Abstract
Dedifferentiated endometrial carcinoma (DDEC) is defined as an undifferentiated carcinoma admixed with differentiated endometrioid carcinoma (Grade 1 or 2). It has poor prognosis compared with Grade 3 endometrioid adenocarcinoma and is often associated with the loss of mismatch repair (MMR) proteins, which is seen in microsatellite instability (MSI)-type endometrial cancer. Recent studies have shown that the effectiveness of immune checkpoint inhibitor therapy is related to MMR deficiency; therefore, we analyzed the immunophenotype (MMR deficient and expression of PD-L1) of 17 DDEC cases. In the undifferentiated component, nine cases (53%) were deficient in MMR proteins and nine cases (53%) expressed PD-L1. PD-L1 expression was significantly associated with MMR deficiency (p = 0.026). In addition, the presence of tumor-infiltrating lymphocytes (CD8+) was significantly associated with MMR deficiency (p = 0.026). In contrast, none of the cases showed PD-L1 expression in the well-differentiated component. Our results show that DDEC could be a target for immune checkpoint inhibitors (anti PD-L1/PD-1 antibodies), especially in the undifferentiated component. As a treatment strategy for DDEC, conventional paclitaxel plus carboplatin and cisplatin plus doxorubicin therapies are effective for those with the well-differentiated component. However, by using immune checkpoint inhibitors in combination with other conventional treatments, it may be possible to control the undifferentiated component and improve prognosis.
1. Introduction
Dedifferentiated endometrial carcinoma (DDEC) is a rare and more aggressive type of endometrioid carcinoma than high grade endometrioid carcinoma [1,2,3]. In 2006, Silva et al reported cases of endometrial carcinoma in which low-grade endometrioid carcinoma was combined with undifferentiated carcinoma and designated them as dedifferentiated endometrial carcinoma [3,4]. A total of 50%–58% of patients with DDEC present with advanced stage disease, and 40% of these patients die within half a month to 20 months from the disease [3,5]. For this reason, it is urgently required to develop therapies, such as immunotherapy, that fit molecular subgroups.
DDEC was suggested to be related to the deficiency of mismatch repair (MMR) proteins, mutL protein homolog 1 (MLH1), postmeiotic segregation increased 2 (PMS2), mutS protein homolog 2 (MSH2), and mutS protein homolog 6 (MSH6), resulting in microsatellite instability (MSI) [5]. MMR deficiency has been reported in 58% of cases by immunohistochemistry (IHC) and occurs more frequently than in common endometrial cancer, with 25%–30% of cases showing MMR deficiency [5,6,7,8]. MMR-deficient tumors are burdened with somatic mutations due to a defective DNA MMR system. It has been reported that tumors with higher numbers of somatic mutations are more immunogenic and have immune escape mechanisms, such as the programmed cell death-1 (PD-1) and PD-1 ligand 1 (PD-L1) pathways [9,10,11]. Clinical trials of immune checkpoint inhibitors for MMR-deficient tumors have been studied in many carcinomas including colorectal cancer and melanoma [12]. As a biomarker for the effectiveness of immune checkpoint inhibitors, PD-L1 expression on IHC, cytotoxic T lymphocyte (CD8+ T cell), and neo antigen (mutation burden rich) are shown in existing reports [12,13,14,15]. Specifically, when assessing the anti-PD-1 antibody for melanoma, infiltration of CD8+ T cells correlate with response to them [14]. It has been suggested that immune checkpoint inhibitors may be effective when there is a high infiltration of CD8+ T cells into the tumor [16,17,18]. Therefore, immune checkpoint inhibitors are thought to be effective for MMR-deficient tumors. However, the relationship between MMR deficiency and the expression of PD-L1 and CD8+ T cell tumor-infiltration remains poorly understood in DDEC. We hypothesized that prognosis may be improved by the use of immune checkpoint inhibitors in DDEC with MMR deficiency. In the present study, we investigated the relationship between the expression of PD-L1 protein and CD8+ T cell tumor-infiltration and MMR deficiency in DDEC.
