Abstract
Novel sulfonamidoindole-based hydrazones with a 2-(hydrazinocarbonyl)-3-phenyl-1H-indole-5-sulfonamide scaffold were synthesized and tested in enzyme inhibition assays against the tumor-associated carbonic anhydrase isoforms, hCA IX and XII, and the off-targets, hCA I and II. The compounds showed selectivity against hCA IX and XII over hCA I and II. Six compounds showed KI values lower than 10 nM against hCA IX or XII. Molecular modeling studies were performed to suggest binding interactions between the ligand and the hCA active sites.
1. Introduction
Carbonic anhydrases (CA) are metalloenzymes that catalyze the reversible hydration of CO2 to HCO3− and H+. This simple but important reaction is widespread amongst many if not all organisms [1]. Many of the six structurally different classes of CAs (i.e., α, β, γ, δ, ζ, and ηCAs) contain a Zinc ion (Zn2+) at the active site that is essential for catalysis [2]. CAs have several important physiological and pathological functions, such as respiration and transporting CO2/HCO3− between metabolizing tissues and lungs, electrolyte secretion [3], pH regulation [4], and biosynthetic reactions such as gluconeogenesis, lipogenesis, ureagenesis [5] and carcinogenicity [6].
The human carbonic anhydrases (hCAs) are a member of the αCAs, which are divided into 16 different isozymes (hCA I-XVI) [7]. These enzymes share similar structure, especially in the active site, but show different catalytic activities and tissue distributions [8]. Cytosolic hCA isoforms I and II (hCA I and hCA II) are widespread in the human body and are targets of clinically used diuretics [9], antiglaucoma [10], drugs, and anticonvulsants [11]. In contrast, transmembrane isoforms, hCA IX and hCA XII, which have an active site on the extracellular part of the cell membrane, are located mainly on hypoxic tumor cells [12] and are validated drug targets for the design of anticancer agents specific for solid hypoxic tumors [13]. Proliferation and survival of tumor cells seem to be closely related to overexpression of both enzymes [14]. Thus, selective inhibition of hCA IX and hCA XII with well characterized CA inhibitors (CAIs) may result in new drugs in chemotherapy.
Sulfonamides, a main class of CAIs, show their effect by binding to the Zn2+ ion of the hCA active site. Many compounds with a sulfonamide moiety are in clinical use as CAIs or at the development process. Most of the sulfonamides act as strong CAIs for many hCAs—including isoforms I and II—causing a wide range of side effects. However, the design of new CAIs, which show selective inhibition for tumor-associated isoenzymes, hCA IX and XII, and weak affinity for hCA I and II, currently receives great attention in medicinal chemistry research [15,16,17]. Indole based sulfonamide derivatives were investigated during the last years and have been evaluated as selective inhibitors of several classes of carbonic anhydrases, including human/mammalian isoforms and pathogens [18,19]. Members of our group investigated 2-(hydrazinocarbonyl)-3-phenyl-1H-indole-5-sulfonamide for their interaction with 12 carbonic anhydrase isoforms in the search of compounds with good inhibitory activity against isozymes, such as CA I, II, VA, VB, VII, IX, and XII, among others [20]. Thus, we explore here the synthesis and structure–activity relationship (SAR) for the inhibition of four CA isoforms (hCA I, II, IX, and XII) with hydrazone derivatives of these compounds as putative CAIs.
2. Results
2.1. Chemistry
As was previously reported by our group, 3-phenyl-5-sulfonamido-1H-indole-2-carbohydrazide 1 was prepared from sulfanilamide as outlined in Scheme 1. After diazotization of the sulfanilamide, condensation of diazonium salt with ethyl 2-benzylacetoacetate 2 led to an intermediate, which was cyclized in acidic medium with formation of the ethyl ester derivative of 3, which was converted to 3 by treatment with hydrazine [20]. Further treatment of 3 with an appropriate carbonyl compound (ketone or aldehyde) yielded hydrazone derivatives 4-24 (Scheme 1).
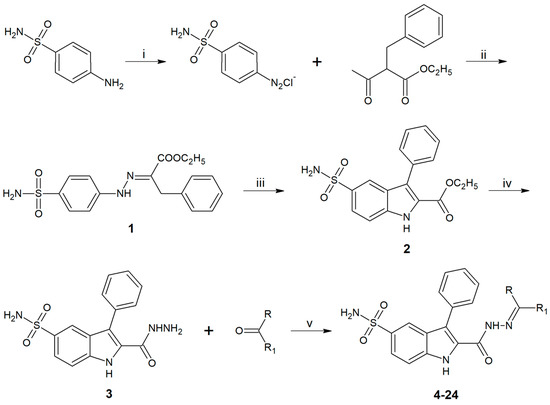
Scheme 1.
Reagents and conditions: (i) NaNO2, HCl, 0 °C; (ii) KOH, 0 °C; (iii) 37% HCl, reflux, 4 h; (iv) H2NNH2.H2O, EtOH, reflux, 6 h; (v) reflux, 6–12 h.
Newly synthesized hydrazones were characterized with melting points and spectral analyses. The IR spectra of compounds 4-24 showed NH stretching bands of the hydrazide-hydrazone group and sulfonamide moiety at the indole ring at 3423–3138 cm−1. The carbonyl functionalities of the new compounds were confirmed by strong C=O stretching bands observed in the 1643–1685 cm−1, while compound 3 showed a band of 1627 cm−1, as expected. Asymmetric and symmetric SO2 stretching vibrations of the sulfonamide group had absorption bands in the 1344–1311 cm−1 and 1176–1147 cm−1 [21,22].
The lH-NMR spectrum of compounds displayed the N-H, the C4-H, the C6-H, and the C7-H protons of the indole ring at 12.39–12.14 ppm (for 9, the second indole ring N-H proton was at 11.59 ppm) as a singlet and 8.85–7.15 ppm, 7.75–7.71, and 7.65–7.61 ppm, respectively. The N-H protons of the hydrazide structure exhibited the expected singlets at the 11.75–9.01 ppm [19]. The SO2NH2 protons had signals at 7.21–7.15 ppm. For compounds 4-11, the azomethine protons resonated at 9.24–8.09 ppm except 10. A broad resonance with 2H integration value at 8.21 and 8.24 ppm was assigned to the two azomethine protons of 10. All the other protons were observed in the expected regions. In the 13C NMR spectrum of compounds, carbon atoms of the hydrazide carbonyl (CONH) and the hydrazone (C=N) groups were observed at in the order of 167.18–157.75 and 157.50–143.19 ppm.
2.2. Enzyme Inhibition Assays
All synthesized compounds were tested in enzyme inhibition assays against the widespread and cytosolic hCA I and II enzymes and the tumor-associated and transmembrane hCA IX and XII enzymes with extracellular catalytic domains (Table 1, Figure 1). The compounds showed selectivity for hCA IX and XII over hCA I and II (Table 1, Figure 1). For hCA IX, 14 of the tested compounds had KI values smaller than 100 nM, including one compound with a KI value of less than 10 nM (i.e., compound 23). Of the 14 compounds, seven had a similar or lower KI value compared to acetazolamide (AAZ). For hCA XII, five of the tested compounds had a similar or lower KI value compared to acetazolamide. Compound 23 was selective for hCA IX with ~seven-fold selectivity over hCA XII and at least ~33-fold selectivity over the off-targets, hCA I and II. Additionally, this compound had a lower KI value compared to acetazolamide (AAZ).

Table 1.
Inhibition data of hCA I, II, IX, XII with compounds 4-24 and the standard inhibitor acetazolamide (AAZ) by a stopped flow CO2 hydrase assay.
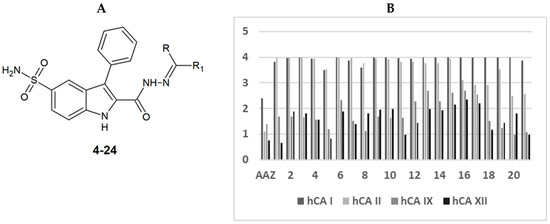
Figure 1.
