Abstract
Time to flower, a process either referring to juvenile–adult phase change or vegetative–reproductive transition, is strictly controlled by an intricate regulatory network involving at least both FT/TFL1 and the micro RNA (miR)156-regulated SPL family members. Despite substantial progresses recently achieved in Arabidopsis and other plant species, information regarding the involvement of these genes during orchid development and flowering competence is still limited. Dendrobium catenatum, a popular orchid species, exhibits a juvenile phase of at least three years. Here, through whole-genome mining and whole-family expression profiling, we analyzed the homologous genes of FT/TFL1, miR156, and SPL with special reference to the developmental stages. The FT/TFL1 family contains nine members; among them, DcHd3b transcribes abundantly in young and juvenile tissues but not in adult, contrasting with the low levels of others. We also found that mature miR156, encoded by a single locus, accumulated in large quantity in protocorms and declined by seedling development, coincident with an increase in transcripts of three of its targeted SPL members, namely DcSPL14, DcSPL7, and DcSPL18. Moreover, among the seven predicted miR156-targeted SPLs, only DcSPL3 was significantly expressed in adult plants and was associated with plant maturation. Our results might suggest that the juvenile phase change or maturation in this orchid plant likely involves both the repressive action of a TFL1-like pathway and the promotive effect from an SPL3-mediated mechanism.
1. Introduction
Flowering, the reproductive process of higher plants, is an important trait vital to agriculture practice. The timing of flower initiation is determined by measuring the day-length (photoperiod) and/or low temperature (vernalization) in many annual plants, including the model plants Arabidopsis and rice [1]. These annual plants may acquire competence to flower shortly after seed germination. However, perennials, especially woody trees, take longer periods, lasting from a few weeks to many years for vegetative growth in order to achieve flowering, even under favorable conditions, a prolonged stage called juvenile phase [1,2].
The Arabidopsis flowering locus T (FT) [3] and its rice ortholog Hd3a [4] induce flowering in a photoperiod-dependent manner, whereas their homolog terminal flower 1 (TFL1) [5] from the same phosphatidylethanolamine-binding protein (PEBP) gene family delays flowering. These proteins are mainly expressed in leaves and move to the shoot apices to activate or repress floral bud development in response to environmental and developmental cues including day-length, cold signals, and maturity status; hence, the molecular identity as the long-sought florigen and anti-florigen is assigned [6,7,8,9]. The FT/TFL1-mediated photoperiod flowering pathway is widespread in angiosperm species [6,10], and homologous members of the family also exist in gymnosperms where they seem to be involved in regulation of growth rhythm, bud dormancy, and reproductive development process [10]. The FT/TFL1 family can be divided into three subfamilies: FT-like, TFL1-like, and MFT-like. The MFT (mother of FT and TFL1) homologs were identified in genomes of some bryophytes, which lack the other FT/TFL1-like genes [11]. MFT-like genes, together with mixed FT/TFL1-genes, also present in gymnosperm species [11,12]. It is, therefore, suggested that MFT-like genes are ancestral to both FT-like and TFL1-like genes and that the separated functions of FT and TFL1 were evolutionarily attained after divergence of gymnosperms and angiosperms (see Reference [6] for a review). Works in Arabidopsis reveal that, at the shoot apex, the mobile FT forms a complex with both a 14-3-3 protein (a G-box containing factor) [13] and a bZIP transcription factor FD which is apex-specific. This FT/FD/14-3-3 complex activates several MADS box genes or floral meristem identity genes, including Apetala 1 (AP1), Fruitfull (FUL), suppressor of overexpression of Constans 1 (SOC1) and the plant-specific transcription factor Leafy (LFY) to initiate vegetative to floral transition [8,14,15,16]. The antagonistic TFL1 proteins also interact competitively with FD and 14-3-3 protein to repress downstream genes for flowering [13,17], although this opposing mechanism in controlling flowering time is still not fully explained [9,10,18,19].
In parallel to and independent of the FT/TFL1 pathway, several of the miRNA156-regulated squamosa promoter binding protein-like (SPL) factors that specifically expressed at the shoot apex also act upstream of some of these MADS-box-containing floral regulators, albeit in an age-dependent manner [20,21,22]. MiR156 belongs to a group of microRNAs (miRNAs) that function as negative regulators for expression of protein-coding genes, by transcript cleavage and translational inhibition upon base-pairing to their targeted messenger RNAs (mRNAs) [23,24,25]. MicroRNAs are short non-coding eukaryotic RNAs, typically 20–24 nt, which are derived from pri-miRNAs through the action of the conserved small RNA machinery [26]. In Arabidopsis, mature miR156 may be produced from eight precursor genes, namely miR156A through miR156H. By targeting the expression of several SPL genes, miR156 plays pivotal roles in vegetative phase change and shoot development as first demonstrated in Arabidopsis, e.g., overexpression of miR156 led to a prolonged juvenile to adult phase [27,28,29].
An age-associated reduction in the abundance of miR156 and the resultant increased levels in its target SPL mRNAs are necessary to induce vegetative phase change and flowering in Arabidopsis, maize, perennial A. alpina, and Brassicaceae species [21,22,27,28,30]. Among the ten SPL genes that are targeted by miR156 in Arabidopsis, SPL9/SPL15 and SPL13 are more relevant to both the juvenile-to-adult vegetative transition and shoot growth, while SPL3/SPL4/SPL5 do not play a major role in vegetative phase change or floral induction, but do promote the floral meristem identity transition through synergistic action with the FT–FD module under long days [31,32]. Expression analysis and transgenic plants for gain-of-function or loss-of-function studies revealed that AtSPL9 promotes flowering by activating floral meristem identity gene AP1, whereas AtSPL15 is responsible for floral induction in short days through activation of the MADS-box gene FUL and another microRNA gene miR172b, which targets and inhibits several flowering repressor genes such as AP2, Schlafmutze (SMZ), Schnarchzapfen (SNZ), target of EAT1 (TOE1), TOE2, and TOE3 [28,33]. Both SPL9 and SPL15 interact with GA signaling protein DELLA and converge with the GA-mediated flowering pathways [34,35,36,37]. Thus, the miR156/SPLs module in Arabidopsis represents the endogenous flowering control axis. Nevertheless, fewer studies addressed the molecular pathways controlling flowering induction and competence to flower in most perennials.
