Abstract
The rearrangement of 4-methyl-3,5-diaryl-4H-1,2,4-triazoles to the corresponding 1-methyl-3,5-diaryl-1H-1,2,4-triazoles showed regioselectivity comparable to that observed for the alkylation of 3,5-diaryl-1H-1,2,4-triazoles. This lends support to a proposed mechanism for the rearrangement that involves consecutive nucleophilic displacements steps.
Introduction
Previous studies of the thermal rearrangement reactions of 4-alkyl substituted 4H-1,2,4-triazoles to the corresponding 1-alkyl-1H-1,2,4-triazoles provided evidence supporting the existence of intermolecular nucleophilic displacement reaction pathways, proceeding through the formation of dialkyltriazolium triazolate ion-pair intermediates or neutral molecules in a chain reaction [1]. The nucleophilic nature of these rearrangements therefore suggests that electronic effects may play a role in their outcome. Alkylation of 3,5-disubstituted-1H-1,2,4-triazoles has also been reported to take place exclusively at the ring 1-position [2]. On alkylation of unsymmetrical substituted triazoles Uda et al. [3] found that the 1-2 selectivity was affected by the nature of the substituents, and that alkylation takes place preferentially at the nitrogen next to the ring-carbon with the most electron donating group [3]. The selectivity was assumed to be a result of stereoelectronic control. The nucleophilic nature we have proposed for the rearrangement of 4-alkyl-3,5-diphenyl-4H-1,2,4-triazoles to the corresponding 1-alkyl-3,5-diphenyl-1H-1,2,4-triazoles should therefore also exhibit sensitivity to electronic effects. In this paper we report a study of the regioselectivity for the rearrangement of a series of unsymmetrically substituted triazoles.
Results and Discussion
A series of triazoles of the general structures 1 and 2 were required for this study. They were readily prepared by a modification of a procedure using unsymmetric bis(α-alkylaminobenzylidene)-hydrazines, 5, which has been described earlier [4] and is outlined in Scheme 1. Compounds 5 were formed from the corresponding N,N′-diarylhydrazides, 6, which in turn were obtained by reacting aroyl chlorides 7 with monohydrazide 8. In the synthesis of compound 2 a 40% aqueous methylamine solution was used. For the synthesis of triazole 1 saturated NH3 in 2-propanol was used, which was found to be superior to a procedure described previously by Stollè et al. [5].
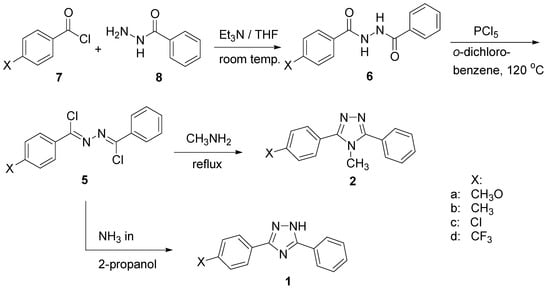
Scheme 1.
The synthetic results are summarized in Table 1. The yields are those of the pure products obtained after recrystallization or sublimation.

Table 1.
Synthesis of unsymmetrically substituted of 3,5-diaryl-1,2,4-triazoles
| Substituent 4-X | Product 5 % | Product 1 % | Product 2 % |
| a: CH3O | 49 | 53 | 72 |
| b: CH3 | 28 | 74a | 53 |
| c: Cl | 57 | 81 | 57 |
| d: CF3 | 75 | 78 | 79 |
a Yield after sublimation
A series of neat unsymmetrical substituted 4-methyl-3,5-diaryl-4H-1,2,4-triazoles, 2, were next thermolyzed at 330 °C for 30 min to investigate the regioselectivity as a function of the electronic conditions induced by the para-substituents in one of the phenyl rings. These results were also compared with those obtained for the selectivity upon alkylation of the corresponding unsymmetrical 3,5-diaryl-1H-1,2,4-triazoles, 1, with methyl iodide. The general reactions are shown in Scheme 2.
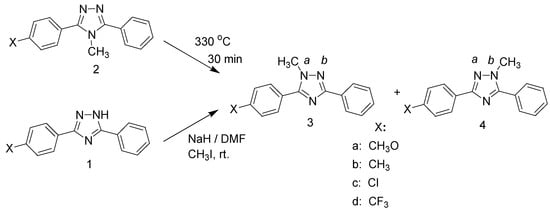
Scheme 2.
