Reactions with Hydrazonoyl Halides. 31. Synthesis of Some New Pyrrolidino[3,4-c]pyrazolines, Pyrazoles, and Pyrazolo[3,4-d]pyridazines
Abstract
:Introduction
Results and Discussion
Experimental
General
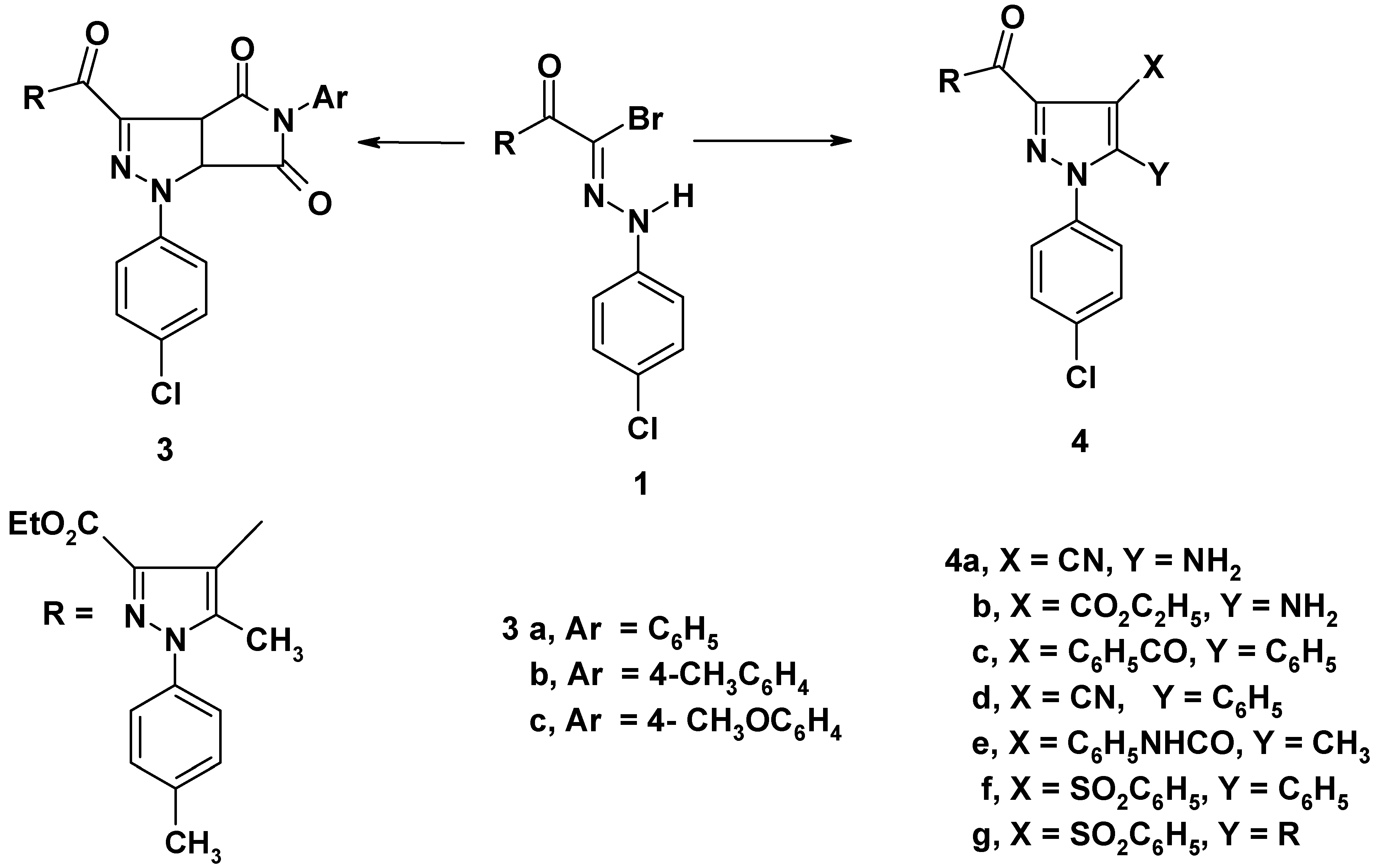
General procedure for the synthesis of 5-aryl-1-(4-chlorophenyl)-3-[3'-ethoxycarbonyl-5'-methyl-1'- (4-tolyl)-4'-pyrazoloyl]pyrrolidino[3,4-c]pyrazoline-4,6-diones (3a-c)
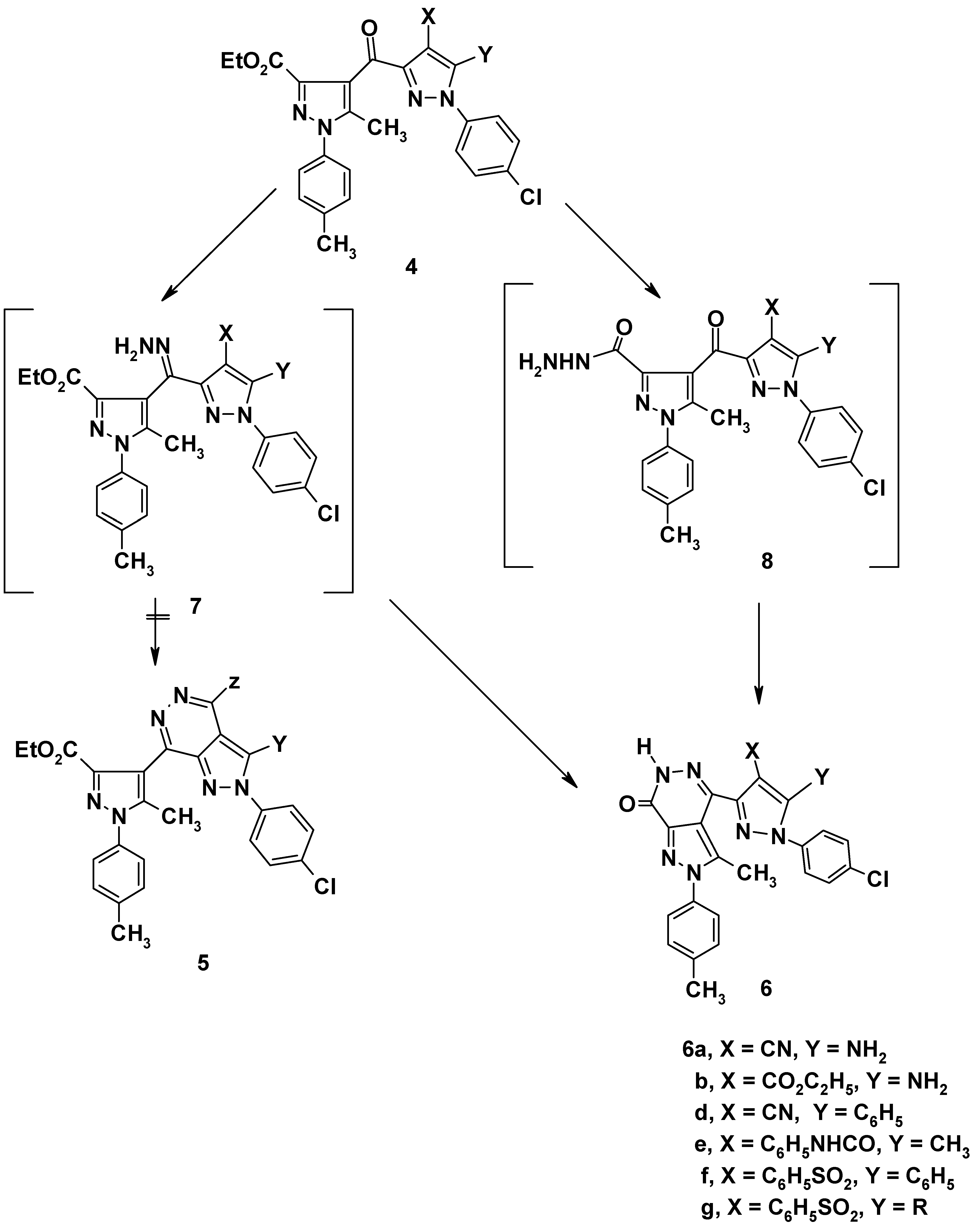
General procedure for the synthesis of 4,5-disubstituted 1-(4-chlorophenyl)- 3-[3'-ethoxy-carbonyl-5'- methyl-1'-(4-tolyl)-4'-pyrazoloyl]pyrazoles (4a-g)
General procedure for the synthesis of 4,5-disubstituted 4-[1-(4-chlorophenyl)-3-pyrazolyl]-2,6- dihydro-3-methyl-2-(4-tolyl)-pyrazolo[3,4-d]pyridazin-7-ones (6a,b,d-g)
References and Notes
- Abdelhamid, A.O.; Rateb, N.M.; Dawood, K.M. Phosph., Sulfur, Silicon, and Related Elements 2000, in press.
