Abstract
Mutations in the insulin receptor (INSR) gene underlie rare severe INSR-related insulin resistance syndromes (SIR), including insulin resistance type A, Rabson–Mendenhall syndrome and Donohue syndrome (DS), with DS representing the most severe form of insulin resistance. Treatment of these cases is challenging, with the majority of DS patients dying within the first two years of life. rhIGF-I (mecasermin) has been reported to improve metabolic control and increase lifespan in DS patients. A case report and literature review were completed. We present a case involving a male patient with DS, harbouring a homozygous mutation in the INSR gene (c.591delC). Initial rhIGF-I application via BID (twice daily) injection was unsatisfactory, but continuous subcutaneous rhIGF-I infusion via an insulin pump improved weight development and diabetes control (HbA1c decreased from 10 to 7.6%). However, our patient died at 22 months of age during the course of a respiratory infection in in Libya. Currently available data in the literature comprising more than 30 treated patients worldwide seem to support a trial of rhIGF-I in SIR. rhIGF-I represents a treatment option for challenging SIR cases, but careful consideration of the therapeutic benefits and the burden of the disease is warranted. Continuous application via pump might be advantageous compared to single injections.
1. Introduction
The etiology and clinical presentation of severe insulin resistance is highly variable [1], with severe insulin receptor (INSR)-related insulin resistance syndromes (SIR) exhibiting a peculiar phenotypical spectrum. Within SIR, there is a continuum of insulin resistance, ranging from Donohue syndrome (DS) with no remaining insulin receptor function to the milder phenotypes of Rabson–Mendenhall syndrome (RMS) and type A insulin resistance [2,3].
Donohue syndrome was initially described in 1948 and 1954 by Donohue and Uchida [4]. Patients with DS exhibit severe intrauterine and postnatal growth retardation. Infants present with typical facial feature, resembling Leprechaun’s elves of Irish fairy tales, with lipoatrophy, acanthosis nigricans, hypertrichosis and severe hyperinsulinism, postprandial hyperglycemia and fasting hypoglycemia (for further clinical characteristics see Table 1). Most children with DS die within the first two years of life, mostly during the course of intercurrent infections of the upper airways, hypoglycemia or cardiomyopathy [3,5,6,7,8]. To date, there is no causative therapy for this very rare disease available (prevalence of DS < 1:1 Mio [9]). Rabson–Mendenhall syndrome was first described 1956 in three siblings with dental and skin abnormalities [10]. Children suffering from Rabson–Mendenhall syndrome typically show a milder phenotype [1], including impaired growth, abnormal nails and dentition as well as insulin resistance with acanthosis nigricans and hirsutism. At birth, patients with RMS also show fasting hypoglycemia due to severely increased insulin levels, but with progression of the disease, insulin levels decline [11]. In contrast to DS, patients develop recurrent diabetic ketoacidosis and microvascular complications during the second decade of life [2,5].

Table 1.
Characteristic clinical features of subjects with Donohue syndrome (DS), Rabson–Mendenhall Syndrome (RMS) and Type A Insulin Resistance (Type A IR) [3,5,6,7,8,10,49,50,56,57,58,59,60,61,62,63].
Diabetic ketoacidosis does not seem to occur in patients with Donohue syndrome [12], at least not in the first years of life [13]. Type A insulin resistance represents the least severe end of the spectrum [2]. It is usually diagnosed in teenagers or young adults who are not obese, but have severe insulin resistance, acanthosis nigricans, and hyperandrogenism in females. In some, but not all cases, a heterozygous genetic defect in the INSR gene has been reported [3].
Any condition of severe insulin resistance is therapeutically challenging with only limited treatment options being available. High dose insulin [14,15] and metformin [16] have been used in patients with insulin resistance syndromes. Metreleptin has been reported to improve blood glucose levels individuals with Rabson–Mendenhall syndrome [17,18].
Due to the structural similarity of insulin, proinsulin, insulin-like growth factor-I (IGF-I) and IGF-II and resemblances of the insulin and IGF-I receptor, IGF-I was proposed as another treatment option for patients with severe insulin resistance [19,20,21]. IGF-I use was first reported in the 1990s as an experimental treatment for patients with SIR, mostly with type A insulin resistance [22,23,24,25,26,27,28]. In the majority of the available case descriptions of SIR, an improvement in glucose homeostasis and a reduction in HbA1c was reported [21]. In addition to type A insulin resistance, mecasermin treatment of few patients with congenital insulin resistance syndromes with biallelic mutations within the insulin receptor gene (INSR) has been reported to improve glycemic control and weight gain [29,30,31,32] with variable results. In addition to the treatment option for SIR, rhIGF-I has been used previously in patients with growth hormone insensitivity syndrome [33,34,35,36,37,38,39,40,41,42]. Since 2008, rhIGF-I has been approved for the treatment of so-called “severe primary IGF-I deficiency” (SPGFD) [43,44].
This paper adds a case description, in which mecasermin treatment via an insulin pump successfully improved glucose homeostasis in a patient with DS and gives an update on the currently available knowledge on the clinical picture, treatment options and complications of these rare insulin resistance syndromes.
2. Materials and Methods
A case report and review of existing knowledge regarding the efficiency and safety of mecasermin in insulin receptor-related severe insulin resistance syndromes were completed using a systematic literature review with utilization of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines. We focused on the treatment of patients with DS, and performed a literature research in the Pubmed database using the following search terms:
“mecasermin”, “insulin resistance and recombinant IGF-I”, “Insulin resistance and recombinant IGF1”, “Donohue syndrome and IGF-I”, “Donohue syndrome and IGF1”, “Rabson–Mendenhall syndrome and IGF-I”, “Rabson–Mendenhall syndrome and IGF1”, “type A insulin resistance and IGF-I”, type A insulin resistance and IGF1”.
Thereby, we identified 189 records through database searching and 49 additional records which were identified through other sources, e.g., reference lists of other articles. The removal of duplicates led to 112 remaining records. After screening these records, 81 full-text articles were assessed for eligibility.
All articles were included in the synthesis of this paper. Because all studies reported only small numbers or presented single cases, a systematic meta-analysis could not be performed.
