Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect
Abstract
1. Introduction
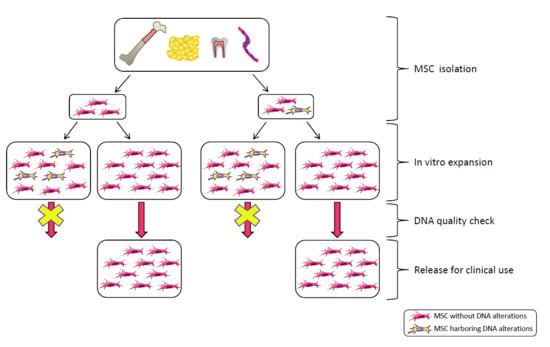
2. MSC Clinical Use
3. The Safety Issue
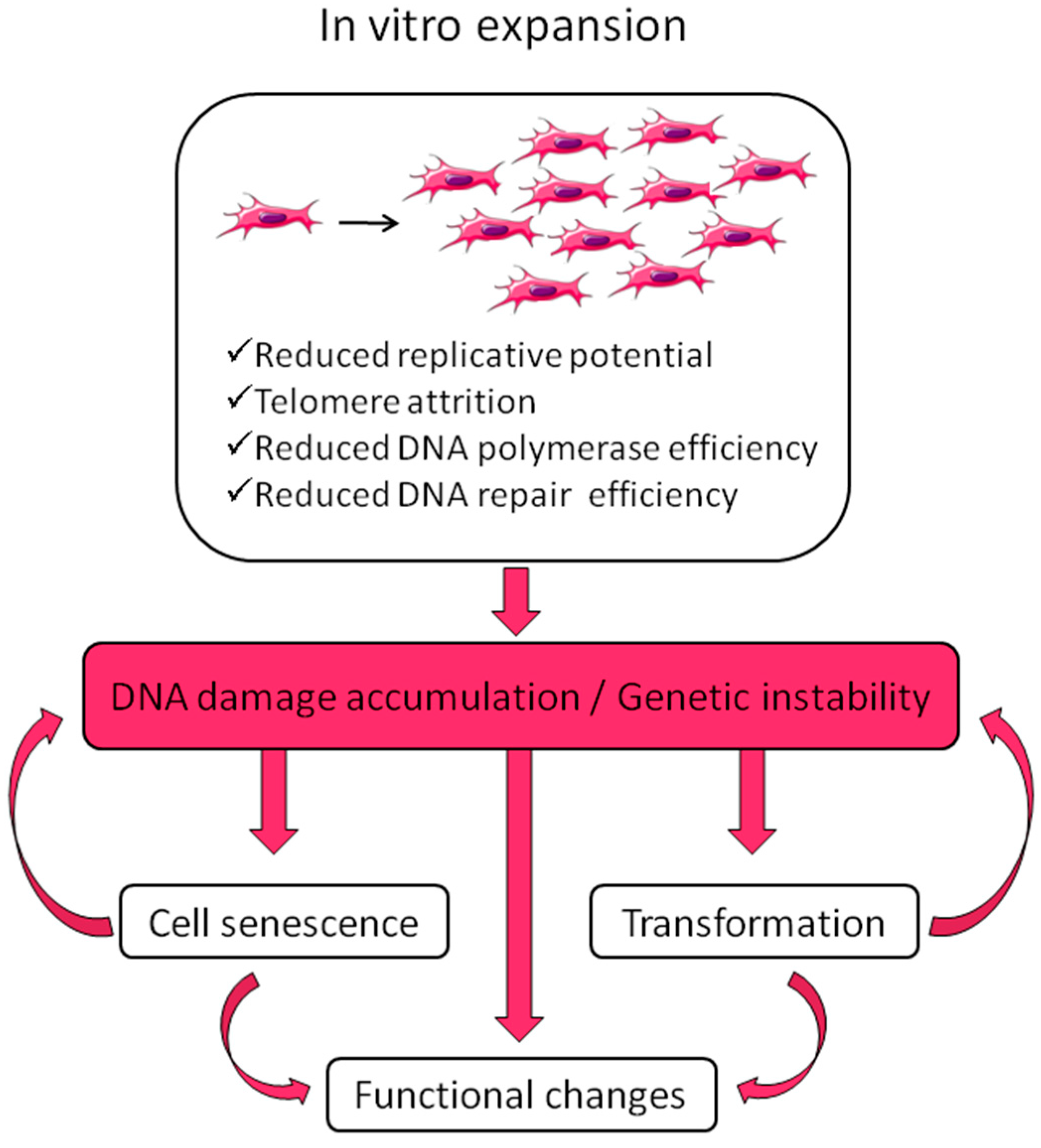
4. Senescence
5. Tumorigenicity
6. Genetic Stability
Methodological Approaches to Assess Genetic Stability
7. Regulatory Aspects
8. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Okita, K.; Nakagawa, M.; Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007, 2, 3081–3089. [Google Scholar] [CrossRef] [PubMed]
- Liras, A. Future research and therapeutic applications of human stem cells: General, regulatory, and bioethical aspects. J. Transl. Med. 2010, 8, 131. [Google Scholar] [CrossRef]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Von Bahr, L.; Batsis, I.; Moll, G.; Hagg, M.; Szakos, A.; Sundberg, B.; Uzunel, M.; Ringden, O.; Le Blanc, K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012, 30, 1575–1578. [Google Scholar] [CrossRef]
- Kalinina, N.; Kharlampieva, D.; Loguinova, M.; Butenko, I.; Pobeguts, O.; Efimenko, A.; Ageeva, L.; Sharonov, G.; Ischenko, D.; Alekseev, D.; et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. 2015, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Mun, C.H.; Kang, M.I.; Shin, Y.D.; Kim, Y.; Park, Y.B. The expression of immunomodulation-related cytokines and genes of adipose- and bone marrow-derived human mesenchymal stromal cells from early to late passages. Tissue Eng. Regen. Med. 2018, 15, 771–779. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise review: Msc-derived exosomes for cell-free therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Moretta, L.; Uccelli, A.; Pistoia, V. Mesenchymal stromal cells and immunity: Introductory overview. Immunol. Lett. 2015, 168, 127–128. [Google Scholar] [CrossRef]
- Zhao, K.; Lou, R.; Huang, F.; Peng, Y.; Jiang, Z.; Huang, K.; Wu, X.; Zhang, Y.; Fan, Z.; Zhou, H.; et al. Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 2015, 21, 97–104. [Google Scholar] [CrossRef] [PubMed]
- English, K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013, 91, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.R.; Parreira, R.C.; Fonseca, E.A.; Amaya, M.J.; Tonelli, F.M.; Lacerda, S.M.; Lalwani, P.; Santos, A.K.; Gomes, K.N.; Ulrich, H.; et al. Human adult stem cells from diverse origins: An overview from multiparametric immunophenotyping to clinical applications. Cytom. Part A 2014, 85, 43–77. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Baer, P.C. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J. Stem Cells 2014, 6, 256–265. [Google Scholar] [CrossRef]
- De Francesco, F.; Ricci, G.; D’Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Human adipose stem cells: From bench to bedside. Tissue Eng. Part B Rev. 2015, 21, 572–584. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. 2015, 6, 55. [Google Scholar] [CrossRef]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of isolation, characterization and expansion of human adipose-derived stem cells (ascs): An overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef]
- Davies, J.E.; Walker, J.T.; Keating, A. Concise review: Wharton’s jelly: The rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl. Med. 2017, 6, 1620–1630. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef] [PubMed]
- Amati, E.; Sella, S.; Perbellini, O.; Alghisi, A.; Bernardi, M.; Chieregato, K.; Lievore, C.; Peserico, D.; Rigno, M.; Zilio, A.; et al. Generation of mesenchymal stromal cells from cord blood: Evaluation of in vitro quality parameters prior to clinical use. Stem Cell Res. 2017, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, E.; David, A.L. Placental stem cells. Best Pract. Res. Cl. Ob. Gynaecol. 2016, 31, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.G.; Piekarski, B.L.; Huang, K.; Emani, S.; Wong, J.Y.; Emani, S.M. Evaluation of placental mesenchymal stem cell sheets for myocardial repair and regeneration. Tissue Eng. Part A 2018. [Google Scholar] [CrossRef] [PubMed]
- Phermthai, T.; Suksompong, S.; Tirawanchai, N.; Issaragrisil, S.; Julavijitphong, S.; Wichitwiengrat, S.; Silpsorn, D.; Pokathikorn, P. Epigenetic analysis and suitability of amniotic fluid stem cells for research and therapeutic purposes. Stem Cells Dev. 2013, 22, 1319–1328. [Google Scholar] [CrossRef]
- Roselli, E.A.; Lazzati, S.; Iseppon, F.; Manganini, M.; Marcato, L.; Gariboldi, M.B.; Maggi, F.; Grati, F.R.; Simoni, G. Fetal mesenchymal stromal cells from cryopreserved human chorionic villi: Cytogenetic and molecular analysis of genome stability in long-term cultures. Cytotherapy 2013, 15, 1340–1351. [Google Scholar] [CrossRef]
- Meng, Z.; Chen, G.; Chen, J.; Yang, B.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; Tian, W. Tumorigenicity analysis of heterogeneous dental stem cells and its self-modification for chromosome instability. Cell Cycle 2015, 14, 3396–3407. [Google Scholar] [CrossRef] [PubMed]
- Stanko, P.; Altanerova, U.; Jakubechova, J.; Repiska, V.; Altaner, C. Dental mesenchymal stem/stromal cells and their exosomes. Stem Cells Int. 2018, 2018, 8973613. [Google Scholar] [CrossRef]
- Vinogradov, A.E.; Shilina, M.A.; Anatskaya, O.V.; Alekseenko, L.L.; Fridlyanskaya, I.I.; Krasnenko, A.; Kim, A.; Korostin, D.; Ilynsky, V.; Elmuratov, A.; et al. Molecular genetic analysis of human endometrial mesenchymal stem cells that survived sublethal heat shock. Stem Cells Int. 2017, 2017, 2362630. [Google Scholar] [CrossRef] [PubMed]
- Pombero, A.; Garcia-Lopez, R.; Martinez, S. Brain mesenchymal stem cells: Physiology and pathological implications. Dev. Growth Differ. 2016, 58, 469–480. [Google Scholar] [CrossRef]
