Abstract
Galectins are a family of galactoside-recognizing proteins involved in different galectin-subtype-specific inflammatory and tumor-promoting processes, which motivates the development of inhibitors that are more selective galectin inhibitors than natural ligand fragments. Here, we describe the synthesis and evaluation of 3-C-methyl-gulopyranoside derivatives and their evaluation as galectin inhibitors. Methyl 3-deoxy-3-C-(hydroxymethyl)-β-d-gulopyranoside showed 7-fold better affinity for galectin-1 than the natural monosaccharide fragment analog methyl β-d-galactopyranoside, as well as a high selectivity over galectin-2, 3, 4, 7, 8, and 9. Derivatization of the 3-C-hydroxymethyl into amides gave gulosides with improved selectivities and affinities; methyl 3-deoxy-3-C-(methyl-2,3,4,5,6-pentafluorobenzamide)-β-d-gulopyranoside had Kd 700 µM for galectin-1, while not binding any other galectin.
1. Introduction
Galectins are an evolutionary ancient family of small soluble proteins with affinity for β-d-galactopyranoside-containing glycoconjugates and a conserved amino acid sequence motif [1,2]. By their carbohydrate-binding activity they can cross-link glycoproteins, resulting in a variety of effects, such as regulation of cell adhesion, intracellular glycoprotein traffic, and cell signaling [3,4,5]. These effects in turn affect cell behavior in inflammation, immunity and cancer, and galectins appear to be rate limiting in some such pathophysiological conditions, e.g., based on effects in null mutant mice and other model systems [6,7,8,9,10]. This has stimulated development of galectin inhibitors as potential drug candidates, but different galectins have a different tissue distribution and function. Although all bind glycoconjugates containing β-galactose residues, each galectin may have a different affinities for larger natural glycans and for artificial small molecule ligands. Hence, there is an important need for selective galectin-inhibitors, that, for example, distinguish between the two most studied galectins in humans, galectin-1 and galectin-3.
The carbohydrate binding site of galectins is a concave groove and long enough to hold about a tetrasaccharide and based on this the carbohydrate binding site of galectins has been described as a combination of four subsites (A–D) together with an additional one less defined fifth subsite E [3]. Within this groove, subsite C is conserved among galectins, made up of the defining amino acid sequence motif and binds β-galactopyranosides by H-bond interaction with 4-OH, 6-OH and the ring 5-O, and CH-π interaction of the α-side of the pyranose ring with a Trp residue. The neighbouring sites, however, vary among galectins, and can be targeted for selective inhibitor development. To do this, previous inhibitor design has derivatized the positions on galactose not engaged by subsite C, namely C1, C2, and C3 [11]. Gulose is a rare saccharide not found in mammals, but can potentially bind galectins because it is structurally similar to galactose with the only difference being the stereoconfiguration at C3. Hence, the C3 is epimeric with the OH axial instead of equatorial in the galectin bound pyranose form. Here, we show that derivatization at C3 in gulose offers a new space for galectin inhibitor design and surprisingly selective inhibitors of galectin-1. In particular, amide-functionalised C3-methyl gulopyranosides are shown to be apparently selective towards human galectin-1.
2. Results and Discussion
2.1. Synthesis of Methyl 3-Deoxy-3-C-(methyl)-β-d-gulopyranosides and galectin inhibition evaluation
The synthesis of the 3-C-methyl-gulo derivatives was initiated by Dess–Martin periodinane oxidation [12] of the known methyl 2,4,6-tri-O-benzyl-β-d-galactopyranoside 11 to afford the corresponding keto derivative 12 in 84% yield (Scheme 1). Methylenation of 12 with Petasis reagent gave the olefin 13 in 79% yield. Next, the olefin 13 was subjected to hydroboration with 9-borabicyclo-[3.3.1]nonane (9-BBN) [12], followed by oxidative cleavage of the carbon-boron bond with alkaline hydrogen peroxide to afford the corresponding gulo and galacto isomers 14a (36%) and 14b (24%), which were separated by flash column chromatography at a ratio 3:2. Both the gulo and galacto derivatives 14a and 14b were separately subjected to hydrogenation [13] in the presence of Pd(OH)2-C to give the desired methyl 3-deoxy-3-C-(hydroxymethyl)-β-d-gulopyranoside 1a and methyl 3-deoxy-3-C-(hydroxymethyl)-β-d-galactopyranoside 1b in yields of 51% and 63%, respectively. Evaluation of 1a and 1b as inhibitors of human galectin-1, 2, 3, 4N (N-terminal domain) 4C (C-terminal domain), 7, 8N, 8C, 9N, and 9C in a reported competitive fluorescence anisotropy assay [14,15] revealed that the gulo derivative 1a was selective for galectin-1 with a dissociation constant of 1300 µM, which is about an order of magnitude better than for the virtually unselective reference compound methyl β-d-galactopyranoside 32 (Figure 1, Table 1).
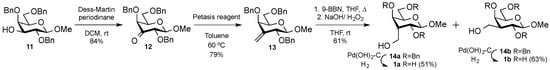
Scheme 1.
Synthesis of methyl 3-deoxy-3-C-(hydroxymethyl)-β-d-gulopyranoside 1a, methyl 3-deoxy-3-C-(hydroxymethyl)-β-d-galactopyranoside 1b.
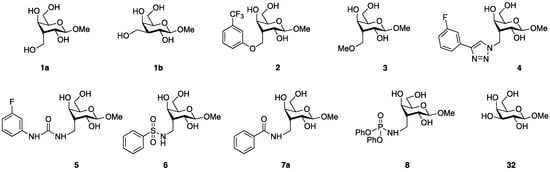
Figure 1.
Structures of the tested compounds 1–8 and reference compound 32.

Table 1.
Kd-values (mM)a of compounds 1a–1b, 2–3, 7a, 8, and the reference methyl β-d-galactopyranoside 32 against human galectin-1, 2, 3, 4N, 4C, 7, 8N, 8C, 9N, and 9C in a competitive fluorescence polarization assay [15,16].
In stark contrast, the galacto derivative 1b did not bind any galectin tested, except for a weak binding to galectin-4N. This observation encouraged us to further explore the 3-C-methyl gulopyranoside scaffold for the discovery of galectin-1-selective inhibitors. Hence, we initiated synthetic efforts toward replacing the hydroxymethyl of 1a with amide, ether, urea, and triazole functionalities. An aryl ether was synthesized following a recently reported iodonium-salt mediated reaction [20] to give the aryl ether 15, which after hydrogenolysis [13] of the benzyl protecting groups gave 2 (Scheme 2). The hydroxymethyl 14a was methylated with methyl iodide to give the methyl ether 16, which after debenzylation gave the 3-methoxymethyl guloside 3. Treatment of 14a with methanesulfonyl chloride furnished the corresponding gulo mesylate, which was then directly treated with NaN3 in dry DMF at 80 °C to provide the gulo azide, 17 in 83% yield. The gulo azide 17 was treated with 1-ethynyl-3-fluorobenzene in the presence of the CuI and DIPEA catalytic system [21] in dry dichloromethane to give the triazole 18 within 48 h in 86% yield. Debenzylation provided the desired triazole-derived methyl guloside 4. The urea 20 was obtained via reduction of the azide 17 to give the amine 19, followed by reaction with 3-fluorophenylisocyanate. Debenzylation [13] of 20 afforded the target gulo urea derivative 5 in 66% yield. The amine 19 was treated with benzensulfonyl chloride, benzoyl chloride, and diphenyl phosphoryl chloride in the presence of Et3N to give the protected sulfonamide 21, amide 22a, and diphenylphosphonamide 23, which were subjected for hydrogenolysis [13] in the presence of Pd(OH)2 to get the unprotected amides 6, 7a, and 8.
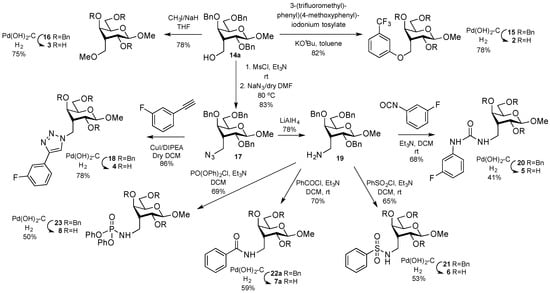
Scheme 2.
Synthesis of methyl 3-deoxy-3-C-methyl-β-d-gulopyranoside ether 2–3, triazole 4, urea 5, sulfonamide 6, amide 7a, and phosphonamide 8 derivatives.
Evaluation of aryl ether 2, methyl ether 3, triazole 4, urea 5, sulfonamide 6, benzamide 7a, and phosphonamide 8 derived methyl gulosides’ affinities for the human galectin-1, 2, 3, 4N, 4C, 7, 8N, 8C, 9N, and 9C showed that most of the gulo derivatives were inactive as ligands for galectins, the benzamide 7a displayed moderate affinity, similar to that of 1a, for galectin-1 and with excellent selectivity (Table 1). Particularly noteworthy was that 7a also had a significantly better affinity for galectin-1 than the simple reference monosaccharide methyl β-d-galactopyranoside 32. Furthermore, the hydroxylmethyl group of 1a plays an important role in the interaction with galectin-1, as the corresponding methyl ether 3 binds galectin-1 significantly worse than 1a does.
2.2. Synthesis and Optimization of 3-Deoxy-3-C-Amidomethyl-β-d-Gulopyranoside Derivatives as Galectin-1 Inhibitors
The observation that the amide 7a showed moderate affinity but high selectivity for galectin-1 prompted us to prepare a series 7c–7l of benzamide analogs carrying selected different substituents at different positions, including four fluorbenzamide expected to possess improved metabolic stability and pharmacokinetic properties, as well as a reference acetamide analog 7b (Scheme 3). Furthermore, in order to investigate the role of the gulo 3-C-methyl substituent, the 3-OH 9 and 3-benzamido 10 gulosides were synthesized (Scheme 3). Hydrolysis of the known 4,6-O-benzylidene gulose derivative, 24 [22] with 80% AcOH at 80 °C gave the diol 25 in 91% yield, which upon Zemplen de-O-acetylation [23] afforded the target methyl β-d-gulopyranoside 9 in 93% yield. Selective 3-O-triflation of methyl 4,6-O-benzylidene-β-d-galactopyranoside 26 [24], followed by one-pot benzoylation of 2-O-hydroxyl gave 27. The crude triflate 27 was subsequently converted into the 3-azido-3-deoxy-guloside 28 by treatment with sodium azide in DMF. De-benzylidenation with 80% AcOH at 80 °C and subsequent benzoylation afforded 29 in 43% yield over four steps from 26. Azide hydrogenation gave 30, which upon benzoylation and de-O-benzoylation gave the benzamide 10.
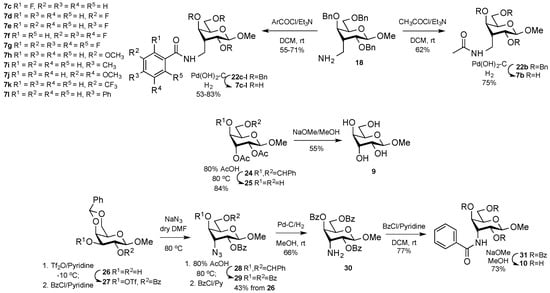
Scheme 3.
Synthesis of 3-deoxy-3-C-amidomethyl-β-d-gulo derivatives 7b–7l, methyl β-d-gulopyranoside 9, and methyl 3-deoxy-3-N-benzamido-β-d-gulopyranoside 10.
An immediate observation upon evaluating the affinities of 7b–7l and 9–10 (Figure 2, Table 2) was that the acetamide 7b displays a similar affinity for galectin-1 as the benzamides 7a and 7c–7k. Hence, the phenyl moieties of 7a and 7c–7k do not contribute to enhancing the affinity for galectin-1. However, the phenyl moieties and substitution patterns of 7a and 7c–7k influence the selectivity over other galectins, as six substituted amides (7a, 7d, and 7f–7i) retained high selectivity for galectin-1 over the other galectins. The pentafluorophenyl 7g turned out to be the best β-d-gulopyranoside-based monosaccharide inhibitor for human galectin-1 with 14-fold improved affinity over the reference methyl β-d-galactopyranoside 32. The larger biphenyl 7l did not bind galectin-1, which suggests that the galectin-1 site accommodating the axial gulo substituent is limited in size. Evaluation of the guloside 9 revealed that while it is similar to the reference galactoside 32 in the affinity for galectin-1, it displays a much higher selectivity in that it is inactive against the other galectins under the evaluation conditions used. Unfortunately, extensive molecular dynamics and docking analyses to explain the selective galectin-1 binding to 3-C-methyl-gulosides were inconclusive as such calculations cannot provide reliable relative affinities of bound ligands. Hence, it remains to find a plausible structural explanation for this selectivity. Interestingly, the benzamide 10 showed no binding to galectin-1 under the assay conditions but instead had improved binding to and selectivity for galectin-4N. Hence, while 3-C-methyl gulosides represent an interesting structural class for the discovery of selective galectin-1 inhibitors, 3-C-amido gulosides may represent a novel structural class for galectin-4 inhibitor discovery.
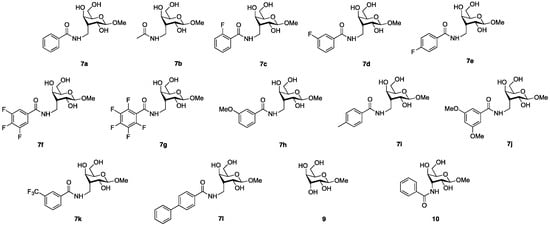
Figure 2.
Structures of all tested compounds 7a–7l and 9–10.

Table 2.
Kd-values (mM)a of compounds 7a–7l, 9, and 10 against human galectin-1, 2, 3, 4N, 4C, 7, 8N, 8C, 9N, and 9C in a competitive fluorescence polarization assay.
3. Materials and Methods
3.1. General Methods Experimental Procedures
All reactions were carried out in oven-dried glassware. All solvents and reagents were mainly purchased from Sigma-Aldrich or Fluka and were used without further purification or synthesized via the literature protocol. TLC analysis was performed on pre-coated Merck silica gel 60 F254 plates using UV light and charring solution (10 mL conc. H2SO4/90 mL EtOH). Flash column chromatography was done on SiO2 purchased from Aldrich (technical grade, 60 Å pore size, 230–400 mesh, 40–63 μm). All NMR spectra were recorded with the Bruker DRX 400 MHz spectrometer (400 MHz for 1H, 100 MHz for 13C (125 MHz 13C for compound 7k with the Bruker Avance III 500 MHz spectrometer equipped with a broadband observe SMART probe, Fällanden, Switzerland), 376 MHz for 19F, 162 MHz for 31P, ESI) at ambient temperature using CDCl3 or CD3OD as solvents. Chemical shifts are given in ppm relative to the residual solvent peak (1H NMR: CDCl3 δ 7.26; CD3OD δ 3.31; 13C NMR: CDCl3 δ 77.16; CD3OD δ 49.00) with multiplicity (b = broad, s = singlet, d = doublet, t = triplet, q = quartet, quin = quintet, sext = sextet, hept = heptet, m = multiplet, td = triplet of doublets, dt = doublet of triplets), coupling constants (in Hz) and integration. Copies of nmr spectra are provided in the supplementary information. High-resolution mass analysis was obtained using the Micromass Q-TOF mass spectrometer. Analytical data is given if the compound is novel or not fully characterized in the literature. Final compounds were further purified via HPLC before evaluation of galectin affinity. All tested compounds were >95% pure according to the analytical HPLC analysis.