3. Case Report
We report on a male infant, who was born at term with a birth weight of 1300 g (−3.86 standard deviation scores; SDS) in Tripolis, Libya. At birth, typical dysmorphic features of DS could already be observed. In particular, muscle hypotonia and hyperglycemia with associated hyperinsulinism led to a clinical suspicion of an insulin receptor (IR)-related severe insulin resistance syndrome. His parents stated that they were very distantly related but were unable to provide more detailed information. Molecular analysis revealed a homozygous mutation of the insulin receptor gene in exon 2 (c.591delC). This frameshift mutation led to a nonsense mRNA and to a non-functioning insulin receptor, explaining the clinical picture of DS. Initial therapy consisted of a trial with metformin and insulin (no dosages available), which did not exert a relevant effect on glucose homeostasis. The patient presented first at our hospital at an age of 8 months, with a weight of 4020 g (<1st percentile, −5.5 SDS) and a height of 56 cm (<1st percentile, −5.61 SDS). He was in poor general health and in an extremely dystrophic nutritional state. Despite reduced subcutaneous fat tissue; he exhibited pronounced acanthosis nigricans and hypertrichosis. His skin texture throughout his whole body was remarkably dry and thick. Biochemical analysis revealed an HbA1c concentration of 10.0% (86 mmol/mol), with very high insulin (>1700 mU/L) and C-peptide (>4.5 ng/mL) levels, accompanied by undetectable IGF-I (<−25 ng/mL) and IGFBP-3 (<0.5 μg/mL) concentrations.
An abdominal ultrasound demonstrated hyperechogenic kidneys. Echocardiography showed a small, but hypertrophic left ventricle. Ophthalmological examination and otolaryngeal status, including auditory brainstem response audiometry, was without pathological findings, except for mucous obstruction of the upper airways.
Based on previous publications of patients suffering from either type A insulin resistance or DS, who were successfully treated with rhIGF-I [1,12,30], we decided to start a mecasermin trial. The parents’ informed consent was obtained after they were given detailed information on the therapy and medication.
The initial treatment consisted of rhIGF-I application via BID injection. The initial dosage consisted of 2 × 200 μg mecasermin, which was escalated to a final dose of 2 × 1 mg/day, corresponding to a dosage of 0.5 mg/kg body weight per day over a period of one week. However, since we found no relevant effect on diurnal glucose control, and rather, an aggravation of fasting hypoglycemia during the night, we decided to test continuous subcutaneous mecasermin infusion using an insulin pump. Mecasermin was diluted with physiological NaCl infusion solution, with a final rhIGF-I dosage of 1.8 mg/day (see Figure 1).
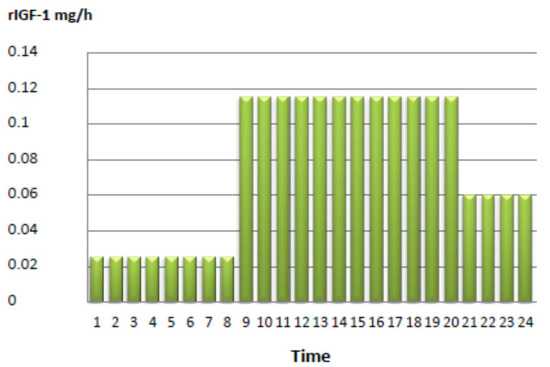
Figure 1.
Mecasermin delivery in mg/h via an insulin pump in the reported patient.
After discharge, the family travelled back to Libya. Because of the distance to the family’s home country and the difficult political situation in Libya, it was not possible to see the patient and his parents more frequently than every four to six months. During these follow-up examinations anthropometry, clinical status, abdominal and thymus ultrasound, bone age, echocardiography, otolaryngeal and ophthalmological status as well as several biochemical analyses (IGF-I, IGFBP-3, glucose homeostasis, insulin level, HbA1c, cholesterol, growth hormone, TSH, ft4, prolactin, blood count, electrolytes, renal and liver parameters) were monitored. In between visits, we had regular email correspondence with the parents, and the child was seen by a diabetologist in his home country.
We saw the patient again at an age of 13 months. Even though the family was highly motivated to use the rhIGF-I infusion, intermittent therapy was not possible because the family could not get the medication in their home country due to logistical problems. Meanwhile the patient lost weight, now weighing 3300 g. His HbA1c was still 10%. We reinitiated the therapy, and this time the family took the medication from Germany to Libya for the next 6 months. At an age of 18 months, after completion of six months of continuous subcutaneous rhIGF-I infusion via insulin pump, the patient presented again in our hospital. His weight was 5250 g (−5.65 SDS). His HbA1c had improved from 10.0 to 7.6%. Blood glucose trends were more stable than they had been without mecasermin therapy (see Figure 2).
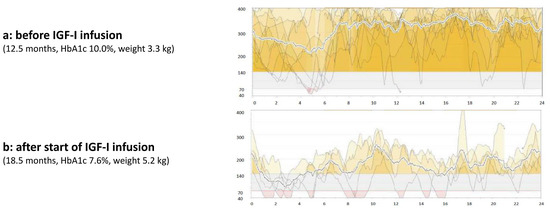
Figure 2.
Glucose profile using a continuous glucose monitoring system (CGMS) before (a) and after (b) initiation of continuous subcutaneous mecasermin infusion via an insulin pump.
In addition, we saw a moderate improvement in motor function and muscular strength. The renal ultrasound, echocardiography and ophthalmological examination results were unchanged relative to those collected before treatment with mecasermin. The otolaryngeal examination revealed adenoid hypertrophy as a potential adverse event of rhIGF-I therapy, leading to an intermittent nocturnal oxygen requirement. Adenoidectomy and tympanic paracentesis were performed. Postoperatively, weaning from ventilation and extubation was complicated by rapid desaturation and hypoxia, requiring cardiopulmonary reanimation (CPR) and subsequent ventilation for several days. Following stabilization, the patient was discharged, and continued with continuous rhIGF-I infusion as described above. Unfortunately, four months after discharge at an age of 22 months the patient died during the course of a respiratory infection in a hospital in Libya.