- Chinnici, C.M.; Amico, G.; Monti, M.; Motta, S.; Casalone, R.; Petri, S.L.; Spada, M.; Gridelli, B.; Conaldi, P.G. Isolation and characterization of multipotent cells from human fetal dermis. Cell Transpl. 2014, 23, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, E.B.; Casado, P.L.; Moura Neto, V.; Duarte, M.E.; Aguiar, D.P. Synovial fluid and synovial membrane mesenchymal stem cells: Latest discoveries and therapeutic perspectives. Stem Cell Res. 2014, 5, 112. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Liu, J.; Pang, C.; Zhang, J.; Li, Y.; Fu, X. Location, isolation, and identification of mesenchymal stem cells from adult human sweat glands. Stem Cells Int. 2018, 2018, 2090276. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Lee, W.; Park, S.H.; Lee, H.J.; Lee, D.C.; Lim, M.H.; Back, S.A.; Yun, B.G.; Jeun, J.H.; Lim, J.Y.; et al. Evaluation of characteristic of human turbinate derived mesenchymal stem cells cultured in the serum free media. PLoS ONE 2017, 12, e0186249. [Google Scholar] [CrossRef]
- Confalonieri, D.; Schwab, A.; Walles, H.; Ehlicke, F. Advanced therapy medicinal products: A guide for bone marrow-derived msc application in bone and cartilage tissue engineering. Tissue Eng. Part B Rev. 2018, 24, 155–169. [Google Scholar] [CrossRef]
- Carrow, J.K.; Cross, L.M.; Reese, R.W.; Jaiswal, M.K.; Gregory, C.A.; Kaunas, R.; Singh, I.; Gaharwar, A.K. Widespread changes in transcriptome profile of human mesenchymal stem cells induced by two-dimensional nanosilicates. Proc. Natl. Acad. Sci. USA 2018, 115, E3905–E3913. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, B.; Kasoju, N.; Ma, J.; Yang, A.; Cui, Z.; Wang, H.; Ye, H. Differential and interactive effects of substrate topography and chemistry on human mesenchymal stem cell gene expression. Int. J. Mol. Sci. 2018, 19, 2344. [Google Scholar] [CrossRef]
- Zhang, B.; Kasoju, N.; Li, Q.; Ma, J.; Yang, A.; Cui, Z.; Wang, H.; Ye, H. Effect of substrate topography and chemistry on human mesenchymal stem cell markers: A transcriptome study. Int. J. Stem Cells 2019, 12, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Graf, T. Covering the stem cell explosion at the 2017 isscr conference in boston. Stem Cell Rep. 2017, 9, 1017–1023. [Google Scholar] [CrossRef][Green Version]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical trials with mesenchymal stem cells: An update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.Y. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Drela, K.; Stanaszek, L.; Nowakowski, A.; Kuczynska, Z.; Lukomska, B. Experimental strategies of mesenchymal stem cell propagation: Adverse events and potential risk of functional changes. Stem Cells Int. 2019, 2019, 2362630. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Dazzi, F.; Dominici, M.; Schlenke, P.; Wagner, W. Clinical perspectives of mesenchymal stem cells. Stem Cells Int. 2012, 2012, 684827. [Google Scholar] [CrossRef]
- Kouroupis, D.; Sanjurjo-Rodriguez, C.; Jones, E.; Correa, D. Mesenchymal stem cell functionalization for enhanced therapeutic applications. Tissue Eng. Part B Rev. 2019, 25, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Kim, S.N.; Lee, H.J.; Kim, J.; Cho, Y.K.; Shin, D.H.; Tak, S.J.; Moon, S.H.; Kang, J.E.; Ji, I.M.; et al. Manufacture of clinical-grade human clonal mesenchymal stem cell products from single colony forming unit-derived colonies based on the subfractionation culturing method. Tissue Eng. Part C Methods 2015, 21, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Selich, A.; Daudert, J.; Hass, R.; Philipp, F.; von Kaisenberg, C.; Paul, G.; Cornils, K.; Fehse, B.; Rittinghausen, S.; Schambach, A.; et al. Massive clonal selection and transiently contributing clones during expansion of mesenchymal stem cell cultures revealed by lentiviral rgb-barcode technology. Stem Cells Transl. Med. 2016, 5, 591–601. [Google Scholar] [CrossRef]
- Martin, I.; de Boer, J.; Sensebe, L. A relativity concept in mesenchymal stromal cell manufacturing. Cytotherapy 2016, 18, 613–620. [Google Scholar] [CrossRef]
- Oberbauer, E.; Steffenhagen, C.; Wurzer, C.; Gabriel, C.; Redl, H.; Wolbank, S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell Regen 2015, 4, 7. [Google Scholar] [CrossRef]
- Neri, S.; Cattini, L.; Facchini, A.; Pawelec, G.; Mariani, E. Microsatellite instability in in vitro ageing of t lymphocyte clones. Exp. Gerontol. 2004, 39, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Pawelec, G.; Facchini, A.; Ferrari, C.; Mariani, E. Altered expression of mismatch repair proteins associated with acquisition of microsatellite instability in a clonal model of human t lymphocyte aging. Rejuvenation Res. 2008, 11, 565–572. [Google Scholar] [CrossRef]
- Neri, S.; Pawelec, G.; Facchini, A.; Mariani, E. Microsatellite instability and compromised mismatch repair gene expression during in vitro passaging of monoclonal human t lymphocytes. Rejuvenation Res. 2007, 10, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Bourin, P.; Peyrafitte, J.A.; Cattini, L.; Facchini, A.; Mariani, E. Human adipose stromal cells (asc) for the regeneration of injured cartilage display genetic stability after in vitro culture expansion. PLoS ONE 2013, 8, e77895. [Google Scholar] [CrossRef]
- Sperka, T.; Wang, J.; Rudolph, K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC. Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.R.; Aoyama, T.; Shima, Y.; Fukiage, K.; Otsuka, S.; Furu, M.; Kohno, Y.; Ito, K.; Fujibayashi, S.; Neo, M.; et al. Expression of the p16ink4a gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells 2007, 25, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Miao, X.; Li, Y.; Smith, C.; Tsang, K.; Cheng, L.; Wang, Q.F. Whole-genome sequencing identifies genetic variances in culture-expanded human mesenchymal stem cells. Stem Cell Rep. 2014, 3, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Mani, C.; Reddy, P.H.; Palle, K. DNA repair fidelity in stem cell maintenance, health, and disease. BBA Mol. Basis Dis. 2019. [Google Scholar] [CrossRef]