The thermolyses were carried out with samples of the appropriate triazoles in sealed glass tubes under nitrogen at ca. 330 °C for 30 minutes. The unsymmetrically substituted triazoles rearrange, as expected, to yield mixtures of the two possible regioisomers, i.e., the methyl group migrated to the a- and b- ring positions respectively, as indicated in Scheme 2, thus forming compounds 3 and 4. The product distributions are compiled in Table 2. The results of alkylation of triazole 1 with methyl iodide in DMF using sodium hydride as base are also shown.

Table 2.
Distribution of regioisomers 3 and 4 on thermolysis of triazoles 2 and alkylation of triazoles 1 with methyl iodide.
| Substituent 4-X | Thermolysis: Product distibution 3 : 4 | Alkylation: Product distribution 3 : 4 |
| a: CH3O | 51 : 49 | 54 : 46 |
| b: CH3 | 52 : 48 | 54 : 46 |
| c: Cl | 42 : 58 | 40 : 60 |
| d: CF3 | 42 : 58 | 36 : 64 |
The regioisomer ratios were determined by 400 MHz proton NMR spectroscopy of the crude reaction mixtures by measuring the intensities of characteristic signals. The assignment of the regioisomers was based on comparison of the NMR spectra to those of authentic samples of triazoles 3 [8], which were prepared by independent syntheses. Triazoles 4 were not prepared independently. The experiments were repeated and results were found to be reproducible within 1-2% of the values reported in the Table.
Authentic samples of the methyl substituted triazoles 3 were prepared using a modification of a method developed by Huisgen and coworkers [6], by reacting 2-methyl-5-phenyl-1H-1,2,3,4-tetrazole [7] with the appropriate 4-substituted benzonitriles, 4-X-Ph-CN, which in general gave better yields than were reported before.
The results reported in Table 2 provide support for the proposed mechanism for the rearrangement reactions. The data from the thermolysis series and the alkylation series, respectively, exhibited the very same trend with respect to regioselectivity. This supports the feasibility of a nucleophilic type mechanism in the rearrangements. The ratios were not identical. However, first of all, the reaction conditions were very different, as were the systems that were reacted. At high temperatures, selectivity will usually diminish. In the two systems, i.e., triazole 1 and 2, the electronic effects of the substituents may also be transmitted to a different extent to the reaction sites. Thus, it was shown by X-ray crystallography, that due to steric interactions with the methyl group, the phenyl ring in 2 are twisted approx. 44 - 48 degrees out of the triazole ring plane, thereby diminishing the overlap of the π-electron systems [8]. Triazole 1, on the other hand is nearly planar, with the phenyl ring only 8-9 degrees out of plane [9]. Therefore, the trends are similar, but the transmission of electronic effects is diminished for triazoles 2. Electron donating substituents in the aryl group resulted in an increased electron density and therefore increased nucleophilicity at the a-position (Scheme 2). As a result of this, products 3 were slightly predominant for aryl groups para-substituted with methoxy- and methyl groups respectively. The opposite effect, as expected, was clearly observed for the electron withdrawing substituents (Cl-, CF3-).
Conclusions
The rearrangement of 4-methyl-3,5-diaryl-4H-1,2,4-triazoles to the corresponding 1-methyl-3,5- diaryl-1H-1,2,4-triazoles exhibited regioselectivity comparable to that for the alkylation of 3,5-diaryl-1H-1,2,4-triazoles, providing further support to the previously proposed mechanism for the rearrangement [19], which would involve consecutive nucleophilic displacements steps (Scheme 3).
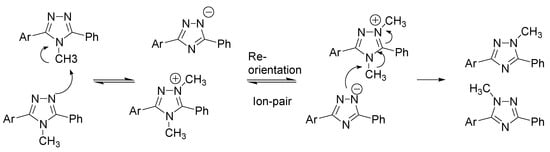
Scheme 3.
Experimental
General
1H- and 13C-NMR spectra were recorded on a JEOL JNM-EX400 FT NMR spectrometer, using CDCl3 as the solvent (unless otherwise indicated) and tetramethylsilane (TMS) as the internal standard. IR and GC-IR spectra were obtained on a Nicolet 20-SXC FT-IR (GC Carlo Erba 5160, 25 m, CP-Sil-5 CB). Mass spectra were recorded on a AEI MS-902 spectrometer at 70 eV (IP). GC measurements were performed on a Varian 3700 capillary gas chromatograph.