(A) New 2-(hydrazinocarbonyl)-3-phenyl-1H-indole-5-sulfonamide based hydrazone derivatives (substitutions are shown in Table 1). (B) Bar-graph showing the log KI values of AAZ (acetazolamide) and the tested compounds.
Compound 17 had a cyclohexyl moiety, which was not very well tolerated (Table 1, Figure 1). Adding flexible aliphatic substituents to the cyclohexyl moiety increased the KI value of the ligand for hCA IX, while adding an aromatic phenyl or bulky tert-butyl substituent (compounds 21 and 22, respectively) decreased the KI values (Table 1, Figure 1). A cyclopentyl instead of a cyclohexyl moiety was also not tolerated.
Compound 18, the analog of compound 23 without a cationic nitrogen atom, showed a ~45-fold higher KI value compared to compound 23. This may indicate that a cationic charge was favorable in the ligand binding interactions.
The compounds had, in general, lower KI values for hCA XII, with 18 compounds showing KI smaller than 100 nM, including five compounds with KI smaller than 10 nM. Compound 7 had the lowest measured KI value for hCA XII (KI: 1.4 nM).
The 2-(hydrazinocarbonyl)-3-phenyl-1H-indole-5-sulfonamide was previously tested by members of our group against the tumor-associated hCA IX and XII and the off-targets, hCA I and II [20]. Compared to this previous compound, the newly synthesized hydrazon compounds had higher selectivity for hCA IX/XII compared to the off-targets.
2.3. Molecular Modeling Studies of hCA IX
Compound 23 displayed the lowest KI value for hCA IX of 9.2 nM. The sulfonamide moiety formed direct interactions with the Zn2+ ion (tetrahedral orientation) and the backbone of Thr199 (Figure 2; 23 pose in orange). The indole group of the ligand was situated between Gln92 and Leu198. The side chain hydroxyl group of Thr200 pointed with its hydrogen atom towards the centroid of the ligand’s phenyl group to interact with the π-cloud. A hydrogen bond was formed between the ligand’s carbonyl group and the side chain of Gln67. The cationic aliphatic ring of the ligand was located between Gln67, Leu91, and Val131. The latter cationic group did not form any interactions with the active site residues. Detailed investigation of the ligand’s docked pose and the active site suggested that the ligand cationic aliphatic group could adopt an extended conformation towards Asp132 (Figure 1; 23 pose in purple and Asp132 rotamer in green). The latter residue could adopt another rotameric conformation that reached for the ligand’s cationic group, and both a hydrogen bond and an electrostatic interaction could have occurred (distance cationic nitrogen of the ligand and the anionic oxygen atom of Asp132 2.750 Å). Such an interaction would be consistent with the enzyme inhibition data for hCA IX in which 23 and 24, compounds with a cationic group, had the lowest KI values. Compound 18 was an analog of 24 without the cationic nitrogen atom with a much higher KI value. Additional analogs such as 16, 17, and 19–21 similarly had higher KI values compared to 23.
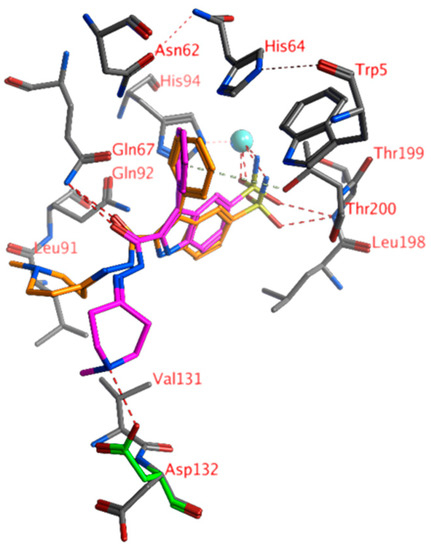
Figure 2.
The docked pose of compound 23 (orange and purple) in the active site of hCA IX (3iai). Hydrogen bonds and interactions with the zinc ion are indicated in red dashed lines. H-arene interactions are indicated in yellow dashed lines. The zinc ion is indicated as a turquoise sphere. The Asp132 rotamer that was able to form a hydrogen bond with the ligand is indicated in green sticks.
2.4. Molecular Modeling Studies of hCA XII
The lowest KI value for hCA XII was measured for compound 7 (KI: 1.4 nM). Again, the sulfonamide moiety formed direct interactions with the Zn2+ ion (tetrahedral orientation) and the backbone of Thr199 (Figure 3). The phenyl and the pyridine groups of the ligands formed H-arene interactions with the hydrogen atoms of Asn62. Further hydrophobic interactions were formed with the side chains of His94, Val121, and Leu198. No hydrogen bonds were formed between the ligand and the active site residues. However, the side chain of Gln92 could adopt a conformation in which a hydrogen bond with the ligand carbonyl group was possible (Figure 3).
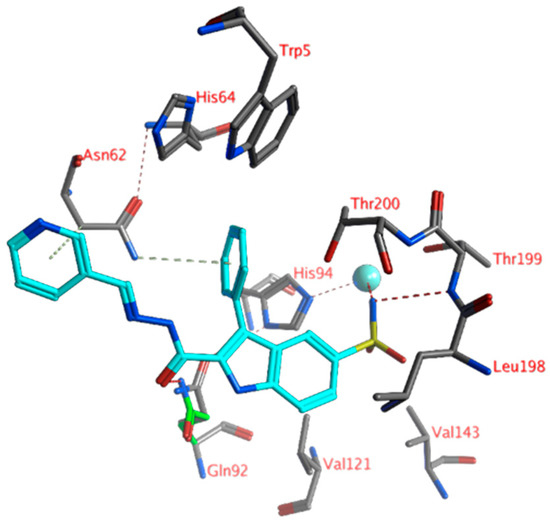
Figure 3.
The docked pose of compound 7 (turquoise) in the active site of hCA XII (1jd0). Hydrogen bonds and interactions with the zinc ion are indicated in red dashed lines. H-arene interactions are indicated in yellow dashed lines. The zinc ion is indicated as a turquoise sphere. The Gln92 rotamer that was able to form a hydrogen bond with the ligand is indicated in green sticks.
In general, the docked poses of the compounds, especially the placement of the sulfonamide and the indole parts of the ligands, were similar due to structural similarity between the compounds and the requirements to form an interaction between the sulfonamide and the zinc ion.
Compounds 18, 19, and 20 had bulky hydrophobic groups that could not form hydrophobic interactions with the active site. This may explain the higher KI values for these ligands. Remarkably, compound 21 had a lower KI value even though it also had a bulky hydrophobic substituent. The phenyl group of the ligand may have formed hydrophobic interactions with the side chain of Asn69 and a Thr91 rotamer (with the methyl group pointing to the ligand instead of the hydroxyl group; Figure 4). It seems this interaction was not possible for compounds 18, 19, and 20.
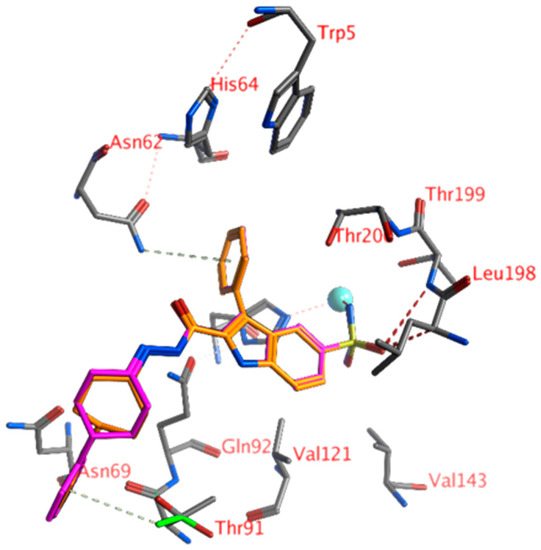
Figure 4.