4. Overview of Current Knowledge on the Use of rhIGF-I in SIR
4.1. Clinical Picture of SIR
As described above, INSR-related severe insulin resistance compromises a phenotypical continuum, with the most severe phenotype seen in DS (leprechaunism) and a milder phenotype in RMS [3], with type A insulin resistance being a relatively mild form of SIR. The INSR gene maps to the short arm of chromosome 19 and is composed of 22 exons [45].
DS and RMS are caused by autosomal recessive mutations of the INSR gene. There is a limited correlation between genotype and phenotype [45]. The most severe phenotypes result from mutations that markedly impair insulin binding. Mutations in the insulin receptor that retain residual insulin-binding are correlated with prolonged survival [45,46]. However, a definitive genotype–phenotype correlation for INSR defects is difficult to establish, primarily due to the rarity of these syndromes [46]. Dysmorphic features of DS and RMS resemble each other (see Table 1), but are less apparent in RMS. Some clinical aspects, like hirsutism or genital enlargement sometimes seem to appear later in RMS [3].
Children with DS present frequently with severe global developmental delay [8], which might be caused by recurrent severe hypoglycemic episodes [47]. Without intervention, death occurs within the first two years of life. Respiratory infections, hypoglycemia [5,6] and cardiomyopathy [7,8] seem to be the major causes of death [3]. Patients who were characterized as leprechaunism and showed long-term survival and normal psychomotor development [48] might have had benefit from early rhIGF-I therapy or should rather have been classified as a patient with RMS [3].
The manifestation of type A insulin resistance occurs commonly around puberty. Patients are normally not obese (in contrast to the majority of polycystic ovary syndrome (PCOS) subjects) and suffer from hyperandrogenism (PCOS in females, hirsutism, acne) and insulin resistance (diabetes, acanthosis nigricans). In subjects with type A insulin resistance, growth in infancy and childhood is normal. There is no intellectual impairment [12,49] (Table 1). Some cases can be attributed to a detectable heterozygous genetic defect in the INSR gene (most cases are autosomal-dominant, but autosomal-recessive cases have been reported as well).
Simpkin et al. [50] reported about 17 patients (eight with a complete dataset) with INSR mutations and a clinical diagnosis of DS or RMS. INSR dysfunction was associated with hypercalciuria and nephrocalcinosis, but no other consistent abnormality of renal function was noted. These results were in contrast to results in genetically modified mice, which showed elevated blood pressure and progressive diabetic nephropathy [51]. Differences between mice and men have also been observed for other aspects of the phenotype of insulin receptor deficiency [50]. INSR knockout mice die soon after birth because of ketoacidosis [52], whereas diabetic ketoacidosis does not seem to occur in human patients with Donohue syndrome [53], at least not in the first years of life [13]. Complete deletion of the insulin receptor gene is compatible with life in men [54].
To achieve the best possible treatment of children with DS and RMS, a close collaboration between several subspecialties is required. Paediatric endocrinologists are typically in charge of assessing glycemic control and respective treatment regimens, paediatric cardiologists monitor hypertrophic cardiomyopathy, and neuropaediatricians are involved in monitoring neurological development and the initiation of supportive care. A screening protocol regarding the occurrence of nephrocalcinosis and/or impaired renal function as well as liver function should be initiated. Adequate nutrition and caloric intake are of paramount importance to improve blood sugar concentrations, weight gain and growth. Routine ultrasound examinations should be performed to control the morphology of ovaries in girls as well as the morphology of the kidneys, liver and spleen in both sexes [3]. In subjects in whom rhIGF-I treatment is started, repetitive fundoscopy and otolaryngeal examinations should be scheduled. Because of dental crowding, orthodontic treatment could be necessary [55,56].
4.2. Treatment Options in SIR Other Than rhIGF-I
Hyperglycaemia in patients with insulin receptor mutations is extremely difficult to treat [12,18], and patients are at risk for early morbidity and mortality from the microvascular complications of diabetes [18,57]. In particular, DS has a poor prognosis with only few therapeutic options being available. In subjects with type A insulin resistance or RMS, the use of insulin sensitizing drugs, such as metformin or rosiglitazone, should be first-line therapy. However, previous reports have indicated that a positive therapeutic impact is only be observed in a fraction of patients and that, over time, the effect of these drugs seems to diminish [64]. Frequently, affected patients require multidrug therapy [64,65].
Moreira et al. [65] reported a successful reduction of HbA1c in a patient with RMS using a combination of nutritional counseling, physical activity, and the following medications: metformin, pioglitazone, vildagliptin and acarbose. The authors mentioned that the use of dipeptidylpeptidase-4 inhibitors might be a promising new approach for patients with RMS.
In cases for whom insulin therapy is initiated, patients require very high insulin doses and therefore, in most cases, U-500 insulin is used for treatment [12,14,66,67]. In patients treated with U-500 insulin, continuous subcutaneous insulin infusion (CSII) is a viable option [12]. While common therapeutic targets may not be achievable in SIR patients, large doses of insulin can improve hyperglycemia, catabolic state and weight loss and reduce the risk of microvascular complications [14]. Metreleptin has been reported to improve glucose metabolism in subjects with genetic lipodystrophic syndromes, and it has been reported to be a potential therapeutic option in patients with RMS [17,18]. In that regard, Brown et al. [18] treated five patients with RMS with metreleptin and were able to show a decrease in HbA1c, from 11.4% at baseline to 9.3% after six months and 9.7% after 12 months of metreleptin treatment. However, the authors also reported that these beneficial effects of metreleptin on HbA1c concentration appeared to wane over time, and metreleptin treatment, with or without insulin, in the long-term was insufficient to achieve targeted HbA1c levels. There has been a single observation in a patient with type A insulin resistance available which reported a remarkable improvement of glycemic control after starting a suppressive dose of levothyroxine [68]. Since the thyroid hormone is an activator of brown adipose tissue and because positron emission tomography (PET) showed the presence of brown adipose tissue in this patient, the activation of brown adipose tissue might be a new therapeutic target of SIR [69]. In DS, metformin and insulin treatment usually do not improve glycaemic control [12,57]. To the best of our knowledge, successful metreleptin treatment has not been reported in DS yet. Frequent or continuous gastric feeding seems to be efficient for preventing fasting hypoglycaemia in patients with DS [12]. Table 2 summarizes the different treatment options in patients with SIR.