- Behrens, A.; van Deursen, J.M.; Rudolph, K.L.; Schumacher, B. Impact of genomic damage and ageing on stem cell function. Nat. Cell Biol. 2014, 16, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.L.; Schumacher, B. DNA damage responses and p53 in the aging process. Blood 2018, 131, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Takahashi, A.; Mann, D.J.; Hara, E. Cellular senescence: A double-edged sword in the fight against cancer. Exp. Derm. 2012, 21, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lamm, N.; Ben-David, U.; Golan-Lev, T.; Storchova, Z.; Benvenisty, N.; Kerem, B. Genomic instability in human pluripotent stem cells arises from replicative stress and chromosome condensation defects. Cell Stem Cell 2016, 18, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.; Munoz-Najar, U.; de Wit, J.; Renard, A.J.; Hoeijmakers, J.H.; Sedivy, J.M.; Van Blitterswijk, C.; De Boer, J. A link between the accumulation of DNA damage and loss of multi-potency of human mesenchymal stromal cells. J. Cell Mol. Med. 2010, 14, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Qiu, L.; Ma, J.; Zhang, H.; Cheng, M.; Li, W.; Zhao, X.; Liu, K. Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts. Mol. Biol. Rep. 2011, 38, 5161–5168. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, S.; Alessio, N.; Acar, M.B.; Mert, E.; Omerli, F.; Peluso, G.; Galderisi, U. Unbiased analysis of senescence associated secretory phenotype (sasp) to identify common components following different genotoxic stresses. Aging 2016, 8, 1316–1329. [Google Scholar] [CrossRef]
- Mieczkowska, A.; Schumacher, A.; Filipowicz, N.; Wardowska, A.; Zielinski, M.; Madanecki, P.; Nowicka, E.; Langa, P.; Deptula, M.; Zielinski, J.; et al. Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells. Sci. Rep. 2018, 8, 11339. [Google Scholar] [CrossRef]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in human mesenchymal stem cells: Functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef]
- Peffers, M.J.; Collins, J.; Fang, Y.; Goljanek-Whysall, K.; Rushton, M.; Loughlin, J.; Proctor, C.; Clegg, P.D. Age-related changes in mesenchymal stem cells identified using a multi-omics approach. Eur. Cell Mater. 2016, 31, 136–159. [Google Scholar] [CrossRef]
- Kim, J.A.; Im, K.O.; Park, S.N.; Kwon, J.S.; Kim, S.Y.; Oh, K.; Lee, D.S.; Kim, M.K.; Kim, S.W.; Jang, M.; et al. Cytogenetic heterogeneity and their serial dynamic changes during acquisition of cytogenetic aberrations in cultured mesenchymal stem cells. Mutat. Res. 2015, 777, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Parsch, D.; Fellenberg, J.; Brummendorf, T.H.; Eschlbeck, A.M.; Richter, W. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes. J. Mol. Med. 2004, 82, 49–55. [Google Scholar] [PubMed]
- Redaelli, S.; Bentivegna, A.; Foudah, D.; Miloso, M.; Redondo, J.; Riva, G.; Baronchelli, S.; Dalpra, L.; Tredici, G. From cytogenomic to epigenomic profiles: Monitoring the biologic behavior of in vitro cultured human bone marrow mesenchymal stem cells. Stem Cell Res. 2012, 3, 47. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Chi, Y.; Zhang, Q.; Xu, F.; Yang, Z.; Meng, L.; Yang, S.; Yan, S.; Mao, A.; et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013, 4, e950. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Im, K.; Park, S.N.; Kwon, J.; Kim, J.A.; Choi, Q.; Hwang, S.M.; Han, S.H.; Kwon, S.; Oh, I.H.; et al. Asymmetric aneuploidy in mesenchymal stromal cells detected by in situ karyotyping and fluorescence in situ hybridization: Suggestions for reference values for stem cells. Stem Cells Dev. 2015, 24, 77–92. [Google Scholar] [CrossRef]
- Sensebe, L.; Bourin, P. Mesenchymal stem cells for therapeutic purposes. Transplantation 2009, 87, S49–S53. [Google Scholar] [CrossRef]
- Sarugaser, R.; Hanoun, L.; Keating, A.; Stanford, W.L.; Davies, J.E. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS ONE 2009, 4, e6498. [Google Scholar] [CrossRef] [PubMed]
- Yannarelli, G.; Pacienza, N.; Cuniberti, L.; Medin, J.; Davies, J.; Keating, A. Brief report: The potential role of epigenetics on multipotent cell differentiation capacity of mesenchymal stromal cells. Stem Cells 2013, 31, 215–220. [Google Scholar] [CrossRef]
- Schellenberg, A.; Hemeda, H.; Wagner, W. Tracking of replicative senescence in mesenchymal stem cells by colony-forming unit frequency. Methods Mol. Biol. 2013, 976, 143–154. [Google Scholar]
- Di, G.H.; Liu, Y.; Lu, Y.; Liu, J.; Wu, C.; Duan, H.F. Il-6 secreted from senescent mesenchymal stem cells promotes proliferation and migration of breast cancer cells. PLoS ONE 2014, 9, e113572. [Google Scholar] [CrossRef]
- Neri, S.; Guidotti, S.; Lilli, N.L.; Cattini, L.; Mariani, E. Infrapatellar fat pad-derived mesenchymal stromal cells from osteoarthritis patients: In vitro genetic stability and replicative senescence. J. Orthop. Res. 2017, 35, 1029–1037. [Google Scholar] [CrossRef]
- Jin, Y.; Kato, T.; Furu, M.; Nasu, A.; Kajita, Y.; Mitsui, H.; Ueda, M.; Aoyama, T.; Nakayama, T.; Nakamura, T.; et al. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem. Biophys Res. Commun. 2010, 391, 1471–1476. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chen, Y.J.; Yew, T.L.; Chen, L.L.; Wang, J.Y.; Chiu, C.H.; Hung, S.C. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of e2a-p21 by hif-twist. Blood 2011, 117, 459–469. [Google Scholar] [CrossRef]
- Bigot, N.; Mouche, A.; Preti, M.; Loisel, S.; Renoud, M.L.; le Guevel, R.; Sensebe, L.; Tarte, K.; Pedeux, R. Hypoxia differentially modulates the genomic stability of clinical-grade adscs and bm-mscs in long-term culture. Stem Cells 2015, 33, 3608–3620. [Google Scholar] [CrossRef]
- Frati, P.; Scopetti, M.; Santurro, A.; Gatto, V.; Fineschi, V. Stem cell research and clinical translation: A roadmap about good clinical practice and patient care. Stem Cells Int. 2017, 2017, 5080259. [Google Scholar] [CrossRef]