Orchids are important horticultural plants with high commercial value. Many orchids have long life cycles, as well as characteristic slow-growing habits, and take more than two years to achieve flowering [38]. Recently, homologs of FT and of other floral meristem identity genes were cloned and functionally studied in Dendrobium Chao Praya Smile [39], Oncidium Gower Ramsey [40], and other orchids [41]. In these studies, FT homologs were shown to correlate with flower organ development and accelerated flowering when overexpressed in Arabidopsis [40] and Chao Praya Smile [39]. However, whether these FTs have a role in regulation of flowering time per se is still elusive, and information regarding the involvement of miR156/SPL in these unique species is lacking. Here, we took advantage of a currently available version of the Dendrobium catenatum genome sequence [42] and utilized a genome-mining approach to identify members of both the FT/TFL1 and the miR156/SPLs gene families. The genome of D. catenatum encodes nine FT/TFL1 members and 12 SPL genes, where seven of the latter are predicted targets of miR156. A BLAST search of the whole-genome sequences resulted in only a single locus of the putative miR156 precursor gene. We further determined the expression profiles of these genes in the three developmental stages: protocorms, one-year-old young (juvenile) plants, and three-year-old (adult) plants. We found in this orchid that a TFL1-like gene, DcHd3b, is highly expressed in the seedling and juvenile phase, while the DcSPL3 is predominant in the adult phase.
2. Results
2.1. Sequence Analysis of FT/TFL1 and SPL Family Members in D. catenatum
To identify genes belonging to the FT/TFL1 family, we used the AtFT and AtTFL1 sequences to BLAST against the genome of D. catenatum and ended up with nine homologous loci, which were annotated in the database either as heading date 3A-like or FT-like genes. The deduced amino-acid sequences showed well-conserved PEBP (phosphatidylethanolamine-binding protein) signature motifs and could be assigned within the three subfamilies: FT-like, TFL1-like, and MFT-like with those from Arabidopsis, rice, and two other orchid species, Phalaenopsis equestris and Apostasia shenzhenica (Figure 1a). DcHd3a is the closest ortholog to the founder florigens, OsHd3a and AtFT, with overall amino-acid identities of 78% and 71%, respectively, and it shares 90.91%, 59.88%, and 95.45% identity with the reported OnFT, OnTFL1 [40], and DoFT [39], respectively. DcHd3a also contains the highly conserved amino-acid residues in fragment B, which forms the external loop in the conserved PEBP protein structures as revealed among other inducer FTs [7,9]. Another member, DcHd3b, shares 70% amino-acid identity with DcHd3a. Alignment of these Dendrobium sequences with known-function FT/TFL1 orthologous proteins from Arabidopsis and rice showed that the four critical amino acids at the positions of Tyr85, Tyr134, Trp138, and Gln140 (numbering according to AtFT sequence) were roughly conserved among Dendrobium sequences (Figure 1b). These four residues confer either an inducer or repressor activity of FT/TFL1 [6,7,43]. Similar to the situations in Arabidopsis and rice, DcMFT is somehow deviated and separated from the other members of the family (Figure 1a,b). In terms of family size in gnomons, the three orchids, D. catenatum, P. equestris, and A. shenzhenica, contain the same number of FT/TFL1 genes (Figure 1c).
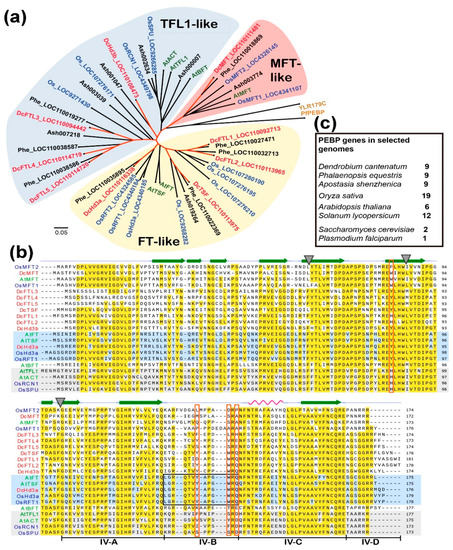
Figure 1.
The nine FT/TFL1 proteins encoded by the phosphatidylethanolamine-binding protein (PEBP) gene family in the Dendrobium cantenatum genome. (a) A phylogenetic tree of FT proteins from D. cantenatum (highlighted in red), Arabidopsis thaliana (highlighted in green), and other species. Only selected FTs from Oryza sativa (Os, in blue), Phalaenopsis equestris (Phe), and Apostasia shenzhenica (Ash) are included; (b) sequence alignment for motif comparisons. The secondary structures are indicated at top of the alignment; the conserved exon boundaries are marked by triangles. The four segments (A, B, C, and D) of the fourth exon important for ligand binding and protein–protein interaction in FT/TFL1, as revealed by Ahn et al., 2006, are line-labeled under the sequences. Known activator FTs and repressor TFL1-like proteins are shaded with light-blue and light-gray, respectively. Red-boxed residues correspond to positions at 85, 134, 138, and 140 for AtFT, and the black-boxed residues denote the highly conserved fragment B in inducer FTs but divergent in the repressor TFLs [7,43]; (c) numbers of PEBP genes identified by BLAST survey in some plant species and the single-cell eukaryotes.