The docked pose of compound 20 (orange) and 21 (purple) in the active site of hCA XII (1jd0). Hydrogen bonds and interactions with the zinc ion are indicated in red dashed lines. H-arene interactions are indicated in yellow dashed lines. The zinc ion is indicated as a turquoise sphere. The Thr91 rotamer that was able to form an H-arene interaction with 21 is indicated in green sticks.
2.5. Prediction of ADMET Properties
The potential mutagenicity [23], the reactivity [24] and Lipinski’s parameters (weight, hydrogen bond donor, and acceptor count) of the compounds were calculated using the MOE software package (v2018.0101, Chemical Computing Group Inc., Montreal), and the LogP value [25] was calculated using the ALogPs 2.1 webserver [26]. No violation of Lipinski’s rule of five was found [27,28], indicating that the compounds may have passed cell membranes by passive diffusion. Compounds 7, 20, 21, and 23 were not predicted to be mutagenic, but of these four compounds, only compound 7 was not predicted to be reactive. It should be noted, however, that these were theoretical predictions, and experimental validation is still needed.
3. Materials and Methods
Melting points were estimated with a Büchi B-540 (BÜCHI Labortechnik AG, Flawil, Switzerland) melting point apparatus in open capillaries and are presented as uncorrected. Elemental analyses were performed on a Thermo Finnigan Flash EA 1112 (Thermo Electron GmbH, Bremen, Germany) elemental analyzer. IR spectra were recorded on KBr discs using a Perkin-Elmer (Waltham, MA, USA) Model 1600 FT-IR spectrometer. 1H NMR, 13C-NMR (decoupled), and HSQC-2D spectra were obtained on Varian UNITY INOVA 500, Varian Mercury (Agilent, Palo Alto, CA, USA) 400 MHz FT-NMR spectrophotometers using DMSO-d6. The mass spectra were taken on a Waters ZQ micromass LC-MS spectrometer (Waters Corporation, Milford, MA, USA) by using ESI (+) method.
3.1. Synthetic Procedures
3.1.1. 2-Benzyl-2-[N-(4-sulfonamidophenyl)hydrazono]ethanoate (1).
To a solution of 0.01 mole sulfanilamide in 4 mL of 37% HCl, 10 mL of 8% NaNO2 aqueous solution was added dropwise at 0 °C. The solution containing the diazonium salt was poured into an ice-cold mixture of 2.3 g (a little excess of 0.01 mole) ethyl 2-benzylacetoacetate, 10 mL of C2H5OH, 20 mL of H2O, and 2.7 g of KOH. The mixture was kept cold overnight. The hydrazone, produced as an oil, was separated, dissolved in (C2H5)2O, washed with H2O, and dried over anhydrous Na2SO4. (C2H5)2O was distilled, and the oily residue was treated with 5 mL of 37% HCl and set aside for 5 h at room temperature. The resulting solid substance was recrystallized from EtOH.
3.1.2. Ethyl 5-(aminosulfonyl)-3-phenyl-1H-indole-2-carboxylate (2).
A mixture of 0.01 mole ethyl 2-benzyl-2-[N-(4-sulfonamidophenyl)hydrazono]ethanoate and about 10 mL of 37% HCl was heated on a water bath for 4 h, cooled, and poured into 100 mL of H2O. The crude product was filtered, washed with H2O, and recrystallized from EtOH.
3.1.3. 2-(hydrazinylcarbonyl)-3-phenyl-1H-indole-5-sulfonamide (3).
For this compound, 3.45 g (0.01 mole) ethyl 5-(aminosulfonyl)-3-phenyl-1H-indole-2-carboxylate was dissolved in 20 mL of C2H5OH, then 4 mL of H2NNH2-H2O was added and refluxed for 6 h, cooled, and kept cold overnight. The resulting crystals were filtered off, washed with (C2H5)2O, and recrystallized from EtOH /DMF.
3.1.4. General procedure for the synthesis of 3-phenyl-5-sulfamoyl-N’-[(heteroaryl)methylidene]-1H-indole-2-carbohydrazide/3-phenyl-5-sulfamoyl-N’-[(non)substituedcycloalkylidene/alkylidene]-1H-indole-2-carbohydrazide derivatives (4-24).
A mixture of 3-phenyl-5-sulfonamidoindole-2-carbohydrazide (3) (0.005 mole), an appropriate aldehyde/heteroaromatic aldehyde/ketone/cyclic ketone (0.005 mole), was refluxed in 20 mL absolute ethanol for 6–12 h. The precipitate obtained was purified either by recrystallization from ethanol or by washing with hot ethanol.
3.1.5. 3-Phenyl-2-{[2-(thiophene-2-ylmethylidene)hydrazinyl]carbonyl}-1H-indole-5-sulfonamide (4)
Yellow powder (25%); mp 309–310 °C; IR(KBr): υ 3400, 3329, 3250 (NH), 1654 (C=O); 1319, 1147 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 7.13 (1H, s, thiophene C4-H), 7.20 (2H, s, SO2NH2), 7.39 (1H, br s, thiophene C3-H), 7.44 (1H, br s, thiophene C5-H), 7.50-7.57 (5H, m, 3-phenyl C-H), 7.64 (1H, d, J=8.79 Hz, indole C7-H), 7.74 (1H, d, J=8.79 Hz, indole C6-H), 8.14 (1H, s, indole C4-H), 8.31 (1H, s, N=CH), 11.44 (1H, s, CONH), 12.35 (1H, s, indole NH). 13C-NMR (HSQC-2D, DMSO-d6, 125 MHz) δ (ppm): 113.19 [ind. C7 - 7.64 (1H, d, J=8.79 Hz, indole C7-H)], 119.06 [ind. C4 - 8.14 (1H, s, indole C4-H)], 121.79 [ind. C6 - 7.74 (1H, d, J=8.79 Hz, indole C6-H)], 127.59 [thiophene C3 - 7.39 (1H, br s, thiophene C3-H),], 128.32 [thiophene C4 - 7.13 (1H, s, thiophene C4-H)], 129.05, 129.77, 130,16 [3-Phenyl C - 7.50-7.57 (5H, m, 3-phenyl C-H)], 131.88 [thiophene C5 - 7.44 (1H, br s, thiophene C5-H)], 143.19 [N=CH - 8.31 (1H, s, N=CH)]. Anal. Calcd. for C20H16N4O3S2 (424.496): C, 56.59; H, 3.80; N, 13.20; S, 15.11. Found: C, 56.27; H, 3.60; N, 11.61; S, 15.43.
3.1.6. 2-({2-[(5-Bromothiophene-2-yl)methylidene]hydrazinyl}carbonyl)-3-phenyl-1H-indole-5-sulfonamide (5)
Yellow powder (30%); mp 272–273 °C; IR(KBr): υ 3423, 3300, 3217 (NH), 1654 (C=O); 1334, 1153 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 7.20 (2H, s, SO2NH2), 7.26 (1H, br s, thiophene C4-H), 7.31 (1H, br s, thiophene C3-H), 7.40 (1H, br s, 3-phenyl C-H), 7.52 (4H, br s, 3-phenyl C-H), 7.64 (1H, d, J=8.78 Hz, indole C7-H), 7.73 (1H, dd, J=9.04, 1.46 Hz, indole C6-H), 8.13 (1H, s, indole C4-H), 8.24 (1H, s, N=CH), 11.52 (1H, s, CONH), 12.32 (1H, s, indole NH). Anal. Calcd. for C20H15BrN4O3S2 (503.392): C, 47.72; H, 3.00; N, 11.13; S, 12.74. Found: C, 47.82; H, 3.30; N, 11.41; S, 12.90.
