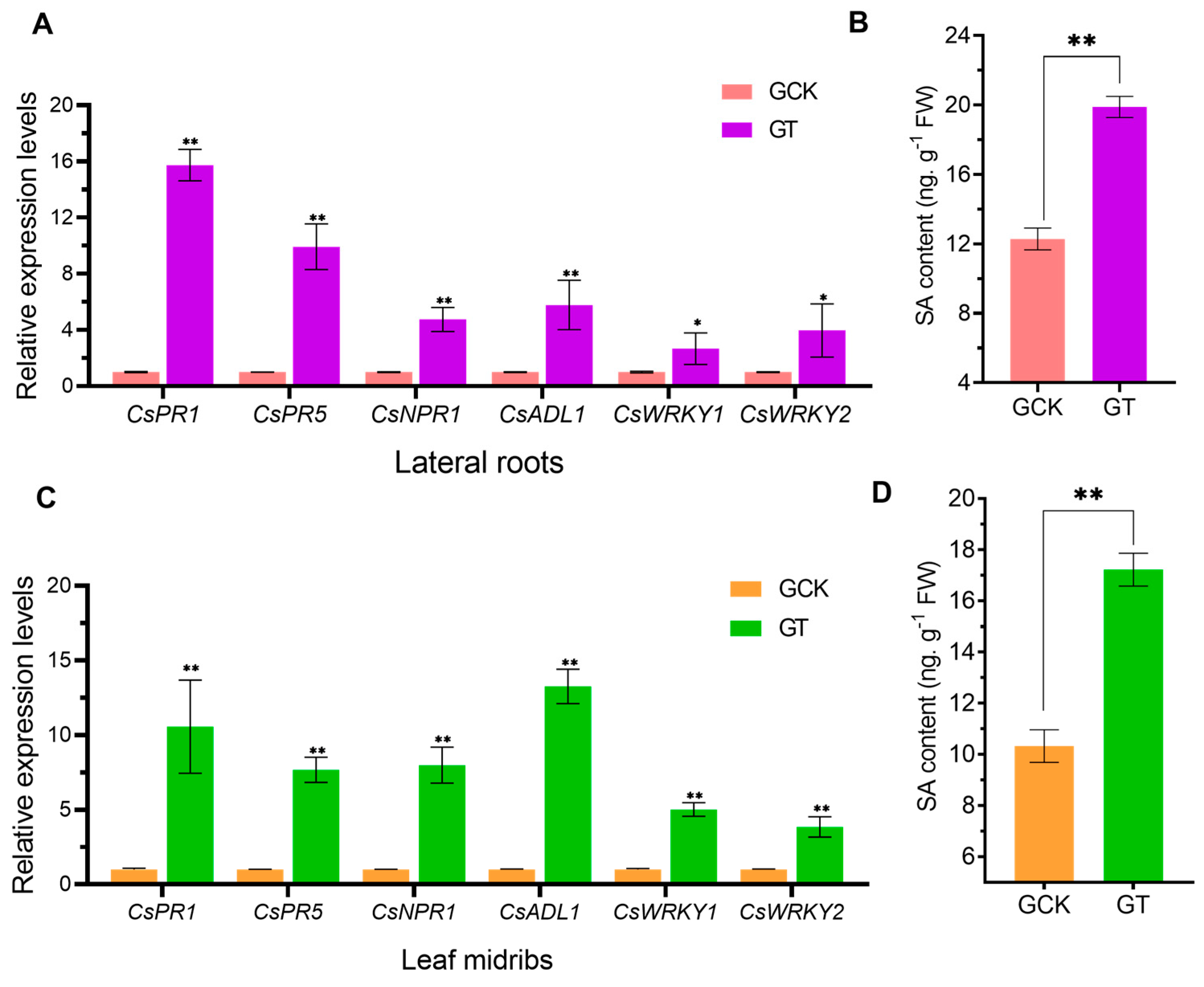
2.4. Effect of Soil pH on the SA Content and Expression of the Resistance-Related Genes in the HLB-Infected Plants
2.5. Proteome Characteristics of Midribs of HLB-Infected Plants Grown in Different Soil pH Conditions
3. Discussion
4. Materials and methods
4.1. Plant Materials and Soil Treatment in Field Trial
4.2. Plant Materials and Soil Treatment in Greenhouse Trial
4.3. Measurement of Soil pH
4.4. Detection of CLas Bacterium
4.5. Titres Measurement of CLas Bacterium
4.6. HLB Disease Index Assessment
4.7. Chlorophyll Determination
4.8. Fruit Yield, Fruit Diameter and Fruit Quality
4.9. Electron Microscopy Observation of Citrus Vascular Bundles
4.10. Measurement of Root Activity Using TTC Method
4.11. Determining the Endogenous Levels of SALICYLIC acid (SA)
4.12. Gene Expression Analysis
4.13. Protein Extraction
4.14. Proteomic TMT and Data Analysis
4.15. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, N. The Citrus Huanglongbing Crisis and Potential Solutions. Mol. Plant 2019, 12, 607–609. [Google Scholar] [CrossRef]
- da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Ferrarezi, R.S.; Qureshi, J.A.; Wright, A.L.; Ritenour, M.A.; Macan, N.P.F. Citrus Production Under Screen as a Strategy to Protect Grapefruit Trees From Huanglongbing Disease. Front. Plant Sci. 2019, 10, 1598. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; He, P.; Wu, Y.; He, P.; Khan, S.; Huang, M.; Cui, W.; He, P.; He, Y. Huanglongbing Control: Perhaps the End of the Beginning. Microb. Ecol. 2018, 76, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.C.; Xia, Y.L.; Lin, X.J.; Hu, H.Q.; Wang, X.D.; Ruan, C.Q.; Lu, L.M.; Bo, L.I.U. Evaluation of thermotherapy against Huanglongbing (citrus greening) in the greenhouse. J. Integr. Agric. 2016, 15, 119. [Google Scholar] [CrossRef]
- Zhou, C. Reconsideration on the control strategy of Citrus Huanglongbing. Plant Prot. 2018, 5, 30–33. [Google Scholar]
- Blaustein, R.A.; Lorca, G.L.; Teplitski, M. Challenges for Managing Candidatus Liberibacter spp. (Huanglongbing Disease Pathogen): Current Control Measures and Future Directions. Phytopathology 2018, 108, 424–435. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Powell, C.A.; Avery, P.B.; Wang, J.; Huang, Y.; Duan, Y. Field evaluation of integrated management for mitigating citrus huanglongbing in Florida. Front. Plant Sci. 2019, 9, 1890. [Google Scholar] [CrossRef]
- Wang, N.; Stelinski, L.L.; Pelz-Stelinski, K.S.; Graham, J.H.; Zhang, Y. Tale of the Huanglongbing disease pyramid in the context of the citrus microbiome. Phytopathology 2017, 107, 380–387. [Google Scholar] [CrossRef]
