Abstract
Endoglucanases (EGLs) are important components of multienzyme cocktails used in the production of a wide variety of fine and bulk chemicals from lignocellulosic feedstocks. However, a low thermostability and the loss of catalytic performance of EGLs at industrially required temperatures limit their commercial applications. A structure-based disulfide bond (DSB) engineering was carried out in order to improve the thermostability of EGLII from Penicillium verruculosum. Based on in silico prediction, two improved enzyme variants, S127C-A165C (DSB2) and Y171C-L201C (DSB3), were obtained. Both engineered enzymes displayed a 15–21% increase in specific activity against carboxymethylcellulose and β-glucan compared to the wild-type EGLII (EGLII-wt). After incubation at 70 °C for 2 h, they retained 52–58% of their activity, while EGLII-wt retained only 38% of its activity. At 80 °C, the enzyme-engineered forms retained 15–22% of their activity after 2 h, whereas EGLII-wt was completely inactivated after the same incubation time. Molecular dynamics simulations revealed that the introduced DSB rigidified a global structure of DSB2 and DSB3 variants, thus enhancing their thermostability. In conclusion, this work provides an insight into DSB protein engineering as a potential rational design strategy that might be applicable for improving the stability of other enzymes for industrial applications.
1. Introduction
Cellulases are highly attractive enzymes for various industrial applications, such as lignocellulosic biomass conversion, cotton and paper manufacturing, juice extraction, and use as detergent enzymes and animal feed additives [1,2,3]. Cellulases are classified into three main groups according to their activities, including endo-1-4-β-glucanases (or simply endoglucanases), exo-cellobiohydrolases, and β-glucosidases. Endoglucanases cleave cellulose molecules internally, thus exposing new chain ends to the processive action of exo-cellobiohydrolases, which release cellobiose molecules into the solution. β-Glucosidases finally hydrolyze cellobiose to glucose. Endoglucanases have drawn much attention from researchers as an essential component of cellulase multienzyme cocktails for biomass degradation or as individual biocatalysts in some abovementioned applications [4,5,6,7].
The practical applications of endoglucanases are often limited because of the loss of enzymatic activity at industrially required temperatures. The increase in temperature by a few degrees above the enzyme temperature optimum leads to structural changes and the unfolding of the protein, thus affecting its function [8,9]. Therefore, the enzyme thermal stabilization is an essential requirement for its efficient use in biotechnology [10]. Protein engineering holds a potential to develop thermostable enzymes as biocatalysts.
Recent advances in enzyme engineering through directed evolution [11] and “KnowVolution” [12] have successfully been applied to improve the thermostability of several enzymes, including cellulases [13,14], phytase [15], xylanases [16], and lipase [17]. However, using only the experimental techniques for the engineering of the enzyme stability is rather costly and time-consuming. Computer-assisted rational design is an attractive alternative to accelerate the enzyme engineering process. In the context of computational design to improve the thermostability of a biocatalyst, several approaches are widely used, such as the consensus sequence (comparing amino acid sequences with a higher thermostability), B-factor/RMSF analysis, molecular dynamics (MD) simulations, constraint network analysis (CNA), disulfide bonds (DSBs) design, and stabilizing salt-bridge design based on the sequence and structure of enzymes [18,19,20,21,22,23].
DSBs play an important role in protein folding and stability [24], enhance the stability of proteins that function in a fluctuating cellular environment, and contribute to the activity of many proteins by stabilizing them in their active conformations [25,26]. In recent years, a few studies have applied the DSB design approach to improve the thermostability of various enzymes, such as alkaline α-amylase [27], cellulases [28], and amadoriase [29]. Recently, the DSB engineering (N31C-T187C/P102C-N125C) of a mesophilic β-glucanase from Bacillus terquilensis showed an enhancement of the thermostability by 48.3% in increasing the half-life at 60 °C and a 4.1 °C rise in melting temperature compared to wild type [22]. However, DSB engineering to improve the thermostability remains to be unexplored for endoglucanase II (EGLII) from P. verruculosum.
In this work, we used the DSB engineering approach to improve the thermostability of EGLII from P. verruculosum belonging to the glycoside hydrolase family 5 [30,31]. EGLII is one of the major enzymes of the P. verruculosum extracellular multienzyme system, and it is an important component of the commercial enzyme preparation Agrocell Plus produced by the Agroferment company (Russia) for using as a feed additive. Although EGLII is one of the most thermostable enzymes of the P. verruculosum cellulase complex [30], its thermostability still needs to be improved since the industrial production of the animal feeds involves the pelletizing stage proceeding at approx. 80 °C. The beneficial variants of EGLII were chosen by computer simulation studies, allowing the characterization of the stability of the enzyme-engineered forms. Identified residue pairs were subjected to site-directed mutagenesis to generate DSBs. This report demonstrates that DSB engineering is an efficient rational designing approach for stabilizing enzymes in biomass bioprocessing applications.
2. Results
DSB engineering was performed as an emerging approach to increase the thermostability of EGLII (Cel5A) from P. verruculosum. The results obtained are divided into three parts. In the first part, we describe the design and selection of DSBs obtained from Schrödinger’s BioLuminate software [32]. Secondly, we provide experimental data demonstrating that the engineered EGLII variants exhibited a higher thermostability in comparison with the wild-type enzyme (EGLII-wt). Finally, we explain the effect of DSBs on the overall enzyme structural stability, as well as the stability of DSBs, using MD simulations.