Table 2.
Therapeutic efficiency of available treatment options in SIR.
4.3. Use of rhIGF-I in Insulin Receptor-Related Severe Insulin Resistance Syndromes
Because of its previously reported effect on glucose homeostasis, rhIGF-I has been postulated as a potential therapeutic option in SIR syndromes. The IGF-I gene is a major target of growth hormone (GH) and an important mediator of GH-stimulated growth, but exerts different effects on carbohydrate, lipid and protein metabolism compared to GH. IGF-I stimulates a reduction in blood glucose and circulating insulin levels and therefore, can cause hypoglycaemia [70]. This effect is in part mediated through IGF-I’s structural similarity with insulin and its affinity to the insulin and IGF-I receptors, which share common post-receptor signaling pathways [19,20]. In vitro studies using adenoviral IGF-I gene transfer into ß-cells have shown the promotion of ß-cell survival and proliferation [71]. Furthermore, islet cell exposure towards IGF-I seems to sustain or increase insulin synthesis [72]. Therefore, an increase in the circulating insulin concentration under conditions where a residual insulin effect is maintained, either through insulin/IGF-hybrid receptors or through mutated insulin receptors with residual IR function, might lead to an improvement in glucose homeostasis. In SIR, the effects of rhIGF-I on glucose homeostasis are thought to occur mainly through increased glucose uptake into peripheral tissues (e.g., muscle) and a reduction in hepatic glucose production (see Figure 3).
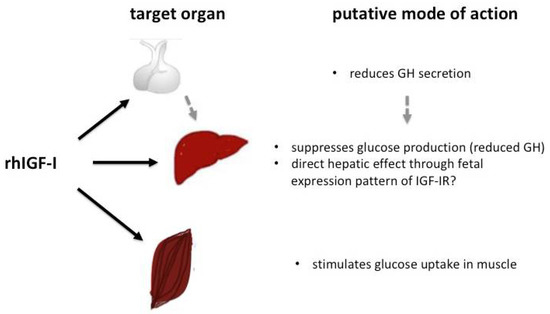
Figure 3.
Putative effects of rhIGF-I on glucose homeostasis.
The first reports of rhIGF-I use in patients with SIR were published in the 1990s. Single-dose intravenous administration of rhIGF-I with a dosage of 100 μg/kg/day was associated with declines in blood glucose, plasma insulin, C-peptide and GH concentrations [23,24]. These initial trials were followed by trials using subcutaneous application of rhIGF-I [22,25,26,27,28,63,73]. Kuzuya [73] first described the usage of rhIGF-I over a period of several months (up to 16 months) as leading to a decrease in HbA1c concentration in patients with SIR. Most publications are either single case reports or include only few (up to six) patients with an SIR syndrome, with pronounced differences in clinical phenotypes (from DS over RMS to Type A IR). Therefore, a direct comparison of the respective treatment effects between the mentioned case reports/small studies is not possible. Table 3 gives a summary of different rhIGF-I trials reporting either short or long-term effects of rhIGF-I in SIR, including trials with no apparent benefit of rhIGF-I in the treatment of patients with SIR syndromes [74,75]. Backeljauw [75] and coworkers treated two infants classified as DS with intravenous rhIGF-I over a period of 66 and 62 h, respectively, and could not see any apparent glucose lowering or a decrease in insulin concentration. A potential explanation for this might be that the effect of rhIGF-I on glucose metabolism in SIR is just as heterogenous as the syndrome itself [74]. In addition to SIR, there are some single case reports regarding successful long-term use of rhIGF-I in patients with leprechaunism [29,31]. In the patient reported above, we were not able to see an improvement of glucose homeostasis by administration of BID mecasermin application. However, we cannot exclude that a higher dosage or more prolonged trial of mecasermin application might have led to a more favourable response [29,31,32] (see also Table 3). Still, the observation of more satisfactory results on blood glucose homeostasis using a continuous application of rhIGF-I via an insulin pump is in accordance with other reports [30,76]. The half-life of insulin-like growth factor I seems to be decreased in patients with insulin receptor defects, which, in part, can be explained by drastically decreased or absent IGF binding protein-3 concentrations. In order to analyse why rhIGF-I is not consistently effective in patients with Rabson–Mendenhall syndrome, Longo et al. [63] measured IGF-I concentrations in a patient after subcutaneous injections. The IGF-I half-life was dramatically reduced (1.3–3 h) compared to the normal range of 17–22 h in healthy subjects. This might partly explain why the use of “moderate” mecasermin dosages of 0.5 mg/kg/day, or even less via continuous subcutaneous infusion, can lead to results that are as satisfactory as those from the use of a very high dose of rhIGF-I through a single injection (for example 1.6 mg/kg/day [29]).

Table 3.
Treatment trials with rhIGF-I in patients with severe INSR-related insulin resistance syndromes (SIR).
In addition to pharmacokinetic influences on treatment success, functional analyses of the INSR gene can aid in the prediction of the severity of an INSR mutation and its associated disease phenotype [76] and thereby, might aid in predicting the effectiveness of rhIGF-I treatment. Furthermore, the timing of treatment has been associated with the treatment response. Early treatment with rhIGF-I has been reported to increase the lifespan of patients with DS or Rabson–Mendenhall syndrome [29,76]. However, the effectiveness of rhIGF-I therapy seems to decrease with progression of the disease [77].
Mecasermin use has been associated with a number of adverse effects, in particular hypoglycaemia, pain at injection side and lymphoid tissue hypertrophy. Lymphoid tissue hypertrophy has been described in about 25% of all patients who were treated with mecasermin and is associated with the requirement of surgery in >10% of patients [20,80] (see Table 4).

Table 4.
Adverse effects of mecasermin treatment in conditions other than SIR and in patients with SIR.