- Tardy, V.; Mathieu, O.; Lévêque, J.; Terrat, S.; Chabbi, A.; Lemanceau, P.; Ranjard, L.; Maron, P.A. Stability of soil microbial structure and activity depends on microbial diversity. Env. Microbiol. Rep. 2014, 6, 173–183. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, S.; Liu, X.; Jiang, Q.; Ding, W. Soil acidification amendments change the rhizosphere bacterial community of tobacco in a bacterial wilt affected field. Appl. Microbiol. Biotechnol. 2018, 102, 9781–9791. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major chinese croplands. Science 2010, 327, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.C.; Semenov, A.M.; Van Diepeningen, A.D.; De Vos, O.J.; Blok, W.J. Relation between soil health, wave-like fluctuations in microbial populations, and soil-borne plant disease management. Proc. Eur. J. Plant Pathol. 2006, 115, 105–122. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Wang, J.; Yang, L.; Zhang, S.; Xu, C.; Ding, W. Soil acidification aggravates the occurrence of bacterial wilt in south China. Front. Microbiol. 2017, 8, 703. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Z.; Zhou, T.; Xin, Z.; Wu, L.; Luo, Y.; Christie, P. Aluminum toxicity decreases the phytoextraction capability by cadmium/zinc hyperaccumulator Sedum plumbizincicola in acid soils. Sci. Total Env. 2020, 711, 134591. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification - A critical review. Sci. Total Env. 2017, 1, 601–611. [Google Scholar] [CrossRef]
- Wan, W.; Tan, J.; Wang, Y.; Qin, Y.; He, H.; Wu, H.; Zuo, W.; He, D. Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Sci. Total Env. 2020, 700, 134418. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Pang, Z.; Kolbasov, V.G.; Ehsani, R.; Carter, E.W.; Wang, N. Developing citrus huanglongbing (HLB) management strategies based on the severity of symptoms in HLB-endemic citrus-producing regions. Phytopathology 2019, 109, 582–592. [Google Scholar] [CrossRef]
- Ayadi, M.; Hanana, M.; Kharrat, N.; Merchaoui, H.; Marzoug, R.B.; Lauvergeat, V.; Rebaï, A.; Mzid, R. The WRKY Transcription Factor Family in Citrus: Valuable and Useful Candidate Genes for Citrus Breeding. Appl. Biochem. Biotechnol. 2016, 180, 516–543. [Google Scholar] [CrossRef]
- Larkin, R.P. Soil Health Paradigms and Implications for Disease Management. Annu. Rev. Phytopathol. 2015, 53, 199–221. [Google Scholar] [CrossRef]
- Bennett, J.A.; Klironomos, J. Mechanisms of plant–soil feedback: Interactions among biotic and abiotic drivers. New Phytol. 2019, 222, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.E.; Bennett, A.E.; Newton, A.C.; White, P.J.; McKenzie, B.M.; George, T.S.; Pakeman, R.J.; Bailey, J.S.; Fornara, D.A.; Hayes, R.C. Liming impacts on soils, crops and biodiversity in the UK: A review. Sci. Total Env. 2018, 610–611, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Huang, X.; Lu, Y.; Wen, D.; Xue, J.; Li, X.; Zhong, B.; Peng, Z. Survey of soil available iron content at navel orange orchards in southern Jiangxi province. South. China Fruits 2015, 44, 53–55. [Google Scholar]