3.2. Methyl 2,4,6-Tri-O-Benzyl-β-d-Xylo-Hex-3-Ulopyranoside 12
Into a solution of alcohol 11 (8.1 g, 17.45 mmol) in dry dichloromethane (250 mL) Dess–Martin periodinane (9.62 g, 22.68 mmol, 1.3 equiv.) was added, under nitrogen atmosphere and the reaction mixture was stirred for 4 h (TLC heptane/EtOAc, 3:1, Rf 0.5). After that, a saturated NaHCO3 solution (400 mL) was added and the mixture was stirred for 30 min. Then, the organic layer was collected and washed successively with the saturated Na2S2O3 solution (2 × 250 mL). The organic layer was collected, dried over Na2SO4, filtered and concentrated in vacuo. Flash chromatography of the crude material (heptane/EtOAc, 7:2) afforded ketone 12 (6.45 g, 13.955 mmol, yield 80%) as a white solid. −72.3 (c 1.4, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.47–7.21 (m, 15H, ArH), 4.76 (d, 1H, J 12.0 Hz, CH2Ph), 4.73 (d, 1H, J 12.0 Hz, CH2Ph), 4.58 (d, 1H, J 12.0 Hz, CH2Ph), 4.51 (d, 1H, J 12.0 Hz, CH2Ph), 4.48 (d, 1H, J1,2 7.6 Hz, H-1), 4.44 (d, 1H, J1,2 7.6 Hz, H-2), 4.43 (d, 1H, J 11.6 Hz, CH2Ph), 4.35 (d, 1H, J 11.6 Hz, CH2Ph), 3.95 (d, 1H, J 1.2 Hz, H-4), 3.83–3.75 (m, 3H, H-5, H-6a, H-6b), 3.61 (s, 3H, OCH3). 13C NMR (CDCl3, 100 MHz): 203.8, 137.7, 137.2, 136.4, 128.29, 128.27, 127.26, 128.0, 127.8, 127.6, 127.5, 104.9, 82.1, 80.7, 73.5, 73.4, 72.3, 72.1, 67.5, 57.1. HRMS calcd for C28H30O6+NH4+ (M+NH4)+: 480.2386, found: 480.2378.
3.3. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-Methylene-β-d-Xylo-Hex-3-Ulopyranoside 13
Into a solution of ketone 12 (6.3 g, 13.63 mmol) in dry toluene (100 mL) bis (cyclopentadienyl) dimethyltitanium was added, 5 wt% in toluene (125 mL, 30 mmol, 2.2 equiv.), under nitrogen atmosphere and the reaction mixture was stirred for 48 h at 65 °C in the dark. After that, the reaction mixture (TLC heptane/EtOAc, 4:1, Rf 0.47) was concentrated in vacuo and flash chromatography of the crude material (heptane/EtOAc, 10:1–5:1) afforded methylene derivative 13 (4.6 g, 9.99 mmol, yield 71%) as a light-yellow oil. −40.3 (c 1.1, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.49–7.28 (m, 15H, ArH), 5.61 (t, 1H, J2,H-7a 2.0 Hz, CH2), 5.20 (t, 1H, J2,H-7b 2.0 Hz, CH2), 5.00 (d, 1H, J 12.0 Hz, CH2Ph), 4.78 (d, 1H, J 12.0 Hz, CH2Ph), 4.65 (d, 1H, J 12.0 Hz, CH2Ph), 4.58 (d, 1H, J 12.0 Hz, CH2Ph), 4.56 (d, 1H, J 12.0 Hz, CH2Ph), 4.36 (d, 1H, J 7.6 Hz, H-1), 4.28 (d, 1H, J 12.0 Hz, CH2Ph), 4.14 (dt, 1H, J1,2 7.6 Hz, J2,H-7a, J2,H-7b 2.0 Hz, H-2), 4.03 (d, 1H, J 0.4 Hz, H-4), 3.91–3.79 (m, 3H, H-5, H-6a, H-6b), 3.65 (s, 3H, OCH3). 13C NMR (CDCl3, 100 MHz): 142.2, 138.5, 138.2, 137.9, 128.4, 128.3, 128.2, 128.0, 127.9, 127.7, 127.62, 127.59, 127.5, 113.7, 104.9, 77.7, 77.3, 76.6, 73.7, 73.6, 69.2, 69.0, 56.7. HRMS calcd for C29H32O5+NH4+ (M+NH4)+: 478.2593, found: 478.2607.
3.4. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-Hydroxymethyl-β-d-Gulopyranoside 14a and Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-Hydroxymethyl-β-d-Galactopyranoside 14b
A solution of 13 (4.6 g, 9.99 mmol) in dry THF (150 mL) was treated with a 9-BBN solution in THF (0.5 M, 125 mL) and heated to reflux for 24 h. After that, the solution was cooled to 0 °C and a 10% aqueous sodium hydroxide solution (100 mL) and a 30% hydrogen peroxide solution (100 mL) were added simultaneously within 5 min and stirring continued for another 30 min. Then, diethyl ether (200 mL) was added followed by careful addition of a 20% aqueous sodium hydrogen sulfite solution (7 mL). This mixture was stirred further for 60 min and extracted with diethyl ether, and the combined organic layers were dried with Na2SO4, filtered, and concentrated in vacuo (TLC heptane/EtOAc, 2:1 (double run), Rf 0.48 for 14a, Rf 0.4 for 14b). Flash chromatography (Heptane/EtOAc, 8:1 to 2:1) of the residue afforded a gulo-isomer, 14a (1.74 g, 3.638 mmol) and galacto-isomer, 14b (1.16 g, 2.426 mmol) to be ≈3:2 in favor of the guloisomer at an overall yield of 61% (2.9 g, 6.064 mmol). Gulo-isomer 14a: −25.7 (c 1.3, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.36–7.20 (m, 15H, ArH), 4.82 (d, 1H, J 12.0 Hz, CH2Ph), 4.65 (d, 1H, J 6.4 Hz, H-1), 4.57 (d, 1H, J 11.6 Hz, CH2Ph), 4.54 (d, 1H, J 11.6 Hz, CH2Ph), 4.52 (d, 1H, J 11.6 Hz, CH2Ph), 4.47 (d, 1H, J 12.0 Hz, CH2Ph), 4.41 (d, 1H, J 11.6 Hz, CH2Ph), 3.95–3.88 (m, 2H, H-4, H-5), 3.73 (dd, 1H, J1,2 6.4 Hz, J2,3 5.2 Hz, H-2), 3.74–3.57 (m, 4H, H-6a, H-6b, CH2OH), 3.54 (s, 3H, OCH3), 2.53–2.47 (m, 1H, H-3), 2.35 (bs, 1H, CH2OH). 13C NMR (CDCl3, 100 MHz): 138.2, 138.1, 138.0, 128.6, 128.52, 128.47, 128.2, 128.1, 128.03, 127.96, 127.9, 127.8, 101.2, 77.2, 74.8, 73.8, 73.6, 73.4, 71.9, 69.5, 62.0, 56.5, 41.6. HRMS calcd for C29H34O6+NH4+ (M+NH4)+: 496.2699, found: 496.2700. Galacto-isomer 14b: −13.4 (c 0.9, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.39–7.28 (m, 15H, ArH), 4.92 (d, 1H, J 11.2 Hz, CH2Ph), 4.65 (d, 1H, J 11.2 Hz, CH2Ph), 4.60–4.52 (m, 4H, CH2Ph), 4.41 (d, 1H, J1,2 7.6 Hz, H-1), 3.90(d, 1H, J3,4 2.8 Hz, H-4), 3.82 (dd, 1H, J 4.8 Hz, J 7.2 Hz, CH2OH), 3.73–3.55 (m, 8H, H-5, H-6a, H-6b, H-2, CH2OH, OCH3), 2.04 (bs, 1H, CH2OH), 1.87–1.82 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 138.5, 138.1, 137.8, 128.6, 128.53, 128.52, 128.4, 128.3, 128.03, 127.98, 127.8, 106.4, 76.5, 76.2, 74.8, 74.7, 74.6, 73.7, 68.6, 62.2, 56.8, 47.3. HRMS calcd for C29H34O6+H+ (M+H)+: 479.2434, found: 479.2434.
3.5. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(3-Trifluoromethylphenoxymethyl)-β-d-Gulopyranoside 15
Compound 14a (80 mg, 0.17 mmol) was stirred in a 25 mL round-bottom flask in toluene (2 mL) for 3 min. A mixture of 3-(trifluoromethyl)phenyl)(4-methoxyphenyl)iodonium tosylate (140 mg, 0.25 mmol) and potassium tert-butoxide (28.5 mg, 0.25 mmol) were added under air and the mixture turned yellow. The reaction was stirred for 3 h, when the TLC showed almost complete consumption of the starting material (TLC heptane/EtOAc, 3:1, Rf 0.48). The mixture was then diluted with EtOAc (10 mL) and filtered. Then the volatiles were removed under reduced pressure, and the residue was subjected to column chromatography (heptane/EtOAc, 8:1 to 4:1) to provide the purified product 15 (92.6 mg, 0.15 mmol, 89%) as a colorless oil. −70.9 (c 0.8, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.40–7.22 (m, 17H, ArH), 7.08 (bs, 1H, ArH), 7.02 (dd, 1H, J 8.0 Hz, J 2.4 Hz, ArH), 4.79 (d, 1H, J 12.0 Hz, CH2Ph), 4.62 (d, 1H, J 6.0 Hz, H-1), 4.58 (d, 1H, J 12.0 Hz, CH2Ph), 4.57 (d, 1H, J 11.6 Hz, CH2Ph), 4.54 (d, 1H, J 12.4 Hz, CH2Ph), 4.50 (s, 2H, CH2Ph), 4.24 (dd, 1H, J 6.0 Hz, J 9.6 Hz, H-3a’), 4.19–4.15 (m, 1H, H-5), 4.08 (dd, 1H, J 9.6 Hz, J 8.0 Hz, H-3b’), 3.87 (dd, 1H, J 5.2 Hz, J 2.8 Hz, H-4), 3.85 (t, 1H, J 6.0 Hz, H-2), 3.80 (dd, 1H, J 10.0 Hz, J 6.8 Hz, H-6a), 3.72 (dd, 1H, J 10.0 Hz, J 5.2 Hz, H-6b), 3.57 (s, 1H, OCH3), 2.76–2.70 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 158.8, 138.30, 138.27, 137.9, 131.9 (q, J 32.1 Hz), 130.0, 128.49, 128.46, 128.3, 128.0, 127.9, 127.83, 127.77, 124.1 (q, J 271 Hz), 118.0, 117.6 (q, J 3.8 Hz), 111.5 (q, J 3.7 Hz), 101.3, 74.7, 73.6, 73.5, 73.3, 72.8, 71.9, 69.8, 64.6, 56.4, 39.6. 19F NMR (CDCl3, 376 MHz): −62.6. HRMS calcd for C36H41F3NO6+NH4+ (M+NH4)+: 640.2886, found: 640.2895.
3.6. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-Methoxymethyl-β-d-Gulopyranoside 16
Compound 14a (57 mg, 0.12 mmol) was stirred in a 5 mL round-bottom flask in dry THF (2 mL) for 5 min at 0 °C. Into the solution, NaH (6 mg, 0.24 mmol) was added and the stirring was continued at 0 °C for 5 min. Then, into the reaction mixture iodomethane dropwise was added and the reaction temperature increased to rt gradually. Stirring continued overnight when the TLC showed almost complete consumption of the starting material (TLC heptane/EtOAc, 3:2, Rf 0.53). Then, NaH was quenched with EtOAc and the volatiles were removed under reduced pressure. The residue was subjected to column chromatography (heptane/EtOAc, 6:1 to 3:1) to provide the purified product 16 (46 mg, 0.09 mmol, 78%). −62.5 (c 1.2, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.35–7.20 (m, 15H, ArH), 4.76 (d, 1H, J 12.0 Hz, CH2Ph), 4.58 (d, 1H, J 12.0 Hz, CH2Ph), 4.57 (d, 1H, J 11.6 Hz, CH2Ph), 4.53 (d, 1H, J1,2 6.4 Hz, H-1), 4.48 (d, 2H, J 12.4 Hz, CH2Ph), 4.37 (d, 1H, J 11.6 Hz, CH2Ph), 4.11–4.07 (m, 1H, H-5), 3.75–3.71 (m, 3H, H-2, H-4, H-6a), 3.67–3.61 (m, 2H, H-6b, CH2OCH3), 3.56–3.50 (m, 4H, CH2OCH3, OCH3), 3.29 (s, 3H, CH2OCH3), 2.58–2.52 (m, 3H, H-3). 13C NMR (CDCl3, 100 MHz): 138.8, 138.43, 138.39, 128.5, 128.43, 128.36, 128.1, 127.94, 128.90, 127.8, 127.74, 127.69, 127.66, 101.8, 75.1, 74.7, 73.7, 73.3, 73.2, 71.8, 70.2, 69.2, 59.0, 56.4, 40.2. HRMS calcd for C17H21NO6+H+ (M+H)+: 335.1369, found: 335.1369.
3.7. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-Azidomethyl-β-d-Gulopyranoside 17
Into a stirred solution of 14a (1.6 g, 3.35 mmol) in DCM (25 mL) containing Et3N (890 μL, 6.69 mmol) at 0 °C MsCl (390 μL, 5.02 mmol) was added dropwise over 5 min, and the solution was stirred for 4 h at rt (TLC heptane/EtOAc, 1:1, Rf 0.31). The solution was extracted with 1N HCl (2 × 50 mL) followed by sat’d NaHCO3 (2 × 50 mL), and the organic layer was dried (Na2SO4). The solvent was removed by rotary evaporation to give a yellow liquid that was dissolved in dry DMF (10 mL). Sodium azide (1.3 g, 20.08 mmol) was added and the solution was heated at 80 °C for 6 h to give a yellowish-brown mixture. The mixture was cooled at rt, water (50 mL) was added, and the mixture was extracted with EtOAc (2 × 40 mL). The organic layer was washed with brine (50 mL) and dried (Na2SO4). The solvent was removed by rotary evaporation to give a yellow liquid that was then purified by flash chromatography (Heptane/EtOAc 8:1 to 3:1) to give compound 17 (1.4 g, 2.78 mmol, 83% from 14a) as a colorless liquid. −5.2 (c 0.8, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.38–7.20 (m, 15H, ArH), 4.76 (d, 1H, J 12.0 Hz, CH2Ph), 4.56 (d, 1H, J 12.0 Hz, CH2Ph), 4.55 (d, 1H, J 11.6 Hz, CH2Ph), 4.51 (d, 1H, J1,2 6.4 Hz, H-1), 4.48 (d, 1H, J 12.4 Hz, CH2Ph), 4.44 (d, 1H, J 12.0 Hz, CH2Ph), 4.41 (d, 1H, J 12.0 Hz, CH2Ph), 4.09–4.05 (m, 1H, H-5), 3.77–3.64 (m, 5H, H-2, H-4, H-6a, H-6b, CH2N3), 3.50 (s, 3H, OCH3), 3.39 (dd, 1H, J 12.4 Hz, J 8.4 Hz, CH2N3), 2.42–2.36 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 138.4, 138.3, 137.9, 128.53, 128.52, 128.50, 128.2, 128.02, 127.95, 127.9, 127.8, 100.8, 75.0, 73.7, 73.6, 73.4, 72.5, 72.0, 69.6, 56.4, 48.6, 39.7. HRMS calcd for C29H33N3O5+NH4+ (M+NH4)+: 521.2764, found: 521.2775.
