Abstract
The salt overly sensitive 1 (SOS1) gene encodes the plasma membrane Na+/H+ antiporter, SOS1, that is mainly responsible for extruding Na+ from the cytoplasm and reducing the Na+ content in plants under salt stress and is considered a vital determinant in conferring salt tolerance to the plant. However, studies on the salt tolerance function of the TrSOS1 gene of recretohalophytes, such as Tamarix, are limited. In this work, the effects of salt stress on cotton seedlings transformed with tobacco-rattle-virus-based virus-induced gene silencing (VIGS) of the endogenous GhSOS1 gene, or Agrobacterium rhizogenes strain K599-mediated TrSOS1-transgenic hairy root composite cotton plants exhibiting VIGS of GhSOS1 were first investigated. Then, with Arabidopsis thaliana AtSOS1 as a reference, differences in the complementation effect of TrSOS1 or GhSOS1 in a yeast mutant were compared under salt treatment. Results showed that compared to empty-vector-transformed plants, GhSOS1-VIGS-transformed cotton plants were more sensitive to salt stress and had reduced growth, insufficient root vigor, and increased Na+ content and Na+/K+ ratio in roots, stems, and leaves. Overexpression of TrSOS1 enhanced the salt tolerance of hairy root composite cotton seedlings exhibiting GhSOS1-VIGS by maintaining higher root vigor and leaf relative water content (RWC), and lower Na+ content and Na+/K+ ratio in roots, stems, and leaves. Transformations of TrSOS1, GhSOS1, or AtSOS1 into yeast NHA1 (Na+/H+ antiporter 1) mutant reduced cellular Na+ content and Na+/K+ ratio, increased K+ level under salt stress, and had good growth complementation in saline conditions. In particular, the ability of TrSOS1 or GhSOS1 to complement the yeast mutant was better than that of AtSOS1. This may indicate that TrSOS1 is an effective substitute and confers enhanced salt tolerance to transgenic hairy root composite cotton seedlings, and even the SOS1 gene from salt-tolerant Tamarix or cotton may have higher efficiency than salt-sensitive Arabidopsis in regulating Na+ efflux, maintaining Na+ and K+ homeostasis, and therefore contributing to stronger salt tolerance.
1. Introduction
Soil salinity is one of the major abiotic stress factors that adversely affects plant growth and development, thus reducing crop quality and yield [1,2]. NaCl is the most soluble and abundant salt released into the soil and is the major cause of salt stress on plants or crops [3]. Ionic toxicity, osmotic stress, nutritional imbalance, and oxidative damage are the main causes of salt injury in plants. Under salt stress, plant cells can be protected through several strategies, such as Na+ extrusion out of the plasma membrane, ionic imbalances in the vacuole, stress signal transduction, and expression of effector genes encoding ion transporters, channels, enzymes involved in osmolyte biosynthesis and antioxidant systems, etc. and regulatory genes encoding transcription factors, protein kinases, phosphatases, and proteases involved in transcriptional and post-transcriptional regulation as well as in signaling pathways [3,4,5]. In general, ionic toxicity by the accumulation of Na+ and Cl− is the primary and dominant factor of salt injury in plants [6], furthermore, crops such as cotton, rice, and barley, are more sensitive to Na+ than to Cl− [7]. Many studies have reported on the mechanisms of maintaining low Na+ in the cytoplasm from two synergistic aspects, i.e., Na+ extrusion out of the cytoplasm into the apoplast and vacuolar compartmentalization through membrane-bound Na+/H+ antiporters, such as the plasma-membrane-localized salt overly sensitive 1 (SOS1) protein, and the tonoplast-localized NHX1 protein [8].
The SOS1 gene was originally identified in Arabidopsis as one main component of the SOS (salt overly sensitive) signal transduction pathway (also including SOS2 and SOS3) and involved in maintaining cellular Na+ homeostasis in plants under salt stress [9,10,11]. SOS1, the activity of which is regulated by the phosphorylation of the SOS2/SOS3 kinase complex, is responsible for extruding Na+ from the roots and reducing the Na+ content in plants under salt stress. Therefore, SOS1 is vital for the salt tolerance of plants and has been considered as a superior salt tolerance determinant [12,13]. The physiological roles of the SOS1 gene have been widely investigated in more than 40 types of plants except Arabidopsis thaliana, including glycophytes such as Oryza sativa [14], Solanum lycopersicum [15], Chrysanthemum morifolium [16], Gossypium hirsutum [17], Glycine max, and Glycine soja [18,19] and halophytes such as Thellungiella salsuginea [20], Chenopodium quinoa [21], Salicornia brachiata [22], and Sesuvium portulacastrum [23]. Upland cotton (G. hirsutum) is one of the most economically important fiber and oil seed crops and can tolerate relatively high salinity and drought stresses compared to other major crops [17,24]. Although the salt tolerance of cotton is moderate and relatively limited, the growth of cotton under salt stress is severely suppressed, especially at the stages of seed germination and young seedlings [25]. The main cause of salinity damage to cotton plants is sodium ion accumulation, which is lethal to most organisms [26]. Chen et al. [17] first cloned the plasma membrane Na+/H+ antiporter gene GhSOS1, and found that GhSOS1-transgenic Arabidopsis plants displayed enhanced salt tolerance, as indicated by a decreased Na+/K+ ratio and malondialdehyde (MDA) content in the leaves of the salt-stressed plants. By RNA-Seq analysis of the roots and leaves of diploid cotton species Gossypium davidsonii under salt stress and normal conditions, Zhang et al. [27] found lots of differentially expressed genes (DEGs) involved in the maintenance of ion homeostasis and oxidation balance.
Compared to glycophytes, halophytes more efficiently control the influx of Na+ into roots, compartmentation of Na+ into vacuoles, and distribution of Na+ into various tissues. In addition, halophytes have evolved unique anatomical adaptations, such as salt glands and bladders, to excrete Na+ out of their body [3,28]. Since halophytes are more capable of overcoming and adapting to soil salinity than glycophytes, studies on the mechanism of the halophytes’ SOS1 gene in salt tolerance display more academic and practical applications. Oh et al. [20] suggested that, although both ThSOS1 and AtSOS1 from the halophyte T. salsuginea and its relative species glycophyte A. thaliana, respectively, could enhance the salt tolerance of the yeast mutant, the complementary effect of ThSOS1 was stronger than that of AtSOS1 under high salinity. Zhou et al. [23] reported that the SpSOS1 gene from halophyte S. portulacastrum complemented the salt sensitivity of Arabidopsis sos1 mutant plants, and SpSOS1 may have a stronger ability to extrude Na+ than the glycophyte Arabidopsis. This reveals that the SOS1 gene of halophytes may play a more critical role in the adaptation to salt stress than that of glycophytes. Therefore, the discovery of salt-tolerance genes from halophytes may have more important theoretical and practical significance that of than those from glycophytes for the in-depth study of the molecular mechanism of plant salt tolerance, especially for the molecular genetic improvement of salt tolerance in crop plants.
Tamarix, a family of woody recretohalophyte species, is widely distributed in the saline soils of drought-stricken areas of Central Asia and China [28]. Our previous studies on three types of Tamarix plants (T. ramosissima, T. gansuensis, and T. leptostachys) showed that the growth of T. ramosissima was relatively less inhibited, and the expression of TrSOS1 was upregulated both in roots and shoots under salt stress [29]. However, the unique role of TrSOS1 in the adaptation of recretohalophyte T. ramosissima to high-saline environments remains unclear. Agrobacterium rhizogenes-mediated transformation for generating hairy root composite plants has become a powerful tool for studying target gene function and root biology due to the rapidity and simplicity of the method [30,31]. Virus-induced gene silencing (VIGS) is a plant RNA-silencing technique that as a powerful tool for genetic analysis uses viral vectors carrying a fragment of a gene of interest to generate double-stranded RNA, which initiates the silencing of the target gene [32]. In the present work, the effects of TrSOS1 on the salt sensitivity of A. rhizogenes strain K599-mediated TrSOS1-transgenic hairy root composite cotton plants, which also exhibited tobacco rattle virus (TRV)-based VIGS of the endogenous GhSOS1 gene, were first investigated. Then, with A. thaliana AtSOS1 as a reference, the differences in the complementation effects of the yeast mutant by TrSOS1 or GhSOS1 were also analyzed and compared under salt treatment. The objective of this study is to provide a novel theoretical basis for the exploitation of the superior salt tolerance function of the halophyte SOS1 gene and its future prior utilization in germplasm innovation and genetic improvement of salt tolerant plants or crops by genetic engineering.