2.1. Computational Design and Screening of Disulfide Bonds
A native EGLII contains one DSB between C221 and C258. To increase the enzyme thermal stability, an introduction of extra DSBs into a protein structure was proposed. Based on the results of calculations with the BioLuminate software [32], three pairs of amino acid substitutions that could result in the formation of new DSBs were chosen (Figure 1). The substitutions of amino acid residues S12 and A270 (S12C-A270C, DSB1) take into account natively unfolded regions from the N- and C-termini of the protein. A disordered region in the middle of EGLII (amino acid residues 212–219) could not be used for mutagenesis because of its proximity to the enzyme active site. Therefore, a location close to this region but not affecting the active site and presumably capable of introducing the additional DSB (substitutions Y171C-L201C, DSB3) was chosen. The variant S127C-A165C (DSB2) was consistent with the parameters, except that the residues chosen for mutations were not part of the natively disordered regions.
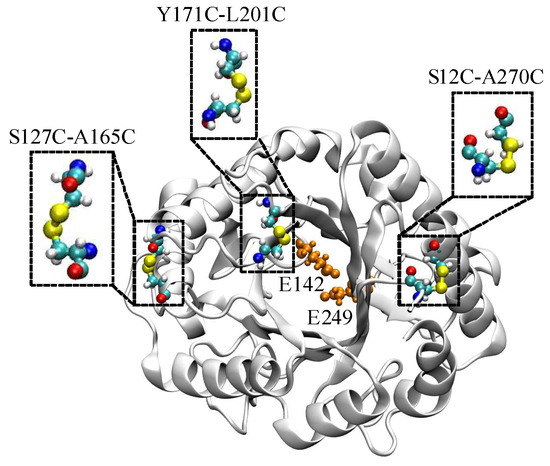
Figure 1.
The three potential disulfide bonds in EGLII (PDB ID: 5L9C, chain A) used for the experimental studies: Catalytic residues E142 and E249 in the enzyme active site are shown in orange.
2.2. Production, Purification, and Biochemical Characterization of the EGLII Variants
Genes encoding EGLII and its mutant forms carrying the abovementioned substitutions were cloned into the Penicillium canescens PCA-10 (niaD-) auxotrophic strain and was expressed under the control of a xyl1 gene promoter. Recombinant EGLII-wt and its DSB2 and DSB3 variants were successfully expressed and then purified for a characterization using the sequential anion exchange and hydrophobic-interaction chromatography. However, the expression of the DSB1 variant was not successful, although a sequencing of the corresponding gene confirmed the presence of the target mutations. Figure 2 shows the SDS-PAGE of proteins from the culture fluids expressed by recombinant strains (a) and the purified EGLII forms (b).
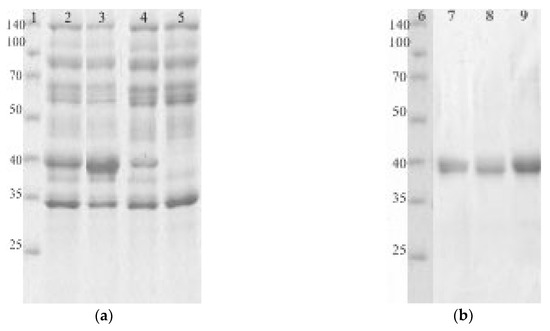
Figure 2.
The SDS-PAGEs of culture fluids containing the recombinant EGLII forms (a) and purified enzymes (b). Lanes 1 and 6 are markers (kDa); lane 2 is the EGLII-DSB2 recombinant strain; lane 3 is the EGLII-DSB3 recombinant strain; lane 4 is the EGLII-wt recombinant strain; lane 5 is the control strain (PCA-10); lane 7 is EGLII-DSB2; lane 8 is EGLII-DSB3; and lane 9 is EGLII-wt.
The enzymatic activities toward carboxymethylcellulose (CMC) and barley β-glucan were determined for both the mutant forms of EGLII (DSB2 and DSB3) and the wild-type enzyme (Table 1). The engineered variants of EGLII exhibited higher specific activities against both substrates (by 15–21%) compared to the EGLII-wt.

Table 1.
The specific activities (U/mg protein) of recombinant EGLII forms.
2.3. Thermostability of Recombinant EGLII Forms
To determine the thermostability of the EGLII recombinant forms, the enzyme solutions were incubated in a thermostat at 70 and 80 °C, and the residual activity against CMC and β-glucan was assayed after different times of incubation. The choice of conditions for the study of thermal stability was due to the fact that the EGLII is an important component of an industrial enzyme preparation for use as a feed additive (see Introduction), which the production of involves the pelletizing stage proceeding at approx. 80 °C. The inactivation curves for recombinant EGLII-wt and its DSB2 and DSB3 variants are shown in Figure 3 and Figure 4.
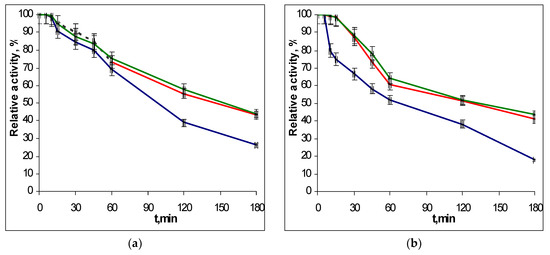
Figure 3.
The thermoinactivation of EGLII-wt (blue line) and its DSB2 (red) and DSB3 (green) variants at pH 5.0 and 70 °C: (a) The residual activity against CMC and (b) the residual activity against β-glucan. The enzyme initial activity is taken as 100%.