Preparation of Substituted N,N′- Dibenzoylhydrazines (6a-d)
These compounds were prepared by reacting benzhydrazide 8 with the appropriate aroyl chlorides 7a-d in dry THF and triethylamine at reflux for 3 hr.
2-(4-Methoxybenzoyl)benzhydrazide (6a). Obtained in 74% yield with m.p. 190-91 °C (lit. [10] 186-87 °C). The IR spectrum was in agreement with literature values [11]. 1H-NMR (100 MHz, DMSO-d6): δ 3.84 (s, 3H), 7.05 (d, 2H, J = 8.8 Hz), 7.50-7.65 (m, 3H), 7.92 (d, 4H, J = 8.3 Hz), 10.33 and 10.41 (2H, NH) ppm; MS [m/z (% rel. int.)]: 270 (6, M+), 252 (6), 135 (100).
2-(4-Methylbenzoyl)benzhydrazide (6b). Obtained in 76% yield with m.p. 226-27 °C (lit.[12] 220-21 °C). 1H-NMR (100 MHz, DMSO-d6): δ 2.41 (s, 3H), 7.21-7.28 (m, 2H), 7.35-7.60 (m, 3H), 7.9 (m, 4H), 9.42 (2H, NH) ppm. IR (KBr): 3203, 3053, 3003, 1667, 1630, 1536, 1489, 1285, 877, 835, 704 cm−1. MS [m/z (% rel. int.)]: 254 (12, M+), 119 (100), 105 (29).
2-(4-Chlorobenzoyl)benzhydrazide (6c). Obtained in 71% yield with m.p. 230-230.5 °C (lit.[13] 232.5-233.0 °C). The IR spectrum was in agreement with literature values [10]. 1H-NMR (100 MHz, DMSO-d6): δ 7.58-7.65 (m, 5H), 7.88-8.00 (m, 4H), 10.50 and 10.58 (2H, NH) ppm. MS [m/z (% rel. int.)]: 276 (5), 275 (2), 274 (11, M+), 139 (43), 105 (100).
2-(4-Trifluoromethylbenzoyl)benzhydrazide (6d). Obtained in 76% yield with m.p. 205.0-206.5 °C. 1H- NMR (100 MHz, DMSO-d6): δ 7.40-7.60 (m, 3H), 7.89-8.05 (m, 4H), 8.05-8.20 (m, 2H), 10.65 (2H, NH) ppm. IR (KBr): 3251, 3085, 1676, 1631, 1582, 1521, 1486, 1331, 1287, 1173, 1121, 1070, 860, 772 cm−1. MS [m/z (% rel. int.)]: 308 (5, M+), 173 (10), 105 (100). Calculated for C15H11F3N2O2: M+ 308.0773. Found: 308.0768.
General Procedure for the Synthesis of Substituted Di(α-chlorobenzylidene)hydrazines (5)
40 mmol of the appropriate dibenzoylhydrazine were added portionwise over 1.5 hr to a stirred mixture of 80 mmol of PCl5 in 30 mL of 1,2-dichlorobenzene. Evaporation of the solvent gave the desired product, which was recrystallized.
4-Methoxy-N-(chlorophenylmethylene)benzencarbohydrazonoyl chloride (5a). Obtained in 49% yield with m.p. 118-20 °C. 1H-NMR (100 MHz): δ 3.88 (s, 3H), 6.96 (d, 2H, J = 8.8 Hz), 7.40-7.54 (m, 3H), 8.03-8.18 (m, 4H) ppm. IR (KBr): 1600, 1590, 1580, 1510, 1486, 1462, 1446, 1420, 1306, 1264, 1220, 1176, 1120, 1029, 1010, 1000, 920, 837, 792, 770 cm−1. MS [m/z (% rel. int.)]: 306 (73, M+). Calculated for C15H12Cl2N2O: M+ 306.0327. Found: 306.0331
4-Methyl-N-(chlorophenylmethylene)benzencarbohydrazonoyl chloride (5b). Obtained in 28% yield with m.p. 85-86 °C. 1H-NMR (100 MHz): δ 2.41 (s, 3H), 7.26 (d, 2H, J = 7.8 Hz), 7.40-7.54 (m, 3H), 7.98-8.17 (m, 4H) ppm. IR (KBr): 1601, 1575, 1563, 1504, 1488, 1445, 1227, 1212, 1177, 915, 822, 791, 764 cm−1. MS [m/z (% rel. int.)]: 290 (68, M+). Calculated for C15H12Cl2N2: M+ 290.0377. Found: 290.0379
4-Chloro-N-(chlorophenylmethylene)benzencarbohydrazonoyl chloride (5c). This compound, previously reported by Cronin et al. [18], was obtained in 57% yield with m.p. 87-88 °C. 1H-NMR (100 MHz): δ 7.39-7.54(m, 5H), 8.03-8.20 (m, 4H) ppm. IR (KBr): 1605, 1573, 1490, 1450, 1402, 1228, 1174, 1096, 1019, 929, 836, 766, 733 cm−1.