- Cakouros, D.; Gronthos, S. Epigenetic regulation of bone marrow stem cell aging: Revealing epigenetic signatures associated with hematopoietic and mesenchymal stem cell aging. Aging Dis. 2019, 10, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.; Xie, Z.; Song, P.; Zhao, R.C.; Guo, L.; Liu, Z.; Wu, Y. Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS ONE 2011, 6, e20526. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, Y.; Galderisi, U. The impact of epigenetics on mesenchymal stem cell biology. J. Cell Physiol. 2016, 231, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Song, X.; Han, F.; Li, Y.; Wei, J.; Liu, X. Alteration of histone acetylation pattern during long-term serum-free culture conditions of human fetal placental mesenchymal stem cells. PLoS ONE 2015, 10, e0117068. [Google Scholar] [CrossRef] [PubMed]
- Toraño, E.G.; Bayón, G.F.; del Real, A.; Sierra, M.I.; García, M.G.; Carella, A.; Belmonte, T.; Urdinguio, R.G.; Cubillo, I.; García-Castro, J.; et al. Age-associated hydroxymethylation in human bone marrow mesenchymal stem cells. J. Transl. Med. 2016, 14, 207. [Google Scholar] [CrossRef]
- Bork, S.; Pfister, S.; Witt, H.; Horn, P.; Korn, B.; Ho, A.D.; Wagner, W. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell 2010, 9, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W. Epigenetic aging clocks in mice and men. Genome Biol. 2017, 18, 107. [Google Scholar] [CrossRef] [PubMed]
- Bentivegna, A.; Miloso, M.; Riva, G.; Foudah, D.; Butta, V.; Dalpra, L.; Tredici, G. DNA methylation changes during in vitro propagation of human mesenchymal stem cells: Implications for their genomic stability? Stem Cells Int. 2013, 2013, 192425. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, A.; Lin, Q.; Schuler, H.; Koch, C.M.; Joussen, S.; Denecke, B.; Walenda, G.; Pallua, N.; Suschek, C.V.; Zenke, M.; et al. Replicative senescence of mesenchymal stem cells causes DNA-methylation changes which correlate with repressive histone marks. Aging 2011, 3, 873–888. [Google Scholar] [CrossRef]
- Wagner, W. Implications of long-term culture for mesenchymal stem cells: Genetic defects or epigenetic regulation? Stem Cell Res. 2012, 3, 54. [Google Scholar] [CrossRef][Green Version]
- Koch, C.M.; Joussen, S.; Schellenberg, A.; Lin, Q.; Zenke, M.; Wagner, W. Monitoring of cellular senescence by DNA-methylation at specific cpg sites. Aging Cell 2012, 11, 366–369. [Google Scholar] [CrossRef]
- Schellenberg, A.; Mauen, S.; Koch, C.M.; Jans, R.; de Waele, P.; Wagner, W. Proof of principle: Quality control of therapeutic cell preparations using senescence-associated DNA-methylation changes. BMC Res. Notes 2014, 7, 254. [Google Scholar] [CrossRef] [PubMed]
- Franzen, J.; Zirkel, A.; Blake, J.; Rath, B.; Benes, V.; Papantonis, A.; Wagner, W. Senescence-associated DNA methylation is stochastically acquired in subpopulations of mesenchymal stem cells. Aging Cell 2017, 16, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Grezella, C.; Fernandez-Rebollo, E.; Franzen, J.; Ventura Ferreira, M.S.; Beier, F.; Wagner, W. Effects of senolytic drugs on human mesenchymal stromal cells. Stem Cell Res. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N.; Mann, D.J.; Hara, E. Cellular senescence: Its role in tumor suppression and aging. Cancer Sci. 2009, 100, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X.; Wang, L.; Liu, G.; Wu, X.; Jing, Y.; Li, H.; Wang, G. Senescent mesenchymal stem cells promote colorectal cancer cells growth via galectin-3 expression. Cell Biosci. 2015, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Torre, M.L.; Lucarelli, E.; Guidi, S.; Ferrari, M.; Alessandri, G.; De Girolamo, L.; Pessina, A.; Ferrero, I. Ex vivo expanded mesenchymal stromal cell minimal quality requirements for clinical application. Stem Cells Dev. 2015, 24, 677–685. [Google Scholar] [CrossRef]
- Bellotti, C.; Capanni, C.; Lattanzi, G.; Donati, D.; Lucarelli, E.; Duchi, S. Detection of mesenchymal stem cells senescence by prelamin a accumulation at the nuclear level. Springerplus 2016, 5, 1427. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Liu, T.; Gao, F.; Xie, H.; Sun, L.; Zhao, A.; Ren, W.; Guo, H.; Zhang, L.; Wang, H.; et al. Microvesicles as potential biomarkers for the identification of senescence in human mesenchymal stem cells. Theranostics 2017, 7, 2673–2689. [Google Scholar] [CrossRef]
- Heslop, J.A.; Hammond, T.G.; Santeramo, I.; Tort Piella, A.; Hopp, I.; Zhou, J.; Baty, R.; Graziano, E.I.; Proto Marco, B.; Caron, A.; et al. Concise review: Workshop review: Understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl. Med. 2015, 4, 389–400. [Google Scholar] [CrossRef]
- Takeuchi, M.; Higashino, A.; Takeuchi, K.; Hori, Y.; Koshiba-Takeuchi, K.; Makino, H.; Monobe, Y.; Kishida, M.; Adachi, J.; Takeuchi, J.; et al. Transcriptional dynamics of immortalized human mesenchymal stem cells during transformation. PLoS ONE 2015, 10, e0126562. [Google Scholar]
- Garcia, S.; Bernad, A.; Martin, M.C.; Cigudosa, J.C.; Garcia-Castro, J.; de la Fuente, R. Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp. Cell Res. 2010, 316, 1648–1650. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, A.; Rosland, G.V.; Bjerkvig, R. Spontaneous transformation of stem cells in vitro and the issue of cross-contamination. Int. J. Biol. Sci. 2012, 8, 1051–1054. [Google Scholar] [CrossRef]
- Torsvik, A.; Rosland, G.V.; Svendsen, A.; Molven, A.; Immervoll, H.; McCormack, E.; Lonning, P.E.; Primon, M.; Sobala, E.; Tonn, J.C.; et al. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: Putting the research field on track—Letter. Cancer Res. 2010, 70, 6393–6396. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Fouraschen, S.M.; de Ruiter, P.E.; Dinjens, W.N.; Kwekkeboom, J.; Tilanus, H.W.; van der Laan, L.J. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp. Biol. Med. 2014, 239, 105–115. [Google Scholar] [CrossRef]
- Andrews, P.W.; Ben-David, U.; Benvenisty, N.; Coffey, P.; Eggan, K.; Knowles, B.B.; Nagy, A.; Pera, M.; Reubinoff, B.; Rugg-Gunn, P.J.; et al. Assessing the safety of human pluripotent stem cells and their derivatives for clinical applications. Stem Cell Rep. 2017, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Arad, G.; Weissbein, U.; Mandefro, B.; Maimon, A.; Golan-Lev, T.; Narwani, K.; Clark, A.T.; Andrews, P.W.; Benvenisty, N.; et al. Aneuploidy induces profound changes in gene expression, proliferation and tumorigenicity of human pluripotent stem cells. Nat. Commun. 2014, 5, 4825. [Google Scholar] [CrossRef]