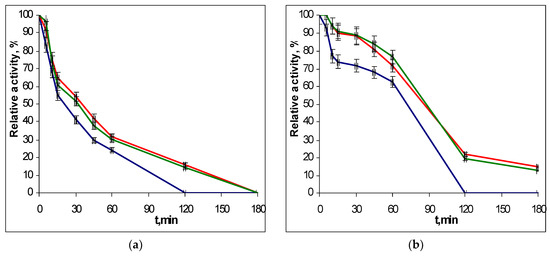
Figure 4.
The thermoinactivation of EGLII-wt (blue line) and its DSB2 (red) and DSB3 (green) variants at pH 5.0 and 80 °C: (a) Residual activity against CMC and (b) the residual activity against β-glucan. The enzyme initial activity is taken as 100%.
After incubation at 70 °C for 2 h, the engineered variants retained 52–58% of their activity toward both substrates, while the EGLII-wt retained only 38% of its activity. At 80 °C, the DSB2 and DSB3 forms retained 15–22% of their activity after 2 h, whereas EGLII-wt was completely inactivated after the same incubation time.
2.4. Analysis of Structural Stability
To understand the molecular mechanism of the increased thermostability caused by the introduction of DSBs, changes in the protein flexibility were analyzed by MD simulations. The MD simulations of EGLII-wt, DSB2, and DSB3 at 26.85, 70, and 80 °C showed that the DSBs in the engineered variants improved the thermostability by increasing the overall compactness of structures. This observation was evaluated by analyzing two key parameters, including root mean square deviation (RMSD) and (radius of gyration) Rg. A higher RMSD typically indicates a more flexible and potentially less thermostability of the overall structure. The RMSD fluctuation remained quite similar in all EGLII forms at 26.85 and 70 °C; however, the engineered variants showed a higher stability at 80 °C in comparison with EGLII-wt (Figure 5). A similar phenomenon was observed in analyzing the Rg of the enzyme forms. At 80 °C, the structure compactness was retained in the DSB2 and DSB3 variants unlike in the EGLII-wt, implying that the DSBs increased the compactness of the enzyme (Figure S1 in the Supplementary Materials).
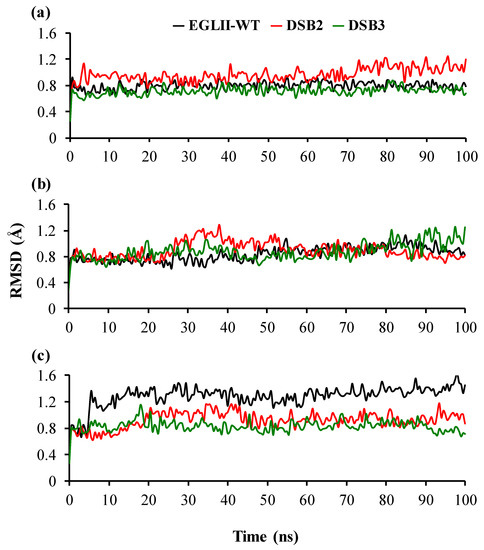
Figure 5.
The structural stability of the EGLII forms based on RMSD: (a,b) At 26.85 and 70 °C, the RMSD of the backbone indicated that the DSB2 and DSB3 variants and EGLII-wt had similar structural stabilities. (c) At 80 °C, the RMSD of the backbone of EGLII-wt showed an instability, whereas the structures of the DSB2 and DSB3 variants remained stable.
As the designed DSBs improved the thermostability of the engineered variants, we evaluated the stability of the -S-S- distances (<2.15 Å) [33] through MD simulations. The distribution of C127SG-C165SG and the C171SG-C201SG distances were investigated to assess their stability during 100-ns simulations as retain in naturally occurring disulfides of enzymes (Figure S2 in the Supplementary Materials). In the case of both DSB2 and DSB3 variants, the analyzed distances were retained within 2.15 Å in 200 data points of 100-ns simulations (Figure S2 in the Supplementary Materials). Thus, the simulation results showed that the designed DSBs remained stable throughout the 100 ns.
3. Discussion
Due to the covalent nature, the DSBs have strong effects on the folding and stability of a protein [34]. Nature has created the DSBs as a strategy to stabilize a protein in both intracellular and extracellular environments [25,35]. The effects of stabilization are expected to exert from the cross-linking of cysteine residues separated by a large number of intervening amino acids along the polypeptide chain [35]. Naturally, the DSB formation between cysteine residues occurs during the folding of proteins in their secretory pathway catalyzed by members of the protein disulfide isomerase family [36]. The introduction of extra DSBs through protein engineering without affecting the catalytic efficacy of enzymes was considered to improve the thermostability of mesophilic enzymes [22,37]. Therefore, in this study, we used rational DSB engineering to improve the thermostability of the EGLII from P. verruculosum.
Protein engineering based on directed evolution [11] and/or “KnowVolution” [12] has successfully been applied to improve the thermostability of different enzymes, although these techniques remain costly and time-consuming. Because of the rapidly increasing demand of highly thermostable enzymes, computer-assisted rational design and its experimental validation are desired. In the area of structure-based engineering, the DSB engineering offers several important advantages over the creation of extra hydrogen bonds, salt bridges, and hydrophobic cores because the DSBs are structurally well-defined and they are easily characterized [19,38]. The selection of the appropriate residue pairs for DSB introduction remains challenging as the new DSBs sometime impair enzyme activity while not contributing much to protein stability [22,32,39].