3.8. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-[4-(3-Fluorophenyl)-1H-1,2,3-Triazol-1-Yl-Methyl]-β-d-Gulopyranoside 18
A solution of azide 17 (53 mg, 0.10 mmol) in dichloromethane (2 mL), 1-Ethynyl-3-fluorobenzene (18.1 μL, 0.16 mmol), CuI (10 mol%, 2 mg) and DIPEA (28 μL, 0.16 mmol) were added and the mixture was stirred at room temperature for 48 h (TLC heptane/EtOAc, 2:1, Rf 0.58). The solvent was removed under reduced pressure, and the residue was dissolved in EtOAc (10 mL) and the solution was washed with sat. NH4Cl (10 mL), brine (10 mL), dried over Na2SO4 and concentrated in vacuo. The product was purified by flash column chromatography (heptane/EtOAc, 6:1 to 1:1) to give the corresponding triazole, 18 as white amorphous solid (56.4 mg, 0.09 mmol, 86%). −63 (c 0.6, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.52–7.04 (m, 20H, ArH), 4.80 (d, 1H, J 11.6 Hz, CH2Ph), 4.63 (dd, 1H, J 6.8 Hz, J 14.0 Hz, H-3′), 4.60–4.44 (m, 7H, H-1, H-3′, CH2Ph), 4.35 (d, 1H, J 11.6 Hz, CH2Ph), 4.26–4.22 (m, 1H, H-5), 3.79 (dd, 1H, J 10.0 Hz, J 7.2 Hz, H-6a), 3.74 (dd, 1H, J 6.4 Hz, J 3.2 Hz, H-4), 3.71 (dd, 1H, J 10.0 Hz, J 5.2 Hz, H-6b), 3.79 (t, 1H, J 4.8 Hz, H-2), 3.51 (s, 1H, OCH3), 2.68–2.61 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 163.3 (d, J 244 Hz), 146.8, 138.19, 138.16, 137.4, 132.8 (d, J 8.3 Hz), 130.5 (d, J 8.4 Hz), 128.7, 128.52, 128.51, 128.3, 128.2, 128.0, 127.9, 127.8, 121.3 (d, J 2.7 Hz), 120.6, 115.0 (d, J 22 Hz), 112.7 (d, J 23 Hz), 100.1, 75.2, 73.52, 73.47, 73.1, 72.5, 72.2, 69.4, 56.4, 47.4, 41.1. 19F NMR (CDCl3, 376 MHz): −112.7. HRMS calcd for C37H38FN3O5+H+ (M+H)+: 624.2874, found: 624.2884.
3.9. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(Aminomethyl)-β-d-Gulopyranoside 19
Into a stirred solution of 17 (1.31 g, 2.60 mmol) in dry THF (20 mL) at 0 °C, LiAlH4 (148 mg, 3.9 mmol) was added in portions over 5 min under nitrogen atmosphere, and the solution was stirred for 1 h at rt (TLC DCM/MeOH, 15:1, Rf 0.44). After 30 min, TLC was checked which shows complete conversion of the azide into amine. Then, the reaction was quenched EtOAc and the reaction mixture was filtered through a pad of Celite® (St. Louis, MO, USA). Then, the filtrate was concentrated in vacuo and the crude was purified by column chromatography (DCM:MeOH 25:1) to give compound 19 (969 mg, 2.03 mmol, yield 78%) as a colorless oil. −36.2 (c 1.1, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.35–7.22 (m, 15H, ArH), 4.80 (d, 1H, J 12.0 Hz, CH2Ph), 4.57–4.54 (m, 3H, H-1, CH2Ph), 4.51 (d, 1H, J 12.0 Hz, CH2Ph), 4.47 (d, 1H, J 12.0 Hz, CH2Ph), 4.42 (d, 1H, J 11.6 Hz, CH2Ph), 3.97–3.93 (m, 1H, H-5), 3.74–3.65 (m, 4H, H-2, H-4, H-6a, H-6b), 3.52 (s, 3H, OCH3), 3.08 (dd, 1H, J 6.4 Hz, J 12.8 Hz, CH2NH2), 2.68 (dd, 1H, J 12.8 Hz, J 6.4 Hz, CH2NH2), 2.32–2.27 (m, 1H, H-3), 1.99 (bs, 2H, CH2NH2). 13C NMR (CDCl3, 100 MHz): 138.6, 138.3, 138.1, 128.53, 128.49, 128.4, 128.2, 128.0, 127.9, 127.83, 127.81, 127.77, 101.3, 76.2, 75.2, 73.6, 73.4, 72.9, 71.7, 69.7, 56.4, 42.7, 39.7. HRMS calcd for C29H35NO5+H+ (M+H)+: 478.2593, found: 478.2603.
3.10. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(3-Fluorophenylureidomethyl)-β-d-Gulopyranoside 20
A solution of amine 19 (61 mg, 0.13 mmol) in dry dichloromethane (2 mL), Et3N (35.6 μL, 0.26 mmol) was added and the mixture was stirred at room temperature for 5 min under N2 atmosphere. Then into the solution phenyl isocyanate (29.2 μL, 0.26 mmol) was added and the solution was stirred at rt for 12 h (TLC heptane/EtOAc, 1:1, Rf 0.32). The solvent was removed under reduced pressure, and the residue was dissolved in EtOAc (10 mL) and the solution was washed with brine (10 mL), dried over Na2SO4 and concentrated in vacuo. The product was purified by flash column chromatography (heptane/EtOAc, 5:1 to 2:1) to give the corresponding semicarbazide 20 as a colorless oil (53.4 mg, 0.09 mmol, yield 68%). −83.1 (c 0.8, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.39–7.12 (m, 17H, ArH), 6.85 (dd, 1H, J 1.2 Hz, J 8.0 Hz, ArH), 6.71–6.66 (m, 1H, ArH), 6.11 (bs, 1H, NHCONHC6H4F), 5.30 (bs, 1H, NHCONHC6H4F), 4.79 (d, 1H, J 11.6 Hz, CH2Ph), 4.59 (d, 1H, J 6.0 Hz, H-1), 4.55 (d, 1H, J 12.0 Hz, CH2Ph), 4.49 (d, 1H, J 12.4 Hz, CH2Ph), 4.48–4.42 (m, 3H, CH2Ph), 4.03–3.99 (m, 1H, H-5), 3.74–3.65 (m, 4H, H-2, H-4, H-6a, H-6b), 3.51 (s, 1H, OCH3), 3.42 (dd, 1H, J 14.0 Hz, J 5.6 Hz, CH2NHCONHC6H4F), 3.34 (dd, 1H, J 14.0 Hz, J 7.6 Hz, CH2NHCONHC6H4F), 2.42–2.36 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 163.3 (d, J 243 Hz), 154.9, 140.6 (d, J 11 Hz), 138.4, 138.2, 137.9, 130.2 (d, J 9.5 Hz), 128.8, 128.5, 128.3, 128.2, 128.0, 127.9, 127.8, 114.8 (d, J 2.7 Hz), 109.7 (d, J 21.2 Hz), 106.9 (d, J 26 Hz), 100.7, 75.3, 73.54, 73.49, 73.0, 72.1, 69.4, 56.5, 39.8, 39.1. 19F NMR (CDCl3, 376 MHz): −111.6. HRMS calcd for C36H40FN2O6+H+ (M+H)+: 615.2886, found: 615.2870.
3.11. General Procedure for the Synthesis of Amides 21, 22a–22l, and 23
To a solution of the amine (1 eq) in dry DCM (2 mL per 0.1 mmol) Et3N (2 eq) was added. Into the solution, acid chloride or anhydride (1.5 eq) was added and the solution was stirred at rt for 8 h. After that, 1(N) HCl solution was added to the reaction mixture and extracted with DCM and washed successively with saturated NaHCO3. After evaporating the solvents in vacuo, the crude material thus obtained was purified by flash chromatography using heptane–EtOAc (5:1 to 1:1) to give pure amides as colorless oils.
3.11.1. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-Phenylsulfonamidomethyl-β-d-Gulopyranoside 21
Compound 21 (TLC heptane/EtOAc, 2:1, Rf 0.21) was prepared according to the general procedure 3.11 from the amine 19 (55 mg, 0.12 mmol). Obtained as a colorless oil in 65% yield (46.2 mg, 0.07 mmol). −55.7 (c 0.7, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.73–7.17 (m, 20H, ArH), 5.23 (dd, 1H, J 5.2 Hz, J 6.8 Hz, CH2NHSO), 4.75 (d, 1H, J 11.6 Hz, CH2Ph), 4.53 (d, 1H, J 12.0 Hz, CH2Ph), 4.50 (d, 1H, J 12.0 Hz, CH2Ph), 4.45 (d, 1H, J 12.0 Hz, CH2Ph), 4.42 (d, 1H, J 6.0 Hz, H-1), 4.39 (d, 1H, J 11.2 Hz, CH2Ph), 4.36 (d, 1H, J 11.2 Hz, CH2Ph), 3.90–3.87 (m, 1H, H-5), 3.70–3.60 (m, 4H, H-2, H-4, H-6a, H-6b), 3.47 (s, 3H, OCH3), 3.17–3.10 (m, 1H, CH2NHSO), 3.00–2.93 (m, 1H, CH2NHSO), 2.41–2.35 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 139.7, 138.1, 137.9, 137.6, 132.6, 129.1, 128.7, 128.5, 128.2, 128.1, 127.94, 127.91, 127.8, 127.1, 100.5, 76.3, 74.8, 73.54, 73.50, 72.8, 71.8, 69.3, 56.3, 42.2, 39.1. HRMS calcd for C35H39NO7S+NH4+ (M+NH4)+: 635.2788, found: 635.2791.
3.11.2. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(Benzamidomethyl)-β-d-Gulopyranoside 22a
Compound 22a (TLC heptane/EtOAc, 2:1, Rf 0.27) was prepared according to the general procedure 3.11 from the amine 19 (43 mg, 0.09 mmol). Obtained as a colorless oil in 70% yield (37 mg, 0.06 mmol). −42.4 (c 0.8, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.43–7.22 (m, 20H, ArH), 7.03 (t, 1H, J 5.6 Hz, CH2NHCO), 4.89 (d, 1H, J 11.2 Hz, CH2Ph), 4.69 (d, 1H, J1,2 6.0 Hz, H-1), 4.57 (d, 1H, J 12.0 Hz, CH2Ph), 4.55–4.46 (m, 4H, CH2Ph), 4.07–4.03 (m, 1H, H-5), 3.82 (dd, 1H, J1,2 6.0 Hz, J2,3 4.8 Hz, H-2), 3.79–3.68 (m, 4H, H-4, H-6a, H-6b, CH2NHCO), 3.60–3.53 (m, 4H, OCH3, CH2NHCO), 2.51–2.46 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 166.8, 138.3, 138.2, 137.8, 134.2, 131.2, 128.7, 128.53, 128.51, 128.47, 128.3, 128.2, 128.0, 127.8, 126.9, 100.9, 77.9, 75.9, 74.3, 73.5, 73.1, 72.2, 69.4, 56.5, 39.8, 39.3. HRMS calcd for C36H39NO6+H+ (M+H)+: 582.2856, found: 582.2851.
3.11.3. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(Acetamidomethyl)-β-d-Gulopyranoside 22b
Compound 22b (TLC heptane/EtOAc, 1:1, Rf 0.4) was prepared according to the general procedure 3.11 from the amine 19 (49 mg, 0.10 mmol). Obtained as a colorless oil in 62% yield (33 mg, 0.06 mmol). −31.6 (c 0.8, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.39–7.21 (m, 15H, ArH), 6.05 (bs, 1H, NHCOCH3), 4.83 (d, 1H, J 11.6 Hz, CH2Ph), 4.59 (d, 1H, J1,2 6.4 Hz, H-1), 4.55 (d, 1H, J 12.0 Hz, CH2Ph), 4.52–4.42 (m, 4H, CH2Ph), 3.98 (td, 1H, J 6.0 Hz, J 2.8 Hz, H-5), 3.74–3.64 (m, 4H, H-2, H-4, H-6a, H-6b), 3.53 (s, 3H, OCH3), 3.51–3.45 (m, 1H, CH2NHCO), 3.32–3.26 (s, 1H, CH2NHCO), 2.36–2.30 (m, 1H, H-3), 1.74 (s, 3H, NHCOCH3). 13C NMR (CDCl3, 100 MHz): 169.9, 138.4, 138.3, 137.9, 128.7, 128.5, 128.2, 128.12, 128.09, 127.94, 127.86, 127.7, 100.8, 77.1, 75.4, 73.8, 73.5, 73.0, 72.0, 69.4, 56.5, 39.7, 38.4, 23.2. HRMS calcd for C31H37NO6+H+ (M+H)+: 520.2699, found: 520.2704.
3.11.4. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(2-Fluorobenzamidomethyl)-β-d-Gulopyranoside 22c
Compound 22c (TLC heptane/EtOAc, 2:1, Rf 0.19) was prepared according to the general procedure 3.11 from the amine 19 (49 mg, 0.10 mmol). Obtained as a colorless oil in 59% yield (31.5 mg, 0.06 mmol). −41.7 (c 0.7, CHCl3). 1H NMR (CDCl3, 400 MHz): 8.05–8.01 (td, 1H, J 8.0 Hz, J 1.6 Hz, ArH), 7.48–7.43 (m, 1H, ArH), 7.36–7.16 (m, 17H, ArH), 7.07–7.02 (m, 1H, ArH), 4.84 (d, 1H, J 11.6 Hz, CH2Ph), 4.65 (d, 1H, J1,2 6.8 Hz, H-1), 4.62 (d, 1H, J 12.0 Hz, CH2Ph), 4.57 (d, 1H, J 12.0 Hz, CH2Ph), 4.48 (d, 1H, J 12.0 Hz, CH2Ph), 4.46 (d, 1H, J 11.6 Hz, CH2Ph), 4.40 (d, 1H, J 11.6 Hz, CH2Ph), 4.07 (td, 1H, J 6.0 Hz, J 2.8 Hz, H-5), 3.79–3.58 (m, 6H, H-2, H-4, H-6a, H-6b, CH2NHCO), 3.54 (s, 3H, OCH3), 2.49–2.43 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 163.2 (d, J 3.0 Hz), 160.6 (d, J 247 Hz), 138.3, 138.2, 137.8, 133.1 (d, J 9.0 Hz), 132.0 (d, J 2.0 Hz), 128.46, 128.45, 128.4, 128.3, 128.2, 127.9, 127.8, 127.7, 124.7 (d, J 3.1 Hz), 121.4 (d, J 12 Hz), 116.1 (d, J 24 Hz), 101.0, 76.3, 75.1, 73.6, 73.5, 72.9, 72.0, 69.5, 56.5, 40.0, 38.5. 19F NMR (CDCl3, 376 MHz): -113.4. HRMS calcd for C36H38FNO6+NH4+ (M+NH4)+: 617.3027, found: 617.3025.
