Abstract
A variety of acetals and thioacetals 2 are deprotected to the corresponding parent carbonyl compounds 3 under solvent-free conditions using benzyltriphenyl-phosphonium peroxymonosulfate (1) in the presence of aluminum chloride.
Introduction
Carbonyl compounds are often protected as thioacetals in organic synthesis [1], particularly in multistep natural product synthesis [2], due to their stability in both acidic and basic conditions. Many procedures are available in the literature for preparing thioacetals [3], but their deprotection to the parent carbonyl compounds is not always an easy process. Therefore, various hydrolytic [4] or oxidative methods [5] have been documented in recent years for dethioacetalization of thioacetals. Recently, the use of manganese-based oxidants (KMnO4, BaMnO4 and MnO2) in aprotic solvents for selective deprotection of thioacetals has been reported [6]. Carbonyl derivatives such as acetals and ketals are also of importance for the characterization and purification of carbonyl compounds [1], and extensive studies on the deprotection of these derivatives to their parent carbonyl compounds have been carried out [7]. However, some of the methods mentioned for deprotection of acetals and thioacetals invariably require high temperatures, long reaction times and often involve toxic metal ions and solvents, which are detrimental to the environment. Therefore, there is a need for a simple, less expensive and safer method for deprotection of acetals and thioacetals.
Oxone® (2KHSO5·KHSO4·K2SO4) is a stable, inexpensive and water-soluble oxidizing reagent that is commercially available, but this reagent is insoluble in organic solvents and buffering is needed due to its acidity [8]. Recently, we have reported the use of benzyltriphenylphosphonium peroxymonosulfate (PhCH2Ph3PHSO5, 1), prepared from Oxone® and benzyltriphenylphosphonium chloride, as a mild, inexpensive and efficient oxidizing reagent for a variety of reactions, including the oxidation of alcohols to aldehydes and ketones under aprotic [9a] or solvent-free conditions [9b], oxidative deprotection of trimethylsilyl and tetrahydropyranyl ethers under non-aqueous conditions [9c], conversion of oximes, phenylhydrazones, 2,4-dinitrophenylhydrazones and semicarbazones to carbonyl compounds in aprotic solvent [9d], oxidation of urazoles to triazolinediones in a solventless system [9e] and selective oxidation of sulfides and thiols to the corresponding sulfoxides and disulfides under solvent-free conditions [9f].
In recent years, there has been an increasing interest in reactions that proceed in the absence of solvents due to their reduced pollution, low costs and simplicity in process and handling [10]. Because of our interest in development of solvent-free reactions [11], we now report the use of benzyl-triphenylphosphonium peroxymonosulfate (1) as an efficient and selective reagent for the deprotection of thioacetals 2a-j (1,3-dithiolanes and 1,3-dithianes) and ethylene acetals 2k-p to the corresponding carbonyl compounds 3a-p under solvent-free conditions.
Benzyltriphenylphosphonium peroxymonosulfate (1), a mild, efficient, stable and cheap reagent, is a white powder, which is quite soluble in dichloromethane, chloroform, acetone and acetonitrile and insoluble in non-polar solvents such as carbon tetrachloride, n-hexane and diethyl ether. This reagent is readily prepared in quantitative yield by the dropwise addition at room temperature of an aqueous solution of Oxone® to an aqueous solution of benzyltriphenylphosphonium chloride and could be stored for months without losing its potency [9]. The amounts of HSO5- in this reagent have been determined by an iodometric titration method [12] and the measurements are consistent with almost 99% by weight of active oxidising agent.
Results and Discussion
Deprotection of acetals and thioacetals with reagent 1 proceeds readily under solid-phase conditions. Initially, we decided to explore the role of the reagent 1 in the presence of hydrated and anhydrous metal salts in a solventless system for the deprotection of 2-(3-nitrophenyl)-1,3-dithiane (2d) used as a model compound. Addition of metal salts under solid-phase condition in the absence of reagent 1 did not effect any dethioacetalization of the model compound 2d, even after prolonged reaction times. Since deprotection of model compound 2d with reagent 1 failed in the absence of metal salt, the effect of several metal salts such as ZnCl2, FeCl3·6H2O, AlCl3 and BiCl3 was thoroughly investigated. Surprisingly, only AlCl3 was effective. An optimum (1:1:2) molar ratio of thioacetal, to AlCl3 to oxidant 1 was established for complete deprotection of thioacetals 2a-j to carbonyl compounds 3a-j while the reaction was incomplete with lesser amounts of oxidant (i.e. ratios of 1:1:1 and 1:1:1.5).
