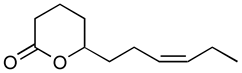
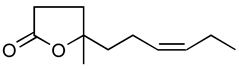
Abstract
This paper presents applicability of commonly available furan derivatives, like 2-methyl furan, furaldehyde and 5-methylfuraldehyde in syntheses of jasmanoides.
Introduction
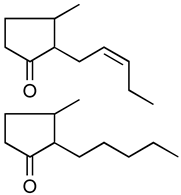
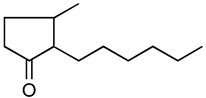
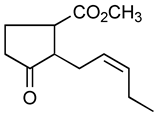
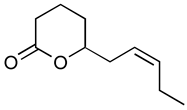
Jasmonoides are a relatively narrow, but very important group of synthetic fragrance substances. Owing to their relatively high prices, jasmonoides are only added to some more valuable fragrances compositions. Table 1 shows major representatives of that group [1,2].

Table 1.
Major jasmonoides.
Numerous methods of synthesis of jasmonoides have been developed due to their practical importance [3,4,5]. Most of these methods have only preparative significance. Among the many methods of synthe-sis, the most interesting ones are those involving furan derivatives. An advantage of these is the fact that they often use inexpensive intermediates, in particular furan intermediates such as furfuryl alcohol, furaldehyde and 2-methylfuran.
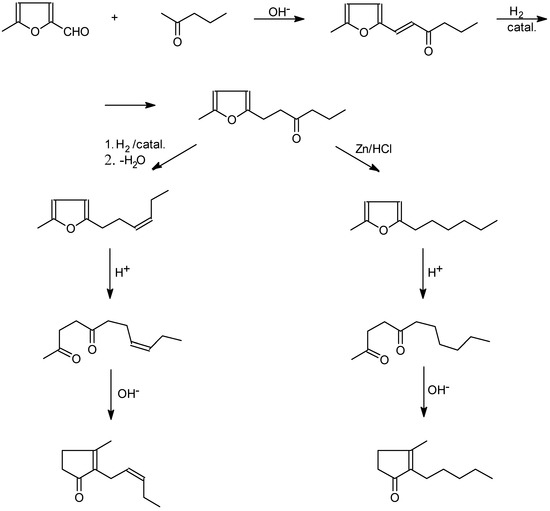
Such a possibility was first indicated in1942 by Hunsdiecker, who developed complete synthesis of jasmone and dihydrojasmone from 5-methylfuraldehyde [6,7] (Scheme 1).
Scheme 1.
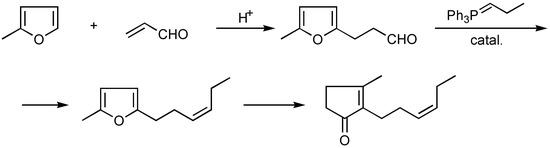
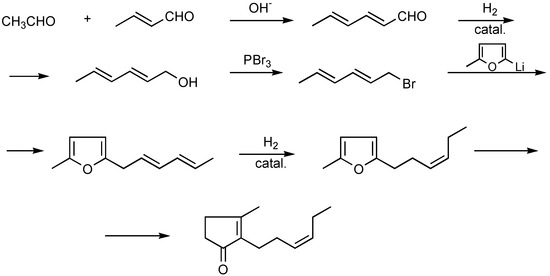
Nowadays, that method is only of historical interest, with its low yield of only 30% for the final products. Quite different was the method proposed by Zefirov et al., based on the readily available raw materials methylfuran and acrolein. Step I of the reaction produced a good yield of 5-methyl-furylpropionic aldehyde, which reacts with an ylide intermediate in a Wittig-Horner reaction to afford a corresponding 5-methyl-2-alkenylfuran, which in turn, after a sequence of conventional reactions, affords cis-jasmone [8,9] (Scheme 2).
Scheme 2.