2. Results
2.1. The Clinicopathological Features
The clinicopathological features of the 17 cases of DDEC are summarized in Table 1. The patient ages ranged from 52 to 78 years (median of 62 years). By the International Federation of Gynecology and Obstetrics staging criteria, the number of cases in stages I, II, III, and IV were 5 (29.4%), 1 (5%), 7 (41.2%), and 4 (23.5%), respectively.

Table 1.
Clinicopathologic features of 17 dedifferentiated endmetorial carcinoma.
2.2. Immunohistochemical Findings
The immunohistochemical findings are shown in Figure 1, Figure 2 and Figure 3. Some cases were differentially stained according to the well-differentiated and the undifferentiated components. Out of 17 cases, 11 (64.7%) were MMR-deficient; loss of MMR proteins was observed in the well-differentiated component for 8 cases (MLH1, 8 cases; PMS2, 4 cases; MSH2, 2 cases; and MSH6, none), in the undifferentiated component for 9 cases (MLH1, 6 cases; PMS2, 5 cases; MSH2, 2 cases; and MSH6, 1 case). Overall, 6 cases out of 17 cases had MMR deficiency in both the well-differentiated component and undifferentiated components. Furthermore, 11 cases (64.7%) had PD-L1 expression. PD-L1 expression was observed only in the undifferentiated component (Table 1).
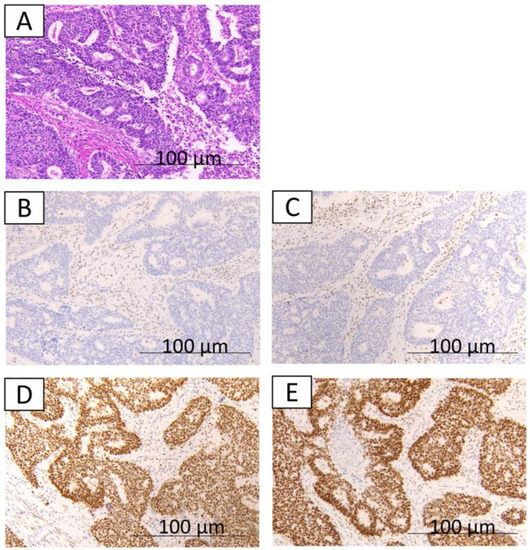
Figure 1.
The immunohistochemical findings from case 1. A,B,C,D,E: well-differentiated component; hematoxylin and eosin staining show well-differentiated endometrioid glands (A); loss of expression of MLH1 (B); loss of expression of PMS2 (C); expression of MSH2 (D); expression of MSH6 (E).
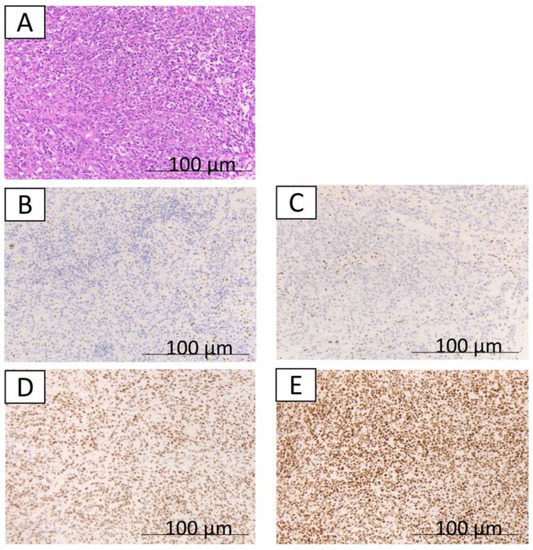
Figure 2.
The immunohistochemical findings from case 1. A,B,C,D,E indicate a typical undifferentiated component; hematoxylin and eosin staining show the undifferentiated component (A); loss of expression of MLH1 (B); loss of expression of PMS2 (C); expression of MSH2 (D); expression of MSH6 (E).