3.11.5. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(3-Fluorobenzamidomethyl)-β-d-Gulopyranoside 22d
Compound 22d (TLC heptane/EtOAc, 2:1, Rf 0.24) was prepared according to the general procedure 3.11 from the amine 19 (46 mg, 0.10 mmol). Obtained as a colorless oil in 67% yield (48 mg, 0.06 mmol). −61.8 (c 0.7, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.36–6.99 (m, 20H, NHCO, ArH), 4.89 (d, 1H, J 11.2 Hz, CH2Ph), 4.69 (d, 1H, J1,2 6.0 Hz, H-1), 4.58 (d, 1H, J 12.0 Hz, CH2Ph), 4.54–4.47 (m, 4H, CH2Ph), 4.07 (td, 1H, J 6.4 Hz, J 2.8 Hz, H-5), 3.82 (dd, 1H, J1,2 6.0 Hz, J2,3 4.8 Hz, H-2), 3.81–3.66 (m, 4H, H-4, H-6a, H-6b, CH2NHCO), 3.60–3.54 (m, 4H, CH2NHCO, OCH3), 2.50–2.44 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 165.5 (d, J 2.2 Hz), 162.7 (d, J 246 Hz), 138.2, 138.0, 137.7, 136.6 (d, J 6.8 Hz), 130.0 (d, J 7.8 Hz), 128.7, 128.53, 128.50, 128.4, 128.29, 128.25, 128.0, 127.9, 127.8, 122.1 (d, J 3.0 Hz), 118.2 (d, J 22 Hz), 114.4 (d, J 23 Hz), 100.8, 78.8, 75.8, 74.3, 73.5, 73.0, 72.2, 69.3, 56.5, 39.6, 39.5. 19F NMR (CDCl3, 376 MHz): −111.9. HRMS calcd for C36H38FNO6+H+ (M+H)+: 600.2761, found: 600.2772.
3.11.6. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(4-Fluorobenzamidomethyl)-β-d-Gulopyranoside 22e
Compound 22e (TLC heptane/EtOAc, 2:1, Rf 0.2) was prepared according to the general procedure 3.11 from the amine 19 (51 mg, 0.11 mmol). Obtained as a colorless oil in 71% yield (45.4 mg, 0.08 mmol). +51.9 (c 0.6, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.36–7.23 (m, 17H, ArH), 7.05 (t, 1H, J 5.6 Hz, NHCO), 6.89–6.84 (m, 2H, ArH), 4.90 (d, 1H, J 11.2 Hz, CH2Ph), 4.70 (d, 1H, J1,2 6.0 Hz, H-1), 4.58 (d, 1H, J 12.0 Hz, CH2Ph), 4.54–4.48 (m, 4H, CH2Ph), 4.05 (td, 1H, J 6.0 Hz, J 2.8 Hz, H-5), 3.83 (dd, 1H, J1,2 6.0 Hz, J2,3 4.8 Hz, H-2), 3.80–3.67 (m, 4H, H-4, H-6a, H-6b, CH2NHCO), 3.59–3.52 (m, 4H, CH2NHCO, OCH3), 2.52–2.46 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 165.7, 164.5 (d, J 250 Hz), 138.2, 138.1, 130.3 (d, J 3.0 Hz), 129.1 (d, J 8.9 Hz), 128.8, 128.6, 128.53, 128.52, 128.33, 128.28, 128.0, 127.9, 127.8, 115.4 (d, J 22 Hz), 100.8, 78.1, 76.0, 74.4, 73.5, 73.1, 72.2, 69.3, 56.5, 39.61, 39.58. 19F NMR (CDCl3, 376 MHz): −108.8. HRMS calcd for C36H38FNO6+NH4+ (M+NH4)+: 617.3027, found: 617.3038.
3.11.7. Methyl2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(3,4,5-Trifluorobenzamidomethyl)-β-d-Gulopyranoside 22f
Compound 22f (TLC heptane/EtOAc, 2:1, Rf 0.18) was prepared according to the general procedure 3.11 from the amine 19 (49 mg, 0.10 mmol). Obtained as a colorless oil in 53% yield (34.6 mg, 0.06 mmol). −73.6 (c 0.8, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.36–6.93 (m, 18H, ArH), 4.87 (d, 1H, J 11.2 Hz, CH2Ph), 4.68 (d, 1H, J1,2 5.6 Hz, H-1), 4.58 (d, 1H, J 11.6 Hz, CH2Ph), 4.51–4.48 (m, 4H, CH2Ph), 4.07 (td, 1H, J 6.4 Hz, J 3.6 Hz, H-5), 3.82–3.53 (m, 9H, H-2, H-4, H-6a, H-6b, OCH3, CH2NHCO), 2.46–2.40 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 163.7, 151.0 (ddd, J 3.4 Hz, J 10.2 Hz, J 251 Hz), 141.9 (dt, J 15.2 Hz, J 255 Hz), 138.2, 137.9, 137.7, 130.4–130.2 (m), 128.8, 128.6, 128.53, 128.47, 128.29, 128.27, 128.2, 127.90, 127.85, 111.5 (dd, J 6.1 Hz, J 16 Hz), 100.5, 78.1, 75.8, 74.3, 73.6, 73.0, 72.3, 69.3, 56.5, 40.0, 39.3. 19F NMR (CDCl3, 376 MHz): -132.1 (d, J 20 Hz), −155.7 (t, J 20 Hz). HRMS calcd for C36H36F3NO6+NH4+ (M+NH4)+: 653.2838, found: 653.2845.
3.11.8. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(2,3,4,5,6-Pentafluorobenzamidomethyl)-β-d-Gulopyranoside 22g
Compound 22g (TLC heptane/EtOAc, 2:1, Rf 0.17) was prepared according to the general procedure 3.11 from the amine 19 (45 mg, 0.09 mmol). Obtained as a colorless oil in 49% yield (31 mg, 0.05 mmol). −75.7 (c 0.5, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.36–7.20 (m, 15H, ArH), 6.81 (t, 1H, J 5.6 Hz, CH2NHCO), 4.89 (d, 1H, J 11.2 Hz, CH2Ph), 4.65 (d, 1H, J1,2 6.0 Hz, H-1), 4.57 (d, 1H, J 12.0 Hz, CH2Ph), 4.51 (d, 1H, J 11.2 Hz, CH2Ph), 4.48 (d, 1H, J 12.0 Hz, CH2Ph), 4.47 (d, 1H, J 12.0 Hz, CH2Ph), 4.42 (d, 1H, J 12.0 Hz, CH2Ph), 4.06 (td, 1H, J 6.8 Hz, J 3.6 Hz, H-5), 3.81 (dd, 1H, J1,2 6.0 Hz, J2,3 3.6 Hz, H-2), 3.78–3.69 (m, 3H, H-4, H-6a, H-6b), 3.63–3.58 (m, 2H, CH2NHCO), 3.54 (s, 1H, OCH3), 2.45–3.39 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 156.9, 145.1–144.9 (m), 142.6–142.4 (m), 140.9–140.6 (m), 138.8–138.5 (m), 138.2, 137.9, 137.7, 136.3–136.0 (m), 128.5, 128.2, 128.1, 128.0, 127.9, 127.8, 111.9–111.5 (m), 106.4, 100.3, 77.8, 75.4, 73.9, 73.5, 72.4, 69.3, 56.5, 39.8, 38.9. 19F NMR (CDCl3, 376 MHz): −140.5 to −140.6 (m, 2F), −151.7 (t, 1F, J 21 Hz), −160.1 to −160.3 (m, 2F). HRMS calcd for C36H34F5NO6+NH4+ (M+NH4)+: 689.2650, found: 689.2656.
3.11.9. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(3-Methoxybenzamidomethyl)-β-d-Gulopyranoside 22h
Compound 22h (TLC heptane/EtOAc, 1:1, Rf 0.45) was prepared according to the general procedure 3.11 from the amine 19 (47 mg, 0.10 mmol). Obtained as a colorless oil in 51% yield (30.7 mg, 0.05 mmol). −43.2 (c 0.5, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.35–7.21 (m, 17H, ArH), 7.08 (t, 1H, J 8.0 Hz, ArH), 7.00 (m, 2H, CH2NHCO, ArH), 6.75–6.72 (m, 1H, ArH), 4.87 (d, 1H, J 11.6 Hz, CH2Ph), 4.68 (d, 1H, J1,2 6.4 Hz, H-1), 4.57 (d, 1H, J 11.6 Hz, CH2Ph), 4.54 (d, 1H, J 11.6 Hz, CH2Ph), 4.51 (d, 1H, J 12.8 Hz, CH2Ph), 4.48 (d, 1H, J 11.2 Hz, CH2Ph), 4.45 (d, 1H, J 11.2 Hz, CH2Ph), 4.05 (td, 1H, J 6.4 Hz, J 2.8 Hz, H-5), 3.83 (dd, 1H, J1,2 6.4 Hz, J2,3 5.2 Hz, H-2), 3.79 (s, 3H, C6H4OCH3), 3.78–3.67 (m, 5H, H-2, H-4, H-6a, H-6b, CH2NHCO), 3.59–3.53 (m, 4H, CH2NHCO, OCH3), 2.50–2.45 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 166.8, 159.9, 138.3, 138.2, 137.8, 129.5, 128.7, 128.5, 128.4, 128.3, 128.1, 128.0, 127.9, 127.8, 118.4, 117.7, 112.3, 100.9, 77.6, 75.8, 74.2, 73.5, 73.1, 72.1, 69.4, 56.5, 55.5, 39.8, 39.2. HRMS calcd for C37H41NO7+H+ (M+H)+: 612.2961, found: 612.2972.
3.11.10. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(p-Toluamidomethyl)-β-d-Gulopyranoside 22i
Compound 22i (TLC heptane/EtOAc, 2:1, Rf 0.24) was prepared according to the general procedure 3.11 from the amine 19 (51 mg, 0.11 mmol). Obtained as a colorless oil in 61% yield (38.8 mg, 0.07 mmol). −56.2 (c 0.5, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.35–7.21 (m, 17H, ArH), 7.04 (d, 1H, J 7.6 Hz, ArH), 6.96 (t, 1H, J 6.0 Hz, CH2NHCO), 4.88 (d, 1H, J 11.2 Hz, CH2Ph), 4.68 (d, 1H, J1,2 6.4 Hz, H-1), 4.57 (d, 1H, J 12.0 Hz, CH2Ph), 4.53 (d, 1H, J 11.2 Hz, CH2Ph), 4.51 (d, 1H, J 12.0 Hz, CH2Ph), 4.48 (d, 1H, J 12.0 Hz, CH2Ph), 4.45 (d, 1H, J 11.6 Hz, CH2Ph), 4.04 (td, 1H, J 6.4 Hz, J 2.8 Hz, H-5), 3.82 (dd, 1H, J1,2 6.4 Hz, J2,3 5.2 Hz, H-2), 3.78–3.67 (m, 3H, H-4, H-6a, H-6b, CH2NHCO), 3.58–3.52 (m, 4H, CH2NHCO, OCH3), 2.51–2.45 (m, 1H, H-3), 2.36 (s, 3H, CH3). 13C NMR (CDCl3, 100 MHz): 166.8, 141.6, 138.3, 138.2, 137.9, 131.4, 130.3, 129.24, 129.16, 128.7, 128.52, 128.51, 128.4, 128.3, 128.1, 128.0, 127.9, 127.8, 126.9, 101.0, 77.8, 75.9, 74.2, 73.5, 73.1, 72.1, 69.4, 56.5, 39.9, 39.2, 21.5. HRMS calcd for C37H41NO6+H+ (M+H)+: 596.3012, found: 596.3019.
3.11.11. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(3,5-Dimethoxybenzamidomethyl)-β-d-Gulopyranoside 22j
Compound 22j (TLC heptane/EtOAc, 2:1, Rf 0.22) was prepared according to the general procedure 3.11 from the amine 19 (53 mg, 0.11 mmol). Obtained as a colorless oil in 67% yield (48 mg, 0.07 mmol). −39.5 (c 0.8, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.35–7.18 (m, 15H, ArH), 6.82 (t, 1H, J 6.0 Hz, NHCO), 6.72 (d, 2H, J 2.4 Hz, ArH), 6.54 (t, 1H, J 2.4 Hz, ArH), 4.84 (d, 1H, J 11.6 Hz, CH2Ph), 4.65 (d, 1H, J1,2 6.4 Hz, H-1), 4.57 (d, 1H, J 12.0 Hz, CH2Ph), 4.56 (d, 1H, J 12.0 Hz, CH2Ph), 4.48 (d, 1H, J 11.6 Hz, CH2Ph), 4.47 (d, 1H, J 11.6 Hz, CH2Ph), 4.43 (d, 1H, J 11.6 Hz, CH2Ph), 4.03 (td, 1H, J 6.4 Hz, J 2.8 Hz, H-5), 3.78 (dd, 1H, J1,2 6.0 Hz, J2,3 4.8 Hz, H-2), 3.75–3.65 (m, 10H, H-4, H-6a, H-6b, CH2NHCO, 2 × OCH3), 3.57–3.52 (m, 4H, CH2NHCO, OCH3), 2.47–2.41 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 167.1, 160.9, 163.3, 138.3, 138.2, 137.8, 136.8, 128.7, 128.5, 128.2, 128.1, 128.0, 127.93, 127.88, 127.8, 104.9, 103.6, 100.9, 77.2, 75.6, 73.9, 73.5, 73.1, 72.1, 69.5, 56.5, 55.6, 39.9, 39.0. HRMS calcd for C38H43NO8+H+ (M+H)+: 642.3066, found: 642.3067.
3.11.12. Methyl 2,4,6-tri-O-Benzyl-3-Deoxy-3-C-(3-Trifluoromethylbenzamidomethyl)-β-d-Gulopyranoside 22k
Compound 22k (TLC heptane/EtOAc, 2:1, Rf 0.25) was prepared according to the general procedure 3.11 from the amine 19 (43 mg, 0.09 mmol). Obtained as a colorless oil in 55% yield (32.2 mg, 0.05 mmol). −38.7 (c 0.8, CHCl3). 1H NMR (CDCl3, 400 MHz): 8.00 (s, 1H, ArH), 7.69–7.66 (m, 1H, ArH), 7.34–7.21 (m, 17H, ArH), 7.09 (t, 1H, J 6.0 Hz, CH2NHCO), 4.88 (d, 1H, J 11.6 Hz, CH2Ph), 4.69 (d, 1H, J1,2 6.0 Hz, H-1), 4.58 (d, 1H, J 12.0 Hz, CH2Ph), 4.53 (d, 1H, J 11.2 Hz, CH2Ph), 4.51–4.47 (m, 3H, CH2Ph), 4.07 (s td, 1H, J 6.4 Hz, J 3.2 Hz, H-5), 3.83 (dd, 1H, J 6.0 Hz, J 4.8 Hz, H-2), 3.81–3.68 (m, 4H, H-2, H-4, H-6a, H-6b, CH2NHCO), 3.62–3.59 (m, 1H, CH2NHCO), 3.56 (s, 3H, OCH3), 2.50–2.45 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 165.5, 138.2, 138.1, 137.8, 135.1, 133.4 131.2 (q, J 32 Hz), 129.5, 129.2, 129.1, 128.7, 128.54, 128.52, 128.4, 128.31, 128.25, 128.0, 127.9, 127.8, 125.2, 124.6 (q, J 3.7 Hz), 123.7 (q, J 271 Hz), 100.8, 77.8, 75.8, 74.3, 73.6, 73.1, 72.2, 69.3, 56.5, 39.62, 39.61. 19F NMR (CDCl3, 376 MHz): −62.7. HRMS calcd for C37H38F3NO6+H+ (M+H)+: 650.2729, found: 650.2727.
