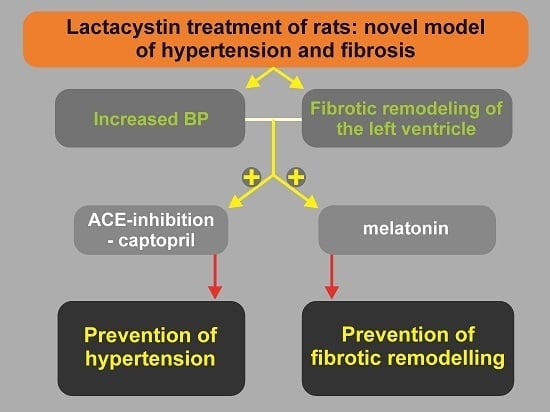
Lactacystin-Induced Model of Hypertension in Rats: Effects of Melatonin and Captopril
Abstract
1. Introduction
2. Results
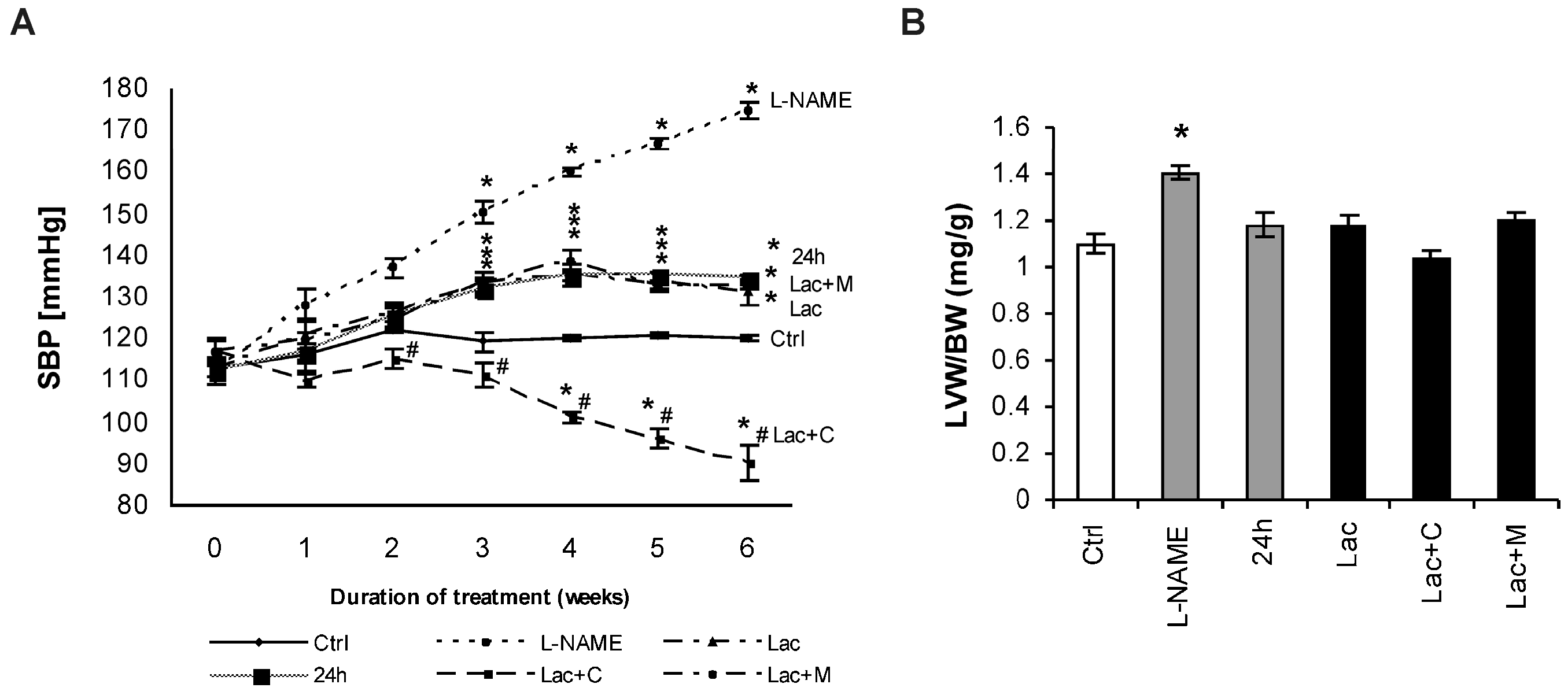
2.1. Cardiovascular Parameters
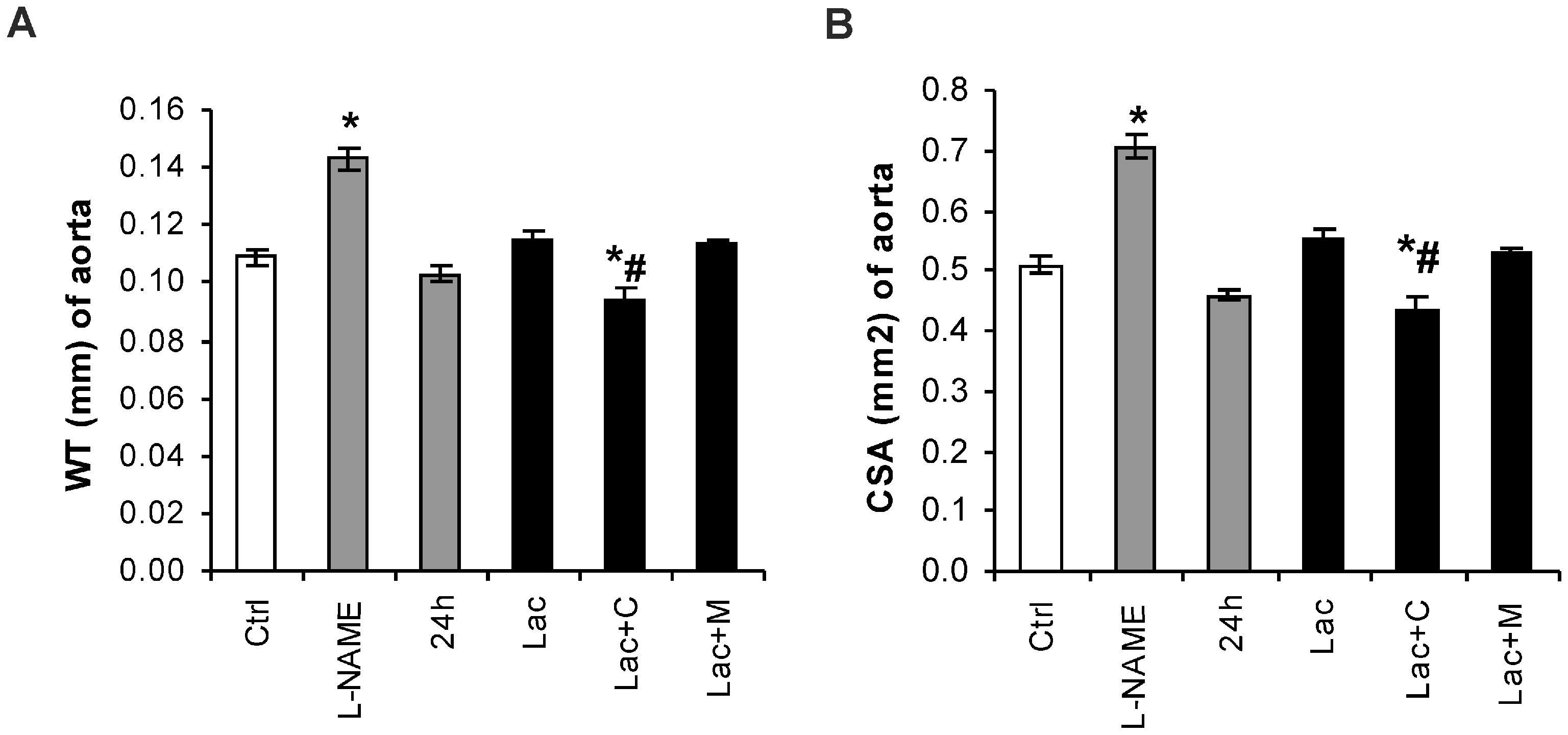
2.2. Morphometry of the Aorta
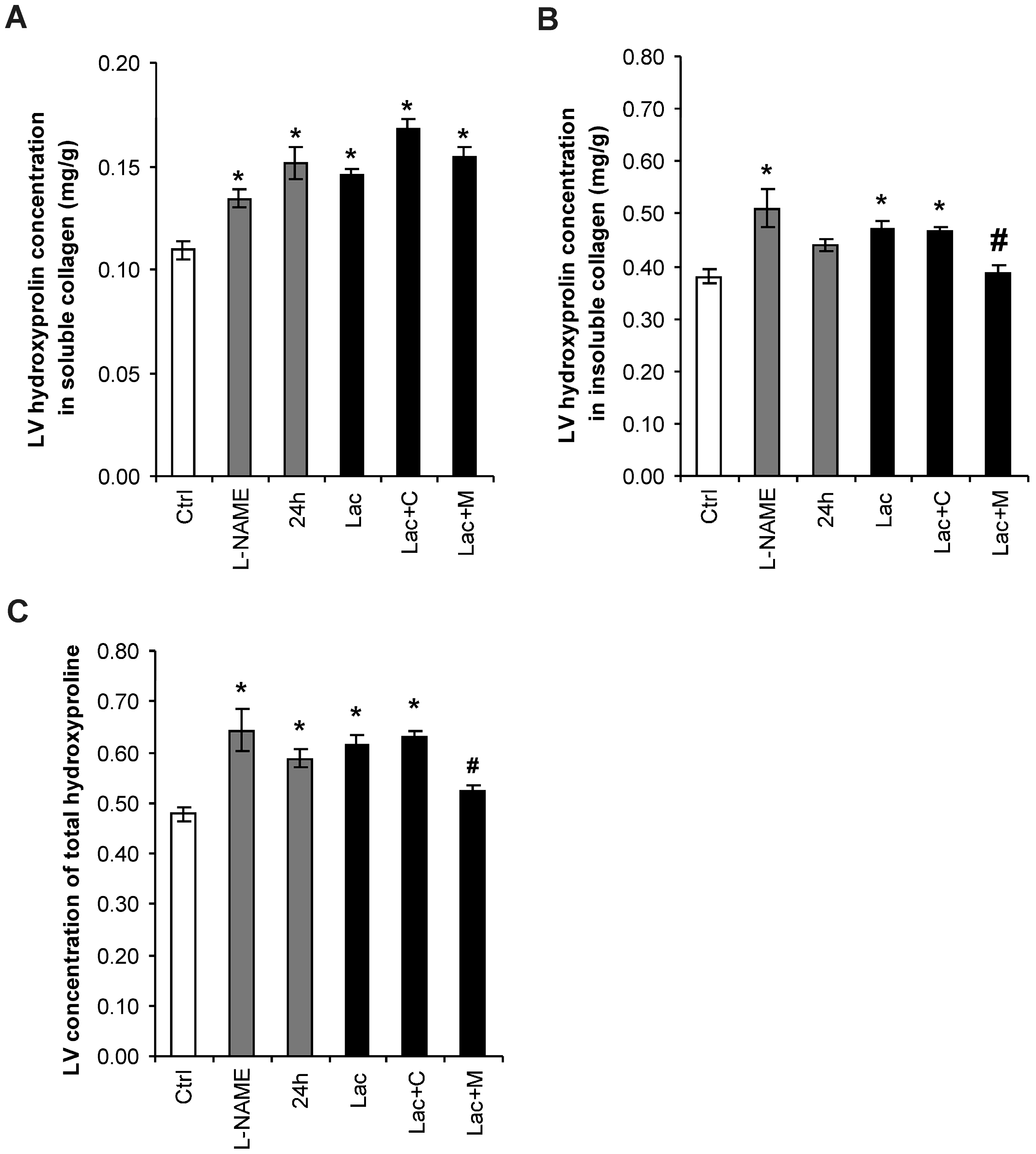
2.3. Hydroxyproline in Soluble and Insoluble Collagenous Fraction in the Left Ventricle (LV)
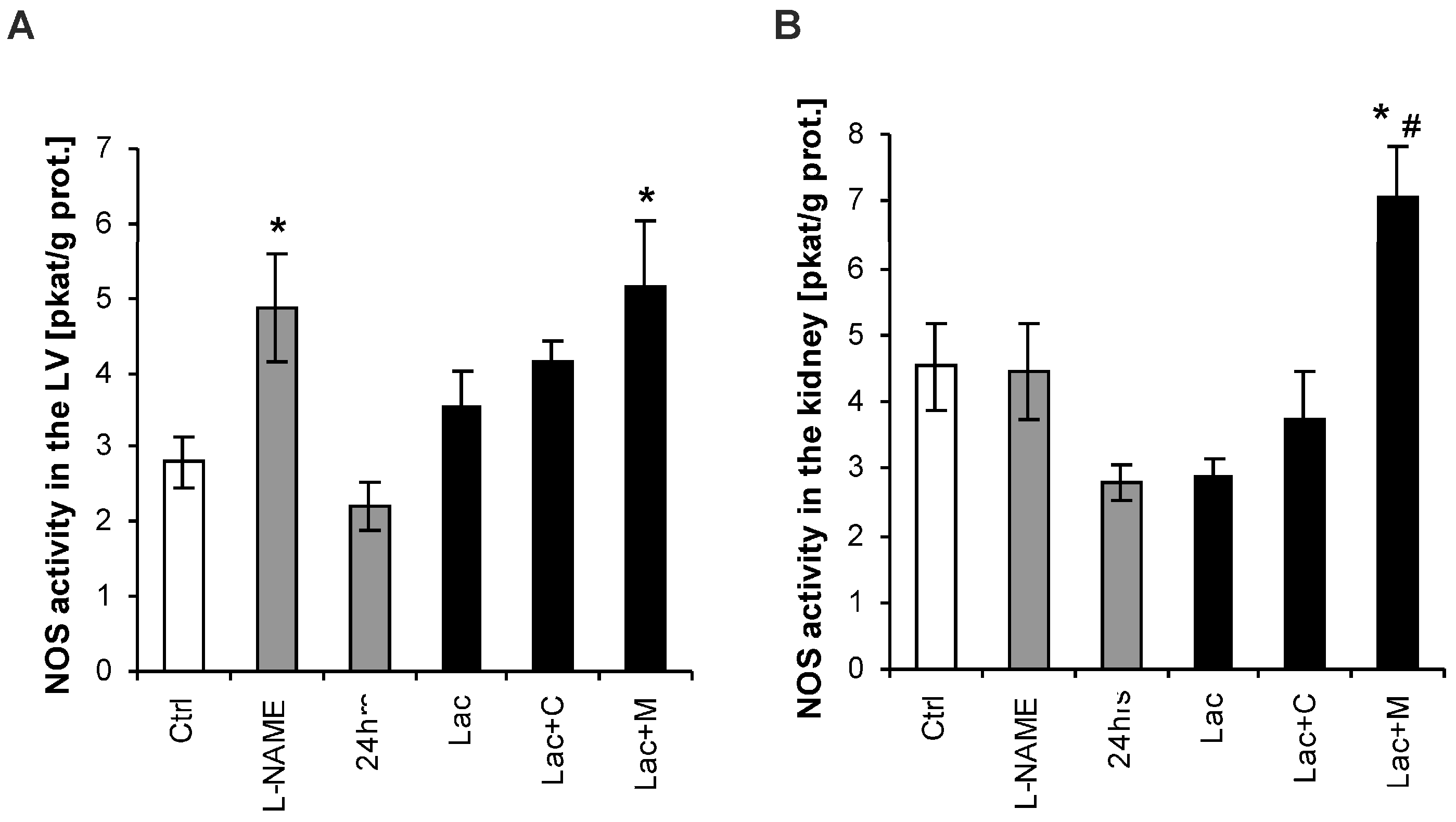
2.4. NO-Synthase (NOS) Activity in the LV and Kidney
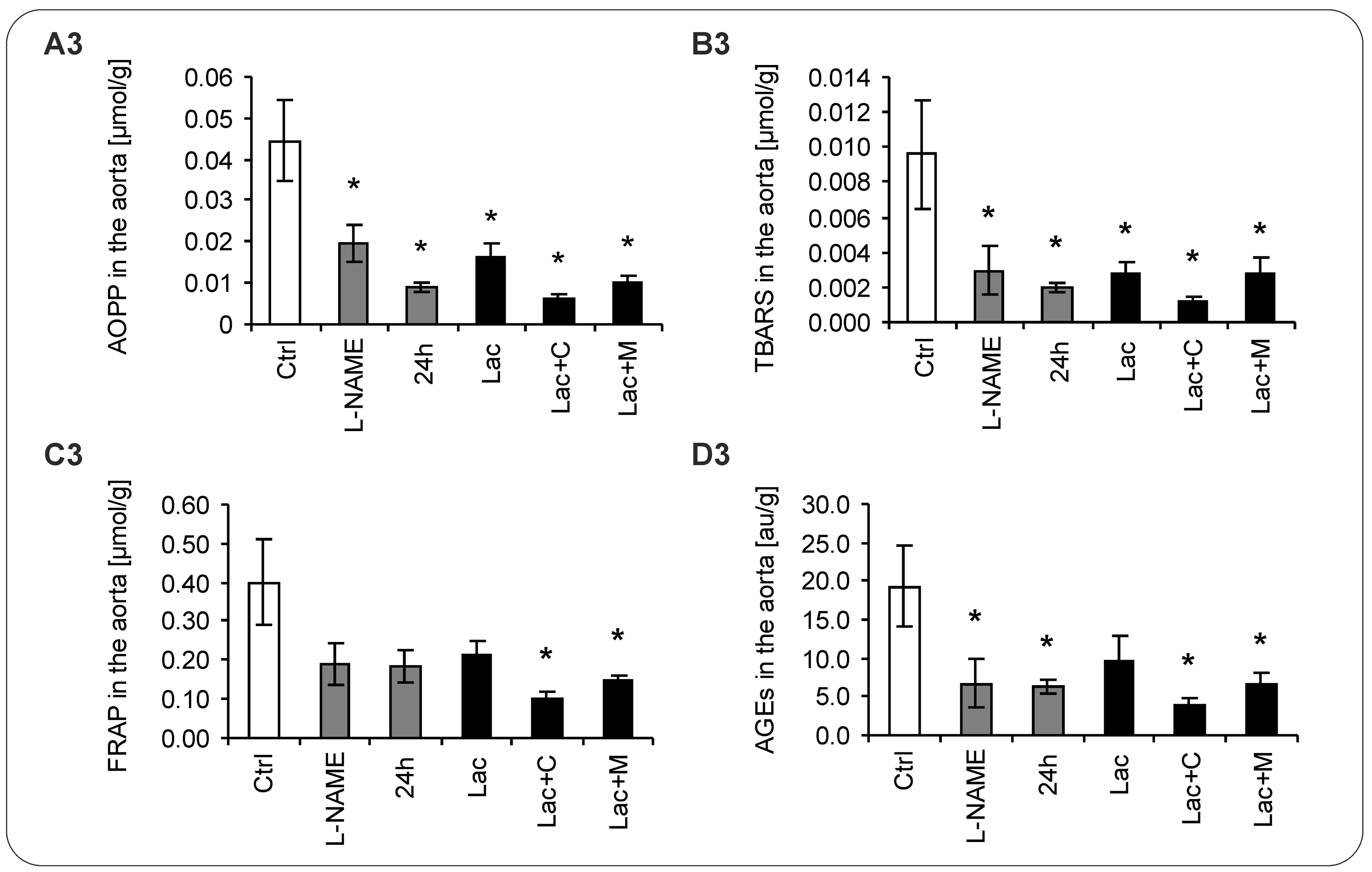
2.5. Oxidative Stress Parameters
2.6. Nuclear Factor-Kappa B (NF-κB) Expression
3. Discussion
4. Experimental Procedures
4.1. Animals and Treatment
4.2. Morphometry of the Aorta
4.3. Determination of Hydroxyproline
4.4. Assay of NO-Synthase (NOS) Activity
4.5. Oxidative Load Measurement
4.6. Western Blotting of NF-κB
4.7. Statistical Analysis
5. Limitations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klingbeil, A.U.; Schneider, M.; Martus, P.; Messerli, F.H.; Schmieder, R.E. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am. J. Med. 2003, 115, 41–46. [Google Scholar] [CrossRef]
- Simko, F. Pathophysiological principles of the relation between myocardial hypertrophy of the left ventricle and its regression. Physiol. Res. 1994, 43, 259–266. [Google Scholar] [PubMed]
- Simko, F. Left ventricular hypertrophy regression as a process with variable biological implications. Can. J. Cardiol. 1996, 12, 507–513. [Google Scholar] [PubMed]
- Weber, K.T.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Gerling, I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013, 10, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Matuskova, J.; Luptak, I.; Krajcirovicova, K.; Kucharska, J.; Gvozdjakova, A.; Babal, P.; Pechanova, O. Effect of simvastatin on remodeling of the left ventricle and aorta in L-NAME-induced hypertension. Life Sci. 2004, 74, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Simko, F. Statins—A prespective for left ventricular hypertrophy treatment (review). Eur. J. Clin. Investig. 2007, 37, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Pechanova, O. Remodelling of the heart and vessels in experimental hypertension: Advances in protection. J. Hypertens. 2010, 28, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Zanchetti, A. Hypertension: Cardiac hypertrophy as a target of antihypertensive therapy. Nat. Rev. Cardiol. 2010, 7, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Simko, J. Heart failure and angiotensin converting enzyme inhibition: Problems and perspectives. Physiol. Res. 1999, 48, 1–8. [Google Scholar] [PubMed]