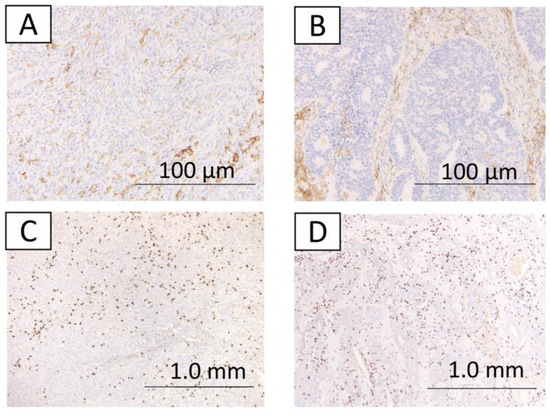
Figure 3.
The immunohistochemical findings from case 1. A,B,C,D indicate no expression of PD-L1 in the well-differentiated component (A). Expression of PD-L1 in the undifferentiated component (B). CD8 expression score of 2 in the well-differentiated component (C). CD8 expression score of 2 in the undifferentiated component (D).
2.3. MSI Analysis
We analyzed genomic MSI in 3 cases that were indicated as having MMR deficiency by IHC (Table 1). All cases were considered as MSI-high based on the MSI analysis. In Figure 4, we present case 1 that was evaluated as MSI-high both in the well-differentiated and undifferentiated components.
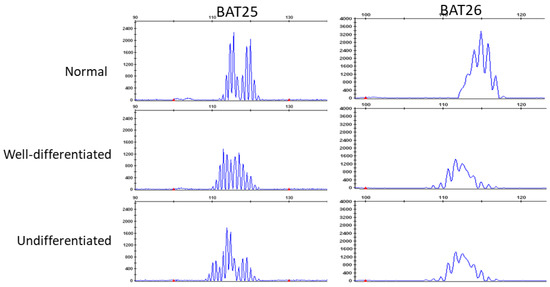
Figure 4.
Microsatellite instability (MSI) analysis of normal (top), well-differentiated components (middle), and undifferentiated components (bottom). Two microsatellite markers (BAT25 and BAT26) show instability and are visible as the shift in well-differentiated and undifferentiated components the size (base pairs) of the amplification products.
2.4. Statistical Analyses
Expression of PD-L1 was significantly associated with MMR deficiency in the undifferentiated component (p = 0.026; Table 2). In contrast, none of the cases showed PD-L1 expression in the well-differentiated component. In the undifferentiated component, the MMR-deficient group had more CD8 positive T cell infiltration than the MMR-proficient group (p = 0.026; Table 3). In the well-differentiated component, there was no significant difference between CD8 positive T cell infiltration and MMR deficiency (p = 0.772; Table 4).

Table 2.
Relationship between status of MMR and PD-L1 expression. (undifferentiated component).

Table 3.
Relationship between status of MMR and CD8 expression. (undeffirentiated component).

Table 4.
Relationship between status of MMR and CD8 expression. (well differentiated component).