In our case report, after six months of mecasermin treatment, our patient underwent surgery for probably treatment-related development of adenoid hypertrophy. Nakae, Kitamei and Jo et al. [29,77,78] reported information about one patient at different time points over the clinical course. The patient was treated with rhIGF-I from an age of six months until 18 years, with further follow-up to an age of 24 years. During this time course, the patient developed several symptoms, which could be interpreted as adverse effects of rhIGF-I therapy, although disease-inherent development of the complications cannot be ruled out: tonsillar hypertrophy (at three years of age), retinal neovascularization (at 12 years of age), mastitis (at 16 years) and endometrial cancer (at 24 years of age). In another patient with DS [30], the development of a granulosa cell tumor was reported, but its relationship to concomitant rhIGF-I therapy remains unclear, since a comparable tumour developed in a girl with leprechaunism who was not treated with rhIGF-I [81]. Other authors did not observe any side effects of rhIGF-I therapy in patients with SIR over a period of five [31] or 10 years [48]. Table 4 summarizes the possible adverse effects of mecasermin therapy in general and the reported side effects of its use in SIR.
In addition to the putative adverse effects depicted in Table 4, children with DS seem to have an increased risk during general anaesthesia [49]. The increase in risk might be related to the presence of restrictive lung disease, abdominal distension and difficult airway conditions. Kirkwood presented a case series of five patients with DS who underwent 12 treatments with general anaesthesia. Two anaesthesia treatments (in two patients) were complicated by cardiac arrest, secondary to difficult manual ventilation, intubation, and hypoxia and required CPR, resembling our case report.
5. Conclusions
The treatment of SIR syndromes is challenging. Even though there are some treatment options available for patients with a Type A insulin resistance phenotype—the mildest form of the INSR-related SIR syndromes—general treatment goals, e.g., long-term normalization of HbA1c cannot be achieved in most cases. The effects of all medications mentioned above (insulin sensitizer, insulin, metreleptin, mecasermin) seem to diminish over time.
The optimal timing, application form and dosage of rhIGF-I still remain unclear, but this seems to be the most effective type of therapy in patients with DS so far. Continuous application via pump might be advantageous compared to single injections.
Further treatment options are clearly needed to improve the quality of life and life expectancy of patients with SIR syndromes. Careful consideration of the therapeutic benefits and the burden of the disease is warranted.
Author Contributions
Michaela Plamper and Joachim Woelfle conceived and designed the study protocol and discussed treatment strategies. Michaela Plamper, Felix Schreiner, Bettina Gohlke and Joachim Woelfle contributed specific parts of the paper. All authors approved the submitted version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McDonald, A.; Williams, R.; Regan, F.M.; Semple, R.K.; Dunger, D.B. IGF-I treatment of insulin resistance. Eur. J. Endocrinol. 2007, 157, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Parker, V.E.R.; Semple, R.K. Genetic Forms of Severe Insulin Resistance: What Endocrinologists Should Know. Eur. J. Endocrinol. 2013, 169, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ben Harouch, S.; Falik Zaccai, T.C.; Klar, A. INSR-Related Severe Syndromic Insulin Resistance. In GeneReviews® [Internet]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2018; Available online: http://www.ncbi.nlm.nih.gov/books/NBK476444/ (accessed on 25 January 2018).
- Donohue, W.L.; Uchida, I. Leprechaunism: A euphemism for a rare familial disorder. J. Pediatr. 1954, 455, 505–519. [Google Scholar] [CrossRef]
- Elders, M.J.; Schedewie, H.K.; Olefsky, J.; Givens, B.; Char, F.; Bier, D.M.; Baldwin, D.; Fiser, R.H.; Seyedabadi, S.; Rubenstein, A. Endocrine-metabolic relationships in patients with leprechaunism. J. Natl. Med. Assoc. 1982, 74, 1195–1210. [Google Scholar] [PubMed]
- De Bock, M.; Hayes, I.; Semple, R. Donohue syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 1416–1417. [Google Scholar] [CrossRef] [PubMed]
- Grasso, V.; Colombo, C.; Favalli, V.; Galderisi, A.; Rabbone, I.; Gombos, S.; Bonora, E.; Massa, O.; Meschi, F.; Cerutti, F.; et al. Six cases with severe insulin resistance (SIR) associated with mutations of insulin receptor: Is a Bartter-like syndrome a feature of congenital SIR? Acta Diabetol. 2013, 50, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Falik Zaccai, T.C.; Kalfon, L.; Klar, A.; Ben Elisha, M.; Hurvitz, H.; Weingarten, G.; Chechik, E.; Fleisher Sheffer, V.; Haj Yahya, R.; Meidan, G.; et al. Two novel mutations identified in familial cases with Donohue syndrome. Mol. Genet. Genom. Med. 2014, 2, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Desbois-Mouthon, C.; Girodon, E.; Ghanem, N.; Caron, M.; Pennerath, A.; Conteville, P.; Magre, J.; Besmond, C.; Goossens, M.; Capeau, J.; et al. Molecular analysis of the insulin receptor gene for prenatal diagnosis of leprechaunism in two families. Prenat. Diagn. 1997, 17, 657–663. [Google Scholar] [CrossRef]
