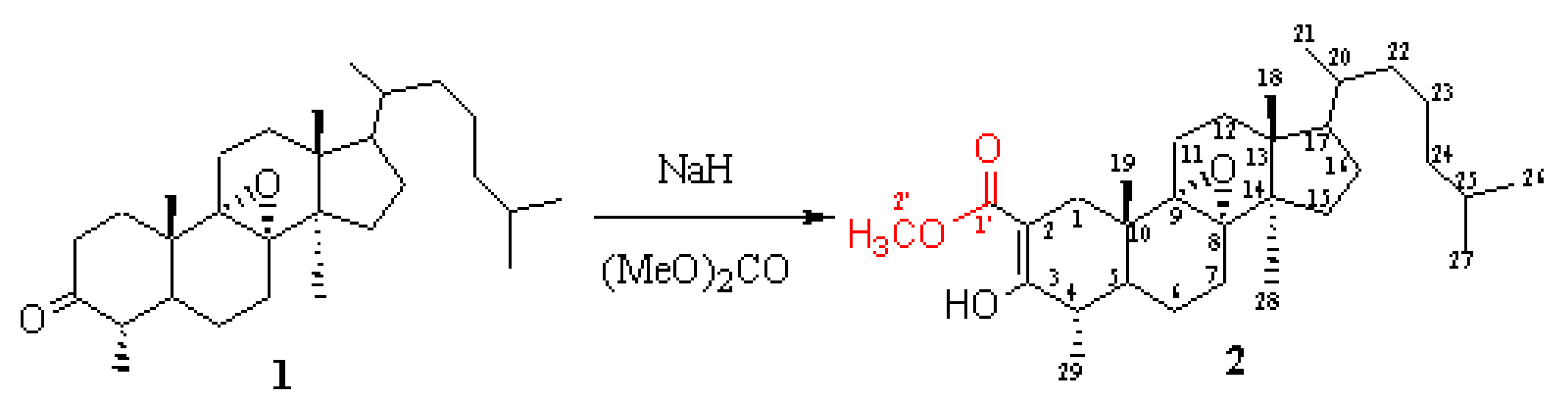
To sodium hydride (0.34 g, 13.96 mmole) carefully washed with anhydrous benzene under nitrogen (to eliminate mineral oil from the commercial product) [1], was added 0.8 ml (9.27 mmol) of dimethylcarbonate (freshly distilled) in dry benzene (20 ml). Under nitrogen atmosphere, the mixture was vigorously stirred and heated at 70 °C. At this temperature was added dropwise 1g (2.33 mmol) of 1 in dry benzene (20 ml) in 2 hours. The agitation was maintained at 100°C during 12 hours. After cooling to 0°C and acidification by 5.5 ml of acetic acid, the mixture was poured on a mixture of 80 ml of ice and 80 ml of HCl (6N). The organic layer was washed with diluted solution of sodium bicarbonate, then dried. After evaporating the solvent in vacuum, the residue was purified by silica gel column chromatography using hexane as eluent to give 2 (0.93 g, 82 %).
IR : 1750 cm-1.
MS (EI, 70eV) : 487.7 (M+.).
1H NMR (200 MHz, CDCl3) : 12.27 (s, OH); 3.66 (s, C2'-H3); 0.72 (s, C18-H3); 0.89 (s, C19-H3); 1.06 (d, J=6 Hz, C21-H3); 0.84 (s, C28-H3); 1.22 (d, J=6 Hz, C29-H3).
13C NMR (50 MHz, CDCl3) : 32.46 (C1); 95.4 (C2); 173.84 (C3); 37.50 (C4); 43.99 (C5); 20.25 (C6);
28.31 (C7); 69.52 (C8); 68.42 (C9); 36.27 (C10); 19.36 (C11); 23.88 (C12); 39.83 (C13); 48.71 (C14);
31.98 (C15); 28.79 (C16); 49.26 (C17); 15.59 (C18); 16.54 (C19); 36.7 (C20); 15.97 (C21); 36.65 (C22);
22.89 (C23); 39.58 (C24); 24.42 (C25); 22.27 (C26); 21.10 (C27); 23.17 (C28); 27.17 (C29); 51.72 (C2');
174.06 (C1').
Supplementary Materials
Reference
- Vander Roest, J. M.; Grieco, P. A. J. Am. Chem. Soc. 1993, 115, 5841. [CrossRef]
Sample Availability: Available from the authors and MDPI. |
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.