2. Results
2.1. Effects of Salt Stress on the Growth and Related Physiological Parameters of GhSOS1-VIGS Cotton Seedlings
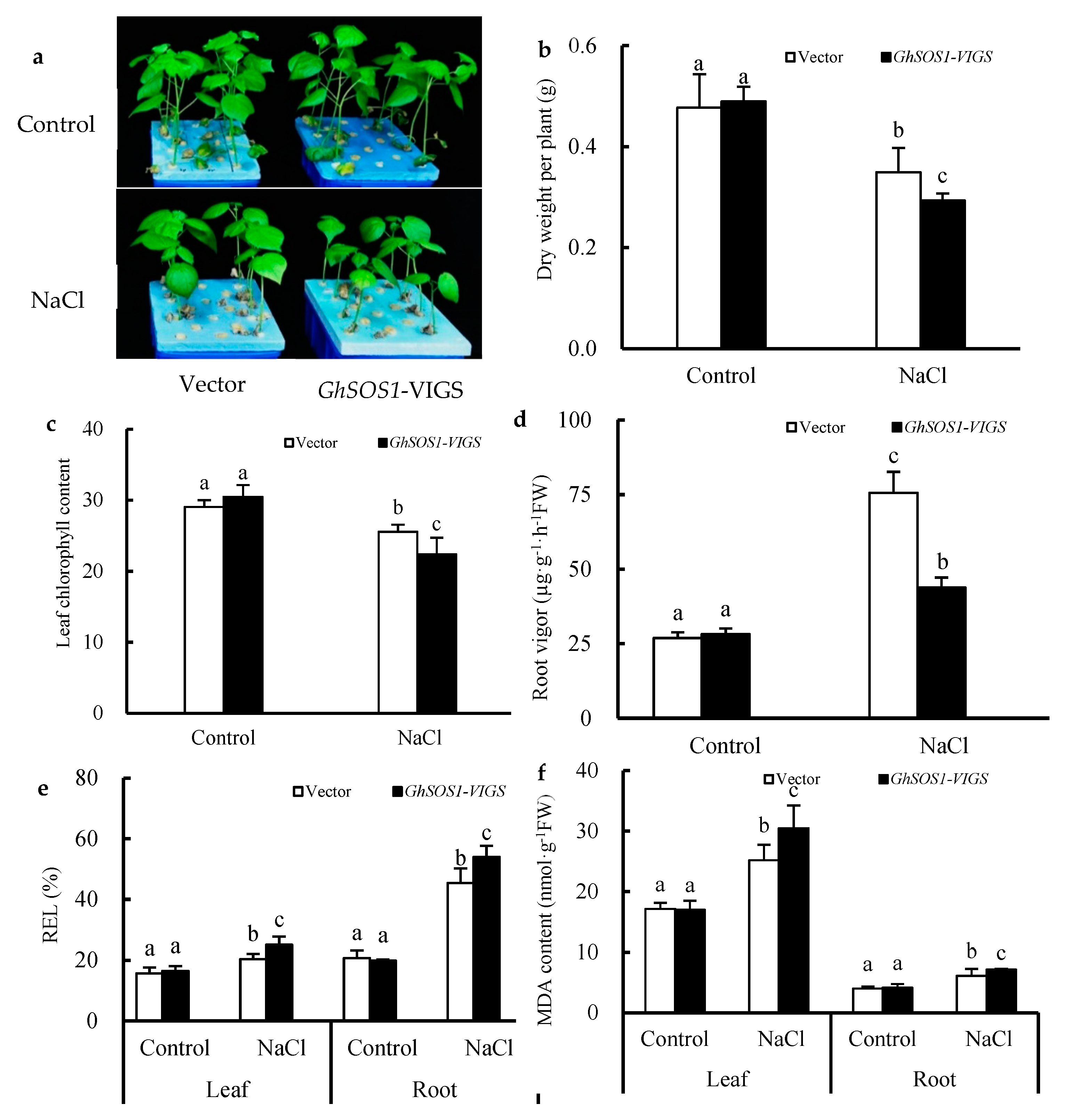
The leaves of cotton seedlings remained green when infected with the empty vector (Vector) for 10 days, while those infected with pTRV2-GhCLA1 (GhCLA1-VIGS) displayed a marked leaf-bleached phenotype in the true leaves (Figure S1a-left), in which, GhCLA1 expression was suppressed (Figure S1a-right). This result indicates that the VIGS technique was successfully established in the cotton seedlings and could be further applied to the functional analysis of the target genes GhSOS1 and TrSOS1 in this study. Under normal conditions, the cotton seedlings transformed with the empty vector or pTRV2-GhSOS1 (GhSOS1-VIGS) also grew well (Figure S1b-left), but GhSOS1 expression was only markedly downregulated in both the true leaves and roots of the latter, thus showing the effective silencing of the GhSOS1 gene (Figure S1b-right).
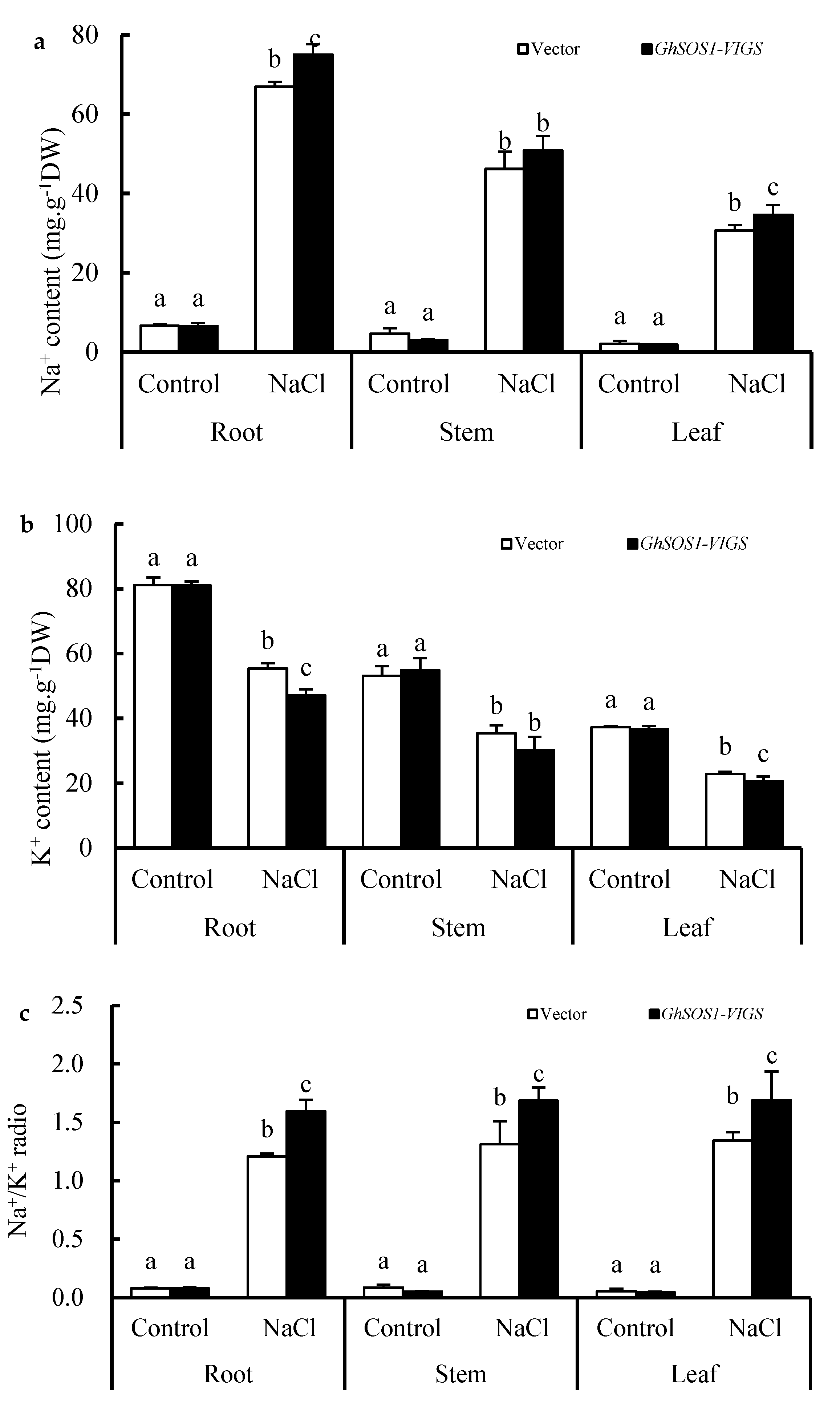
When the empty vector and GhSOS1-VIGS-transformed cotton plants were exposed to 200 mM NaCl for seven days, the growth of both was obviously inhibited, but the latter displayed smaller leaves, lower plant dry weight and leaf chlorophyll content, and a higher relative electrolytic leakage (REL) value and MDA content in the leaves and roots (Figure 1a–f), which suggests more severe salt injury or sensitivity to the GhSOS1-VIGS-transformed cotton plants than the empty-vector-transformed cotton plants. Unlike the changes mentioned above, the root vigor of both cotton plants increased significantly compared with the control, but the increase in the GhSOS1-VIGS plants was less than that in the empty-vector-transformed plants (p ≤ 0.05). In addition, under NaCl stress, the Na+ contents in the roots, stems, and leaves of the empty-vector- and GhSOS1-VIGS-transformed cotton plants were substantially increased, and the K+ content markedly decreased compared with that of the control (p ≤ 0.05). Accordingly, Na+/K+ ratios in the roots, stems, and leaves were substantially increased. Generally, the variations in the GhSOS1-VIGS-transformed plants were substantially larger than those in the empty-vector-transformed plants (Figure 2).