- Aguilar, S.; Nye, E.; Chan, J.; Loebinger, M.; Spencer-Dene, B.; Fisk, N.; Stamp, G.; Bonnet, D.; Janes, S.M. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells 2007, 25, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Centeno, C.J.; Schultz, J.R.; Cheever, M.; Robinson, B.; Freeman, M.; Marasco, W. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr. Stem Cell Res. 2010, 5, 81–93. [Google Scholar] [CrossRef][Green Version]
- Choumerianou, D.M.; Dimitriou, H.; Perdikogianni, C.; Martimianaki, G.; Riminucci, M.; Kalmanti, M. Study of oncogenic transformation in ex vivo expanded mesenchymal cells, from paediatric bone marrow. Cell Prolif. 2008, 41, 909–922. [Google Scholar] [CrossRef]
- Jones, M.; Varella-Garcia, M.; Skokan, M.; Bryce, S.; Schowinsky, J.; Peters, R.; Vang, B.; Brecheisen, M.; Startz, T.; Frank, N.; et al. Genetic stability of bone marrow-derived human mesenchymal stromal cells in the quantum system. Cytotherapy 2013, 15, 1323–1339. [Google Scholar] [CrossRef][Green Version]
- Lopez-Iglesias, P.; Blazquez-Martinez, A.; Fernandez-Delgado, J.; Regadera, J.; Nistal, M.; Miguel, M.P. Short and long term fate of human amsc subcutaneously injected in mice. World J. Stem Cells 2011, 3, 53–62. [Google Scholar] [CrossRef]
- Paula, A.C.; Martins, T.M.; Zonari, A.; Frade, S.P.; Angelo, P.C.; Gomes, D.A.; Goes, A.M. Human adipose tissue-derived stem cells cultured in xeno-free culture condition enhance c-myc expression increasing proliferation but bypassing spontaneous cell transformation. Stem Cell Res. 2015, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Roemeling-van Rhijn, M.; de Klein, A.; Douben, H.; Pan, Q.; van der Laan, L.J.; Ijzermans, J.N.; Betjes, M.G.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Culture expansion induces non-tumorigenic aneuploidy in adipose tissue-derived mesenchymal stromal cells. Cytotherapy 2013, 15, 1352–1361. [Google Scholar] [CrossRef]
- Scheers, I.; Lombard, C.; Paganelli, M.; Campard, D.; Najimi, M.; Gala, J.L.; Decottignies, A.; Sokal, E. Human umbilical cord matrix stem cells maintain multilineage differentiation abilities and do not transform during long-term culture. PLoS ONE 2013, 8, e71374. [Google Scholar] [CrossRef] [PubMed]
- Vilalta, M.; Degano, I.R.; Bago, J.; Gould, D.; Santos, M.; Garcia-Arranz, M.; Ayats, R.; Fuster, C.; Chernajovsky, Y.; Garcia-Olmo, D.; et al. Biodistribution, long-term survival, and safety of human adipose tissue-derived mesenchymal stem cells transplanted in nude mice by high sensitivity non-invasive bioluminescence imaging. Stem Cells Dev. 2008, 17, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, Z.B.; Song, Y.P.; Han, Z.C. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012, 2012, 652034. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Suzuki, K.; Qu, J.; Wang, P.; Zhou, J.; Liu, X.; Ren, R.; Xu, X.; Ocampo, A.; et al. Aging stem cells. A werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 2015, 348, 1160–1163. [Google Scholar] [CrossRef]
- Agostini, F.; Rossi, F.M.; Aldinucci, D.; Battiston, M.; Lombardi, E.; Zanolin, S.; Massarut, S.; Parodi, P.C.; da Ponte, A.; Tessitori, G.; et al. Improved gmp compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media. Stem Cell Res. 2018, 9, 130. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Zaffaroni, N.; Novara, F.; Cometa, A.M.; Avanzini, M.A.; Moretta, A.; Montagna, D.; Maccario, R.; Villa, R.; Daidone, M.G.; et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007, 67, 9142–9149. [Google Scholar] [CrossRef]
- Chen, G.; Yue, A.; Ruan, Z.; Yin, Y.; Wang, R.; Ren, Y.; Zhu, L. Human umbilical cord-derived mesenchymal stem cells do not undergo malignant transformation during long-term culturing in serum-free medium. PLoS ONE 2014, 9, e98565. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.; Santos, J.M.; de Almeida, J.M.; Filipe, M.A.; de Almeida, M.V.; Almeida, S.C.; Agua-Doce, A.; Varela, A.; Gilljam, M.; Stellan, B.; et al. Towards an advanced therapy medicinal product based on mesenchymal stromal cells isolated from the umbilical cord tissue: Quality and safety data. Stem Cell Res. 2014, 5, 9. [Google Scholar] [CrossRef]
- MacIsaac, Z.M.; Shang, H.; Agrawal, H.; Yang, N.; Parker, A.; Katz, A.J. Long-term in-vivo tumorigenic assessment of human culture-expanded adipose stromal/stem cells. Exp. Cell Res. 2012, 318, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Centeno, C.J.; Schultz, J.R.; Cheever, M.; Freeman, M.; Faulkner, S.; Robinson, B.; Hanson, R. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr. Stem Cell Res. 2011, 6, 368–378. [Google Scholar] [CrossRef]
- Veriter, S.; Andre, W.; Aouassar, N.; Poirel, H.A.; Lafosse, A.; Docquier, P.L.; Dufrane, D. Human adipose-derived mesenchymal stem cells in cell therapy: Safety and feasibility in different “hospital exemption” clinical applications. PLoS ONE 2015, 10, e0139566. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Awe, O.; Rao, R.C.; Kirby, P.A.; Hitchon, P.W. Autograft-derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: Case report. J. Neurosurg. Spine 2014, 21, 618–622. [Google Scholar] [CrossRef]
- Amariglio, N.; Hirshberg, A.; Scheithauer, B.W.; Cohen, Y.; Loewenthal, R.; Trakhtenbrot, L.; Paz, N.; Koren-Michowitz, M.; Waldman, D.; Leider-Trejo, L.; et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009, 6, e1000029. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (msc) paradigm: Polarization into a pro-inflammatory msc1 or an immunosuppressive msc2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- Waterman, R.S.; Henkle, S.L.; Betancourt, A.M. Mesenchymal stem cell 1 (msc1)-based therapy attenuates tumor growth whereas msc2-treatment promotes tumor growth and metastasis. PLoS ONE 2012, 7, e45590. [Google Scholar] [CrossRef]