4-(Trifluoromethyl)-N-(chlorophenylmethylene)benzencarbohydrazonoyl chloride (5d). Obtained in 75% yield with m.p. 77-78 °C. 1H-NMR (100 MHz): δ 7.37-7.58 (m, 3H), 7.73 (d, 2H, J = 8.3 Hz), 8.08-8.28 (m, 4H) ppm. IR (KBr): 1600, 1576, 1510, 1488, 1447, 1409, 1330, 1218, 1187, 1161, 1113, 1071, 1015, 922, 768 cm−1. MS [m/z (% rel. int.)]: 344 (40, M+). Calculated for C15H9Cl2N2F3: M+ 344.0095. Found: 344.0092.
Synthesis of Unsymmetric 4-Methyl-3,5-diaryl-4H-1,2,4-triazoles (2)
These compounds were prepared from the corresponding di(α-chlorobenzylidene)hydrazines, 5, as described previously in the literature [14] .
3-(4-Methoxyphenyl)-4-methyl-5-phenyl-4H-1,2,4-triazole (2a). The yield of the pure product was 72% with m.p. 211-13 °C (recryst. from toluene). 1H-NMR (400 MHz): δ 3.69 (s, 3H), 3.88 (s, 3H), 7.05 (d, 2H, J = 8.8 Hz), 7.50-7.55 (m, 3H), 7.67 (d, 2H, J = 8.8 Hz), 7.70-7.75 (m, 2H) ppm. 13C-NMR (100 MHz): δ 33.2, 55.5, 114.3, 119.6, 127.4, 128.8, 129.4, 129.9, 130.3, 155.8, 160.9 ppm. IR (KBr): 3006, 2965, 2937, 1615, 1540, 1483, 1439, 1296, 1255, 1182, 1040, 1029, 834, 772, 741, 701 cm−1. MS [m/z (% rel. int.)]: 266(18), 265 (100, M+), 264 (70), 148 (10), 134 (13), 118 (35). Calculated for C16H15N3O: M+ 265.1215. Found: 265.1213. Analysis: Calc. for C16H15N3O: C, 72.43; H, 5.70; N, 15.84. Found: C, 72.59; H, 5.91; N, 15.78.
3-(4-Methylphenyl)-4-methyl-5-phenyl-4H-1,2,4-triazole (2b). The yield of the pure product was 53% with m.p. 236.5-237.5 °C (recryst. from ethanol). 1H-NMR (400 MHz): δ 2.44 (s, 3H), 3.70 (s, 3H), 7.35 (d, 2H, J = 8.5 Hz), 7.38-7.56 (m, 3H), 7.62 (d, 2H, J = 8.3 Hz), 7.72-7.74 (m, 2H) ppm. 13C- NMR (100 MHz): δ 21.4, 33.2, 124.3, 127.3, 128.1, 128.9, 129.6, 130.0, 135.8, 140.2, 155.9 ppm; IR (KBr): 3051, 3037, 3020, 1686, 1619, 1578, 1526, 1485, 1449, 1075, 832, 771, 736, 710, 700 cm−1. MS [m/z (% rel. int.)]: 250 (17), 249 (100, M+), 248 (93), 132 (18), 118 (42). Calculated for C16H15N3: M+ 249.1266. Found: 249.1268. Analysis: Calc. for C16H15N3: C, 77.08; H, 6.06; N, 16.85. Found: C, 77.25; H, 6.21; N, 17.01.