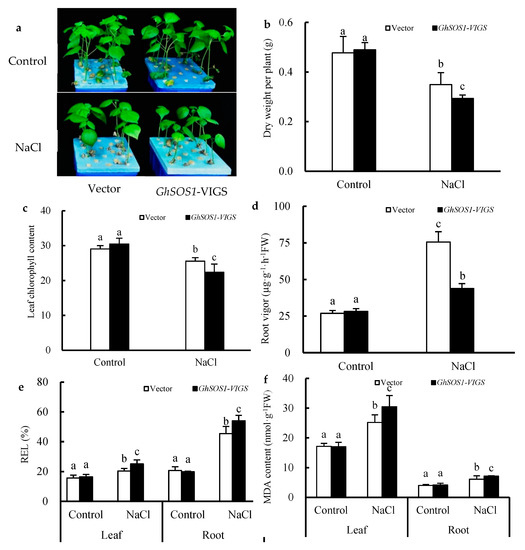
Figure 1.
Effects of salt treatment on the growth (a), dry weight per plant (b), leaf chlorophyll content (c), root vigor (d), relative electrolytic leakage (REL) value (e), and MDA content (f) in the leaves and roots of GhSOS1-VIGS (virus-induced gene silencing) cotton plants. Different lowercases in the same group indicate the significant differences (p ≤ 0.05).
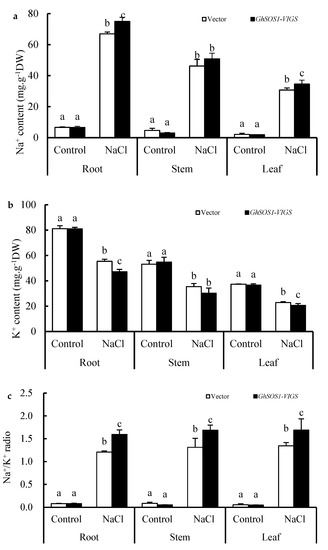
Figure 2.
Changes in contents of Na+ (a) and K+ (b), and Na+/K+ ratio (c) in the roots, stems, and leaves of the GhSOS1-VIGS cotton plants under salt stress. Different lowercases in the same group indicate the significant differences (p ≤ 0.05).
2.2. Effects of Salt Stress on the Growth and Related Physiological Parameters of TrSOS1-Transgenic Hairy Root Composite Cotton Plants Exhibiting VIGS of GhSOS1
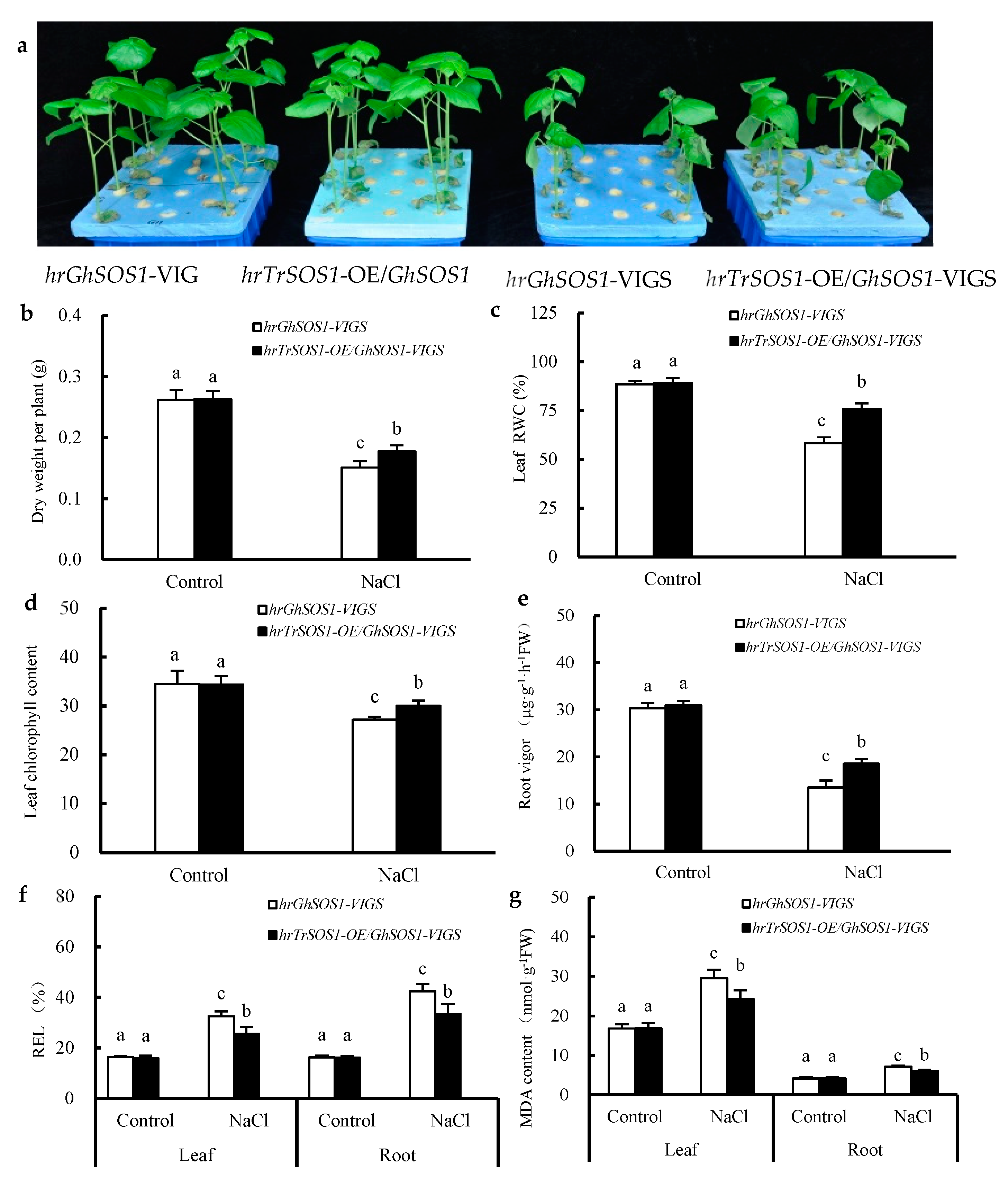
By PCR amplification and identification of DNA (Figure S2a), or RNA (Figure S2b) extracted from the roots and leaves of cotton plants, only the expression of TrSOS1 in the hairy roots and the evident suppression of GhSOS1 in the roots and leaves indicated that the TrSOS1-transgenic hairy root composite cotton plants exhibiting VIGS of GhSOS1 (named as hrTrSOS1-OE/GhSOS1-VIGS) were successfully constructed.