- Cuiffo, B.G.; Karnoub, A.E. Mesenchymal stem cells in tumor development: Emerging roles and concepts. Cell Adh Migr. 2012, 6, 220–230. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Rhee, K.J.; Lee, J.I.; Eom, Y.W. Mesenchymal stem cell-mediated effects of tumor support or suppression. Int. J. Mol. Sci. 2015, 16, 30015–30033. [Google Scholar] [CrossRef]
- Djouad, F.; Plence, P.; Bony, C.; Tropel, P.; Apparailly, F.; Sany, J.; Noel, D.; Jorgensen, C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003, 102, 3837–3844. [Google Scholar] [CrossRef]
- Chen, B.; Yu, J.; Wang, Q.; Zhao, Y.; Sun, L.; Xu, C.; Zhao, X.; Shen, B.; Wang, M.; Xu, W.; et al. Human bone marrow mesenchymal stem cells promote gastric cancer growth via regulating c-myc. Stem Cells Int. 2018, 2018, 9501747. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, L.; Li, Y.; Zhang, X.; Gu, J.; Yan, Y.; Xu, X.; Wang, M.; Qian, H.; Xu, W. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012, 315, 28–37. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, Y.; Jiao, Z.; Wang, X.; Zhao, Y.; Li, Y.; Chen, H.; Yang, L.; Zhu, H. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell Physiol. Biochem. 2017, 42, 2242–2254. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Jiang, Q.; Deng, J.; Xu, F.; Chen, X.; Cheng, F.; Zhang, Y.; Yao, Y.; Xia, Z.; et al. Human adipose-derived mesenchymal stem cell-secreted cxcl1 and cxcl8 facilitate breast tumor growth by promoting angiogenesis. Stem Cells 2017, 35, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Mayshar, Y.; Benvenisty, N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell 2011, 9, 97–102. [Google Scholar] [CrossRef]
- Capelli, C.; Pedrini, O.; Cassina, G.; Spinelli, O.; Salmoiraghi, S.; Golay, J.; Rambaldi, A.; Giussani, U.; Introna, M. Frequent occurrence of non-malignant genetic alterations in clinical grade mesenchymal stromal cells expanded for cell therapy protocols. Haematologica 2014, 99, e94–e97. [Google Scholar] [CrossRef][Green Version]
- Duarte, D.M.; Cornelio, D.A.; Corado, C.; Medeiros, V.K.; de Araujo, L.A.; Cavalvanti, G.B., Jr.; de Medeiros, S.R. Chromosomal characterization of cryopreserved mesenchymal stem cells from the human subendothelium umbilical cord vein. Regen. Med. 2012, 7, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.C.; Torres, Y.; Benguria, A.; Dopazo, A.; Roche, E.; Carrera-Quintanar, L.; Perez, R.A.; Enriquez, J.A.; Torres, R.; Ramirez, J.C.; et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013, 4, e691. [Google Scholar] [CrossRef]
- Lucarelli, E.; Bellotti, C.; Mantelli, M.; Avanzini, M.A.; Maccario, R.; Novara, F.; Arrigo, G.; Zuffardi, O.; Zuntini, M.; Pandolfi, M.; et al. In vitro biosafety profile evaluation of multipotent mesenchymal stem cells derived from the bone marrow of sarcoma patients. J. Transl. Med. 2014, 12, 95. [Google Scholar] [CrossRef]
- Rebuzzini, P.; Zuccotti, M.; Redi, C.A.; Garagna, S. Chromosomal abnormalities in embryonic and somatic stem cells. Cytogenet. Genome. Res. 2015, 147, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sensebe, L.; Tarte, K.; Galipeau, J.; Krampera, M.; Martin, I.; Phinney, D.G.; Shi, Y. Limited acquisition of chromosomal aberrations in human adult mesenchymal stromal cells. Cell Stem Cell 2012, 10, 9–11. [Google Scholar] [CrossRef]
- Stultz, B.G.; McGinnis, K.; Thompson, E.E.; Lo Surdo, J.L.; Bauer, S.R.; Hursh, D.A. Chromosomal stability of mesenchymal stromal cells during in vitro culture. Cytotherapy 2016, 18, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Tarte, K.; Gaillard, J.; Lataillade, J.J.; Fouillard, L.; Becker, M.; Mossafa, H.; Tchirkov, A.; Rouard, H.; Henry, C.; Splingard, M.; et al. Clinical-grade production of human mesenchymal stromal cells: Occurrence of aneuploidy without transformation. Blood 2010, 115, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Weissbein, U.; Ben-David, U.; Benvenisty, N. Virtual karyotyping reveals greater chromosomal stability in neural cells derived by transdifferentiation than those from stem cells. Cell Stem Cell 2014, 15, 687–691. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Guan, L.X.; Zhang, K.; Wang, S.; Cao, P.C.; Wang, Y.H.; Wang, Z.; Dai, L.J. Cytogenetic analysis of human bone marrow-derived mesenchymal stem cells passaged in vitro. Cell Biol. Int. 2007, 31, 645–648. [Google Scholar] [CrossRef]
- Blazquez-Prunera, A.; Diez, J.M.; Gajardo, R.; Grancha, S. Human mesenchymal stem cells maintain their phenotype, multipotentiality, and genetic stability when cultured using a defined xeno-free human plasma fraction. Stem Cell Res. 2017, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, T.; Vaz, I.M.; Senegaglia, A.C.; Rebelatto, C.L.; Brofman, P.R. Genetic evaluation of mesenchymal stem cells by g-banded karyotyping in a cell technology center. Rev. Bras. Hematol. Hemoter. 2014, 36, 202–207. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Xu, X. Biological characteristics and karyotiping of a new isolation method for human adipose mesenchymal stem cells in vitro. Tissue Cell 2017, 49, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Poloni, A.; Maurizi, G.; Babini, L.; Serrani, F.; Berardinelli, E.; Mancini, S.; Costantini, B.; Discepoli, G.; Leoni, P. Human mesenchymal stem cells from chorionic villi and amniotic fluid are not susceptible to transformation after extensive in vitro expansion. Cell Transpl. 2011, 20, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Nikbakht, M.; Malek Mohammadi, A.; Zahed Panah, M.; Ostadali, M.R.; Nasiri, H.; Ghavamzadeh, A. Human platelet lysate as a xeno free alternative of fetal bovine serum for the in vitro expansion of human mesenchymal stromal cells. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 161–171. [Google Scholar] [PubMed]
- Sensebe, L.; Gadelorge, M.; Fleury-Cappellesso, S. Production of mesenchymal stromal/stem cells according to good manufacturing practices: A review. Stem Cell Res. 2013, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Mayshar, Y.; Benvenisty, N. Significant acquisition of chromosomal aberrations in human adult mesenchymal stem cells: Response to sensebe et al. Cell Stem Cell 2012, 10, 10–11. [Google Scholar] [CrossRef][Green Version]