A serious drawback of the method is the use of the Wittig-Horner reaction, which is hard to carry out on a larger scale. Besides, it usually gives a mixture of cis-trans isomers and the trans isomers have a fatty odour which make them is useless in perfume compositions. The later works of Crombie [10] and Fetizon [11,12] would enable reduction of the amount of trans isomer to 5% (using Na-tert-amylate as catalyst of Wittig - Horner reaction ). 5-Methyl-2-(cis)-hexene-3-ylfuran may also be obtained by direct methylfuran alkylation with cis-hexenyl bromide [13,14] ( Scheme 3 ) or by the method described by Furuchata et al. [15], where the following reaction sequence was employed (Scheme 4).
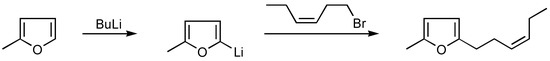
Scheme 3.
Scheme 4.
An important intermediate in the synthesis of jasmonoides is 5-methylfuraldehyde. It is obtained in greater than 90% yields from methylfuran, POCl3 and DMF by the Vilsmeiyer reaction [16].
Jasmone and dihydrojasmone may be synthesised from 5-methylfuraldehyde by two methods. One of them is based on a Grignard reaction with a corresponding alkyl magnesium halide, followed by rear-rangement to a cyclopentanone derivative [17,18,19] (Scheme 5).
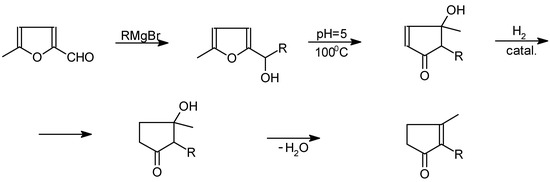
Scheme 5.
The rearrangement reaction is carried out under phase transfer catalyse conditions (PTC) in a slightly a-cidic medium, which enables elimination of the inconvenient step of catalytic hydrogenation [20]. An option is to effect the rearrangement reaction without PTC [21]. The process can be further simplified by omission of the Grignard reaction altogether. Methylfuran is condensed with an excess of aliphatic aldehyde in the presence of an acid catalyst to produce corresponding furylcarbinols [22] (Scheme 6).
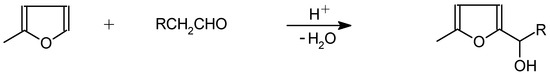
Scheme 6.
The other method is based on aldehyde hydrogenation to 5-methylfurfuryl alcohol, followed by its rearrangement and alkylation of the resulting methylcyclopentenolone [23,24] (Scheme 7).
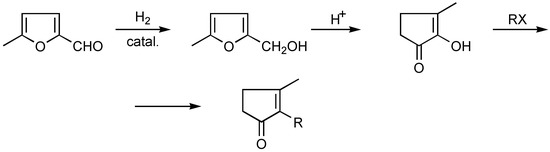
Scheme 7.
Alkylation of methylcyclopentenolone may be effected either conventionally [25] (Scheme 8), or under phase transfer catalysis (PTC) conditions [26,27] (Scheme 9).
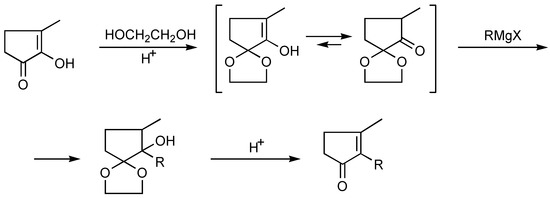
Scheme 8.
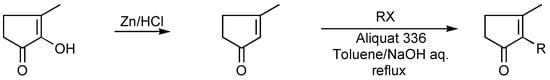
Scheme 9.
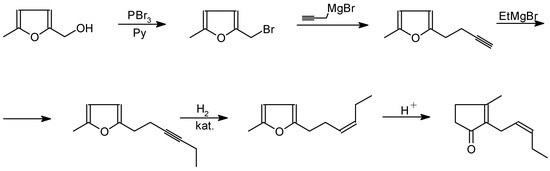
Scheme 10.