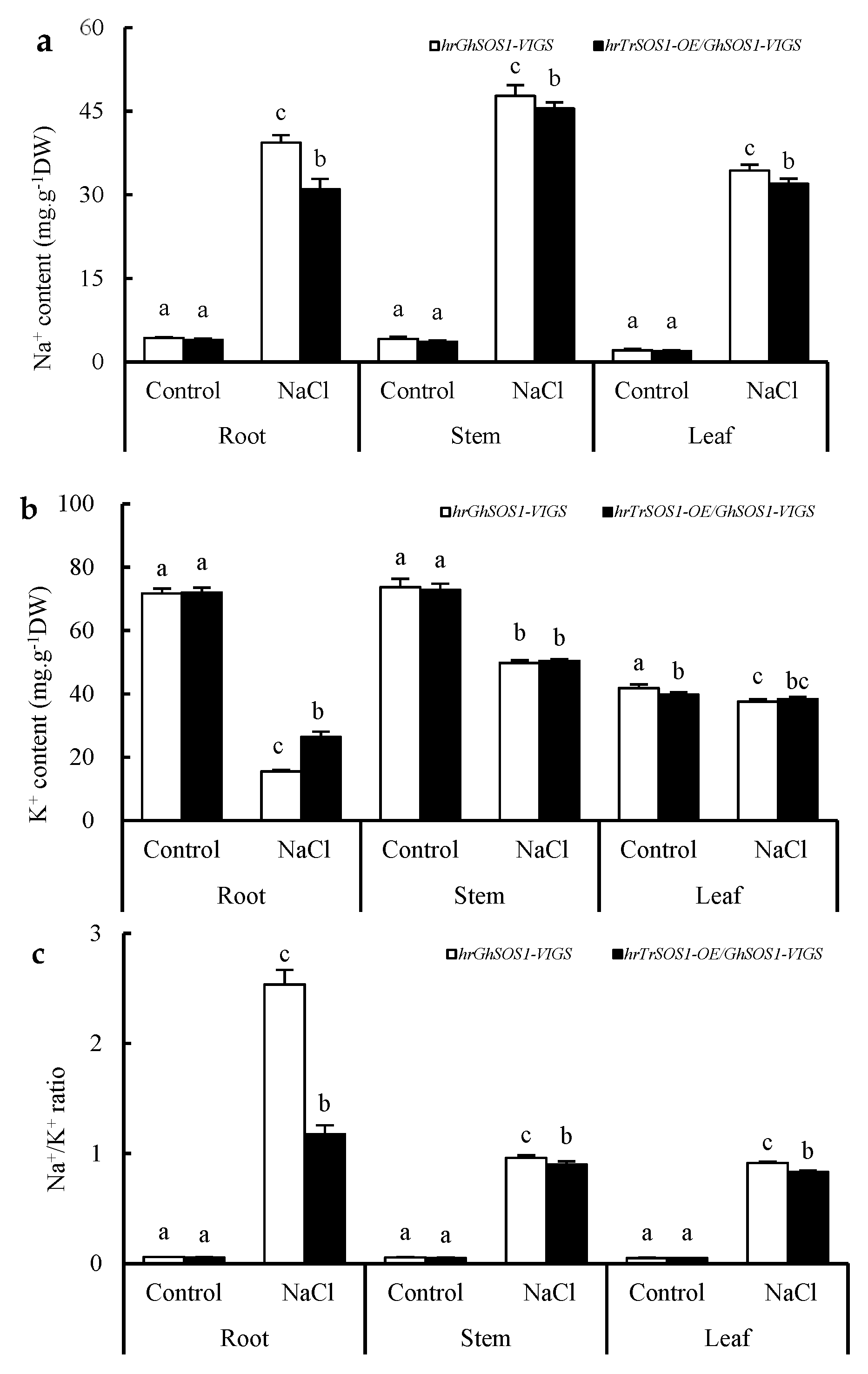
Under normal conditions, both the hrGhSOS1-VIGS and hrTrSOS1-OE/GhSOS1-VIGS cotton plants grew well without significant differences in plant dry weight, leaf relative water content (RWC) and chlorophyll content, root vigor, REL value, and MDA content in the roots and leaves (p ≥ 0.05) (Figure 3). When exposed to 200 mM NaCl for seven days, the growth of both was clearly reduced and partial true leaves lost water and wilted, but the hrTrSOS1-OE/GhSOS1-VIGS plants suffered relatively less salt damage (Figure 3a), displaying sufficient substitution and conferring enhanced salt tolerance of TrSOS1 to the cotton seedlings exhibiting GhSOS1-VIGS. In addition, plant dry weight, leaf RWC and chlorophyll content, and root vigor markedly decreased compared with those of the control, whereas the decline in the hrTrSOS1-OE/GhSOS1-VIGS plants was lower than that of GhSOS1-VIGS (Figure 3b–e). The REL value and MDA content in the leaves and roots of both plants observably increased compared with the those of the control, and yet the increase in the hrTrSOS1-OE/GhSOS1-VIGS plants was also smaller than that of the hrGhSOS1-VIGS plants (p ≤ 0.05) (Figure 3f,g). The Na+ content and Na+/K+ ratio in the roots, stems, and leaves of both salt-stressed cotton plants greatly increased compared with those of the control, and the K+ content in the roots, stems, and leaves visibly decreased. In particular, the increases in the Na+ content and Na+/K+ ratio in the hrTrSOS1-OE/GhSOS1-VIGS plants were clearly inferior to those of the hrGhSOS1-VIGS plants (p ≤ 0.05) (Figure 4).
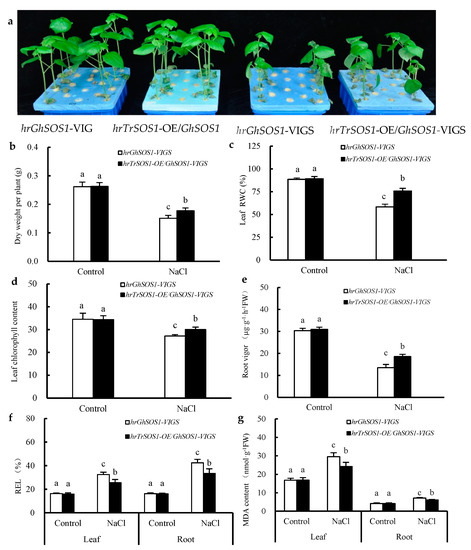
Figure 3.
Effects of salt treatment on growth phenotype (a), dry weight per plant (b), leaf relative water content (RWC) (c), leaf chlorophyll content (d), root vigor (e), REL (f), and MDA content (g) in the leaves and roots of hairy root composite cotton plants of hrGhSOS1-VIGS or hrTrSOS1-OE/GhSOS1-VIGS. Different lowercases in the same group indicate the significant differences (p ≤ 0.05).
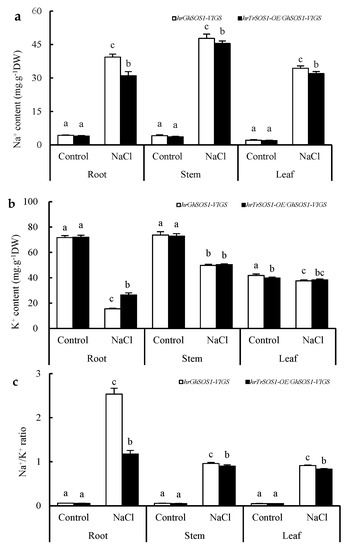
Figure 4.
Effect of salt stress on contents of Na+ (a), K+ (b), and Na+/K+ ratio (c) in the roots, stems, and leaves of hairy root composite cotton plants of hrGhSOS1-VIGS or hrTrSOS1-OE/GhSOS1-VIGS. Different lowercases in the same group indicate the significant differences (p ≤ 0.05).