3-(4-Chlorophenyl)-4-methyl-5-phenyl-4H-1,2,4-triazole (2c). The yield of the pure product was 57% with m.p. 276-77.5 °C (recryst. from toluene). 1H-NMR (400 MHz): δ 3.72 (s, 3H), 7.51-7.56 (m, 5H), 7.68-7.74 (m, 4H) ppm. 13C-NMR (100 MHz): δ 33.2, 125.7, 127.1, 128.9, 129.2, 130.1, 136.4, 154.9, 156.2 ppm. IR (KBr): 3067, 3051, 3025, 2970, 1605, 1480, 1456, 1437, 1402, 1098, 1074, 1014, 840, 827, 770 736, 717, 701 cm−1. MS [m/z (% rel. int.)]: 272 (5), 271 (32), 270 (42), 269 (100, M+), 268 (86), 267 (5), 152 (19), 118 (23). Calculated for C15H12ClN3: 269.0720. Found: 269.0717. Analysis: Calc. for C15H12N3Cl: C, 66.79; H, 4.48; N, 15.58; Cl, 13.14. Found: C, 66.65; H, 4.28; N, 15.40.
3-(4-Trifluoromethylhenyl)-4-methyl-5-phenyl-4H-1,2,4-triazole (2d). The yield of the pure product was 79% with m.p. 293-94 °C (recryst. from toluene). 1H-NMR (400 MHz): δ 3.75 (s, 3H), 7.52-7.58 (m, 3H), 7.72-7.76 (m, 2H), 7.80, 7.83, 7.89, 7.91 (4H, AA′BB′) ppm. IR (KBr): 3074, 3055, 3027, 1481, 1408, 1334, 1319, 1170, 1157, 1116, 1089, 1073, 1061, 1022, 1014, 861, 842, 774, 732, 705 cm−1. MS [m/z (% rel. int.)]: 304 (16), 303 (94, M+), 302 (100), 186 (27), 145 (11), 118 (18), 104 (19). Calculated for C16H12F3N2: 303.0983. Found: 303.0979. Analysis: Calc. for C16H12N3F3: C, 63.36; H, 3.99; N, 13.85; F, 18.79. Found: C, 63.48; H, 4.18; N, 13.67.
General Procedure for the Synthesis of 5-Aryl-3-phenyl-1H-1,2,4-triazoles (1a-d)
The appropriate substituted di(α-chlorobenzylidene)hydrazine, 5, (2 mmol) was dissolved in 2-propanol (100 mL) saturated with NH3, and the resulting mixture was stirred at room temperature overnight and finally refluxed for 30 h. Evaporation of the solvent under reduced pressure gave the crude product, which was further purified by crystallization or sublimation.
3-(4-Methoxyphenyl)-5-phenyl-1H-1,2,4-triazole (1a). Yield = 53% (after crystallization from toluene). M.p. 169.5-72.0 °C. 1H-NMR (100 MHz, DMSO-d6): δ 3.84 (s, 3H), 7.10 (d, 2H, J = 8.8 Hz), 7.49-7.53 (m, 3H), 8.00-8.20 (m, 4H) ppm. 13C-NMR (25 MHz, DMSO-d6): δ 55.2, 114.2, 121.4, 125.9, 127.5, 128.7, 129.3, 129.7, 157.5, 160.3 ppm. IR (KBr): 3500-2500 (broad), 1614, 1508, 1471, 1441, 1408, 1394, 1310, 1304, 1290, 1278, 1260, 1180, 1142, 1034, 982, 836, 749, 722 cm−1. Analysis: Calc. for C15H13N3O: C, 71.70; H, 5.21; N, 16.72; O, 6.37. Found: C, 71.88; H, 5.41; N, 16.67.
3-(4-Methylphenyl)-5-phenyl-1H-1,2,4-triazole (1b). Yield = 74% after sublimation. M.p. 178-80 °C. The spectroscopic properties were all in agreement with those reported in the literature [15].
3-(4-Chlorophenyl)-5-phenyl-1H-1,2,4-triazole (1c). Yield = 81% yield after crystallization from toluene. M.p. 241-42 °C. The spectroscopic properties were all in agreement with those reported in the literature [16].
3-(4-Trifluorophenyl)-5-phenyl-1H-1,2,4-triazole (1d). Yield = 74% yield after crystallization from toluene. M.p. 224-26 °C. All the spectroscopic properties were in agreement with those reported in the literature [17].