This method, however, has only limited preparative importance. Furaldehyde based syntheses are a separate group. In this case as well, the key step is the use of a Grignard reaction to produce alkylfu-rylcarbinols, followed by rearrangement into 2-alkylcyclopentenone derivatives [31,32,33,34] (Scheme 11).
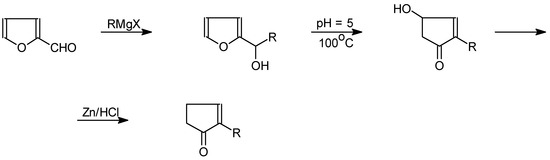
Scheme 11.
Alkylcyclopentenolone can also be obtained from furfuryl alcohol [35] (Scheme 12).
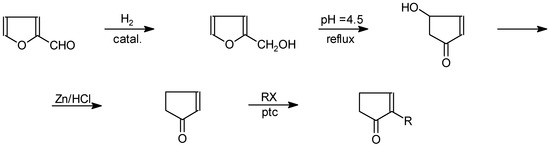
Scheme 12.
The resulting 2-alkylcyclopentenone can be alkylated with methyllithium to afford jasmone and its analogs [36] ( Scheme 13) or more simply, it can be converted directly into jasmonic acid esters, which can be hydrolysed and decarboxylated to give jasmone. This can be effected in a reaction of 2-alkylcyclopentenone and diethyl malonate [37,38] (Scheme 14).
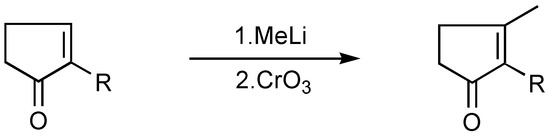
Scheme 13.
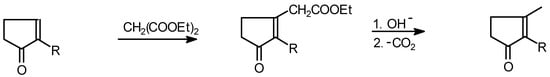
Scheme 14.
Jasmonate analogs are also obtained by 2-alkylcyclopentenone cyanation [39] (Scheme 15).
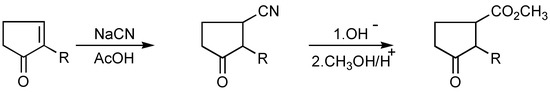
Scheme 15.
After hydrogenation of the double bond the resulting 2-alkylcyclopentanone can be oxidised to give jasmolactone derivatives [40] (Scheme 16).
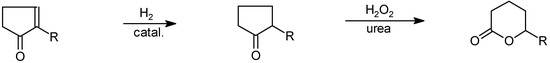
Scheme 16.
An analysis of the above methods of synthesis of jasmonoides from readily available furan derivatives such as furaldehyde, furfuryl alcohol but primarily 2-methylfuran, indicates that such methods may be competitive with traditionally used, commercial processes to synthesise such fragrant substances, especially in cases where inexpensive furan monomers are available. The relatively novel methods based on PTC may well be implemented in the commercial practice.
References
- Bedoukian, P.Z. Perfumery and Flavoring Synthetics; Allured Publ. Corp.: Wheaton, 1986. [Google Scholar]
- Voytkevich, S.A. 846 Dushistych Veshchestv dla Parfum. i Bytovoj Prom.-sti.; Izd.Pishch. Promst: Moscov, 1994. [Google Scholar]
- Van der Gen, A. Parf. Cosmet. Sav. France 1972, 2, 356.
- Kirtany, J.K. Pafai J. 1989, (10-12), 17.
- Kirtany, J.K. Pafa J. 1990, (1-3), 25.
- Hunsdiecker, H. Berichte 1942, 75, 447.
- Hunsdiecker, H. US Patent No. 2,387,587, 1945.
- Yuriev, Yu.K.; Zefirov, N.S. Zh. Obshch. Khim. 1964, 1150.