A homology search with Arabidopsis and tomato sequences identified 12 SPL genes (annotated as SPL1, 3, 7, 8, 9, 10, 12, 14, 15, 16, 18, and 19, by the genome project) and one precursor gene of miR156 in D. catenatum (Figure 2a,b). We grouped the proteins together with those from Arabidopsis and tomato into six clades using alignment with full-length amino-acid sequence, rather than with their nucleotide sequences [44]. The predicted miR156-targeted SPLs could be placed within the IV, V, and VI clades (Figure 2a), suggesting a functional similarity at the protein level. Pairing with the 20-nt sequence of miR156 with its seven target SPL transcripts, as predicted by the online tool psRNATarget, http://plantgrn.noble.org/psRNATarget/, revealed only a mismatch in six mRNAs except DcSPL3, in which the target sequence exhibited three mismatches, suggesting a repression either by transcription cleavage or translation inhibition, or both (Figure 2c).
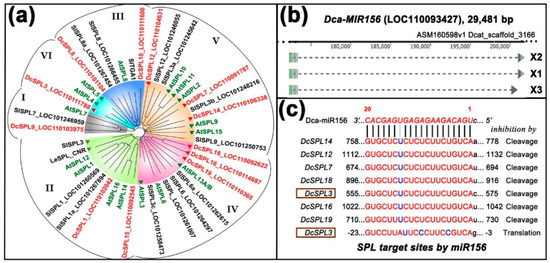
Figure 2.
Twelve SPL genes and one microRNA (miR)156 gene are found in Dendrobium catenatum. (a) A phylogenetic tree of SPL proteins from D. catenatum, A. thaliana, and S. lycopersicum. The genome of D. catenatum encodes 12 SPLs (highlighted in red), seven of which are predicted targets of miR156 (marked with red triangles). Arabidopsis contains 16 SPL proteins (highlighted in green), 10 of which are targeted by miR156 (with green triangles). The tomato genome has more SPL-coding genes than listed here, two of which were experimentally shown to be regulated by miR156 (gray triangles); (b) a single locus was identified in the genome which transcribes into three precursor non-coding RNA (ncRNA) variants. Broken lines denote introns, and light-green boxes denote exons; (c) miR156-targeted sites in messenger RNAs (mRNAs) of SPLs. Note that the DcSPL3 is possibly inhibited by mRNA cleavage and/or translational blockage.
In contrast to Arabidopsis, where mature miR156 was encoded by eight precursor genes, the genome of D. catenatum only encodes a single locus miR156 gene. This gene (LOC110093427) is annotated as uncharacterized and spans a region of 29,481 bp, which produced three non-coding RNA (ncRNA) variants with lengths of 1194 bp, 1332 bp, and 902 bp [42]. Herein, we rename it to DcMIR156 and its transcription to pre-miRNA156 X1/X2/X3 (Figure 2b). To our knowledge, neither SPL nor miR156 were studied in more detail in Dendrobium species [45].
2.2. DcHd3b Is Highly Expressed in Protocorms and Juvenile Plants
To determine whether the expression pattern of these genes would follow a developmental stage-specific manner, we isolated total RNA from undifferentiated (Figure 3a,b) and differentiating (Figure 3c) protocorms, leaves, and stems of young juvenile (Figure 3d) or three-year-old adult plants (Figure 3e). Using the 18S RNA as a reference, the relative abundances of mRNAs were detected by quantitative real-time PCR.
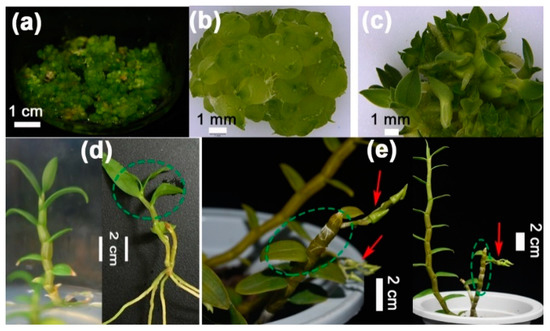
Figure 3.
D. catenatum plants in the three developmental stages. (a) Protocorm clusts on proliferation medium (½ MS + 0.269 μM NAA + 2.22 μM BA + 5% potato pulp + 2.5% sucrose); (b) proliferating protocorms on proliferation medium; (c) differentiating protocorms at 40 days on germination medium (½ MS +2.69 μM NAA +5% banana pulp + 3% sucrose); (d) one-year-old young plants in juvenile stage which were incapable of flowering; (e) adult three-year-old plants cultured in pots. Red arrows indicate the lateral floral bud sets. Green circles indicate sampling positions for RNA isolation from leaves and stems.
Profiling the FT/TFL1 gene expression showed that, in the protocorms, either undifferentiated or at earlier differentiating stage, DcHd3b is the most expressed among the FT/TFL1 genes, followed by DcFTL4 (~5.5-fold lower than DcHd3b) and DcFTL5 (~17-fold lower than DcHd3b). Transcripts of other FT/TFL genes were barely detectable (Figure 4a). No significant change in the expression of FT/TFL1 genes was found during early differentiation of the protocorms, e.g., until leaf primordium opening (Figure 3c). Notably, DcHd3b also showed much higher expression in young juvenile plants. Its mRNA levels were approximately 13.5-fold and 2.6-fold higher in leaves and stems of young plants compared to adult plants, respectively (Figure 4b,c).
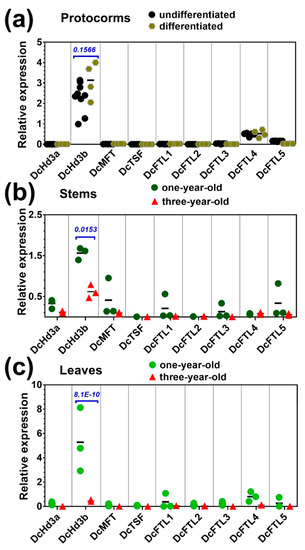
Figure 4.
Expression profiles of FT/TFL1 family members in (a) protocorms at proliferation and differentiation stages, (b) stems of young and adult plants, and (c) leaves of young and adult plants. The 18S gene was used a reference. Statistical significance was determined using a paired Student’s t-test with alpha = 0.05; the adjusted p-value is shown above the data.