3.1.7. 2-({2-[(1-Methyl-1H-pyrrol-2-yl)methylidene]hydrazinyl}carbonyl)-3-phenyl-1H-indole-5-sulfonamide (6)
Yellow powder (40%); mp 298–299 °C; IR(KBr): υ 3419, 3313, 3253, 3213 (NH), 1645 (C=O); 1307, 1157 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 3.84 (3H, s, pyrrol 1-CH3), 6.10 (1H, s, pyrrol C3-H), 6.48 (1H, s, pyrrol C4-H), 6.98 (1H, s, pyrrol C5-H), 7.20 (2H, s, SO2NH2), 7.41 (2H, d, J=6.83 Hz, 3-phenyl C-H), 7.51-7.56 (3H, m, 3-phenyl C-H), 7.64 (1H, d, J=8.78 Hz, indole C7-H), 7.74 (1H, d, J=8.30 Hz, indole C6-H), 8.10 (1H, s, indole C4-H), 8.13 (1H, s, N=CH), 11.13 (1H, s, CONH), 12.31 (1H, s, indole NH). Anal. Calcd. for C21H19N5O3S (421.472): C, 59.84; H, 4.54; N, 16.62; S, 7.61. Found: C, 59.67; H, 4.62; N, 16.81; S, 7.83.
3.1.8. 3-Phenyl-2-{[2-(pyridine-3-ylmethylidene)hydrazinyl]carbonyl}-1H-indole-5-sulfonamide (7)
Ivory powder (60%); mp 287.5–287.8 °C; IR(KBr): υ 3360, 3275, 3190 (NH), 1643 (C=O); 1340, 1159 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 7.19 (2H, s, SO2NH2), 7.32-7.53 (6H, m, 3-phenyl C-H and pyridine C5-H), 7.65 (1H, d, J=8.78 Hz, indole C7-H), 7.74 (1H, d, J=8.30 Hz, indole C6-H), 8.14 (2H, br s, indole C4-H and N=CH), 8.48 (1H, br s, pyridine C4-H), 8.60 (1H, s, pyridine C6-H), 8.82 (1H, s, pyridine C2-H), 11.64 (1H, s, CONH), 12.38 (1H, s, indole NH). Anal. Calcd. for C21H17N5O3S (419.456): C, 60.13; H, 4.09; N, 16.70; S, 7.64. Found: C, 59.80; H, 3.93; N, 16.61; S, 7.43.
3.1.9. 3-Phenyl-2-{[2-(pyridine-4-ylmethylidene)hydrazinyl]carbonyl}-1H-indole-5-sulfonamide (8)
Yellow powder (40%); mp 271.5–271.7 °C; IR(KBr): υ 3236, 3178, 3163 (NH), 1666 (C=O); 1321, 1151 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 7.21 (2H, s, SO2NH2), 7.29-7.58 (7H, m, 3-phenyl C-H and pyridine C3,5-H), 7.65 (1H, d, J=8.30 Hz, indole C7-H), 7.75 (1H, dd, J=8.78, 1.47 Hz, indole C6-H), 8.09 (1H, s, indole C4-H), 8.15 (1H, br s, N=CH), 8.62 (2H, br s, pyridine C2,6-H), 11.75 (1H, s, CONH), 12.38 (1H, s, indole NH). Anal. Calcd. for C21H17N5O3S (419.456): C, 60.13; H, 4.09; N, 16.70; S, 7.64. Found: C, 60.33; H, 4.35; N, 16.91; S, 7.93.
3.1.10. 3-Phenyl-2-{[2-(1H-indole-3-ylmethylidene)hydrazinyl]carbonyl}-1H-indole-5-sulfonamide (9)
Yellow crystal (20%); mp 264–265 °C; IR(KBr): υ 3373, 3313, 3211 (NH), 1643 (C=O); 1319, 1151 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 7.15-7.17 (4H, m, 2.indole C5,6-H and SO2NH2), 7.30-7.45 (2H, m, 3-phenyl C4-H and 2.indole C7-H), 7.51 (2H, t, J=7.81 Hz, 3-phenyl C3,5-H), 7.58 (2H, d, J=7.32 Hz, 3-phenyl C2,6-H), 7.64 (1H, d, J=8.78 Hz, indole C7-H), 7.70 (1H, dd, J=8.78, 1.46 Hz, indole C6-H), 7.79 (1H, d, J=2.93 Hz, 2.indole C2-H), 8.13 (1H, s, indole C4-H), 8.25 (1H, s, N=CH), 8.26 (1H, d, J=7.81 Hz, 2. indole C4-H), 11.07 (1H, s, CONH), 11.59 (1H, s, 2. indole NH), 12.34 (1H, s, indole NH). Anal. Calcd. for C24H19N5O3S (457.504): C, 63.01; H, 4.19; N, 15.31; S, 7.01. Found: C, 59.87; H, 4.50; N, 15.61; S, 7.33.
3.1.11. 2-[(2-Methylidenehydrazinyl)carbonyl]-3-phenyl-1H-indole-5-sulfonamide (10)
White powder (30%); mp 248.5–248.8 °C; IR(KBr): υ 3244 (NH), 1645 (C=O); 1323, 1149 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 7.17 (2H, s, SO2NH2), 7.35-7.49 (5H, m, 3-phenyl C-H), 7.62 (1H, d, J=8.21 Hz, indole C7-H), 7.70 (1H, d, J=8.21 Hz, indole C6-H), 8.10 (1H, s, indole C4-H), 8.21, 8.24 (2H, 2d, J=3.52 Hz, N=CH2), 11.26 (1H, s, CONH), 12.14 (1H, s, indole NH). Anal. Calcd. for C16H14N4O3S (342.372): C, 56.13; H, 4.12; N, 16.36; S, 9.37. Found: C, 56.50; H, 3.93; N, 16.60; S, 9.67.
3.1.12. 2-[(2-Ethylidenehydrazinyl)carbonyl]-3-phenyl-1H-indole-5-sulfonamide (11)
Ivory powder (27%); mp 250–250.1 °C; IR(KBr): υ 3302, 3221 (NH), 1670 (C=O); 1323, 1145 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 1.88 (3H, d, J=3.90 Hz, -CH3), 7.18 (2H, s, SO2NH2), 7.34-7.44 (2H, m, 3-phenyl C4-H and N=CH), 7.46-7.54 (4H, m, 3-phenyl C2,3,5,6-H), 7.61 (1H, d, J=8.30 Hz, indole C7-H), 7.75 (1H, d, J=8.78 Hz, indole C6-H), 8.10 (1H, d, J=1.95 Hz, indole C4-H), 11.00 (1H, s, CONH), 12.31 (1H, s, indole NH). 13CNMR (proton decoupled, DMSO-d6, 150 MHz) δ (ppm): 56.15 (-CH3), 112.69 (ind. C7), 118.64 (ind. C4), 119.20 (ind. C3), 121.26 (ind. C6), 125.46 (ind. C3a), 127.17 (3-Phenyl C4), 128.65 (3-Phenyl C3/5), 129.19 (ind. C2), 129.64 (3-Phenyl C2/6), 133.03 (ind. C5), 136.74 (3-Phenyl C1), 139.05 (ind. C7a), 148.67 (N=C), 157.75 (CONH). Anal. Calcd. for C17H16N4O3S (356.398): C, 57.29; H, 4.52; N, 15.72; S, 9.00. Found: C, 56.96; H, 4.13; N, 16.05; S, 9.37. m/z ESI (positive) 357.36 (M+H)+.
3.1.13. 3-Phenyl-2-{[2-(propan-2-ylidene)hydrazinyl]carbonyl}-1H-indole-5-sulfonamide (12)
White powder (30%); mp 287.9–288 °C; IR(KBr): υ 3334, 3305, 3217 (NH), 1668 (C=O); 1325, 1147 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 1.27 (3H, s, -CH3), 1.89 (3H, s, -CH3), 7.17 (2H, s, SO2NH2), 7.45-7.56 (5H, m, 3-phenyl C-H), 7.62 (1H, d, J=8.30 Hz, indole C7-H), 7.72 (1H, dd, J=8.30, 1.95 Hz, indole C6-H), 7.91 (1H, s, indole C4-H), 9.24 (1H, s, CONH), 12.39 (1H, s, indole NH). Anal. Calcd. for C18H18N4O3S (370.425): C, 58.36; H, 4.90; N, 15.12; S, 8.66. Found: C, 58.76; H, 5.13; N, 15.05; S, 9.01.