In this work. we applied a rational design, screening, and ranking of DSBs considering the energy ΔEi, ΔE, and the Quality parameter [32]. In the DSB1, S12 is located in the N-terminal and A270 is located in the α-helix region; however, this variant did not show an expression in the P. canescens host. The residue pair in the DSB2 is located in the α-helix-loop transition, whereas that in the DSB3 is located in the β-sheet-loop transition regions. The results from the MD simulation studies showed that the introduction of the DSB2 and DSB3 rigidified the global structures of the variants at higher temperatures. The reduction of the overall flexibility enhanced the protein thermostability. Moreover, both DSBs remained structurally stable and contributed to an improved thermostability though rigidifying the overall structures.
The half-life times of EGLII at 70 and 80 °C increased by 1.5–2 times as a result of introducing the DSB2 and DSB3. A similar 1.5-fold effect of increasing the half-life value of a mesophilic β-glucanase from Bacillus terquilensis at 60 °C has been reported for the enzyme mutant forms N31C-T187C and P102C-N125C [22]. In the case of cellulase C (CelC) from Clostridium thermocellum, the enzyme activity half-life for the wild-type CelC and its double mutant I232C-N249C at 65 °C were 0.46 and 2.5 min, respectively [28]. Although in our case the achieved effects of increasing the EGLII thermostability were not very high, they are quite important from a practical point of view. The higher specific activity of the enzyme-engineered forms and the better activity retention at 80 °C, that is, a temperature of a pelletizing stage in the production of animal feeds, allows obtaining the final product with improved properties. In particular, a higher β-glucanase activity in barley-based diets helps to mitigate the antinutritive effect of cereal β-glucans on monogastric animals, particularly in poultry, and therefore, using thermostable enzymes in the preparation of feeds attracts the attention of researchers working in this field [40,41].
4. Materials and Methods
4.1. Disulfide Bond Design
A structure-based design of disulfides was performed through Cys scanning to identify potential mutations that can result in disulfide bonds using Schrödinger’s BioLuminate software [32]. The selection of the most appropriate amino acid pairs for mutations to Cys was decided according to the ΔEi (interaction energy), ΔE (strain energy or the difference between the internal energy before and after relaxation). and the Quality parameter (Good: ΔEi < 0 and ΔStrain E < 20; Bad: ΔEi > 40, ΔStrain E > 40, or ΔEi > 20 and ΔStrain E > 20; Medium: any other values) [32]. Additional criteria for the selection of disulfide bonds includes the distance between Cβ–Cβ atoms of two amino acids that is not more than 5 Å apart (for glycine: from Cα), the arrangement of residues on the surface of the protein or between secondary structures, the distance from the catalytic core, the appropriate conformation of amino acids, and the length of disulfide bonds about 2 Å [33,42].
4.2. Microbial Strains
The P. canescens PCA-10 strain [43] was used as an auxotrophic host strain (niaD-) in transformation. The Escherichia coli MachI T1R strain (Thermo Fisher Scientific Inc., Waltman, MA, USA) was used to obtain competent cells in the subcloning experiments.
4.3. Enzyme Activity Assays
The enzyme activities toward polysaccharides were determined by analyzing the reducing sugars released after the enzymatic reaction using the Nelson–Somogyi assay [44]. The enzyme activities toward carboxymethylcellulose (CMC), birchwood xylan (Sigma, St. Louis, MO, USA), and barley β-glucan (Megazyme, Boronia, Australia) were assayed at pH 5.0 (0.05 M Na-acetate buffer) and 50 °C using a substrate concentration of 5 mg/mL [45].
The enzymatic activities were expressed in international units. One unit of activity corresponded to the quantity of enzyme hydrolyzing 1 µmol of substrate or the release of 1 µmol of reducing sugars (in glucose equivalents) per minute.
4.4. Site-Directed Mutagenesis and Protein Expression
Cloning the P. verruculosum native egl2 gene, encoding EGLII, into the P. canescens PCA-10 (niaD-) auxotrophic strain has been described previously [31]. Six pairs of oligonucleotide primers were constructed to conduct the S12C-A270C, S127C-A165C, and Y171C-L201C substitutions (3 DSBs) in the recombinant EGLII (Table 2).

Table 2.
The sequences of the oligonucleotides.
Mutagenic primers containing the target mutations were designed in the Laboratory of Enzyme Biotechnology (FRC of Biotechnology, RAS) and synthesized in the Syntol laboratory (Moscow, Russia) that specializes in oligonucleotide synthesis. A routine polymerase chain reaction (PCR) was used to perform site-directed mutagenesis. The standard blend of reagents for PCR was supplied by Thermo Fisher Scientific Inc. (Waltman, MA, USA). The isolation of genomic and plasmid DNA as well as the PCR product purification was carried out using QIAGEN Kits (QIAGEN, Valencia, CA, USA). Briefly, in the first round of the PCRs, four DNA fragments for two point mutations were obtained using the genomic DNA of P. verruculosum as a template, and then a full-size egl2 gene (1319 bp), carrying target mutations, was amplified in a second round of PCR with purified fragments as a template. The total number of PCR cycles to introduce two point mutations did not exceed 30. The full-size mutated egl2 gene was cloned into a modified linear PC1 shuttle vector containing a promoter region of xylA gene, encoding xylanase A from P. canescens, and a terminator region encoding endoglucanase III from P. verruculosum [41]. Next, the resulting expression plasmids were transformed into competent E. coli MachI cells, and the produced DNA material was supplied for analysis. The E. coli MachI cells were cultured as described elsewhere [46]. Thus, plasmid constructs pPC1-DSB1, pPC1-DSB2, and pPC1-DSB3, containing S12C-A270C, S127C-A165C, and Y171C-L201C substitutions, respectively, were obtained. The absence of additional mutations, deletions, or insertions in the egl2 gene was confirmed by its sequencing in both directions by the method described by Sanger et al. [47].