- Simko, F.; Simko, J. The potential role of nitric oxide in the hypertrophic growth of the left ventricle. Physiol. Res. 2000, 49, 37–46. [Google Scholar] [PubMed]
- Mandarim-De-Lacerda, C.A.; Pereira, L.M. Effect of telmisartan on preexistent cardiac and renal lesions in spontaneously hypertensive mature rats. Histol. Histopathol. 2004, 19, 727–733. [Google Scholar] [PubMed]
- Mearini, G.; Schlossarek, S.; Willis, M.S.; Carrier, L. The ubiquitin-proteasome system in cardiac dysfunction. Biochim. Biophys. Acta 2008, 1782, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Massaro, M.; de Caterina, R. Insulin potentiates cytokine-induced VCAM-1 expression in human endothelial cells. Biochim. Biophys. Acta 2008, 1782, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Carbajosa, N.A.; Corradi, G.; Verrilli, M.A.; Guil, M.J.; Vatta, M.S.; Gironacci, M.M. Tyrosine hydroxylase is short-term regulated by the ubiquitin-proteasome system in PC12 cells and hypothalamic and brainstem neurons from spontaneously hypertensive rats: Possible implications in hypertension. PLoS ONE 2015, 24, e0116597. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Perez-Giren, J.V.; Hernanz, R.; Palacios, R.; Briones, A.M.; Fortuño, A.; Zalba, G.; Salaices, M.; Alonso, M.J. Peroxisome proliferator-activated receptor-γ activation reduces cyclooxygenase-2 expression in vascular smooth muscle cells from hypertensive rats by interfering with oxidative stress. J. Hypertens. 2012, 30, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Eble, D.M.; Spragia, M.L.; Ferguson, A.G.; Samarel, A.M. Sarcomeric myosin heavy chain is degraded by the proteasome. Cell Tissue Res. 1999, 296, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; de la Torre-Hernandez, J.M.; Gonzalez-Gonzalez, J.; Garcia-Camarero, T.; Consuegra-Sanchez, L.; Garcia-Saiz, M.D.; Aldea-Perona, A.; Virgos-Aller, T.; Azpeitia, A.; et al. Effect of intravenous and intracoronary melatonin as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: Results of the melatonin adjunct in the acute myocaRdial infarction treated with angioplasty trial. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef]