Furthermore, DcMFT, DcFTL5, DcFTL3, and DcFTL1 showed some variation in their mRNA level between young and adult stems, although the difference was not significant, probably due to uneven spatial distributions of these genes in the organs (Figure 4b). Similarly, expression of DcFTL4, DcFTL5, and DcFTL1 in leaves between young and adult plants displayed small differences (Figure 4c). Despite no significance, they all seemed to decline with aging.
Thus, several of the nine FT/TFL1 genes in D. catenatum, namely DcHd3b, DcFTL3, DcFTL4, DcFTL5, DcFTL1, and DcMFT, were preferentially expressed in young juvenile tissues or downregulated in adult tissues. DcHd3b represented a significantly highly expressed gene in young plants, but was repressed in adult plants.
2.3. MiR156 Abundance Correlated Well with Protocorm Germination and Differentiation
To determine whether miR156 is expressed differently during development, we used stem–loop quantitative RT-PCR to measure the level of miR156 in protocorms, differentiating protocorms at 40 days, and juvenile and adult plants. The results showed that miR156 was highly accumulated in protocorms, with a level of 2−Δct of 560.52 relative to 18S ribosomal RNA (rRNA), and declined by a factor of ~4.98 at 40 days following germination and differentiation. Mature miR156 level was further reduced by 33.50- and 193.28-fold in one-year-old leaves and stems, and by 22.35- and 52.58-fold in three-year-old leaves and stems, respectively (Figure 5a). The steady-state levels of miR156 of adult plants were slightly higher than in the juvenile plants (not significantly for leaves); however, a tendency to decrease after protocorm differentiation was obvious (Figure 5a).
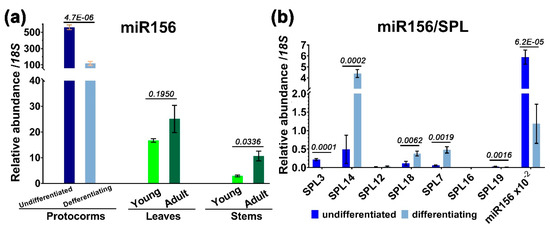
Figure 5.
Expression profiles of miR156 in different tissues and of SPLs in the protocorms. (a) Quantitative stem–loop RT-PCR analysis of miR156 in protocorms, leaves, and stems; (b) RT-qPCR analysis of SPL transcript levels in the protocorms. For comparison, a value of 10−2 × miR156 was also included. Error bars represent the SD for at least three biological replicates. Statistical significance was determined using a paired Student’s t-test with alpha = 0.05; the adjusted p-value is shown above the bars.
The decline of miR156 during earlier protocorm differentiation was concurrent with an increase in transcripts of SPL14, SPL7, and SPL18, three of the seven miR156 targets, up to 9.00, 7.85, and 3.45 folds of reduction, respectively (Figure 5b). However, an opposite expression pattern was also observed for SPL3 and SPL19 during differentiation, although their levels were rather low (Figure 5b). It is, thus, likely that SPL14/SPL7/SPL18 may be positively involved in the regulatory pathway of protocorm differentiation or seedling development.
Since effective inhibition of miR156 on SPL was proposed to require a threshold of at least 100 times greater abundance over its targeted transcripts, as suggested in Arabidopsis [27], we compared the abundance of miR156 to the seven SPL mRNAs. The ratios were well above this value in undifferentiated protocorms. Even in the differentiating protocorms, two of the three upregulated genes, SPL18 and SPL7, also had an mRNA level of 1/311 and 1/246 of miR156, respectively (Figure 5b). The only exception was SPL14, where its transcript was 26.88 times less abundant than miR156. Therefore, at least before the establishment of a seedling, almost all target SPL genes were greatly repressed by miR156, leaving the SPL14 as the least affected gene during seedling development (Figure 5b).
2.4. DcSPL3 Is the Only Significantly Upregulated Gene among the SPL Family in Adult Plants
Although the adult plants did not display a reduced level of mature miR156 in both leaves and stems, as compared with juvenile plants (Figure 5a), a further analysis of the SPL expression between juvenile and adult plants would help explore the miR156/SPL module in Dendrobium flowering control.
Among the five SPLs which were not predicted as targets by miR156, transcripts of SPL15 and SPL1 were more abundant in all three tissues tested, followed by SPL9. Typically, transcripts of these non-target SPL were readily detectable, except SPL10. Higher expression was mainly found in leaves (Figure 6). With regard to differential expression between young and adult plants, transcripts of SPL1 were significantly more expressed in adult leaves than in young ones, whereas SPL8 was preferentially expressed in young stems (Figure 6).
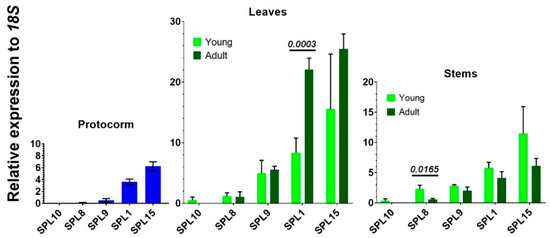
Figure 6.
Expression profiles of five SPLs that were not targeted by miR156 in protocorms, leaves, and stems. Error bars represent the SD for at least three biological replicates. For statistical significance, the adjusted p-values are given on top of the bars.
Except for SPL3, all the other targeted SPLs displayed very low expression levels in adult leaves or stems (Figure 7). Slightly increased expression in young tissues was observed for SPL7 and SPL18, but these little difference in expression levels could not possibly explain their roles in juvenile phase change.
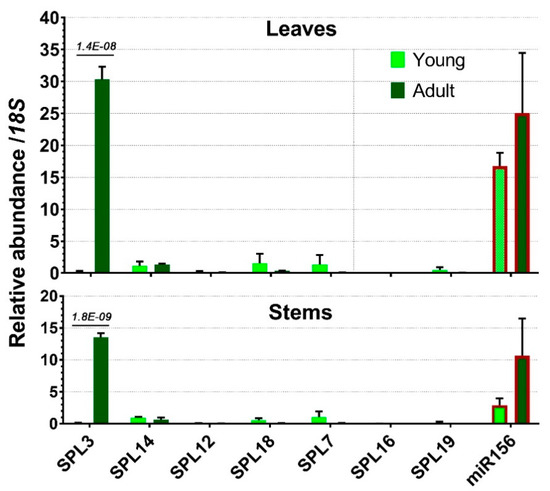
Figure 7.