2.3. Comparison of TrSOS1, GhSOS1, and AtSOS1 complementation in the yeast NHA1 mutant
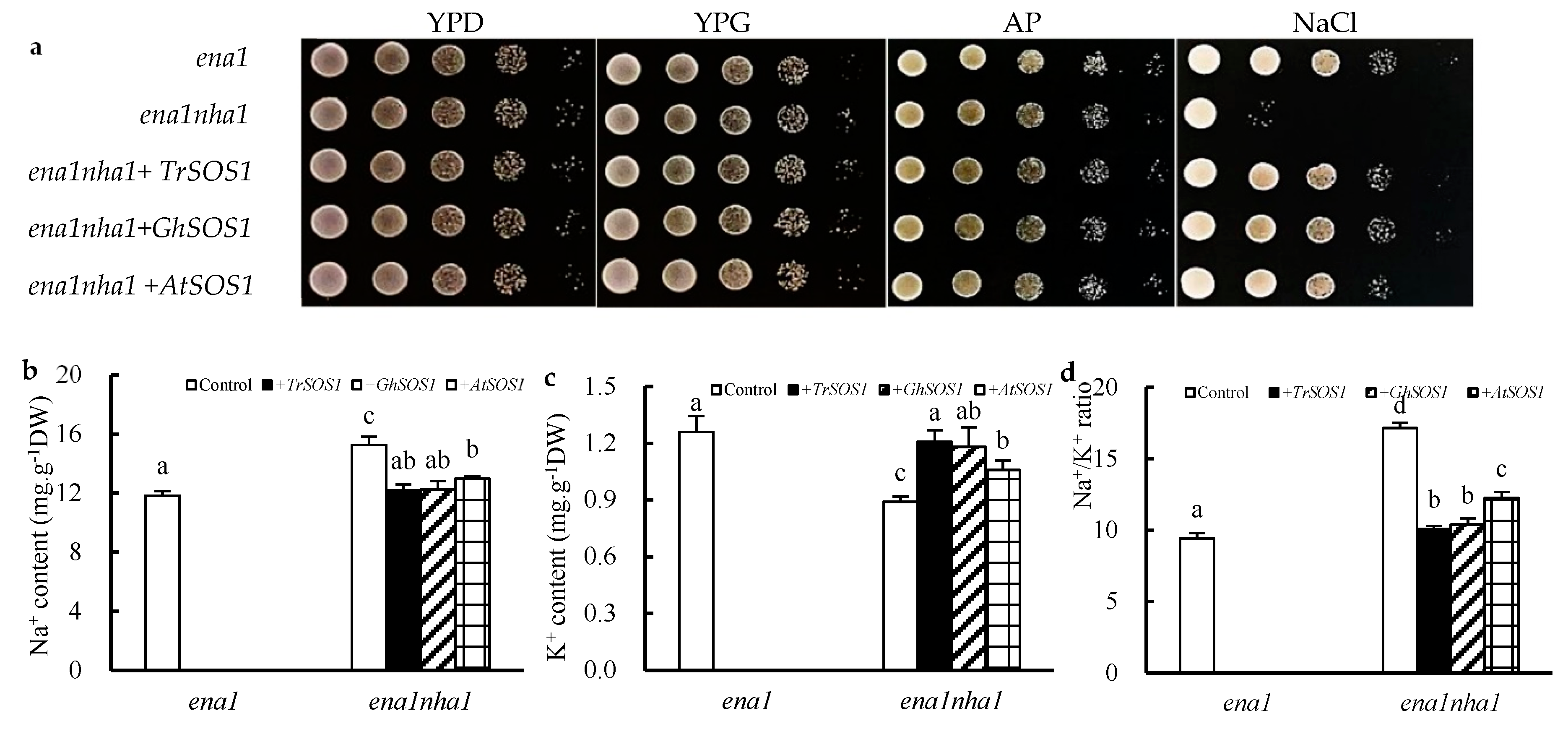
To compare the capacity of TrSOS1, GhSOS1, and AtSOS1 to substitute for the yeast NHA1 antiporter, mutant complementation tests were performed. All yeast cells, including the wild type G19 (ena1), the mutant ANT3 (ena1nha1), and the TrSOS1-, GhSOS1-, or AtSOS1-transformed mutants, grew well in YPD (1% yeast extract, 2% peptone, and 2% dextrose), YPG (1% yeast extract, 2% peptone, and 2% galactose), or AP (arginine–phosphate) medium. When exposed to NaCl stress, the growth of the mutant ena1nha1 was markedly inhibited but was restored by transformation with TrSOS1, GhSOS1, or AtSOS1; however, the recovery of those cells transformed with TrSOS1 or GhSOS1 appeared better than that of the cells transformed with AtSOS1 (Figure 5a). Under NaCl treatment, the cellular Na+ content in the mutant ena1nha1 increased significantly compared with that of the wild type (ena1), and the K+ content decreased. When TrSOS1, GhSOS1, or AtSOS1 was transformed, the increased Na+ content and decreased K+ level in each transformed yeast mutant were restored to the level of ena1; among them, the recovery of the TrSOS1 or GhSOS1 transformants was near the control level (p ≥ 0.05) and better than that of the AtSOS1 transformant. Accordingly, the Na+/K+ ratio in the transformed ena1nha1 cells substantially decreased to the wild type level though it was still significantly higher than the level in the control (p ≤ 0.05), but the recovery of the TrSOS1 or GhSOS1 transformants in the context of Na+/K+ ratio was still superior to that of the AtSOS1 transformant (p ≥ 0.05) (Figure 5b–d).
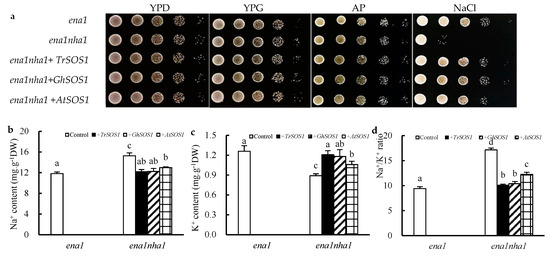
Figure 5.
Effects on the growth phenotype (a), contents of Na+ (b), K+ (c), and Na+/K+ ratio (d) of the yeast strains ena1, ena1nha1, and ena1nha1 complemented by TrSOS1, GhSOS1, and AtSOS1 under NaCl treatment. Different lowercases in the same group indicate the significant differences (p ≤ 0.05).
3. Discussion
There have been many studies on the salt tolerance function of the plant SOS1 gene through Agrobacterium tumefaciens-mediated overexpression or RNA interference (RNAi)-based suppression [17,18,20,22,33]. For the first time, a TRV-based VIGS technique for endogenous GhSOS1 gene silencing, together with the A. rhizogenes-mediated hairy root composite plant for target TrSOS1 gene overexpression, was simultaneously adopted and successfully constructed in this work (Figures S1 and S2). When compared to the empty-vector-transformed plants, the GhSOS1-VIGS-transformed cotton seedlings were more sensitive to salt stress, and displayed reduced growth, insufficient root vigor, and an increased Na+ content and Na+/K+ ratio in their roots, stems, and leaves (Figure 1 and Figure 2). As a glycophytic plant, cotton normally shows higher salt and drought tolerance than other major crops, and is often considered as a moderately salt-tolerant crop [17,24,28]. Our findings further indicate that, as in the case with the SOS1 gene in many other plants, the normal expression of GhSOS1 in the roots and leaves of seedlings is indispensable for the salt tolerance of cotton. Moreover, the overexpression of TrSOS1 from recretohalophyte Tamarix markedly reduced the salt sensitivity and enhanced the salt tolerance of the GhSOS1-VIGS cotton plants by maintaining higher root vigor and leaf RWC, and a lower Na+ content and Na+/K+ ratio in the roots, stems, and leaves (Figure 3 and Figure 4). This demonstrates the enhanced complementary function of TrSOS1-OE on the salt tolerance of the GhSOS1-VIGS cotton plants, and this is also somewhat similar to the complementary effect of the SOS1 gene from many other glycophytes and a few euhalophytes on the salt sensitivity of the A. thaliana sos1 mutant [18,23,34].