3.1.14. 2-{[2-(Butan-2-ylidene)hydrazinyl]carbonyl}-3-phenyl-1H-indole-5-sulfonamide (13)
White powder (78%); mp 285.5–285.6 °C; IR(KBr): υ 3360, 3304, 3224 (NH), 1680 (C=O); 1315, 1151 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 0.93-1.04 (3H, m, -CH3), 1.26 (3H, s, -CH3), 2.20 (2H, d, J=4.88 Hz, -CH2-), 7.17 (2H, s, SO2NH2), 7.45-7.57 (5H, m, 3-phenyl C-H), 7.63 (1H, d, J=8.79 Hz, indole C7-H), 7.71 (1H, dd, J=8.79, 1.46 Hz, indole C6-H), 7.91 (1H, s, indole C4-H), 9.19 (1H, s, CONH), 12.35 (1H, s, indole NH). Anal. Calcd. for C19H20N4O3S (384.452): C, 59.36; H, 5.24; N, 14.57; S, 8.34. Found: C, 59.56; H, 5.36; N, 14.15; S, 8.71.
3.1.15. 2-{[2-(Pentan-3-ylidene)hydrazinyl]carbonyl}-3-phenyl-1H-indole-5-sulfonamide (14)
White powder (60%); mp 288.2–288.3 °C; IR(KBr): υ 3356, 3300, 3221 (NH), 1680 (C=O); 1319, 1151 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 0.63 (3H, s, -CH3), 1.00 (3H, s, -CH3), 1.60 (2H, d, J=6.04 Hz, -CH2-), 2.20 (2H, d, J=6.04 Hz, -CH2-), 7.15 (2H, s, SO2NH2), 7.46-7.58 (5H, m, 3-phenyl C-H), 7.62 (1H, d, J=8.78 Hz, indole C7-H), 7.71 (1H, dd, J=8.23, 1.65 Hz, indole C6-H), 7.85 (1H, s, indole C4-H), 9.20 (1H, s, CONH), 12.39 (1H, s, indole NH). Anal. Calcd. for C20H22N4O3S (398.478): C, 60.28; H, 5.56; N, 14.06; S, 8.05. Found: C, 59.96; H, 5.16; N, 14.40; S, 8.36.
3.1.16. 2-{[2-(4-Methylpentan-2-ylidene)hydrazinyl]carbonyl}-3-phenyl-1H-indole-5-sulfonamide (15)
White powder (50%); mp 255.1–255.2 °C; IR(KBr): υ 3352, 3321, 3265 (NH), 1674 (C=O); 1332, 1151 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 0.83 (6H, d, J=5.49 Hz, -CH(CH3)2), 1.24 (3H, s, -CH3), 1.84-1.88 (1H, m, -CH-), 2.05 (2H, d, J=5.49 Hz, -CH2-), 7.16 (2H, s, SO2NH2), 7.47-7.56 (5H, m, 3-phenyl C-H), 7.62 (1H, d, J=8.78 Hz, indole C7-H), 7.72 (1H, dd, J=8.78, 1.65 Hz, indole C6-H), 7.91 (1H, s, indole C4-H), 9.20 (1H, s, CONH), 12.36 (1H, s, indole NH). Anal. Calcd. for C21H24N4O3S (412.505): C, 61.14; H, 5.86; N, 13.58; S, 7.77. Found: C, 60.83; H, 5.50; N, 13.35; S, 7.56.
3.1.17. 2-[(2-Cyclopentylidenehydrazinyl)carbonyl]-3-phenyl-1H-indole-5-sulfonamide (16)
White powder (84%); mp 313.1–313.2 °C; IR(KBr): υ 3336, 3234, 3138 (NH), 1658 (C=O); 1344, 1153 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 1.50-1.65 (6H, m, -cyclopentyl C-H), 2.30 (2H, m, -cyclopentyl C-H), 7.17 (2H, s, SO2NH2), 7.50-7.59 (5H, m, 3-phenyl C-H), 7.61 (1H, d, J=8.78 Hz, indole C7-H), 7.71 (1H, dd, J=8.78, 0.97 Hz, indole C6-H), 7.89 (1H, s, indole C4-H), 9.01 (1H, s, CONH), 12.39 (1H, s, indole NH). 13CNMR (proton decoupled, DMSO-d6, 150 MHz) δ (ppm): 24.08, 24.11 (cyclopentyl C3/4), 26.62, 32.59 (cyclopentyl C2/5), 112.64 (ind. C7), 118.53 (ind. C4), 121.35 (ind. C6), 126.16 (ind. C3a), 127.93 (3-Phenyl C4), 128.90 (3-Phenyl C3/5), 129.19 (ind. C2), 130.19 (3-Phenyl C2/6), 132.85 (ind. C5), 136.35 (3-Phenyl C1), 136.58 (ind. C7a), 156.90 (N=C), 167.18 (CONH). Anal. Calcd. for C20H20N4O3S (396.462): C, 60.59; H, 5.08; N, 14.13; S, 8.09. Found: C, 60.73; H, 5.10; N, 13.85; S, 7.86. m/z ESI (positive) 396.40 (M+H)+.
3.1.18. 2-[(2-Cyclohexylidenehydrazinyl)carbonyl]-3-phenyl-1H-indole-5-sulfonamide (17)
White powder (80%); mp 285.5–285.6 °C; IR(KBr): υ 3358, 3296, 3147 (NH), 1676 (C=O); 1317, 1149 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 1.34 (2H, s, -cyclohexyl C-H), 1.49 (2H, s, - cyclohexyl C-H), 1.60 (4H, s, -cyclohexyl C-H), 2.28 (2H, s, -cyclohexyl C-H), 7.16 (2H, s, SO2NH2), 7.45-7.57 (5H, m, 3-phenyl C-H), 7.61 (1H, d, J=8.79 Hz, indole C7-H), 7.72 (1H, dd, J=7.32, 1.46 Hz, indole C6-H), 7.94 (1H, s, indole C4-H), 9.52 (1H, s, CONH), 12.36 (1H, s, indole NH). 13CNMR (proton decoupled, DMSO-d6, 150 MHz) δ (ppm): 24.80 (cyclohexyl C4), 25.50, 26.26 (cyclohexyl C3/5), 26.56, 34.69 (cyclohexyl C2/6), 112.56 (ind. C7), 118.46 (ind. C4), 121.18 (ind. C6), 125.95 (ind. C3a), 127.59(3-Phenyl C4), 128.93 (3-Phenyl C3/5), 129.40 (ind. C2), 130.10 (3-Phenyl C2/6), 132.82 (ind. C5), 136.29 (3-Phenyl C1), 136.64 (ind. C7a), 157.26 (N=C), 161.59 (CONH). Anal. Calcd. for C21H22N4O3S (410.489): C, 61.44; H, 5.40; N, 13.65; S, 7.81. Found: C, 61.83; H, 5.17; N, 13.86; S, 7.46. m/z ESI (positive) 411.42 (M+H)+.