The pPC1-DSB1, pPC1-DSB2, and pPC1-DSB3 plasmid constructs were directed into protoplasts of the recipient P. canescens PCA-10 (niaD-) strain together with a plasmid pSTA10 (10:1, µg) using the modified method described by Aleksenko et al. [48]. The efficiency of integration amounted to 30–40 clones per 1 µg of the target DNA that corresponds to a standard frequency of transformation for the fungal genus Penicillium [46]. Stable transformants were grown in flasks for 6 days using a small volume (100 mL) of the liquid culture medium containing 40 g/L soybean hulls, 50 g/L corn extract, and 25 g/L KH2PO4, and then CMCase and β-glucanase activities were determined in the broth as described above.
4.5. Purification and Charactetization of the Thermostable Variants
The purification of EGLII and its mutant forms was carried out as described elsewhere [31]. The isolated enzymes were subjected to a sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) in 12% gel using a Mini Protean II equipment (Bio-Rad Laboratories, Hercules, CA, USA). The staining of protein bands was carried out with Coomassie Blue R-250 (Ferak, Berlin, Germany). The protein concentration in the samples was determined by the modified Lowry method [49], using bovine serum albumin as the standard.
In the thermostability studies, the enzymes were incubated at 70 or 80 °C in a 0.1 M Na-acetate buffer, pH 5.0. At a definite incubation time, an aliquot of the enzyme solution was taken and cooled in cold water, and then the residual activity against β-glucan was assayed under standard conditions as described above. The residual activity was expressed as the percentage of the initial activity.
4.6. Molecular Modelling
In order to understand the role of DSBs in enhancing the enzyme thermostability on a molecular level, classical MD simulations were performed for EGLII-wt and its variants (DSB2 and DSB3) at temperatures 26.85, 70, and 80 °C. Simulations at 26.85 °C were considered to identify the baseline dynamics and a comparison of the structural features of the EGLII-wt and its variants under standard conditions. The temperatures 70 and 80 °C were considered as those used for experimental conditions to observe the structural changes that occured in the thermal unfolding. The starting coordinates of EGLII-wt were taken from the X-ray crystal structure of the enzyme (PDB ID: 5L9C, chain A). The variant structures were generated based on EGLII-wt structures using a FoldX plugin implemented in the YASARA software version 17.8.19 [50,51,52]. For the variants, DSBs were generated using the “interactive SS bridge selection” option implemented in the GROMACS v5.1.2 package [51,52,53,54]. Prior to simulations, water molecules and ligands (cellobiose and N-acetyl-d-glucosamine) present in the crystal structure were removed using the YASARA software version 17.8.19. All MD simulations were performed using the GROMACS v5.1.2 package [53,54,55,56] with the Amber99SB-ILDN force field and extended simple point charge (SPC/E) 216 water model [57,58]. The protonation state of titratable residues was determined based on pKa estimation using the PROPKA method implemented in the PDB2PQR server using AMBER99 charges at pH 5 [59,60,61,62]. Specifically, protonation was assigned to Nδ1 for H96, H102, and H207 based on the possible H-bonds networks, and both Nδ1 and Nɛ2 protonated for H144 based on the PROPKA calculation. Subsequently, the enzymes were inserted in a 416.77 nm3 cubic box and then explicitly solvated in water, and sodium ions were added to neutralize the charge of the systems. The final systems contained in total approx. 41,050 atoms and 12,190 water molecules. The electrostatic interactions were calculated by applying the particle mesh Ewald (PME) method [63,64]. Short-range electrostatic interactions (rcoulomb) and Van der Waals (rvdw) were calculated using a cutoff value of 1.0 nm each. The energy minimization of each system was performed individually using the steepest descent minimization algorithm until the maximum force reached 1000.0 kJ mol−1 nm−1. The final system equilibration was conducted in the NVT and NPT ensembles at three temperatures: 26.85, 70, and 80 °C. The NVT and NPT equilibrations were performed with a time step of 2 fs at three temperatures for 100 ps. In the case of NVT equilibration, initial random velocities were assigned to the atoms of the molecules according to the Maxwell–Boltzmann algorithm at the same temperature. For the NPT equilibration, the Berendsen thermostat and Parrinello–Rahman pressure coupling were used to keep the system at a fixed temperature, time constant (τT) of 0.1 ps and 1 bar pressure, and time constant (τP) of 2 ps. All bonds between hydrogen and heavy atoms were constrained with the LINCS algorithm [55,65,66]. The production run was carried out using the NPT ensemble for 100 ns with a time step of 2 fs for each temperature. The coordinates were saved every 500 ps from simulation trajectories in the 100 ns simulations. The trajectories and the structures were visualized using VMD 1.9.1 [67]. Analyses including the root mean square deviation (RMSD) of backbone atoms of the protein with respect to a minimized crystal structure [68], the radius of gyration (Rg) [69], were performed using tools from the GROMACS software package [55]. The stability of -S-S- distance of variants was analyzed using VMD 1.9.1 [67].