3.11.13. Methyl 2,4,6-Tri-O-Benzyl-3-Deoxy-3-C-(4-Phenylbenzamidomethyl)-β-d-Gulopyranoside 22l
Compound 22l (TLC heptane/EtOAc, 2:1, Rf 0.32) was prepared according to the general procedure 3.11 from the amine 19 (60 mg, 0.13 mmol). Obtained as a colorless oil in 55% yield (45.5 mg, 0.07 mmol). −47.8 (c 0.9, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.61–7.24 (m, 24H, ArH), 7.10 (t, 1H, J 6.0 Hz, CH2NHCO), 4.92 (d, 1H, J 11.2 Hz, CH2Ph), 4.72 (d, 1H, J1,2 6.4 Hz, H-1), 4.59 (d, 1H, J 12.0 Hz, CH2Ph), 4.57–4.48 (m, 4H, CH2Ph), 4.08 (td, 1H, J 6.0 Hz, J 2.8 Hz, H-5), 3.85 (dd, 1H, J1,2 6.0 Hz, J2,3 4.8 Hz, H-2), 3.82–3.70 (m, 4H, H-4, H-6a, H-6b, CH2NHCO), 3.64–3.59 (m, 1H, CH2NHCO), 3.58 (s, 3H, OCH3), 2.55–2.50 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 166.5, 143.9, 140.3, 138.3, 138.2, 137.9, 132.9, 129.0, 128.7, 128.53, 128,49, 128.3, 128.2, 128.02, 127.98, 127.9, 127.8, 127.4, 127.24, 127.15, 100.9, 77.9, 75.9, 74.3, 73.5, 73.1, 72.2, 69.4, 56.5, 39.8, 39.4. HRMS calcd for C42H43NO6+NH4+ (M+NH4)+: 675.3434, found: 675.3433.
3.11.14. Methyl 2,4,6-tri-O-Benzyl-3-Deoxy-3-C-(Diphenylphosphonamidomethyl)-β-d-Gulopyranoside 23
Compound 23 (TLC heptane/EtOAc, 2:1, Rf 0.26) was prepared according to the general procedure 3.11 from the amine 19 (52 mg, 0.11 mmol). Obtained as a colorless oil in 69% yield (53.3 mg, 0.08 mmol). −77.8 (c 0.7, CHCl3). 1H NMR (CDCl3, 400 MHz): 7.36–7.13 (m, 20H, ArH), 4.76 (d, 1H, J 12.0 Hz, CH2Ph), 4.55–49 (m, 3H, H-1, CH2Ph), 4.45 (d, 1H, J 12.0 Hz, CH2Ph), 4.34 (d, 1H, J 11.6 Hz, CH2Ph), 4.28 (d, 1H, J 11.6 Hz, CH2Ph), 3.95–92 (m, 1H, H-5), 3.71–3.58 (m, 5H, H-2, H-4, H-6a, H-6b, NHPO(OPh)2), 3.47 (s, 3H, OCH3), 3.40–3.31 (m, 1H, CH2NHSO), 3.15–3.09 (m, 1H, CH2NHSO), 2.33–2.27 (m, 1H, H-3). 13C NMR (CDCl3, 100 MHz): 150.9 (dd, J 6.7 Hz, J 2.4 Hz), 138.22, 138.21, 137.9, 128.6, 128.51, 128.45, 128.1, 128.0, 127.93, 127.87, 127.8, 125.0 (d, J 4.3 Hz), 120.3 (d, J 5.0 Hz, J 8.0 Hz), 100.8, 76.1, 74.9, 73.6, 73.4, 72.8, 71.8, 69.5, 56.5, 42.1 (d, J 1.8 Hz), 40.2. 31P NMR (CDCl3, 162 MHz): -1.01. HRMS calcd for C41H44PNO8+H+ (M+H)+: 710.2883, found: 710.2889.
3.12. General Procedure the Synthesis of 1a, 1b, 2–6, 7a–7l, and 8
A solution of crude in EtOAc/isopropanol (1:3) was stirred with Pd(OH)2/C (10% wt., 1 mg per 4 mg of crude) under hydrogen atmosphere at room temperature for 12 h. All the hydrogenation reactions were carried out in an EtOAc-isopropanol mixture (1:3, 4 mL). After the completion of the reaction (as indicated by TLC), the reaction mixture was filtered through a Celite bed and washed with methanol. The filtrate was concentrated under reduced pressure and purified through the flash column (DCM:MeOH) to get the desired compounds as white amorphous solids or colorless oils.
3.12.1. Methyl 3-Deoxy-3-C-Hydroxymethyl-β-d-Gulopyranoside 1a
Compound 1a (TLC, DCM/MeOH, 5:1, Rf 0.41) was prepared according to the general procedure 3.12 from the alcohol 14a (63 mg, 0.13 mmol). Obtained as a white amorphous solid in 51% yield (14 mg, 0.07 mmol) from flash column chromatography (DCM:MeOH 12:1–5:1). −50.7 (c 0.6, CH3OH). 1H NMR (CD3OD, 400 MHz): 4.39 (d, 1H, J 7.6 Hz, H-1), 3.96 (dd, 1H, J3,4 4.0 Hz, J4,5 2.0 Hz, H-4), 3.92 (dd, 1H, J 11.2 Hz, J 5.6 Hz, CH2OH), 3.86 (dd, 1H, J1,2 7.6 Hz, J2,3 6.0 Hz H-2), 3.84–3.74 (m, 3H, H-5, H-6a, H-6b), 3.67 (dd, 1H, J 11.2 Hz, J 8.4 Hz, CH2OH), 3.51 (s, 3H, OCH3), 2.32–2.26 (m, 1H, H-3). 13C NMR (CD3OD, 125 MHz): 104.0, 76.1, 69.0, 68.3, 63.1, 59.6, 56.8, 49.0. HRMS calcd for C8H16O6-H+ (M-H)+: 207.0869, found: 207.0865.
3.12.2. Methyl 3-Deoxy-3-C-Hydroxymethyl-β-d-Galactopyranoside 1b
Compound 1b (TLC, DCM/MeOH, 5:1, Rf 0.40) was prepared according to the general procedure 3.12 from the alcohol 14b (46 mg, 0.10 mmol). Obtained as a white amorphous solid in 63% yield (12.6 mg, 0.06 mmol) from flash column chromatography (DCM:MeOH 12:1–6:1). −32.1 (c 0.5, CH3OH). 1H NMR (CD3OD, 500 MHz): 4.16 (d, 1H, J 7.6 Hz, H-1), 3.97 (d, 1H, J3,4 2.4 Hz, H-4), 3.90 (dd, 1H, J 10.4 Hz, J 4.4 Hz, CH2OH), 3.78 (dd, 1H, J 10.8 Hz, J 8.4 Hz, H-2), 3.72 (dd, 1H, J 5.6 Hz, H-6a, H-6b), 3.55–3.51 (m, 4H, H-5, OCH3), 3.44 (dd, 1H, J 10.8 Hz, J 7.6 Hz, CH2OH), 1.73–1.66 (m, 1H, H-3). 13C NMR (CD3OD, 125 MHz): 107.6, 79.8, 68.8, 67.1, 62.7, 61.2, 57.1, 49.0. HRMS calcd for C8H16O6+Na+ (M+Na)+: 231.0845, found: 231.0840.
3.12.3. Methyl 3-Deoxy-3-C-(3-Trifluoromethylphenoxymethyl)-β-d-Galactopyranoside 2
Compound 2 (TLC, DCM/MeOH, 10:1, Rf 0.5) was prepared according to the general procedure 3.12 from the ether 15 (53 mg, 0.09 mmol). Obtained as a colorless oil in 75% yield (22.5 mg, 0.06 mmol) from flash column chromatography (DCM:MeOH 20:1–12:1). −12.8 (c 0.7, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.46 (t, 1H, J 8.0 Hz, ArH), 7.23–7.21 (m, 3H, ArH), 4.50 (d, 1H, J 7.2 Hz, H-1), 4.35 (dd, 1H, J 4.8 Hz, J 10.0 Hz, CH2OH), 4.22 (t, 1H, J 8.8 Hz, CH2OH), 4.08 (bs, 1H, H-4), 3.97–3.94 (m, 2H, H-2, H-5), 3.78 (d, 2H, J 6.0 Hz, H-6a, H-6b), 3.54 (s, 3H, OCH3), 2.64–2.58 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 160.6, 132.8 (q*, J 32 Hz), 131.4, 125.5 (q*, J 270 Hz), 119.3, 118.3 (br q, J 3.7 Hz), 112.4 (br q, J 3.8 Hz), 104.1, 76.1, 68.2, 68.1, 65.9, 62.9, 56.8, 46.1. 19F NMR (CD3OD, 376 MHz): −64.2. HRMS calcd for C15H20F3O6+H+ (M+H)+: 353.1212, found: 353.1208.
*Only two peaks from the q are observed: See Supplementary information page S43)
3.12.4. Methyl 3-Deoxy-3-C-Methoxymethyl-β-d-Galactopyranoside 3
Compound 3 (TLC, DCM/MeOH, 10:1, Rf 0.5) was prepared according to the general procedure 3.12 from 16 (36 mg, 0.07 mmol). Obtained as a colorless oil in 64% yield (10.4 mg, 0.05 mmol). −33.4 (c 0.5, CH3OH) from flash column chromatography (DCM:MeOH 15:1–9:1). 1H NMR (CD3OD, 400 MHz): 4.41 (d, 1H, J 7.6 Hz, H-1), 3.93 (dd, 1H, J4,5 3.2 Hz, J3,4 2.0 Hz, H-4), 3.88–3.82 (m, 2H, H-2, H-5), 3.73 (d, 2H, J 6.0 Hz, H-6a, H-6b), 3.65 (dd, 1H, J 10.0 Hz, J 4.8 Hz, CH2OCH3), 3.57 (dd, 1H, J 10.0 Hz, J 4.8 Hz, CH2OCH3), 3.50 (s, 3H, OCH3), 3.33 (s, 3H, OCH3), 2.38–2.34 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 104.1, 76.3, 70.2, 68.7, 68.5, 63.1, 59.1, 56.8, 46.6. HRMS calcd for C9H18O6+Na+ (M+Na)+: 245.1001, found: 245.1004.
3.12.5. Methyl 3-Deoxy-3-C-[4-(3-Fluorophenyl)-1H-1,2,3-Triazol-1-Ylmethyl]-β-d-Galactopyranoside 4
Compound 4 (TLC, DCM/MeOH, 10:1, Rf 0.43) was prepared according to the general procedure 3.12 from triazole 18 (55 mg, 0.09 mmol). Obtained as a colorless oil in 78% yield (24.3 mg, 0.07 mmol) from flash column chromatography (DCM:MeOH 20:1–9:1). −20.5 (c 0.7, CH3OH). 1H NMR (CD3OD, 400 MHz): 8.40 (s, 1H, ArH), 7.65–7.05 (m, 4H, ArH), 4.80 (dd, 1H, J 14.4 Hz, J 6.0 Hz, CH2N3C8H5F), 4.63 (dd, 1H, J 14.4 Hz, J 9.2 Hz, CH2OH), 4.48 (d, 1H, J 6.4 Hz, H-1), 4.02–3.99 (m, 1H, H-5), 3.82–3.78 (m, 4H, H-2, H-4, H-6a, H-6b), 3.53 (s, 3H, OCH3), 2.72–2.66 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 164.3 (d, J 243 Hz), 147.7 (d, J 3.1 Hz), 134.0 (d, J 8.3 Hz), 131.8 (d, J 8.4 Hz), 123.4, 122.4 (d, J 2.5 Hz), 115.9 (d, J 21 Hz), 113.2 (d, J 23 Hz), 103.6, 75.8, 68.0, 67.4, 62.8, 56.8, 48.2, 46.8. HRMS calcd for C16H20FN3O5+H+ (M+H)+: 354.1465, found: 354.1462.
3.12.6. Methyl 3-Deoxy-3-C-(3-Fluorophenylureido)Methyl-β-d-Galactopyranoside 5
Compound 5 (TLC, DCM/MeOH, 10:1, Rf 0.44) was prepared according to the general procedure 3.12 from 20 (50 mg, 0.0814 mmol). Obtained as a colorless oil in 41% yield (11.5 mg, 0.03 mmol) from flash column chromatography (DCM:MeOH 12:1–5:1). −17.3 (c 0.6, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.34 (dt, 1H, J 12.0 Hz, J 2.0 Hz, ArH), 7.24–6.64 (m, 3H, ArH), 4.44 (d, 1H, J 7.2 Hz, H-1), 3.90–3.83 (m, 3H, H-2, H-4, H-5), 4.22 (t, 1H, J 8.8 Hz, CH2OH), 4.08 (bs, 1H, H-4), 3.97–3.94 (m, 2H, H-2, H-5), 3.78–3.76 (d, 2H, H-6a, H-6b), 3.52 (s, 3H, OCH3), 3.47 (dd, 1H, J 14.4 Hz, J 6.4 Hz, CH2NHCONH), 3.41 (dd, 1H, J 14.4 Hz, J 7.6 Hz, CH2NHCONH), 2.27–2.21 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 164.5 (d, J 240 Hz), 158.0, 143.0 (d, J 11 Hz), 131.0 (d, J 9.8 Hz), 115.2 (d, J 3.2 Hz), 109.4 (d, J 22 Hz), 106.7 (d, J 22 Hz), 103.8, 76.1, 69.2, 69.0, 63.0, 56.9, 46.8, 38.0. HRMS calcd for C15H21FN2O6+H+ (M+H)+: 354.1462, found: 345.1459.
3.12.7. Methyl 3-Deoxy-3-C-(Phenylsufonamido)Methyl-β-d-Galactopyranoside 6
Compound 6 (TLC, DCM/MeOH, 10:1, Rf 0.45) was prepared according to the general procedure 3.12 from amide 21 (39 mg, 0.06 mmol). Obtained as a colorless oil in 53% yield (11.6 mg, 0.03 mmol) from flash column chromatography (DCM:MeOH 20:1–10:1). −21.4 (c 0.6, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.88–7.56 (m, 5H, ArH), 4.26 (d, 1H, J 7.2 Hz, H-1), 3.93 (d, 1H, J 3.6 Hz, H-4), 3.80 (dd, 1H, J1,2 7.2 Hz, J2,3 6.0 Hz, H-2), 3.76–3.70 (m, 3H, H-5, H-6a, H-6b), 3.20 (dd, 1H, J 5.2 Hz, J 11.2 Hz, CH2NH), 3.20 (dd, 1H, J 11.2 Hz, J 10.0 Hz, CH2NH), 2.26–2.21 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 141.7, 133.7, 130.3, 128.0, 103.5, 75.7, 68.4, 67.9, 63.2, 56.8, 46.3, 40.6. HRMS calcd for C14H21NO7S+H+ (M+H)+: 348.1117, found: 348.1115.