3.1.19. 2-{[2-(4-Methylcyclohexylidene)hydrazinyl]carbonyl}-3-phenyl-1H-indole-5-sulfonamide (18)
White powder (75%); mp 295.6–295.7 °C; IR(KBr): υ 3350, 3309, 3165 (NH), 1666 (C=O); 1315, 1147 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 0.87 (3H, d, J=5.37 Hz, 4-CH3), 1.04-1.14 (1H, s, -cyclohexyl C-H), 1.47-1.58 (4H, m, - cyclohexyl C-H), 1.72-1.80 (2H, m, -cyclohexyl C-H), 2.11-2.22 (1H, m, -cyclohexyl C-H), 2.31 (1H, d, J=12.20 Hz, -cyclohexyl C-H), 7.16 (2H, s, SO2NH2), 7.42-7.56 (5H, m, 3-phenyl C-H), 7.61 (1H, d, J=8.79 Hz, indole C7-H), 7.71 (1H, dd, J=8.79 Hz, indole C6-H), 7.93 (1H, s, indole C4-H), 9.51 (1H, s, CONH), 12.35 (1H, s, indole NH). Anal. Calcd. for C22H24N4O3S (424.515): C, 62.24; H, 5.70; N, 13.20; S, 7.55. Found: C, 61.98; H, 5.63; N, 12.85; S, 7.16.
3.1.20. 2-{[2-(4-Ethylcyclohexylidene)hydrazinyl]carbonyl}-3-phenyl-1H-indole-5-sulfonamide (19)
White powder (74%); mp 282.4–282.5 °C; IR(KBr): υ 3348, 3311, 3176 (NH), 1666 (C=O); 1315, 1147 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 0.85 (3H, t, J=7.32 Hz, 4-CH2CH3), 1.16 (1H, t, J=7.32 Hz, -cyclohexyl C-H), 1.21 (2H, t, J=6.83 Hz, 4-CH2CH3), 1.35 (1H, br s, -cyclohexyl C-H), 1.49 (1H, t, J=13.67 Hz, -cyclohexyl C-H), 1.62 (2H, d, J=12.20 Hz, -cyclohexyl C-H), 1.75 (1H, d, J=13.67 Hz, -cyclohexyl C-H), 1.85 (1H, d, J=9.27 Hz, -cyclohexyl C-H), 2.14 (1H, t, J=11.71 Hz, -cyclohexyl C-H), 2.32 (1H, d, J=12.20 Hz, -cyclohexyl C-H), 7.17 (2H, s, SO2NH2), 7.37-7.57 (5H, m, 3-phenyl C-H), 7.62 (1H, d, J=8.79 Hz, indole C7-H), 7.72 (1H, dd, J=8.79 Hz, indole C6-H), 7.93 (1H, s, indole C4-H), 9.50 (1H, s, CONH), 12.37 (1H, s, indole NH). Anal. Calcd. for C23H24N4O3S (424.515): C, 62.24; H, 5.70; N, 13.20; S, 7.55. Found: C, 62.55; H, 5.38; N, 13.63; S, 7.47.
3.1.21. 2-{[2-(4-Propylcyclohexylidene)hydrazinyl]carbonyl}-3-phenyl-1H-indole-5-sulfonamide (20)
White powder (46%); mp 249.7–250 °C; IR(KBr): υ 3354, 3331, 3236 (NH), 1662 (C=O); 1342, 1143 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 0.86 (3H, t, J=7.32 Hz, 4-CH2CH2CH3), 1.06 (1H, t, J=7.32 Hz, -cyclohexyl C-H), 1.15 (2H, d, J=6.34 Hz, 4-CH2CH2CH3), 1.28 (2H, q, J=7.32 Hz, 4-CH2CH2CH3), 1.45-1.51 (3H, m, - cyclohexyl C-H), 1.60 (1H, d, J=12.20 Hz, -cyclohexyl C-H), 1.74 (1H, d, J=13.67 Hz, -cyclohexyl C-H), 1.84 (1H, d, J=14.00 Hz, -cyclohexyl C-H), 2.14 (1H, t, J=10.74 Hz, -cyclohexyl C-H), 2.30-2.36 (1H, m, -cyclohexyl C-H), 7.16 (2H, s, SO2NH2), 7.36-7.57 (5H, m, 3-phenyl C-H), 7.61 (1H, d, J=8.79 Hz, indole C7-H), 7.72 (1H, d, J=8.79 Hz, indole C6-H), 7.93 (1H, s, indole C4-H), 9.50 (1H, s, CONH), 12.36 (1H, s, indole NH). Anal. Calcd. for C24H28N4O3S (452.569): C, 63.69; H, 6.24; N, 12.38; S, 7.09. Found: C, 63.45; H, 6.47; N, 12.78; S, 7.49.
3.1.22. 2-{[2-(4-Phenylcyclohexylidene)hydrazinyl]carbonyl}-3-phenyl-1H-indole-5-sulfonamide (21)
White powder (80%); mp 312.8–313 °C; IR(KBr): υ 3358, 3319, 3280 (NH), 1670 (C=O); 1338, 1159 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 1.28-1.41 (1H, m, -cyclohexyl C-H), 1.58-1.77 (3H, m, -cyclohexyl C-H), 1.87 (1H, d, J=13.18 Hz, -cyclohexyl C-H), 1.96 (1H, br s, -cyclohexyl C-H), 2.26-2.39 (1H, m, -cyclohexyl C-H), 2.46 (1H, d, J=13.18 Hz, -cyclohexyl C-H), 2.78 (1H, m, -cyclohexyl C-H), 7.17 (2H, s, SO2NH2), 7.19-7.21 (3H, m, cyclohexyl 4-phenyl C-H), 7.30 (2H, t, J=7.81 Hz, cyclohexyl 4-phenyl C-H), 7.42-7.48 (1H, m, 3-phenyl C-H), 7.49-7.55 (4H, m, 3-phenyl C-H), 7.63 (1H, d, J=8.79 Hz, indole C7-H), 7.71 (1H, dd, J=8.79, 0.98 Hz, indole C6-H), 7.95 (1H, s, indole C4-H), 9.61 (1H, s, CONH), 12.37 (1H, s, indole NH). Anal. Calcd. for C27H26N4O3S (486.585): C, 66.65; H, 5.39; N, 11.51; S, 6.59. Found: C, 66.38; H, 5.57; N, 11.88; S, 8.94.
3.1.23. 2-{[2-(4-tert-buthylcyclohexylidene)hydrazinyl]carbonyl}-3-phenyl-1H-indole-5-sulfonamide (22)
White powder (55%); mp 284.9–285.2 °C; IR(KBr): υ 3358, 3284, 3161 (NH), 1662 (C=O); 1342, 1143 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 0.83 (9H, s, 3 x CH3), 1.10-1.23 (3H, m, -cyclohexyl C-H), 1.45 (1H, td, J=14.15, 4.88 Hz, -cyclohexyl C-H), 1.64 (1H, d, J=10.74 Hz, -cyclohexyl C-H), 1.80-1.89 (2H, m, -cyclohexyl C-H), 2.13 (1H, t, J=10.74 Hz, -cyclohexyl C-H), 2.36 (1H, d, J=12.69 Hz, -cyclohexyl C-H), 7.16 (2H, s, SO2NH2), 7.47-7.56 (5H, m, 3-phenyl C-H), 7.61 (1H, d, J=8.79 Hz, indole C7-H), 7.71 (1H, d, J=8.79 Hz, indole C6-H), 7.94 (1H, s, indole C4-H), 9.49 (1H, s, CONH), 12.36 (1H, s, indole NH). Anal. Calcd. for C25H30N4O3S (466.595): C, 64.35; H, 6.48; N, 12.01; S, 6.87. Found: C, 64.28; H, 6.35; N, 11.90; S, 6.47.