Expression profiles of miR156-targeted SPLs in leaves and stems of one-year-old and three-year-old plants compared with miR156 levels as in Figure 5a. Error bars represent the SD of at least three biological replicates. For statistical significance, the adjusted p-values are given above the bars.
SPL3 showed a striking difference in gene expression between adult and juvenile plants. Its transcripts were hardly present in young plants but accumulated to a large amount in both adult stems and leaves, with the latter being the most abundant tissue (Figure 7). Since miR156 in both leaves and stems was not significantly different between young and adult plants, the highly accumulated SPL3 transcripts in adult plants suggest that SPL3 mRNA was not sufficiently repressed by miR156 in this stage (Figure 7). Therefore, SPL3 was the only miR156-targeted gene that was significantly associated with adult plants and was expressed strongly in both leaves and stems.
3. Discussion
Time to flower, a process either referring to juvenile–adult phase change or vegetative–reproductive transition, is strictly controlled by an intricate regulatory network involving at least both FT/TFL1 and the miR156-regulated SPL family members. They regulate growth-related downstream genes in response to diverse environmental and developmental signals, and coordinate different flowering pathways to ensure maximal reproductive success [46]. Like most orchid plants, D. catenatum requires at least three years from seeds to reach developmental maturation when flowers are induced under favorable environmental conditions. Protocorm, a middle stage of a seedling establishment, might be induced to further differentiation or kept at the proliferation stage (undifferentiated) under in vitro culture. The one-year-old seedlings represent the juvenile stage, and the three-year-old plants are developmentally ready to reproduce. These three stages showed divergent gene expression patterns for both family members for FT/TFL1 and SPL.
Most notably, under normal growth conditions in the seedling and juvenile stages, the TFL1-like gene DcHd3b from the FT/TFL1 family was predominantly expressed (Figure 4), whereas the negative regulatory RNA, miR156, was highly accumulated in seedling but downregulated during further development, which was coincident with the activation of some of its target SPL genes: SPL14/SPL7/SPL18 in differentiated protocorms and SPL3 in adult plants (Figure 5, Figure 6 and Figure 7). These results provide hints for further deciphering the mechanism of developmental control and flowering competence in this orchid species. Importantly, verification of their putative functions in controlling flowering time would allow the application of biotechnology to assist molecular breeding of the shortened juvenile phase.
3.1. Is there a Repression of Competence to Flower by TFL1-Like Genes in D. catenatum?
The prevailing expression in seedling and young plants but very low detectable transcripts in adult leaves or stems of DcHd3b likely implies that a similar mechanism might exist in D. catenatum. DcHd3b is closer to the TFL1-like proteins such as terminal flower 1 from Arabidopsis and contains a somewhat divergent P-loop region as compared with the floral-promoting FT sequences [43] (Figure 1). Floral-repressing TFL1 paralogs were also reported in several other plants, for example, in gymnosperm species [10], sugar beet [19], strawberry [47], and rose, in which the TFL1 paralog RoKSN was an intuitive repressor of flowering, while its mutated allele exists in continuous-flowering rose species [44].
It was shown in an orchid plant, Oncidium Gower Ramsey, that orthologs of both FT and TFL1 (OnFT and OnTFL1) were able to complement the respective mutant phenotypes of Arabidopsis. Both OnTFL1 and OnFT were expressed highly in pseudobulbs in the reproductive stage and juvenile axillary bud. However, the OnFT mRNA was more abundant than OnTFL1 and it also accumulated in reproductive buds and leaves [40]. In Dendrobium Chao Praya Smile, an ortholog of FT was upregulated in reproductive organs, including inflorescence apices, stems, floral buds, and open flowers. Overexpression of DoFT promoted flowering and notably pseudobulb formation, while suppressing the endogenous DoFT transcripts delayed flowering in the orchid plants [39]. However, these genes seemed to participate in controlling storage or flower organs rather than with juvenile phase transition. Our result of a downregulated expression pattern of TFL-like gene(s) in the adult stage suggests that, in D. catenatum, there might exist a repressed mechanism to prevent flowering during vegetative growth. However, more work is needed to evaluate the functions of DcHd3b.
3.2. MiR156/SPL Involvement in Seedling Development and Maturation in D. catenatum
MiR156 is a “negative” regulator in many developmental processes by repressing specific SPL transcription factors at the mRNA level [48,49,50]. The age-dependent miR156-regulated SPL pathway transforms vegetative shoot apical meristem (SAM) into floral bud and control flower development in response to endogenous signals and external hints [22,28,29,31,51]. In the perennial plant Arabis alpine, downregulation of miR156 by age and de-repression of its target SPL transcription factors in the apices account for its competence to flower under vernalization [52].
We showed that, during Dendrobium protocorm differentiation or early seedling development, the increase in DcSPL14/DcSPL7/DcSPL18 transcript levels is associated with the reduced abundance of miR156 (Figure 5), indicating an involvement of the three SPL genes during these processes. On the other hand, the miR156-targeted DcSPL3 (the closest homolog of AtSPL3/4/5 clade) was actively associated with adult stage in both leaves and stems, where its transcript level exceeded that of miR156 (Figure 7). Thus, we speculate that DcSPL3 might be relevant to the control of plant maturation in D. catenatum.
3.3. Is there a Possible Link between the DcHd3b Pathway and the miR156/DcSPL3 Module in Phase Transition in Dendrobium catenatum?