- Hu, W.; Ma, Z.; Jiang, S.; Fan, C.; Deng, C.; Yan, X.; Di, S.; Lv, J.; Reiter, R.J.; Yang, Y. Melatonin: The dawning of a treatment for fibrosis? J. Pineal Res. 2016, 60, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Yang, Y.; Guo, Y.; Fan, Y.; Zhang, M.; Man, W.; Gao, E.; Hu, W.; Reiter, R.J.; et al. Melatonin alleviates postinfarction cardiac remodeling and dysfunction by inhibiting Mst1. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Pechanova, O.; Pelouch, V.; Krajcirovicova, K.; Mullerova, M.; Bednárova, K.; Adamcova, M.; Paulis, L. Effect of melatonin, captopril, spironolactone and simvastatin on blood pressure and left ventricular remodeling in spontaneously hypertensive rats. J. Hypertens. 2009, 27, S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Pechanova, O.; Repova-Bednarova, K.; Krajcirovicova, K.; Celec, P.; Kamodyova, N.; Zorad, S.; Kucharska, J.; Gvozdjakova, A.; Adamcova, M.; et al. Hypertension and cardiovascular remodelling in rats exposed to continuous light: Protection by ACE-inhibition and melatonin. Mediat. Inflamm. 2014, 2014, 703175. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Pechanova, O. Recent trends in hypertension treatment: Perspectives from animal studies. J. Hypertens. 2009, 27, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Medina, M.E.; Tan, D.X.; Reiter, R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal Res. 2015, 58, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.; González-Candia, A.; Montt, C.; Ebensperger, G.; Chubretovic, M.; Serón-Ferré, M.; Reyes, R.V.; Llanos, A.J.; Herrera, E.A. Melatonin reduces oxidative stress and improves vascular function in pulmonary hypertensive newborn sheep. J. Pineal Res. 2015, 58, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, Y.; Tan, D.X.; Reiter, R.J.; Chan, Z.; He, C. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J. Pineal Res. 2015, 59, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; Sanchez-Sanchez, J.J.; Kaski, J.C.; Reiter, R.J. Melatonin and circadian biology in human cardiovascular disease. J. Pineal Res. 2010, 49, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. Melatonin feedback on clock genes: A theory involving the proteasome. J. Pineal Res. 2015, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Baka, T.; Paulis, L.; Reiter, R.J. Elevated