3.12.8. Methyl 3-Deoxy-3-C-Benzamidomethyl-β-d-Gulopyranoside 7a
Compound 7a (TLC, DCM/MeOH, 10:1, Rf 0.41) was prepared according to the general procedure 3.12 from the amide 22a (35 mg, 0.05 mmol). Obtained as a colorless oil in 59% yield (11 mg, 0.04 mmol) from flash column chromatography (DCM:MeOH 20:1–10:1). −6.5 (c 0.6, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.82–7.80 (m, 2H, ArH), 7.56–7.52 (m, 1H, ArH), 7.49–7.44 (m, 2H, ArH), 4.48 (d, 1H, J1,2 6.8 Hz, H-1), 3.95 (td, 1H, J 6.0 Hz, J 2.4 Hz, H-5), 3.89–3.86 (m, 2H, H-2, H-4), 3.79 (d, 1H, J6a,6b 12.4 Hz, J5,6a 5.6 Hz, H-6a), 3.75 (dd, 1H, J6a,6b 12.4 Hz, J5,6b 5.6 Hz, H-6b), 3.68 (dd, 1H, J 14.0 Hz, J 6.4 Hz, CH2NH), 3.61 (dd, 1H, J 11.2 Hz, J 6.4 Hz, CH2NH), 3.54 (s, 3H, OCH3), 2.42–2.35 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 170.5, 135.6, 132.7, 129.6, 128.2, 103.7, 76.0, 69.0, 68.7, 63.1, 56.8, 46.4, 37.8. HRMS calcd for C14H19NO6+H+ (M+H)+: 289.1291, found: 298.1289.
3.12.9. Methyl 3-Deoxy-3-C-Acetamidomethyl-β-d-Gulopyranoside 7b
Compound 7b (TLC, DCM/MeOH, 10:1, Rf 0.42) was prepared according to the general procedure 3.12 from the amide 22b (27 mg, 0.05 mmol). Obtained as a colorless oil in 75% yield (9.7 mg, 0.04 mmol) from flash column chromatography (DCM:MeOH 15:1–7:1). −1.5 (c 0.6, CH3OH). 1H NMR (CD3OD, 400 MHz): 4.40 (d, 1H, J1,2 6.8 Hz, H-1), 3.88–3.84 (m, 1H, H-5), 3.82–371 (m, 4H, H-2, H-4, H-6a, H-6b), 3.51 (s, 3H, OCH3), 3.44 (dd, 1H, J 14.0 Hz, J 6.0 Hz, CH2NH), 3.38 (dd, 1H, J 14.0 Hz, J 8.8 Hz, CH2NH), 2.24–2.18 (m, 1H, H-3), 1.95 (s, 3H, NHCOCH3). 13C NMR (CD3OD, 100 MHz): 173.6, 103.6, 75.9, 68.7, 68.5, 63.1, 56.8, 46.3, 37.1, 22.6. HRMS calcd for C10H19NO6+H+ (M+H)+: 250.1291, found: 250.1291.
3.12.10. Methyl 3-Deoxy-3-C-(2-Fluorobenzamidomethyl)-β-d-Galactopyranoside 7c
Compound 7c (TLC, DCM/MeOH, 10:1, Rf 0.4) was prepared according to the general procedure 3.12 from the amide 22c (33 mg, 0.06 mmol). Obtained as a colorless oil in 69% yield (12.5 mg, 0.04 mmol) from flash column chromatography (DCM:MeOH 20:1–9:1). −9.3 (c 0.5, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.76 (td, 1H, J 7.6 Hz, J 2.0 Hz, ArH), 7.56–7.50 (m, 1H, ArH), 7.28 (td, 1H, J 7.6 Hz, J 0.8 Hz, ArH), 7.20 (ddd, 1H, J 11.2 Hz, J 8.4 Hz, J 0.8 Hz, ArH), 4.48 (d, 1H, J1,2 7.2 Hz, H-1), 3.93–3.86 (m, 3H, H-2, H-4, H-5), 3.77 (d, 2H, J5,6a, J5,6b 5.6 Hz, H-6a, H-6b), 3.66 (d, 2H, J 7.2 CH2NH), 3.53 (s, 3H, OCH3), 2.42–2.34 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 166.7, 161.4 (d, J 248 Hz), 134.2 (d, J 8.8 Hz), 131.6 (d, J 2.3 Hz), 125.7 (d, J 3.4 Hz), 123.9 (d, J 14 Hz), 131.6 (d, J 23 Hz), 103.7, 76.0, 69.0, 68.8, 63.1, 56.9, 46.1, 38.1. 19F NMR (CD3OD, 376 MHz): −116.0. HRMS calcd for C15H20FNO6+H+ (M+H)+: 330.1353, found: 330.1352.
3.12.11. Methyl 3-Deoxy-3-C-(3-Fluorobenzamidomethyl)-β-d-Galactopyranoside 7d
Compound 7d (TLC, DCM/MeOH, 10:1, Rf 0.45) was prepared according to the general procedure 3.12 from the amide 22d (35 mg, 0.06 mmol). Obtained as a colorless oil in 59% yield (11.3 mg, 0.03 mmol) from flash column chromatography (DCM:MeOH 20:1–10:1). −14.6 (c 0.6, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.65–7.26 (m, 4H, ArH), 4.47 (d, 1H, J 7.2 Hz, H-1), 3.94 (td, 1H, J 6.0 Hz, J 2.0 Hz, H-5), 3.88–3.85 (m, 2H, H-2, H-4), 3.77 (d, 2H, J 5.6 Hz, H-6a, H-6b), 3.67 (dd, 1H, J 14.0 Hz, J 6.4 Hz, CH2OH), 3.67 (dd, 1H, J 14.0 Hz, J 9.2 Hz, CH2OH), 3.53 (s, 3H, OCH3), 2.41–2.35 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 168.9 (d, J 2.7 Hz), 164.1 (d, J 244 Hz), 138.0 (d, J 6.8 Hz), 131.6 (d, J 7.9 Hz), 124.0 (d, J 2.9 Hz), 119.4 (d, J 22 Hz), 115.2 (d, J 23 Hz), 103.6, 75.9, 68.9, 68.6, 63.1, 56.8, 46.4, 37.8. HRMS calcd for C15H20FNO6+H+ (M+H)+: 330.1353, found: 330.1354.
3.12.12. Methyl 3-Deoxy-3-C-(4-Fluorobenzamidomethyl)-β-d-Galactopyranoside 7e
Compound 7e (TLC, DCM/MeOH, 10:1, Rf 0.44) was prepared according to the general procedure 3.12 from the amide 22e (40 mg, 0.07 mmol). Obtained as a colorless oil in 53% yield (11.6 mg, 0.04 mmol) from flash column chromatography (DCM:MeOH 20:1–9:1). −16.4 (c 0.5, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.89–7.84 (m, 2H, ArH), 7.22–7.16 (m, 2H, ArH), 4.47 (d, 1H, J1,2 7.2 Hz, H-1), 3.94 (td, 1H, J 6.0 Hz, J 3.0 Hz, H-5), 3.88–3.85 (m, 2H, H-2, H-4), 3.78 (d, 1H, J6a,6b 11.6 Hz, J5,6a 5.6 Hz, H-6a), 3.75 (d, 1H, J6a,6b 11.6 Hz, J5,6b 5.6 Hz, H-6b), 3.66 (d, 1H, J 14.0 Hz, J 6.4 Hz, CH2NH), 3.61 (d, 1H, J 14.0 Hz, J 6.4 Hz, CH2NH), 3.53 (s, 3H, OCH3), 2.40–2.34 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 169.3, 166.2 (d, J 249 Hz), 131.9 (d, J 3.0 Hz), 130.8 (d, J 8.9 Hz), 116.4 (d, J 22 Hz), 103.7, 75.9, 69.0, 68.6, 63.1, 56.8, 46.4, 37.8. 19F NMR (CD3OD, 376 MHz): −110.7. HRMS calcd for C15H20FNO6+H+ (M+H)+: 330.1353, found: 330.1354.
3.12.13. Methyl 3-Deoxy-3-C-(3,4,5-Trifluorobenzamidomethyl)-β-d-Galactopyranoside 7f
Compound 7f (TLC, DCM/MeOH, 10:1, Rf 0.47) was prepared according to the general procedure 3.12 from the amide 22f (31 mg, 0.05 mmol). Obtained as a colorless oil in 70% yield (12.5 mg, 0.03 mmol) from flash column chromatography (DCM:MeOH 20:1–9:1). −13.5 (c 0.6, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.66–7.59 (m, 2H, ArH), 4.45 (d, 1H, J 6.8 Hz, H-1), 4.00 (td, 1H, J 6.0 Hz, J 2.0 Hz, H-5), 3.87–3.84 (m, 2H, H-2, H-4), 3.76 (d, 2H, J 5.6 Hz, H-6a, H-6b), 3.66 (dd, 1H, J 14.0 Hz, J 6.0 Hz, CH2NH), 3.59 (dd, 1H, J 14.0 Hz, J 9.2 Hz, CH2NH), 3.53 (s, 3H, OCH3), 2.40–2.40–2.34 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 166.7, 152.3 (ddd, 248.3 Hz, J 9.8 Hz, J 3.8 Hz), 143.0 (dt, J 254 Hz, J 16 Hz,), 132.2–132.0 (m), 113.1 (dd, J 17 Hz, J 6.1 Hz), 103.7, 75.9, 68.8, 68.5, 63.1, 56.8, 46.3, 38.0. 19F NMR (CD3OD, 376 MHz): −135.7 (d, J 20 Hz). −159.1 (t, J 20 Hz). HRMS calcd for C15H18F3NO6+H+ (M+H)+: 366.1164, found: 366.1161.
3.12.14. Methyl 3-Deoxy-3-C-(2,3,4,5,6-Pentafluorobenzamidomethyl)-β-d-Galactopyranoside 7g
Compound 7g (TLC, DCM/MeOH, 10:1, Rf 0.38) was prepared according to the general procedure 3.12 from the amide 22g (30 mg, 0.04 mmol). Obtained as a colorless oil in 83% yield (14.9 mg, 0.04 mmol) from flash column chromatography (DCM:MeOH 20:1–8:1). −18.9 (c 0.6, CH3OH). 1H NMR (CD3OD, 400 MHz): 4.46 (d, 1H, J 6.4 Hz, H-1), 3.94–3.85 (m, 4H, H-2, H-4, H-5), 3.79 (dd, 1H, J 6.0 Hz, J 11.2 Hz, H-6a), 3.79 (dd, 1H, J 5.2 Hz, J 11.2 Hz, H-6a), 3.68 (dd, 1H, J 6.4 Hz, J 14.4 Hz, CH2NHCO), 3.62 (dd, 1H, J 14.4 Hz, J 8.8 Hz, CH2NHCO), 3.53 (s, 3H, OCH3), 2.38–2.32 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 159.9, 146.4, 144.0, 140.2, 137.6, 103.6, 75.8, 68.5, 68.4, 63.2, 56.9, 46.3, 37.7. 19F NMR (CD3OD, 376 MHz): −143.8 to −143.2 (m), −155.2 to −155.3 (m), −163.7 to −163.9 (m). HRMS calcd for C15H16F5NO6+H+ (M+H)+: 402.0976, found: 402.0974.
3.12.15. Methyl 3-Deoxy-3-C-(3-Methoxybenzamidomethyl)-β-d-Galactopyranoside 7h
Compound 7h (TLC, DCM/MeOH, 10:1, Rf 0.42) was prepared according to the general procedure 3.12 from the amide 22h (28 mg, 0.03 mmol). Obtained as a colorless oil in 66% yield (10.3 mg, 0.03 mmol) from flash column chromatography (DCM:MeOH 20:1–10:1). −9.5 (c 0.5, CH3OH). 1H NMR (CD3OD, 400 MHz): 8.47 (t, J 5.2 Hz, CONH), 7.38–7.33 (m, 3H, ArH), 7.08 (m, 1H, ArH), 4.47 (d, 1H, J 7.2 Hz, H-1), 3.94 (td, 1H, J 5.6 Hz, J 2.0 Hz, H-5), 3.88–3.85 (m, 2H, H-2, H-4), 3.84 (s, 3H, OCH3), 3.78 (d, 2H, J 5.6 Hz, H-6a, H-6b), 3.66–3.62 (m, 2H, CH2NN), 3.53 (s, 3H, OCH3), 2.41–2.35 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 170.3, 161.3, 137.0, 130.7, 120.3, 118.5, 113.6, 103.7, 75.9, 70.0, 68.6, 63.1, 56.9, 55.9, 46.4, 37.8. HRMS calcd for C16H23NO7+H+ (M+H)+: 342.1553, found: 342.1555.
3.12.16. Methyl 3-Deoxy-3-C-(p-Toluamidomethyl)-β-d-Galactopyranoside 7i
Compound 7i (TLC, DCM/MeOH, 10:1, Rf 0.48) was prepared according to the general procedure 3.12 from the amide 22i (32 mg, 0.05 mmol). Obtained as a colorless oil in 53% yield (11.9 mg, 0.04 mmol) from flash column chromatography (DCM:MeOH 20:1–10:1). −4.8 (c 0.5, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.72–7.69 (m, 2H, ArH), 7.27 (d, 2H, J 8.0 Hz, ArH), 4.47 (d, 1H, J 7.2 Hz, H-1), 3.94 (td, 1H, J 5.6 Hz, J 2.0 Hz, H-5), 3.88–3.85 (m, 2H, H-2, H-4), 3.77 (d, 2H, J 5.6 Hz, H-6a, H-6b), 3.66–3.62 (m, 2H, CH2NN), 3.53 (s, 3H, OCH3), 2.39–2.34 (m, 1H, H-3, CH3). 13C NMR (CD3OD, 100 MHz): 170.4, 143.4, 132.7, 130.2, 128.2, 103.7, 76.0, 69.0, 68.7, 63.2, 56.9, 46.4, 37.7, 21.4. HRMS calcd for C16H23NO6+H+ (M+H)+: 326.1604, found: 326.1603.
3.12.17. Methyl 3-Deoxy-3-C-(3,5-Dimethoxybenzamidomethyl)-β-d-Galactopyranoside 7j
Compound 7j (TLC, DCM/MeOH, 10:1, Rf 0.43) was prepared according to the general procedure 3.12 from the amide 22j (24 mg, 0.04 mmol). Obtained as a colorless oil in 62% yield (10 mg, 0.03 mmol) from flash column chromatography (DCM:MeOH 20:1–9:1). −25.7 (c 0.5, CH3OH). 1H NMR (CD3OD, 400 MHz): 6.96 (d, 1H, J 2.0 Hz, ArH), 6.63 (t, 1H, J 2.0 Hz, ArH), 4.47 (d, 1H, J1,2 7.2 Hz, H-1), 3.94 (td, 1H, J 5.6 Hz, J 2.0 Hz, H-5), 3.88–3.85 (m, 2H, H-2, H-4), 3.82 (s, 6H, 2×OCH3), 3.77 (d, 2H, J 6.0 Hz), 3.67–3.57 (m, 2H, CH2NH), 3.53 (s, 3H, OCH3), 2.40–2.34 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 170.2, 162.4, 137.6, 106.1, 104.5, 103.7, 75.9, 69.0, 68.6, 63.1, 56.8, 56.0, 46.4, 37.8. HRMS calcd for C17H25NO8+H+ (M+H)+: 372.1658, found: 372.1663.