5. Conclusions
Protein engineering based on the DSB design was performed to improve the thermostability of EGLII from P. verruculosum. The experimental results showed that the designed variants, DSB2 and DSB3, contributed significantly to the thermostability of EGLII. Simultaneously, the MD simulations revealed that the introduced DSBs rigidified the overall structures and thereby enhanced the thermostability of EGLII. The overall results demonstrate the applicability of the DSB design as an efficient approach towards increasing the thermostability of EGLII that can be applied in similar enzymes. In the growing need of sustainable social development across the world, the application of thermostable enzymes will have significant socioeconomic benefits. In particular, the higher specific activity and the enhanced thermostability of the engineered EGLII should contribute to its more effective use as an additive to animal feeds as well as to its potential application in the processes of the bioconversion of renewable lignocellulosic biomass for the production of a wide variety of fine and bulk chemicals in different biorefineries.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/7/1602/s1.
Author Contributions
A.B. characterized the native and mutated EGLII forms, measured the thermal stability, and wrote the manuscript. S.P. performed and analyzed the molecular dynamics simulations and the structure–function relationship and wrote the manuscript. P.V. designed the plasmids and made the genetic engineering experiments. A.R. expressed the native and mutated EGLII forms in the Penicillium canescens host. V.N. designed the disulfide bonds. I.Z. purified the homogeneous proteins. A.G. analyzed and discussed the molecular modelling. A.S. discussed the results and revised the manuscript. U.S. discussed the results and provided input. M.D.D. analyzed the results and revised the manuscript.
Funding
This research was funded by the Ministry of Science and High Education (MON) of Russia, project identification number: RFMEFI61617X0081. The RWTH authors thank the German Federal Ministry of Education and Research (BMBF) (FKZ: 031B0506) for the financial support in the framework of BioEconomoy International.
Acknowledgments
M.D.D. thanks the computing resources granted by JARA-HPC from RWTH Aachen University under projects RWTH0116.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CMC | Carboxymethylcellulose |
| DSB | Disulfide bond |
| EGL | Endoglucanase |
| MD | Molecular dynamics |
References
- Lambertz, C.; Garvey, M.; Klinger, J.; Heesel, D.; Klose, H.; Fischer, R.; Commandeur, U. Challenges and advances in the heterologous expression of cellulolytic enzymes: A review. Biotechnol. Biofuels 2014, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Mi, L.; Pontrelli, S.; Luo, S. Fuelling the future: Microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 2016, 14, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Artzi, L.; Bayer, E.A.; Morais, S. Cellulosomes: Bacterial nanomachines for dismantling plant polysaccharides. Nat. Rev. Microbiol. 2017, 15, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Yennamalli, R.M.; Rader, A.J.; Kenny, A.J.; Wolt, J.D.; Sen, T.Z. Endoglucanases: Insights into thermostability for biofuel applications. Biotechnol. Biofuels 2013, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Scapin, S.M.N.; Souza, F.H.M.; Zanphorlin, L.M.; de Almeida, T.S.; Sade, Y.B.; Cardoso, A.M.; Pinheiro, G.L.; Murakami, M.T. Structure and function of a novel GH8 endoglucanase from the bacterial cellulose synthase complex of Raoultella ornithinolytica. PloS ONE 2017, 12, e0176550. [Google Scholar] [CrossRef]
- Jensen, M.S.; Fredriksen, L.; MacKenzie, A.K.; Leiros, I.; Chylenski, P.; Williamson, A.K.; Christopeit, T.; Ostby, H.; Vaaje-Kolstad, G.; Eijsinket, V.G.H. Discovery and characterization of a thermostable two-domain GH6 endoglucanase from a compost metagenome. PloS ONE 2018, 13, e0197862. [Google Scholar] [CrossRef]
- Miotto, L.S.; de Rezende, C.A.; Bernardes, A.; Serpa, V.I.; Tsang, A.; Polikarpovet, I. The characterization of the endoglucanase Cel12A from Gloeophyllum trabeum reveals an enzyme highly active on β-glucan. PLoS ONE 2014, 9, e108393. [Google Scholar] [CrossRef]
- Ghosh, K.; Dill, K. Cellular proteomes have broad distributions of protein stability. Biophys. J. 2010, 99, 3996–4002. [Google Scholar] [CrossRef]
- Park, Y.G.; Jung, M.C.; Song, H.; Jeong, K.W.; Bang, E.; Hwang, G.S.; Kim, Y. Novel structural components contribute to the high thermal stability of acyl carrier protein from Enterococcus faecalis. J. Biol. Chem. 2016, 291, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Fact. 2007, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.S.; Liu, D.R. Methods for the directed evolution of proteins. Nat. Rev. Genet. 2015, 16, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhu, L.; Schwaneberg, U. Directed evolution 2.0: Improving and deciphering enzyme properties. Chem. Commun. 2015, 51, 9760–9772. [Google Scholar] [CrossRef] [PubMed]