It is worth noting that the root vigor of both the salt-stressed empty-vector- and GhSOS1-VIGS- transformed cotton seedlings increased significantly compared with the control, but the rise of the GhSOS1-VIGS plants was less than that of the empty-vector-transformed plants (Figure 1d). However, the root vigor of both the salt-stressed hrGhSOS1-VIGS and hrTrSOS1-OE/GhSOS1-VIGS cotton plants markedly decreased compared with that of the control, whereas the decline in the hrTrSOS1-OE/GhSOS1-VIGS plants was lower than that of the hrGhSOS1-VIGS plants (Figure 3e). The roots of former group of cotton plants were the original roots, which showed a positive salt stress response ability and resulted in enhanced root vigor, while the roots of the latter group were newly grown hairy roots, which showed a weaker salt stress response ability and reduced root vigor. However, further comparison revealed that the root vigor of the GhSOS1-VIGS cotton seedlings in former group was lower than that of the empty-vector-transformed seedlings under salt stress, indicating a positive correlation between the lower root vigor of cotton plants and VIGS-mediated endogenous GhSOS1 suppression. In contrast, the root vigor of the hrTrSOS1-OE/GhSOS1-VIGS cotton plants in latter group was higher than that of the hrGhSOS1-VIGS plants under salt stress, also indicating a positive correlation between higher root vigor of cotton plants and exogenous TrSOS1 overexpression. Generally, the upregulation of the expression level of a functional gene, such as SOS1, is conducive to the activity enhancement of its encoded protein [35]. The expression level of the TrSOS1 gene in the roots and/or leaves of cotton plants, and even the transport activity of the SOS1 protein seem to be positively correlated with root vigor to a certain extent. However, the details remain to be further studied.
Under saline conditions, salt excretion was evidently observed on the surface of the aboveground part of three species of recretohalophyte Tamarix [29]. Peng et al. [25] reported that glandular trichomes (GTs) on the leaves of salt-treated cotton plants could secrete excess salt. With A. thaliana AtSOS1 as a reference, we found that the transformation of TrSOS1, GhSOS1 or AtSOS1 into the yeast mutant ANT3 (ena1nha1) reduced the cellular Na+ content and Na+/ K+ ratio, increased the K+ level under salt stress, and displayed good growth complementation effect in saline conditions. In particular, the complementation ability of TrSOS1 or GhSOS1 in the salt tolerance of the yeast mutant was evidently superior to that of AtSOS1, but the ability of TrSOS1 was basically equal to or slightly better than that of GhSOS1 (Figure 5). This suggests that the contribution of the SOS1 genes from the recretohalophyte Tamarix, salt-tolerant glycophyte cotton, and salt-sensitive glycophyte Arabidopsis to salt tolerance is TrSOS1 ≥ GhSOS1 > AtSOS1 by the yeast mutant complementation test, which may be another innovative discovery in this study. Oh et al. [20] reported that although both ThSOS1 and AtSOS1 from the halophyte T. salsuginea and glycophyte A. thaliana, respectively, could enhance the salt tolerance of the yeast mutant, the complementary effect of ThSOS1 was stronger than that of AtSOS1 under high salinity. Therefore, this study can indicate that the salt-tolerant function of the SOS1 gene of a certain kind of plant, may be related to its habitat or salt-tolerant habit, and the molecular evolutions of the SOS1 gene of halophytes, salt-tolerant and salt-sensitive glycophytes need further attention and study.
4. Materials and Methods
4.1. Plant Materials, Bacteria and Yeast Strains, and Plasmids
Plants including T. ramosissima, G. hirsutum cv. Xinluzao No. 51, and A. thaliana wild type (WT) (Columbia ecotype glabrous 1), Escherichia coli DH5α, A. tumefaciens strain GV3101, A. rhizogenes strain K599, Saccharomyces cerevisiae strain G19 (Δena1::HIS3::ena4, represented as ena1), and its plasma membrane antiporter NHA1 mutant ANT3 (Δena1::HIS3::ena4, Δnha1::LEU2, represented as ena1nha1), the plant transformation binary vector pSuper1300+, the pTRV1 and pTRV2 vectors and yeast expression vector plasmid pYES2 were used in this study.
4.2. TrSOS1, GhSOS1, and AtSOS1 Gene Cloning and Vector Construction
Seeds of T. ramosissima and G. hirsutum cv. Xinluzao No. 51 were sterilized and sown in pots containing a sterilized peat moss and vermiculite mixture and grown in a growth chamber under a 12 h light/12 h dark cycle at 28 ± 2 °C, with 60–70% relative humidity. In addition, seeds of A. thaliana WT were first chilled at 4 °C in the dark for two days, and sown and grown in a growth chamber under a 14 h light/10 h dark cycle at 20 ± 2 °C. Total RNA was extracted from the seedlings of the above plants using the MiniBEST Plant RNA Extraction Kit (TaKaRa, Dalian, China). First-strand cDNAs were synthesized with 2 µg total RNAs using a PrimeScriptTM II first-strand cDNA Synthesis Kit (TaKaRa) according to the manufacturer’s protocol. A 420 bp cDNA fragment corresponding to bases 994–1414 of the GhCLA1 gene (KJ123647) was amplified and the resulting PCR product was cloned into Kpn Ι-cut pTRV2 to obtain the recombinant plasmid pTRV2-GhCLA1. At the same time, a 459 bp cDNA fragment corresponding to bases 281–740 of the GhSOS1 gene (NM-001327028) was amplified and the resulting PCR product was cloned into Kpn Ι-cut pTRV2 to obtain the recombinant plasmid pTRV2-GhSOS1. The full-length coding region of TrSOS1 was amplified from cDNA as described in our previous study [29], and the resulting PCR product was ligated into the plasmid Sma Ι-cut pSuper+1300 to obtain the recombinant plasmid pSuper+1300-TrSOS1. Subsequently, the open reading frames of TrSOS1, GhSOS1, and AtSOS1 (AT2G01980) were amplified from cDNA. Then, the resulting PCR products were ligated into the EcoR Ι-cut pYES2 plasmid to obtain the recombinant plasmids pYES2-TrSOS1, pYES2-GhSOS1, and pYES2-AtSOS1. The primers used for the abovementioned gene amplification are listed in Table 1. After sequence verification, and by the freeze–thaw method [36], the recombinant plasmid pTRV2-GhCLA1 or pTRV2-GhSOS1 was transformed into A. tumefaciens GV3101, and the recombinant plasmid pSuper+1300-TrSOS1 was transformed into A. rhizogenes K599. The recombinant plasmids pYES2-TrSOS1, pYES2-GhSOS1, and pYES2-AtSOS1 were respectively transformed into the NHA1-deleted yeast mutant strain ena1nha1 using the PEG/LiAc procedure and transformants were identified by PCR-based methods [37].

Table 1.
Primers for gene analysis.
4.3. Construction of GhSOS1-VIGS Cotton Plants
GhSOS1-VIGS cotton plants were obtained according to the methods described by Gao et al. [38] using a 1:1 mixture of A. tumefaciens GV3101 containing pTRV1 and pTRV2-GhSOS1 which were pelleted and resuspended in infiltration culture containing 10 mM MgCl2, 10 mM MES, and 200 µM acetosyringone, and two fully expanded cotyledons of one-week-old plants were infiltrated using a needleless syringe. In the same way, the cotton plants infected with A. tumefaciens GV3101 containing pTRV1 and pTRV2 for 10 days were used as the empty-vector control, and the plants infected with A. tumefaciens GV3101 containing pTRV1 and pTRV2-GhCLA1 for 10 days were used as a parallel control (GhCLA1-VIGS), which showed the photo bleaching phenotype.
4.4. Construction of hrTrSOS1-OE/GhSOS1-VIGS Cotton Plants
Cotton plants were first infected with A. rhizogenes strain K599 containing pSuper+1300-TrSOS1 according to the methods of Wei et al. [31], using the transformation with the empty vector pSuper+1300 as a negative control. Total DNA isolated from the roots and leaves of the empty-vector- or TrSOS1-transformed plants was identified by the PCR-based method of An et al. [39]. Then, the empty-vector- or TrSOS1-transformed cotton plants were infected once with A. tumefaciens GV3101 containing pTRV1 and pTRV2-GhSOS1 for 10 days as indicated before to obtain hrGhSOS1-VIGS or hrTrSOS1-OE/GhSOS1-VIGS cotton plants.