- Rabson, S.M.; Mendenhall, E.N. Familial hypertrophy of pineal body, hyperplasia of adrenal cortex and diabetes mellitus. Am. J. Clin. Pathol. 1956, 26, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Wang, Y.; Pasquali, M. Progressive decline in insulin levels in Rabson-Mendenhall syndrome. J. Clin. Endocrinol. Metab. 1999, 84, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Semple, R.; Williams, R.; Dunger, D.B. What is the best management strategy for patients with severe insulin resistance? Clin. Endocrinol. 2010, 73, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Semple, R.K.; Savage, D.B.; Cochran, E.K.; Gorden, P.; O’Rahilly, S. Genetic syndromes of severe insulin resistance. Endocr. Rev. 2011, 32, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Cochran, E.; Musso, C.; Gorden, P. The use of U-500 in patients with extreme insulin resistance. Diabetes Care 2005, 28, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Huggard, D.; Stack, T.; Satas, S.; Gorman, C.O. Donohue syndrome and use of continuous subcutaneous insulin pump therapy. BMJ Case Rep. 2015, 27, 2015. [Google Scholar] [CrossRef] [PubMed]
- Atabek, M.E.; Pirgon, O. Some effect of metformin on insulin resistance in an infant with leprechaunism. J. Pediatr. Endocrinol. Metab. 2006, 19, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Cochran, E.; Young, J.R.; Sebring, N.; DePaoli, A.; Oral, E.A.; Gorden, P. Efficacy of recombinant methionyl human leptin therapy for the extreme insulin resistance of the Rabson-Mendenhall syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Cochran, E.; Gorden, P. Metreleptin improves blood glucose in patients with insulin receptor mutations. J. Clin. Endocinol. Metab. 2013, 98, E1749–E1756. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, A.; Gray, A.; Tam, A.W.; Yanf-Feng, T.; Tsubokawa, M.; Collin, C.; Henzel, W.; Le Bon, T.; Kathuria, S.; Chen, E. Insulin-like growth factor I receptor primary structure: Comparison with insulin receptor suggests structural determinants that define functional specifity. EMBO J. 1986, 5, 2503–2512. [Google Scholar] [PubMed]
- Rosenbloom, A.L. Mecasermin (recombinant human insulin-like growth factor I). Adv. Ther. 2009, 26, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; McDonald, A.; O’Savage, M.; Dunger, D.B. Mecasermin rinfabate: rhIGF-I/rhIGFBP-3 complex: iPLEX. Expert Opin. Drug Metab. Toxicol. 2008, 4, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Froesch, E.R. Treatment of type A insulin resistance with insulin-like growth factor-I. Lancet 1993, 3418859, 1536–1537. [Google Scholar] [CrossRef]
- Quin, J.D.; Fisher, B.M.; Paterson, K.R.; Inoue, A.; Beastall, G.H.; MacCuish, A.C. Acute response to recombinant insulin-like growth factor I in a patient with Mendenhall’s syndrome. N. Engl. J. Med. 1990, 323, 1425–1426. [Google Scholar] [PubMed]
- Schoenle, E.J.; Zenobi, P.D.; Torresani, T.; Werder, E.A.; Zachmann, M.; Froesch, E.R. Recombinant human insulin-like growth factor I (rhIGF I) reduces hyperglycaemia in patients with extreme insulin resistance. Diabetologia 1991, 34, 675–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zenobi, P.D.; Glatz, Y.; Keller, A.; Graf, S.; Jaeggi-Groisman, S.E.; Riesen, W.F.; Schoenle, E.J.; Froesch, E.R. Beneficial metabolic effects of insulin-like growth factor I in patients with severe insulin-resistant diabetes type A. Eur. J. Endocrinol. 1994, 131, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, H.; Rossen, M.; Urhammer, S.A.; Müller, J.; Pedersen, O. Short- and long-term metabolic effects of recombinant human IGF-I treatment in patients with severe insulin resistance and diabetes mellitus. Eur. J. Endocrinol. 1997, 136, 475–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morrow, L.A.; O’Brien, M.B.; Moller, D.E.; Flier, J.S.; Moses, A.C. Recombinant human insulin-like growth factor-I therapy improves glycemic control and insulin action in the type A syndrome of severe insulin resistance. J. Clin. Endocrinol. Metab. 1994, 79, 205–210. [Google Scholar] [PubMed]
- Nakashima, N.; Umeda, F.; Yanase, T.; Nawata, H. Insulin resistance associated with substitution of histidine for arginine 252 in the alpha-subunit of the human insulin receptor: Trial of insulin-like growth factor I injection therapy to enhance insulin sensitivity. J. Clin. Endocrinol. Metab. 1995, 80, 3662–3667. [Google Scholar] [PubMed]
- Nakae, J.; Kato, M.; Murashita, M.; Shinohara, N.; Tajima, T.; Fujieda, K. Long-term effect of recombinant human insulin-like growth factor I on metabolic and growth control in a patient with leprechaunism. JCEM 1998, 83, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Stanescu, D.; Semple, R.; Holland, C.; Magge, S.N. Continuous, subcutaneous IGF-I therapy via insulin pump in patient with Donohue Syndrome. J. Pediatr. Endocrinol. Metab. 2014, 27, 1237–1241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carmody, D.; Ladsaria, S.S.; Buikema, R.K.; Semple, R.K.; Greeley, S.A. Case Report: Successful rhIGF1 treatment for over 5 years in a patient with severe insulin resistance due to homozygous insulin receptor mutation. Diabet. Med. 2016, 33, e8–e12. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kadowaki, H.; Momomura, K.; Fukushima, Y.; Orban, T.; Okai, T.; Taketani, Y.; Akanuma, Y.; Yazaki, Y.; Kadowaki, T. A homozygous kinase-defective mutation in the insulin receptor gene in a patient with leprechaunism. Diabetologia 1997, 40, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Grosse, G.; Hilger, A.; Ludwig, M.; Reutter, H.; Lorenzen, F.; Even, G.; Holterhus, P.M.; Woelfle, J.; German GHI Study Group. Targeted Resequencing of Putative Growth-Related Genes Using Whole Exome Sequencing in Patients with Severe Primary IGF-I Deficiency. Horm. Res. Paediatr. 2017, 88, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, J.; Chia, D.J.; Massart-Schlesinger, M.B.; Moyano, P.; Rotwein, P. Molecular physiology, pathology, and regulation of the growth hormone/insulin-like growth factor-I system. Pediatr. Nephrol. 2005, 20, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, J.; Chia, D.J.; Rotwein, P. Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J. Biol. Chem. 2003, 278, 51261–51266. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, J.; Rotwein, P. In vivo regulation of growth hormone-stimulated gene transcription by STAT5b. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E393–E401. [Google Scholar] [CrossRef] [PubMed]
