Facile and Stereoselective Synthesis of Non-Racemic 3,3,3-Trifluoroalanine
Abstract
:Introduction
Results and Discussion
Experimental
General
Synthesis of N-p-Tolylsulfinyl-Imino-Triphenylphosphorane (S)-2
Reduction with DIBAH: synthesis of 3,3,3-trifluoro-2-(toluene-4-sulfinyl)amino-propionic acid ethyl esters 4,5
Reduction with DIBAH and ZnBr2
Reduction with NaBH4
Reduction with 9-BBN
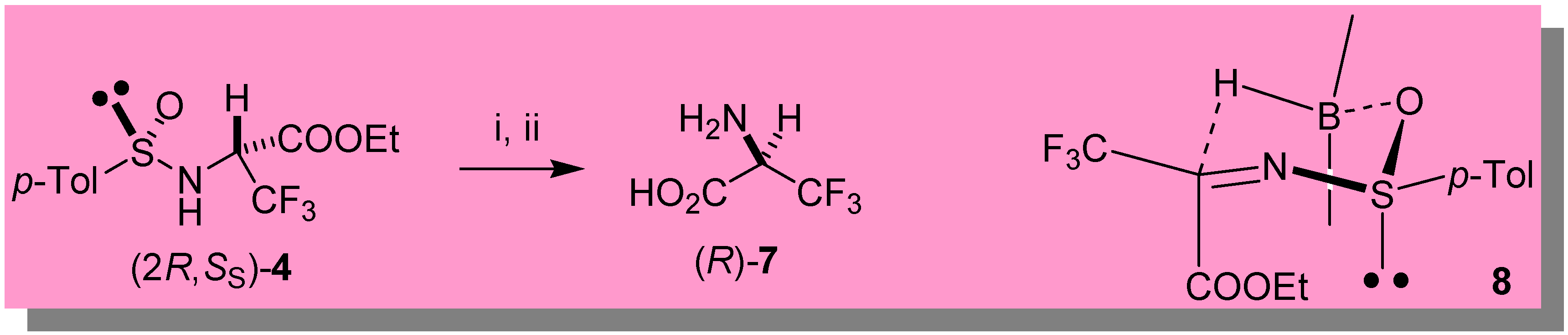
Direct synthesis of (R)-(+)-3,3,3-trifluoro-alanine 7
Acknowledgments
References and Notes
- Fluorine-containing Amino Acids: Synthesis and Properties; Kukhar, V. P.; Soloshonok, V. A. (Eds.) Wiley: Chichester, 1995.
- Welch, J. T.; Eswarakrishnan, S. Fluorine in Bioorganic Chemistry; Wiley: New York, 1991. [Google Scholar] Filler, R.; Kobayashi, Y.; Yagupolskii, L. M. Biomedical Aspects of Fluorine Chemistry; Elsevier: Amsterdam, 1993. [Google Scholar]
- Uneyama, K. Enantiocontrolled Synthesis of Fluoro-Organic Compounds: Stereochemistry, Challenges and Biomedicinal Targets; Soloshonok, V. A., Ed.; Wiley: Chichester, 1999; pp. 391–418. [Google Scholar] For some of our recent efforts in the field: Bravo, P.; Fustero, S.; Guidetti, M.; Volonterio, A.; Zanda, M. J. Org. Chem. 1999, 64, 8731–8735. Crucianelli, M.; Bravo, P.; Arnone, A.; Corradi, E.; Meille, S. V.; Zanda, M. J. Org. Chem. 2000, 65, 2965–2971. [PubMed]
- See Ref. 2a, pp.54-65.
- Bieth, J.; Dimicoli, J. L.; Wermuth, C. G.; Delalande, S. A. Ger. Offen., DE 2806833, 1978; Chem. Abstr. 1979, 90, 72447g. [Google Scholar] Höss, E.; Rudolph, M.; Seymour, L.; Schierlinger, C.; Burger, K. J. Fluorine Chem. 1993, 61, 163–170. and references therein. Osipov, S. N.; Sewald, N.; Kolomiets, A. F.; Fokin, A. V.; Burger, K. Tetrahedron Lett. 1996, 37, 615–618. Dessipri, E.; Tirrell, D. A. Macromolecules 1994, 27, 5463–5470. Bordusa, F.; Dahl, C.; Jakubke, H.-D.; Burger, K.; Koksch, B. Tetrahedron: Asymmetry 1999, 10, 307–313.
- Weygand, F.; Steglich, W.; Fraunberger, F. Angew. Chem. 1967, 79, 822. Weygand, F.; Steglich, W.; Oettmei, W. Chem. Ber. 1970, 103, 818–826. [PubMed]Soloshonok, V. A.; Kukhar, V. P. Tetrahedron 1997, 53, 8307–8314. and references therein. Burger, K.; Hoess, E.; Gaa, K.; Sewald, N.; Schierlinger, C. Z. Naturforsch. 1991, 46b, 361–384. and references therein.
- Sakai, T.; Yan, F.; Kashino, S.; Uneyama, K. Tetrahedron 1996, 52, 233–244. and references therein. Arnone, A.; Bravo, P.; Capelli, S.; Fronza, G.; Meille, S. V.; Zanda, M.; Cavicchio, G.; Crucianelli, M. J. Org. Chem. 1996, 61, 3375, Corrigenda: J. Org. Chem. 1996, 61, 9635.
