Abstract
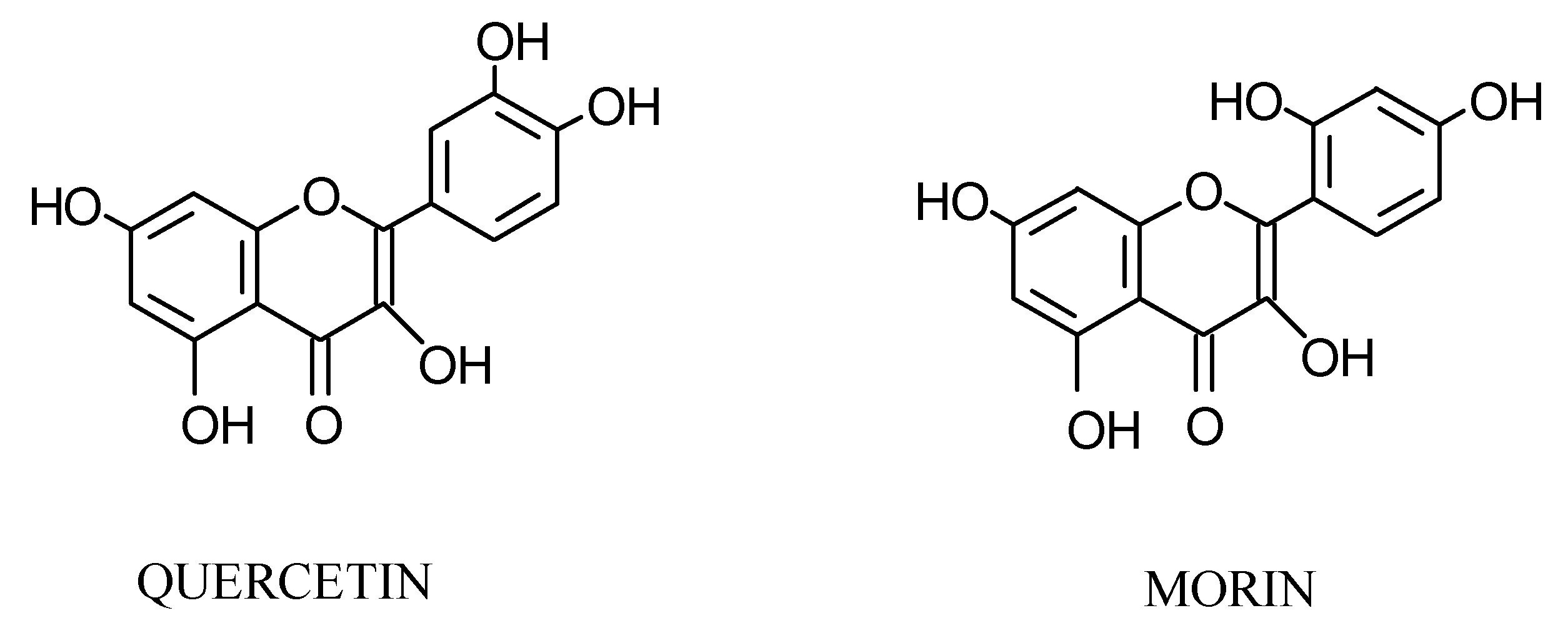
The complexes between some flavonoids and metals (Co(II), Cu(II), Mn(II), Mg(II), Sn(II)) have been studied by spectrophotometric methods in order to determine the stoichiometry and stability constants.
Introduction
Flavonoids are poliphenolic compounds, which are characterized by showing a variety of pharma- cological activities, e.g. antioxidant, antihelmintic, antiinflamatory, antiviral, antitumor, etc. Most of these activities are due to their ability to inhibit enzymes, such as trypsine, protein kinases, topoi- somerases.
The complex formation of these compounds and metals of the active enzyme site or complexes between certain amino acids of the active site and the metals of the medium is probably the reason of this enzymatic inhibition.
This study deals with the determination of the stoichiometry of the complexes of some flavonoids and a variety of metals (Co(II), Cu(II), Mn(II), Mg(II), Sn(II)) as well as the determination of each sta- bility constant.
Experimental
Spectrophotometric methods were used. Solutions of each flavonoid and metal salts in a molar rate (metal mols/total mols) in the range of 0.09 and 0.9 were prepared using methanol.
UV-Visible spectra for each molar rate were performed, and plotting Absorbance vs Molar rate or Initial metal concentration the exact stoichiometry of each complex was determined.
Results and discussion
Formation of species with a 1:1 (metal:ligand) stoichiometry were obtained for the flavonoids tested. The stability constant of each complex was determined.
Acknowledgment:
The authors are indebted to CONICET (Argentine) and the Universidad de Buenos Aires for financial support.
References and Notes
- Phytochemistry 1986, 25(2), 383–385.
- Biochemical Pharmacology 1993, 46(7), 1257–1271.
- J. Biol. Chem. 1996, 271(4), 2262–2270.
- J. Nat. Prod. 1995, 58(6), 823–829.
- Biochem. Pharmacol. 1989, 38(10), 1617–1624.
- Biochem. Pharmacol. 1986, 35(14), 2345–2357.
- Biochem. Pharmacol. 1986, 35(2), 237–245.
- Biochem. Pharmacol. 1987, 36(5), 717–720.
- Biochem. Pharmacol. 1989, 38(17), 2859–2865.
- Can. J. Chem. 1991, 69, 1994–2001.