heart rate and nondipping heart rate as potential targets for melatonin: A review. J. Pineal Res. 2016, 61, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T. From inflammation to fibrosis: A stiff stretch of highway. Hypertension 2004, 43, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K. Oxidative shielding or oxidative stress? J. Pharmacol. Exp. Ther. 2012, 342, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Simko, F. Chronic antioxidant therapy fails to ameliorate hypertension: Potential mechanisms behind. J. Hypertens. 2009, 27, S32–S36. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E. Does increased oxidative stress cause hypertension? Diabetes Care 2008, 3, S185–S189. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 23–33. [Google Scholar]
- Ceriello, A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care 2008, 31, S181–S184. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. Type 2 diabetes as a redox disease. Lancet 2014, 383, 841. [Google Scholar] [CrossRef]
- Lalu, M.M.; Xu, H.; Sankaralingam, S.; Davidge, S.T. Proteasome inhibition decreases inflammation in human endothelial cells exposed to lipopolysaccharide. J. Cardiovasc. Pharmacol. 2012, 60, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Paulis, L.; Simko, F. LA419, a novel nitric oxide donor, prevents cardiac remodeling via the endothelial nitric oxide pathway. NO donors as a means of antiremodeling. Hypertension 2007, 50, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Paulis, L.; Pechanova, O.; Zicha, J.; Krajcirovicova, K.; Barta, A.; Pelouch, V.; Adamcova, M.; Simko, F. Melatonin prevents fibrosis but not hypertrophy development in the left ventricle of NG-nitro-l-arginine-methyl ester hypertensive rats. J. Hypertens. 2009, 27, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Pechanova, O.; Pelouch, V.; Krajcirovicova, K.; Celec, P.; Palffy, R.; Bednarova, K.; Vrankova, S.; Adamcova, M.; Paulis, L. Continuous light and L-NAME—Induced left ventricular remodelling: Different protection by melatonin and captopril. J. Hypertens. 2010, 28, S13–S18. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Bednarova, K.; Krajcirovicova, K.; Hrenak, J.; Celec, P.; Kamodyova, N.; Gajdosechova, L.; Zorad, S.; Adamcova, M. Melatonin reduces cardiac remodeling and improves survival in rats with isoproterenol-induced heart failure. J. Pineal Res. 2014, 57, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Paulis, L. Antifibrotic effect of melatonin—Perspective protection in hypertensive heart disease. Int. J. Cardiol. 