3.1.24. 2-{[2-(1-Methylpiperidine-4-ylidene)hydrazinyl]carbonyl}-3-phenyl-1H-indole-5-sulfonamide (23)
White powder (65%); mp 250.1–250.3 °C; IR(KBr): υ 3352, 3334, 3305 (NH), 1666 (C=O); 1315, 1159 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 1.71 (2H, br s, piperidine C3/5-H), 2.17 (5H, br s, N-CH3 and piperidine C2/6 -H), 2.30 (2H, br s, piperidine C3/5-H), 2.42 (2H, br s, piperidine C2/6 -H), 7.17 (2H, s, SO2NH2), 7.38-7.54 (5H, m, 3-phenyl C-H), 7.61 (1H, d, J=8.79 Hz, indole C7-H), 7.72 (1H, dd, J=8.78, 1.46 Hz, indole C6-H), 7.96 (1H, s, indole C4-H), 9.66 (1H, s, CONH), 12.39 (1H, s, indole NH). 13CNMR (proton decoupled, DMSO-d6, 150 MHz) δ (ppm): 26.42, 33.99 (piperidine C3/5), 45.20 (N-CH3), 53.88, 55.29 (piperidine C2/6), 112.66 (ind. C7), 118.52 (ind. C4), 118.60 (ind. C3), 121.28 (ind. C6), 125.86 (ind. C3a), 127.59 (3-Phenyl C4), 128.93 (3-Phenyl C3/5), 132.93 (ind. C5), 130.19 (3-Phenyl C2/6), 136.36 (3-Phenyl C1), 136.64 (ind. C7a), 157.51 (N=C), 158.57 (CONH). 13C-NMR (HSQC-2D, DMSO-d6, 125 MHz) δ (ppm): 26.40 [piperidine C3/5 - 1.71 (2H, br s, piperidine C3/5-H)], 33.95 [piperidine C3/5- 2.30 (2H, br s, piperidine C3/5-H)], 45.20 [N-CH3 - 2.17 (5H, br s, N-CH3 and piperidine C2/6-H)], 53.87 [piperidine C2/6- 2.17 (5H, br s, N-CH3 and piperidine C2/6 -H)], 55.28 [piperidine C2/6—2.42 (2H, br s, piperidine C2/6 -H)], 112.63 [ind. C7 - 7.61 (1H, d, J=8.79 Hz, indole C7-H)], 118.55 [ind C4 -7.96 (1H, s, indole C4-H)], 121.27 [ind. C6 - 7.72 (1H, dd, J=8.78, 1.46 Hz, indole C6-H)], 127.58 [3-phenyl C4 - 7.38–7.54 (5H, m, 3-phenyl C-H)], 128.92 [3-phenyl C3/5 - 7.38–7.54 (5H, m, 3-phenyl C-H)], 130.14 [3-phenyl C2/6 - 7.38–7.54 (5H, m, 3-phenyl C-H)]. Anal. Calcd. for C21H23N5O3S (425.504): C, 59.28; H, 5.45; N, 16.46; S, 7.54. Found: C, 58.87; H, 5.30; N, 16.20; S, 7.36.
3.1.25. 2-{[2-(1-Benzylpiperidine-4-ylidene)hydrazinyl]carbonyl}-3-phenyl-1H-indole-5-sulfonamide (24)
White powder (70%); mp 251.2–251.4 °C; IR(KBr): υ 3379, 3352, 3265 (NH), 1685 (C=O); 1311, 1176 (S=O); 1H NMR (DMSO-d6/500 MHz δ (ppm): 1.73 (2H, br s, piperidine C-H), 2.26-2.31 (4H, m, piperidine C-H), 2.48 (2H, s, piperidine C-H), 3.37 (2H, s, N-CH2-), 7.16 (2H, s, SO2NH2), 7.25-7.35 (5H, m, benzyl C-H), 7.39-7.45 (1H, m, 3-phenyl C-H), 7.49-7.55 (4H, m, 3-phenyl C-H), 7.61 (1H, d, J=8.30 Hz, indole C7-H), 7.72 (1H, d, J=8.79 Hz, indole C6-H), 7.96 (1H, s, indole C4-H), 9.67 (1H, s, CONH), 12.37 (1H, s, indole NH). 13CNMR (proton decoupled, DMSO-d6, 150 MHz) δ (ppm): 26.42, 33.93 (piperidine C3/5), 51.50, 52.72 (piperidine C2/6), 61.01 (N-CH2-Ph), 112.53 (ind. C7), 118.45 (ind. C4), 122.49 (ind. C6), 125.76 (ind. C3a), 126. 84, 127.40 (2 x Phenyl C4), 128.08, 128.61 (2 x Phenyl C3/5), 128.82 (ind. C2), 129.38, 130.08 (2 x Phenyl C2/6), 132.90 (ind. C5), 136.30, 136.57 (2 x Phenyl C1), 138.30 (ind. C7a), 157.50 (N=C), 158.50 (CONH). Anal. Calcd. for C27H27N5O3S (501.599): C, 64.65; H, 5.43; N, 13.96; S, 6.39. Found: C, 64.87; H, 5.20; N,14.22; S, 6.16. m/z ESI (positive) 502.63 (M+H)+.
3.2. Enzyme Inhibition Studies
A stopped-flow instrument (SX.18 MV-R Applied Photophysics model) was used for assaying the CO2 hydration activity of various CA isozymes, as reported by Khalifah [29]. Phenol red (at a concentration of 0.2 mM) was used as indicator, working at the absorbance maximum of 557 nm with 10 mM Hepes (pH 7.4) as a buffer and 0.1 M NaClO4 (for constantly maintaining the ionic strength; this anion was not inhibitory in the used concentration) following the CA-catalyzed CO2 hydration reaction for a period of 5–10 s. Saturated CO2 solutions in water at 25 °C were used as substrates. Stock solutions of inhibitors were prepared at a concentration of 10 mM (in DMSO/water 1:1, v/v) and dilutions up to 0.01 nM were done with the assay buffer mentioned above. At least seven different inhibitor concentrations were used for measuring the inhibition constant. For allowing the complete formation of the enzyme-inhibitor adduct, the inhibitor and the enzyme were pre-incubated for 15 min. IC50 values were obtained from dose response curves working at seven different concentrations of the test compound (from 0.1 nM to 50 mM) by fitting the curves using PRISM (www.graphpad.com) and non-linear least squares methods, the obtained values representing the mean of at least three different determinations. The inhibition constants (KI) were derived from the IC50 values by using the Cheng-Prusoff equation as follows: KI=IC50/(1+[S]/KM) where [S] represents the CO2 concentration at which the measurement was carried out, and KM represents the concentration of the substrate at which the enzyme activity was at half maximal. As reported earlier, all enzymes used were recombinant and produced in Escherichia Coli. [30,31,32,33]. The concentrations of enzymes used in the assay were: hCA I, 11.9 nM; hCA II, 7.7 nM; hCA IX, 9.1 nM, and hCA XII, 11.5 nM.
3.3. Molecular Modeling Studies
The three-dimensional structures of all ligands were prepared in their lowest energy conformation using the MOE software package (v2018.0101, Chemical Computing Group, Inc, Montreal, Canada). The sulfonamide nitrogen atoms were assigned a negative charge (R-SO2NH-), and the ligands were energy minimized (MMFF94x force field).
All protein structures were obtained from the RCSB protein databank: hCA I in complex with topiramate (pdb: 3lxe, 1.90 Å), hCA II in complex with water (pdb: 4e3d, 1.60 Å), hCA IX in complex with acetazolamide (pdb: 3iai; 2.20 Å), and hCA XII in complex with acetazolamide (pdb: 1jd0; 1.50 Å). The protein atoms and the active site zinc ions were retained, and all other atoms were omitted. The remaining structure was protonated using the protonate 3D functionality of MOE, and subsequently, the obtained structure was energy-minimized (AMBER14:EHT) [34]. Finally, the obtained protein models were superposed on the hCA I structure using the backbone Cα-atoms and all Zn2+-ions, zinc-binding histidines, and the overall backbone atoms superposed well [root-mean-square deviation (RMSD) value: 1.281 Å].
Docking calculations were performed using the FlexX docking tool (v2.3.2; BioSolveIT GmbH, St. Augustin, Germany) within MOE. The binding pocket was defined as all residues within 6.5 Å of the reference ligand, acetazolamide. The sulfonamide tail of the ligands was forced to adopt a similar orientation and interactions to the Zn2+-ion as observed for acetazolamide using a pharmacophore model. All ligands were docked fifty times, and the best scoring three poses were subjected to refinement calculations. To this end, the ligand and the binding pocket residues were energy minimized and rescored using a GBVI/WSA force field [35].