3. Discussion
DDEC is rare, occurring in only 9% of all endometrial carcinomas [4]. The Cancer Genome Atlas stratifies endometrial carcinomas into four distinct molecular groups on the basis of molecular genetic alterations, namely those with Defective DNA polymerase ε (POLE) mutations, those with MSI, those with low copy number alterations, and those with high copy number alterations, including p53 mutations. However, it is not explicitly specified as to which molecular group DDEC is classified to [6]. The above study suggested that progression free survival is better in patients with POLE mutations than in those with MSI. However, recent studies have reported that MSI is associated with several poor prognostic indicators that are routinely used to make decisions for adjuvant therapy use [19,20,21,22]. Previous studies have suggested that DDEC is associated with a deficiency of MMR proteins (MLH1, PMS2, MLH2, and MSH6) [5]. In recent reports, the prevalence of MMR deficiency in endometrial carcinoma is 25%–30% [6,7,8]. DDEC is generally associated with 58% MMR deficiency and is more frequent than endometrial carcinoma [5]. In addition, DDEC has a poorer prognosis as compared with Grade 3 endometrial carcinoma [1,2,3]. Therefore, individual treatment strategies for DDEC, especially MMR-deficient cases, need to be devised. In recent years, it has been reported that tumors with higher numbers of somatic mutations (high mutation burden), such as MSI tumors, are more immunogenic, and immune checkpoint inhibitors are effective for such tumors [10,23,24]. Although the number of cases was small, Hussaini et al. and Liu et al. demonstrated PD-L1 expression in DDEC [25,26]. Therefore, we hypothesized that prognosis may be improved through the use of immune checkpoint inhibitors (anti PD-1/PD-L1 antibodies) in DDEC with MMR deficiency. Recently, we reported that MMR deficiency is a biomarker for predicting the effect of immune checkpoint inhibitors using immunostaining in endometrial carcinoma [27]. In other reports, tumor infiltration of lymphocytes was associated with the responsiveness of immune checkpoint inhibitors [15,16,17]. From there, we thought that it could be a target for immune checkpoint inhibitors by correlating the tumor infiltration of lymphocytes with MMR deficiency and the expression of PD-L1. Based on this, in the present study, we investigated the expression of PD-L1 and the level of tumor-infiltrating CD8 positive T cells in endometrial carcinoma cases. In this research, MMR deficiency observed in either undifferentiated or well-differentiated components was found to be 64.7%, which is consistent with previous reports [5]. Our results showed that MMR deficiency was significantly associated with PD-1 expression (p = 0.026) and the presence of tumor-infiltrating lymphocytes (CD8+) (p = 0.026). Our results suggest that DDEC could therefore be a target for immune checkpoint inhibitors.
DDEC is a very rare and new histopathological concept; as such, the molecular mechanisms are poorly understood [1,3,4]. A recent study reported that the evidence regarding similar mutations in the well-differentiated and undifferentiated components suggests that this tumor progresses from a low-grade endometrioid adenocarcinoma to an undifferentiated carcinoma [28]. Interestingly, even within the same tumor, staining results were different in the undifferentiated and well-differentiated components, and PD-L1 was expressed only in the undifferentiated component in our results. Yokomizo et al. first demonstrated the loss of MMR protein expression in the undifferentiated component [29]. These findings may suggest that DDEC has intra-tumor heterogeneity. Wu et al. showed that the metastatic histology of DDEC was mainly composed of an undifferentiated component [1]. Although the reason for DDEC aggressiveness is not clear, it is most likely due to the undifferentiated component. Although this is a hypothesis, an immune escape mechanism occurs during the process of dedifferentiation. As a treatment strategy for DDEC with MMR deficiency, conventional paclitaxel plus carboplatin and cisplatin plus doxorubicin therapies are effective for the well-differentiated component. However, by using immune checkpoint inhibitors in combination with conventional chemotherapy, it may be possible to control the growth of the undifferentiated component and finally lead to the improvement of prognosis. However, this study is limited by the relatively low number of cases due to the rarity of these tumors. In vitro cytotoxicity assays are needed to determine the actual effectiveness of immune checkpoint inhibitors for DDEC. However, in the future, it is hoped that efficacy assessments will be re-evaluated by accumulating clinical trial results based on other carcinoma investigations.