The stage-specific expression patterns of DcHd3b and DcSPL3, as discussed above, raise an interesting question as to whether and how the two pathways are inter-related. It is well demonstrated in Arabidopsis that the FT–FD–14-3-3 protein complex and the downstream gene SOC1 regulate SPL3, SPL4, and SPL5 by directly binding to their promoters in response to photoperiod signals. This regulation is independent of miR156 [13]. In turn, SPL3/4/5 also directly activated several floral differentiation genes including SOC1, Fruitfull (FUL), Leafy (LFY), Apetala1 (AP1), and FT [21,22]. Evidences from the perennial A. alpine suggested that, in young plants, TFL1 is required to delay flowering through repression of the floral meristem identity gene LFY. Until in the later developmental stage, this blockage of flowering competence was overcome by the upregulated activity of SPL transcription factors which targeted to the common floral meristem identity genes [13,52]. Although not strongly supported yet, a similar mechanism might exist in D. catenatum, given that the opposite pattern of expression exists between DcHd3b and DcSPL3 in young and adult. It is, thus, possible that the convergent antagonistic action of both pathways determines the timing of juvenile phase change in D. catenatum. Of course, involvements of other members from the FT/TFL or SPL family are not exclusive.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Seeds of D. catenatum were germinated aseptically in half-strength MS medium solidified with 3 g/L agar and supplemented with hormones (0.22 μM 6-benzyladenine (BA) + 0.027 μM α-naphthalene acetic acid (NAA) + 0.05 μM indole-3-butyic acid (IBA)). Seed-derived protocorms were proliferated in the liquid proliferation medium (½ MS + 0.269 μM NAA + 2.22 μM BA + 5% potato pulp + 2.5% sucrose) and developed on differentiation medium (½ MS + 2.69 μM NAA + 5% banana pulp + 3% sucrose + 3 g/L agar), both cultured in 25 ± 2 °C, with a 12-h photoperiod and 2000 lux from an LED light source. Seedlings developed from germinating protocorms after ca. four months were grown in pots and maintained in a climate room under long days (14-h light/10-h dark) and 26 °C/22 °C. In our conditions, one-year-old plants were unable to flower; only after three years of growth can plants set flowers. In addition to protocorms at either proliferation or the 40-day differentiation stage, these one-year-old and three-year-old plants were used as materials for RNA isolation.
4.2. Gene Identification and Phylogenetic Analysis
The NCBI genome assembly (ASM160598v2) of D. catenatum was used for BLAST search homologous sequences of FT/TFL1 and SPL families using known proteins from Arabidopsis, rice, and tomato. A BLAST search was also conducted with Apostasia shenzhenica [53] and Phalaenopsis equestris [54] databases. The mature miR156 20-nt sequence was used to BLAST the D. catenatum’s DNA and RNA databases in NCBI to include all putative sequences. All resulting sequences and annotations were manually filtered, selected, and retrieved. For alignments, protein sequences were submitted to MUSCLE (https://www.ebi.ac.uk/Tools/msa/muscle/), and the phylogenetic trees were constructed by MEGA X (https://www.megasoftware.net/) using the maximum-likelihood method with a 1000 bootstrap number. The neighbor-joining trees were generated via Figtree version 1.4.4 downloaded from https://github.com/rambaut/figtree.
4.3. RNA Isolation and Quantitative RT-PCR
For RNA isolation, protocorms, leaves, and stems were collected and frozen in liquid nitrogen. We pooled two plants for leaf and stem samples as one biological replication, and six to 10 protocorms as one. Three biological replications were used. Total RNA was isolated using Trizol (Invitrogen) and treated with DNase (Ambion) following the manufacturer’s instructions. During RNA preparation, approximately 1 g of fresh tissue was ground into fine powder in liquid N2, and about 200 mg of the frozen powder was extracted in 500 mL of extraction buffer. Finally, we merged four extractions from the same sample together. These RNA isolations were kept at −80 °C or used immediately for quantitative RT-PCR measurements of miR156 and FT/TFL1 or SPL genes. Usually, 2 μg of total RNA was used in one RT reaction.
To measure the abundance of miR156, the stem–loop qRT-PCR method with SYBR Green was employed as detailed in Reference [55], using an miRNA156-specific RT primer dca-miR156-rt, 5′–GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGTGCTCA–3′ for synthesis of the templates, and the specific qPCR primers dca-miR156-qF, 5′–GCGGCGGTGACAGAAGAGAGT–3′ and dca-miR156-qRV, 5′–GTGCAGGGTCCGAGGT–3′, for quantification.
For qPCR analysis of transcript levels of FT/TFL1 and SPLs, the first-strand complementary DNA (cDNA) was synthesized with random primes using the Prime Script™ First Strand cDNA Synthesis Kit (Takara Biomedical Technology (Beijing, China) Co., Ltd.). Gene-specific primers were designed with the Primer3+ program (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) (access on February 20, 2017). and are listed in Table S1 (Supplementary Materials). Specificity of all primer pairs was monitored using the melt curve method to ensure a single peak for each reaction. Before the quantitative PCR, annealing temperature optimization was conducted by testing a range of temperatures from 55 to 70 °C.
All qPCR reactions were performed in triplicate for each biological replicate on a Roche Light Cycler 96, using a PCR program set as an initial step of 30 s at 94 °C, followed by 40 cycles of 15 s at 94 °C, and 30 s at 60 °C. The relative expression levels of genes were calculated by the 2 (−ΔΔ C (T)) method [56], using 18S rRNA as an endogenous reference gene (primers in Table S1, Supplementary Materials).
For statistical analysis, either two-way ANOVA or pair-wide multiple t-tests on means of at least three biological replicates was performed using the GraphPad Prism software (version 7). The adjusted p-values were presented in the figures.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/20/11/2725/s1: Table S1: List of primers used for RT-qPCR.
Author Contributions
J.Z. and B.W. designed the experiments. J.Z., Y.M., M.Z., M.L., and Y.Y. performed the experiments. J.Z., M.L., Y.Y., and B.W. analyzed the data. B.W. and J.Z. wrote the manuscript.
Funding
This research was funded by the “Min Jiang Scholar Project” (grant number MJJZ13-005) to B.W. from Fujian A&F University.