Benzyltriphenylphosphonium peroxymonosulfate (1) in the presence of aluminum chloride was also used to convert ethylene acetals 2k-p to the corresponding carbonyl compounds 3k-p in high yields under solid-phase conditions. A (1:1:1) molar ratio of acetal, to AlCl3 to oxidant 1 was found to be ideal for complete conversion. In this method, deprotection of a thioacetal or acetal is achieved by grinding together a mixture of a thioacetal 2a-j or acetal 2k-p, AlCl3 and reagent 1 under solvent-free condition. The reaction time is usually between 5-20 minutes (Table 1 and Scheme 1). The carbonyl compounds 3a-p were isolated by washing the reaction mixture with solvent followed by filtration. Evaporation of the filtrate under vacuum followed by flash chromatography often produces pure carbonyl compounds 3a-p in high yield (Table 1). This method offers a simple, mild, solvent-free and efficient route for converting acetals and thioacetals to the corresponding carbonyl compounds. As evident from the results presented in Table 1, functional groups such as NO2 and MeO increase the reaction times. This could be the effect of complexation between these functional groups and the AlCl3. Notably, aldehydes did not undergo further oxidation to their carboxylic acids under the reaction conditions. To demonstrate the utility of the procedure, a ten-fold scale reaction was carried out under solvent-free condition for the deprotection of 2-(3-nitrophenyl)-1,3-dithiane (2d) to afford 3‑nitrobenzaldehyde in 90% yield. It should be noted that there are no thermal or safety problems were noted during mixing of the starting materials and the reactions.
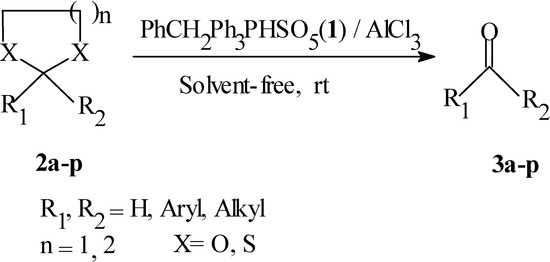
Scheme 1.
A comparison of the results of deprotection of thioacetals by our method with those reported for MnO2/AlCl3 (II) [6], Oxone®/wet Al2O3 (III) [5e] and SeO2 (IV) [5h] is shown in Table 2. This reagent is seen to be superior to the reported reagents in terms of high yields, short reaction times and the amount of oxidant required.

Table 1.
Deprotection of ethylene acetals, 1,3-dithianes and 1,3-dithiolanes with reagent 1 in the presence of AlCl3 in a solventless systema
| Substrate | R1 | R2 | n, X | Subs./AlCl3/Oxidant | Time (min) | Yield (%)b |
|---|---|---|---|---|---|---|
| 2a | Ph | Me | 1, S | 1/1/2 | 12 | 96 |
| 2b | 4-Cl-C6H4 | Me | 1, S | 1/1/2 | 15 | 90 |
| 2c | 2-O2N-C6H4 | H | 2, S | 1/1/2 | 20 | 87 |
| 2d | 3-O2N-C6H4 | H | 2, S | 1/1/2 | 20 | 88 |
| 2e | 4-O2N-C6H4 | H | 2,S | 1/1/2 | 20 | 91 |
| 2f | 4-Cl-C6H4 | H | 2, S | 1/1/2 | 16 | 93 |
| 2g | 4-Br-C6H4 | CH2Br | 2, S | 1/1/2 | 15 | 86 |
2h | 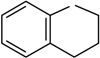 | __ | 2, S | 1/1/2 | 10 | 97 |
| 2i | Ph | H | 2, S | 1/1/2 | 5 | 99 |
| 2j | 2-MeOC6H4 | H | 2, S | 1/1/2 | 12 | 90 |
| 2k | Ph | Me | 1, O | 1/1/1 | 5 | 91 |
| 2l | 2-MeOC6H4 | H | 1, O | 1/1/1 | 10 | 88 |
| 2m | 2-O2N-C6H4 | H | 1, O | 1/1/1 | 12 | 80 |
| 2n | 2-Cl-C6H4 | Me | 1, O | 1/1/1 | 13 | 85 |
2o |  | __ | 1, O | 1/1/1 | 5 | 99 |
| 2p | 4-Ph-C6H4 | Me | 1, O | 1/1/1 | 10 | 92 |
a Products confirmed by comparison with authentic samples (TLC, IR, 1H-NMR, melting and boiling points).b Yields of isolated carbonyl compound after purification.