- Ulrich, H. The Chemistry of Imidoyl Halides; Plenum Press: New York, 1968; p. 173. [Google Scholar]
- Huisgen, R.; Garashey, R.; Sauer, J. The Chemistry of Alkenes; Patai, S., Ed.; Wiley-Interscience: New York, N.Y, 1964; Vol. 1, p. 739. [Google Scholar]
- Abdelhamid, A.O. J. Chem. Res. 1993, (S) 208, (M) 1239.
- Butler, R.N.; Scott, F.L. Chem. Ind. (London) 1970, 1216.
- Hassan, N.M.; Abdelhamid, A.O. J. Chem. Res. 1997, (S) 350, (M) 2254 (1997).
- Hassan, N.M.; Fahmi, A.A.; Abd-El-mageid, F.F.; Abdelhamid, A.O. J. Chin. Chem. Soc. 1996, 43, 493.
- Abdelhamid, A.O.; Abd-El-mageid, F.F.; Hassan, N.M.; Zohdi, H.F. J. Chem. Res. 1995 (S), 492, (M) 3036.
- Abdelhamid, A.O.; Attaby, F.A.; Khalifa, F.A.; Ghabrial, S. S. Arch. Pharm. Res. 1992, 15, 14.
- Abdelhamid, A.O.; Zohdi, H.F.; Sallam, M.M.M.; Ahmed, N.A. Phosph., Sulfur, Silicon, and Related Elements 2000, in press.
- Abdelhamid, A.O.; Ghabrial, S.S. Sulfur Letters 1987, 7, 19.
- Searle, N.F.E. U.S. U.S. Pat, 2,444,536 (to E.I. Dupont De Nemours and Co. Inc.). 1948. [Chem. Abstr. 1949, 42, 7340c].
- Sample Availability: Available from MDPI.
| Compd no. | Color | Yield % | M.P., °C Solvent | Mol.Formula Mol.Wt. | % Analyses, Calcd. /Found | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | |||||
| 3a | Yellowish | 77 | 250-253 | C32H26ClN5O5 | 64.48 | 4.40 | 11.75 | |
| green | EtOH | 596.05 | 64.70 | 4.30 | 11.60 | |||
| 3b | Yellowish | 78 | 228-230 | C33H28ClN5O5 | 64.97 | 4.63 | 11.48 | |
| green | EtOH | 610.07 | 65.10 | 4.50 | 11.30 | |||
| 3c | Yellowish | 82 | 285-287 | C33H28ClN5O6 | 63.31 | 4.51 | 11.19 | |
| green | AcOH | 626.07 | 63.10 | 4.50 | 11.00 | |||
| 4a | Yellow | 85 | 208-10 | C25H21ClN6O3 | 61.41 | 4.33 | 17.19 | |
| EtOH | 488.94 | 61.22 | 4.20 | 17.30 | ||||
| 4b | Colorless | 80 | 243-245 | C27H26ClN5O5 | 60.50 | 4.89 | 13.07 | |
| EtOH | 535.99 | 60.60 | 4.90 | 12.90 | ||||
| 4c | Yellow | 92 | 197-200 | C37H29ClN4O4 | 70.64 | 4.65 | 8.91 | |
| EtOH | 629.12 | 70.60 | 4.40 | 8.80 | ||||
| 4d | Yellow | 76 | 185-187 | C31H24ClN5O3 | 67.70 | 4.40 | 12.73 | |
| EtOH | 550.02 | 67.90 | 4.30 | 12.80 | ||||
| 4e | Yellow | 69 | 175-178 | C32H28ClN5O4 | 66.03 | 4.85 | 12.03 | |
| EtOH | 582.06 | 66.10 | 5.00 | 11.90 | ||||
| 4f | Yellow | 82 | 127-130 | C36H29ClN4O5S | 65.01 | 4.39 | 8.42 | 4.82 |
| EtOH | 665.17 | 65.20 | 4.10 | 8.20 | 4.90 | |||