Acknowledgments
We thank members in the Wu lab for helpful discussions on the research, and Yongyan Zhang and Wei Wang for excellent technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bergonzi, S.; Albani, M.C. Reproductive competence from an annual and a perennial perspective. J. Exp. Bot. 2011, 62, 4415–4422. [Google Scholar] [CrossRef] [PubMed]
- Hackett, W.P. Juvenility, maturation and rejuvenation in woody plants. Hortic. Rev. 1985, 7, 109–155. [Google Scholar]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. Ft protein movement contributes to long-distance signaling in floral induction of arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Matsuo, S.; Wong, H.L.; Yokoi, S.; Shimamoto, K. Hd3a protein is a mobile flowering signal in rice. Science 2007, 316, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kaya, H.; Goto, K.; Iwabuchi, M.; Araki, T. A pair of related genes with antagonistic roles in mediating flowering signals. Science 1999, 286, 1960–1962. [Google Scholar] [CrossRef] [PubMed]
- Wickland, D.P.; Hanzawa, Y. The flowering locus t/terminal flower 1 gene family: Functional evolution and molecular mechanisms. Mol. Plant 2015, 8, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Miller, D.; Winter, V.J.; Banfield, M.J.; Lee, J.H.; Yoo, S.Y.; Henz, S.R.; Brady, R.L.; Weigel, D. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 2006, 25, 605–614. [Google Scholar] [CrossRef]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y. Florigen and anti-florigen: Flowering regulation in horticultural crops. Breed. Sci. 2018, 68, 109–118. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yang, K.Z.; Wei, X.X.; Wang, X.Q. Revisiting the phosphatidylethanolamine-binding protein (PEBP) gene family reveals cryptic flowering locus t gene homologs in gymnosperms and sheds new light on functional evolution. New Phytol. 2016, 212, 730–744. [Google Scholar] [CrossRef]
- Hedman, H.; Källman, T.; Lagercrantz, U. Early evolution of the MFT-like gene family in plants. Plant Mol. Biol. 2009, 70, 359–369. [Google Scholar] [CrossRef]
- Karlgren, A.; Gyllenstrand, N.; Källman, T.; Sundström, J.F.; Moore, D.; Lascoux, M.; Lagercrantz, U. Evolution of the PEBP gene family in plants: Functional diversification in seed plant evolution. Plant Physiol. 2011, 156, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Taoka, K.; Ohki, I.; Tsuji, H.; Furuita, K.; Hayashi, K.; Yanase, T.; Yamaguchi, M.; Nakashima, C.; Purwestri, Y.A.; Tamaki, S.; et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 2011, 476, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Melzer, S.; Lens, F.; Gennen, J.; Vanneste, S.; Rohde, A.; Beeckman, T. Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet. 2008, 40, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, M.; Park, H.; Lee, I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates leafy. Plant J. 2008, 55, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, J.U.; Weigel, D. Building beauty: The genetic control of floral patterning. Dev. Cell 2002, 2, 135–142. [Google Scholar] [CrossRef]
- Randoux, M.; Daviere, J.M.; Jeauffre, J.; Thouroude, T.; Pierre, S.; Toualbia, Y.; Perrotte, J.; Reynoird, J.P.; Jammes, M.J.; Hibrand-Saint Oyant, L.; et al. Roksn, a floral repressor, forms protein complexes with RoFD and RoFT to regulate vegetative and reproductive development in rose. New Phytol. 2014, 202, 161–173. [Google Scholar] [CrossRef]
- Qin, Z.; Bai, Y.; Muhammad, S.; Wu, X.; Deng, P.; Wu, J.; An, H.; Wu, L. Divergent roles of FT-like 9 in flowering transition under different day lengths in brachypodium distachyon. Nat. Commun. 2019, 10, 812. [Google Scholar] [CrossRef]
- Pin, P.A.; Benlloch, R.; Bonnet, D.; Wremerth-Weich, E.; Kraft, T.; Gielen, J.J.; Nilsson, O. An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 2010, 330, 1397–1400. [Google Scholar] [CrossRef]
- Jung, J.H.; Ju, Y.; Seo, P.J.; Lee, J.H.; Park, C.M. The SOC1-SPl module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. Plant J. Cell Mol. Biol. 2012, 69, 577–588. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Wu, M.-F.; Yang, L.; Wu, G.; Poethig, R.S.; Wagner, D. The microRNA-regulated SBP-box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev. Cell 2009, 17, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Czech, B.; Weigel, D. Mir156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; You, C.; Chen, X. The evolution of micrornas in plants. Curr. Opin. Plant Biol. 2017, 35, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Tarver, J.E.; Hiscock, S.J.; Donoghue, P.C.J. Evolutionary history of plant micrornas. Trends Plant Sci. 2014, 19, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Somoza, I.; Weigel, D. Microrna networks and developmental plasticity in plants. Trends Plant Sci. 2011, 16, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microrna biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, M.; Willmann, M.R.; McCormick, K.; Hu, T.; Yang, L.; Starker, C.G.; Voytas, D.F.; Meyers, B.C.; Poethig, R.S. Threshold-dependent repression of SPL gene expression by mir156/mir157 controls vegetative phase change in Arabidopsis thaliana. PLoS Genet. 2018, 14, e1007337. [Google Scholar] [CrossRef]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.W.; Weigel, D.; Poethig, R.S. The sequential action of mir156 and mir172 regulates developmental timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef]
- Wu, G.; Poethig, R.S. Temporal regulation of shoot development in Arabidopsis thaliana by mir156 and its target SPL3. Development 2006, 133, 3539–3547. [Google Scholar] [CrossRef]
- Hou, H.M.; Yan, X.X.; Sha, T.; Yan, Q.; Wang, X.P. The SBP-box gene VpSBP11 from Chinese wild vitis is involved in floral transition and affects leaf development. Int. J. Mol. Sci. 2017, 18, 1493. [Google Scholar] [CrossRef]