Table 2.
Comparison of deprotection of thioacetals by our method with those reported in the literature
| R1 | R2 | n | Yield (%)/Time (min) | |||
|---|---|---|---|---|---|---|
| Ia | IIb | IIIc | IVd | |||
| Ph | H | 2 | 99/5 | 96/90 | 70/50 | __ |
| 4-Cl-C6H4 | H | 2 | 93/16 | 92/120 | __ | __ |
| Ph | Me | 1 | 96/12 | __ | 91/120 | 98/25 |
a Substrate/PhCH2Ph3PHSO5/AlCl3 (1:2:1);b Substrate/MnO2/AlCl3 (1:7:1.5);c Substrate/Oxone®/wet Al2O3 (1:5);d Substrate/SeO2 (1:5);
Conclusions
We report a new and efficient methodology for the regeneration of aldehydes and ketones from acetals and thioacetals at room temperature and in the absence of solvent. The stability, easy preparation of the reagent and easy work-up make this method a novel and useful one compared to the known methodologies for regeneration of carbonyl compounds from acetals and thioacetals. Also, this method does not require a large excess of reagent nor long reaction times.
Acknowledgments
We are thankful to the Isfahan University of Technology (IUT) Research Council for the partial support of this work. Further financial support from Center of Excellency in Chemistry Research (IUT) is gratefully acknowledged.
Experimental
General
All the reported yields refer to isolated products after purification. All of the products were characterized by comparison of their spectral (IR, 1H-NMR and TLC) and physical data (melting and boiling points) with those of authentic samples [13]. All 1H-NMR spectra were recorded at 90 MHz in CDCl3 relative to TMS as an internal standard and IR spectra were recorded on Shimadzu 435 IR spectrometer. The reagent 1 was prepared according to our previous reported procedures [9]. All of the reactions were carried out in the absence of solvent at room temperature in a fumehood with strong ventilation. The carbonyl derivatives were prepared from the corresponding carbonyl compounds and 1,2-ethanediol, 1,2-ethanedithiol and 1,3-propanedithiol according to the reported procedures [3,7b].
Typical Procedure for Solid Phase Deprotection of Acetals and Thioacetals with Reagent 1: Reaction of 2-Methyl-2-(4-chlorophenyl)-1,3-dithiolane (2b).
Benzyltriphenylphosphonium peroxymonosulfate (1) (0.7 g, 2 mmol) was added to a mixture of 2-methyl-2-(4-chlorophenyl)-1,3-dithiolane (2b, 0.231 g, 1 mmol) and aluminum chloride (0.13 g, 1mmol) placed in a mortar. The reaction mixture was ground by pestle at room temperature under solvent-free condition for 15 minutes. After the disappearance of starting material as monitored by TLC, the mixture was washed with cyclohexane and filtered. The filtrate was evaporated under reduced pressure and the resulting crude material was purified by flash chromatography on SiO2 (eluent: CH2Cl2) to afford 4-chloroacetophenone (3b, 0.14 g, 90%); bp 231-232 oC/760 Torr (lit. [13] bp 232 oC/760 Torr ); IR (KBr): υ = 3050 (m), 2890 (m), 1680 (s), 1590 (s), 1400 (m), 1250 (s), 830 (m), 760 (m) cm-1; 1H-NMR (CDCl3, 90 MHz): δ = 2.6 (s, 3H), 7.4-7.7 (d, 2H, J = 6 Hz), 7.9-8.2 (d, 2H, J = 6 Hz) ppm.
References and Notes
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis; Wiley: New York, 1991. [Google Scholar] Komatsu, N.; Uda, M.; Suzuki, H. Synlett 1995, 984.
- Guanti, G.; Banfi, L.; Brusco, S.; Riva, R. Tetrahedron Lett. 1993, 34, 8549.
- Firouzabadi, H.; Iranpoor, N.; Karimi, B. Synthesis 1999, 58. Seebach, D.; Corey, E.J. J. Org. Chem. 1975, 40, 231. Groblel, B.T.; Seebach, D. Synthesis 1977, 357. Hatch, R.P.; Shringarpure, J.; Weinreb, S.M. J. Org. Chem. 1978, 43, 4172. Marshall, J.A.; Belletire, J.L. Tetrahedron Lett. 1971, 871.