- Muhlmann, M.; Kunze, M.; Ribeiro, J.; Geinitz, B.; Lehmann, C.; Schwaneberg, U.; Commandeur, U.; Buchset, J. Cellulolytic RoboLector—Towards an automated high-throughput screening platform for recombinant cellulase expression. J. Biol. Eng. 2017, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Goedegebuur, F.; Dankmeyer, L.; Gualfetti, P.; Karkehabadi, S.; Hansson, H.; Jana, S.; Huynh, V.; Kelemen, B.R.; Kruithof, P.; Larenas, E.A.; et al. Improving the thermal stability of cellobiohydrolase Cel7A from Hypocrea jecorina by directed evolution. J. Biol. Chem. 2017, 292, 17418–17430. [Google Scholar] [CrossRef] [PubMed]
- Shivange, A.V.; Roccatano, D.; Schwaneberg, U. Iterative key-residues interrogation of a phytase with thermostability increasing substitutions identified in directed evolution. Appl. Microbiol. Biotechnol. 2016, 100, 227–242. [Google Scholar] [CrossRef]
- Wang, X.; Ma, R.; Xie, X.; Liu, W.; Tu, T.; Zheng, F.; You, S.; Ge, J.; Xie, H.; Yao, B.; et al. Thermostability improvement of a Talaromyces leycettanus xylanase by rational protein engineering. Sci. Rep. 2017, 7, 15287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Yang, G.; Xie, Y.; Xu, L.; An, J.; Cui, L.; Feng, Y. Modulation of the thermostability and substrate specificity of Candida rugosa lipase1 by altering the acyl-binding residue Gly414 at the α-helix-connecting bend. Enzyme Microb. Technol. 2016, 82, 34–41. [Google Scholar] [CrossRef]
- Modarres, H.P.; Mofrad, M.; Sanati-Nezhad, A. Protein thermostability engineering. RSC Adv. 2016, 6, 115252–115270. [Google Scholar] [CrossRef]
- Kruger, D.M.; Rathi, P.C.; Pfleger, C.; Gohlke, H. CNA web server: Rigidity theory-based thermal unfolding simulations of proteins for linking structure, thermostability, and function. Nucleic Acids Res. 2013, 41, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Yu, T.H.; Wong, K.B. Stabilizing salt-bridge enhances protein thermostability by reducing the heat capacity change of unfolding. PLoS ONE 2011, 6, e21624. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.B.; Dombkowski, A.A. Disulfide by Design 2.0: A web-based tool for disulfide engineering in proteins. BMC Bioinf. 2013, 14, 346. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Zhu, L.; Xu, X.; Li, Q. Rational design of disulfide bonds increases thermostability of a mesophilic 1,3-1,4-β-glucanase from Bacillus terquilensis. PLoS ONE 2016, 11, e0154036. [Google Scholar] [CrossRef]
- Yang, H.; Liu, L.; Li, J.; Chen, J.; Du, G. Rational design to improve protein thermostability: Recent advances and prospects. ChemBioEng. Rev. 2015, 2, 87–94. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Luo, X.; Li, J.; Reed, S.A.; Xiao, H.; Young, T.S.; Schultz, P.G. Enhancing protein stability with extended disulfide bonds. Proc. Natl. Acad. Sci. USA 2016, 113, 5910–5915. [Google Scholar] [CrossRef] [PubMed]
- Hogg, P.J. Disulfide bonds as switches for protein function. Trends Biochem. Sci. 2003, 28, 210–214. [Google Scholar] [CrossRef]
- Kadokura, H.; Katzen, F.; Beckwith, J. Protein disulfide bond formation in prokaryotes. Annu. Rev. Biochem. 2003, 72, 111–135. [Google Scholar] [CrossRef]
- Liu, L.; Deng, Z.; Yang, H.; Li, J.; Shin, H.D.; Chen, R.R.; Du, G.; Chen, J. In silico rational design and systems engineering of disulfide bridges in the catalytic domain of an alkaline α-amylase from Alkalimonas amylolytica to improve thermostability. Appl. Environ. Microbiol. 2013, 80, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Badieyan, S.; Bevan, D.R.; Zhang, C. Study and design of stability in GH5 cellulases. Biotechnol. Bioeng. 2012, 109, 31–44. [Google Scholar] [CrossRef]
- Rigoldi, F.; Donini, S.; Giacomina, f.; Sorana, F.; Redaelli, A.; Bandiera, T.; Parisini, E.; Gautieri, A. Thermal stabilization of the deglycating enzyme Amadoriase I by rational design. Sci. Rep. 2018, 8, 3042. [Google Scholar] [CrossRef] [PubMed]
- Morozova, V.V.; Gusakov, A.V.; Andrianov, R.M.; Pravilnikov, A.G.; Osipov, D.O.; Sinitsyn, A.P. Cellulases of Penicillium verruculosum. Biotechnol. J. 2010, 5, 871–880. [Google Scholar] [CrossRef]
- Dotsenko, A.S.; Rozhkova, A.M.; Gusakov, A.V. Properties and N-glycosylation of recombinant endoglucanase II from Penicillium verruculosum. Moscow Univ. Chem. Bull. 2015, 70, 283–286. [Google Scholar] [CrossRef]
- Salam, N.K.; Adzhigirey, M.; Sherman, W.; Pearlman, D.A. Structure-based approach to the prediction of disulfide bonds in proteins. J. Protein Eng. Des. Sel. 2014, 27, 365–374. [Google Scholar] [CrossRef]
- Dombkowski, A.A.; Sultana, K.Z.; Craig, D.B. Protein disulfide engineering. J. FEBS Lett. 2014, 588, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Feige, M.J.; Hendershot, L.M. Disulfide bonds in ER protein folding and homeostasis. Curr. Opin. Cell Biol. 2011, 23, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Fass, D. Disulfide bonding in protein biophysics. Annu. Rev. Biophys. 2012, 41, 63–79. [Google Scholar] [CrossRef]
- Bulleid, N.J. Disulfide bond formation in the mammalian endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2012, 11, a013219. [Google Scholar] [CrossRef]