2013, 168, 2876–2877. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Reiter, R.J.; Pechanova, O.; Paulis, L. Experimental models of melatonin-deficient hypertension. Front. Biosci. 2013, 18, 616–625. [Google Scholar] [CrossRef]
- Gupta, S.; Young, D.; Maitra, R.K.; Gupta, A.; Popovic, Z.B.; Yong, S.L.; Mahajan, A.; Wang, Q.; Sen, S. Prevention of cardiac hypertrophy and heart failure by silencing of NF-κB. J. Mol. Biol. 2008, 375, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, F.M.; Ehrlich, B.E. Calcium, calpains, and cardiac hypertrophy: A new link. Circ. Res. 2009, 104, e19–e20. [Google Scholar] [CrossRef] [PubMed]
- Timmers, L.; van Keulen, J.K.; Hoefer, I.E.; Meijs, M.F.; van Middelaar, B.; den Ouden, K.; van Echteld, C.J.; Pasterkamp, G.; de Kleijn, D.P. Targeted deletion of nuclear factor κB p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circ. Res. 2009, 104, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Chen, H.; Jiang, J.; Kong, W.; Wang, Y. Silencing MR-1 attenuates inflammatory damage in mice heart induced by Ang II. Biochem. Biophys. Res. Commun. 2010, 391, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Hingtgen, S.D.; Li, Z.; Kutschke, W.; Tian, X.; Sharma, R.V.; Davisson, R.L. Superoxide scavenging and Akt inhibition in myocardium ameliorate pressure overload-induced NF-κB activation and cardiac hypertrophy. Physiol. Genom. 2010, 41, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Zelarayan, L.; Renger, A.; Noack, C.; Zafiriou, M.P.; Gehrke, C.; van der Nagel, R.; Dietz, R.; de Windt, L.; Bergmann, M.W. NF-κB activation is required for adaptive cardiac hypertrophy. Cardiovasc. Res. 2009, 84, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Badshah, H.; Kim, T.H.; Kim, M.O. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model. J. Pineal Res. 2015, 58, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Jumnongprakhon, P.; Govitrapong, P.; Tocharus, C.; Tocharus, J. Melatonin promotes blood-brain barrier integrity in methamphetamine-induced inflammation in primary rat brain microvascular endothelial cells. Brain Res. 2016, 1, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Q.; Li, H.; Tian, T.; Lu, Y.X.; Bai, L.; Chen, L.Z.; Sheng, H.; Mo, H.Y.; Zeng, J.B.; Deng, W.; et