4.5. Semi-Quantitative RT-PCR Analysis
For semi-quantitative RT-PCR analysis of GhCLA1 or GhSOS1 gene silencing, total RNA was isolated from the leaves of GhCLA1-VIGS cotton plants, the roots and leaves of GhSOS1-VIGS, hrGhSOS1-VIGS or hrTrSOS1-OE/GhSOS1-VIGS cotton plants, respectively, then the first-strand cDNAs were synthesized as indicated previously. The housekeeping gene GhActin9 was used as an internal control. The amplification program for this work was performed at 94 °C for 3 min, followed by 30 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 40 s, and a final extension of 72 °C for 10 min. The primers used for GhCLA1, GhSOS1, and GhActin9 are listed in Table 1.
4.6. Salt-Tolerance Tests of GhSOS1-VIGS or TrSOS1-OE/GhSOS1-VIGS Cotton Plants
The above-obtained vector and GhSOS1-VIGS, hrGhSOS1-VIGS and hrTrSOS1-OE/GhSOS1-VIGS cotton plants were treated with 1/2 Hoagland solution containing 0 or 200 mM NaCl for seven days. After being photographed, the plants were fully rinsed in distilled water, fixed at 105 °C for 10 min, and dried to a constant weight at 80 °C to measure the dry weight per plant. Leaf chlorophyll content (SPAD value) was measured with a chlorophyll meter (SPAD-502PLUS, Konica Minolta Holdings, Inc., Tokyo, Japan), and leaf relative water content (RWC) was determined using the following formula: RWC = (FW − DW)/(TW − DW) × 100%, and relative electrolytic leakage (REL) in roots and leaves was calculated as: (C1 − Cw)/(C2 − Cw) × 100%, both as described by Tian et al. [40]. The malondialdehyde (MDA) contents in the leaves and roots were measured according to the method described by Jouve et al. [41], and the amount of MDA was calculated from the following formula: C = 6.45(A532 − A600) − 0.56A450. Na+ and K+ contents in the roots, stems, and leaves of cotton plants were measured according to the method of Chen et al. [42], using a flame spectrophotometer (AP1200 type, Shanghai, China).
4.7. Complementation Test of TrSOS1, GhSOS1, and AtSOS1 in Yeast Mutant
According to our previous methods [19] with minor modifications, ten-fold serial dilutions (starting at OD550 ≈ 0.5) of each yeast culture, including G19 (ena1) as a positive control, and the mutants ANT3 (ena1nha1), ANT3-TrSOS1 (ena1nha1+ TrSOS1), ANT3-GhSOS1 (ena1nha1+GhSOS1), and ANT3-AtSOS1 (ena1nha1+ AtSOS1), were plated on YPD (1% yeast extract, 2% peptone, and 2% dextrose) medium, YPG (1% yeast extract, 2% peptone, and 2% galactose) medium, or arginine–phosphate medium (AP medium: 10 mM L-arginine, 8 mM H3PO4, 2 mM MgSO4, 0.2 mM CaCl2, 2% glucose, vitamins, and trace elements, pH 6.5) plus NaCl (130 mM) [43] and KCl (1 mM), and the plates were incubated at 30 °C for three days and then photographed. For Na+ and K+ content measurements, the yeast cells were grown in liquid AP medium plus NaCl (130 mM) and KCl (1 mM) and collected during the exponential growth phase (OD550 ≈ 0.2). The Na+ and K+ contents in the yeast cells were determined using a flame spectrophotometer as described above.
4.8. Statistical Analyses
Data were expressed as the mean ± SD for each treatment (n = 3, except for the measurement of dry weight per plant, where n = 6, semi-quantitative RT-PCR for GhSOS1 expression in the roots and leaves of cotton plants, where n = 5) using SPSS software (ver. 20.0., International Business Machines Corporation, Armonk, NY, USA), and differences among means were determined by Duncan’s test at p ≤ 0.05.
5. Conclusions
The recretohalophyte Tamarix TrSOS1 gene conferred enhanced salt tolerance to transgenic hairy root composite cotton seedlings exhibiting GhSOS1-VIGS by maintaining enhanced root vigor and leaf RWC and a reduced Na+ content and Na+/K+ ratio in the roots, stems, and leaves. A yeast mutant complementation test elucidated the contribution of SOS1 genes from the recretohalophyte Tamarix salt-tolerant glycophyte cotton, and salt-sensitive glycophyte Arabidopsis to salt tolerance: TrSOS1 ≥ GhSOS1 > AtSOS1. Therefore, the contribution of the recretohalophyte Tamarix TrSOS1 gene to salt tolerance is very similar to that of other plants’ SOS1 gene: The direct contribution is the participation in the enhancement of Na+ extrusion in plants (mainly through roots, and possibly through leaves) under salt stress and the regulation of Na+ and K+ homeostasis, while the indirect contribution is the improvement of root vigor, reduction in the cell membrane damage in the roots and leaves, and thus the promotion of plant growth. Moreover, this study effectively indicates that the salt-tolerant function of the SOS1 gene of a certain kind of plant may be related to its habitat or salt-tolerant habit. This finding suggests that the SOS1 gene of halophytes or salt-tolerant crops should be given preference for utilization in the germplasm innovation and genetic improvement of salt-tolerant plants or crops by genetic engineering in the future.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/12/2930/s1.
Author Contributions
B.C., C.C., and J.F. conducted the experiments, collected, and analyzed all data. B.Y. and L.J. designed the experiments. B.Y., B.C., C.C., and Y.L. interpreted the data and wrote the manuscript. All authors read and approved the final version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (U1603111, 31671604), and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX18_0746).
Acknowledgments
We are deeply indebted to the entire research team for their valuable effort during the period of this research work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Volkov, V. Salinity tolerance in plants. Quantitative approach to ion transport starting from halophytes and stepping to genetic and protein engineering for manipulating ion fluxes. Front. Plant Sci. 2015, 6, 873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Wan, Q.; He, X.L.; Ning, L.H.; Huang, Y.H.; Xu, Z.L.; Liu, J.; Shao, H.B. Genome-wide characterization of the ankyrin repeats gene family under salt stress in soybean. Sci. Total Environ. 2016, 568, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Choi, J.; An, G.; Kim, S.R. Ectopic expression of OsSta2 enhances salt stress tolerance in rice. Front. Plant Sci. 2017, 8, 316. [Google Scholar] [CrossRef] [PubMed]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Adem, G.D.; Roy, S.J.; Zhou, M.X.; Bowman, J.P.; Shabala, S. Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol. 2014, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.N.; Zhou, Q.; Yu, B.J. Effects of Zn2+ and niflumic acid on photosynthesis in Glycine soja and Glycine max seedlings under NaCl stress. Environ. Exp. Bot. 2009, 65, 304–309. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Shi, H.Z.; Ishitani, M.; Kim, C.S.; Zhu, J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef]
- Yu, B.J.; Liu, Y.L. SOS genes family and plant salt tolerance. Plant Physiol. Commun. 2004, 4, 409–413. [Google Scholar]