General Procedure for the Synthesis of 1-Methyl-3-aryl-5-phenyl-1H-1,2,4-triazoles (3a-d)
The method described by Huisgen et al. was applied [6]. Thus, a solution of 2-methyl-5-phenyl-2H-1,2,3,4-tetrazole [7], (2.5 mmol) in the appropriate substituted benzonitrile, X-Ph-CN, (2 mL) was refluxed for 3-5 h under nitrogen. The nitrile was then removed under reduced pressure by Kugelrohr distillation, and the crude product was recrystallized.
1-Methyl-5-(4-methoxyphenyl)-3-phenyl-1H-1,2,4-triazole (3a). Yield = 39% of a product of 98% purity (GC) and with m.p. 82.5-85.5 °C. 1H-NMR (400 MHz): δ 3.88 (s, 3H), 4.00 (s, 3H), 7.04 (d, 2H, J = 8.8 Hz), 7.37-7.46 (m, 3H), 7.68 (d, 2H, J = 8.8 Hz), 8.14 (d, 2H, J = 6.8 Hz) ppm. 13C-NMR (100 MHz, CDCl3): δ 36.9, 55.3, 114.4, 120.3, 126.2, 128.5, 129.0, 130.1, 131.1, 155.4, 160.9 ppm. IR (KBr): 3040, 3023, 3009, 1611, 1545, 1486, 1473, 1444, 1396, 1355, 1298, 1256, 1186, 1172, 1131, 1033, 1023, 835, 790, 747, 724, 702 cm−1. MS [m/z (% rel. int.)]: 266 (19), 265 (100, M+), 264 (9), 132 (60), 131 (19), 105 (18), 104 (25). Calculated for C16H15N3O: 265.1215. Found: 265.1213. Analysis: Calc. for C16H15N3O: C, 72.43; H, 5.70; N, 15.84. Found: C, 72.28; H, 5.77; N, 15.65.
1-Methyl-5-(4-methylphenyl)-3-phenyl-1H-1,2,4-triazole (3b). Yield = 55% of a product of 96% purity (GC) and with m.p. 78-81 °C. 1H-NMR (400 MHz): δ 2.44 (s, 3H), 4.01 (s, 3H), 7.32 (d, 2H, J = 7.3 Hz), 7.37-7.46 (m, 3H), 7.62 (d, 2H, J = 8.3), 8.14 (d, 2H, J = 7.3) ppm. 13C-NMR (100 MHz, DMSO-d6): δ 21.4, 36.9, 125.1, 126.3, 128.5, 128.6, 129.0, 129.4, 131.1, 140.2, 155.7, 161.0 ppm. IR (KBr): 3072, 3024, 2946, 1610, 1482, 1472, 1447, 1353, 1138, 1070, 1014, 823, 749, 735, 713 cm−1. MS [m/z (% rel. int.)]: 250(19), 249(100, M+), 248(8), 132(62), 131(16), 104(19). Calculated for C16H15N3: 249.1266. Found: 249.1268. Analysis: Calc. for C16H15N3: C, 77.08; H, 6.06; N, 16.85. Found: C, 77.20; H, 6.19; N, 16.78.
1-Methyl-5-(4-chlorophenyl)-3-phenyl-1H-1,2,4-triazole (3c). Yield = 83% of a product with >99 % purity (GC) and m.p. 122.0-23.5 °C. All spectroscopic properties were in agreement with those reported in the literature [6].
1-Methyl-5-(4-trifluoromethylphenyl)-3-phenyl-1H-1,2,4-triazole (3d). Yield = 58% of a product of 99% purity (GC) and m.p. 131.5-33.5 °C (lit.[17] 115-17 °C). 1H-NMR (400 MHz): δ 4.05 (s, 3H), 7.26-7.45 (m, 3H), 7.79, 7.81, 7.88, 7.90 (4H, -AA′BB′), 8.14 (d, 2H, J = 7.6) ppm. 13C-NMR (100 MHz, CDCl3): δ 37.1, 123.7, (q, JCF = 270 Hz), 125.7, 125.9, 126.3, 128.6, 129.1, 129.3, 130.7, 131.5, 132.6, 154.2, 161.4 ppm. IR (KBr): 3074, 3058, 2955, 2927, 1623, 1472, 1447, 1437, 1331, 1314, 1165, 1122, 1078, 1057, 1023, 1014, 849, 789, 757, 748, 732, 704 cm−1. MS [m/z (% rel. int.)]: 304 (19), 303 (100, M+), 132 (69), 131 (23), 104 (28). Calculated for C16H12F3N3: 303.0983. Found: 303.0981; Analysis: Calc. for C16H12F3N3: C, 63.36; H, 3.99; N, 13.85; F, 18.79. Found: C, 63.61; H, 7.21; N, 13.51.