al. Melatonin overcomes gemcitabine resistance in pancreatic ductal adenocarcinoma by abrogating nuclear factor-κB activation. J. Pineal Res. 2016, 60, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Lee, L.M.; Lee, W.J.; Chu, C.Y.; Tan, P.; Yang, Y.C.; Chen, W.Y.; Yang, S.F.; Hsiao, M.; Chien, M.H. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J. Pineal Res. 2016, 60, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.M.; Lin, W.Y.; Shen, C.C.; Pan, H.C.; Keh-Bin, W.; Chen, Y.C.; Jan, Y.J.; Lai, D.W.; Tang, S.C.; Tien, H.R.; et al. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPβ and NF-κB cleavage. J. Pineal Res. 2016, 60, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Forman, K.; Vara, E.; García, C.; Kireev, R.; Cuesta, S.; Acuña-Castroviejo, D.; Tresguerres, J.A. Beneficial effects of melatonin on cardiological alterations in a murine model of accelerated aging. J. Pineal Res. 2010, 49, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. Melatonin as a proteasome inhibitor. Is there any clinical evidence? Life Sci. 2014, 12, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. The KEAP1-Nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol. Cell. Endocrinol. 2015, 5, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brasier, A.R. Angiotensinogen gene activation by angiotensin II is mediated by the Rel A (nuclear factor-κB p65) transcription factor: One mechanism for the renin angiotensin system positive feedback loop in hepatocytes. Mol. Endocrinol. 1996, 10, 252–264. [Google Scholar] [PubMed]
- Sekiguchi, K.; Li, X.; Coker, M.; Flesch, M.; Barger, P.M.; Sivasubramanian, N.; Mann, D.L. Cross-regulation between the renin-angiotensin system and inflammatory mediators in cardiac hypertrophy and failure. Cardiovasc. Res. 2004, 15, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Presa, M.; Bustos, C.; Ortego, M.; Tuñon, J.; Renedo, G.; Ruiz-Ortega, M.; Egido, J. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-κB activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation 1997, 18, 1532–1541. [Google Scholar] [CrossRef]