- Xu, M.; Hu, T.; Zhao, J.; Park, M.Y.; Earley, K.W.; Wu, G.; Yang, L.; Poethig, R.S. Developmental functions of mir156-regulated squamosa promoter binding protein-like (SPL) genes in Arabidopsis thaliana. PLoS Genet. 2016, 12, e1006263. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, H.J.; Ryu, J.Y.; Park, C.M. SPL3/4/5 integrate developmental aging and photoperiodic signals into the FT-FD module in Arabidopsis flowering. Mol. Plant 2016, 9, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Aukerman, M.J.; Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its apetala2-like target genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.; Richter, R.; Vincent, C.; Martinez-Gallegos, R.; Porri, A.; Coupland, G. Multi-layered regulation of SPL15 and cooperation with soc1 integrate endogenous flowering pathways at the Arabidopsis shoot meristem. Dev. Cell 2016, 37, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Winter, C.M.; Wu, M.F.; Kanno, Y.; Yamaguchi, A.; Seo, M.; Wagner, D. Gibberellin acts positively then negatively to control onset of flower formation in Arabidopsis. Science 2014, 344, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, Q.; Yao, T.; Fu, X. Shedding light on integrative GA signaling. Curr. Opin. Plant Biol. 2014, 21, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Galvao, V.C.; Zhang, Y.C.; Horrer, D.; Zhang, T.Q.; Hao, Y.H.; Feng, Y.Q.; Wang, S.; Schmid, M.; Wang, J.W. Gibberellin regulates the Arabidopsis floral transition through mir156-targeted squamosa promoter binding-like transcription factors. Plant Cell 2012, 24, 3320–3332. [Google Scholar] [CrossRef]
- da Silva, J.A.T.; Aceto, S.; Liu, W.; Yu, H.; Kanno, A. Genetic control of flower development, color and senescence of Dendrobium orchids. Sci. Hortic-Amst. 2014, 175, 74–86. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Song, S.; Li, Y.; Shen, L.; Yu, H. DOFT and DOFTIP1 affect reproductive development in the orchid Dendrobium chao praya smile. J. Exp. Bot. 2017, 68, 5759–5772. [Google Scholar] [CrossRef]
- Hou, C.J.; Yang, C.H. Functional analysis of FT and TFL1 orthologs from orchid (oncidium gower ramsey) that regulate the vegetative to reproductive transition. Plant Cell Physiol. 2009, 50, 1544–1557. [Google Scholar] [CrossRef]
- Wang, H.-M.; Tong, C.-G.; Jang, S. Current progress in orchid flowering/flower development research. Plant Signal Behav. 2017, 12, e1322245. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Xu, Q.; Bian, C.; Tsai, W.C.; Yeh, C.M.; Liu, K.W.; Yoshida, K.; Zhang, L.S.; Chang, S.B.; Chen, F.; et al. The Dendrobium catenatum lindl. Genome sequence provides insights into polysaccharide synthase, floral development and adaptive evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.W.; Weigel, D. Structural features determining flower-promoting activity of Arabidopsis flowering locus T. Plant Cell 2014, 26, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.C.; Hileman, L.C. Functional evolution in the plant squamosa-promoter binding protein-like (SPL) gene family. Front. Plant Sci. 2013, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yu, D.; Xue, J.; Lu, J.; Feng, S.; Shen, C.; Wang, H. A transcriptome-wide, organ-specific regulatory map of Dendrobium officinale, an important traditional Chinese orchid herb. Sci. Rep. 2016, 6, 18864. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.; Richter, R.; Coupland, G. Competence to flower: Age-controlled sensitivity to environmental cues. Plant Physiol. 2017, 173, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Mouhu, K.; Kurokura, T.; Koskela, E.A.; Albert, V.A.; Elomaa, P.; Hytonen, T. The fragaria vesca homolog of suppressor of overexpression of constans1 represses flowering and promotes vegetative growth. Plant Cell 2013, 25, 3296–3310. [Google Scholar] [CrossRef] [PubMed]
- Gandikota, M.; Birkenbihl, R.P.; Hohmann, S.; Cardon, G.H.; Saedler, H.; Huijser, P. The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J. Cell Mol. Biol. 2007, 49, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wu, C.; Xiong, L. Genomic organization, differential expression, and interaction of squamosa promoter-binding-like transcription factors and microrna156 in rice. Plant Physiol. 2006, 142, 280–293. [Google Scholar] [CrossRef]
- Yu, N.; Cai, W.J.; Wang, S.; Shan, C.M.; Wang, L.J.; Chen, X.Y. Temporal control of trichome distribution by microrna156-targeted SPL genes in Arabidopsis thaliana. Plant Cell 2010, 22, 2322–2335. [Google Scholar] [CrossRef]
- Wang, J.W.; Park, M.Y.; Wang, L.J.; Koo, Y.; Chen, X.Y.; Weigel, D.; Poethig, R.S. Mirna control of vegetative phase change in trees. PLoS Genet. 2011, 7, e1002012. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, S.; Albani, M.C.; Ver Loren van Themaat, E.; Nordstrom, K.J.; Wang, R.; Schneeberger, K.; Moerland, P.D.; Coupland, G. Mechanisms of age-dependent response to winter temperature in perennial flowering of Arabis alpina. Science 2013, 340, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Liu, K.W.; Li, Z.; Lohaus, R.; Hsiao, Y.Y.; Niu, S.C.; Wang, J.Y.; Lin, Y.C.; Xu, Q.; Chen, L.J.; et al. The apostasia genome and the evolution of orchids. Nature 2017, 549, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, X.; Vanneste, K.; Proost, S.; Tsai, W.C.; Liu, K.W.; Chen, L.J.; He, Y.; Xu, Q.; Bian, C.; et al. The genome sequence of the orchid Phalaenopsis equestris. Nat. Genet. 2015, 47, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Varkonyi-Gasic, E. Stem-loop QRT-PCR for the detection of plant micrornas. Methods Mol. Biol. 2017, 1456, 163–175. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).