- Chia, D.J.; Ono, M.; Woelfle, J.; Schlesinger-Massart, M.; Jiang, H.; Rotwein, P. Characterization of distinct Stat5b binding sites that mediate growth hormone-stimulated IGF-I gene transcription. J. Biol. Chem. 2006, 281, 3190–3197. [Google Scholar] [CrossRef] [PubMed]
- Vidal, O.M.; Merino, R.; Rico-Bautista, E.; Fernandez-Perez, L.; Chia, D.J.; Woelfle, J.; Ono, M.; Lenhard, B.; Norstedt, G.; Rotwein, P.; et al. In vivo transcript profiling and phylogenetic analysis identifies suppressor of cytokine signaling 2 as a direct signal transducer and activator of transcription 5b target in liver. Mol. Endocrinol. 2007, 21, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, J.; Billiard, J.; Rotwein, P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J. Biol. Chem. 2003, 278, 22696–22702. [Google Scholar] [CrossRef] [PubMed]
- Rotwein, P.; Billiard, J.; Woelfle, J. Molecular physiology of IGF-I expression. J. Pediatr. Endocrinol. Metab. 2002, 15 (Suppl. 5), 1455–1458. [Google Scholar] [PubMed]
- Woelfle, J. Wachstumshormoninsensitivität und schwerer primärer Mangel an insulinähnlichem Wachstumsfaktor-1: Behandlung im Kindesalter. Monatsschrift Kinderheilkunde 2014, 162, 309–314. [Google Scholar] [CrossRef]
- Schreiner, F.; Schoenberger, S.; Koester, B.; Domené, H.M.; Woelfle, J. Novel acid-labile subunit (IGFALS) mutation p.T145K (c.434C > A) in a patient with ALS deficiency, normal stature and immunological dysfunction. Horm. Res. Paediatr. 2013, 80, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Bang, P.; Polak, M.; Woelfle, J.; Houchard, A.; EU IGFD Registry Study Group. Effectiveness and Safety of rhIGF-1 Therapy in Children: The European Increlex® Growth Forum Database Experience. Horm. Res. Paediatr. 2015, 83, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B.; Wölfle, J.; Schnabel, D.; Bettendorf, M. Treatment of dwarfism with recombinant human insulin-like growth factor-1. Dtsch. Arztebl. Int. 2009, 106, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Ardon, O.; Procter, M.; Tvrdik, T.; Longo, N.; Mao, R. Sequencing analysis of insulin receptor defects and detection of two novel mutations in INSR gene. Mol. Genet. Metab. Rep. 2014, 1, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Wang, Y.; Smith, S.A.; Langley, S.D.; DiMeglio, L.A.; Giannella-Neto, D. Genotype–phenotype correlation in inherited severe insulin resistance. Hum. Mol. Genet. 2002, 11, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdelaziz, R.; Ben Chehida, A.; Azzouz, H.; Boudabbous, H.; Lascols, O.; Ben Turkia, H.; Tebib, N. A novel homozygous missense mutation in the insulin receptor gene results in an atypical presentation of Rabson-Mendenhall syndrome. Eur. J. Med. Genet. 2016, 59, 16–19. [Google Scholar] [CrossRef] [PubMed]
- De Kerdanet, M.; Caron-Debarle, M.; Nivot, S.; Gaillot, T.; Lascols, O.; Fremont, B.; Bonnaure-Mallet, M.; Gie, S.; Massart, C.; Capeau, J. Ten-year improvement of insulin resistance and growth with recombinant human insulin-like growth factor 1 in a patient with insulin receptor mutations resulting in leprechaunism. Diabetes Metab. 2015, 41, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, A.; Stuart, G.; Harding, L. Donohue syndrome: A review of literature, case series, and anesthetic considerations. Pediatr. Anesth. 2018, 28, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Simpkin, A.; Cochran, E.; Cameron, F.; Dattani, M.; de Bock, M.; Dunger, D.B.; Forsander, G.; Guran, T.; Harris, J.; Isaac, I.; et al. Insulin receptor and the kidney: Nephrocalcinosis in patients with recessive INSR mutations. Nephron Physiol. 2014, 128, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Sharma, N.; Gill, P.S.; Igarashi, P.; Kahn, C.R.; Wade, J.B.; Ecelbarger, C.M. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 6469–6474. [Google Scholar] [CrossRef] [PubMed]
- Accili, D.; Drago, J.; Lee, E.J.; Johnson, M.D.; Cool, M.H.; Salvatore, P.; Asico, L.D.; José, P.A.; Taylor, S.I.; Westphal, H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat. Genet. 1996, 12, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Ogilvy-Stuart, A.L.; Soos, M.A.; Hands, S.J.; Anthony, M.Y.; Dunger, D.B.; O’Rahilly, S. Hypoglycemia and resistance to ketoacidosis in a subject without functional insulin receptors. J. Clin. Endocrinol. Metab. 2001, 86, 3319–3326. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, E.; Lu, S.; Backeljauw, P.F.; Davenport, M.L.; Taylor, S.I. Homozygous deletion of the human insulin receptor gene results in leprechaunism. Nat. Genet. 1993, 5, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, T.; Murakami, T.; Tanaka, H.; Miyawaki, S.; Yamashiro, T.; Takano-Yamamoto, T. Dental and craniofacial characteristics in a patient with leprechaunism treated with insulin-like growth factor-I. Angle Orthod. 2008, 78, 745–751. [Google Scholar] [CrossRef]