3.12.18. Methyl 3-Deoxy-3-C-(3-Trifluoromethylbenzamidomethyl)-β-d-Galactopyranoside 7k
Compound 7k (TLC, DCM/MeOH, 10:1, Rf 0.51) was prepared according to the general procedure 3.12 from the amide 22k (25 mg, 0.04 mmol). Obtained as a colorless oil in 66% yield (11.2 mg, 0.03 mmol) from flash column chromatography (DCM:MeOH 20:1–10:1). −3.9 (c 0.6, CH3OH). 1H NMR (CD3OD, 400 MHz): 8.13 (s, 1H, ArH), 8.08 (t, 1H, J 8.0 Hz, ArH), 7.85 (d, 1H, J 8.0 Hz, ArH), 7.68 (t, 1H, J 8.0 Hz, ArH), 4.47 (d, 1H, J1,2 7.2 Hz, H-1), 3.95 (td, 1H, J 6.0 Hz, J 2.0 Hz, H-5), 3.89–3.86 (m, 2H, H-2, H-4), 3.79 (dd, 1H, J6a,6b 12.0 Hz, J5,6a 6.0 Hz, H-6a), 3.76 (dd, 1H, J6a,6b 12.0 Hz, J5,6b 6.0 Hz, H-6b), 3.69 (dd, 1H, J 13.6 Hz, J 5.6 Hz, CH2NH), 3.63 (dd, 1H, J 13.6 Hz, J 9.2 Hz, CH2NH), 3.54 (s, 3H, OCH3), 2.43–2.37 (m, 1H, H-3). 13C NMR (CD3OD, 125 MHz): 168.7, 136.7, 132.0 (q, J 32 Hz), 131.9, 130.6, 129.2 (q J 3.6 Hz), 125.4 (q, J 270 Hz), 125.1 (q, J 4.0 Hz), 103.7, 75.9, 68.9, 68.6, 63.1, 56.9, 46.5, 37.8. 19F NMR (CD3OD, 376 MHz): −64.2. HRMS calcd for C16H20F3NO6+H+ (M+H)+: 380.1321, found: 380.1321.
3.12.19. Methyl 3-Deoxy-3-C-(4-Phenylbenzamidomethyl)-β-d-Galactopyranoside 7l
Compound 7l (TLC, DCM/MeOH, 10:1, Rf 0.54) was prepared according to the general procedure 3.12 from the amide 22l (39 mg, 0.06 mmol). Obtained as a colorless oil in 67% yield (15.4 mg, 0.04 mmol) from flash column chromatography (DCM:MeOH 20:1–12:1). −21.4 (c 0.7, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.70 (dd, 2H, J 6.8 Hz, J 2.0 Hz, ArH), 7.71 (dd, 2H, J 6.8 Hz, J 2.0 Hz, ArH), 7.65 (m, 2H, ArH), 7.48–7.44 (m, 2H, ArH), 7.40–7.35 (m, 1H, ArH), 4.49 (d, 1H, J1,2 7.2 Hz, H-1), 3.96 (td, 1H, J 6.0 Hz, J 2.4 Hz, H-5), 3.82–3.75 (m, 2H, H-6a, H-6b), 3.73–3.62 (m, 2H, CH2NH), 3.54 (s, 3H, OCH3), 2.44–2.38 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 170.1, 145.7, 141.2, 134.2, 130.0, 129.1, 128.8, 128.11, 128.07, 103.7, 76.0, 69.0, 68.7, 63.2, 56.9, 46.4, 37.8. HRMS calcd for C21H25NO6+H+ (M+H)+: 388.1760, found: 388.1761.
3.12.20. Methyl 3-Deoxy-3-C-(Diphenylphosphonamidomethyl)-β-d-Galactopyranoside 8
Compound 8 (TLC, DCM/MeOH, 10:1, Rf 0.45) was prepared according to the general procedure 3.12 from the amide 23 (43 mg, 0.06 mmol). Obtained as a colorless oil in 50% yield (13.3 mg, 0.03 mmol) from flash column chromatography (DCM:MeOH 15:1–9:1). −18.6 (c 0.7, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.40 (t, 4H, J 8.0 Hz, ArH), 7.29–7.21 (m, 6H, ArH), 4.36 (d, 1H, J 7.2 Hz, H-1), 3.90 (dd, 1H, J 4.0 Hz, J 2.0 Hz, H-4), 3.83 (dd, 1H, J 7.2 Hz, J 5.6 Hz, H-2), 3.79–3.76 (m, 1H, H-5), 3.71 (dd, 1H, J5,6a 10.4 Hz, J6a,6b 4.4 Hz, H-6a), 3.71 (dd, 1H, J5,6b 10.8 Hz, J6a,6b 4.4 Hz, H-6b), 3.51 (s, 3H, OCH3), 3.50–3.44 (m, 1H, CH2NH), 3.17–3.08 (m, 1H, CH2NH), 2.28–2.22 (m, 1H, H-3). 13C NMR (CD3OD, 100 MHz): 152.2 (dd, J 6.2 Hz, J 2.7 Hz), 130.9, 126.3 (d, J 3.1 Hz), 121.4 (dd, J 4.6 Hz, J 11.1 Hz), 103.6, 75.7, 68.7, 68.1, 63.2, 56.8, 47.4 (d, 5.7 Hz), 39.2. 31P NMR (CD3OD, 162 MHz): −1.0. HRMS calcd for C20H26PNO8+H+ (M+H)+: 440.1474, found: 440.1470.
3.13. Methyl 2,3-Di-O-Acetyl-β-d-Gulopyranoside 25
Compound 24 (300 mg, 0.82 mmol) was dissolved in 80% aqueous AcOH (5 mL) and the solution was stirred at 80 °C for 2 h. When the TLC (TLC, heptane/EtOAc, 1:2, Rf 0.39) showed complete consumption of the starting material, the solvents were evaporated under reduced pressure and co-evaporated twice with toluene (10 mL). Then, the crude was purified via flash chromatography (Heptane/EtOAc, 3:1–1:2) to obtain pure compound 25 (191 mg, 0.69 mmol, 84%) as a white foam. −30.4 (c 0.8, CHCl3). 1H NMR (CDCl3, 400 MHz): 5.35 (t, 1H, J2,3 3.6 Hz, H-3), 5.10 (dd, 1H, J1,2 8.0 Hz, J2,3 3.6 Hz, H-2), 4.68 (d, 1H, J 8.0 Hz, H-1), 3.93–3.89 (m, 4H, H4, H-5, H-6a, H-6b), 3.52 (s, 3H, OCH3), 2.11 (s, 3H, COCH3), 2.03 (s, 3H, COCH3). 13C NMR (CDCl3, 100 MHz): 170.0, 169.9, 99.9, 73.3, 70.6, 69.0, 68.4, 62.8, 56.9, 20.98, 20.95. HRMS calcd for C11H18O8+Na+ (M+Na)+: 301.0899, found: 301.0898.
3.14. Methyl β-d-Gulopyranoside 9
Compound 25 (120 mg, 0.43 mmol) was dissolved in MeOH (3 mL). NaOMe (1.0 mL, 0.5 M in MeOH) was added and the solution was stirred at room temperature for 2 h (TLC, DCM/MeOH, 5:1, Rf 0.3). The solution was neutralized with DOWEX 50 W H+ resin, filtered and the solvents were evaporated under reduced pressure and the crude was purified via flash chromatography (DCM/MeOH, 7:1–3:1) to obtain pure compound 9 (46 mg, 0.23 mmol, 55%) as a colorless oil. −19.2 (c 0.9, CH3OH). 1H NMR (D2O, 400 MHz): 4.60 (d, 1H, J1,2 8.4 Hz, H-1), 4.05 (t, 1H, J3,4 3.6 Hz, H-4), 4.00–3.96 (m, 1H, H-5), 3.80 (dd, 1H, J3,4 3.6 Hz, J4,5 1.2 Hz, H-4), 3.75 (dd, 1H, J6a,6b 12.0 Hz, J5,6a 6.4 Hz, H-6a), 3.76 (dd, 1H, J6a,6b 12.0 Hz, J5,6a 4.8 Hz, H-6b), 3.76 (dd, 1H, J1,2 8.4 Hz, J2,3 3.6 Hz, H-2), 3.56 (s, 3H, OCH3). 13C NMR (D2O, 100 MHz): 101.5, 73.8, 71.1, 69.3, 68.0, 61.0, 56.9. HRMS calcd for C7H14O6+Na+ (M+Na)+: 217.0688, found: 217.0687.
3.15. Methyl 3-Azido-2,4,6-Tri-O-Benzoyl-3-Deoxy-β-d-Gulopyranoside 29
Triflic anhydride (235 μL, 1.4 mmol) was added dropwise to a stirred solution of 26 (400 mg, 1.4 mmol) in DCM (10 mL) and pyridine (451 μL, 5.6 mmol) at −30 °C and under N2 atmosphere after which the reaction was allowed to reach rt under 2 h. BzCl (179 μL, 1.54 mmol) was added and the reaction was stirred for another 2 h before the reaction was diluted with DCM (25 mL) and washed with saturated NaHCO3 (2 × 25 mL). The combined aqueous phases were extracted with DCM (40 mL). The pooled organic phases were dried over MgSO4 and concentrated to give crude 27. Sodium azide (637 mg, 9.8 mmol) was added to the crude 27 (≤1.4 mmol) in DMF (15 mL) and the reaction was stirred overnight at 70 °C under N2 atmosphere. The reaction was concentrated to give crude 28, which was dissolved in 90% AcOH (20 mL) and heated at 80 °C for 3 h. The solvent was evaporated in vacuo and co-evaporated with toluene to remove the residual AcOH. The residue was dissolved in pyridine (15 mL), into the solution catalytic amount of DMAP and benzoyl chloride (488 μL, 4.2 mmol) was added subsequently. The solution was stirred at room temperature for 4 h when TLC (heptane/EtOAc, 4:1, Rf 0.48) showed complete conversion of the starting material to a faster moving spot. The solvents were evaporated in vacuo and co-evaporated with toluene to remove residual pyridine. The solid residue thus obtained was dissolved in EtOAc (50 mL) and washed with 1 N HCl (50 mL), followed by saturated NaHCO3 and brine (50 mL). The organic layer was collected, dried (Na2SO4), filtered and evaporated in vacuo. The crude was purified by flash chromatography using heptane/EtOAc (6:1 to 5:2) as the eluent to afford pure compound 29 (324 mg, 0.61 mmol, 43% over four steps) as a white amorphous mass. −45.3 (c 0.7, CHCl3). 1H NMR (CDCl3, 400 MHz): 8.14–7.39 (m, 15H, ArH), 5.51 (dd, 1H, J1,2 7.6 Hz, J2,3 4.0 Hz, H-2), 5.41 (dd, 1H, J3,4 4.0 Hz, J3,4 0.8 Hz, H-4), 5.00 (d, 1H, J 7.6 Hz, H-1), 4.66–4.61 (m, 1H, H-5), 4.52–4.45 (m, 3H, H-3, H-6a, H-6b), 3.58 (s, 3H, OCH3). 13C NMR (CDCl3, 100 MHz): 166.0, 165.3, 165.2, 133.8, 133.6, 133.2, 130.02, 129.98, 129.7, 129.5, 129.0, 128.7, 128.6, 128.5, 128.4, 99.6, 70.3, 70.1, 69.5, 62.4, 60.1, 57.0. HRMS calcd for C28H25N3O8+NH4+ (M+NH4)+: 549.1985, found: 549.1989.
3.16. Methyl 3-Amino-2,4,6-Tri-O-Benzoyl-3-Deoxy-β-d-Gulopyranoside 30
A solution of 29 (201 mg, 0.3784 mmol) in MeOH (7 mL) was stirred with Pd(OH)2/C (10% wt., 1 mg per 5 mg of crude, 40 mg) under hydrogen atmosphere at room temperature for 2 h. After the completion of the reaction (as indicated by TLC, heptane/EtOAc, 1:1, Rf 0.26), the reaction mixture was filtered through a Celite bed and washed with methanol. The filtrate was concentrated under reduced pressure and purified through flash column (heptane/EtOAc, 4:1–1:1) to get the desired compound as a white amorphous solid. Yield: 126 mg (0.2494 mmol, 66%). −39.9 (c 0.8, CHCl3). 1H NMR (CDCl3, 400 MHz): 8.13–7.38 (m, 15H, ArH), 5.38 (dd, 1H, J1,2 7.2 Hz, J2,3 4.0 Hz, H-2), 5.29 (dd, 1H, J3,4 4.4 Hz, J4,5 2.4 Hz, H-4), 5.11 (d, 1H, J 7.2 Hz, H-1), 4.83–4.79 (m, 1H, H-5), 4.64 (dd, 1H, dd, 1H, J6a,6b 11.2 Hz, J5,6a 6.8 Hz, H-6a), 4.51 (dd, 1H, dd, 1H, J6a,6b 11.2 Hz, J5,6b 6.0 Hz, H-6b), 3.90 (t, 1H, J3,4, J2,3 4.0 Hz, H-3), 3.57 (s, 3H, OCH3), 1.97 (bs, 1H, NH2). 13C NMR (CDCl3, 100 MHz): 166.3, 165.9, 165.3, 133.7, 133.5, 133.2, 130.1, 129.9, 129.8, 128.69, 128.67, 128.5, 99.3, 72.1, 71.7, 70.2, 63.3, 57.1, 50.6. HRMS calcd for C28H27NO8+H+ (M+H)+: 506.1815, found: 506.1817.
3.17. Methyl 3-Benzamido-2,4,6-Tri-O-Benzoyl-3-Deoxy-β-d-Gulopyranoside 31
Compound 30 was dissolved in pyridine (3 mL), into the solution catalytic amount of DMAP and benzoyl chloride (29 μL, 0.2464 mmol) was added subsequently. The solution was stirred at room temperature for 3 h when TLC (heptane/EtOAc, 1:1, Rf 4.8) showed complete conversion of the starting material to a faster moving spot. The solvents were evaporated in vacuo and co-evaporated with toluene to remove residual pyridine. The solid residue thus obtained was dissolved in EtOAc (7 mL) and washed with 1 (N) HCl (5 mL), followed by saturated NaHCO3 and brine (5 mL). The organic layer was collected, dried over Na2SO4, filtered and evaporated in vacuo. The crude was purified by flash chromatography using heptane/EtOAc (7:1 to 3:1) as the eluent to afford pure compound 31 (77 mg, 0.1265 mmol, 77%) as a white amorphous solid. −48.8 (c 0.6, CHCl3). 1H NMR (CDCl3, 400 MHz): 8.11–7.29 (m, 20H, ArH), 6.60 (d, 1H, J3,NHCOPh 8.4 Hz, NHCOPh), 5.96 (dd, 1H, J 10.8 Hz, J 6.0 Hz, H-4), 5.55 (t, 1H, J 2.8 Hz, H-2), 5.34–5.29 (m, 1H, H-3), 4.99 (d, 1H, J1,2 2.8 Hz, H-1), 4.92 (dd, 1H, J6a,6b 11.6 Hz, J5,6a 5.6 Hz, H-6a), 4.86 (dd, 1H, J6a,6b 11.6 Hz, J5,6b 6.4 Hz, H-6a), 4.77 (dd, 1H, J6a,6b 12.4 Hz, J4,5 6.0 Hz, H-5), 3.61 (s, 3H, OCH3). 13C NMR (CDCl3, 100 MHz): 167.4, 166.8, 166.2, 165.5, 133.9, 133.8, 133.7, 133.0, 131.7, 130.0, 129.9, 129.7, 129.6, 129.1, 128.7, 128.59, 128.57, 128.3, 127.0, 99.5, 72.4, 71.8, 68.4, 64.5, 60.4, 56.8, 46.3. HRMS calcd for C35H31NO9+H+ (M+H)+: 610.2077, found: 610.2081.