Recent molecular studies have reported that the inactivation of core components of the switch/sucrose non-fermentable (SWI/SNF) chromatin remodeling complex proteins, BRG1 inactivation, INI1 inactivation, or ARID1A/ARID1B co-inactivation are associated with histological dedifferentiation [30,31,32,33]. In these reports, the patients with dedifferentiated or undifferentiated endometrial carcinoma with SWI/SNF complex deficiency were defined as a highly aggressive subset [32]. It was suggested that therapies targeting chromatin remodeling resulting in epigenetic control might be effective [32]. Although undifferentiated carcinoma was included in the study population in this report, only one out of 34 cases had a POLE mutation [32]. In other studies, seven out of 13 DDEC cases had POLE exonuclease domain mutations [34]. Furthermore, the patients with POLE-mutated dedifferentiated and undifferentiated endometrial carcinomas had a favorable outcome. On the other hand, DDEC tended to be associated with MSI due to abnormalities in MLH1/PMS2 [34]. A case of DDEC with MLH1 promoter hypermethylation, high MSI status, and high PD-L1 expression was reported very recently [26]. Our results also showed that most MSI cases were deficient in MLH1/PMS2. In patients with colorectal cancer, tumors with a poor differentiation, Crohn-like lymphoid reaction, and PD-L1 expression occurred more frequently in sporadic MSI cases than in Lynch syndrome-associated cases [35]. In the present study, we did not examine MLH1 promoter methylation status, MMR germline status, POLE mutation, or mutation burden status. However, the current results and a recent case report [33] speculated that DDEC had a high mutation burden, resulting in high immunogenicity. Therefore, a pathological diagnosis of DDEC is a potential predictive factor for a good response to immunotherapy targeting. So far, there is little consideration for histopathological-specific therapies. In cases of histopathological specificity according to a special clinical course like DDEC, it is necessary to find individual treatment strategies. To that end, if possible, more cases should be investigated, and the development of cancer genomic medicine using sequencing technology for DDEC in a clinical setting should be further evaluated [36].
In summary, the current results that indicated a high expression of PD-L1 and CD8 positive T cells in the dedifferentiated component of MMR deficient tumors suggest that DDEC deficiency could be a target for immune checkpoint inhibitors (anti PD-1/PD-L1 antibodies), and the presence of MMR deficiency may be a biomarker for a good response to PD-L1 immunotherapy in DDEC.
4. Materials and Methods
4.1. Study Samples
We searched the pathology databases of Shimane University, Shimane Prefectural Central Hospital, Seirei Hamamatsu General Hospital from 2007 to 2017. Samples were collected from 17 patients who were diagnosed with low-grade endometrioid carcinoma (Grade 1–2) that contained an undifferentiated component. Patients were diagnosed based on the 2014 World Health Organization (WHO) Classification of Tumors of the Female Genital Organs. Some patients were diagnosed with high-grade endometrioid carcinoma [37]. Furthermore, we evaluated the distinction between particularly confusing DDEC and high-grade endometrial carcinoma with reference to the literature by Han et al. Specifically, the undifferentiated component of DDEC represented a solid sheet constructed by tumor cells lacking intercellular adhesion without glandular formation. Furthermore, DDEC has clear boundaries for undifferentiated and well-differentiated components. In contrast, high-grade endometrial carcinoma has a glandular structure and often shifts gradually from low grade [38]. All cases were independently diagnosed by pathologists (Yoshihiro Otsuki and Hideyuki Onuma) at each hospital and reviewed by a gynecologic pathologist (Noriyoshi Ishikawa). Samples were collected after obtaining written consent from all patients with the approval of the Facility Ethical Committee (Shimane University Hospital, Izumo, Japan; approval No. 2004–0381, 5 March 2007).
4.2. Immunohistochemistry
The expression of MMR proteins (MLH1, PMS2, MSH2, and MSH6), CD8, PD-L1, were examined by immunostaining. The method used for immunostaining was described in detail in our previous report [27]. Briefly, formalin-fixed and paraffin-embedded sections (4-µm thick) were dewaxed in xylene and hydrated in graded alcohol. After antigen retrieval in a sodium citrate buffer, slides were incubated overnight at 4 °C with antibodies against MLH1 (1:50; Dako, Santa Clara, CA, United States), PMS2 (1:40; Dako, Santa Clara, CA, United States), MSH2 (1:50; Dako, Santa Clara, CA, United States), MSH6 (1:50; Dako, Santa Clara, CA, United States), CD8 (1:100; Roche, Basel, Switzerland), PD-L1 (ab205921, Abcam, Cambridge, CAM, United Kingdom). Immunostaining was evaluated using a double-blind method by two researchers (R.O. and K.N.). We evaluated the differentiated and undifferentiated components of each case. Tumors were considered to be MMR deficient if at least one of the four MMR proteins (MLH1, PMS2, MSH2, or MSH6) was deficient. The level of tumor infiltrating lymphocytes was classified into four categories by CD8 expression: 0, undetectable; 1+, weakly positive (percentage of CD8 positive cells per tumor cells 0–30%); 2+, moderately positive (30−60%); and 3+, strongly positive (≥60%). Cases that were 2+ or 3+ were counted as positive in our analysis. Based on the cut-off value used in many clinical trials including Nivolumab’s 3rd clinical trial on malignant melanoma, IHC of PD-L1 was evaluated as positive if more than 5% of the tumor cells were stained [39,40].