- Zefirov, N.S.; Kost’eykin, P.W.; Yuriev, Yu.K. Zh. Obshch. Chim. 1964, 1069.
- Crombie, L.; Hemesley, P.; Pattenden, G. J. Chem. Soc. 1969, 1025.
- Fetizon, M.; Chalbar, J. La France Perf. 1969, 57, 330.
- Schablar, J. DE Patent No. 1,959,513, 18 Jun 1969.
- Buchi, G.; Wuest, H. J. Org. Chem. 1966, 31, 977.
- Shono, T.; Matsumura, Y.; Hamaguchi, H.; Nakamura, K. Chem. Lett. 1976, 1249.
- Furuchata, A.; Onishi, K.; Fujita, A.; Kogami, K. Agric. Biol. Chem. 1982, 46, 1757.
- Joule, J.A.; Smith, G.F. Chemistry and Technology of Heterocyclic Compounds; PWN: Warsaw, 1984. [Google Scholar]
- Screti, A.; Piancatelli, G.; D’Auria, M.; David, G. Tetrahedron 1979, 35, 135.
- Minai, M. DE Patent No. 3,122,995, 25 Mar 1982.
- Saito, K.; Yamachika, H. EP Patent No. 35,060, 11 May 1983.
- Saito, K. EP Patent No. 31,909, 15 Jun 1981.
- Minai, M. JP Patent No. 99,546, 21 Jun 1982. CA 1982, 97, 197,855.
- Minai, M.; Moriyama, S.; Katsura, T.; Suita, O. DE Patent No. 3,338,853, 21 Oct 1984.
- Minai, M. JP Patent No. 136,544, 23 Aug 1982. CA 1983, 98, 71546.
- Shono, T.; Matsumura, Y. J. Chem. Soc. Chem. Commun. 1970, 20, 712.
- Erickson, J.L.; Collins, F.E., Jr. J. Org. Chem. 1965, 30, 1050. [CrossRef]
- Wiegers, W.J.; Hall, J.B. US Patent No. 4,045,489, 30 Aug 1976.
- Wiegers, W.J.; Hall, J.B. US Patent No. 4,265,836, 5 May 981.
- Sarkar, K.A. J.Chem. Res. Synops. 1992, (12), 418.
- Sisido, K.; Kawasamo, Y. Parf. Ess. Oil Report 1966, (6), 364.
- Shishino, K.; Kawashima, Y. JP Patent No. 14,216, 27 Apr 1972. CA 1972, 77, 88724.
- Shishino, K.; Kawashima, Y. JP Patent No. 14,217, 27 Apr 1972. CA 1972, 77, 62176.
- Piancatelli, G.; Screti, A. Tetrahedron Lett. 1976, 39, 3555. [CrossRef]
- Minai, M. JP Patent No. 188,540, 19 Nov 1982. CA 1983, 98, 178812.
- Saito, K.; Takisawa, Y.; Yamachika, H. EP Patent No. 53,842, 25 Jun 1982.
- Minai, M. JP Patent No. 62,236, 15 Apr 1982. CA 1983, 98, 106860.
- Zhujin, L.; Guobin, R. Synth. Commun. 1986, 16, 871.
- Trost, B.M.; Verhoeven, J. J. Am. Chem. Soc. 1980, 102, 4730. [CrossRef]
- Kitahara, T.; Hamaguchi, K.; Warita, Y.; Takagaki, Y.; Mori, K. Agric. Biol. Chem. 1986, 50, 1867.
- Mookherjee, B.D.; Wilson, R.A.; Schmitt, F.L.; Vock, M.H. US Patent No. 4,331,611, 23 Oct 1980.
- Jingyao, Z.; Qing, Y. Huaxue Shijie 1986, 27, 207, CA 1987, 107, 119614.
© 2000 by MDPI (http://www.mdpi.org). All rights reserved