- Li, R.; Sonik, A.; Stindl, R.; Rasnick, D.; Duesberg, P. Aneuploidy vs. Gene mutation hypothesis of cancer: Recent study claims mutation but is found to support aneuploidy. Proc. Natl. Acad. Sci. USA 2000, 97, 3236–3241. [Google Scholar] [CrossRef]
- Zimonjic, D.; Brooks, M.W.; Popescu, N.; Weinberg, R.A.; Hahn, W.C. Derivation of human tumor cells in vitro without widespread genomic instability. Cancer. Res. 2001, 61, 8838–8844. [Google Scholar] [PubMed]
- Prockop, D.J.; Keating, A. Relearning the lessons of genomic stability of human cells during expansion in culture: Implications for clinical research. Stem Cells 2012, 30, 1051–1052. [Google Scholar] [CrossRef]
- Binato, R.; de Souza Fernandez, T.; Lazzarotto-Silva, C.; Du Rocher, B.; Mencalha, A.; Pizzatti, L.; Bouzas, L.F.; Abdelhay, E. Stability of human mesenchymal stem cells during in vitro culture: Considerations for cell therapy. Cell Prolif. 2013, 46, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Buscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies—Bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhonde, R. Mesenchymal stromal cells are genetically stable under a hostile in vivo-like scenario as revealed by in vitro micronucleus test. Cytotherapy 2015, 17, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhonde, R. Influence of nuclear blebs and micronuclei status on the growth kinetics of human mesenchymal stem cells. J. Cell Physiol. 2015, 230, 657–666. [Google Scholar] [CrossRef]
- Ben-David, U.; Mayshar, Y.; Benvenisty, N. Virtual karyotyping of pluripotent stem cells on the basis of their global gene expression profiles. Nat. Protoc. 2013, 8, 989–997. [Google Scholar] [CrossRef]
- Von der Heide, E.K.; Neumann, M.; Vosberg, S.; James, A.R.; Schroeder, M.P.; Ortiz-Tanchez, J.; Isaakidis, K.; Schlee, C.; Luther, M.; Johrens, K.; et al. Molecular alterations in bone marrow mesenchymal stromal cells derived from acute myeloid leukemia patients. Leukemia 2017, 31, 1069–1078. [Google Scholar] [CrossRef]
- Azuma, K.; Umezu, T.; Imanishi, S.; Asano, M.; Yoshizawa, S.; Katagiri, S.; Ohyashiki, K.; Ohyashiki, J.H. Genetic variations of bone marrow mesenchymal stromal cells derived from acute leukemia and myelodysplastic syndrome by targeted deep sequencing. Leuk. Res. 2017, 62, 23–28. [Google Scholar] [CrossRef]
- Kouvidi, E.; Stratigi, A.; Batsali, A.; Mavroudi, I.; Mastrodemou, S.; Ximeri, M.; Papadaki, H.A.; Pontikoglou, C.G. Cytogenetic evaluation of mesenchymal stem/stromal cells from patients with myelodysplastic syndromes at different time-points during ex vivo expansion. Leuk. Res. 2016, 43, 24–32. [Google Scholar] [CrossRef]
- Mantelli, M.; Avanzini, M.A.; Rosti, V.; Ingo, D.M.; Conforti, A.; Novara, F.; Arrigo, G.; Boni, M.; Zappatore, R.; Lenta, E.; et al. Comprehensive characterization of mesenchymal stromal cells from patients with fanconi anaemia. Br. J. Haematol. 2015, 170, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, T.; Solarewicz, M.M.; Vaz, I.M.; Daga, D.; Rebelatto, C.L.; Senegaglia, A.C.; Ribeiro, E.; Cavalli, I.J.; Brofman, P.S. Emergence of clonal chromosomal alterations during the mesenchymal stromal cell cultivation. Mol. Cytogenet. 2015, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Bollag, R.J.; Yu, J.C.; Isales, C.M.; Eroglu, A. Chemically defined and xeno-free cryopreservation of human adipose-derived stem cells. PLoS ONE 2016, 11, e0152161. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Rahman, M.T.; Abu Kasim, N.H.; Alabsi, A.M. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Sci. World J. 2013, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Yong, K.W.; Safwani, W.; Xu, F.; Zhang, X.; Choi, J.R.; Abas, W.; Omar, S.Z.; Azmi, M.A.N.; Chua, K.H.; Pingguan-Murphy, B. Assessment of tumourigenic potential in long-term cryopreserved human adipose-derived stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 2217–2226. [Google Scholar] [CrossRef]
- Nomani, A.; Chen, X.; Hatefi, A. Evaluation of genotoxicity and mutagenic effects of vector/DNA nanocomplexes in transfected mesenchymal stem cells by flow cytometry. Acta Biomater. 2018, 74, 236–246. [Google Scholar] [CrossRef]
- Park, J.S.; Yi, S.W.; Kim, H.J.; Oh, H.J.; Lee, J.S.; Go, M.; Shim, S.H.; Park, K.H. Verification of long-term genetic stability of hmscs during subculture after internalization of sunflower-type nanoparticles (sf-nps). Theranostics 2018, 8, 5548–5561. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, D. The dangers of unregulated stem-cell marketing. Lancet 2017, 390, 1823–1824. [Google Scholar] [CrossRef]
- Sensebe, L.; Bourin, P.; Tarte, K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum. Gene 2011, 22, 19–26. [Google Scholar] [CrossRef]
- Ancans, J. Cell therapy medicinal product regulatory framework in europe and its application for msc-based therapy development. Front. Immunol. 2012, 3, 253. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, H.T.; D’Apote, L.; Schneider, C.K.; Herberts, C. The evolution of nonclinical regulatory science: Advanced therapy medicinal products as a paradigm. Mol. Ther. 2013, 21, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Frobel, J.; Goetzke, R. Epigenetic quality check—How good are your mesenchymal stromal cells? Epigenomics 2016, 8, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Daley, G.Q.; Hyun, I.; Apperley, J.F.; Barker, R.A.; Benvenisty, N.; Bredenoord, A.L.; Breuer, C.K.; Caulfield, T.; Cedars, M.I.; Frey-Vasconcells, J.; et al. Setting global standards for stem cell research and clinical translation: The 2016 isscr guidelines. Stem Cell Rep. 2016, 6, 787–797. [Google Scholar] [CrossRef]