4.3. DNA Extraction and MSI Analysis
We performed MSI analysis for three cases that were indicated as MMR deficient according to IHC. DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissues according to protocols for the isolation of total DNA. Well-differentiated components and undifferentiated components were separately collected macroscopically with reference to Hematoxylin-Eosin (HE) staining and interstitial tissue was collected to use as a control in the analysis. We digested tumor tissues (0.01 M NaCl; 0.5 M Tris-HCl, pH 8.0; 20 mM EDTA; 0.05% Tween-20; 0.1 mg/mLproteinase K) for 1 h at 56 ˚C or until the sample indicated complete lysis. To inactivate the proteinase K, we heated the tissues to 90 ˚C for 1 h. Following this, DNA was extracted with phenol/chloroform treatment and ethanol precipitation. The MSI status was determined using eight microsatellite markers (BAT25, BAT26, D2S123, D5S346, D17S250, NR21, MONO27, and NR2). We analyzed the amplicons on the ABI PRISM 310 Genetic Analyzer and evaluated allelic sizes by GeneMapper (Appleid Biosystems, Thermo Fisher K.K Yokohama Japan). Tumors with instability at two or more markers were considered as MSI-high.
4.4. Statistical Analyses
The relationships between MMR status and the expression of CD8 and PD-L1 were assessed using a Chi-squared test. We examined the well-differentiated and undifferentiated components separately.
Author Contributions
K.N. and R.O. designed the study. K.N. supervised all experiments. All other authors contributed to data collection. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research was funded by grants from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (18K09229).
Acknowledgments
The authors wish to thank the Department of Pathology for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, E.S.; Shih I, M.; Diaz-Montes, T.P. Dedifferentiated endometrioid adenocarcinoma: An under-recognized but aggressive tumor? Gynecol. Oncol. Case Rep. 2013, 5, 25–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Altrabulsi, B.; Malpica, A.; Deavers, M.T.; Bodurka, D.C.; Broaddus, R.; Silva, E.G. Undifferentiated carcinoma of the endometrium. Am. J. Surg. Pathol. 2005, 29, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.G.; Deavers, M.T.; Bodurka, D.C.; Malpica, A. Association of low-grade endometrioid carcinoma of the uterus and ovary with undifferentiated carcinoma: A new type of dedifferentiated carcinoma? Int. J. Gynecol. Pathol. 2006, 25, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.G.; Deavers, M.T.; Malpica, A. Undifferentiated carcinoma of the endometrium: A review. Pathology 2007, 39, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Tafe, L.J.; Garg, K.; Chew, I.; Tornos, C.; Soslow, R.A. Endometrial and ovarian carcinomas with undifferentiated components: Clinically aggressive and frequently underrecognized neoplasms. Mod. Pathol. 2010, 23, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [PubMed]