4. Conclusions
A total of 21 new indole-based sulfonamide derivatives were synthesized and tested against the tumor-associated hCA IX and XII and the widespread off-targets, hCA I and II. The compounds showed potent inhibition in the lower nanomolar range and at least ~six-fold selectivity for hCA IX and XII over hCA I and II. Molecular modeling studies were subsequently performed to gather insight in the possible binding modes between the newly developed CAIs and their respective target hCAs. This information may result in more potent and more selective CAIs for hCA IX and XII.
Author Contributions
Conceptualization, Ö.G.A. and C.T.S.; Synthesis, K.D.Y., N.M.A. and Ö.G.A., Molecular modeling, A.A. and K.D.Y.; Enzyme inhibition assays: S.B., C.T.S. Writing—Original Draft Preparation, K.D.Y., A.A. Ö.G.A.; Writing—Review & Editing, A.A. and Ö.G.A.; Supervision, Ö.G.A.; Project Administration, Ö.G.A.; Funding Acquisition, Ö.G.A.
Funding
This research was funding by the Istanbul University Scientific Research Projects Department under project numbers TSA-2019-30535.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| CA | Carbonic Anhydrases |
| hCA | Human Carbonic Anhydrases |
| CAI | Carbonic Anhydrase Inhibitor |
References
- Supuran, C.T. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med. Chem. 2011, 3, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Scozzafava, A. Carbonic anhydrase inhibitors and their therapeutic potential. Expert Opin. Ther. Pat. 2005, 10, 575–600. [Google Scholar] [CrossRef]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumors as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef]
- Akdemir, A.; Güzel-Akdemir, Ö. The Structure, Physiological Role, and Potential Medicinal Applications of Carbonic Anhydrase V. In Carbonic Anhydrases as Biocatalysts: From Theory to Medical and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 125–138. ISBN 9780444632630. [Google Scholar]
- Supuran, C.T.; Scozzafava, A. Carbonic anhydrases as targets for medicinal chemistry. Bioorganic Med. Chem. 2007, 15, 4336–4350. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Briganti, F.; Tilli, S.; Chegwidden, W.R.; Scozzafava, A. Carbonic anhydrase inhibitors: Sulfonamides as antitumor agents? Bioorganic Med. Chem. 2001, 9, 703–714. [Google Scholar] [CrossRef]
- Carta, F.; Supuran, C.T. Diuretics with carbonic anhydrase inhibitory action: A patent and literature review (2005–2013). Expert Opin. Ther. Pat. 2013, 23, 681–691. [Google Scholar] [CrossRef]
- Masini, E.; Carta, F.; Scozzafava, A.; Supuran, C.T. Antiglaucoma carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Scozzafava, A.; Supuran, C.T. Which carbonic anhydrases are targeted by the antiepileptic sulfonamides and sulfamates? Chem. Biol. Drug Des. 2009, 74, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Akdemir, A.; Güzel-Akdemir, Ö.; Scozzafava, A.; Capasso, C.; Supuran, C.T. Inhibition of tumor-associated human carbonic anhydrase isozymes IX and XII by a new class of substituted-phenylacetamido aromatic sulfonamides. Bioorganic Med. Chem. 2013, 21, 5228–5232. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic Anhydrase Inhibition and the Management of Hypoxic Tumors. Metabolites 2017, 7, 48. [Google Scholar] [CrossRef]
- Nocentini, A.; Supuran, C.T. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: A patent review (2008–2018). Expert Opin. Ther. Pat. 2018, 28, 729–740. [Google Scholar] [CrossRef]
- Puccetti, L.; Fasolis, G.; Vullo, D.; Chohan, Z.H.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, IX, and XII with Schiff’s bases incorporating chromone and aromatic sulfonamide moieties, and their zinc complexes. Bioorganic Med. Chem. Lett. 2005, 15, 3096–3101. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzyme Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [PubMed]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Vullo, D.; Scozzafava, A.; T. Supuran, C.; Guzela, O.; Innocenti, A. 3-Phenyl-1H-Indole-5-Sulfonamides: Structure-Based Drug Design of a Promising Class of Carbonic Anhydrase Inhibitors. Curr. Pharm. Des. 2010, 16, 3317–3326. [Google Scholar]
- Güzel, Ö.; Innocenti, A.; Scozzafava, A.; Salman, A.; Parkkila, S.; Hilvo, M.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis and inhibition studies against mammalian isoforms I-XV with a series of 2-(hydrazinocarbonyl)-3-substituted-phenyl-1H-indole-5-sulfonamides. Bioorganic Med. Chem. 2008, 16, 9113–9120. [Google Scholar] [CrossRef]
- Güzel, Ö.; Temperini, C.; Innocenti, A.; Scozzafava, A.; Salman, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Interaction of 2-(hydrazinocarbonyl)-3-phenyl-1H-indole-5-sulfonamide with 12 mammalian isoforms: Kinetic and X-ray crystallographic studies. Bioorganic Med. Chem. Lett. 2008, 18, 152–158. [Google Scholar] [CrossRef]
- Ergenç, N.; Salman, A.; Gürsoy, A.; Pharmazie, G. Synthesis and antifungal evaluation of some 3-phenyl-2, 5-disubstituted indoles derived from new ethyl-2-benzyl-2-[N-(aryl)hydrazono] ethanoates. Die Pharm. 1990, 45, 346–347. [Google Scholar] [CrossRef]
- Ergenç, N.; Günay, N.S.; Demirdamar, R. Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives. Eur. J. Med. Chem. 1998, 33, 143–148. [Google Scholar] [CrossRef]
- Kazius, J.; Mcguire, R.; Bursi, R. Derivation and Validation of Toxicophores for Mutagenicity Prediction. J. Med. Chem. 2005, 48, 312–320. [Google Scholar] [CrossRef]
- TI, O. Property distribution of drug-related chemical databases. J. Comput. Aided. Mol. Des. 2000, 14, 251–264. [Google Scholar]
- Tetko, I.V.; Tanchuk, V.Y. Application of Associative Neural Networks for Prediction of Lipophilicity in ALOGPS 2.1 Program. J. Chem. Inf. Comput. Sci. 2002, 42, 1136–1145. [Google Scholar] [CrossRef]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual computational chemistry laboratory—Design and description. J. Comput. Aided. Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]
- Christopher, A.L. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Khalifah, R.G. The Carbon Dioxide Hydration Activity of Carbonic Anhydrase. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [PubMed]
- Lou, Y.; McDonald, P.C.; Oloumi, A.; Chia, S.; Ostlund, C.; Ahmadi, A.; Kyle, A.; Auf Dem Keller, U.; Leung, S.; Huntsman, D.; et al. Targeting tumor hypoxia: Suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011, 71, 3364–3376. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Morgan, P.E.; Vullo, D.; Scozzafava, A.; Mastrolorenzo, A.; Supuran, C.T. Carbonic Anhydrase Inhibitors. Design of Selective, Membrane-Impermeant Inhibitors Targeting the Human Tumor-Associated Isozyme IX. J. Med. Chem. 2004, 47, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Tars, K.; Vullo, D.; Kazaks, A.; Leitans, J.; Lends, A.; Grandane, A.; Zalubovskis, R.; Scozzafava, A.; Supuran, C.T. Sulfocoumarins (1,2-benzoxathiine-2,2-dioxides): A class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. J. Med. Chem. 2013, 56, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbon- versus sulphur-based zinc binding groups for carbonic anhydrase inhibitors? J. Enzyme Inhib. Med. Chem. 2018, 33, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Labute, P. Protonate3D: Assignment of ionization states and hydrogen coordinates to macromolecular structures. Proteins Struct. Funct. Bioinforma. 2009, 75, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Labute, P. The generalized born/volume integral implicit solvent model: Estimation of the free energy of hydration using London dispersion instead of atomic surface area. J. Comput. Chem. 2008, 29, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).