| 4g | Brown | 78 | 140-143 | C44H39ClN6O7S | 63.57 | 4.73 | 10.11 | 3.86 |
| EtOH | 831.35 | 63.40 | 4.73 | 10.20 | 3.70 | |||
| 6a | Orange | 72 | 200-203 | C23H17ClN8O | 60.46 | 3.75 | 24.52 | |
| EtOH | 456.90 | 60.20 | 3.90 | 24.70 | ||||
| 6b | Yellow | 62 | 227-230 | C22H22ClN7O3 | 59.58 | 4.40 | 19.46 | |
| EtOH | 503.95 | 59.50 | 4.40 | 19.60 | ||||
| 6d | Colourless | 70 | 310-12 | C29H20ClN7O | 67.25 | 3.89 | 18.93 | |
| EtOH | 517.98 | 67.30 | 4.10 | 18.80 | ||||
| 6e | Colourless | 66 | 307-10 | C30H24ClN7O2 | 65.51 | 4.40 | 17.83 | |
| EtOH | 550.02 | 65.50 | 4.50 | 17.90 | ||||
| 6f | Yellow | 73 | 197-200 | C34H25ClN6O3S | 64.50 | 3.98 | 13.27 | 5.60 |
| EtOH | 633.13 | 64.40 | 4.20 | 13.40 | 5.40 | |||
| 6g | Yellow | 78 | 320-22 | C42H35ClN8O5S | 63.11 | 4.41 | 14.02 | 4.44 |
| EtOH | 799.31 | 63.30 | 4.20 | 14.20 | 4.30 | |||
| Comp | IR (cm-1) | 1H NMR (δ ppm) |
|---|---|---|
| no. | ||
| 3a | 1740-1720 and 1710-1690 (CO’s) | 1.11 (t, 3H, CH2CH3); 2.33 (s, 3H, CH3); 2.41 (s, 3H, CH3); 4.12 (q, 2H, CH2CH3); 5.20 (d, 1H, pyrazoline H-4); 5.49 (d, 1H, pyrazoline H-5) and 7.16-7.46 (m, 13H, ArH). |
| 3b | 1740-1720 and 1710-1690 (CO’s) | 1.11 (t, 3H, CH2CH3); 2.33 (s, 3H, CH3); 2.41 (s, 6H, 2CH3); 4.12 (q, 2H, CH2CH3); 5.02 (d, 1H, pyrazoline H-4); 5.49 (d, 1H, pyrazoline H-5) and 7.14-7.46 (m, 12H, ArH). |
| 3c | 1740-1720 and 1710-1690 (CO’s) | 1.11 (t, 3H, CH2CH3); 2.33 (s, 3H, CH3); 2.41 (s, 3H, CH3); 4.92 (s, 3H, OCH3); 4.12 (q, 2H, CH2CH3); 5.02 (d, 1H, pyrazoline H-4); 5.49 (d, 1H, pyrazoline H-5) and 7.14-7.46 (m, 12H, ArH). |
| 4a | 3292,3176 (NH2); 2229 (CN); 737, 1659 (CO’s). | 1.02 (t, 3H, CH2CH3); 2.40 (s, 3H, CH3); 2.43 (s, 3H, CH3); 4.01 (q, 2H, CH2CH3); 7.14 (s, br., 2H, NH2) and 7.37-7.68 (m, 8H, ArH’s). |
| 4b | 3272, 3185 (NH2), 1730, 1647 (CO’s). | 1.02 (t, 3H, CH2CH3); 1.10 (t, 3H, CH2CH3); 2.40 (s, 3H, CH3); 2.43 (s, 3H, CH3); 4.01 (q, 2H, CH2CH3); 4.21 (q, 2H, CH2CH3); 7.14 (s, br., 2H, NH2) and 7.37-7.68 (m, 8H, ArH). |
| 4c | 1720, 1685, 1660 (CO's) | 1.23 (t, 3H, CH2CH3); 2.32 (s, 3H, CH3); 2.39 (s, 3H, CH3); 4.24 (q, 2H, CH2CH3); 7.19-8.09 (m, 18H, ArH). |
| 4d | 2236 (CN); 1724, 1658 (CO’s). | 1.07 (t, 3H, CH2CH3); 2.42 (s, 3H, CH3); 2.49 (s, 3H, CH3); 4.13 (q, 2H, CH2CH3); and 7.17-7.50 (m, 13H, ArH). |
| 4e | 3240 (NH); 1735, 1668 (CO’s). | 1.05 (t, 3H, CH2CH3); 2.41 (s, 3H, CH3); 2.50 (s, 3H, CH3); 2.75 (s, 3H, CH3); 4.03 (q, 2H, CH2CH3); 7.32-7.88 (m, 13H, ArH) and 11.64 (s, br., 1H, NH). |