- McMeekin, D.S.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Lankes, H.A.; Geller, M.A.; Powell, M.A.; Backes, F.J.; Landrum, L.M.; Zaino, R.; et al. Clinicopathologic Significance of Mismatch Repair Defects in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2016, 34, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, D.D.; Tan, Y.Y.; Walsh, M.D.; Clendenning, M.; Metcalf, A.M.; Ferguson, K.; Arnold, S.T.; Thompson, B.A.; Lose, F.A.; Parsons, M.T.; et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J. Clin. Oncol. 2014, 32, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Arum, C.J.; Anderssen, E.; Viset, T.; Kodama, Y.; Lundgren, S.; Chen, D.; Zhao, C.M. Cancer immunoediting from immunosurveillance to tumor escape in microvillus-formed niche: A study of syngeneic orthotopic rat bladder cancer model in comparison with human bladder cancer. Neoplasia 2010, 12, 434–442. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.; Garattini, S.K.; Bonotto, M.; Ongaro, E.; Casagrande, M.; Fanotto, V.; de Carlo, E.; Loupakis, F.; Urbano, F.; Nergi, F.V.; et al. Immunotherapy for colorectal cancer: Where are we heading? Expert Opin. Biol. 2017, 17, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Gilligan, B.M.; Yuan, J.; Li, T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J. Hematol. Oncol. 2016, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Adners, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Tumehs, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of inflammatory Response with B7-H1 Expression in Human Melanocytic Lesions Supports an Adaptive Resistance Mechanism of Immune Escape. Sci. Transl. Med. 2012, 4, 127ra37. [Google Scholar] [CrossRef]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Diaz, L.A.; Armstrong, D.K.; Tanner, E.J.; Uram, J.; Eyring, A.; Wang, H.; Fisher, G.; Grenten, T.; Le, D. Preliminary results of a phase II study: PD-1 blockade in mismatch repair–deficient, recurrent or persistent endometrial cancer. Gynecol. Oncol. 2016, 141, 206–207. [Google Scholar] [CrossRef]
- Mackay, H.J.; Gallinger, S.; Tsao, M.S.; McLachlin, C.M.; Tu, D.; Keiser, K.; Eisenhauer, E.A.; Oza, A.M. Prognostic value of microsatellite instability (MSI) and PTEN expression in women with endometrial cancer: Results from studies of the NCIC Clinical Trials Group (NCIC CTG). Eur. J. Cancer 2010, 46, 1365–1373. [Google Scholar] [CrossRef]
- Kanopiene, D.; Smailyte, G.; Vidugiriene, J.; Bacher, J. Impact of microsatellite instability on survival of endometrial cancer patients. Medicina 2014, 50, 216–221. [Google Scholar] [CrossRef]
- Diaz-Padilla, I.; Romero, N.; Amir, E.; Matias-Guiu, X.; Vilar, E.; Muggia, F.; Garcia-Donas, J. Mismatch repair status and clinical outcome in endometrial cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2013, 88, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Kuhn, E.; Ayhan, A.; Bahadirli-Talbott, A.; Zhao, C.; Shih I, M. Molecular characterization of undifferentiated carcinoma associated with endometrioid carcinoma. Am. J. Surg. Pathol. 2014, 38, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, M.; Lataifeh, I.; Jaradat, I.; Abdeen, G.; Otay, L.; Badran, O.; Abu Sheikha, A.; Dayyat, A.; El Khaldi, M.; Ashi Al-Loh, S. Undifferentiated Endometrial Carcinoma, an Immunohistochemical Study Including PD-L1Testing of a Series of Cases From a Single Cancer Center. Int. J. Gynecol. Pathol. 2018, 37, 564–574. [Google Scholar] [PubMed]
- Liu, X.; Liu, X.; Wang, X.; Wu, R.; Zhang, K.; Liu, B.; Liu, Q.; Shao, Y.; Tang, R.; You, J.; et al. A novel case of endometrial dedifferentiated adenocarcinoma associated with MLH1 promotor hypermethylation and microsatellite instability. Pathol. Res. Pract. 2018, 214, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Nakayama, K.; Ishikawa, M.; Nakamura, K.; Ishibashi, T.; Sanuki, K.; Ono, R.; Sasamori, H.; Minamoto, T.; Iida, K.; et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget 2018, 9, 5652–5664. [Google Scholar] [CrossRef] [PubMed]