- Barker, R.A.; Carpenter, M.K.; Forbes, S.; Goldman, S.A.; Jamieson, C.; Murry, C.E.; Takahashi, J.; Weir, G. The challenges of first-in-human stem cell clinical trials: What does this mean for ethics and institutional review boards? Stem Cell Rep. 2018, 10, 1429–1431. [Google Scholar] [CrossRef]
| Method | Detected Alterations | Resolution | Target | Characteristics and Limits | |
|---|---|---|---|---|---|
| Conventional Karyotype (G, Q banding) |  | Chromosomal rearrangements, aneuploidies, deletions, duplications | 5–10 Mb | Whole genome | Low throughput Only gross alterations. Interphase cells and low-frequency alterations not detected. |
| SKY (Spectral Karyotype) | 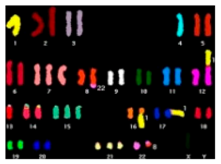 | Structural abnormalities (small and complex rearrangements), aneuploidies | 1–2 Mb | Whole genome | Inversions, deletions and duplications in the same chromosome not detected. Interphase cells and low-frequency alterations not detected. |
| Array-CGH (comparative genomic hybridization) |  | Deletions duplications | ≤50 kb | Whole genome | Low mosaicism (20%–30%) and balanced rearrangements not detected. |
| Virtual Karyotype (e-karyotype) | 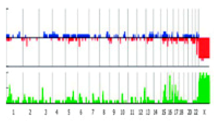 | Deletions, duplications | 20kb–1 Mb | Whole genome | Comparative large scale expression analysis. Can compare different dataset. |
| FISH Fluorescent in situ hybridization | 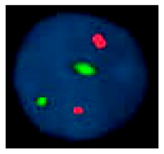 | Structural abnormalities/aneuploidies | 3–10 kb | Specific targets identified by probes | Can be performed in interphase cells. |
| Microsatellite instability (MSI) analysis | 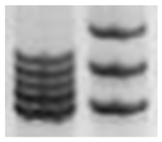 | Repeated sequences variations | 2–6 bp | Repeated sequences throughout the genome | Indirect indication of genomic instability |
| Single nucleotide polymorphism (SNP) array | 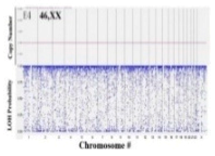 | Single base modifications | 1 bp | SNP throughout the genome | Detects single base variations in potentially hundreds of thousands of loci |
| gammaH2AX | 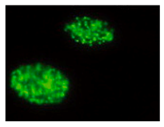 | Histone phosphorylation | Whole nuclei | Indirect measure of double-strand breaks | |
| Telomere length by Southern | 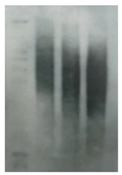 | Telomere attrition | 0.5–10 kb | All telomeres | Labor intensive and time-consuming. |
| Sanger sequencing | 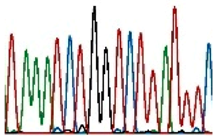 | Single base modifications/small deletions and duplications | 1 bp | Specific regions identified by primers | Do not detect low-frequency alterations. |
| Next generation sequencing (NGS) | 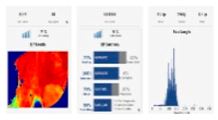 | Single base modifications/small deletions and duplications | 1 bp | Whole genome, exome, specific regions | High throughput, scalable. |
| Micronuclei test |  | Micronuclei and blebs | Whole nuclei | Macroscopic alterations, labor intensive and time-consuming, only semi-quantitative | |
| Comet assay | 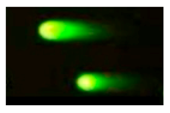 | Aspecific DNA fragmentation | Whole cells | Aspecific indication of DNA damage | |
| Droplet digital PCR |  | Single base variations | 1 bp | Specific regions identified by primers | Very high sensitivity (0.01%) |
| European Reference Legislation | |
| Regulation (EC) N° 1394/2007 implemented by regulation EC N° 668/2009 | Definition of ATMPs. Legal basis for authorization procedure of ATMPs. |
| Commission directive 2009/120/EC (amending directive 2001/83/EC) | Updated definition and detailed scientific technical requirements for gene-therapy and somatic cell therapy medicinal products combined ATMPs and tissue engineered products. |
| Commission directive 2004/23/EC implemented by directives 2006/17/EC and 2006/86/EC | Definition of quality and safety standards for donation, procurement and testing of human tissues and cells. |
| Regulation EU 536/2014 repealing directive 2001/20/EC | Implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. |
| Guidelines and Recommendations | |
| EMEA/CHMP/410869/2006 | Guideline on human cell-based medicinal products adopted in 2008. General overview of the requirements to license ATMPs. |
| EMEA/149995/2008 | Guideline on safety and efficacy follow-up and risk management of ATMPs. |
| EMA/630043/2008 | Update of the procedure for the evaluation of ATMPs marketing authorization, adopted in 2018. |
| EMA/CAT/571134/2009 | Reflection paper on stem cell-based medicinal products adopted in 2011. Focused on stem cell-based products. |
| EMA/763463/2009 | Public statement on concerns over unregulated medicinal products containing stem cells. |
| EMA/CAT/600280/2010 rev.1 | Revision of the reflection paper on the classification of ATMPs. Major points: what constitutes a substantial manipulation of cells or tissues; what is considered as a non-homologous use of cells or tissues. |
| EMA/CAT/CPWP/686637/2011 | Guideline on the risk-based approach for ATMPs’, adopted in 2013. |
| C (2017) 7694 Guidelines | Guideline on GMP specific for ATMPs. Recommendations: risks and effectiveness based on current scientific knowledge; level of effort and documentation commensurate with the risk. |
| EMA/CAT/327664/2018 | CAT work plan 2019 including the development of a new guideline on requirements for ATMPs in clinical trials and on ATMP comparability. |
| In preparation | EMA guideline on investigational ATMPs to create common standards for the assessment of novel ATMP products.Public consultation for the draft revised guideline EMEA/149995/2008) closed. Outcome expected in 2019. |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. https://doi.org/10.3390/ijms20102406
Neri S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. International Journal of Molecular Sciences. 2019; 20(10):2406. https://doi.org/10.3390/ijms20102406
Chicago/Turabian StyleNeri, Simona. 2019. "Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect" International Journal of Molecular Sciences 20, no. 10: 2406. https://doi.org/10.3390/ijms20102406
APA StyleNeri, S. (2019). Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. International Journal of Molecular Sciences, 20(10), 2406. https://doi.org/10.3390/ijms20102406