- Chang, M.; Chu, X.; Lv, J.; Li, Q.; Tian, J.; Wu, N. Improving the thermostability of acidic pullulanase from Bacillus naganoensis by rational design. PloS ONE 2016, 11, e0165006. [Google Scholar] [CrossRef]
- Wedemeyer, W.J.; Welker, E.; Naravan, M.; Scheraga, H.A. Disulfide bonds and protein folding. Biochemistry 2000, 39, 4207–4216. [Google Scholar] [CrossRef]
- Eijsink, V.; Vriend, G.; Van den Burg, B. Engineering a hyperstable enzyme by manipulation of early steps in the unfolding process. Biocatal. Biotransf. 2001, 19, 443–458. [Google Scholar] [CrossRef]
- Ribeiro, T.; Lordelo, M.M.S.; Ponte, P.I.P.; Maçãs, B.; Prates, J.A.M.; Fontes, M.A.; Falcão, L.; Freire, J.P.B.; Ferreira, L.M.A.; Fontes, C.M.G.A. Levels of endogenous -glucanase activity in barley affect the efficacy of exogenous enzymes used to supplement barley-based diets for poultry. Poultry Sci. 2011, 90, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Luo, H.; Shi, P.; Huang, H.; Bai, Y.; Yao, B. A highly-active endo-1,3-1,4-β-glucanase from thermophilic Talaromyces emersonii CBS394.64 with application potential in the brewing and feed industries. Process Biochem. 2014, 49, 1448–1456. [Google Scholar] [CrossRef]
- Singh, R. A review of algorithmic techniques for disulfide-bond determination. Brief. Funct. Genomics 2008, 7, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Sinitsyn, A.P.; Rozhkova, A.M. Penicillium canescens host as the platform for development of a new recombinant strain producers of carbohydrases. In Microorganisms in Biorefineries; Kamm, B., Ed.; Springer: Berlin, Germany, 2015; pp. 1–19. [Google Scholar]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of sugars. J. Biol. Chem. 1944, 153, 375–379. [Google Scholar]
- Sinitsyna, O.A.; Bukhtoyarov, F.E.; Gusakov, A.V.; Okunev, O.N.; Bekkarevitch, A.O.; Vinetsky, Y.P.; Sinitsyn, A.P. Isolation and properties of major components of Penicillium canescens extracellular enzyme complex. Biochemistry (Moscow) 2003, 68, 1200–1209. [Google Scholar] [CrossRef]
- Dotsenko, A.S.; Gusakov, A.V.; Volkov, P.V.; Rozhkova, A.M.; Sinitsyn, A.P. N-linked glycosylation of recombinant cellobiohydrolase I (Cel7A) from Penicillium verruculosum and its effect on the enzyme activity. Biotechnol. Bioeng. 2016, 113, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Chase, A.R. DNA Sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Aleksenko, A.; Makarova, N.; Nikolaev, I.; Clutterbuck, A. Integrative and replicative transformation of Penicillium canescens with a heterologous nitrate-reductase gene. Curr. Genet. 1995, 28, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal. Biochem. 1979, 100, 201–220. [Google Scholar] [CrossRef]
- Van Durme, J.; Delgado, J.; Stricher, F.; Serrano, L.; Schymkowitz, J.; Rousseau, F. A graphical interface for the FoldX forcefield. Bioinformatics 2011, 27, 1711–1712. [Google Scholar] [CrossRef]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX web server: An online force field. Nucleic Acids Res. 2005, 33, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins Struct. Funct. Bioinf. 2009, 77, 114–122. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Pall, S.; Abraham, M.J.; Kutzner, C.; Hess, B.; Lindahl, E. Tackling exascale software challenges in molecular dynamics simulations with GROMACS. In Proceedings of the International Conference on Exascale Applications and Software (EASC), Stockholm, Sweden, 2–3 April 2014; Markidis, S., Laure, E., Eds.; Springer: Basel, Switzerland, 2015; pp. 3–27. [Google Scholar]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: A package for molecular simulation and trajectory analysis. J. Mol. Model. 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Kusalik, P.G.; Svishchev, I.M. The spatial structure in liquid water. Science 1994, 265, 1219–1221. [Google Scholar] [CrossRef]
- Fyta, M.; Netz, R.R. Ionic force field optimization based on single-ion and ion-pair solvation properties: Going beyond standard mixing rules. J. Chem. Phys. 2012, 136, 124103. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, 665–667. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35, 522–525. [Google Scholar] [CrossRef]
- Onufriev, A.V.; Alexov, E. Protonation and pK changes in protein-ligand binding. Q. Rev. Biophys. 2013, 46, 181–209. [Google Scholar] [CrossRef]
- Wang, J.; Cieplak, P.; Kollman, P.A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 2000, 21, 1049–1074. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Norberg, J.; Nilsson, L. On the truncation of long-range electrostatic interactions in DNA. Biophys. J. 2000, 79, 1537–1553. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Hess, B. P-LINCS: A parallel linear constraint solver for molecular simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Maiorov, V.N.; Crippen, G.M. Size-independent comparison of protein three-dimensional structures. Proteins Struct. Funct. Bioinf. 1995, 22, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, M.Y.; Bogatyreva, N.S.; Galzitskaya, O.V. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. (Moscow) 2008, 42, 623–628. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).