- Li, Z.F.; Huang, C.; Pan, Z.T.; Chen, X.X.; Tang, Z.; Chen, C.L.; Chen, J.Y. Fertility characters and relationship between soil organic matter and nutrients of navel orange orchards in Fuchuan of Guangxi. J. South. Agric. 2015, 46, 996–1001. [Google Scholar]
- Lin, M.; Feng, X.; Zhang, W.; Ping, X.; Yao, Z.; Wang, Y.; Wang, T. Environmental Quality and Evaluation of Soil Heavy Metals in Main Citrus Producting Areas of Taizhou. J. Zhejiang Agric. Sci. 2012, 1, 1301–1305. [Google Scholar]
- Tao, M.; Ying, H.; Sun, Q.; Tan, Q.; Li, C.; Hu, X. Soil and Plant Nutrient Status of Citrus Orchard in Hubei and Hunan Province. J. Hubei Agric. Sci. 2016, 13, 3289–3292. [Google Scholar]
- Chen, L.; Chen, Y.; Cui, H.; Xie, X.; Chen, J.; Yao, Q. Analysis of Nutrient Status of Soil and Plants in‘Shatangju’’ Orchards in Main Production Areas of Guangdong Province. South. China Fruits 2013, 1, 3. [Google Scholar]
- Qian, X.; Lin, X.; Xiao, J.; Li, F. Study on Quality Evaluation of Soil pH, Nutrient Relationship and Soil Fertility in Fujian Orchards. Fujian Sci. Technol. Trop. Crop. 2017, 42, 9–17. [Google Scholar]
- Su, T.; Zhou, X.; Xu, M.; Wu, T.; Gao, A.; Shi, X. Study on Nutrient Status of Citrus Orchard Soil in Chongqing. Soils 2017, 49, 897–902. [Google Scholar]
- Zhou, X.; Shi, X.; Sun, P.; Li, W.; Dai, H.; Peng, L.; Chun, C. Status of soil fertility in citrus orchards of Chongqing Sanxia Reservoir Area. J. Plant. Nutr. Fertil. 2010, 16, 817–823. [Google Scholar]
- Yan, S.; Wu, C.; Meng, L.; Ding, X.; Dong, P.; Deng, H.; Lei, J.; Gong, Y.; Bao, L. An analysis of soil nutrient status of kiwifruit orchard in Qianjiang, Chongqing. Geophys. Geochem. Explor. 2019, 43, 1123–1130. [Google Scholar]
- Heyburn, J.; McKenzie, P.; Crawley, M.J.; Fornara, D.A. Long-term belowground effects of grassland management: The key role of liming: The. Ecol. Appl. 2017, 27, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Nwugo, C.C.; Lin, H.; Duan, Y.; Civerolo, E.L. The effect of ‘Candidatus Liberibacter asiaticus’ infection on the proteomic profiles and nutritional status of pre-symptomatic and symptomatic grapefruit (Citrus paradisi) plants. Bmc Plant. Biol. 2013, 13, 59. [Google Scholar] [CrossRef]
- Ramrez-Rodrguez, V.; Lpez-Bucio, J.; Herrera-Estrella, L. Adaptive Responses in Plants to Nonoptimal Soil pH. In Plant Abiotic Stress; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 145–170. ISBN 1405122382. [Google Scholar]