- Lebouc, A.; Simonet, J.; Gelas, J.; Dehbi, A. Synthesis 1987, 320.
- Firouzabadi, H.; Iranpoor, N.; Zolfigol, M.A. Bull. Chem. Soc. Jpn. 1998, 71, 2169. Varma, R.S.; Saini, R.K. Tetrahedron Lett. 1997, 38, 2633. Meshram, H.M.; Reddy, G.S.; Yadav, J.S. Tetrahedron Lett. 1997, 38, 8891. Curini, M.; Marcotullio, M.C.; Pisani, E.; Rosati, O. Synlett 1997, 769. Curini, M.; Ceccherelli, P.; Marcotullio, M.C.; Epifano, F.; Rosati, O. Synlett 1996, 767. Komatsu, N.; Taniguchi, A.; Uda, M.; Suzuki, H. Chem. Commun. 1996, 1847. Schmittel, M.; Levis, M. Synlett 1996, 315. Haroutounian, S.A. Synthesis 1995, 39. Hirano, M.; Ukawa, K.; Yakabe, S.; Clark, J.H.; Morimoto, T. Synthesis 1997, 858. Lee, J.G.; Hwang, J.P. Chem. Lett. 1995, 507. Meshram, H.M.; Reddy, G.S.; Sumitra, G.; Yadav, J.S. Synth. Commun. 1999, 29, 1113.
- Firouzabadi, H.; Hazarkhani, H.; Karimi, B.; Niroumand, U.; Ghassamipour, S. Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), September 1-30, 2000.
- Mohammadpoor-Baltork, I.; Nourozi, A.R. Synthesis 1999, 487. Meskens, F.A.J. Synthesis 1981, 501. Gros, P.; Perchec, P.L.; Senet, J.P. J. Chem. Res. (S) 1995, 196. Marcantoni, E.; Nobili, F. J. Org. Chem. 1997, 62, 4183.
- For some recent work using Oxone® as an oxidant, see: Hajipour, A.R. Indian J. Chem. 1997, 36B, 1069. Hajipour, A.R.; Mahboubkhah, N. Org. Prep. Proced. Int. 1999, 31, 112. Meunier, B. New J. Chem. 1992, 16, 203.
- Hajipour, A.R.; Mallakpour, S.E.; Adibi, H. Phosphorus, Sulfur and Silicon 2000, 167, 71. Hajipour, A.R.; Mallakpour, S.E.; Adibi, H. Chem. Lett. 2000, 460. Hajipour, A.R.; Mallakpour, S.E.; Mohammadpoor-Baltork, I.; Adibi, H. Phosphorus, Sulfur and Silicon 2000, 165, 155. Hajipour, A.R.; Mallakpour, S.E.; Mohammadpoor-Baltork, I.; Adibi, H. Synth. Commun. 2001, 31, 3401. Hajipour, A.R.; Mallakpour, S.E.; Adibi, H. Chem. Lett. 2001, 164. Hajipour, A.R.; Mallakpour, S.E.; Adibi, H. Phosphorus, Sulfur and Silicon 2002, in press.
- Toda, F. Acc. Chem. Res. 1995, 28, 480. Toda, F.; Tanaka, K. Chem. Rev. 2000, 100, 1025.
- Hajipour, A.R.; Mallakpour, S.E.; Khoee, S. Chem. Lett. 2000, 120. Hajipour, A.R.; Mallakpour, S.E.; Imanzadeh, Gh. Indian J. Chem. 2001, 40B, 237. Hajipour, A.R.; Mallakpour, S.E.; Afrousheh, A. Phosphorus, Sulfur and Silicon 2000, 160, 67. Hajipour, A.R.; Mallakpour, S.E.; Khoee, S. Synlett 2000, 740. Hajipour, A.R.; Mallakpour, S.E.; Afrousheh, A. Tetrahedron 1999, 55, 2311. Hajipour, A.R.; Mallakpour, S.E.; Mohammadpoor-Baltork, I.; Khoee, S. Synth. Commun. 2001, 31, 1138. Hajipour, A.R.; Mallakpour, S.E. Mol. Cryst. and Liq. Cryst. 2001, 55, 356.
- Trost, B.M.; Braslau, R. J. Org. Chem. 1988, 53, 532. [CrossRef]
- Dictionary of Organic Compounds, 6th ed.; Chapman and Hall: London, 1982.
- Sample availability: Samples are available from the authors
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.