- Strehl, J.D.; Wachter, D.L.; Fiedler, J.; Heimerl, E.; Beckmann, M.W.; Hartmann, A.; Agaimy, A. Pattern of SMARCB1 (INI1) and SMARCA4 (BRG1) in poorly differentiated endometrioid adenocarcinoma of the uterus: Analysis of a series with emphasis on a novel SMARCA4-deficient dedifferentiated rhabdoid variant. Ann. Diagn. Pathol. 2015, 19, 198–202. [Google Scholar] [CrossRef]
- Yokomizo, R.; Yamada, K.; Iida, Y.; Kiyokawa, T.; Ueda, K.; Saito, M.; Yanaihara, N.; Nakamura, M.; Okamoto, A. Dedifferentiated endometrial carcinoma: A report of three cases and review of the literature. Mol. Clin. Oncol. 2017, 7, 1008–1012. [Google Scholar] [CrossRef]
- Karnezis, A.N.; Hoang, L.N.; Coatham, M.; Ravn, S.; Almadani, N.; Tessier-Cloutier, B.; Irving, J.; Meng, B.; Li, X.; Chow, C.; et al. Loss of switch/sucrose non-fermenting complex protein expression is associated with dedifferentiation in endometrial carcinomas. Mod. Pathol. 2016, 29, 302–314. [Google Scholar] [CrossRef]
- Kobel, M.; Hoang, L.N.; Tessier-Cloutier, B.; Meng, B.; Soslow, R.A.; Stewart, C.J.R.; Lee, C.H. Undifferentiated Endometrial Carcinomas Show Frequent Loss of Core Switch/Sucrose Nonfermentable Complex Proteins. Am. J. Surg. Pathol. 2018, 42, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Coatham, M.; Li, X.; Karnezis, A.N.; Hoang, L.N.; Tessier-Cloutier, B.; Meng, B.; Soslow, R.A.; Blake Gilks, C.; Huntsman, D.G.; Stewart, C.J.; et al. Concurrent ARID1A and ARID1B inactivation in endometrial and ovarian dedifferentiated carcinomas. Mod. Pathol. 2016, 29, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, I.; Lee, C.H.; D’Angelo, E.; Palacios, J.; Prat, J. Undifferentiated and Dedifferentiated Endometrial Carcinomas With POLE Exonuclease Domain Mutations Have a Favorable Prognosis. Am. J. Surg. Pathol. 2017, 41, 1121–1128. [Google Scholar] [CrossRef]
- Garg, K.; Leitao, M.M., Jr.; Kauff, N.D.; Hansen, J.; Kosarin, K.; Shia, J.; Soslow, R.A. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am. J. Surg. Pathol. 2009, 33, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Yamaguchi, T.; Iijima, T.; Wakaume, R.; Takao, M.; Koizumi, K.; Hishima, T.; Horiguchi, S.I. Differences in histological features and PD-L1 expression between sporadic microsatellite instability and Lynch-syndrome-associated disease in Japanese patients with colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Dumbrava, E.E.I.; Litzenburger, B.; Khotskaya, Y.B.; Johnson, A.M.; Yap, T.A.; Rodon, J.; Zeng, J.; Shufean, M.A.; Bailey, A.M.; et al. Precision Oncology Decision Support: Current Approaches and Strategies for the Future. Clin. Cancer Res. 2018, 24, 2719–2731. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO classification of tumours of female reproductive organs. IARC 2014, 2014, 122–133. [Google Scholar]
- Han, J.I.; Ki, E.Y.; Rha, S.E.; Hur, S.; Lee, A. Dedifferentiated endometrioid carcinoma of the uterus: Report of four cases and review of literature. World J. Surg. Oncol. 2017, 15, 17. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Black, D.; Soslow, R.A.; Levine, D.A.; Tornos, C.; Chen, S.C.; Hummer, A.J.; Bogomolniy, F.; Olvera, N.; Barakat, R.R.; Boyd, J. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J. Clin. Oncol. 2006, 24, 1745–1753. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).