- Grumbach, I.M.; Chen, W.; Mertens, S.A.; Harrison, D.G. A negative feedback mechanism involving nitric oxide and nuclear factor κB modulates endothelial nitric oxide synthase transcription. J. Mol. Cell. Cardiol. 2005, 39, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Pechanova, O.; Simko, F. The role of nuclear factor κB and nitric oxide interaction in heart remodelling. J. Hypertens. 2010, 28, S39–S44. [Google Scholar] [CrossRef] [PubMed]
- Paulis, L.; Matuskova, J.; Adamcova, M.; Pelouch, V.; Simko, J.; Krajcirovicova, K.; Potacova, A.; Hulin, I.; Janega, P.; Pechanova, O.; et al. Regression of left ventricular hypertrophy and aortic remodelling in NO-deficient hypertensive rats: Effect of l-arginine and spironolactone. Acta Physiol. 2008, 194, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Pelouch, V.; Milerova, M.; Ostadal, B.; Samánek, M.; Hucín, B. Protein profiling of human atrial and ventricular musculature: The effect of normoxaemia and hypoxaemia in congenital heart diseases. Physiol. Res. 1993, 42, 235–242. [Google Scholar] [PubMed]
- Pelouch, V.; Milerova, M.; Ostadal, B.; Hucín, B.; Samánek, M. Differences between atrial and ventricular protein profiling in children with congenital heart disease. Mol. Cell. Biochem. 1995, 147, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.; Enwemeka, C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef]
- Bredt, D.S.; Snyder, S.H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. USA 1990, 87, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Munch, G. Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Bhatwadekar, A.D.; Ghole, V.S. Rapid method for the preparation of an AGE-BSA standard calibrator using thermal glycation. J. Clin. Lab. Anal. 2005, 19, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Behuliak, M.; Palffy, R.; Gardlik, R.; Hodosy, J.; Halcak, L.; Celec, P. Variability of thiobarbituric acid reacting substances in saliva. Dis. Mark. 2009, 26, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Helenius, M.; Hänninen, M.; Lehtinen, S.K.; Salminen, A. Aging-induced upregulation of nuclear binding activities of oxidative stress responsive NF-κB transcription factor in mouse cardiac muscle. J. Mol. Cell. Cardiol. 1996, 28, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Welinder, C.; Ekblad, L. Coomassie staining as loading control in Western blot analysis. J. Proteome Res. 2011, 10, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Racchumi, G.; Iadecola, C. cis-Acting, element-specific transcriptional activity of differentially phosphorylated nuclear factor-κB. J. Biol. Chem. 2005, 7, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Post-translational modifications regulating the activity and function of the nuclear factor κB pathway. Oncogene 2006, 30, 6717–6730. [Google Scholar] [CrossRef] [PubMed]