- Burnham, M.B.; Cumming, J.R.; Adams, M.B.; Peterjohn, W.T. Soluble soil aluminum alters the relative uptake of mineral nitrogen forms by six mature temperate broadleaf tree species: Possible implications for watershed nitrate retention. Oecologia 2017, 185, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of “Candidatus Liberibacter asiaticus” root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant. Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Nejat, N.; Mantri, N. Plant Immune System: Crosstalk Between Responses to Biotic and Abiotic Stresses the Missing Link in Understanding Plant Defence. Curr. Issues Mol. Biol. 2017, 23, 1–16. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. Salicylic Acid and its Function in Plant Immunity. J. Integr. Plant. Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, J.; Wang, N. Control of citrus huanglongbing via trunk injection of plant defense activators and antibiotics. Phytopathology 2018, 108, 186–195. [Google Scholar] [CrossRef]
- Li, J.; Pang, Z.; Trivedi, P.; Zhou, X.; Ying, X.; Jia, H.; Wang, N. “Candidatus liberibacter asiaticus” encodes a functional salicylic acid (SA) hydroxylase that degrades SA to suppress plant defenses. Mol. Plant.-Microbe Interact. 2017, 30, 620–630. [Google Scholar] [CrossRef]
- Hamamouch, N.; Li, C.; Seo, P.J.; Park, C.M.; Davis, E.L. Expression of Arabidopsis pathogenesis-related genes during nematode infection. Mol. Plant. Pathol. 2011, 12, 355–364. [Google Scholar] [CrossRef]
- Corbin, C.; Drouet, S.; Markulin, L.; Auguin, D.; Lainé, É.; Davin, L.B.; Cort, J.R.; Lewis, N.G.; Hano, C. A genome-wide analysis of the flax (Linum usitatissimum L.) dirigent protein family: From gene identification and evolution to differential regulation. Plant Mol. Biol. 2018, 97, 73–101. [Google Scholar] [CrossRef] [PubMed]
- Zubieta, C.; Ross, J.R.; Koscheski, P.; Yang, Y.; Pichersky, E.; Noel, J.P. Structural Basis for Substrate Recognition in the Salicylic Acid Carboxyl Methyltransferase Family. Plant. Cell 2003, 15, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.J.; Green, S.A.; Chen, X.; Bailleul, E.J.D.; Matich, A.J.; Wang, M.Y.; Atkinson, R.G. Functional Genomics Reveals That a Compact Terpene Synthase Gene Family Can Account for Terpene Volatile Production in Apple. Plant. Physiol. 2013, 161, 787–804. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Yuan, J.S.; Köllner, T.G.; Chen, Y.; Guo, Y.; Zhuang, X.; Chen, X.; Zhang, Y.J.; Fu, J.; et al. The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae. Plant. Biotechnol. J. 2018, 16, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Mugford, S.T.; Qi, X.; Bakht, S.; Hill, L.; Wegel, E.; Hughes, R.K.; Papadopoulou, K.; Melton, R.; Philo, M.; Sainsbury, F.; et al. A serine carboxypeptidase-like acyltransferase is required for synthesis of antimicrobial compounds and disease resistance in oats. Plant. Cell 2009, 21, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Michlmayr, H.; Schweiger, W.; Malachova, A.; Shin, S.; Huang, Y.; Dong, Y.; Wiesenberger, G.; McCormick, S.; Lemmens, M.; et al. A barley UDP-glucosyltransferase inactivates nivalenol and provides Fusarium Head Blight resistance in transgenic wheat. J. Exp. Bot. 2017, 68, 2187–2197. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of huanglongbing or greening disease on orange juice quality, a review. Front. Plant. Sci. 2019, 9, 1976. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, J.; Zhu, Q.; Zhang, Z.; Lin, X. pH is the primary determinant of the bacterial community structure in agricultural soils impacted by polycyclic aromatic hydrocarbon pollution. Sci. Rep. 2017, 7, 40093. [Google Scholar] [CrossRef]
- Trivedi, P.; He, Z.; Van Nostrand, J.D.; Albrigo, G.; Zhou, J.; Wang, N. Huanglongbing alters the structure and functional diversity of microbial communities associated with citrus rhizosphere. Isme J. 2012, 6, 363–383. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Mahony, S.; Baldock, J.A.; Butterly, C.R. Factors affecting the measurement of soil pH buffer capacity: Approaches to optimize the methods. Eur. J. Soil Sci. 2015, 66, 53–64. [Google Scholar] [CrossRef]
- Tatineni, S.; Sagaram, U.S.; Gowda, S.; Robertson, C.J.; Dawson, W.O.; Iwanami, T.; Wang, N. In planta distribution of “Candidatus Liberibacter asiaticus” as revealed by polymerase chain reaction (PCR) and real-time PCR. Phytopathology 2008, 98, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, T.R.; Graça, J.V.d.; Bassanezi, R.B. Citrus Huanglongbing: The Pathogen and Its Impact. Plant. Heal. Prog. 2007, 8, 31. [Google Scholar] [CrossRef]
- El-Zeftawi, B.M. Regulating pre-harvest fruit drop and the duration of the harvest season of grapefruit with 2,4-D and GA. J. Hortic. Sci. 1980, 55, 211–217. [Google Scholar] [CrossRef]
- Baldwin, E.; Plotto, A.; Manthey, J.; Mccollum, G.; Bai, J.; Irey, M.; Cameron, R.; Luzio, G. Effect of liberibacter infection (Huanglongbing disease) of citrus on orange fruit physiology and fruit/fruit juice quality: Chemical and physical analyses. J. Agric. Food Chem. 2010, 58, 1247–1262. [Google Scholar] [CrossRef]
- Yamauchi, T.; Watanabe, K.; Fukazawa, A.; Mori, H.; Abe, F.; Kawaguchi, K.; Oyanagi, A.; Nakazono, M. Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J. Exp. Bot. 2014, 65, 261–273. [Google Scholar] [CrossRef]
- Matsuura, H.; Aoi, A.; Satou, C.; Nakaya, M.; Masuta, C.; Nabeta, K. Simultaneous UPLC MS/MS analysis of endogenous jasmonic acid, salicylic acid, and their related compounds. Plant. Growth Regul. 2009, 57, 293–301. [Google Scholar] [CrossRef]
- Schmittgen, D.T.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]

| FT | FCK | |
|---|---|---|
| Production index | ||
| Yield (kg × tree−1) | 57.6 ± 2.40 * | 52 ± 3.00 |
| Fruit diameter (cm) | 7.86 ± 0.19 * | 7.25 ± 0.31 |
| Fruit drop rate (%) | 22.25 ± 1.90 ** | 47.43 ± 3.40 |
| Fruit quality index | ||
| Soluble solids (%) | 14.5 ± 0.62 * | 11.3 ± 1.21 |
| Vitamin C (mg × 100 g−1) | 38.4 ± 0.92 | 39.8 ± 2.32 |