3.18. Methyl 3-Benzamido-3-Deoxy-β-d-Gulopyranoside 10
Compound 31 (54 mg, 0.0886 mmol) was dissolved in MeOH (2 mL). NaOMe (0.5 mL, 0.5 M in MeOH) was added and the solution was stirred at room temperature for 12 h (TLC, DCM/MeOH, 10:1, Rf 0.4). The solution was neutralized with DOWEX 50 W H+ resin, filtered and the solvents were evaporated under reduced pressure and the residue was purified by a short flash column using DCM–MeOH (9:1) to afford the compound 10 (19.2 mg, 0.0646 mmol, 73%). −18.3 (c 0.6, CH3OH). 1H NMR (CD3OD, 400 MHz): 7.83 (d, 2H, J 7.6 Hz, ArH), 7.57–7.44 (m, 3H, ArH), 4.70 (d, 1H, J1,2 8.4 Hz, H-1), 4.48–4.44 (m, 1H, H-3), 4.01 (dd, 1H, J3,4 3.6 Hz, J4,5 1.2 Hz, H-4), 3.94 (dd, 1H, J1,2 7.6 Hz, J2,3 5.2 Hz, H-2), 3.84 (td, 1H, J5,6a, J5,6a 6.0 Hz, J4,5 1.6 Hz, H-5), 3.77 (dd, 1H, J6a,6b 11.2 Hz, J5,6a 6.0 Hz, H-6a), 3.74 (dd, 1H, J6a,6b 11.2 Hz, J5,6a 6.0 Hz, H-6b), 3.57 (s, 3H, OCH3). 13C NMR (CD3OD, 100 MHz): 171.4, 164.6, 135.9, 132.7, 129.5, 128.6, 103.4, 75.7, 68.8, 67.8, 62.6, 56.9, 56.0. HRMS calcd for C14H19NO6+H+ (M+H)+: 298.1291, found: 298.1289.
3.19. Expression Constructs, Expression, and Purification of Recombinant Galectins
Human galectin-1 [25], galectin-2 [26], galectin-3 [27], galectin-4N [19], galectin-4C [19], galectin-8N [28], galectin-8C [28], galectin-9N [29], and galectin-9C [30], were expressed and purified as described earlier. Human galectin-7 was expressed using a pET3c plasmid in E. coli BL21-star. The plasmid containing expression optimized DNA encoding the full human galectin-7 sequence (NCBI Reference Sequence: NP_002298.1) was obtained from GenScript (Piscataway, NJ, USA). Bacterial culture and induction, and galectin purification was essential as described for galectin-3 expressed with the same vector [27]; a typical yield was 1.5–2 mg/L culture. Lactose was removed by chromatography on a PD-10 column (Amersham Biosciences) with repeated ultrafiltration with Centriprep (Amicon).
3.20. Fluorescence Polarization Assay
Fluorescence polarization experiments were carried out either with a POLARStar plate reader and FLUOstar Galaxy software or with a PheraStarFS plate reader and PHERAstar Mars version 2.10 R3 software (BMG, Offenburg, Germany). The dissociation constant (Kd) values were determined in PBS as described earlier [18,19]. Specific conditions for galectin-1, 2, 3, 4N, 4C, 8N, 8C, 9N, and 9C were kept as reported [29]. Experiments were performed at room temperature with human galectin-7 at 5 µM and the fluorescent probe β-d-galactopyranosyl-(1–4)-2-acetamido-2-deoxy-β-d-glucopyranosyl-(1–3)-β-d-galactopyranosyl-(1–4)-(N1-fluorescein-5-yl-carbonylaminomethylcarbonyl)-β-d-glucopyranosylamine [29] at 0.02 µM. All the compounds in Table 1 except 32 were dissolved in a neat DMSO at 100 mM and diluted in PBS to three to six different concentrations to be tested in duplicate. Average Kd values and SEMs were calculated from 2–8 single-triple point measurements showing between 30%–70% inhibition.
4. Conclusions
In summary, we report the synthesis and discovery of 3-C-methyl-guloside derivatives as highly selective galectin-1 inhibitors with 3-C-benzamidomethyl-3-deoxy-gulosides being the most selective structural class. The reason for the exceptional galectin-1-selectivites discovered remains to be elucidated as molecular modelling failed to provide insight into this and experimental structural studies by X-ray diffraction or nmr spectroscopy are likely necessary. Although the galectin-1 affinities are in the high-µM to low mM range, they are significantly higher affinity than that of simple galactosides, such as methyl β-d-galactopyranoside, and thus points towards a novel structural class and synthetic route towards the discovery of galectin-1 inhibitors with high selectivity. This is important in light of the roles of galectin-1 in tumor progression and immune regulation [31,32].
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/15/3786/s1.
Author Contributions
K.B.P. and M.M. contributed equally to the synthesis and characterization of all compounds. K.B.P wrote the major part of the manuscript. H.L. supervised and analyzed the result of the fluorescence polarization assay. U.J.N. conceived the study, analyzed the data and co-wrote the paper. The manuscript was written with contributions from all authors. All authors have given consent to the final version of the manuscript.
Funding
We thank the Swedish Research Council (Grant No. 621-2016-03667), a project grant awarded by the Knut and Alice Wallenberg Foundation (KAW 2013.0022), and Galecto Biotech AB, Sweden for financial support.
Acknowledgments
We thank Barbro Kahl-Knutson for excellent assistance with determining affinities by fluorescence polarization and Sofia Essén for excellent assistance with hrms and analytical hplc experiments.
Conflicts of Interest
H.L. and U.J.N. are shareholders in Galecto Biotech AB, Sweden, a company developing galectin inhibitors.
Abbreviations
| Ac | Acetyl |
| Bn | Benzyl |
| DCM | Dichloromethane |
| THF | Tetrahydrofuran |
| DMF | Dimethylformamide |
| DIPEA | Diisopropylethylamine |
| AcOH | AcOH |
| EtOAc | EtOAc |
| TLC | Thin layer chromatography |
| HPLC | High-performance liquid chromatography |
| HRMS | High resolution mass spectrometry |
| DMSO | Dimethylsulfoxide |
| µM | Micromolar |
| mM | Milimolar |
| 9-BBN | 9-Borabicyclo[3.3.1]nonane |
| DMAP | 4-Dimethylaminopyridine |
References
- Lella, S.D.; Sundblad, V.; Cerliani, J.P.; Guardia, C.M.; Estrin, D.A.; Vasta, G.R.; Rabinovich, G.A. When Galectins Recognize Glycans: From Biochemistry to Physiology and Back Again. Biochemistry 2011, 50, 7842–7857. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.-Y.; Rabinovich, G.A.; Liu, F.-T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Croci, D.O. Regulatory Circuits Mediated by Lectin-Glycan Interactions in Autoimmunity and Cancer. Immunity 2012, 36, 322–335. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.A. Turning “sweet” on immunity: Galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef]
- Costa, A.F.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular galectin-3 in tumor progression and metastasis. Front Oncol. 2014, 4, 1–9. [Google Scholar]
- Cousin, J.M.; Cloninger, M.J. The Role of Galectin-1 in Cancer Progression, and Synthetic Multivalent Systems for the Study of Galectin-1. Int. J. Mol. Sci. 2016, 17, 1566. [Google Scholar] [CrossRef]
- Chang, W.-A.; Tsai, M.-J.; Kuo, P.-L.; Hung, J.-Y. Role of galectins in lung cancer. Oncol Lett. 2017, 14, 5077–5084. [Google Scholar]
- Diao, B.; Liu, Y.; Zhang, Y.; Xie, J.; Gong, J. The role of galectin-3 in the tumorigenesis and progression of pituitary tumors. Oncol. Lett. 2018, 15, 4919–4925. [Google Scholar] [CrossRef]
- Califice, S.; Castronovo, V.; Brule, F.V.D. Galectin-3 and cancer. Int. J. Oncol. 2004, 25, 983–992. [Google Scholar]
- Laaf, D.; Bojarová, P.; Elling, L.; Kren, V. Galectin-Carbohydrate Interactions in Biomedicine and Biotechnology. Trends Biotechnol. 2019, 37, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Notz, W.; Hartel, C.; Waldscheck, B.; Schmidt, R.R. De Novo Synthesis of a Methylene-Bridged Neu5Ac-r-(2,3)-Gal C-Disaccharide. J. Org. Chem. 2001, 66, 4250–4260. [Google Scholar] [CrossRef] [PubMed]
- Manickam, G.; Ghoshal, A.; Subramaniam, P.; Li, Y. More Efficient Palladium Catalyst for Hydrogenolysis of Benzyl Groups. Synthetic Commun. 2006, 36, 925–928. [Google Scholar]
- Sörme, P.; Kahl-Knutson, B.; Wellmar, U.; Nilsson, U.J.; Leffler, H. Fluorescence Polarization to Study Galectin–Ligand Interactions. Methods Enzymol. 2003, 362, 504–512. [Google Scholar] [PubMed]
- Sörme, P.; Kahl-Knutson, B.; Huflejt, M.U.; Nilsson, U.J.; Leffler, H. Fluorescence polarization as an analytical tool to evaluate galectin–ligand interactions. Anal. Biochem. 2004, 334, 36–47. [Google Scholar]
- Cumpstey, I.; Carlsson, S.; Leffler, H.; Nilsson, U.J. Synthesis of a phenyl thio-β-d-galactopyranoside library from 1,5-difluoro-2,4-dinitrobenzene: Discovery of efficient and selective monosaccharide inhibitors of galectin-7. Org. Biomol. Chem. 2005, 3, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Öberg, C.-T.; Blanchard, H.; Leffler, H.; Nilsson, U.J. Protein subtype-targeting through ligand epimerization: Talose-selectivity of galectin-4 and galectin-8. Bioorg. Med. Chem. Lett. 2008, 18, 3691–3694. [Google Scholar] [CrossRef]
- Pal, K.B.; Mahanti, M.; Huang, X.; Persson, S.; Sundin, A.P.; Zetterberg, F.R.; Oredsson, S.; Leffler, H.; Nilsson, U.J. Quinoline–galactose hybrids bind selectively with high affinity to a galectin-8 N-terminal domain. Org. Biomol. Chem. 2018, 16, 6295–6305. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Rajput, V.K.; Sundin, A.P.; Leffler, H.; Mukhopadhyay, B.; Nilsson, U.J. Galactose-amidine derivatives as selective antagonists of galectin-9. Can. J. Chem. 2016, 94, 936–939. [Google Scholar] [CrossRef]
- Tolnai, G.L.; Nilsson, U.J.; Olofsson, B. Efficient O-Functionalization of Carbohydrates with Electrophilic Reagents. Angew. Chem. Int. Ed. Engl. 2016, 55, 11226–11230. [Google Scholar] [CrossRef]
- Peterson, K.; Kumar, R.; Stenström, O.; Verma, P.; Verma, P.R.; Håkansson, M.; Kahl-Knutsson, B.; Zetterberg, F.; Leffler, H.; Akke, M.; et al. Systematic Tuning of Fluoro-galectin-3 Interactions Provides Thiogalactoside Derivatives with Single-Digit nM Affinity and High Selectivity. J. Med. Chem. 2018, 61, 1164–1175. [Google Scholar] [CrossRef]
- Öberg, C.T.; Noresson, A.-L.; Delaine, T.; Larumbe, A.; Tejler, J.; von Wachenfeldt, H.; Nilsson, U.J. Synthesis of 3-azido-3-deoxy-β-d-galactopyranosides. Carbohydr. Res. 2009, 344, 1282–1284. [Google Scholar]
- Zemplén, G. Abbau der reduzierenden Biosen, I. Direkte Konstitutionsermitt-lung der cellobiose. Ber. Dtsch. Chem. Ges. 1926, 59, 1254. [Google Scholar]
- Wong, C.-H.; Maris-Varas, F.; Hung, S.-C.; Marron, T.G.; Lin, C.-C.; Gong, K.W.; Weitz-Schimidt, G. Small Molecules as Structural and Functional Mimics of Sialyl Lewis X Tetrasaccharide in Selectin Inhibition: A Remarkable Enhancement of Inhibition by Additional Negative Charge and/or Hydrophobic Group. J. Am. Chem. Soc. 1997, 119, 8152–8158. [Google Scholar] [CrossRef]
- Salomonsson, E.; Larumbe, A.; Tejler, J.; Tullberg, E.; Rydberg, H.; Sundin, A.; Khabut, T.; Frejd, T.; Lobsanov, Y.D.; Rini, J.M.; et al. Monovalent Interactions of Galectin-1. Biochemistry 2010, 49, 9518–9532. [Google Scholar] [CrossRef]
- Gitt, M.A.; Massa, S.M.; Leffler, H.; Barondes, S.H. Isolation and expression of a gene encoding L-14-II, a new human soluble lactose-binding lectin. J. Biol. Chem. 1992, 267, 10601–10606. [Google Scholar]
- Massa, S.M.; Cooper, D.N.; Leffler, H.; Barondes, S.H. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry 1993, 32, 260–267. [Google Scholar] [CrossRef]
- Carlsson, S.; Öberg, C.T.; Carlsson, M.C.; Sundin, A.; Nilsson, U.J.; Smith, D.; Cummings, R.D.; Almkvist, J.; Karlsson, A.; Leffler, H. Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology 2007, 17, 663–676. [Google Scholar] [CrossRef]
- Öberg, C.T.; Carlsson, S.; Fillion, E.; Leffler, H.; Nilsson, U.J. Efficient and Expedient Two-Step Pyranose-Retaining Fluorescein Conjugation of Complex Reducing Oligosaccharides: Galectin Oligosaccharide Specificity Studies in a Fluorescence Polarization Assay. Bioconj. Chem. 2003, 14, 1289–1297. [Google Scholar] [CrossRef]
- Delaine, T.; Collins, P.; MacKinnon, A.; Sharma, G.; Stegmayr, J.; Rajput, V.K.; Mandal, S.; Cumpstey, I.; Larumbe, A.; Salameh, B.A.; et al. Galectin 3-Binding Glycomimetics that Strongly Reduce Bleomycin-Induced Lung Fibrosis and Modulate Intercellular Glycan Recognition. ChemBioChem 2016, 17, 1759–1770. [Google Scholar] [CrossRef]
- Méndez-Huergo, S.P.; Blidner, A.G.; Rabinovich, G.A. Galectins: Emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr. Opin. Immun. 2017, 45, 8–15. [Google Scholar] [CrossRef]
- Kaltner, H.; Toegel, S.; Caballero, G.G.; Manning, J.C.; Ledeen, R.W.; Gabius, H.-J. Galectins: Their network and roles in immunity/tumor growth control. Histochem. Cell. Biol. 2016, 147, 239–256. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).