Thermolysis of 4-methyl-3-aryl-5-phenyl-4H-1,2,4-triazoles (1)
The triazole (30 mg) was placed under nitrogen in a sealed glass tube and then heated in an oven at 323-35 °C for 30 min. The crude product contained the compounds 3 and 4. The compositions of the reaction mixtures were determined by 400 MHz NMR spectroscopy by comparison with those of the authentic samples. The regioisomers could not be separated by either GC or TLC.
Methylation of 3-aryl-5-phenyl-1H-1,2,4-triazoles (1)
An appropriate 3-aryl-5-phenyl-1H-1,2,4-triazole, 1 (30 mmol), was dissolved in dry DMF (1 mL), 0.3 g NaH were added and the mixture was stirred under nitrogen for 1 h. Then CH3I (0.30 mmol) was added and the reaction mixture stirred for 48 h. Water was added and the mixture extracted with CH2Cl2, yielding the crude product which was analyzed by NMR as described above.
Acknowledgements
The authors wish to thank The Norwegian Research Council, NFR, for financial support.
References
- Carlsen, P.H.J.; Gautun, O.R. Acta Chem. Scand. 1990, 44, 485.
- Lee, L.A.; Evans, R.; Wheeler, J.W. J. Org. Chem. 1972, 37, 343.Atkinson, M.R.; Polya, J.B. J. Chem. Soc. 1954, 141.
- Uda, M.; Hisazumi, Y.; Sato, K.; Kubota, S. Chem. Pharm. Bull. 1975, 24, 3103.
- Gautun, O.R.; Carlsen, P.H.J. Acta Chem. Scand. 1991, 45, 609.
- Stollè, R.; Thimä, K. J. Prakt. Chem. 1906, 73, 288.
- Fliege, W.; Grashey, R.; Huisgen, R. Chem. Ber. 1984, 117, 1194.Huisgen, R.; Seidel, M.; Sauer, J.; McFarland, J.W.; Wallbillich, G. J. Org. Chem. 1959, 24, 892.
- Henry, R.A. J. Am. Chem. Soc. 1951, 73, 4470.
- Carlsen, P.H.J.; Jørgensen, K.B.; Gautun, O.R.; Jagner, S.; Håkansson, M. Acta Chem. Scand. 1994, 49, 676.
- Carlsen, P.H.J.; Gautun, O.R.; Samuelsen, E.J.; Mårdalen, J.; Helgesson, G.; Jagner, S. PhysicaScripta 1991, 44, 214.
- Grekov, A.P.; Kulakova, L.N.; Shvaika, O.P. J. Gen. Chem., USSR. 1966, 29, 3020.
- Zadorozhnyi, B.A.; Ishcenko, I.K.; Mnatsakanova, T.R.; Shvaika, O.P. J. Org. Chem., USSR. 1966, 2, 431.
- Gilbert, E.C. J. Am. Chem. Soc. 1927, 49, 286.
- Beck, H.; Kroner, J. Chem. Ber. 1966, 99, 2039.
- Gautun, O.R.; Carlsen, P.H.J. Acta Chem. Scand. 1991, 45, 609.
- Whitfield, L.A.; Papadopoulos, E.P. J. Heterocycl. Chem. 1981, 18, 1197.
- Pèrez, M.A.; Dorado, C.A.; Soto, J.L. Synthesis 1983, 483.
- Lin, Y.-I; Hlavka, J.J.; Bitha, P.; Lang, S.A. J. Heterocycl. Chem. 1983, 20, 1693.
- Cronin, J.; Hegarty, A.F.; Cashell, P.A.; Scott, F.L. J. Chem. Soc., Perkin Trans II. 1973, 1708.
- Gautun, O.R.; Carlsen, P.H.J. Eur. J. Org. Chem. 2000, 3749.
- Sample Availability: Available from the authors.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.