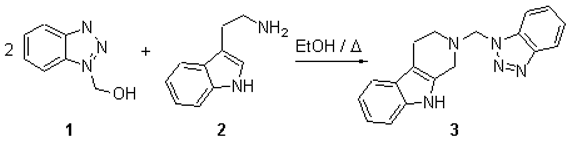
Katritzky's well-established chemistry of benzotriazole [1] was applied to the synthesis of 1,2,3,4- tetrahydro-b-carboline, which have been traditionally prepared by Pictet-Spengler condensation2, Fischer cyclisation and other methods3. This facile reaction of 1-hydroxymethylbenzotriazole 1 with tryptamine 2 yielded very high quantities of crystalline N-benzotriazol-1-yl-methyl-1,2,3,4-tetrahydro-b-carboline 3.
Tryptamine 2 (1.02g, 6.34mmol) and 1-hydroxymethylbenzotriazole 1 (1.94g, 12.99mmol) were dissolved in ethanol (50mL) and refluxed for two hours. A crystalline solid dropped out of the solution while refluxing. The mixture was chilled and vacuum filtered to obtain a pale tan crystalline compound (1.70g, 89%), then recrystallised from ethanol to give N-benzotriazol-1-yl-methyl-1,2,3,4-tetrahydro-b-carboline 3 as a pale tan powder (1.581g, 82%)..
M.p. 200-202 °C. (EtOH, uncorrected).
1H-NMR (200 MHz; DMSO-d6; Me4Si): 10.76 (1H, s, NH), 8.13-8.07, 7.66-7.58, 7.48-7.44, 7.38-7.34,
7.30-7.26, 7.05-6.94 (8H, m, Ar), 5.87 (1H, s, NCH2Bt), 3.87 (2H, s, InCH2N), 3.02 (2H, s, CCH2N), 2.74 (2H, s, InCH2C)
13C-NMR (50 MHz; DMSO-d6): 21.3 (InCH2C), 46.7 (InCH2N), 48.3 (InCH2CH2N), 68.2 (NCH2Bt),
106.2, 117.6, 118.5, 120.7, 126.8, 136.2 (Ar), 111.1(C=CN), 111.4,119.3,124.2, 127.7, 132.2, 145.3 (Ar),
134.2 (C=CN).
Analysis cal. for C18H17N5 (303.36): C 71.27, H 5.65, 23.09; Found: C 70.99, H 5.64; N, 22.83.
References
- Katritzky, A.R.; Lan, X.; Yang, J.Z.; Denisko, O.V. Chem. Rev. 1998, 98, 409.
- Abramovich, R.A.; Spenser, I.D. Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Academic Press: New-York, 1973; Volume 3, pp. 80–207. [Google Scholar]
- Cox, E.D.; Cook, J.M. Chem. Rev. 1995, 1797–1842. [CrossRef]
Sample Availability: Available from the authors and MDPI. |
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.