| 4f | 1730,1667 (CO’s); 1314,1140 (SO2). | 1.05 (t, 3H, CH2CH3); 2.34 (s, 3H, CH3); 2.44 (s, 3H, CH3); 4.03 (q, 2H, CH2CH3); and 7.32-7.88 (m, 18H, ArH). |
| 4g | 1730, 1667 (CO’s) and 1314, 1140 (SO2). | 1.04 (t, 3H, CH2CH3); 1.14 (t, 3H, CH2CH3); 2.39 (s, 6H, 2CH3); 2.43 (s, 6H, CH3); 4.02 (q, 2H, CH2CH3); 4.12 (q, 2H, CH2CH3) and 7.26-8.04 (m, 17H, ArH). |
| 6a | 3340, 3292, 3176 (NH2, NH); 2220 (CN); 1668 (CO) | 2.43 (s, 3H, CH3); 2.55 (s, 3H, CH3); 6.90 (s, 2H, NH2); 7.04-7.65 (m, 8H, ArH) and 11.63 (s, 1H, NH). |
| 6b | 3244 (NH); 1710, 1666 (CO). | 1.33 (t, 3H, CH2CH3); 2.39 (s, 3H, CH3); 2.67 (s, 3H, CH3); 4.12 (q, 2H, CH2CH3); 6.22 (s, 2H, NH2) and 7.00-7.79 (m, 8 H, ArH); 10.62 (s, 1H, NH). |
| 6d | 3345 (NH); 2233 (CN); 1674 (CO). | 2.40 (s, 3H, CH3); 2.67 (s, 3H, CH3); 7.21-7.73 (m, 13H, ArH) and 12.65 (s, 1H, NH). |
| 6e | 3244 (NH); 1666 (CO) | 2.39(s, 3H, CH3); 2.67(s, 3H, CH3); 2.42(s, 3H, CH3); 7.00-7.79(m, 13H, ArH); 10.62(s, 1H, NH) and 102.35(s, 1H, NH) |
| 6f | 3222 (NH); 1663 (CO); 1314, 1140 (SO2). | 2.34 (s, 3H, CH3); 2.44 (s, 3H, CH3); 7.13-7.54 (m, 18H, ArH) and 10.33 (s, 1H, NH). |
| 6g | 3222 (NH); 1663 (CO); 1314, 1140 (SO2). | Insoluble |
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Abdelhamid, A.O.; Zohdi, H.F.; Sallam, M.M.M.; Ahmed, N.A. Reactions with Hydrazonoyl Halides. 31. Synthesis of Some New Pyrrolidino[3,4-c]pyrazolines, Pyrazoles, and Pyrazolo[3,4-d]pyridazines. Molecules 2000, 5, 967-973. https://doi.org/10.3390/50700967
Abdelhamid AO, Zohdi HF, Sallam MMM, Ahmed NA. Reactions with Hydrazonoyl Halides. 31. Synthesis of Some New Pyrrolidino[3,4-c]pyrazolines, Pyrazoles, and Pyrazolo[3,4-d]pyridazines. Molecules. 2000; 5(7):967-973. https://doi.org/10.3390/50700967
Chicago/Turabian StyleAbdelhamid, Abdou O., Hussien F. Zohdi, Mohamed M. M. Sallam, and Nagla A. Ahmed. 2000. "Reactions with Hydrazonoyl Halides. 31. Synthesis of Some New Pyrrolidino[3,4-c]pyrazolines, Pyrazoles, and Pyrazolo[3,4-d]pyridazines" Molecules 5, no. 7: 967-973. https://doi.org/10.3390/50700967
APA StyleAbdelhamid, A. O., Zohdi, H. F., Sallam, M. M. M., & Ahmed, N. A. (2000). Reactions with Hydrazonoyl Halides. 31. Synthesis of Some New Pyrrolidino[3,4-c]pyrazolines, Pyrazoles, and Pyrazolo[3,4-d]pyridazines. Molecules, 5(7), 967-973. https://doi.org/10.3390/50700967