- Sakai, T.; Yan, F.-Y.; Uneyama, K. Synlett 1995, 753–754. and references therein.
- Uneyama et al. described the enantioselective reduction of N-p-methoxyphenyl-imines of trifluoropyruvate with oxazaborolidine catalysts (see Ref. 7a and 8), producing (R)-7 with 62% e.e.
- Bravo, P.; Crucianelli, M.; Vergani, B.; Zanda, M. Tetrahedron Lett. 1998, 39, 7771–7774. Racemic 2 was also described: Senning, A.; Kelly, P. Naturwissenschaften 1968, 55, 543, Chem. Abstr. 70: 47555v. For an overview of the Staudinger (aza-Wittig) reaction: Staudinger, H.; Meyer, J. Helv. Chim. Acta 1919, 2, 635–646. Molina, P.; Vilaplana, M. J. Synthesis 1994, 1197–1218. Johnson, A. W.; Kasha, W. C.; Starzewsky, K. A. O.; Dixon, D. A. «Iminophosphoranes and Related Compounds». In Ylides and Imines of Phosphorus; New York: Wiley, 1993; pp. 403–483. [Google Scholar] (f) The use of benzene freshly distilled from Na was found to be important for minimizing the racemization of the sulfinimine 3 upon reaction with a variety of Grignard reagents (see Ref. 10a). For this reason the same conditions were used in the present work.
- Davis, F. A.; Reddy, R. E.; Szewczyk, J. M.; Reddy, G. V.; Portonovo, P. S.; Zhang, H.; Fanelli, D.; Reddy, R. T.; Zhou, P.; Carroll, P. J. J. Org. Chem. 1997, 62, 2555–2563. [PubMed] For a review on sulfinimines and sulfinamides: Davis, F. A.; Zhou, P.; Chen, B.-C. Chem. Soc. Rev. 1998, 27, 13–18.
- Bittner, S.; Assaf, Y.; Krief, P.; Pomerantz, M.; Ziemnicka, B. T.; Smith, C. G. J. Org. Chem. 1985, 50, 1712–1718.
- Brown, H. C.; Krishnamurthy, S. J. Org. Chem. 1975, 40, 1864–1865.
- A similar side-reaction has been observed for related fluorine-containing compounds: see Ref. 7b.
- Asensio, A.; Bravo, P.; Crucianelli, M.; Farina, A.; Fustero, S.; Soler, J. C.; Meille, S. V.; Panzeri, W.; Viani, F.; Volonterio, A.; Zanda, M. submitted for publication. See also: Fustero, S.; Navarro, A.; Pina, B.; Asensio, A.; Bravo, P.; Crucianelli, M.; Volonterio, A.; Zanda, M. J. Org. Chem. 1998, 63, 6210–6219. [PubMed]Bravo, P.; Fustero, S.; Guidetti, M.; Volonterio, A.; Zanda, M. J. Org. Chem. 1999, 64, 8731–8735.
- Hua, D.; Lagneau, N.; Wang, H.; Chen, J. Tetrahedron: Asymmetry 1995, 6, 349–352.
- In our hands, free 3,3,3-trifluoroalanine 7 was poorly stable. We observed complete decomposition of solid samples of 7, purified by ion-exchange chromatography, after one week at 4 °C. Racemic 7 is known to undergo progressive elimination of HF at pH > 6: Weygand, F.; Steglich, W.; Oettmeier, W. Chem. Ber. 1970, 103, 1655–1663. [PubMed] Therefore, we cannot rule out that a partial racemization of free 7 might take place during the purification or storage, even if short.
- Sample Availability: Samples of iminophosphoranes (R)- and (S)-2 are available from the authors.

| Entry | [H] | Conditions | Yield (%)a | D.r. 4/5 |
|---|---|---|---|---|
| 1 | DIBAH | THF, −70 °C | 52 | 4:1 |
| 2 | 9-BBN | THF, 0 °C | 78 | 1:20 |
| 3 | DIBAH/ZnBr2 | THF, r.t. to –70 °C | 58 | 2:1 |
| 4 | NaBH4 | Methanol, −70 °C | /b,c | /b,c |
© 2000 MDPI. All rights reserved.
Share and Cite
Crucianelli, M.; Battista, N.; Bravo, P.; Volonterio, A.; Zanda, M. Facile and Stereoselective Synthesis of Non-Racemic 3,3,3-Trifluoroalanine. Molecules 2000, 5, 1251-1258. https://doi.org/10.3390/51201251
Crucianelli M, Battista N, Bravo P, Volonterio A, Zanda M. Facile and Stereoselective Synthesis of Non-Racemic 3,3,3-Trifluoroalanine. Molecules. 2000; 5(12):1251-1258. https://doi.org/10.3390/51201251
Chicago/Turabian StyleCrucianelli, Marcello, Natalia Battista, Pierfrancesco Bravo, Alessandro Volonterio, and Matteo Zanda. 2000. "Facile and Stereoselective Synthesis of Non-Racemic 3,3,3-Trifluoroalanine" Molecules 5, no. 12: 1251-1258. https://doi.org/10.3390/51201251
APA StyleCrucianelli, M., Battista, N., Bravo, P., Volonterio, A., & Zanda, M. (2000). Facile and Stereoselective Synthesis of Non-Racemic 3,3,3-Trifluoroalanine. Molecules, 5(12), 1251-1258. https://doi.org/10.3390/51201251