- Bathi, R.J.; Parveen, S.; Mutalik, S.; Rao, R. Rabson-Mendenhall syndrome: two case reports and a brief review of the literature. Odontology 2010, 98, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.; Cochran, E.; Moran, S.A.; Skarulis, M.C.; Oral, E.A.; Taylor, S.; Gorden, P. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): A 30-year prospective. Medicine 2004, 83, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Watanabe, T. Renal tubular dysfunction in patients with molecular defects of the insulin receptor gene. Eur. J. Pediatr. 2014, 173, 263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abe, Y.; Sato, T.; Takagi, M.; Watanabe, T.; Nagayama, Y.; Hasegawa, T.; Abe, T. A case of Rabson-Mendenhall syndrome with a novel mutation in the tyrosine kinase domain of the insulin receptor gene complicated by medullary sponge kidney. J. Pediatr. Endocrinol. Metab. 2012, 25, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Baqir, Z.S.; Al-Lawati, T.T.; Al Hussaini, S.O.; Al-Sinani, A.; Al-Said, K.; Al-Rashdi, I. A novel leprechaunism mutation, Cys807Arg, in an Arab infant: a rare cause of hypoglycaemia. Paediatr. Int. Child Health 2012, 32, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Hovnik, T.; Brantanic, N.; Podkrajšek, K.T.; Kovač, J.; Paro, D.; Podnar, T.; Bratina, N.; Battelino, T. Severe progressive obstructive cardiomyopathy and renal tubular dysfunction in Donohue syndrome with decreased insulin receptor autophosphorylation due to a novel INSR mutation. Eur. J. Pediatr. 2013, 172, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Desbois-Mouthon, C.; Magré, J.; Duprey, J.; Caron, M.; Blivet-Van Eggelpoel, M.J.; Daubas, C.; Gourmelen, M.; Chevallier, B.; Rizkalla, S.; Robert, J.J.; et al. Major circadian variations of glucose homeostasis in a patient with Rabson-Mendenhall syndrome and primary insulin resistance due to a mutation(Cys284 → Tyr) in the insulin receptor alpha-subunit. Pediatr. Res. 1997, 42, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Singh, R.; Elsas, L.J. Decreased half-life of insulin-like growth factor I in Rabson-Mendenhall syndrome. J. Inherit. Metab. Dis. 2001, 24, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Carrasco de la Fuente, M.; Barrio Castellanos, R.; Alonso Blanco, M.; de la Calle Blasco, H. Long survival in Rabson-Mendenhall syndrome. Diabetes Res. Clin. Pract. 2010, 89, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.O.; Zagury, R.L.; Nascimento, T.S. Multidrug therapy in a patient with Rabson-Mendenhall syndrome. Diabetologia 2010, 53, 2454–2455. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.H.; Taylor, B.J.; Wheeler, B.J. Renal manifestations of severe Rabson-Mendenhall syndrome: A case report. J. Diabetes Metab. Disord. 2013, 12, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moore, M.M.; Bailey, A.M.; Flannery, A.H.; Baum, R.A. Treatment of diabetic ketoacidosis with intravenous U-500 insulin in a patient with Rabson-Mendenhall syndrome: A case report. J. Pharm. Pract. 2017, 30, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Skarulis, M.C.; Celi, F.S.; Mueller, E.; Zemskova, M.; Malek, R.; Hugendubler, L.; Cochran, C.; Solomon, J.; Chen, C.; Gorden, P. Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. JCEM 2010, 95, 256–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gorden, P.; Zadeh, E.S.; Cochran, E.; Brown, R.J. Syndromic insulin resistance: Models for the therapeutic basis of the metabolic syndrome and other targets of insulin resistance. Endocr. Pract. 2012, 18, 763–771. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Yakar, S. Mechanisms of disease: Metabolic effects of growth hormone and insulin-like growth factor 1. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Li, T.; Chen, Z.B.; Luo, B.; Sun, R.P. Prevention of beta cell dysfunction and apoptosis by adenoviral gene transfer of rat insulin-like growth factor 1. Chin. Med. J. 2009, 122, 2159–2164. [Google Scholar] [PubMed]
- Escribano, O.; Guillén, C.; Nevado, C.; Gómez-Hernández, A.; Kahn, C.R.; Benito, M. Beta-Cell hyperplasia induced by hepatic insulin resistance: Role of a liver-pancreas endocrine axis through insulin receptor A isoform. Diabetes 2009, 584, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, H.; Matsuura, N.; Sakamoto, M.; Makino, H.; Sakamoto, Y.; Kadowaki, T.; Suzuki, Y.; Kobayashi, M.; Akazawa, Y.; Nomura, M.; et al. Trial of insulin like growth factor I therapy for patients with extreme insulin resistance syndromes. Diabetes 1993, 42, 696–70572. [Google Scholar] [CrossRef] [PubMed]
- Bondy, C.A.; Underwood, L.E.; Clemmons, D.R.; Guler, H.P.; Bach, M.A.; Skarulis, M. Clinical uses of insulin-like growth factor I. Ann. Intern. Med. 1994, 120, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Backeljauw, P.F.; Alves, C.; Clemmons, D.R.; Guler, H.P.; Bach, M.A.; Skarulis, M. Effect of intravenous insulin-like growth factor I in two patients with leprechaunism. Pediatr. Res. 1994, 36, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Nishimura, R.; Utsunomiya, A.; Kagawa, R.; Funata, H.; Fujimoto, M.; Hanaki, K.; Kanzaki, S. Leprechaunism (Donohue syndrome): A case bearing novel compound heterozygous mutations in the insulin receptor gene. Endocr. J. 2013, 60, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Sudo, S.; Nakamura, A.; Endo, D.; Konno, Y.; Ishizu, K.; Tajima, T. Development of endometrial carcinoma in a patient with leprechaunism (donohue syndrome). Clin. Pediatr. Endocrinol. 2013, 22, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kitamei, H.; Yokoi, M.; Kase, M.; Ohno, S. Retinal neovascularization during treatment with IGF-1 for insulin resistance syndrome. Graefes Arch. Clin. Exp. Opthalmol. 2005, 243, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Regan, F.M.; Williams, R.M.; McDonald, A.; Umpleby, A.M.; Acerini, C.L.; O’Rahilly, S.; Hovorka, R.; Semple, R.K.; Dunger, D.B. Treatment with recombinant human insulin-like growth factor (rhIGF)-I/rhIGF binding protein-3 complex improves metabolic control in subjects with severe insulin resistance. JCEM 2010, 95, 2113–2122. [Google Scholar] [PubMed]
- Ranke, M.B. Insulin-like growth factor-I treatment of growth disorders, diabetes mellitus and insulin resistance. Trends Endocrinol. Metab. 2005, 16, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Brisigotti, M.; Fabbretti, G.; Pesce, F.; Gatti, R.; Cohen, A.; Parenti, G.; Callea, F. Congenital bilateral juvenile granulosa cell tumor of the ovary in leprechaunism: A case report. Pediatr. Pathol. 1993, 13, 549–558. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).