| Glucose (g × 100 g−1) | 2.51±0.21 * | 1.51 ± 0.10 |
| Sucrose (g × 100 g−1) | 4.40 ± 0.11 ** | 0.98 ± 0.08 |
| Fructose (g × 100 g−1) | 2.40 ± 0.10 ** | 1.50 ± 0.12 |
| Maltose (g × 100 g−1) | 0.43 ± 0.13 | ND |
| UniProtKB ID | Fold Change | p-Value | Description | Species |
|---|---|---|---|---|
| Defence/stress responses | ||||
| A0A067H9X4 | 2.58 | 0.0039 | Thaumatin-like protein (PR5) | Dorcoceras hygrometricum |
| A0A067DC18 | 1.6 | 0.0001 | Pathogenesis-related protein 1 | Arabidopsis thaliana |
| A0A067FFK4 | 1.51 | 0.0001 | Lipoxygenase | Citrus sinensis |
| A0A067DHQ0 | 1.53 | 0.0001 | Like serine/threonine-protein kinase | Citrus sinensis |
| Secondary metabolism | ||||
| A0A067FUD3 | 2.53 | 0.0007 | Salicylate carboxymethyltransferase | Handroanthus impetiginosus |
| A0A067DZ96 | 1.51 | 0.0007 | SABATH methyltransferase 3 | Lonicera japonica var. chinensis |
| A0A067E1I5 | 1.84 | 0.0003 | Caffeic acid 3-O-methyltransferase | Zea mays |
| A0A067DNI8 | 1.51 | 0.009 | Caffeic acid 3-O-methyltransferase | Zea mays |
| A0A067DIU3 | 1.55 | 0.0001 | Orcinol O-methyltransferase | Medicago truncatula |
| A0A067DU63 | 1.82 | 0.0011 | Terpene synthase | Citrus sinensis |
| A0A067F9Y6 | 1.72 | 0.0039 | Dirigent protein | Citrus sinensis |
| A0A067FU00 | 1.67 | 0.0001 | Dirigent protein | Citrus sinensis |
| A0A067F6J2 | 1.62 | 0.0001 | Dirigent protein | Citrus sinensis |
| A0A067D7I5 | 1.71 | 0.0003 | Serine-type carboxypeptidase | Citrus sinensis |
| A0A067EBY4 | 1.54 | 0.009 | Serine-type carboxypeptidase | Citrus sinensis |
| A0A067D666 | 1.51 | 0.0001 | Serine-type carboxypeptidase | Citrus sinensis |
| A0A067GBM2 | 1.51 | 0.0001 | Putative UDP-glucosyltransferase | Citrus paradisi |
| A0A067FCS1 | 1.51 | 0.0001 | Beta_HSD domain-containing protein | Citrus sinensis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Wang, S.; Zhang, Y.; Qiu, D. Acid Soil Improvement Enhances Disease Tolerance in Citrus Infected by Candidatus Liberibacter asiaticus. Int. J. Mol. Sci. 2020, 21, 3614. https://doi.org/10.3390/ijms21103614
Li B, Wang S, Zhang Y, Qiu D. Acid Soil Improvement Enhances Disease Tolerance in Citrus Infected by Candidatus Liberibacter asiaticus. International Journal of Molecular Sciences. 2020; 21(10):3614. https://doi.org/10.3390/ijms21103614
Chicago/Turabian StyleLi, Bo, Shuangchao Wang, Yi Zhang, and Dewen Qiu. 2020. "Acid Soil Improvement Enhances Disease Tolerance in Citrus Infected by Candidatus Liberibacter asiaticus" International Journal of Molecular Sciences 21, no. 10: 3614. https://doi.org/10.3390/ijms21103614
APA StyleLi, B., Wang, S., Zhang, Y., & Qiu, D. (2020). Acid Soil Improvement Enhances Disease Tolerance in Citrus Infected by Candidatus Liberibacter asiaticus. International Journal of Molecular Sciences, 21(10), 3614. https://doi.org/10.3390/ijms21103614