- Bartels, D.; Dinakar, C. Balancing salinity stress responses in halophytes and non-halophytes: A comparison between Thellungiella and Arabidopsis thaliana. Funct. Plant Biol. 2013, 40, 819–831. [Google Scholar] [CrossRef]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, I.; Iqbal, M.; Hakvoort, H.W.J.; Bliek, M.; de Boer, B.; Schat, H. Expression levels and promoter activities of candidate salt tolerance genes in halophyte and glycophytic Brassicaceae. Environ. Exp. Bot. 2014, 99, 59–66. [Google Scholar] [CrossRef]
- Razzaque, S.; Elias, S.M.; Biswas, S.; Haque, T.; Seraj, Z.I. Cloning of the plasma membrane sodium/hydrogen antiporter SOS1 for its over expression in rice. Plant Tissue Cult. Biotechnol. 2013, 23, 263–273. [Google Scholar] [CrossRef]
- Olías, R.; Eljakaoui, Z.; Li, J.; De Morales, P.A.; Marı’n-Manzano, M.C.; Pardo, J.M.; Belver, A. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ. 2009, 32, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Sun, J.; Cao, P.P.; Ren, L.P.; Liu, C.; Chen, S.M.; Chen, F.D.; Jiang, J.F. Variation in tissue Na+ content and the activity of SOS1 genes among two species and two related genera of Chrysanthemum. BMC Plant Biol. 2016, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.G.; Lu, X.K.; Shu, N.; Wang, D.L.; Wang, S.; Wang, J.J.; Guo, L.X.; Guo, X.N.; Fan, W.L.; Lin, Z.X.; et al. GhSOS1, a plasma membrane Na+/H+ antiporter gene from upland cotton, enhances salt tolerance in transgenic Arabidopsis thaliana. PLoS ONE 2017, 12, e0181450. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.X.; Xu, L.; Yu, B.J. A putative soybean GmsSOS1 confers enhanced salt tolerance to transgenic Arabidopsis sos1-1 mutant. Protoplasma 2015, 252, 127–134. [Google Scholar] [CrossRef]
- Zhao, X.F.; Wei, P.P.; Liu, Z.; Yu, B.J.; Shi, H.Z. Soybean Na+/H+ antiporter GmsSOS1 enhances antioxidant enzyme activity and reduces Na+ accumulation in Arabidopsis and yeast cells under salt stress. Acta Physiol. Plant. 2017, 39, 19. [Google Scholar] [CrossRef]
- Oh, D.H.; Leidi, E.; Zhang, Q.; Hwang, S.M.; Li, Y.; Quintero, F.J.; Jiang, X.Y.; D’Urzo, M.P.; Lee, S.Y.; Zhao, Y.X.; et al. Loss of halophytism by interference with SOS1 expression. Plant Physiol. 2009, 151, 210–222. [Google Scholar] [CrossRef]
- Maughan, P.J.; Turner, T.B.; Coleman, C.E.; Elzinga, D.B.; Jellen, E.N.; Morales, J.A.; Udall, J.A.; Fairbanks, D.J.; Bonifacio, A. Characterization of Salt Overly Sensitive 1 (SOS1) gene homologous in quinoa (Chenopodium quinoa Willd.). Genome 2009, 52, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.S.; Shukla, P.S.; Jha, A.; Agarwal, P.K.; Jha, B. The SbSOS1 gene from the extreme halophyte Salicornia brachiata enhances Na+ loading in xylem and confers salt tolerance in transgenic tobacco. BMC Plant Biol. 2012, 12, 188. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, X.C.; Duan, R.J.; Hu, Y.P.; Xie, Q.; Li, R.M.; Zhu, B.B.; Fu, S.P.; Guo, J.C.; Jiang, X.Y. The Sesuvium portulacastrum Plasma Membrane Na+/H+ Antiporter SpSOS1 Complemented the Salt Sensitivity of Transgenic Arabidopsis sos1 Mutant Plants. Plant Mol. Biol. Rep. 2018, 36, 553–563. [Google Scholar] [CrossRef]
- Abid, M.A.; Liang, C.; Malik, W.; Meng, Z.G.; Tao, Z.; Meng, Z.G.; Ashraf, J.; Guo, S.D.; Zhang, R. Cascades of ionic and molecular networks involved in expression of genes underpin salinity tolerance in cotton. J. Plant Growth Regul. 2018, 37, 668–679. [Google Scholar] [CrossRef]
- Peng, Z.; He, S.P.; Sun, J.L.; Pan, Z.E.; Gong, W.F.; Lu, Y.L.; Du, X.M. Na+ compartmentalization related to salinity stress tolerance in upland cotton (Gossypium hirsutum) seedlings. Sci. Rep. 2016, 6, 34548. [Google Scholar] [CrossRef] [PubMed]
- Leidi, E.O.; Saiz, J. Is salinity tolerance related to Na accumulation in upland cotton (Gossypium hirsutum) seedlings? Plant and Soil 1997, 190, 67–75. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, G.Z.; Du, L.; Shang, X.G.; Cheng, C.Z.; Yang, B.; Hu, Y.; Cai, C.P.; Guo, W.Z. Genetic regulation of salt stress tolerance revealed by RNA-Seq in cotton diploid wild species, Gossypium davidsonii. Sci. Rep. 2016, 6, 20682. [Google Scholar] [CrossRef]
- Wang, N.; Qiao, W.Q.; Liu, X.H.; Shi, J.B.; Xu, Q.H.; Zhou, H.; Yan, G.T.; Huang, Q. Relative contribution of Na+/H+, homeostasis, photochemical efficiency and antioxidant defense system to differential salt tolerance in cotton (Gossypium hirsutum L.) cultivars. Plant Physiol. Biochem. 2017, 119, 121–131. [Google Scholar] [CrossRef]
- Liu, Y.M.; Cheng, C.; Jiang, L.; Yu, B.J. Comparisons on growth, salt ion distribution, and relative expression of SOS1 gene in three species of Tamarix Linn. under NaCl stress. J. Plant Resour. Environ. 2019, 28, 1–9. [Google Scholar]
- Cao, D.; Hou, W.S.; Song, S.K.; Sun, H.B.; Wu, C.X.; Gao, Y.S.; Han, T.F. Assessment of conditions affecting Agrobacterium rhizogenes-mediated transformation of soybean. Plant Cell Tissue Organ Cult. 2009, 96, 45–52. [Google Scholar] [CrossRef]
- Wei, P.P.; Wang, L.C.; Liu, A.L.; Yu, B.J.; Lam, H.M. GmCLC1 confers enhanced salt tolerance through regulating chloride accumulation in soybean. Front. Plant Sci. 2016, 7, 1082. [Google Scholar] [CrossRef] [PubMed]
- Burch-Smith, T.M.; Schiff, M.; Liu, Y.L.; Dinesh-Kumar, S.P. Efficient virus-induced gene silencing in Arabidopsis. Plant Physiol. 2006, 142, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Lee, B.H.; Wu, S.J.; Zhu, J.K. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat. Biotechnol. 2003, 21, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Feki, K.; Quintero, F.J.; Khoudi, H.; Leidi, E.O.; Masmoudi, K.; Pardo, J.M.; Brini, F. A constitutively active form of a durum wheat Na+/H+ antiporter SOS1 confers high salt tolerance to transgenic Arabidopsis. Plant Cell Rep. 2014, 33, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.; Glazebrook, J. Transformation of Agrobacterium Using the Freeze-Thaw Method. Cold Spring Harb Protoc. 2006, 7, 1031–1036. [Google Scholar] [CrossRef]
- Gietz, R.D. Yeast Transformation by the LiAc/SS Carrier DNA/PEG Method. Methods Mol. Biol. 2014, 1163, 33–44. [Google Scholar] [CrossRef]
- Gao, X.Q.; Wheeler, T.; Li, Z.H.; Kenerley, C.M.; He, P.; Shan, L.B. Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt. Plant J. 2011, 66, 293–305. [Google Scholar] [CrossRef]
- An, J.; Hu, Z.M.; Che, B.N.; Chen, H.Y.; Yu, B.J.; Cai, W.M. Heterologous expression of Panax ginseng PgTIP1 confers enhanced salt tolerance of soybean cotyledon hairy roots, composite, and whole plants. Front. Plant Sci. 2017, 8, 1232. [Google Scholar] [CrossRef]
- Tian, F.; Jia, T.J.; Yu, B.J. Physiological regulation of seed soaking with soybean isoflavones on drought tolerance of Glycine max and Glycine soja. Plant Growth Regul. 2014, 74, 229–237. [Google Scholar] [CrossRef]
- Jouve, L.; Jacques, D.; Douglas, G.C.; Hoffmanna, L.; Hausman, J.F. Biochemical characterization of early and late bud flushing in common ash (Fraxinus excelsior L.). Plant Sci. 2007, 172, 962–969. [Google Scholar] [CrossRef]
- Chen, X.Q.; Yu, B.J. Ionic effects of Na+ and Cl− on photosynthesis in Glycine max seedlings under isoosmotic salt stress. J. Plant Physiol. Mol. Biol. 2007, 33, 294–300. [Google Scholar]
- Li, Y.; Takano, T.; Liu, S.K. Discovery and Characterization of Two Novel Salt-Tolerance Genes in Puccinellia tenuiflora. Int. J. Mol. Sci. 2014, 15, 16469–16483. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).