- Mikenberg, I.; Widera, D.; Kaus, A.; Kaltschmidt, B.; Kaltschmidt, C. Transcription factor NF-κB is transported to the nucleus via cytoplasmic dynein/dynactin motor complex in hippocampal neurons. PLoS ONE 2007, 11, e589. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.N.; Bae, J.W.; Gao, E.; Koch, W.J. Regulation of nuclear factor κB (NF-κB) in the nucleus of cardiomyocytes by G protein-coupled receptor kinase 5 (GRK5). J. Biol. Chem. 2013, 13, 35683–35689. [Google Scholar] [CrossRef] [PubMed]
- Paulis, L.; Pechanova, O.; Zicha, J.; Barta, A.; Gardlik, R.; Celec, P.; Kunes, J.; Simko, F. Melatonin interactions with blood pressure and vascular function during L-NAME-induced hypertension. J. Pineal Res. 2010, 48, 102–108. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simko, F.; Pechanova, O.; Repova, K.; Aziriova, S.; Krajcirovicova, K.; Celec, P.; Tothova, L.; Vrankova, S.; Balazova, L.; Zorad, S.; et al. Lactacystin-Induced Model of Hypertension in Rats: Effects of Melatonin and Captopril. Int. J. Mol. Sci. 2017, 18, 1612. https://doi.org/10.3390/ijms18081612
Simko F, Pechanova O, Repova K, Aziriova S, Krajcirovicova K, Celec P, Tothova L, Vrankova S, Balazova L, Zorad S, et al. Lactacystin-Induced Model of Hypertension in Rats: Effects of Melatonin and Captopril. International Journal of Molecular Sciences. 2017; 18(8):1612. https://doi.org/10.3390/ijms18081612
Chicago/Turabian StyleSimko, Fedor, Olga Pechanova, Kristina Repova, Silvia Aziriova, Kristina Krajcirovicova, Peter Celec, Lubomira Tothova, Stanislava Vrankova, Lucia Balazova, Stefan Zorad, and et al. 2017. "Lactacystin-Induced Model of Hypertension in Rats: Effects of Melatonin and Captopril" International Journal of Molecular Sciences 18, no. 8: 1612. https://doi.org/10.3390/ijms18081612
APA StyleSimko, F., Pechanova, O., Repova, K., Aziriova, S., Krajcirovicova, K., Celec, P., Tothova, L., Vrankova, S., Balazova, L., Zorad, S., & Adamcova, M. (2017). Lactacystin-Induced Model of Hypertension in Rats: Effects of Melatonin and Captopril. International Journal of Molecular Sciences, 18(8), 1612. https://doi.org/10.3390/ijms18081612